Introduction
Idiopathic scoliosis (IS) represents a three-dimensional deformity of the spine and thorax that can occur at any age in growing children and progresses especially during the pubertal rapid growth spurt [
1]. Depending on the age of occurrence, it can be classified as infantile (0-3), juvenile (4-9) and adolescent idiopathic scoliosis (10-18) [
2]. Many studies have been conducted regarding aetiopathogenesis of idiopathic scoliosis [
3,
4], suggesting there is a multifactorial cause including genetic [
5] and epigenetic factors [
6], central nervous system (CNS), skeletal spinal growth and bone metabolism, metabolic pathways, biomechanics, and other factors yet its aetiology still remains unclear [
7,
8].
Risk of scoliosis progression is related to growth potential that can be determined by the assessment of skeletal maturity. Bone age as the essential parameter to evaluate the remaining growth during puberty can be determinated on pelvis and upper limb radiographs (elbow, hand, wrist and humeral head ossification [
9]. One of the most important and used indicators of skeletal maturity is the Risser sign [
10], due to the fact it can often be assessed on the same spine radiograph as the Cobb angle. Risser sign is assessed by the ossification of the iliac apophysis divided in five stages (0 to V) and is displayed on full AP or PA spine radiographs. The most growth potential is expected between Risser 0 and 2, whereas Risser 0 covers about two-thirds of puberty and therefore can be misleading [
11]. Therefore, Risser sign proved to have limited sensitivity during peak growth velocity and the possibility of high mismatch risk and mistreatment [
12]. For this reason, there is a need for additional parameters that can help in the assessment of growth potential and risk of scoliosis progression.
There are also some other skeletal maturity indicators, such as Sanders [
13] and elbow triradiate cartilage classification [
14] that are not widely utilized in clinical practice due to additional radiation (need for the radiograph of the wrist or elbow). There is a clear correlation between growth velocity and skeletal maturation [
15].
Among the clinical parameters, data about age at menarche is considered to be important, along with biometric measurements and Tanner stages [
16]. The age at the menarche onset is one of the indicators of the remaining growth potential in girls [
17].
Some authors state that it is considered to be even more reliable than a Risser sign [
18].
The first physical sign of puberty occurs about 2 years before menarche, and final height is usually achieved 2.5–3 years after menarche. In girls menarche occurs at age 13.5 years of bone age, usually at Risser grade I [
11]. After girls experience menarche, there is a gradual decrease in the scoliosis progression risk. This is due to the fact that at the time of menarche, approximately 2/3 of the period of pubescent growth spurt and the peak of growth has passed. The potential for scoliosis progression is much lower after the spinal growth and skeletal maturation is complete [
2].
Late onset of menarche correlates with delayed skeletal maturity and higher prevalence of AIS. For this reason, in girls who experience menarche later there is a prolonged period of spine vulnerability and greater possibility of scoliosis progression [
19].
Taking everything above into consideration, data about menarche in girls can provide the clinicians additional information about the remaining growth, help them assess the risk of progression and therefore guide them in treatment decisions.
For this reason, the age of menarche onset is a parameter documented during the clinical assessment and follow up of scoliotic patients in many clinics and during school screening programs.
There are a few studies showing that melatonin may play a role in the pathogenesis of scoliosis, supporting the neuroendocrine hypothesis [
20]. Melatonin as a hormone produced mainly in the pineal gland but also in retina is called “the light of the night”, since its synthesis and release is stimulated by the darkness and inhibited by the light. Among many other roles, melatonin influences sexual maturation process in a way that it reduces secretion of gonadotropins, mainly LH. This is related to the occurrence of menarche, which arises as the melatonin levels decrease during the growth, influencing the onset of puberty [
21,
22,
23].
This can explain why different sunlight exposure depending on the geographical location and equatorial distance influences the melatonin secretion and modifies age at menarche. Therefore, the role of melatonin can be extended to the findings that menarche depends on the geographical latitude [
24,
25,
26,
27,
28].
This might be a reason why reported AIS prevalence in the literature increases in the norther geographic latitudes and decreases with the latitude approaching the equator [
29,
30,
31,
32,
33,
34,
35,
36,
37,
38,
39,
40,
41,
42,
43,
44,
45].
This claim is supported by the epidemiological data on AIS prevalence performed in different countries, showing that Finland has the highest geographical latitude and AIS prevalence of 12% and Singapore has the lowest geographical latitude and AIS prevalence of 0,93%. When assessing the influence of the geographic factors, it is important to distinguish them from socioeconomic circumstances [
46].
There is also a higher prevalence of scoliosis in a population of blind women (42,3%) compared to the prevalence in the general population of the same region (2,9%) [
47]. This finding contributes to the possible role of melatonin in IS etiopathogenesis.
The only country in Balkan region that published data on AIS prevalence by now is Greece [
48].
When comparing scoliotic and non-scoliotic girls in the Mediterranean region, no statistically significant difference has been found in the age of menarche. On the other hand, there was a statistically significant difference between menarche positive and menarche negative scoliotic girls in relation to the laterality of scoliotic curves [
17]. The laterality of the curve is defined as the deviation of the spine in the frontal plane and is represented by the Cobb angle, measured on the spine radiograph. It is the most documented measurement, frequently used in scoliotic patients for the follow-up and the treatment outcome and is also considered to be one of the main predictors of progression [
49].
To our knowledge, there is no data about age of menarche in scoliotic and non-scoliotic girls in Balkan countries Serbia and Bosnia and Herzegovina.
The aims of the study were to determine whether the age of menarche differs in scoliotic and non-scoliotic Balkan girls, to compare the results to the findings from another countries and to assess the relation between menarche and laterality of scoliotic curves.
Patients and methods
Study design
A retrospective study based on prospectively collected clinical data was conducted in Bosnia and Herzegovina and Serbia.
Participants
Participants selected for the study were among 2000 patients of the Institute for physical and rehabilitation medicine and orthopaedic surgery “Dr Miroslav Zotovic” in Banja Luka (Bosnia and Herzegovina) and healthy girls, randomly selected during screening in sport clubs in Novi Sad (Serbia). Patients data collection and analysis was approved by the Ethical committee of the Institute.
Methods
Criteria for the inclusion in the study were gender and Cobb angle. Since our research is based on menarche, the boys were excluded from the study. The girls were referred to our clinic due to clinical trunk asymmetry detected during school screening or by other health professionals or parents. The girls with asymmetry in the Adam’s bending test and scoliometer readings ≥5° were referred to further diagnostics which consisted of full spine PA radiograph done in our clinic. Scoliosis was defined by the SRS definition as the curve with Cobb angle ≥10°. Scoliosis was excluded if the Cobb angle was <10°. The girls in Serbia were examined as part of the regular screening program in amateur sport clubs where they have been engaged in some type of sport training up to 4 times a week. In the control group we included the girls with no trunk asymmetry in standing position or Adam’s bending test or with any signs of scoliosis.
According to the above described parameters, the participants were divided into three groups: scoliotic group, non-scoliotic group and control group.
The scoliotic group consisted out of 494 patients, mean age 12,57 ± 2,4 years (interval 7 to 18 years) and average Cobb angle 20,44±10,88°. The non-scoliotic group consisted out of 523 patients, mean age 11,51±2,71 years (interval 7 to 18 years) and average Cobb angle 6,71±1,76°. The control group consisted out of 86 healthy girls, mean age 13,63±1,54 years (interval 10 to 17 years).
The limitations of the study were relatively small number of girls in the control group (86 participants). In the future, we plan to include more healthy girls in the control group of the study and also to do additional analysis according to age of the participants in different groups.
Statistical analysis
Statistical analysis was performed using the program SPSS Inc. Released 2016. SPSS for Windows, Version 24.0. Chicago, IL.
The results were presented as frequency and percentage (for all participants in three main groups – scoliotics, nonscoliotics and control and subgroups – according to age of menarche and BMI level), mean and standard deviation SD (for average age and body height of the participants), median and interquartile range – IQR (for Risser sign). One-way ANOVA was used to compare the groups and determine if there were statistically significant differences between them, measured on interval scale, with normal distribution. Tukey’s HSD test was used as post hoc test if there was significant difference between groups. Fisher’s test was used to compare the groups measured on nominal or ordinal scale (BMI level). Chi-squared test was used when the frequency in the categories was less than 5. Statistical significance was defined as p<0,05.
Results
There were 1017 girls selected among patients for the first two groups and 86 healthy girls in the control group.
Data about height in all participants and according to the age of menarche, as well as comparison between the groups is presented in
Table 1.
There was a significant difference in height between the three groups (p<0.001). The girls in the control group were significantly taller than the participants in other two groups (scoliotics and non-scoliotics). With post hoc analysis and according to the age of menarche, significant difference was found in premenarchal girls between control and non-scoliotic group (p<0.001), as well as in postmenarchal girls between control and scoliotic group (p=0.020).
To form BMI categories for the participants, the CDC growth reference chart for girls was used with five categories according to percentiles: Underweight (<5 percentiles), Healthy Weight (5 to 85 percentiles), Overweight (85 to 95 percentiles), Obesity (> 95 percentiles), Severe Obesity (> 95 percentiles x 1.2)
Table 2.
There was no statistically significant difference between scoliotic, non-scoliotic and control group according to BMI,
Table 2, for all girls (p=0.073), premenarchal girls (p=0.560) and postmenarchal girls (p=0.128). There was also no statistically significant difference in the scoliotic group between premenarchal and postmenarchal girls (Chi Square test, p=0.371).
Risser sign was homogenized and there was no significant difference in Risser sign between the groups (Chi-square test, p=0.461).
Data about age of participants and age at menarche in all three groups is presented in
Table 3.
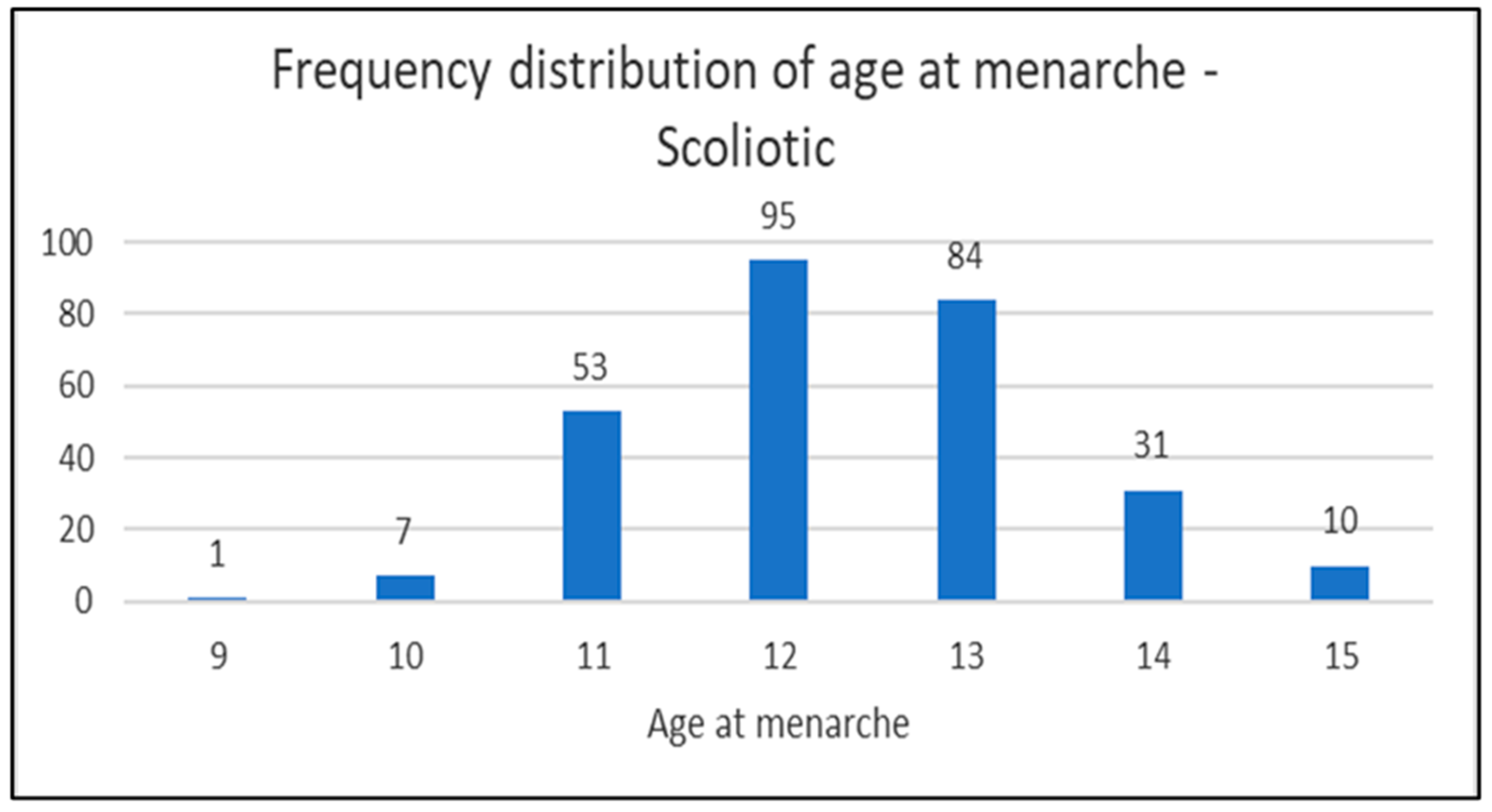
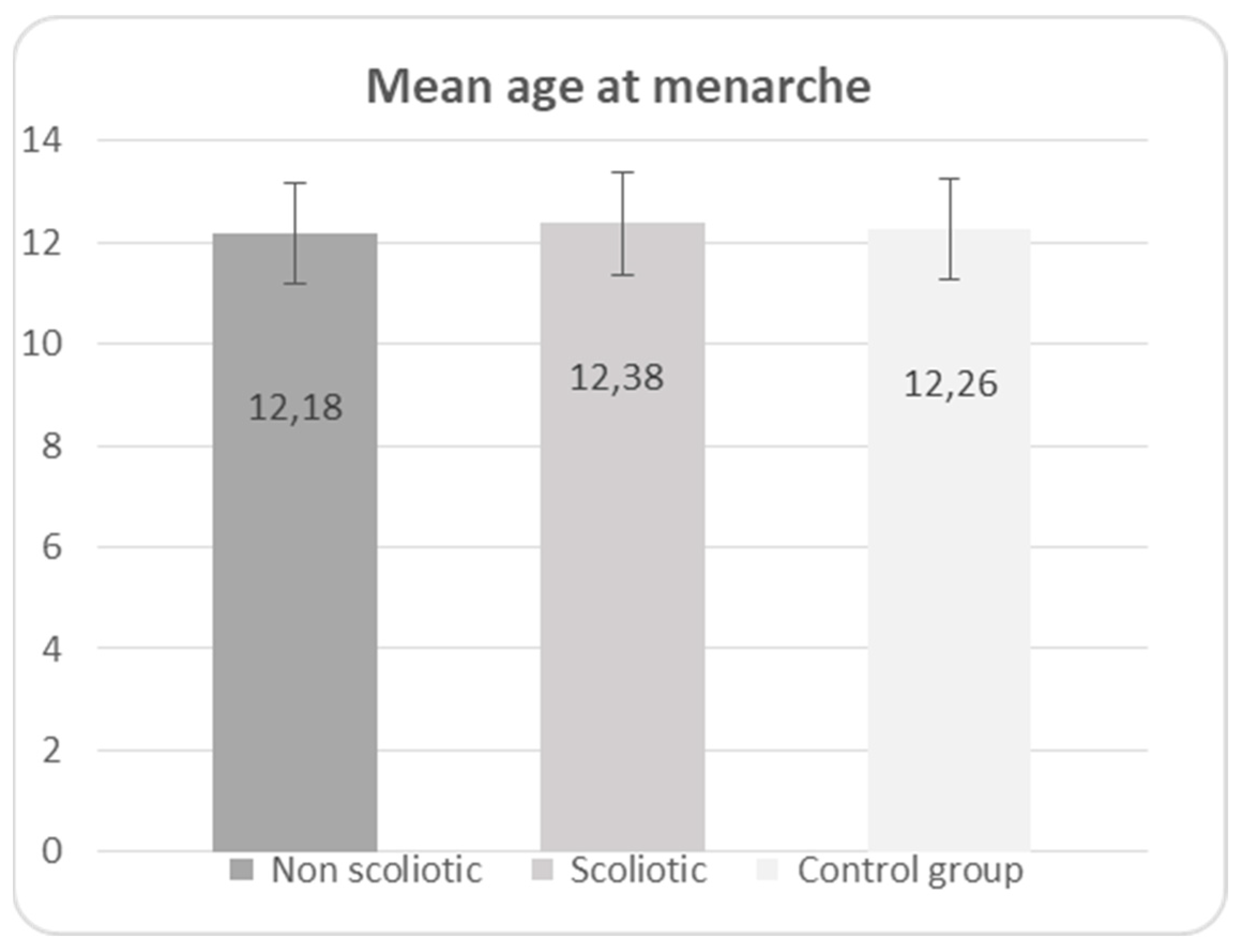
In scoliotic group, 281 girls (56,88%), mean age 14,04±1,50 (range 10-18) were postmenarchal (mean age at menarche 12,38±1,11), while 213 girls (43,12%), mean age 10,63±1,95 (range 7-16) were premenarchal.
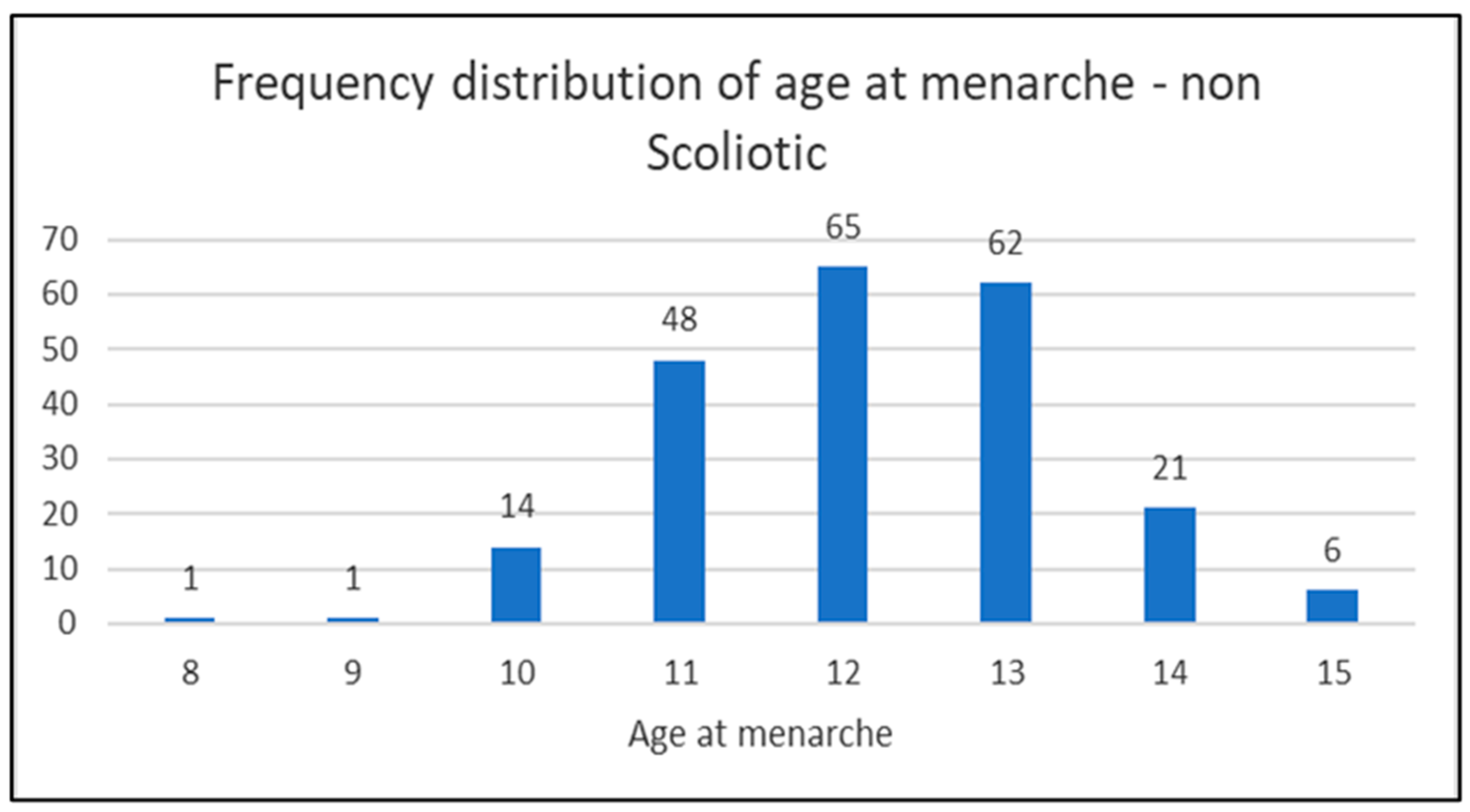
In non-scoliotic group, 218 girls (41,68%), mean age 13,92±1,69 (range 11-18) were postmenarchal (mean age at menarche 12,18±1,22), while 305 girls (58,32%) mean age 9,78 ±1,85 (range 7-15 years) were premenarchal.
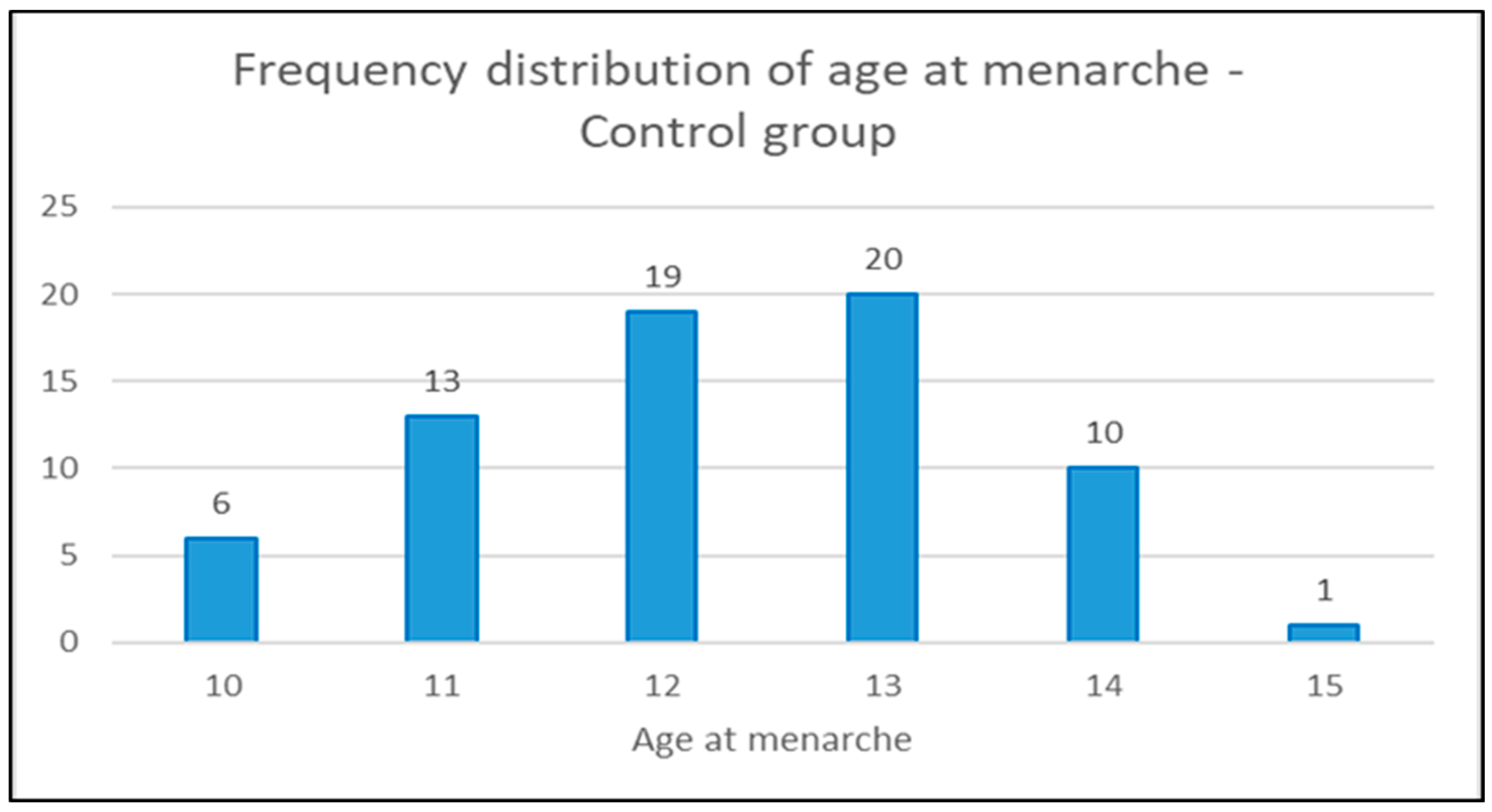
In control group, 69 girls (80,23%), mean age 14,07±1,28 (interval 12-17) were postmenarchal (mean age at menarche 12,26±1,22 (range 10-15), while 17 girls were premenarchal (mean age at menarche 11,82±1,19 (range 10-14).
Table 3.
Age and age at the onset of menarche in all three groups of girls: scoliotics, non-scoliotics and healthy girls.
Table 3.
Age and age at the onset of menarche in all three groups of girls: scoliotics, non-scoliotics and healthy girls.
| |
|
Menarche |
TOTAL |
| |
|
Yes |
No |
| Scoliotics |
N |
281 |
213 |
494 |
| % |
56,88% |
43,12% |
100% |
| |
Age |
Age at menarche |
Age |
Age |
| Mean |
14,04 |
12,38 |
10,63 |
12,57 |
| SD |
1,50 |
1,11 |
1,95 |
2,40 |
| Min-max |
10-18 |
9-15 |
7-16 |
7-18 |
| Non scoliotics |
N |
218 |
305 |
523 |
| % |
41,68% |
58,32% |
100% |
| |
Age |
Age at menarche |
Age |
Age |
| Mean |
13,92 |
12,18 |
9,78 |
11,51 |
| SD |
1,69 |
1,22 |
1,85 |
2,71 |
| Min-max |
11-18 |
8-15 |
7-15 |
7-18 |
| Control group |
N |
69 |
17 |
86 |
| % |
80,23% |
19,77% |
100% |
| |
Age |
Age at menarche |
Age |
Age |
| Mean |
14,07 |
12,26 |
11,82 |
13,63 |
| SD |
1,28 |
1,22 |
1,19 |
1,54 |
| Min-max |
12-17 |
10-15 |
10-14 |
10-17 |
In the diagrams below (1,2 and 3) the distribution of the age of menarche is shown according to the groups and in diagram 4 for all three groups (scoliotic, non scoliotic and control).
Diagram 1.
Distribution of the age of menarche by age in non-scoliotic group.
Diagram 1.
Distribution of the age of menarche by age in non-scoliotic group.
Diagram 2.
Distribution of the age of menarche by age in scoliotic group.
Diagram 2.
Distribution of the age of menarche by age in scoliotic group.
Diagram 3.
Distribution of the age of menarche by age in control group.
Diagram 3.
Distribution of the age of menarche by age in control group.
Diagram 4.
Mean age at menarche in non-scoliotic, scoliotic and control groups.
Diagram 4.
Mean age at menarche in non-scoliotic, scoliotic and control groups.
No statistically significant difference was found in age of menarche between the three groups (p=0,168), presented in
Table 4.
In the scoliotic group of girls a statistically significant relation was found between the age at menarche and laterality of the primary scoliotic curve (χ2(1,n=494) = 10,18, p<0,01, phi =0,148).
In postmenarchal scoliotic girls, primary right curve was dominant in 54,80%, while in scoliotic premenarchal girls primary left curve was dominant 60,09% (p<0,01). These results are shown below in the Tables 5 and 6.
Table 5.
Distribution of the laterality of primary curves in scoliotic postmenarchal group.
Table 5.
Distribution of the laterality of primary curves in scoliotic postmenarchal group.
| 281 SCOLIOTIC GIRLS WITH MENARCHE |
LATERALITY OF PRIMARY CURVE |
TOTAL |
| Left |
Right |
| LOCATION OF PRIMARY CURVE |
Lumbar |
Count |
61 |
23 |
84 |
| % Within LOCATION OF PRIMARY CURVE |
72,62% |
27,38% |
100% |
| % Of Total |
21,71% |
8,18% |
29,89% |
| Thoracic |
Count |
13 |
80 |
93 |
| % Within LOCATION OF PRIMARY CURVE |
13,98% |
86,02% |
100% |
| % Of Total |
4,63% |
28,47% |
33,10% |
Thoraco
Lumbar
|
Count |
53 |
51 |
104 |
| % Within LOCATION OF PRIMARY CURVE |
50,96% |
49,04% |
100% |
| % Of Total |
18,86% |
18,15% |
37,01% |
| TOTAL |
Count |
127 |
154 |
281 |
| % Within LOCATION OF PRIMARY CURVE |
45,20% |
54,80% |
100% |
| % Of Total |
45,20% |
54,80% |
100% |
Table 6.
Distribution of the laterality of primary curves in scoliotic premenarchal group.
Table 6.
Distribution of the laterality of primary curves in scoliotic premenarchal group.
| 213 SCOLIOTIC GIRLS WITHOUT MENARCHE |
LATERALITY OF PRIMARY CURVE |
TOTAL |
| Left |
Right |
| LOCATION OF PRIMARY CURVE |
Lumbar |
Count |
38 |
9 |
47 |
| % Within LOCATION OF PRIMARY CURVE |
80,85% |
19,15% |
100% |
| % Of Total |
17,84% |
4,22% |
22,06% |
| Thoracic |
Count |
14 |
48 |
62 |
| % Within LOCATION OF PRIMARY CURVE |
22,58% |
77,42% |
100% |
| % Of Total |
6,57% |
22,54% |
29,11% |
Thoraco
Lumbar
|
Count |
76 |
28 |
104 |
| % Within LOCATION OF PRIMARY CURVE |
73,08% |
26,92% |
100% |
| % Of Total |
35,68% |
13,15% |
48,83% |
| TOTAL |
Count |
128 |
85 |
213 |
| % Within LOCATION OF PRIMARY CURVE |
60,09% |
39,91% |
100% |
| % Of Total |
60,09% |
39,91% |
100% |
Discussion
Menarche in girls is widely considered to be one of the important parameters in the assessment of risk of scoliosis progression and one of the influencing factors in the etiopathogenesis of IS. The age of menarche can indicate the remaining growth potential in girls [
12] and be even more reliable than a Risser sign [
18].
However, Risser sign as a skeletal maturity indicator has been widely used by the professionals in the clinical settings. Nonetheless, its accuracy in the assessment of risk of scoliosis progression has been undermined lately due to reported limitations, especially during the growth spurt [
12].
Regardless of its significance, there are only a few studies that highlighted the importance of menarche in girls.
In 2002, Grivas et al. [
17] answered the pending question whether there is a difference in the age of menarche between scoliotics and non-scoliotics among Mediterranean girls. They found no statistically significant difference of the age at menarche between scoliotic and non-scoliotic girls. These results are in accordance with the results of our study, which can be explained by similar geographical latitude of the Balkan countries (Greece 38° 16' 29.82" N, 22° 00' E, Bosnia and Herzegovina 43.9159° N, 17.6791° E, Serbia 44.0165° N, 21.0059° E) and supports the findings that the distance from Equator affects the age of menarche.
19 There is also an association between AIS prevalence and age at menarche in different geographic latitudes [
50,
51,
52,
53,
54,
55,
56,
57,
58,
59,
60].
The scoliotic girls in northern countries, with greater distance from the Equator, experience menarche in later age which is connected to higher prevalence of IS in these countries. This concept seems to be actual in southern globe countries as well [
61]. This suggests the possible role of geography in the pathogenesis of IS. Unfortunately, since there is no official data about scoliosis prevalence in Serbia and Bosnia and Herzegovina, we were not able to contribute to these findings.
The growth parameters in terms of height and weight were also considered.
Our results showed significant difference in height between the groups in terms of premenarchal girls in control group being taller than non-scoliotic girls, as well as postmenarchal girls in control group being taller than scoliotic girls.
This finding is not in accordance with some previously published results [
62,
63,
64,
65,
66] and can possibly be explained by the sample size of our control group and the age difference between the groups, that is the girls in the control group being older (13,63 years of age) compared to scoliotic (12,57 years of age) and non-scoliotic girls (11,51 years of age).
Goldberg et al. [
18] found that scoliotic girls are taller when they are younger, but not in the adolescence, while Duval-Beaupère [
66] and Grivas et al [
67] didn’t find any difference in height between scoliotics and healthy children.
When analyzing the weight according to BMI groups, the majority of participants in all groups had healthy weight (normal BMI). In scoliotic group, there were more girls with low BMI compared to non-scoliotic and control group. However, no significant difference in weight according to BMI groups was found (p=0.073). Grivas et al [
64] reported that scoliotic girls from the age of 8 to 2 years are heavier compared to their non-scoliotic counterparts, but are thinner after the age of 13, while results from another studies showed no difference in weight between scoliotics and non-scoliotics [
18,
62].
The significant difference in laterality of scoliotic curves in premenarchal and postmenarchal scoliotic girls was found and is in accordance with previously published results [
12]. The menarche positive scoliotic girls showed predominantly right sided primary curves, while the menarche negative scoliotic girls had mainly left sided primary curves. They showed that primary right curve was dominant in menarche positive girls in 61%, while primary left curve was dominant in menarche negative girls in 64,3% which is similar to our findings (54,80% in menarche positive girls and 60,09% in menarche negative girls).
In menarche positive girls, primary right curve was dominant in 54,80%, while in menarche negative girls primary left curve was dominant 60,09% (p<0,01), which represents patterns reflecting developmental theory and scoliosis by Goldberg et al [
18].
The reason for this finding could also be in the similar underlying mechanism of IIS, since left curves are dominant both in IIS and premenarchal scoliotic girls. The development of thorax in IIS is asymmetrical due to developmental delay of upper ribs leading to the funnel shaped rib cage and causing the spinal deformity due to the inability of the upper rib system to act as spinal rotation-defending system in the trunk to the pelvic rotation-inducing system during gait. This hypothesis may also be relevant to the Nottingham concept of IS aetiology [
68].
Conclusions
In Bosnia & Herzegovina and Serbia Balkan girls, there was no significant difference in age of menarche between scoliotic and non-scoliotic girls. However, a significant difference was found in laterality of primary curve in premenarchal and postmenarchal scoliotic girls. Furthermore, the results showed a significant difference in height between scoliotic and non-scoliotic girls. Further research is needed to put these results in the context of scoliogeny.
Author Contributions
Conceptualization: SP, TBG; Data curation: SP; Statistical analysis, Literature Investigation: SP, TBG, NJ; Project administration: TBG, SP, NJ; Writing—original draft: SP. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement and Informed Consent Statement
The study was approved by the Ethical Committee of the Institute for physical and rehabilitation medicine and orthopedic surgery "dr Miroslav Zotovic" (Protocol code: 135-01-4971-2/21, approved on 14 May 2021).
Data Availability Statement
Data are available on demand.
Conflicts of Interest
The authors declare that they have no conflict of interest concerning this article.
Abbreviations
AIS – adolescent idiopathic scoliosis
AP – anteroposterior
BMI – body mass index
IS – idiopathic scoliosis
IIS – infantile idiopathic scoliosis
LH – luteinizing hormone
PA – posteroanterior
SRS – Scoliosis Research Society
References
- Negrini, S.; Aulisa, L.; Ferraro, C.; Fraschini, P.; Masiero, S.; Simonazzi, P.; et al. Italian guidelines on rehabilitation treatment of adolescents with scoliosis or other spinal deformities. Eura Medicophys 2005, 41, 183–201. [Google Scholar] [PubMed]
- Negrini, S.; et al. 2011 SOSORT guidelines: Orthopaedic and rehabilitation treatment of idiopathic scoliosis during growth. Scoliosis 2012, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Burwell, R.G.; Dangerfield, P.H.; Moulton, A.; Grivas, T.B.; Cheng, J.C. Whither the etiopathogenesis (and scoliogeny) of adolescent idiopathic scoliosis? Incorporating presentations on scoliogeny at the 2012 IRSSD and SRS meetings. Scoliosis 2013, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Grivas, T.B.; Vasiliadis, E.S.; Mihas, C.; Savvidou, O. The effect of growth on the correlation between the spinal and rib cage deformity: implications on idiopathic scoliosis pathogenesis. Scoliosis 2007, 2, 11. [Google Scholar] [CrossRef] [PubMed]
- Grauers, A.; Einarsdottir, E.; Gerdhem, P. Genetics and pathogenesis of idiopathic scoliosis. Scoliosis and spinal disorders 2016, 11, 45. [Google Scholar] [CrossRef] [PubMed]
- Fadzan, M.; Bettany-Saltikov, J. Ethiological theories of Adolscent Idiopathic Scoliosis: past and present. Open Orthop J 2017, 11, 1466–1489. [Google Scholar] [CrossRef]
- Wang, W.J.; Yeung, H.Y.; Chu, W.; Tang, N.L.-S.; Lee, K.M.; Qiu, Y.; Burwell, R.G.; Cheng, J. Top theories for the etiopathogenesis of adolescent idiopathic scoliosis. Pediatr. Orthoped. 2011, 31, S14–S27. [Google Scholar] [CrossRef] [PubMed]
- Lowe, T.G.; Edgar, M.; Margulies, J.Y.; Miller, N.H.; Raso, V.J.; Reinker, K.A.; Rivard, C.H. Etiology of idiopathic scoliosis: Current trends in research. J Bone Joint Surg Am 2000, 82, 1157–1168. [Google Scholar] [CrossRef] [PubMed]
- Li, D.T.; Cui, J.J.; DeVries, S.; Nicholson, A.D.; Li, E.; Petit, L.; Kahan, J.B.; et al. Humeral Head Ossification Predicts Peak Height Velocity Timing and Percentage of Growth Remaining in Children. J Pediatr Orthop. 2018, 38, e546–e550. [Google Scholar] [CrossRef]
- Little, D.G.; Sussman, M. The Risser sign: a critical analysis. J Pediatr Orthop 1994, 14, 569–575. [Google Scholar] [CrossRef]
- Canavese, F.; Dimeglio, A. Progression or not progression? How to deal with adolescent idiopathic scoliosis during puberty. J Child Orthop 2013, 7, 43–49. [Google Scholar]
- Minkara, A.; et al. High risk of mismatch between Sanders and Risser staging in AIS: Are we guiding treatment using the wrong classification? J Pediatr Orthop 2020, 40, 60–64. [Google Scholar] [CrossRef]
- Sanders, J.O.; Khoury, J.G.; Kishan, S.; et al. Predicting scoliosis progression from skeletal maturity: a simplified classification during adolescence. J Bone Joint Surg Am 2008, 90, 540–553. [Google Scholar] [CrossRef]
- Charles, Y.P.; Dimeglio, A.; Canavese, F.; Daures, J.P. Skeletal age assessment from the olecranon for idiopathic scoliosis at Risser grade 0. J Bone Joint Surg Am 2007, 89, 2737–2744. [Google Scholar] [CrossRef] [PubMed]
- Little, D.G.; Song, K.M.; Katz, D.; Herring, A. Relationship of peak height velocity to other maturity indicators in idiopathic scoliosis. J Bone Joint Surg Am 2000, 82, 685–693. [Google Scholar] [CrossRef]
- Tanner, J.M.; Whitehouse, R.H.; Marshall, W.A.; Healy, M.J.R. Assessment of skeletal maturity and prediction of adult height (TW2-Method). Academic Press, London 1975.
- Grivas, T.B.; Samelis, P.; Pappa, A.S.; Stavlas, P.; Polyzois, D. Menarche in scoliotic and nonscoliotic Mediterranean girls. Is there any relation between menarche and laterality of scoliotic curves? Stud Health Technol Inform 2002, 88, 30–36. [Google Scholar] [PubMed]
- Goldberg, C.J. Dowling FE. Fogarty EE. Adolescent idiopathic scoliosis – early menarche, normal growth. Spine 1993;18(5): 529-535. Grivas TB, Vasiliadis E, Savvidou O, Mouzakis V, Koufopoulos G. Geographic latitude and prevalence of adolescent idiopathic scoliosis. Stud Health Technol Inform 2006, 123, 84–89.
- Grivas, T.B.; Savvidou, O.D. Melatonin the “light of night” in human biology and adolescent idiopathic scoliosis. Scoliosis 2007, 2, 6. [Google Scholar] [CrossRef] [PubMed]
- Cardinali, D.P.; Ladizesky, M.G.; Boggio, V.; Cutrera, R.A.; Mautalen, C. Melatonin effects on bone: experimental facts and clinical perspectives. J Pineal Res 2003, 34, 81–87. [Google Scholar] [CrossRef]
- Pandi-Perumal, S.R.; Srinivasan, V.G.; Maestroni, G.J.M.; Cardinali, D.P.; Poeggeler, B.; Hardeland, R. Melatonin. FEBS J 2006, 273, 2813–2838. [Google Scholar] [CrossRef]
- Grivas, T.B.; Vasiliadis, E.S.; Triantafyllopoulos, G.; Kaspiris, A.; Burwel, R.G. Age variations of melatonin level and its hormesis; implications for AIS and osteoporosis. Scoliosis 2009, 4 (Suppl 2), O8. [Google Scholar] [CrossRef]
- Rosenberg, M. Menarheal age for Norwegian women born 1830–1960. Ann Hum Biol 1991, 18, 207–219. [Google Scholar] [CrossRef]
- Helm, P.; Grolund, L. A halt in the secular trend towards earlier menarche in Denmark. Acta Obstet Gynecol Scand 1998, 77, 198–200. [Google Scholar] [CrossRef] [PubMed]
- Mul, D.; Fredriks, A.M.; Van Buuren, S.; Oostdijk, W.; Verloove-Vanhorick, S.P.; Wit, J.M. Pubertal development in The Netherlands 1965–1997. Pediatr Res 2001, 50, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Vercauteren, M.; Susanne, C. Current age at menarche in Belgium. Anthropol Anz 1984, 42, 211–217. [Google Scholar]
- Largo, R.H.; Prader, A. Pubertal development in Swiss girls. Helv Paediatr Acta 1983, 38, 229–243. [Google Scholar]
- Dober, I.; Kiralyfalvi, L. Pubertal development in south-Hungarian boys and girls. Ann Hum Biol 1993, 20, 714. [Google Scholar] [CrossRef] [PubMed]
- Prebeg, Z.; Bralic, I. Changes in menarcheal age in girls exposed to war conditions. Am J Hum Biol 2000, 12, 503–508. [Google Scholar] [CrossRef]
- Danubio, M.E.; De Simone, M.; Vecchi, F.; Amicone, E.; Altobelli, E.; Gruppioni, G. Age at menarche and age of onset of pubertal characteristics in 6–14 years old girls from the Province of L'Aquila. Am J Hum Biol 2004, 16, 4708. [Google Scholar] [CrossRef]
- Marrodan, M.D.; Mesa, M.S.; Arechiga, J.; Perez-Magdaleno, A. Trend in menarcheal age in Spain. Ann Hum Biol 2000, 27, 313–319. [Google Scholar]
- Padez, C.; Rocha, M.A. Age at menarche in Coimbra (Portugal) school girls. Ann Hum Biol 2003, 30, 622–632. [Google Scholar] [CrossRef]
- Chumlea, W.C.; Schubert, C.M.; Roche, A.F.; Kulin, H.E.; Lee, P.A.; Himes, J.H.; Sun, S.S. Age at menarche and Racial Comparisons in US girls. Pediatrics 2003, 111, 110–113. [Google Scholar] [CrossRef] [PubMed]
- Floris, G.; Murgia, E.; Sanciu, G.M.; Sanna, E. Age at menarche in Sardinia (Italy). Ann Hum Biol 1987, 14, 285–286. [Google Scholar] [CrossRef] [PubMed]
- Dacou-Vouletakis, C.; Klontza, D.; Lagos, P.; Tzonou, A.; Katsarou, E.; Antoniadis, S.; Papazisis, G.; Papadopoulos, G.; Matsaniotis, N. Age of pubertal stages including menarche in Greek girls. Ann Hum Biol 1983, 10, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Vadocz, E.A.; Siegel, S.R.; Malina, R.M. Age at menarche in competitive figure skaters: variation by competency and discipline. J Sports Sci 2002, 20, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Freedman, D.S.; Khan, L.K.; Serdula, M.K.; Dietz, W.H.; Srinivasan, S.R.; Berenson, G.S. Relation of age at menarche to race, time period, and anthropometric dimensions: the Bogalusa Heart Study. Pediatrics 2002, 110, 43. [Google Scholar] [CrossRef] [PubMed]
- Montero, P.; Bernis, C.; Loukid, M.; Hilali, K.; Baali, A. Characteristics of menstrual cycles in Moroccan girls: prevalence of dysfunctions and associated behaviours. Ann Hum Biol 1999, 26, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Malina, R.M.; Ryan, R.C.; Bonci, C.M. Age at menarche in athletes and their mothers and sisters. Ann Hum Biol 1994, 21, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K. Age at menarche in northwest Indian females and a review of Indian data. Ann Hum Biol 1990, 17, 159–162. [Google Scholar] [CrossRef]
- Singh, S.P.; Malhotra, P. Secular shift in menarcheal age of Patiala (India) schoolgirls between 1974 and 1986. Ann Hum Biol 1988, 15, 77–80. [Google Scholar] [CrossRef]
- Attallah NL: Age at menarche of schoolgirls in Egypt. Ann Hum Biol 1978, 5, 185–189. 42. Ayatollahi SM, Dowlatabadi E, Ayatollahi SA: Age at menarche in Iran. Ann Hum Biol 2002, 29, 355–362.
- Hesketh, T.; Ding, Q.J.; Tomkins, A. Growth status and menarche in urban and rural China. Ann Hum Biol 2002, 29, 348–352. [Google Scholar] [CrossRef]
- Huen, K.F.; Leung, S.S.; Lau, J.T.; Cheung, A.Y.; Leung, N.K.; Chiu, M.C. Secular trend in the sexual maturation of southern Chinese girls. Acta Paediatr 1997, 86, 1121–1124. [Google Scholar] [CrossRef] [PubMed]
- Grivas, T.B.; Vasiliadis, E.; Mouzakis, V.; Mihas, C.; Koufopoulos, G. Association between adolescent idiopathic scoliosis prevalence and age at menarche in different geographic latitudes. Scoliosis 2006, 1, 9. [Google Scholar] [CrossRef] [PubMed]
- Grivas, T.B.; Saviddou, O.D.; Vasiliadis, E.; Psarakis, S.; Koufopoulos, G. Prevalence of scoliosis in women with visual deficiency. Stud Health Technol Inform 2006, 123, 52–56. [Google Scholar]
- Koukourakis, I.; Giaourakis, G.; Kouvidis, G.; Kivernitakis, E.; Blazos, J.; Koukourakis, M. Screening school children for scoliosis on the island of Crete. J Spinal Disord 1997, 10, 527–531. [Google Scholar] [CrossRef]
- Alfrahait, A.; Samdani, A.F.; Balasubramanian, S. Predicting curve progression for adolescent idiopathic scoliosis using random forest model. PLoS ONE 2022, 17, e0273002. [Google Scholar] [CrossRef]
- Nissinen, M.; Heliovaara, M.; Ylikoski, M.; Poussa, M. Trunk asymmetry and screening for scoliosis: a longitudinal cohort study of pubertal schoolchildren. Acta Paediatr 1993, 82, 77–82. [Google Scholar] [CrossRef]
- Willner, S.; Uden, A. A prospective prevalence study of scoliosis in Southern Sweden. Acta Orthop Scand 1982, 53, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Laulund, T.; Sojbjerg, J.O.; Horlyck, E. Moire topography in school screening for structural scoliosis. Acta Orthop Scand 1982, 53, 765–768. [Google Scholar] [CrossRef]
- Dickson RA: Scoliosis in the community. Br Med J 1983, 19(6365):615-8. 286 Br Med J (Clin Res Ed), 1983 Feb 19;286(6365):615-8. [CrossRef]
- Morais, T.; Bernier, M.; Turcotte, F. Age- and sex-specific prevalence of scoliosis and the value of school screening programs. Am J Public Health 1985, 75, 1377–1380. [Google Scholar] [CrossRef]
- BP, *!!! REPLACE !!!*; Yawn, R.A.; Hodge, D.; Kurland, M.; Shaughnessy, W.J.; Ilstrup, D.; Jacobsen, S.J. A population-based study of school scoliosis screening. JAMA 1999, 282, 1472–1474. [Google Scholar]
- Gore, D.R.; Passehl, R.; Sepic, S.; Dalton, A. Scoliosis screening: results of a community project. Pediatrics 1981, 67, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Cucek-Plenicar M: 3-DEtiological prognostic aspects Screening for scoliosis in Slovenia: Results of 15 years In Three-dimensional Analysis of Spinal Deformities Edited by: D'Amico, M.; Merolli, A.; Santambrogio, G.C. Amsterdam, Netherlands: IOS Press; 1995, 275-277.
- Rogala, E.J.; Drummond, D.S.; Gurr, J. Scoliosis: incidence and natural history. A prospective epidemiological study. J Bone Joint Surg Am 1978, 60, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Strayer, L.M. The incidence of scoliosis in post-partum female on Cape Cod. J Bone Joint Surg Am 1973, 55A, 436. [Google Scholar]
- Brooks, H.L.; Azen, S.P.; Gerberg, E.; Brooks, R.; Chan, L. Scoliosis: A prospective epidemiological study. J Bone Joint Surg Am 1975, 57, 968–972. [Google Scholar] [CrossRef]
- Carcamo, M.; Espinoza, P.; Rodas, M.; Urrejola, O.; Betany-Saltikov, J.; Grivas, T.B. Prevalence, risk of progression and quality of life assessment in adolescents undergoing school screening for adolescent idiopathic scoliosis. Andes pediatr 2023, 94, 78–85. [Google Scholar]
- Buric, M.; Momcilovic, B. Groth pattern and skeletal age in schoolgirls with idiopathic scoliosis. Clin Orthop 170, 238-2-12, 1982.
- Dickson, R.A.; Sevitt, E.A. Growth and idiopathic scoliosis: a longitudinal cohort study. J Bone Joint Surg. 64-B, 385, 1982.
- Grivas, T.B.; Arvaniti, A.; Maziotou, C.; Manesioti, M.; Fergadi, A. Comparison of body weight and height between normal and scoliotic children. Stud Health Technol Inform. 2002, 91, 47–53. [Google Scholar] [PubMed]
- Nicolopoulos, K.S.; Burwell, R.G.; Webb, J.K. Stature and its components in Adolescent Idiopathic Scoliosis, J Bone Joint Surg, 67-B: 594-601, !985.
- Loncar-Dusek, M.; Pecina, M.; Prebeg, Z. A longitudinal study of Growth. Velocity and Development of Secontary Gender Characteristics Versus Onset of Idiopathic Scoliosis. Clin Orthop 1991, 270, 278–282. [Google Scholar] [CrossRef]
- Duval- Beaupère G: Pathogenic relationship between scoliosis and growth. Jn Zorab PA (ed): Proceedings of a Third Symposium London, Churchill Livingstone, 1971 pp. 58–64.
- Grivas, T.B. Thorax and Idiopathic scoliosis. Int. J. Adv. Res 2023, 11, 1252–1290. [Google Scholar] [CrossRef]
- Βurwell, R.G.; Cole, A.A.; Cook, T.A.; Grivas, T.B.; Kiel, A.W.; Moulton, A.; Thirlwall, A.S.; Upadhyay, S.S.; Webb, J.K.; Wemyss-Holden, S.A.; Whitwell, D.J.; Wojcik, A.S.; Wythers, D.J. 1991-1992. Pathogenesis of Idiopathic Scoliosis. The Nottingham Concept. Acta Orthop Belgica. 1992, 58 (Suppl. 1), 33–58. [Google Scholar]
Table 1.
Comparison between scoliotic, non-scoliotic and control group according to height.
Table 1.
Comparison between scoliotic, non-scoliotic and control group according to height.
| Weight (cm) |
Scoliotic |
Non-scoliotic |
Control |
p value |
| All patients |
159.1 ± 12.5 |
153.8 ± 14.1 |
166.7 ± 9.7 |
p<0.001a
|
| Premenarchal |
150.2 ± 12.8 |
145.2 ± 11.7 |
154.3 ± 6.0 |
p<0.001a
|
| Postmenarchal |
165.9 ± 6.7 |
165.7 ± 6.3 |
169.8 ± 7.3 |
p<0.001a
|
Table 2.
Comparison between scoliotic, non-scoliotic and control group according to BMI.
Table 2.
Comparison between scoliotic, non-scoliotic and control group according to BMI.
| |
Under weight |
Healthy Weight |
Over weight |
Obesity |
Severe Obesity |
p value |
| All patients |
|
|
|
|
|
|
| Scoliotic |
52 (10,5%) |
377 (76,3%) |
42 (8,5%) |
22 (4,5%) |
1 (0,2%) |
p=0.073a
|
| Non-scoliotic |
32 (6,1%) |
404 (77,2%) |
58 (11,1%) |
26 (5,0%) |
3 (0,6%) |
| Control |
4 (4,7%) |
75 (87,2%) |
6 (7,0%) |
1 (1,2%) |
0 (0,0%) |
| Premenarchal |
|
|
|
|
|
|
| Scoliotic |
25 (11,7%) |
155 (72,8%) |
23 (10,8%) |
10 (4,7%) |
0 (0,0%) |
|
| Non-scoliotic |
24 (7,9%) |
226 (74,1%) |
36 (11,8%) |
18 (5,9%) |
1 (0,3%) |
p=0.560a
|
| Control |
2 (11,8%) |
15 (88,2%) |
0 (0,0%) |
0 (0,0%) |
0 (0,0%) |
|
| Postmenarchal |
|
|
|
|
|
|
| Scoliotic |
27 (9,6%) |
222 (79,0%) |
19 (6,8%) |
12 (4,3%) |
1 (0,4%) |
p=0.128a
|
| Non-scoliotic |
8 (3,7%) |
178 (81,7%) |
22 (10,1%) |
8 (3,7%) |
2 (0,9%) |
| Control |
2 (2,9%) |
60 (87,0%) |
6 (8,7%) |
1 (1,4%) |
0 (0,0%) |
Table 4.
Difference in age of menarche in scoliotic and non-scoliotic girls.
Table 4.
Difference in age of menarche in scoliotic and non-scoliotic girls.
| |
Scoliotic |
Non scoliotic |
Control group |
p value |
| N |
281 |
218 |
69 |
0.168 a
|
| Age (years) |
12.38 ± 1.11 |
12.18 ± 1.22 |
12.26 ± 1.22 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).