Submitted:
26 October 2023
Posted:
30 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Setting, Period, and Design
2.2. Sample processing for Light-emitting diode fluorescent microscopy
2.3. Expectorated Sputum Sample processing for Xpert MTB/RIF assay
2.4. Lymph nodes and other tissues sample processing for Xpert MTB/RIF assay
2.5. Processing of Non-sterile Lymph nodes and other tissues for Xpert MTB/RIF assay
2.6. Sterile collection of Lymph nodes and other tissues for Xpert/MTB/RIF assay
2.7. Processing of CSF samples for Xpert MTB/RIF assay
2.8. DNA Extraction using GenoLyse for MTBDRplus VER 2.0 assay
2.9. Hybridization for First-line drugs
2.10. DNA Extraction using GenoLyse for MTBDRsl VER 2.0 assay
2.11. Hybridization for second line drugs
3. Results
4. Discussion
- Early diagnosis.
- Novel case-finding methods beyond healthcare facilities.
- Shorter and simpler successful treatment regimens for drug-sensitive and drug-resistant tuberculosis.
- A greater focus on prevention strategies.
- Steps to reduce mortality and transmission in adults and children.
5. Conclusions
Author Contributions
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Global tuberculosis report 2022. Geneva: World Health Organization; 2022 (https://www.who.int/publications/i/item/9789240037021).
- Dean, A.S.; et al. 25 years of surveillance of drug-resistant tuberculosis: achievements, challenges, and way forward. Lancet Infect Dis .2022, 22(7), e191-E196. [CrossRef]
- Global tuberculosis report 2020. Geneva: World Health Organization; 2020 (https://www.who.int/publications/i/item/9789240037021).
- Nandlal, L.; Perumal, R.; Naidoo, K. Rapid Molecular Assays for the Diagnosis of Drug-Resistant Tuberculosis. Infect Drug Resist. 2022, 15, 4971-4984. [CrossRef]
- Tamirat, K.S.; Kebede, F.B.;Baraki, A.G.; Akalu, T.Y. The Role of GeneXpert MTB/RIF in Reducing Treatment Delay Among Multidrug Resistance Tuberculosis Patients: A Propensity Score Matched Analysis. Infect Drug Resist. 2022, 15,285-294. [CrossRef]
- Pongpeeradech,N.;Kasetchareo,Y.; Chuchottaworn,C.; Lawpoolsri,S.; Silachamroon, U.; Kaewkungwal, J. (2022) Evaluation of the use of GeneXpert MTB/RIF in a zone with high burden of tuberculosis in Thailand. PLoS ONE.2022, 17(7), e0271130. [CrossRef]
- International Union against Tuberculosis and Lung Disease. The Public Health Service National Tuberculosis Reference Laboratory and the National Laboratory Network. Paris: International Union Against Tuberculosis and Lung Disease; 1998.
- Kabir, S.; Parash, M.T.H.; Emran, N.A.; Hossain, A.B.M.T.; Shimmi, S.C. Diagnostic challenges and Gene-Xpert utility in detecting Mycobacterium tuberculosis among suspected cases of Pulmonary tuberculosis. PLoS ONE.2021, 16(5): e0251858. [CrossRef]
- Mukhida, S.; Vyawahare, C.R.; Mirza, S.B.; Gandham, N.R; Khan, S.; Kannuri, S.; Role of GeneXpert MTB/RIF assay for the diagnosis of cervical lymph node tuberculosis and rifampicin resistance. Tzu Chi Med J. 2022, 34(4),418-422. [CrossRef]
- Xpert MTB/RIF system for the diagnosis of pulmonary and extra-pulmonary TB and rifampicin resistance in adults and children. A pre-publication version of the policy guidance may be accessed at: http://www.stoptb.org/wg/gli/assets/documents/WHO Policy Statement on Xpert MTB-RIF 2013 pre-publication 22102013.pdf.
- Smita, S.S.; Venkatesh, K.; Usharani, B.; Anbazhagi, S.; Vidya Raj,C.K.; Chitra,A.; Muthuraj, M. Prevalence and factors associated with multidrug-resistant tuberculosis in South India.Sci Rep.2020, 10,17552. [CrossRef]
- C.K. Vidyaraj,C.K.; Chitra,A.; Smita,S.; Muthuraj,M.; Govindarajan,S.; Usharani,B.; Anbazhagi,A. Prevalence of rifampicin-resistant Mycobacterium tuberculosis among human-immunodeficiency-virus-seropositive patients and their treatment outcomes. J Epidemiol Glob Health.2017,7(4), 289 - 294. [CrossRef]
- Aaina, M.; Venkatesh, K.; Usharani, B.; Anbazhagi, M.; Rakesh, G.; Muthuraj, M. Risk Factors and Treatment Outcome Analysis Associated with Second-Line Drug-Resistant Tuberculosis. J Respir. 2022, 2, 1–12. [CrossRef]
- Sinshaw, W.; Kebede ,A.; Bitew, A.; Tesfaye, E.; Tadesse, M.; Mehamed, Z. Prevalence of tuberculosis, multidrug resistant tuberculosis and associated risk factors among smear negative presumptive pulmonary tuberculosis patients in Addis. BMC Infect Dis. BMC Infect Dis. 2019, 19, 1-15. [CrossRef]
- Wasihun, A.G.; Dejene, T.A.; Hailu, G.G. Frequency of MTB and rifampicin resistance MTB using Xpert-MTB/RIF assay among adult presumptive tuberculosis patients in Tigray, Northern Ethiopia: A cross sectional study. PLoS ONE.2020, 15(11), e0240361. [CrossRef]
- Shiv, K.S.; Pramod, R.B.; Anjana, S.; Deepak, D.; Deepa, G.; Renu, S.; Rifampicin-resistant Mycobacterium tuberculosis by GeneXpert MTB/RIF and Associated Factors among Presumptive Pulmonary Tuberculosis Patients in Nepal. Infect Drug Resist. 2020,13, 2011-2019. [CrossRef]
- William,A.; Yogita Rai,Y.; Ravinder Kaur,R. Evaluation of Rifampicin-resistant Tuberculosis in Pediatric Patients by GeneXpert MTB/RIF. J Microbiol Infect Dis. 2021, 11 (2), 81-87. [CrossRef]
- Zhua,W.; Wanga,Y.; Lic,T.; Chenc,W.; Wang,W. Gap to End-TB targets in eastern China: A joinpoint analysis from population-based notification data in Zhejiang Province, China, 2005–2018. Int J Infect Dis.2021, 104, 407-414. [CrossRef]
- Ibrahim, M.M.; Tom, M.I.; Umoru, M.A.; Jidda, B.U.; Mustafa A. I.; Adam, M.; Akbar, S.Trends in the incidence of Rifampicin resistant Mycobacterium tuberculosis infection in northeastern Nigeria. Sci Afr, 2022, 17, e01341. [CrossRef]
- Peter, O.; Ikuabe. I.D.E. Assay in patients with pulmonary tuberculosis in Yenagoa, Nigeria. Pan Afr Med J. 2018, 29, 1-4.
- Nemagouda, S.K. A three-year experience with genexpert MTB/RIF assay in tuberculosis control programme (RNTCP)- a clinical study. J Evolution Med Dent Sci. 2019,8(41),3080-3083. [CrossRef]
- Anwar, S.K.; Sajid Ali , Muhammad Tahir Khan , Sajjad Ahmed , Yasir Khattak, Abduljabbar , Muhammad Irfan , Wasim Sajjad 2018. Comparison of GeneXpert MTB/RIF assay and LED-FM microscopy for the diagnosis of extra pulmonary tuberculosis in Khyber Pakhtunkhwa, Pakistan. Braz J microbiol. 2018, 4(9), 909–913. [CrossRef]
- Elbrolosy, A.M.; Helbawy,R.H.E.; Mansour,O.M.; Latif,R.A. Diagnostic utility of GeneXpert MTB/RIF assay versus conventional methods for diagnosis of pulmonary and extrapulmonary tuberculosis. BMC Microbiol.2021, 21,144. [CrossRef]
- Mulengwa,D.L.; Maropeng Charles Monyama,M.C.; Lebelo,S.L. Evaluation of the GeneXpert MTB/RIF assay performance in sputum samples with various characteristics from presumed pulmonary tuberculosis patients in Shiselweni region, Eswatini. Infect Dis.2022, 54:3, 170-177. [CrossRef]
- Raina, C.; Sabita, B.; Alina,S.; Manoj, P.; Brajendra, S.; Yengkokpam, S.; Ranjit, S.; Zareena, F.; Rachana, M.; Ali, A.R.; Alfonso, J.; Rodriguez,M.; Kuldeep, D. Diagnostic performance of GeneXpert MTB/RIF assay compared to conventional Mycobacterium tuberculosis culture for diagnosis of pulmonary and extra pulmonary tuberculosis, Nepal. Narra J. 2021, 1(2): e33. [CrossRef]
- Li, S.; Liu, B.; Peng, M.; Chen, M.; Yin, W.; Tang, H. Diagnostic accuracy of Xpert MTB/RIF for tuberculosis detection in different regions with different endemic burden: a systematic review and meta-analysis. PLoS One. 2017,12. [CrossRef]
- Opota, O.; Zakham, F.; Mazza-Stalder, J.; Nicod, L.; Greub, G.; Jaton, K. Added Value of Xpert MTB/RIF Ultra for Diagnosis of Pulmonary Tuberculosis in a Low-Prevalence Setting. J Clin Microbiol. 2019, 57, e01717-18. [CrossRef]
- Tang, T.; Liu, F.; Lu, X.; Huang, Q. Evaluation of GeneXpert MTB/RIF for detecting mycobacterium tuberculosis in a hospital in China. J Int Med Res. 2017, 45,816-822. [CrossRef]
- Faria, M.G.B.F.; Andrade, R.L.P.; Camillo, A.J.G.; Leite, K.F.S.; Saita, N.M.; Bollela, V.R. Effectiveness of GeneXpert in the diagnosis of tuberculosis in people living with HIV/AIDS. Rev Saude Publica. 2021,55,89. [CrossRef]
- Cox, H.; Dickson,H.; Ndjeka, N.; Hoog, A.G.; Cobelens, A.F.; Stevens, W. Delays and loss to follow-up before treatment of drug-resistant tuberculosis following implementation of Xpert MTB/RIF in South Africa: A retrospective cohort study. PLoS Med. 2017, 14:2, e1002238. [CrossRef]
- Rimal, R.; Shrestha, D.; Pyakurel, S.; Poudel, R.; Shrestha ,P.; Rai, K R, et al. Diagnostic performance of GeneXpert MTB/RIF in detecting MTB in smear-negative presumptive TB patients. BMC Infect Dis. 2022, 22,321. [CrossRef]
- Habte, D.; Melese, M.; Hiruy, N.; Gashu, Z.; Jerene, D.; Moges, F.; Yifru, S.; Girma, B.; Kassie, Y.; Haile, Y.K.; Suarez, P.G.; Tessema, B. The additional yield of GeneXpert MTB/RIF test in the diagnosis of pulmonary tuberculosis among household contacts of smear positive TB cases. Int J Infect Dis. 2016, 49,179-184. [CrossRef]
- Gebretsadik, D.; Ahmed, N.; Kebede, E.; Mohammed, E.; Belete, M.A. Prevalence of Tuberculosis by Automated GeneXpert Rifampicin Assay and Associated Risk Factors among Presumptive Pulmonary Tuberculosis Patients at Ataye District Hospital, North East Ethiopia. Infect Drug Resist. 2020,13,1507-1516. [CrossRef]
- Gurung, S.C.; Dixit, K.; Paudel, R.; Sah, M.K.; Pandit, R.N.; Aryal, T.P.; Khatiwada, S.U.; Majhi, G.; Dhital, R.; Paudel, P.R.; et al. Comparing Additionality of Tuberculosis Cases Using GeneXpert or Smear-Based Active TB CaseFinding Strategies among Social Contacts of Index Cases in Nepal. Trop. Med. Infect. Dis. 2023, 8, 369. [CrossRef]
- Kalra, A.; Parija, D.; Raizada, N.; Sachdeva, K.S.; Rao, R.; Swaminathan, S. Upfront Xpert MTB/RIF for diagnosis of pediatric TB—Does it work? Experience from India. PLoS ONE.2020, 15(8), e0236057. [CrossRef]
- Sasikumar,C.; Utpat,K.; Desai,U.; oshi,J. The role of Genexpert in the diagnosis of Mycobacterium tuberculosis. Eur Respi J.2019, 54, PA3003. [CrossRef]
- Worku,M.; Agonafir,M.; Yassin,M.A.; Yassin,M.A.; Datiko,D.G.; Theobald,S.; Cuevas,L.E. Use of Xpert MTB/RIF for the Identification of TB and Drug Resistance among Smear-Negative and Re-Treatment Cases in Rural Areas of Ethiopia. Open Microbiol J. 2019, 13, 188-192. [CrossRef]
- Farra,A.; Manirakiza,A.; Yambiyo,B.M.; Zandanga,G.; Lokoti,B.; Arthaud,A.B.; Ngaya,G.; Hermana,G.; Ourandji,L.M.; Ignaleamoko,A.;Nzonzo,A.D.K.; Simelo,J.P.; Iragena,J.D. Surveillance of Rifampicin Resistance With GeneXpert MTB/RIF in the National Reference Laboratory for Tuberculosis at the Institut Pasteur in Bangui, 2015–2017.Open Forum Infect Dis.2023. [CrossRef]
- Zhou R et al (2022). Drug resistance characteristics of Mycobacterium tuberculosis isolates obtained between 2018 and 2020 in Sichuan, China. Epidemiol Infect.2022, 150, e27, 1–9. [CrossRef]
- Dreyer, V.; Mandal, A.; Dev,P.; Merker.M.; Barilar.I.; Utpatel,C.; Nilgiriwala,K.; Rodrigues,C.;Crook,D.W.et al.high fluoroquinolone resistance proportions among multidrug-resistant tuberculosis driven by dominant L2M.tuberculosis clones in the Mumbai Metropolitan region. Genome Med.2022, 14, 95. [CrossRef]
- WHO-Global tuberculosis Report 2020[internet].WHO.World Health Organization; [cited 2020 November 4].Available from:http://www.eho.int/ tb/ publications/ global_report/en/.
- Lee, H.W.; Yim, J.J. Fluoroquinolone resistance in multidrug-resistant tuberculosis patients. Korean J Intern Med. 2019,34,286-287. [CrossRef]
- Rohini Sharma,R.; Singha,B.K.; Kumar,K.; Ramachandran,R.; Jorwa,P. Presence of Fluoroquinolone mono-resistance among drug-sensitive Mycobacterium tuberculosis isolates: An alarming trend and implications. Clinl Epidemiol Glob Health.2019, 7, 363-366. [CrossRef]
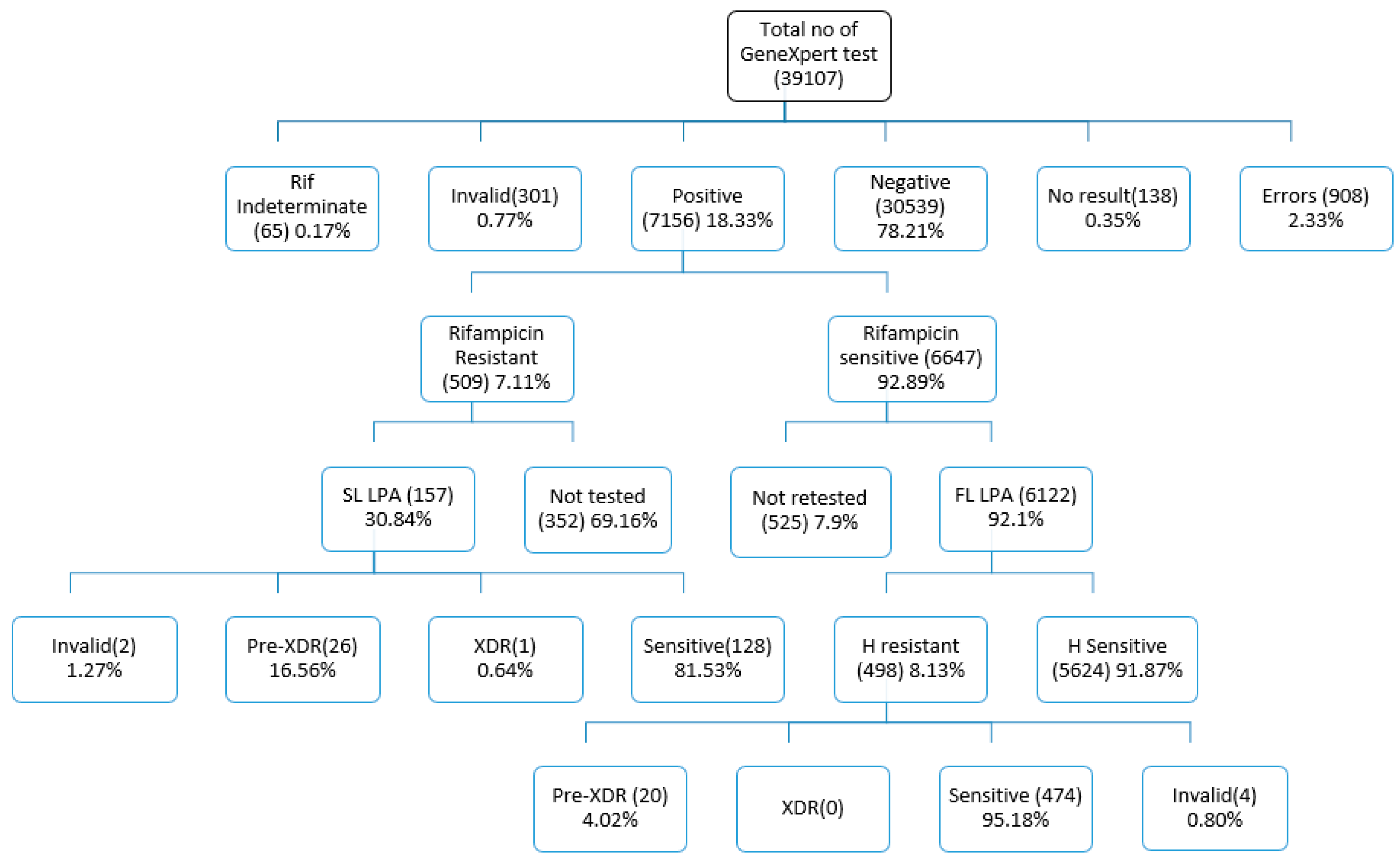
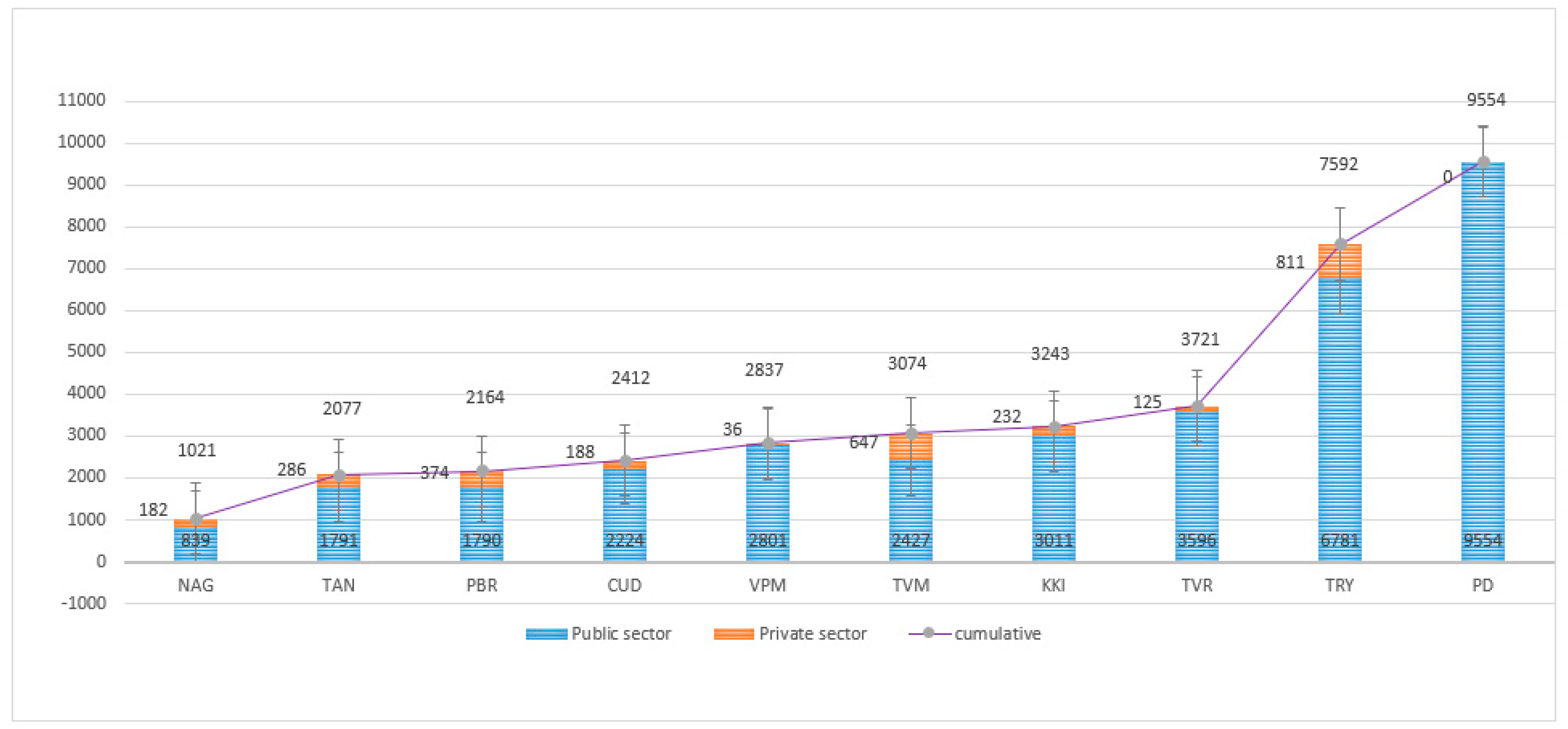
| Stratification of patients | Total | MTB not detected (MTB-) | MTB detected (MTB+) | RIF resistance not detected | RIF resistance detected | % of MTB Positive | % of RIF Resistant |
|
| Presumptive TB | PLHIV out of presumptive TB | 2374 | 2195 | 179 | 155 | 10 | 7.54 | 5.59 |
| Paediatric out of presumptive TB | 2257 | 2207 | 50 | 49 | 0 | 2.22 | 0.00 | |
| Smear Negative, X-ray suggestive of TB | 11233 | 8927 | 2306 | 2088 | 113 | 20.53 | 4.90 | |
| Other Vulnerable group | 1820 | 1557 | 263 | 197 | 10 | 14.45 | 3.80 | |
| Contacts of TB & DRTB patients | 595 | 494 | 101 | 77 | 24 | 16.97 | 23.76 | |
| EP TB | 7639 | 6892 | 747 | 669 | 26 | 9.78 | 3.48 | |
| Upfront Molecular test offered | 4026 | 3427 | 599 | 191 | 6 | 14.88 | 1.00 | |
| Presumptive DRTB (Pulmonary) | Notified TB patients (New)- UDST | 3846 | 2273 | 1573 | 1210 | 268 | 40.90 | 17.04 |
| Notified TB patients (Pre-treated) -UDST | 462 | 275 | 187 | 165 | 19 | 40.48 | 10.16 | |
| Non-responders (DS TB & Hr TB) | 562 | 93 | 469 | 282 | 14 | 83.45 | 2.99 | |
| Private sector | Pulmonary TB | 1939 | 1430 | 509 | 472 | 15 | 26.25 | 2.95 |
| EPTB | 942 | 769 | 173 | 134 | 4 | 18.37 | 2.31 | |
| 37695 | 30539 | 7156 | 5689 | 509 | 18.98 | 7.11 | ||
| Number | % | Sensitivity (%) with 95% CI | Specificity (%) with 95% CI | PPV (%) with 95% CI |
NPV (%) with 95% CI |
Prevalence (%) with 95% CI | Accuracy (%) with 95% CI | Kappa with 95% CI | |
| PTB | 29114 | 74.45 | 99.87 (0.12-0.07) |
99.92 (0.04-0.03) |
99.71 (0.17-0.11) |
99.97 (0.04-0.01) |
21.38 (0.46-0.48) |
99.91 (0.04-0.03) |
0.99735 (0.99633-0.99837) |
| EPTB | 8581 | 21.94 | 99.45 (0.72- 0.37) |
99.84 (0.11-0.08) |
98.70 (0.97-0.55) |
99.93 (0.09-0.04) |
10.64 (0.65-0.67) |
99.80 (0.12-0.08) |
0.98962(0.98469-0.99455) |
| Presumptive TB | 32825 | 87.08 | 99.82 (0.17-0.10) |
99.91 (0.04-0.03) |
99.51 (0.23-0.16) |
99.97 (0.03-0.01) |
14.96 (0.38-0.39) |
99.93 (0.04-0.02) |
0.99605(0.99471-0.99740) |
| Presumptive DRTB (Pulmonary) | 4870 | 12.92 | 99.82 (0.28-0.13) |
99.77 (0.26-0.15) |
99.73 (0.33-0.15) |
99.85 (0.25-0.09) |
45.73 (1.41-2.41) |
99.92 (0.10-0.05) |
0.99586(0.99330-0.99842) |
| Presumptive TB: | |||||||||
| PLHIV out of presumptive TB | 2374 | 6.07 | 99.44 (2.53-0.55) |
99.91 (0.24-0.08) |
98.88 (3.20-0.84) |
99.95 (0.27-0.04) |
7.50 (1.03-1.13) |
99.87 (0.24-0.10) |
0.99091(0.98064-1.00000) |
| Paediatric out of presumptive TB | 2257 | 5.77 | 97.96 (8.81-1.99.) |
99.91 (0.24-0.08) |
96.00 (10.28-2.27) |
99.95 (0.26-0.04) |
2.17 (0.56-0.69) |
99.87 (0.26-0.10) |
0.96902(0.93400-1.00000) |
| Smear Negative, X-ray suggestive of TB | 11233 | 28.72 | 100.00 (0.16-0.0) |
99.99 (0.05-0.01) |
99.96 (0.27-0.03) |
100.00 (0.04-0.00) |
20.52 (0.74-0.76) |
99.99 (0.04-0.01) |
0.99973(0.99919-1.00000) |
| Other Vulnerable group | 1820 | 4.65 | 99.62 (1.73-0.37) |
99.87 (0.33-0.11) |
99.24 (2.21-0.57) |
99.94 (0.39-0.05) |
14.40 (1.59-1.69) |
99.84 (0.32-0.13) |
0.99332(0.98577-1.00000) |
| Contacts of TB & DRTB patients | 595 | 1.52 | 100.00 (3.69-0.00) |
99.40 (1.15-0.48) |
97.03 (5.67-1.99) |
100.00 (0.74-0.00) |
16.47 (2.89-3.23) |
99.50 (0.97-0.40) |
0.98190(0.96147-1.00000) |
| EP TB | 7639 | 19.53 | 99.60 (0.78-0.32) |
99.90 (0.11-0.06) |
99.06 (1.00-0.39) |
99.96 (0.09-0.03) |
9.73 (0.66-0.68) |
99.87 (0.11-0.07) |
0.99256(0.98796-0.99717) |
| Upfront Molecular test offered | 4026 | 10.29 | 99.83 (0.76-0.17) |
99.97 (0.13-0.03) |
99.83 (1.00-0.15) |
99.97 (0.18-0.03) |
14.88 (1.09-1.54) |
99.95 (0.13-0.04) |
0.99804(0.99532-1.00000) |
| Presumptive DRTB (Pulmonary): | |||||||||
| Notified TB patients (New)- UDST | 3846 | 9.83 | 99.94 (0.34-0.06) |
99.87 (0.25-0.10) |
99.81 (0.40-0.13) |
99.96 (0.27-0.03) |
40.85 (1.56-1.57) |
99.90 (0.17-0.07) |
0.99785(0.99574-0.99996) |
| Notified (Previously treated) -UDST | 462 | 1.18 | 99.47 (2.41-0.52) |
99.64 (1.65-0.35) |
99.47 (3.13-0.45) |
99.64 (2.15-0.31) |
40.48 (4.51-4.63) |
99.57 (1.12-0.38) |
0.99102(0.97859-1.00000) |
| Non-responders (DS TB & H Resistant TB) |
562 | 1.44 | 99.57 (1.10-0.38) |
97.85 (5.40-1.89) |
99.57 (1.23-0.32) |
97.85 (5.91-1.60) |
83.45 (3.33-2.98) |
99.29 (1.10-0.62) |
0.97423(0.94907-0.99939) |
| Private sector: | |||||||||
| Pulmonary TB | 1939 | 4.96 | 100.00 (0.78-0.00) |
99.93 (0.32-0.07) |
99.80 (0.18-0.17) |
100.00 (0.26-0.00) |
26.20 (1.95-2.02) |
99.95 (0.24-0.05) |
0.99867(0.99606-1.00000) |
| EPTB | 942 | 2.41 | 98.82 (3.01-1.04) |
99.35 (0.85-0.44) |
97.11 (3.77-1.66) |
99.74 (0.76-0.19) |
18.05 (1.41-2.60) |
99.26 (0.79-0.44) |
0.97505(0.95664-0.99346) |
| Xpert results | R resistant detected | Resistant Probes | |||||
| Probe A | Probe B | Probe C | Probe D | Probe E | Δ CT value >4 | ||
| Very low | 212(41.65%) | 09 | 27 | 19 | 22 | 29 | 106 |
| Low | 163(32.02%) | 13 | 15 | 17 | 21 | 28 | 69 |
| Medium | 98(19.25%) | 13 | 12 | 09 | 12 | 10 | 42 |
| High | 36(7.07%) | 2 | 1 | 4 | 2 | 1 | 26 |
| Total | 509 | 37(7.27%) | 55(10.81%) | 49(9.63%) | 57(11.20%) | 68(13.36%) | 243(47.74%) |
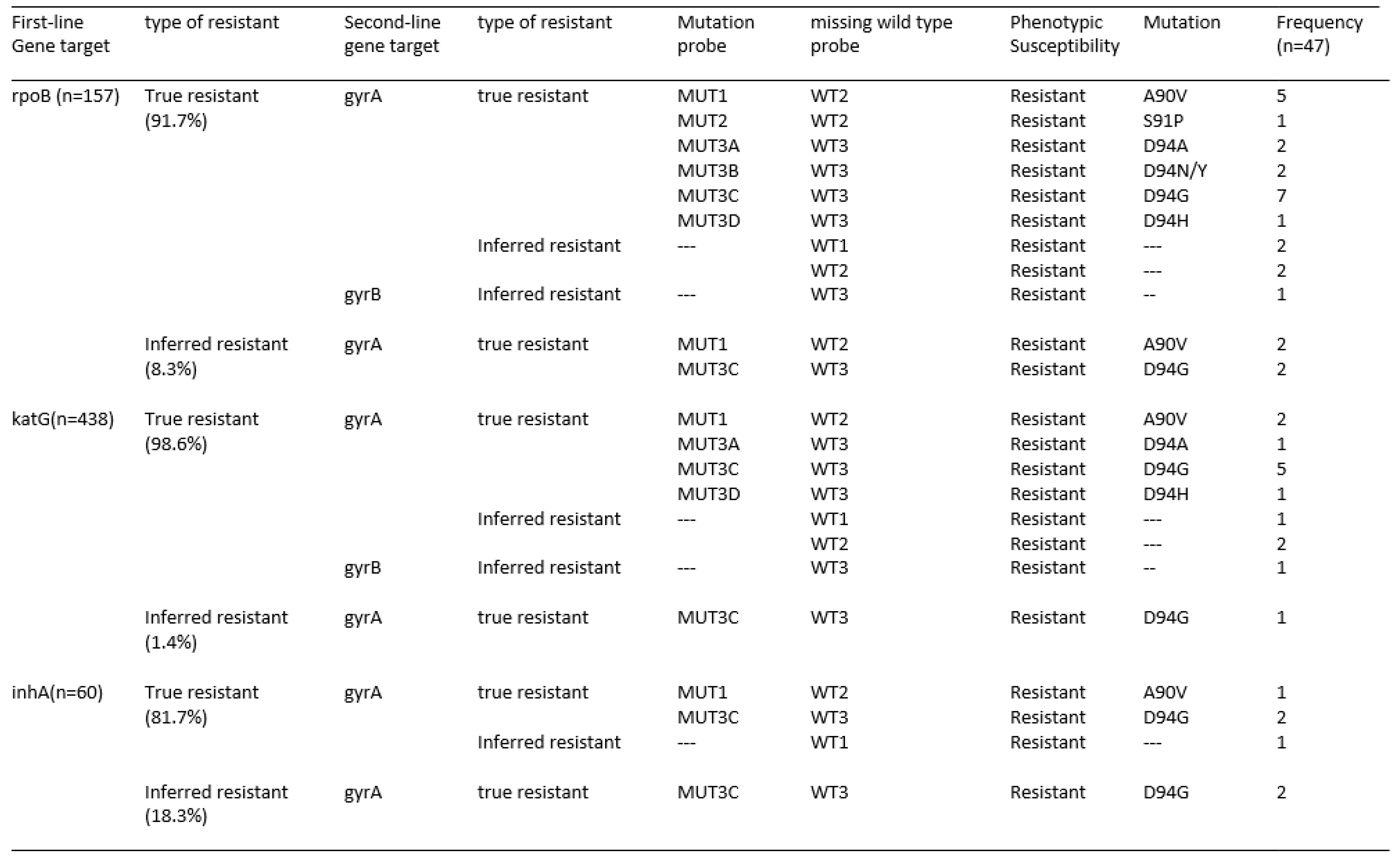
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




