1. Introduction
Despite the advancement of medical therapy, a significant proportion of patients with Crohn’s disease (CD) undergo surgery, most commonly ileocolectomy during their disease course.[
1,
2] Unfortunately, post-operative anastomotic recurrence (POR) is the rule, leading to additional surgery in up to a third of patients at 10 years.[2-4] Endoscopic post-operative recurrence (ePOR) assessed by the Rutgeerts score [
5] precedes and predicts clinical symptoms and progression to surgery.[
6]
Smoking, penetrating disease, submucosal myenteric plexitis, previous small bowel resections and histologic inflammation in the resected bowel margins were identified, among others, as risk factors for early disease recurrence.[7-10] The American Gastroenterological Association institute guidelines suggests post-operative early pharmacological prophylactic treatment with thiopurines or TNF-α inhibitors (TNFis) over endoscopic monitoring.[
11] The first surveillance colonoscopy is recommended 6-12 months following surgery. Clinical studies comparing prophylaxis with thiopurines and TNFis, yielded conflicting evidence.[12-16] We aimed to compare rates of ePOR in patients treated early with prophylactic 6-mercaptopurine (6-MP) or adalimumab.
2. Materials and Methods
2.1. Study design and population
We conducted a prospective, single center, randomized, open label, clinical study aimed to compare the efficacy of adalimumab to 6-MP for the maintenance of post-operative remission in patients with CD.
Patients with CD who underwent their first ileocecectomy due to active disease were screened 2 weeks post-operatively. Inclusion criteria: 18-70 years; clinical remission, i.e., Crohn’s disease activity index (CDAI) ≤ 150; eligible for treatment with adalimumab or 6MP. Exclusion criteria: residual inflammatory disease, i.e., residual ileal or colonic inflammation or perianal disease; experienced prior non-response or intolerance to TNFi agents; received antibiotics or mesalamine at baseline; had evidence of current infectious disease or severe or progressive systemic disease.
Eligible consecutive patients were allocated randomly in a 1:1 ratio to adalimumab or 6-MP treatment groups.
2.2. Study interventions
Treatment was started within 4 weeks following randomization. 6-MP was administered orally starting at a dose of 50 mg/day with escalating doses every 1-2 weeks as tolerated, to a target dose of 1-1.5 mg/kg. Adalimumab was administered as subcutaneous injections (SC), using pre-filled syringes (40 mg/syringe). Dosing regimen was as follows: Induction: week 0 – 160mg.; week 2 – 80mg; week 4- 40mg, maintenance: 40mg every other week. No treatment optimizations were performed.
2.3. Follow up
Scheduled clinic visits were conducted at baseline, and at 18, 32, 42 and 58 weeks post surgery, thereafter. Pathological data, including maximal small bowel diameter and inflammation of margins, was extracted from the surgical pathology report at baseline. At each visit patients were assessed for body weight, disease activity by calculating CDAI score and quality of life using the Inflammatory Bowel Disease Questionnaire (IBDQ) and the SF-36 health survey. Clinical activity was defined by CDAI score (<150 for clinical remission, 150-220 for mild clinical activity and >220 for moderately to severely active disease). Blood and stool samples were collected and analyzed for blood count, C-reactive protein and fecal calprotectin, respectively. Fecal calprotectin was assessed using IDK® Calprotectin ELISA test (Immundiagnostik AG, Stubenwald-Allee 8a, 64625 Bensheim, Germany) as recommended by the manufacturer. All patients were scheduled to undergo colonoscopies at weeks 32 and 58 post-operatively, to assess for disease recurrence using the Rutgeerts endoscopic scoring system. A Rutgeerts score of ≥ i2 was regarded as ePOR and was followed by withdrawal from the study. Two physicians, who were blinded to the patients' study group performed the endoscopies and graded the anastomotic appearance.
Treatment failure, resulting in withdrawal from the study was defined in patients with CD exacerbation during a 1-year treatment period as an increase of ≥70 points in CDAI compared to baseline and a total CDAI score ≥ 220; Rutgeerts score ≥ i2; patients requiring re-operation or starting steroid therapy; patients experiencing significant hypersensitivity reactions (e.g., bronchospasm, anaphylaxis or urticaria) to study medications; or if the patient was not compliant with the study protocol.
2.4. Study Outcomes
The primary endpoint was the proportion of patients with ePOR (Rutgeerts score ≥i2) at 58 weeks post-operatively.
The study’s secondary endpoints were ePOR rate at 32 weeks; proportion of patients at clinical remission at 32 and 58 weeks; time to clinical relapse; mean serum C-reactive protein (CRP) levels; mean serum TNFi levels, proportion of patients experiencing adverse events, and quality of life scores (IBDQ and SF-36 health survey).
Study protocol was approved by the Institutional Review Board of the Tel Aviv Medical Center, and all participants provided informed consent prior to the study enrollment. The study was listed as a clinical trial with a ClinicalTrials.gov Identifier: NCT01629628.
2.5. Statistical Analysis
We aimed to recruit a total of 100 patients, with a 1:1 ratio between treatment arms. We assumed an endoscopic recurrence rate of 60% at 1 year on 6MP therapy, and a 50% decrease in endoscopic recurrence on TNFi therapy. A total sample size of 100 patients was calculated to provide a power of 0.8 (α = 0.05). Unfortunately, patient enrollment did not meet expectations, partially due to approval of additional CD therapies by the Israeli ministry of health, offering alternative post-operative therapeutic options.
Descriptive statistics were calculated for each variable measured and are reported as means, medians, or proportions. Univariate analyses for differences in patients’ characteristics and demographics were determined using paired t tests or Wilcoxon rank-sum tests for means and McNemar tests for categorical variables. A p-value < 0.05 was considered significant. Kaplan-Meier estimate curves were plotted for relapse-free survival. Hazard ratios (HRs) for relapse-free survival were estimated by Cox regression, stratified by treatment arm and variable found to be statistically significant for predicting POR.
3. Results
Forty-one patients with CD who underwent first ileocolonic surgical resection for active CD during 2012-2018 were screened. The study was terminated by the PIs after enrollment of 41% of the intended cohort due to futility in recruitment rate. Patients were excluded for early post-operative complications (n=1), CD involving proximal small bowel (n=1) and withdrawal of consent (n=4), leaving 35 study eligible patients, 25 males, mean age 35±1.4 years, randomized to receive either 6-MP (n=16) or adalimumab (n=19). Study arms were comparable in baseline demographic parameters, BMI, smoking status, clinical scores and surgical findings as seen in
Table 1. Preoperative therapy was also comparable between the study groups. Specifically, 11(31%) patients were treated with TNFis preoperatively, of these 7(64%) were treated with adalimumab during the study and 4(36%) were started on 6-MP.
3.1. Study timeline
The actual study timeline was similar to the timeline projected in the protocol. Following surgery, patients were screened for the study at 4 (median, range: 1.7-8.9) weeks, commenced therapy with allocated treatment at 6.4 (median, range: 2.6-15.2) weeks and underwent surveillance endoscopies at 32.9 (median, range:25.6-42) weeks and 58.4 (median, range: 51.6-86) weeks.
3.2. ePOR at week 32
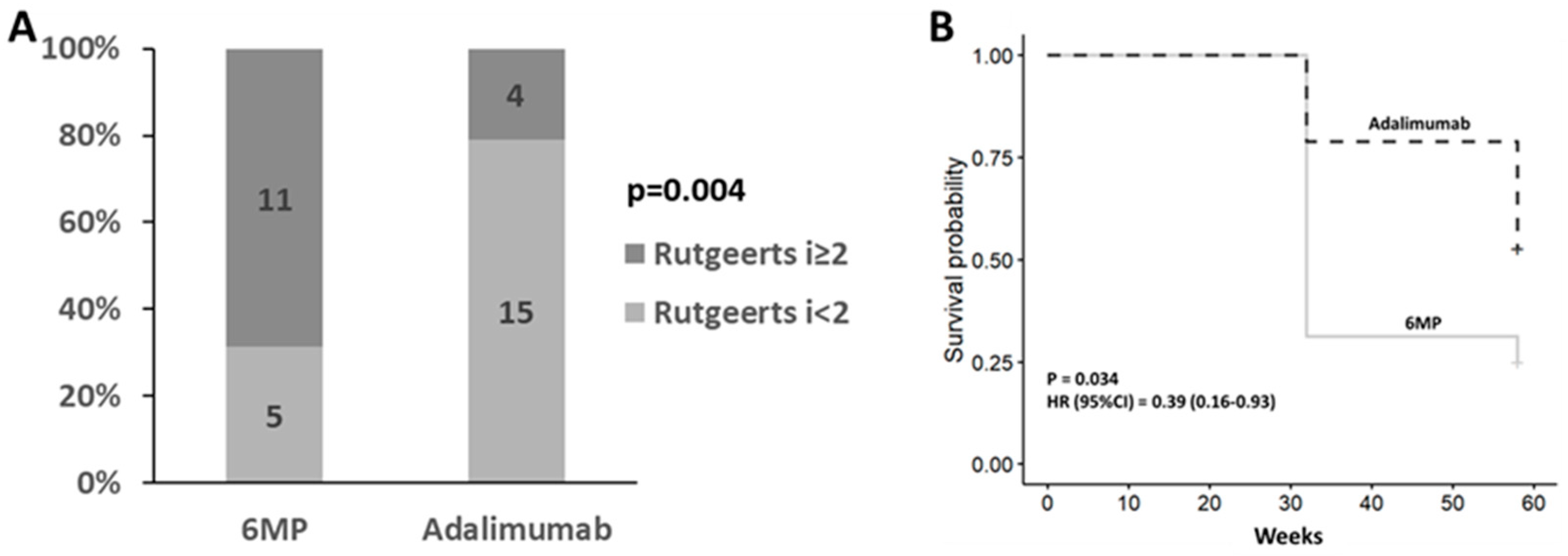
All 35 patients underwent Ileocolonoscopy 32.9 weeks following surgery. Of these, 15 (43%) patients were determined to have ePOR (Rutgeerts ≥ i2).
Post-operative treatment with adalimumab was associated with a lower recurrence rate compared with 6-MP therapy (4[21%] vs 11[69%], p=0.004) with a relative risk of 0.3 (95% CI: 0.12 – 0.78) (
Figure 1A).
3.3. Endoscopic recurrence at week 58
In the intention to treat cohort, 20 (63%) patients experienced ePOR at week 58. Of these, 12 (75%) in the 6MP study arm and 9 (47%) in the adalimumab arm. Post-operative treatment with adalimumab was associated with a significantly lower recurrence rate compared with 6-MP (p=0.03, HR=0.39, 95% CI=0.16-0.93) (
Figure 1B).
3.4. Factors Associated with Endoscopic POR
3.4.1. Small bowel diameter
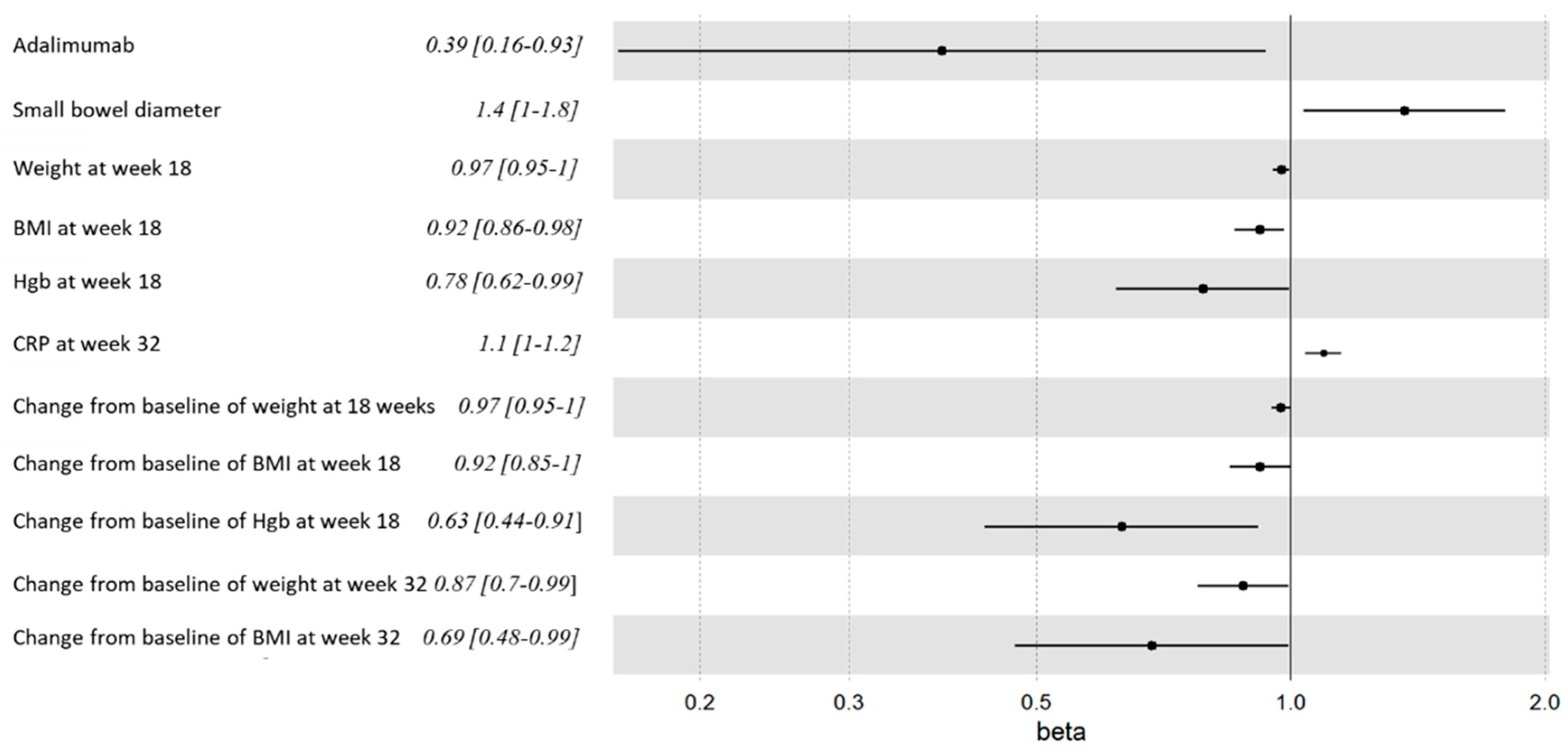
Increased resected small bowel diameter, as measured in the pathology lab, was associated with ePOR at weeks 32 and 58 (3.5±1.8 cm vs 3.1±0.8 cm, p=0.05) and (p=0.023, HR=1.4, 95% CI=1-1.8) (
Table 2,
supplement Table S1 and
Figure 2).
3.4.2. Inflammatory indices
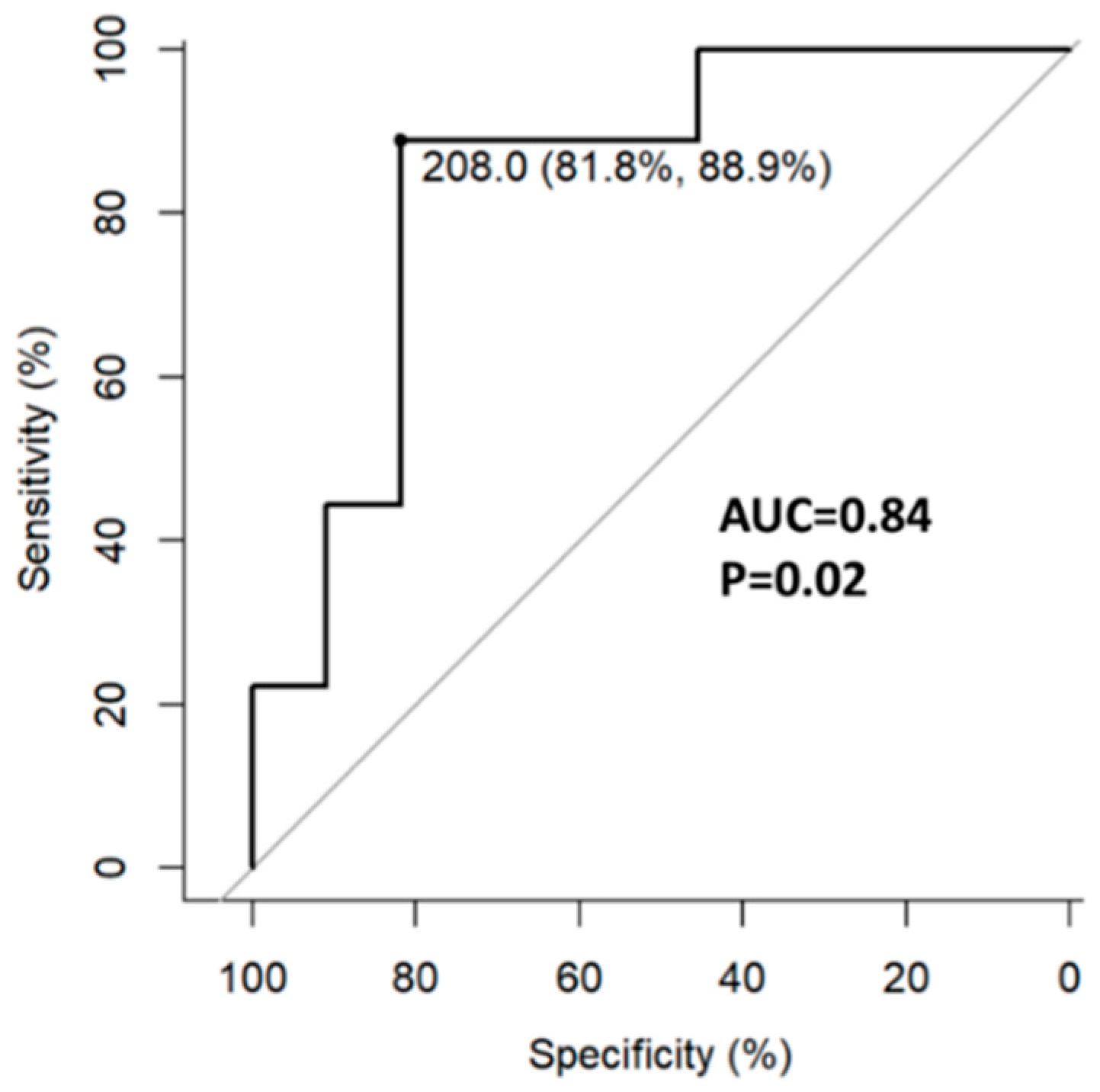
All 35 patients had week 18 and 58 labs. Higher week 18 fecal calprotectin concentration was associated with week 32 ePOR. The optimal cut-off point associated with ePOR was 208 µg/gr (AUC=0.84, 95%CI 0.65-1.0) with a sensitivity of 89% and specificity of 82% (
Figure 3).
Higher week 32 CRP levels were associated with week 58 CD recurrence (p=0.019, HR=1.1, 95%CI=1-1.2).
3.4.3. Weight and BMI
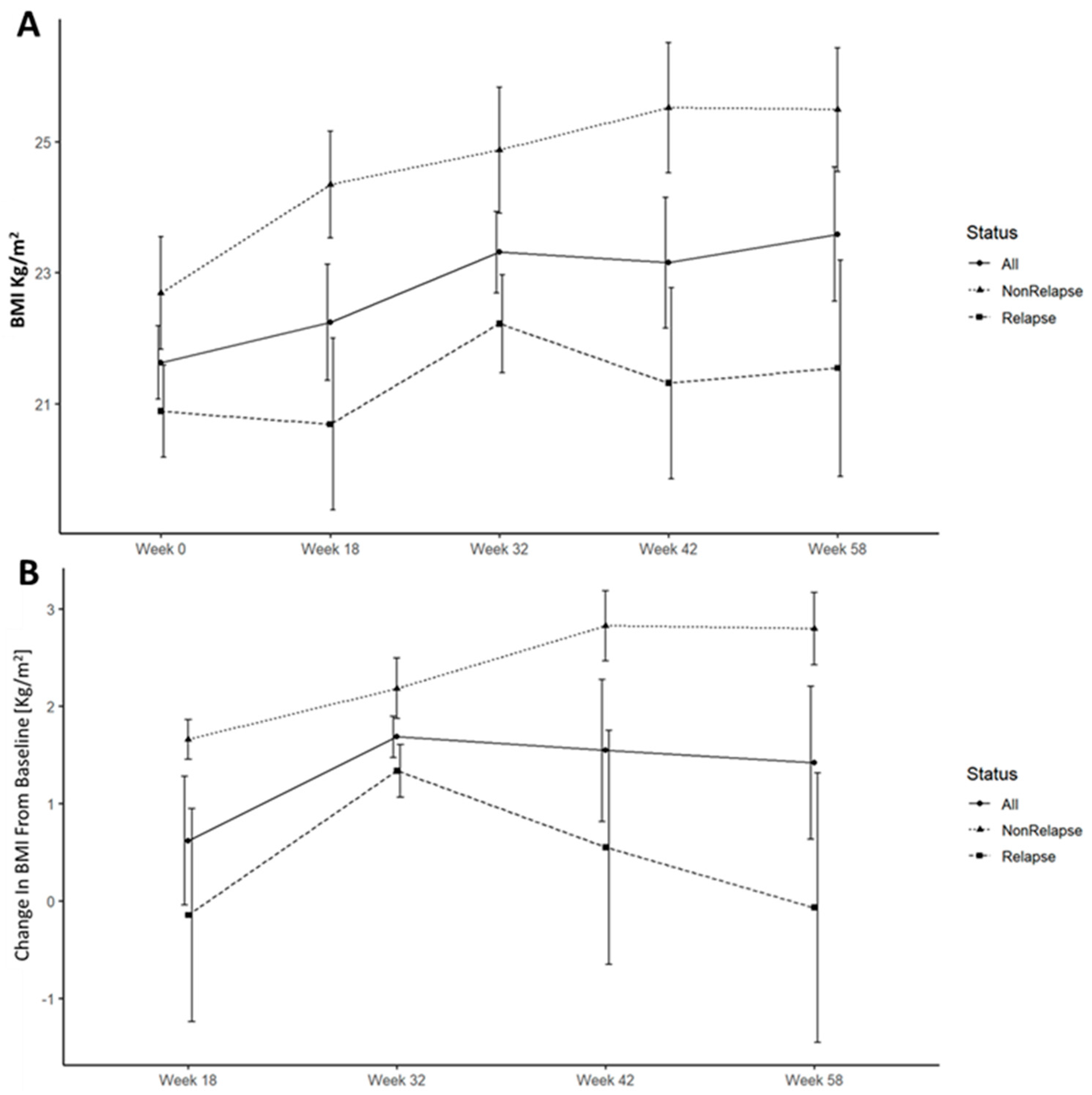
Lower weight and BMI were associated with increased risk of ePOR. This was noticed throughout the study duration and became statistically significant at weeks 18 and 32. (
Figure 2 and
Figure 4A). All patients gained weight and increased in BMI following surgery, however, this trend was significantly accentuated and durable in patients who remained disease free at week 58 (
Figure 4B).
3.4.4. Hemoglobin
Lower absolute haemoglobin (Hgb) levels at week 18, and a decrease of Hgb levels at week 18 compared to baseline were associated with ePOR at weeks 32 and 58 (
Table 3 and supplement table S2,
Figure 2).
3.4.5. Alanine aminotransferase
Higher alanine aminotransferase (ALT) levels at week 18 were associated with week 32 ePOR.
The associations of ALT and Hgb with ePOR remained even after excluding the 6-MP arm. Other baseline variables, previously described as impactful, such as disease extent, smoking status or inflammation of the resected bowel margins, were not associated with disease recurrence in our patient cohort (
Table 2).
3.5. Clinical recurrence
Clinical recurrence occurred in 3 patients and clinical scores, and quality of life questionnaires were comparable for both study arms at 32 and 58 weeks (
Table 3). Two patients progressed from clinical remission to mildly active clinical disease and one patient (adalimumab arm) progressed from mildly active disease to severely active clinical disease.
3.6. Safety
Adverse events and severe adverse events rates were comparable in the two study arms. Overall, 8 patients in the adalimumab arm reported 11 adverse events (examination with seton placement, neuropathy, rash, respiratory infections, folliculitis, psoriasis, alopecia) and 2 severe adverse events (abdominal pain, acute gastroenteritis), compared to the 6-MP arm where 9 patients reported 13 adverse events (leukopenia, hepatitis, respiratory infections) and 6 severe adverse events (leukopenia, hepatitis, dermatitis, abdominal wall infection, pneumonia, iatrogenic colon perforation).
No drug intolerance or infusion reactions leading to drug or study withdrawal were reported.
4. Discussion
In this study, Post OPerative Adalimumab Recurrance Trial (POPART), we aimed to compare rates of POR in patients treated early with prophylactic 6-mercaptopurine (6-MP) or adalimumab. Recruiting 35 patients and following them clinically and using laboratory and endoscopic biomarkers we demonstrated adalimumab superiority over 6-MP in preventing POR. We identified small bowel dilatation of the surgical specimen and lower post-operative weight/BMI as risk factors for ePOR. In addition, we identified several predictors of ePOR, including rate of weight/BMI gain, week 18 serum Hgb, ALT and fecal calprotectin levels, and week 32 CRP level.
International society guidelines recommend thiopurines and TNFis for prophylaxis of POR, regardless of patient risk stratification.[
17] The recommendation is without preference of drug mechanism and infers equivalence in efficacy. Prospective, randomized studies comparing thiopurines and TNFis as post-operative prophylaxis strategy are scarce with inconsistent results.
In a pilot study, Armuzzi et al compared 1-year ePOR in 22 patients with high-risk for POR randomized for treatment with infliximab or azathioprine. Infliximab was numerically superior to azathioprine; however, the study was underpowered and did not achieve statistical significance. Savarino et al performed a controlled, non-blinded study with 51 patients randomly allocated to three study arms comparing adalimumab, azathioprine and mesalamine.[
16] The study included high-risk patients (smokers with previous small bowel resection) and looked at 1-year endoscopic and clinical recurrence. Surprisingly, only 6.3% (one patient) of the adalimumab treated patients were found with ePOR compared with 64.7% and 83.3% in the azathioprine and mesalamine arms, respectively. Adalimumab was also significantly better at prevention of clinical recurrence with 12.5% recurrence rate, compared with 64.7% and 50% in the azathioprine and mesalamine groups. The GETECCU study, by Lopez-Sanroman et al, was a randomized, controlled, open-label study, comparing adalimumab and azathioprine in 61 patients, of which approximately 60% were high-risk for POR. All patients were treated with metronidazole for 3 months. ePOR rates at 58 weeks were comparable between adalimumab and azathioprine (29.7% vs 33.3%, p=0.76). A sub-analysis of the POCER study, compared adalimumab and azathioprine in high-risk patients, treated also with metronidazole for 3 months, and scoped for ePOR at 6 months.[
14] Adalimumab was superior to azathioprine in the prevention of POR (45% vs 21%, p=0.028). While the POCER study compared endoscopic-driven management strategies, we aimed to compare treatment efficacy. The difference in study goals manifested in different design and study populations.[
12] All the patients in the POCER study were treated with antibiotics, and only high-risk patients received early post-operative medical prophylaxis. Azathioprine was the default treatment for the high-risk patients, and only thiopurine intolerant patients were treated with adalimumab. We avoided initial antibiotic therapy in the POPART study to avoid short-term reduction in POR which may interfere with direct comparison of the efficacy of 6MP and adalimumab at 6 and 12 months. In addition, in contrast to the patient risk stratification in POCER, all patients in the POPART study were randomly allocated to early treatment with 6MP or adalimumab. Furthermore, approximately 60% of the patients in the POCER study had preoperative bowel perforation, 40% were smokers and 30-43% underwent prior surgeries. In comparison, the POPART study population had milder disease course, no prior surgeries and only 20% were smokers. Many of these patients would have not been eligible for early medical prophylaxis if they were enrolled to POCER. In short, in contrast to the POCER study, we randomly allocated mixed high and low risk populations, without stratification and without initial antibiotic therapy, to early prophylactic treatment with 6MP or adalimumab. We found adalimumab was more effective than 6-MP in the prevention of POR at 32 and 58 weeks post-operatively. We were also able to show that disease recurrence was associated with increased resected small bowel diameter which may be a marker of pre-stenotic dilatation. However, patients who suffered from fibrostenotic disease without an active inflammatory disease were not included in our study.
Regarding biomarkers, increased 6 months fecal calprotectin was associated with ePOR in the POCER study.[
18] Subsequent studies showed 3 months fecal calprotectin level to be predictive of endoscopic recurrence.[
19,
20] Our results replicate these findings with fecal calprotectin ≥218 µg/gr having a sensitivity of 88% and specificity of 82% for disease recurrence. Another finding was the association of lower initial post-operative weight and BMI with endoscopic recurrence. In addition, ePOR was associated with lower weight/BMI increase during the study. These findings may be interpreted either as evidence of worse surgical outcomes resulting from poor pre-surgical dietary status, or as persistent subacute inflammation in a more refractory patient population leading to initial and consequent lower weight and BMI.
Furthermore, we found that at 3 months decreased Hgb and increased ALT levels were predictive of ePOR. Decreased Hgb levels may represent a subclinical inflammation with subsequent iron loss and diminished absorption. Both increased ALT and decreased Hgb levels remained predictive of recurrence even when excluding the 6-MP arm, precluding 6-MP shunting as a cause of this finding.
We found no association between clinical and endoscopic recurrence, and overall showed very low clinical recurrence rates. These findings are in line with previous studies showing the disconnection between clinical and endoscopic findings and the lag of clinical following endoscopic recurrence, lending support to the choice of endoscopic recurrence as a monitoring metric in POR.[
6] Longer study follow up may have shown higher clinical recurrence rates.
Our study has merit, including its prospective study design and random allocation to study treatment. The study was performed in a single tertiary medical center and patients were screened, enrolled and treated in a similar fashion. Two physicians performed and graded all the endoscopies adding to the study procedures uniformity. We enrolled only patients undergoing ileocecectomy as their first surgical intervention and treated them without risk stratification or antibiotic treatment. This design not only offer direct comparison of the effects of prophylaxis but is also streamlined and closer to real-world practice avoiding complex patient stratification and biomarker monitoring with early therapy commencement.
However, our study is not without limitations, including the study open label design, introducing patient and physician bias in evaluating clinical and endoscopic disease activity. We did, however, blind the physician who performed the endoscopies and graded the anastomoses. Additional weakness is a low patient recruitment rate, probably due to the introduction of novel advanced therapies and due to the single center design, which may limit the generalizability of our findings. These led to the study termination before achieving the required number of patients. We were also unable to test for serum thiopurine metabolites and serum adalimumab levels, which means that our therapy was not optimized proactively, possibly underutilizing our medical options and increasing recurrence rates.
5. Conclusions
In conclusion, we show that adalimumab is more effective than 6-MP in post-operative CD prophylaxis. Increased surgical operative small bowel diameter and lower post-operative weight/BMI were associated with ePOR. Three months serum Hgb and ALT levels, and stool calprotectin concentration are predictive of ePOR. Our findings may improve the treatment of post-operative CD patients, especially in the low-risk patient group, and assist clinicians in the choice of pharmacologic prophylaxis.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
Conceptualization, Erez Scapa, Iris Dotan and Nitsan Maharshak; methodology, Erez Scapa, Iris Dotan and Nitsan Maharshak.; validation, Ayal Hirsch; formal analysis, Ayal Hirsch and Naomi Fliss-Isakov; investigation, Erez Scapa, Hagit Tulchinsky, Eran Itzkowitz, Yehuda Kariv, Yulia Ron, Henit Yanai, Ian White, Eli Brazovski, Iris Dotan, Nitsan Maharshak; data curation, Ayal Hirsch, Sharif Yasin; writing—original draft preparation, Ayal Hirsch; writing—review and editing, all authors.; visualization, Ayal Hirsch; supervision, Nitsan Maharshak and Iris Dotan; project administration, Erez Scapa, Nitsan Maharshak and Iris Dotan; funding acquisition, Erez Scapa, Nitsan Maharshak and Iris Dotan. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by by Abbvie. Abbvie, its employees or consultant did not take part or influenced the study design, data collection and analyses, and manuscript drafting.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of Tel Aviv Sourasky Medical Center. The study was listed as a clinical trial with a ClinicalTrials.gov Identifier: NCT01629628.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.
Conflicts of Interest
The authors received previously honoraria, speaking fees and educational and research grants from ABBVIE, the manufacturer of Humira and funder of this trial. Ayal Hirsch: honoraria, speaking fees and educational grant. Hagit Tulchinsky: speaking fees. Iris Dotan: honoraria, speaking fees and educational and research grants. Nitsan Maharshak: honoraria, speaking fees and educational and research grants. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Dittrich, A.E.; Sutton, R.T.; Haynes, K.; Wang, H.; Fedorak, R.N.; Kroeker, K.I. Incidence Rates for Surgery in Crohn's Disease Have Decreased: A Population-based Time-trend Analysis. Inflamm. Bowel Dis. 2020, 26, 1909–1916. [Google Scholar] [CrossRef] [PubMed]
- Stoss, C.; Berlet, M.; Reischl, S.; Nitsche, U.; Weber, M.C.; Friess, H.; Wilhelm, D.; Neumann, P.A. Crohn's disease: a population-based study of surgery in the age of biological therapy. Int. J. Color. Dis. 2021, 36, 2419–2426. [Google Scholar] [CrossRef]
- Peyrin-Biroulet, L.; Harmsen, W.S.; Tremaine, W.J.; Zinsmeister, A.R.; Sandborn, W.J.; Loftus, E.V., Jr. Surgery in a population-based cohort of Crohn's disease from Olmsted County, Minnesota (1970-2004). Am J Gastroenterol 2012, 107, 1693–1701. [Google Scholar] [CrossRef] [PubMed]
- Szanto, K.; Nyari, T.; Balint, A.; Bor, R.; Milassin, A.; Rutka, M.; Fabian, A.; Szepes, Z.; Nagy, F.; Molnar, T.; et al. Biological therapy and surgery rates in inflammatory bowel diseases - Data analysis of almost 1000 patients from a Hungarian tertiary IBD center. PLoS One 2018, 13, e0200824. [Google Scholar] [CrossRef]
- Rutgeerts, P.; Geboes, K.; Vantrappen, G.; Beyls, J.; Kerremans, R.; Hiele, M. Predictability of the postoperative course of Crohn's disease. Gastroenterology 1990, 99, 956–963. [Google Scholar] [CrossRef] [PubMed]
- Ble, A.; Renzulli, C.; Cenci, F.; Grimaldi, M.; Barone, M.; Sedano, R.; Chang, J.; Nguyen, T.M.; Hogan, M.; Zou, G.; et al. The Relationship Between Endoscopic and Clinical Recurrence in Postoperative Crohn's Disease: A Systematic Review and Meta-analysis. J Crohns Colitis 2022, 16, 490–499. [Google Scholar] [CrossRef]
- Cottone, M.; Rosselli, M.; Orlando, A.; Oliva, L.; Puleo, A.; Cappello, M.; Traina, M.; Tonelli, F.; Pagliaro, L. Smoking habits and recurrence in Crohn's disease. Gastroenterology 1994, 106, 643–648. [Google Scholar] [CrossRef] [PubMed]
- McLeod, R.S.; Wolff, B.G.; Ross, S.; Parkes, R.; McKenzie, M.; Investigators of the, C.T. Recurrence of Crohn's disease after ileocolic resection is not affected by anastomotic type: results of a multicenter, randomized, controlled trial. Dis Colon Rectum 2009, 52, 919–927. [Google Scholar] [CrossRef] [PubMed]
- Avidan, B.; Sakhnini, E.; Lahat, A.; Lang, A.; Koler, M.; Zmora, O.; Bar-Meir, S.; Chowers, Y. Risk factors regarding the need for a second operation in patients with Crohn's disease. Digestion 2005, 72, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Pascua, M.; Su, C.; Lewis, J.D.; Brensinger, C.; Lichtenstein, G.R. Meta-analysis: factors predicting post-operative recurrence with placebo therapy in patients with Crohn's disease. Aliment Pharmacol Ther 2008, 28, 545–556. [Google Scholar] [CrossRef]
- Adamina, M.; Bonovas, S.; Raine, T.; Spinelli, A.; Warusavitarne, J.; Armuzzi, A.; Bachmann, O.; Bager, P.; Biancone, L.; Bokemeyer, B.; et al. ECCO Guidelines on Therapeutics in Crohn's Disease: Surgical Treatment. J Crohns Colitis 2020, 14, 155–168. [Google Scholar] [CrossRef] [PubMed]
- De Cruz, P.; Kamm, M.A.; Hamilton, A.L.; Ritchie, K.J.; Krejany, E.O.; Gorelik, A.; Liew, D.; Prideaux, L.; Lawrance, I.C.; Andrews, J.M.; et al. Crohn's disease management after intestinal resection: a randomised trial. Lancet 2015, 385, 1406–1417. [Google Scholar] [CrossRef] [PubMed]
- Armuzzi, A.; Felice, C.; Papa, A.; Marzo, M.; Pugliese, D.; Andrisani, G.; Federico, F.; De Vitis, I.; Rapaccini, G.L.; Guidi, L. Prevention of postoperative recurrence with azathioprine or infliximab in patients with Crohn's disease: an open-label pilot study. J. Crohn's Colitis 2013, 7, e623–e629. [Google Scholar] [CrossRef]
- De Cruz, P.; Kamm, M.A.; Hamilton, A.L.; Ritchie, K.J.; Krejany, E.O.; Gorelik, A.; Liew, D.; Prideaux, L.; Lawrance, I.C.; Andrews, J.M.; et al. Efficacy of thiopurines and adalimumab in preventing Crohn's disease recurrence in high-risk patients - a POCER study analysis. Aliment. Pharmacol. Ther. 2015, 42, 867–879. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Sanroman, A.; Vera-Mendoza, I.; Domenech, E.; Taxonera, C.; Vega Ruiz, V.; Marin-Jimenez, I.; Guardiola, J.; Castro, L.; Esteve, M.; Iglesias, E.; et al. Adalimumab vs Azathioprine in the Prevention of Postoperative Crohn's Disease Recurrence. A GETECCU Randomised Trial. J Crohns Colitis 2017, 11, 1293–1301. [Google Scholar] [CrossRef] [PubMed]
- Savarino, E.; Bodini, G.; Dulbecco, P.; Assandri, L.; Bruzzone, L.; Mazza, F.; Frigo, A.C.; Fazio, V.; Marabotto, E.; Savarino, V. Adalimumab is more effective than azathioprine and mesalamine at preventing postoperative recurrence of Crohn's disease: a randomized controlled trial. Am. J. Gastroenterol. 2013, 108, 1731–1742. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, G.C.; Loftus, E.V., Jr.; Hirano, I.; Falck-Ytter, Y.; Singh, S.; Sultan, S.; Committee, A.G.A.I.C.G. American Gastroenterological Association Institute Guideline on the Management of Crohn's Disease After Surgical Resection. Gastroenterology 2017, 152, 271–275. [Google Scholar] [CrossRef]
- Wright, E.K.; Kamm, M.A.; De Cruz, P.; Hamilton, A.L.; Ritchie, K.J.; Krejany, E.O.; Leach, S.; Gorelik, A.; Liew, D.; Prideaux, L.; et al. Measurement of fecal calprotectin improves monitoring and detection of recurrence of Crohn's disease after surgery. Gastroenterology 2015, 148, 938–947. [Google Scholar] [CrossRef] [PubMed]
- Boube, M.; Laharie, D.; Nancey, S.; Hebuterne, X.; Fumery, M.; Pariente, B.; Roblin, X.; Peyrin-Biroulet, L.; Minet-Quinard, R.; Pereira, B.; et al. Variation of faecal calprotectin level within the first three months after bowel resection is predictive of endoscopic postoperative recurrence in Crohn's disease. Dig Liver Dis 2020, 52, 740–744. [Google Scholar] [CrossRef] [PubMed]
- Veyre, F.; Boschetti, G.; Meunier, C.; Cuerq, C.; Gay, C.; Charlois, A.L.; Duclaux-Loras, R.; Danion, P.; Cotte, E.; Kepenekian, V.; et al. Low Levels of Fecal Calprotectin 3 Months After Surgery Predict Subsequent Endoscopic Postoperative Remission in Crohn's Disease. Dig Dis Sci 2021, 66, 4429–4435. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).