Submitted:
30 October 2023
Posted:
31 October 2023
You are already at the latest version
Abstract
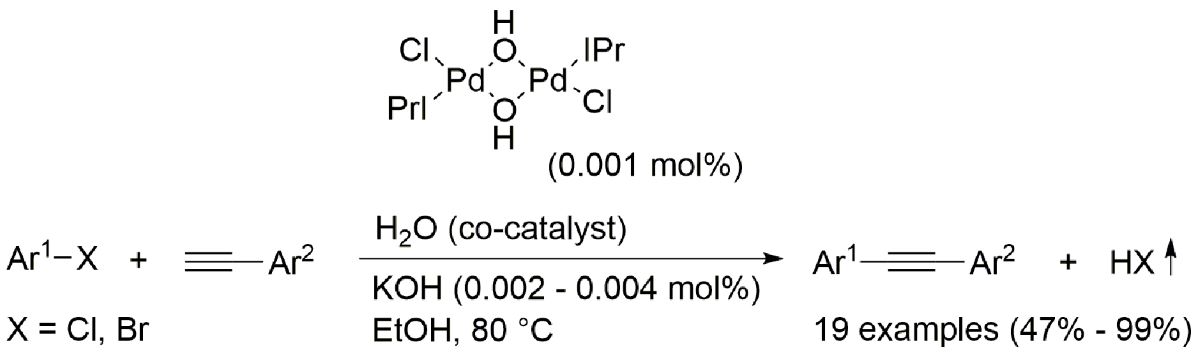
Keywords:
1. Introduction
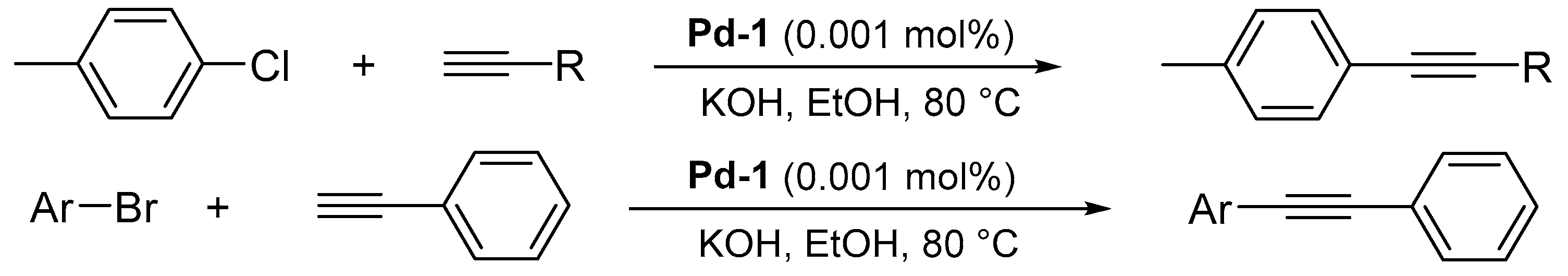
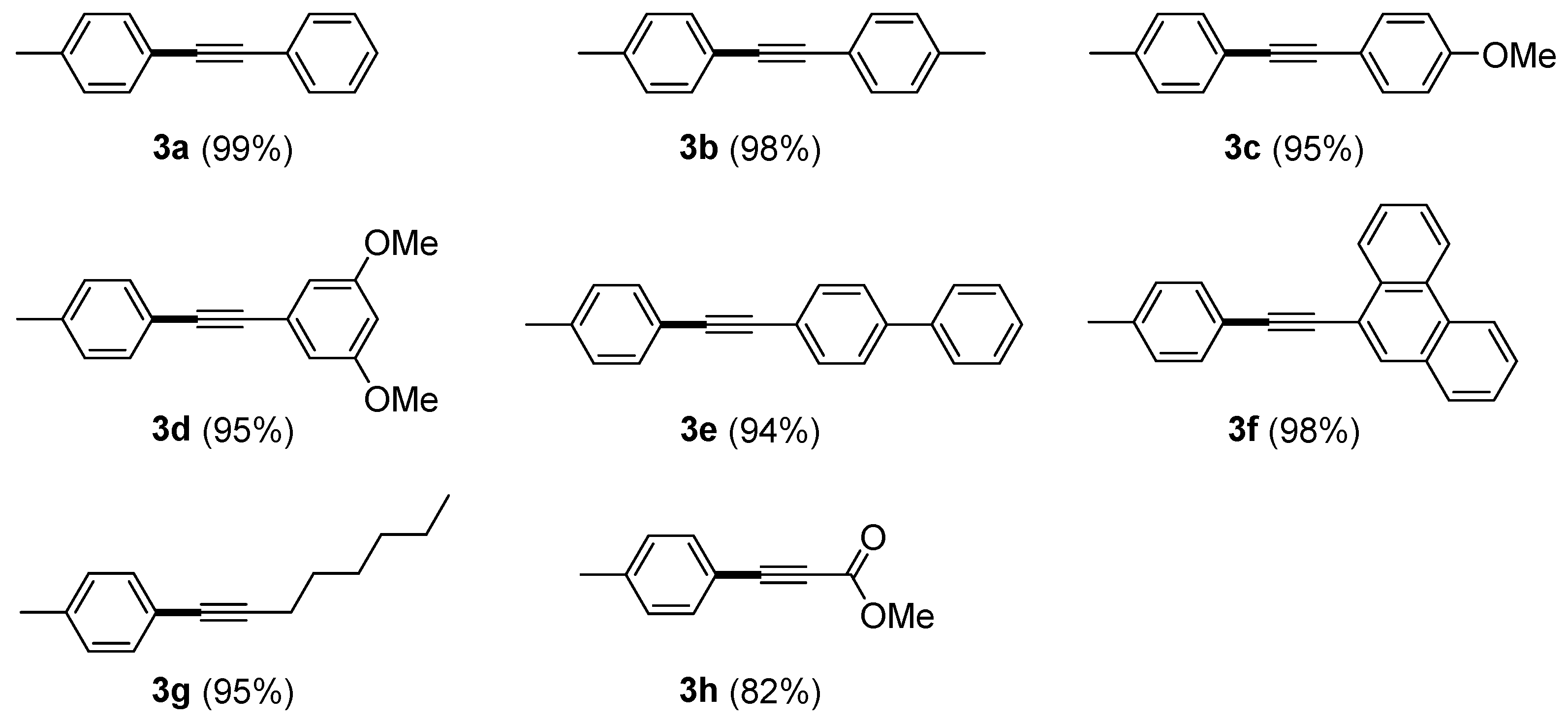
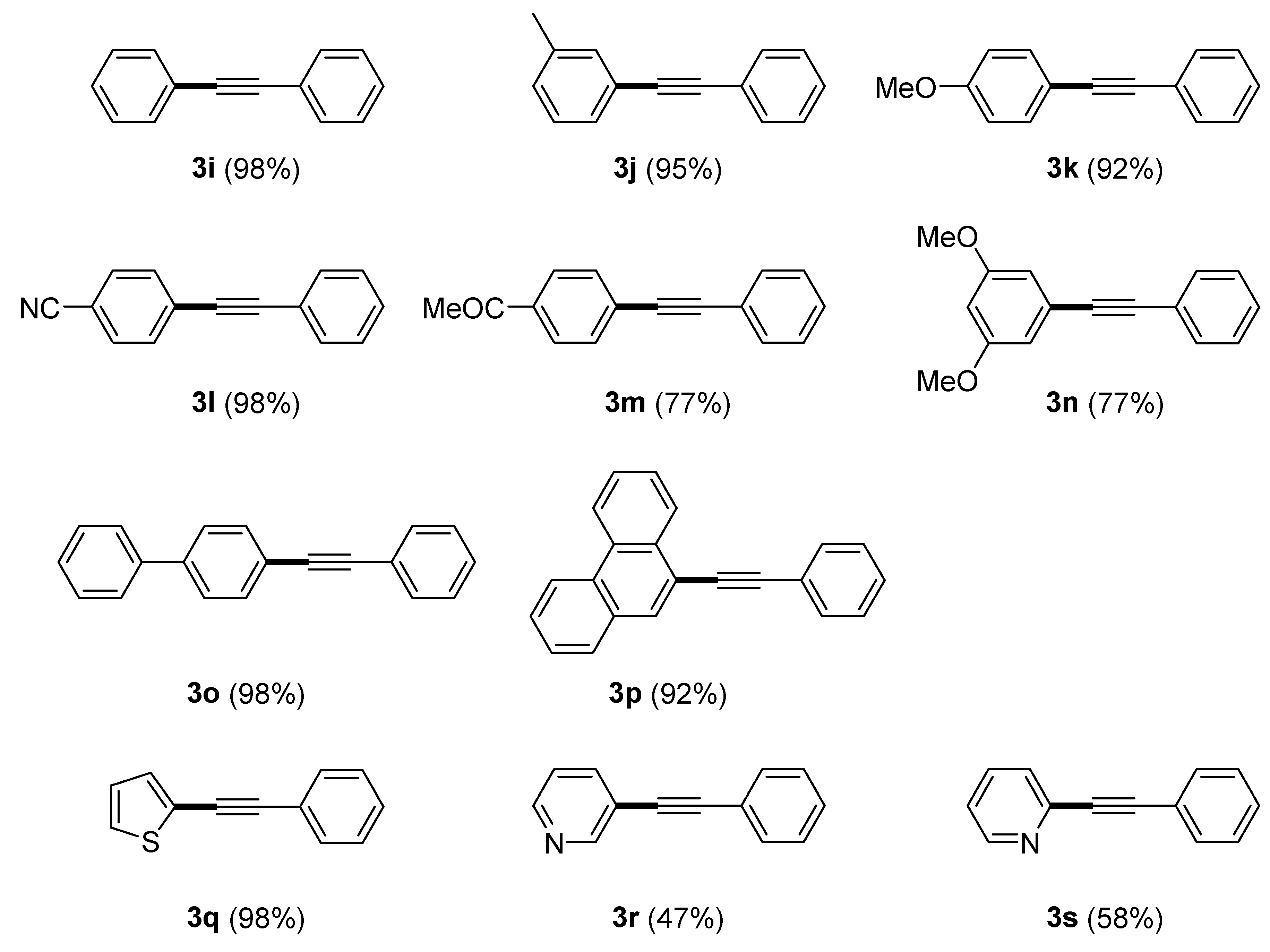
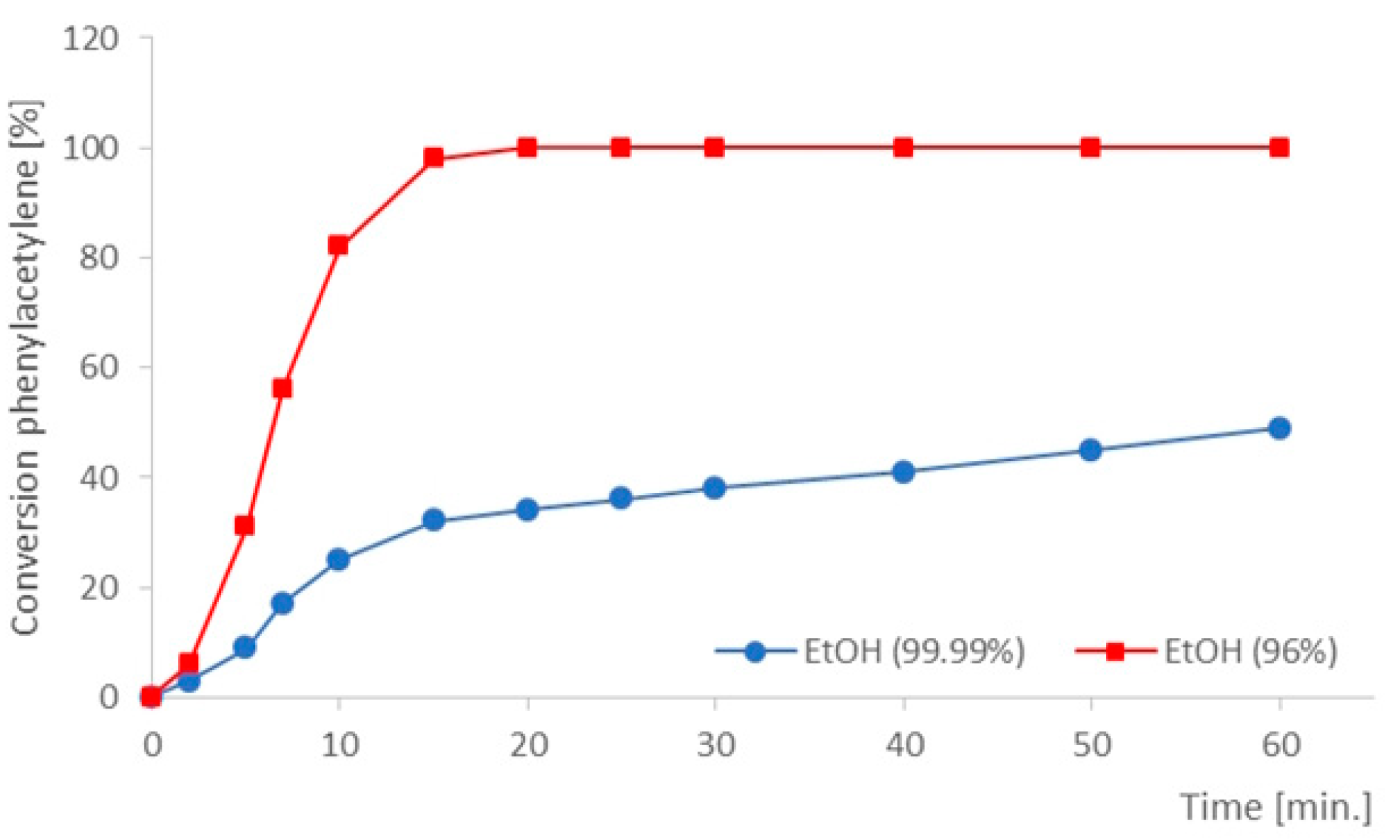
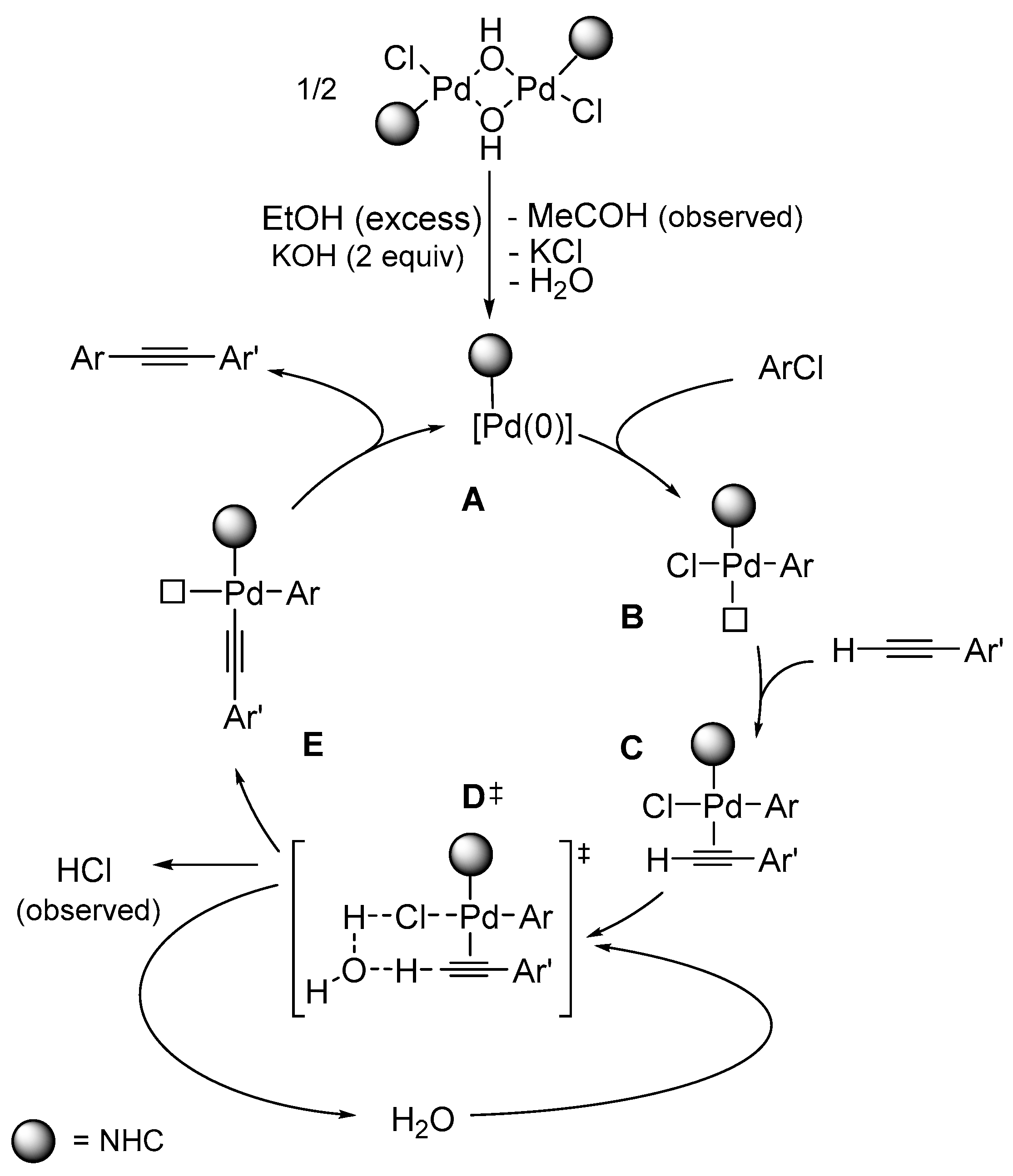
2. Results and discussion
3. Conclusions
4. Experimental
Representative procedure for catalytic test.
General procedure for the synthesis of Sonogashira cross-coupling products
General procedure for the synthesis of Sonogashira cross-coupling products
References
- Chinchilla, R.; Najera, C. The Sonogashira reaction: A booming methodology in synthetic organic chemistry. Chem. Rev. 2007, 107, 874–922. [Google Scholar] [CrossRef] [PubMed]
- Chinchilla, R.; Najera, C. Recent advances in Sonogashira reactions. Chem. Soc. Rev. 2011, 40, 5084–5121. [Google Scholar] [CrossRef]
- Karak, M.; Barbosa, L.C.A.; and Hargaden, G.C. Recent mechanistic developments and next generation catalysts for the Sonogashira coupling reaction. RSC Adv. 2014, 4, 53442–53466. [Google Scholar] [CrossRef]
- Kanwal, I.; Mujahid, A.; Rasool, N.; Rizwan, K.; Malik, A.; Ahmad, G.; Shah, S.A.A.; Rashid, U.; Nasir, N.M. Palladium and copper catalyzed Sonogashira cross coupling an excellent methodology for C-C bond formation over 17 years: A review. Catalysts 2020, 10, 443–491. [Google Scholar] [CrossRef]
- Choy, P.Y.; Gan, K.B.; Kwong, F.Y. Recent expedition in Pd catalyzed Sonogashira coupling and related processes. Chinese J. Chem. 2023, 41, 1099–1118. [Google Scholar] [CrossRef]
- Akhtar, R.; Zahoor, A.F.; Parveen, B.; Suleman, M. Development of environmental friendly synthetic strategies for Sonogashira cross coupling reaction: An update. Synth. Commun. 2019, 49, 167–192. [Google Scholar] [CrossRef]
- Roy, D.; Uozumi, Y. Recent advances in palladium-catalyzed cross-coupling reactions at ppm to ppb molar catalyst loadings. Adv. Synth. Catal. 2018, 360, 602–625. [Google Scholar] [CrossRef]
- Deraedt, C.; Astruc, D. “Homeopathic” palladium nanoparticle catalysis of cross carbon-carbon coupling reactions. Acc. Chem. Res. 2014, 47, 494–503. [Google Scholar] [CrossRef]
- Mohajer, F.; Heravi, M.M.; Zadsirjan, V.; Poormohammad, N. Copper-free Sonogashira cross-coupling reactions: an overview. RSC Adv. 2021, 11, 6885–6925. [Google Scholar] [CrossRef]
- Cassar, L. Synthesis of aryl- and vinyl-substituted acetylene derivatives by the use of nickel and palladium complexes. J. Organomet. Chem. 1975, 93, 253–257. [Google Scholar] [CrossRef]
- Dieck, H.A.; Heck, F.R. Palladium catalyzed synthesis of aryl, heterocyclic, and vinylic acetylene derivatives. J. Organomet. Chem. 1975, 93, 259–263. [Google Scholar] [CrossRef]
- Bakherad, M. Recent progress and current applications of Sonogashira coupling reaction in water. Appl. Organomet. Chem. 2013, 27, 125–140. [Google Scholar] [CrossRef]
- Struwe, J.; Ackermann, L.; Gallou, F. Recent progress in copper-free Sonogashira-Hagihara cross-couplings in water. Chem. Catal. 2023, 3, 100485. [Google Scholar] [CrossRef]
- Gelman, D.; Buchwald, S.L. Efficient palladium-catalyzed coupling of aryl chlorides and tosylates with terminal alkynes: Use of a copper cocatalyst inhibits the reaction. Angew. Chem. Int. Ed. 2003, 42, 5993–5996. [Google Scholar] [CrossRef]
- Sabounchei, S.J.; Ahmadi, M. An efficient protocol for copper- and amine-free Sonogashira reactions catalyzed by mononuclear palladacycle complexes containing bidentate phosphine ligands. Catal. Commun. 2013, 37, 114–121. [Google Scholar] [CrossRef]
- Zhou, R.; Wang, W.; Jiang, Z.-J.; Fu, H.-Y.; Zheng, X.-L.; Zhang, C.-C.; Chen, H.; Li, R.-X. Pd/tetraphosphine catalytic system for Cu-free Sonogashira reaction “on water”. Catal. Sci. Technol. 2014, 4, 746–751. [Google Scholar] [CrossRef]
- Zhou, R.; Wang, W.; Jiang, Z.-J.; Wang, K.; Zheng, X.-L.; Fu, H.-Y.; Chen, H.; Li, R.-X. One-pot synthesis of 2-substituted benzo[b]furans via Pd–tetraphosphine catalyzed coupling of 2-halophenols with alkynes. Chem. Commun. 2014, 50, 6023–6026. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-H.; Kwon, Y.-J.; Jin, M.-J. Highly Active palladium catalyst for the Sonogashira coupling reaction of unreactive aryl chlorides. Adv. Synth. Catal. 2011, 353, 3090–3094. [Google Scholar] [CrossRef]
- Lee, D.-H.; Qiu, H.; Cho, M.-H.; Lee, I.-M.; Jin, M.-J. Efficient Sonogashira coupling reaction catalyzed by Palladium(II) β-oxoiminatophosphane complexes under mild conditions. Synlett 2008, 11, 1657–1660. [Google Scholar] [CrossRef]
- Feuerstein, M.; Doucet, H.; Santelli, M. Sonogashira cross-coupling reactions of aryl chlorides with alkynes catalysed by a tetraphosphine–palladium catalyst. Tetrahedron Lett. 2004, 45, 8443–8446. [Google Scholar] [CrossRef]
- Roy, S.; Plenio, H. Sulfonated N-heterocyclic carbenes for Pd-catalyzed Sonogashira and Suzuki–Miyaura coupling in aqueous solvents. Adv. Synth. Catal. 2010, 352, 1014–1022. [Google Scholar] [CrossRef]
- Liu, Q.-X.; Cai, K.-Q.; Zhao, Z.-X. Synthesis, structure and catalysis of a NHC–Pd(II) complex based on a tetradentate mixed ligand. RSC Adv. 2015, 5, 85568–85578. [Google Scholar] [CrossRef]
- Ostrowska, S.; Lorkowski, J.; Kubicki, M.; Pietraszuk, C. [{Pd(μ-OH)Cl(IPr)}2] - A highly efficient precatalyst for Suzuki–Miyaura coupling also able to act under base-free conditions. ChemCatChem 2016, 8, 3580–3583. [Google Scholar] [CrossRef]
- Pietraszuk, C.; Ostrowska, S.; Rogalski, S.; Lorkowski, J.; Walkowiak, J. Efficient Homocoupling of aryl- and alkenylboronic acids in the presence of low loadings of [{Pd(μ–OH)Cl(IPr)}2]. Synlett 2018, 29, 1735–1740. [Google Scholar] [CrossRef]
- Ostrowska, S.; Szymaszek, N.; Pietraszuk, C. Selective dimerization of terminal acetylenes in the presence of PEPPSI precatalysts and relative chloro- and hydroxo-bridged N-heterocyclic carbene palladium dimers. J. Organomet. Chem. 2018, 856, 63–69. [Google Scholar] [CrossRef]
- Hadei, N.; Kantchev, E.A.B.; O'Brien, C.J.; Chass, G.; Hunter, H.H.; Penner, G.; Hopkinson, A.C.; Organ, M.G. Rational catalyst design and its application in sp3-sp3 couplings. 230th ACS National Meeting. Washington, DC, American Chemical Society, 2005, pp Abstract 308.
- Nasielski, J.; Hadei, N.; Achonduh, G.; Kantchev, E.A.B.; O’Brien, C.J.; Organ, M.G. Structure–activity relationship analysis of Pd–PEPPSI complexes in cross-couplings: A close inspection of the catalytic cycle and the precatalyst activation model. Chem. Eur. J. 2010, 16, 10844–10853. [Google Scholar] [PubMed]
- Hussain, A.; Yousuf, S.K.; Kumar, D.; Lambu, M.; Singh, B.; Maity, S.; Mukherjee, D. Adv. Synth. Catal. 2012, 354, 1933–1940. [CrossRef]
- Min, Q.; Miao, P.; Chu, D.; Liu, J.; Qi, M.; Kazemnejadi, M. Introduction of a recyclable basic ionic solvent with bis-(NHC) ligand property and the possibility of immobilization on magnetite for ligand- and base-free Pd-catalyzed Heck, Suzuki and Sonogashira cross-coupling reactions in water. Catal. Lett. 2021, 151, 1–18. [Google Scholar] [CrossRef]
- Tong, Z.; Gao, P.; Deng, H.; Zhang, L. Xu, P.-F.; Zhai, H. Propylene oxide assisted Sonogashira coupling reaction. Synlett 2008, 20, 3239–3241. [Google Scholar]
- Dewan, A.; Sarmah, M.; Bora, U.; Thakur, A. J. A green protocol for ligand, copper and base free Sonogashira cross-coupling reaction. Tetrahedron Lett. 2016, 57, 3760–3763. [Google Scholar] [CrossRef]
- Armarego, W.L.F.; Chai, C.L.L. Purification of laboratory chemicals. Elsevier: Amsterdam, 2003, pp 231–232. (Reaction with magnesium ethoxide).
- García-Melchor, M.; Pacheco, M.C.; Nájera, C.; Lledós, A.; Ujaque, G. Mechanistic exploration of the Pd-catalyzed copper-free Sonogashira reaction. ACS Catal. 2012, 2, 135–144. [Google Scholar] [CrossRef]
- Melvin, P.R.; Balcells, D.; Hazari, N.; Nova, A. Understanding Precatalyst Activation in Cross-Coupling Reactions: Alcohol Facilitated Reduction from Pd(II) to Pd(0) in Precatalysts of the Type (η3-allyl)Pd(L)(Cl) and (η3-indenyl)Pd(L)(Cl). ACS Catal. 2015, 5, 5596–5606. [Google Scholar] [CrossRef]
- Raja, G.C.E.; Irudayanathan, F.M.; Kim, H.; Kim, J.; Lee, S. Nickel-catalyzed Hiyama-type decarboxylative coupling of propiolic acids and organosilanes. J. Org. Chem. 2016, 81, 5244–5249. [Google Scholar] [CrossRef] [PubMed]
- Truong, T.; Daugulis, O. Transition-metal-free alkynylation of aryl chlorides. Org. Lett. 2011, 13, 4172–4175. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Jeong, K.H.; Kim, Y.; Noh, T.; Choi, J.; Ham, J. Three-component one-pot synthesis of unsymmetrical diarylalkynes by thermocontrolled sequential Sonogashira reactions using potassium ethynyltrifluoroborate. Eur. J. Org. Chem. 2017, 2017, 2425–2431. [Google Scholar] [CrossRef]
- Li, X.; Yang, F.; Wu, Y. Palladacycle-catalyzed decarboxylative coupling of alkynyl carboxylic acids with aryl chlorides under air. J. Org. Chem. 2013, 78, 4543–4550. [Google Scholar] [CrossRef]
- Sardarian, A.R.; Kazemnejadi, M.; Esmaeilpour, M. Bis-salophen palladium complex immobilized on Fe3O4@SiO2 nanoparticles as a highly active and durable phosphine-free catalyst for Heck and copper-free Sonogashira coupling reactions. Dalton Trans. 2019, 48, 3132–3145. [Google Scholar] [CrossRef]
- Gallop, C.W.D.; Chen, M.; Navarro, O. Sonogashira couplings catalyzed by collaborative (N-heterocyclic carbene)-copper and -palladium complexes. Org. Lett. 2014, 16, 3724–3727. [Google Scholar] [CrossRef]
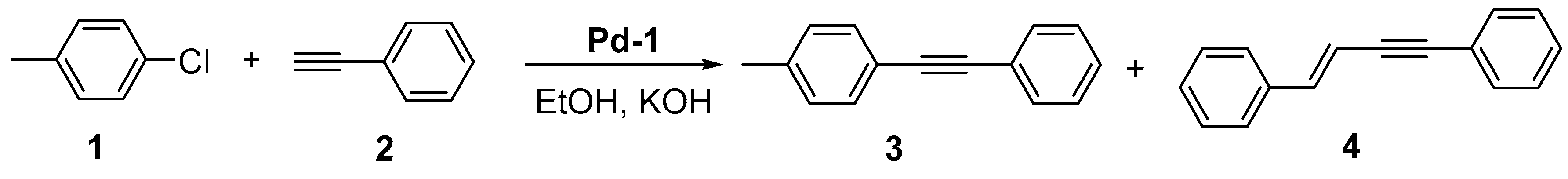
| Entry | [C7H7Cl]/[≡] | [Pd]/[KOH] | Temp. [°C] |
Time [h] |
Conv. of 2 [%] |
Yield [%] |
|---|---|---|---|---|---|---|
| 1 | 1/1 | base-free | 22 | 24 | 0 | - |
| 2 | 1/1 | base-free | 80 | 24 | 84 | 4 (100) |
| 3 | 1/1 | 1/2 | 22 | 24 | 91 | 4 (100) |
| 4 | 5/1 | 1/2 | 22 | 24 | 91 | 4 (100) |
| 5 | 5/1 | 1/2 | 40 | 24 | 93 | 3 (traces); 4 (98) |
| 6 | 5/1 | 1/2 | 70 | 24 | 98 | 3 (18); 4 (80), |
| 7 | 5/1 | 1/2 | 80 | 0.25 | >99 | 3 (100) |
| 8 | 2/1 | 1/2 | 80 | 0.25 | >99 | 3 (60); 4 (40) |
| Entry | Pd-1 [mol%] |
[Pd]/[KOH] | Time [h] |
Conv. of 2 [%] |
Selectivity of 3 [%] | TON |
|---|---|---|---|---|---|---|
| 1 | 0.1 | 1/2 | 0.25 | >99 | 100 | 1000 |
| 2 | 0.01 | 1/2 | 0.25 | >99 | 100 | 10000 |
| 3 | 0.001 | 1/4 | 24 | 97 | 100 | 100000 |
| 4 | 0.0001 | 1/4 | 24 | 56 | 100 | 560000 |
| Entry | Solvent | Time [h] |
Conversiona [%] |
Selectivity of 3 [%] |
|---|---|---|---|---|
| 2 | EtOH | 0.25 | 100 | 100 |
| 3 | EtOH/H2O (1/1) | 0.25 | 100 | 100 |
| 4 | MeOHb | 15 | 94 | 100 |
| 5 | i-PrOH | 24 | 99 | 100 |
| 6 | DMF | 0.25 | 89 | 100 |
| 7 | THF | 24 | 21 | 10 |
| 8 | CHCl3b | 24 | 11 | 30 |
| 9 | toluene/H2O (9/1) | 24 | 14 | 100 |
| Entry | Base | Time [h] | Conversiona [%] | Selectivity of 3 [%] |
|---|---|---|---|---|
| 1 | KOH | 0.25 | 100 | 100 |
| 2 | NaOH | 24 | 0 | - |
| 3 | K2CO3 | 24 | 12 | 40 |
| 4 | Cs2CO3 | 24 | 21 | 100 |
| 5 | TBAF | 24 | 9 | - |
| 6 | KOt-Bu | 24 | 74 | - |
| Entry | Catalyst | Conv. of 2 [%] | Selectivity of 3 [%] |
|---|---|---|---|
| 1 | [{Pd(μ-OH)(Cl)(IPr)}2] (Pd-1) | 100a | 100a |
| 2 | [{Pd(μ-OH)Cl(SIPr)}2] (Pd-2) | 100a | 100a |
| 3 | [{Pd(μ-OH)Cl(IMes)}2] (Pd-3) | 68a (88) | 100 |
| 4 | [{Pd(μ-OH)Cl(SIMes)}2] (Pd-4) | 48a (61) | 100 |
| 5 | [{Pd(μ-Cl)Cl(IPr)}2] (Pd-5) | 73 | 100 |
| 6 | [{Pd(μ-Cl)Cl(SIPr)}2] (Pd-6) | 82 | 100 |
| 7 | [{Pd(μ-Cl)Cl(IMes)}2] (Pd-7) | 44 | 10; 4 (90) |
| 8 | [{Pd(μ-Cl)Cl(SIMes)}2] (Pd-8) | 45 | 4 (100) |
| 9 | [PdCl2(IPr)(3-chloropyridine)] (Pd-9) | 55 | 30; 4 (70) |
| 10 | [PdCl2(SIPr)(3-chloropyridine)] (Pd-10) | 58 | 50; 4 (50) |
| 11 | [PdCl2(IMes)(3-chloropyridine)] (Pd-11) | 59 | 30; 4 (70) |
| 12 | [PdCl2(SIMes)(3-chloropyridine)] (Pd-12) | 53 | 4 (100) |
| 13 | PdCl2 | 0 | - |
| 14 | Pd(OAc)2 | 0 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





