Submitted:
21 October 2023
Posted:
23 October 2023
You are already at the latest version
Abstract
Keywords:
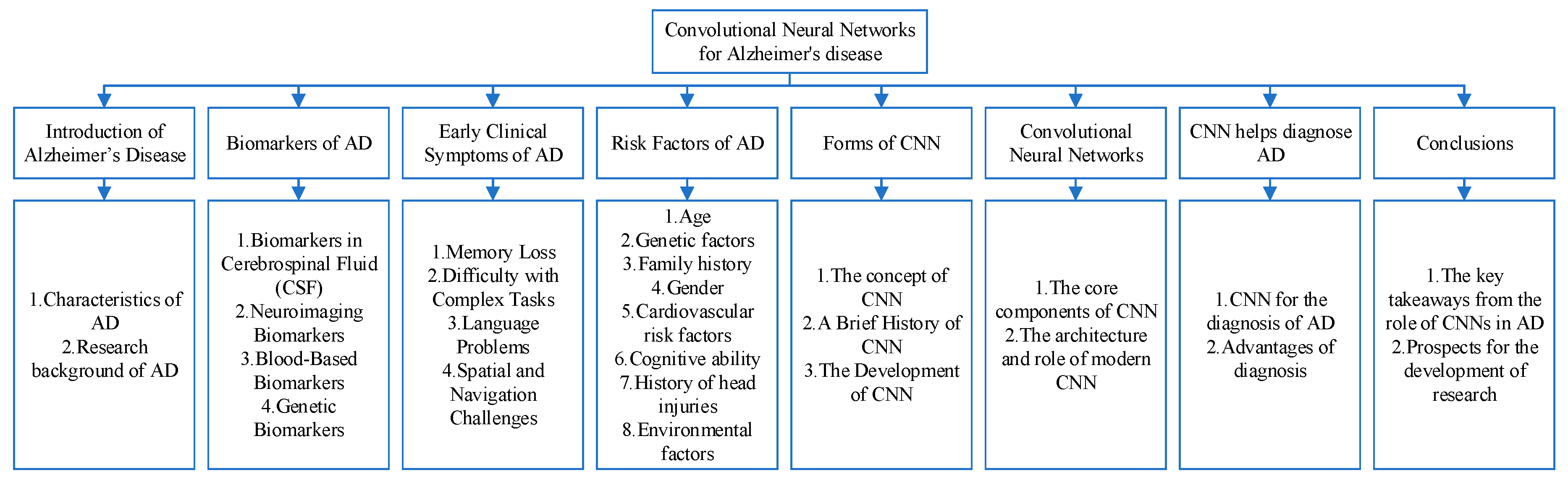
Introduction of Alzheimer’s Disease
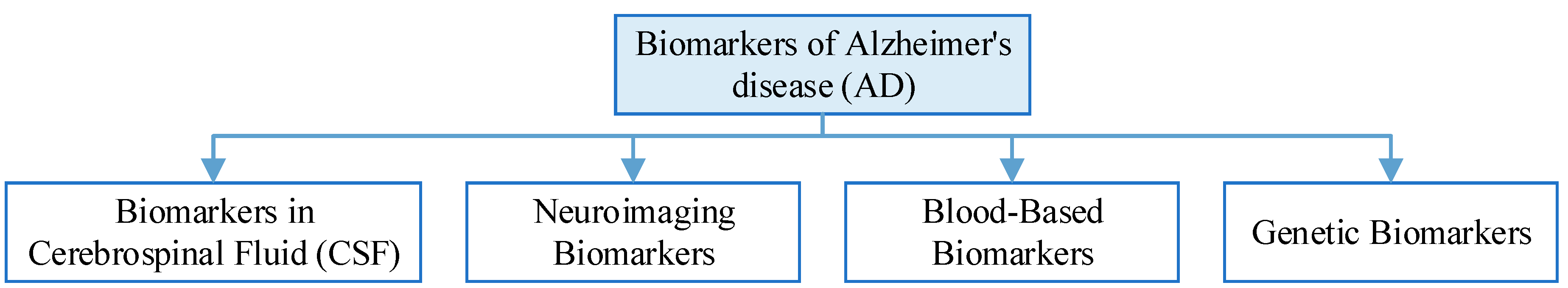
Biomarkers of AD
Early Clinical Symptoms of AD
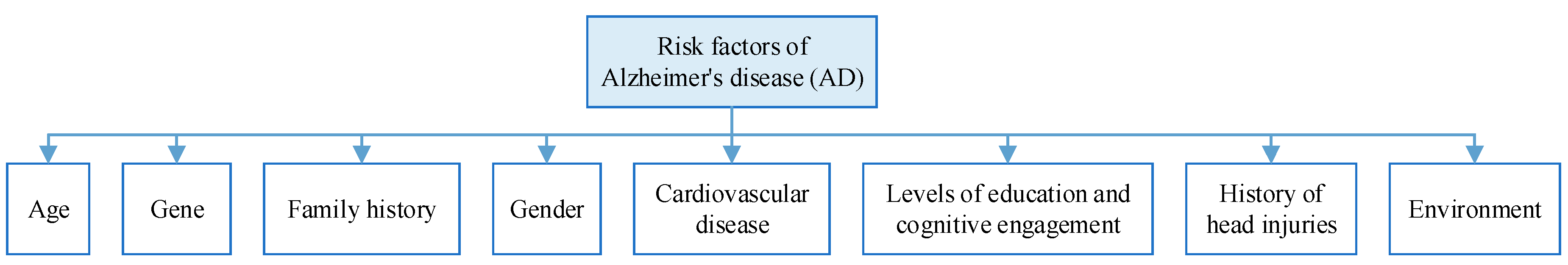
Risk Factors of AD
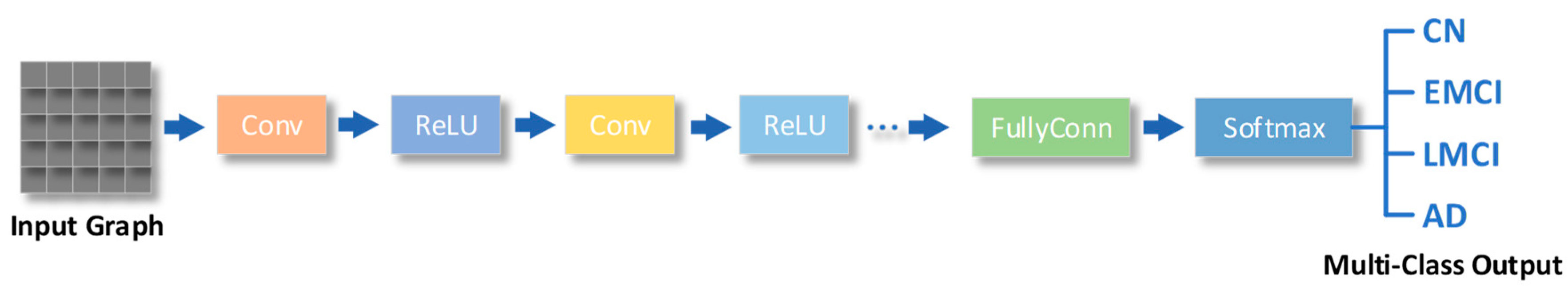
Convolutional Neural Networks
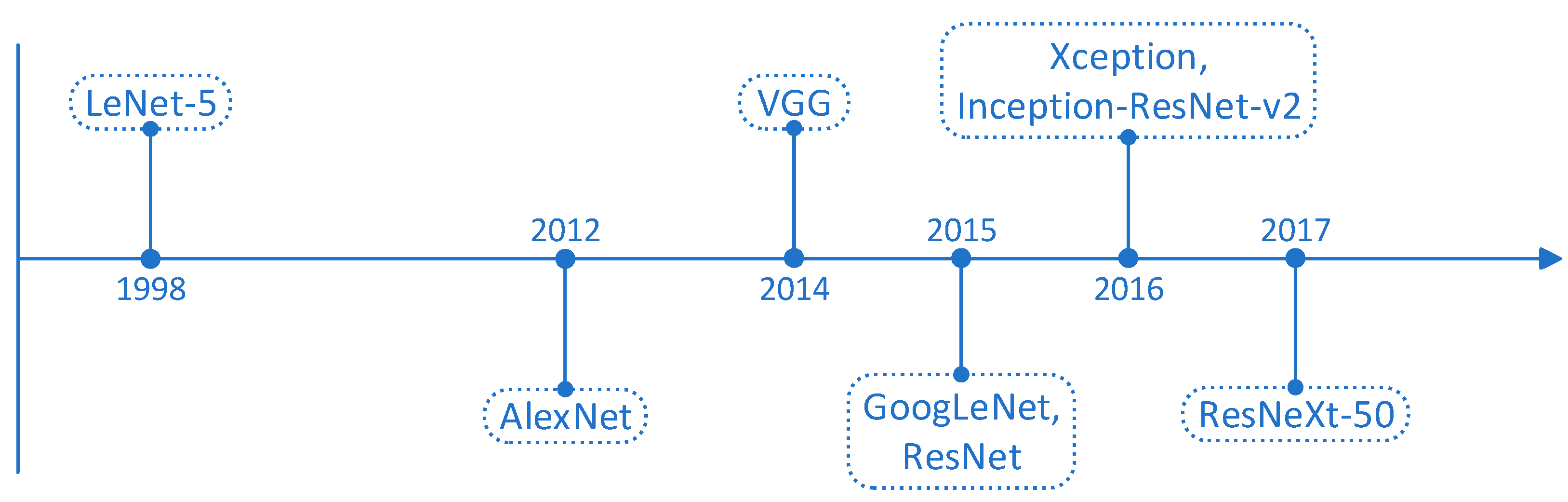
Forms of CNN
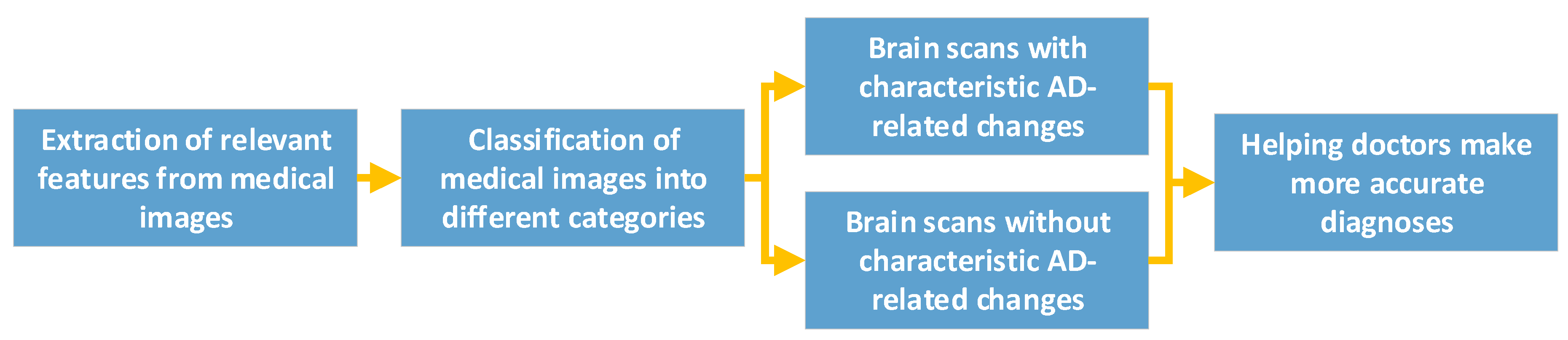
CNN Helps Diagnose AD
Conclusions
Funding
Acknowledgment
References
- Lu, Y.; Li, J.; Hu, T. Analysis of correlation between serum inflammatory factors and cognitive function, language, and memory in alzheimer’s disease and its clinical significance. Computational and Mathematical Methods in Medicine 2022, 2022. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.-R.; Huang, J.-B.; Yang, S.-L.; Hong, F.-F. Role of cholinergic signaling in Alzheimer’s disease. Molecules 2022, 27, 1816. [Google Scholar] [CrossRef]
- Zhang, Y. Prediction of MCI to Alzheimer’s conversion based on tensor-based morphometry and kernel support vector machine. Alzheimer’s & Dementia 2015, 11, 702. [Google Scholar]
- Li, X.; Feng, X.; Sun, X.; Hou, N.; Han, F.; Liu, Y. Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2019. Frontiers in Aging Neuroscience 2022, 14, 937486. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, M. Temporal patterns of the burden of Alzheimer’s disease and their association with Sociodemographic Index in countries with varying rates of aging 1990–2019. Aging Medicine 2023, 6, 281–289. [Google Scholar] [CrossRef]
- Zhang, Y. Three-Dimensional Eigenbrain for the Detection of Subjects and Brain Regions Related with Alzheimer’s Disease. Journal of Alzheimer’s Disease 2016, 50, 1163–1179. [Google Scholar] [CrossRef]
- Hampel, H.; Broich, K.; Hoessler, Y.; Pantel, J. Biological markers for early detection and pharmacological treatment of Alzheimer’s disease. Dialogues in clinical neuroscience 2022. [CrossRef] [PubMed]
- Klyucherev, T.O.; Olszewski, P.; Shalimova, A.A.; Chubarev, V.N.; Tarasov, V.V.; Attwood, M.M.; et al. Advances in the development of new biomarkers for Alzheimer’s disease. Translational Neurodegeneration 2022, 11, 1–24. [Google Scholar] [CrossRef]
- Wang, B.; Zhong, X.; Fields, L.; Lu, H.; Zhu, Z.; Li, L. Structural proteomic profiling of cerebrospinal fluids to reveal novel conformational biomarkers for Alzheimer’s disease. Journal of the American Society for Mass Spectrometry 2023, 34, 459–471. [Google Scholar] [CrossRef]
- Virgilio, E.; Vecchio, D.; Crespi, I.; Puricelli, C.; Barbero, P.; Galli, G.; et al. Cerebrospinal fluid biomarkers and cognitive functions at multiple sclerosis diagnosis. Journal of Neurology 2022, 269, 3249–3257. [Google Scholar] [CrossRef]
- Wang, S.-H. Alzheimer’s Disease Detection by Pseudo Zernike Moment and Linear Regression Classification. CNS & Neurological Disorders - Drug Targets 2017, 16, 11–15. [Google Scholar]
- Zhou, J.; Benoit, M.; Sharoar, M.G. Recent advances in pre-clinical diagnosis of Alzheimer’s disease. Metabolic Brain Disease 2022, 37, 1703–1725. [Google Scholar] [CrossRef] [PubMed]
- McKay, N.S.; Gordon, B.A.; Hornbeck, R.C.; Dincer, A.; Flores, S.; Keefe, S.J.; et al. Positron emission tomography and magnetic resonance imaging methods and datasets within the Dominantly Inherited Alzheimer Network (DIAN). Nature Neuroscience 2023, 26, 1449–1460. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jin, C.; Zhou, J.; Zhou, R.; Tian, M.; Lee, H.J.; et al. PET molecular imaging for pathophysiological visualization in Alzheimer’s disease. European Journal of Nuclear Medicine and Molecular Imaging 2023, 50, 765–783. [Google Scholar] [CrossRef] [PubMed]
- Brickman, A.M.; Manly, J.J.; Honig, L.S.; Sanchez, D.; Reyes-Dumeyer, D.; Lantigua, R.A.; et al. Correlation of plasma and neuroimaging biomarkers in Alzheimer’s disease. Annals of clinical and translational neurology 2022, 9, 756–761. [Google Scholar] [CrossRef] [PubMed]
- Teunissen, C.E.; Verberk, I.M.; Thijssen, E.H.; Vermunt, L.; Hansson, O.; Zetterberg, H.; et al. Blood-based biomarkers for Alzheimer’s disease: towards clinical implementation. The Lancet Neurology 2022, 21, 66–77. [Google Scholar] [CrossRef]
- Varesi, A.; Carrara, A.; Pires, V.G.; Floris, V.; Pierella, E.; Savioli, G.; et al. Blood-based biomarkers for alzheimer’s disease diagnosis and progression: An overview. Cells 2022, 11, 1367. [Google Scholar] [CrossRef]
- Li, R.-X.; Ma, Y.-H.; Tan, L.; Yu, J.-T. Prospective biomarkers of Alzheimer’s disease: A systematic review and meta-analysis. Ageing Research Reviews 2022, 101699. [Google Scholar] [CrossRef]
- Fernández-Calle, R.; Konings, S.C.; Frontiñán-Rubio, J.; García-Revilla, J.; Camprubí-Ferrer, L.; Svensson, M.; et al. APOE in the bullseye of neurodegenerative diseases: Impact of the APOE genotype in Alzheimer’s disease pathology and brain diseases. Molecular Neurodegeneration 2022, 17, 62. [Google Scholar] [CrossRef]
- Maurya, R.; Bhattacharjee, G.; Khambhati, K.; Gohil, N.; Singh, P.; Mani, I.; et al. Amyloid precursor protein in Alzheimer’s disease. Progress in Molecular Biology and Translational Science 2023, 196, 261–270. [Google Scholar]
- Hansson, O.; Edelmayer, R.M.; Boxer, A.L.; Carrillo, M.C.; Mielke, M.M.; Rabinovici, G.D.; et al. The Alzheimer’s Association appropriate use recommendations for blood biomarkers in Alzheimer’s disease. Alzheimer’s & Dementia 2022, 18, 2669–2686. [Google Scholar]
- Zhu, Z.; Ma, X.; Wu, J.; Xiao, Z.; Wu, W.; Ding, S.; et al. Altered gut microbiota and its clinical relevance in mild cognitive impairment and Alzheimer’s disease: Shanghai Aging Study and Shanghai Memory Study. Nutrients 2022, 14, 3959. [Google Scholar] [CrossRef]
- Wang, S. Detection of Alzheimer’s Disease by Three-Dimensional Displacement Field Estimation in Structural Magnetic Resonance Imaging. Journal of Alzheimer’s Disease 2016, 50, 233–248. [Google Scholar] [CrossRef] [PubMed]
- Mirakhori, F.; Moafi, M.; Milanifard, M.; Tahernia, H. Diagnosis and Treatment Methods in Alzheimer’s Patients Based on Modern Techniques: The Orginal Article. Journal of Pharmaceutical Negative Results, 1907. [Google Scholar]
- Jahn, H. Memory loss in Alzheimer’s disease. Dialogues in clinical neuroscience 2022. [Google Scholar] [CrossRef] [PubMed]
- Corriveau-Lecavalier, N.; Machulda, M.M.; Botha, H.; Graff-Radford, J.; Knopman, D.S.; Lowe, V.J.; et al. Phenotypic subtypes of progressive dysexecutive syndrome due to Alzheimer’s disease: A series of clinical cases. Journal of Neurology 2022, 269, 4110–4128. [Google Scholar] [CrossRef] [PubMed]
- Polsinelli, A.J.; Apostolova, L.G. Atypical Alzheimer disease variants. CONTINUUM: Lifelong Learning in Neurology 2022, 28, 676–701. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.; Martínez, M.C. Spatial memory deficits in Alzheimer’s disease and their connection to cognitive maps’ formation by place cells and grid cells. Frontiers in Behavioral Neuroscience 2023, 16, 1082158. [Google Scholar] [CrossRef]
- Gillis, C.; Montenigro, P.; Nejati, M.; Maserejian, N. Estimating prevalence of early Alzheimer’s disease in the United States, accounting for racial and ethnic diversity. Alzheimer’s & Dementia 2023, 19, 1841–1848. [Google Scholar]
- Jabeen, K.; Rehman, K.; Akash, M.S.H. Genetic mutations of APOEε4 carriers in cardiovascular patients lead to the development of insulin resistance and risk of Alzheimer’s disease. Journal of biochemical and molecular toxicology 2022, 36, e22953. [Google Scholar] [CrossRef]
- Mielke, M.M.; Aggarwal, N.T.; Vila-Castelar, C.; Agarwal, P.; Arenaza-Urquijo, E.M.; Brett, B.; et al. Consideration of sex and gender in Alzheimer’s disease and related disorders from a global perspective. Alzheimer’s & dementia 2022, 18, 2707–2724. [Google Scholar]
- Pasqualetti, G.; Thayanandan, T.; Edison, P. Influence of genetic and cardiometabolic risk factors in Alzheimer’s disease. Ageing Research Reviews 2022, 101723. [Google Scholar] [CrossRef]
- Mielke, M.M.; Ransom, J.E.; Mandrekar, J.; Turcano, P.; Savica, R.; Brown, A.W. Traumatic brain injury and risk of Alzheimer’s disease and related dementias in the population. Journal of Alzheimer’s disease 2022, 88, 1049–1059. [Google Scholar] [CrossRef]
- Rezende-Teixeira, P.; Dusi, R.G.; Jimenez, P.C.; Espindola, L.S.; Costa-Lotufo, L.V. What can we learn from commercial insecticides? Efficacy, toxicity, environmental impacts, and future developments. Environmental Pollution 2022, 300, 118983. [Google Scholar] [CrossRef]
- Zhang, Y. Image processing methods to elucidate spatial characteristics of retinal microglia after optic nerve transection. Scientific Reports 2016, 6, 21816. [Google Scholar] [CrossRef]
- Taki, O.; Rhazi, K.S.; Mejdoub, Y. Stirling engine optimization using artificial neural networks algorithm. in ITM Web of Conferences 2023, 02010. [Google Scholar] [CrossRef]
- Turhan, G.; Küçük, H.; Isik, E.O. Spatio-temporal convolution for classification of alzheimer disease and mild cognitive impairment. Computer Methods and Programs in Biomedicine 2022, 221, 106825. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, H.; Wan, Z. Ore image classification based on improved CNN. Computers and Electrical Engineering 2022, 99, 107819. [Google Scholar] [CrossRef]
- Nugraha, G.S.; Darmawan, M.I.; Dwiyansaputra, R. Comparison of CNN’s Architecture GoogleNet, AlexNet, VGG-16, Lenet-5, Resnet-50 in Arabic Handwriting Pattern Recognition. Kinetik: Game Technology, Information System, Computer Network, Computing, Electronics, and Control 2023. [Google Scholar] [CrossRef]
- Zhang, Y.D.; Satapathy, S. A seven-layer convolutional neural network for chest CT-based COVID-19 diagnosis using stochastic pooling. IEEE Sensors Journal 2022, 22, 17573–17582. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-D. Improving ductal carcinoma in situ classification by convolutional neural network with exponential linear unit and rank-based weighted pooling. Complex & Intelligent Systems 2021, 7, 1295–1310. [Google Scholar]
- Chen, H. Hardware Implementation for Convolutional Neural Networks in Artificial Intelligence. Highlights in Science, Engineering and Technology 2023, 62, 73–77. [Google Scholar] [CrossRef]
- Nazir, S.; Dickson, D.M.; Akram, M.U. Survey of explainable artificial intelligence techniques for biomedical imaging with deep neural networks. Computers in Biology and Medicine 2023, 106668. [Google Scholar] [CrossRef]
- Torres, M.; Cantú, F. Learning to see: Convolutional neural networks for the analysis of social science data. Political Analysis 2022, 30, 113–131. [Google Scholar] [CrossRef]
- Cao, J.; Li, Y.; Sun, M.; Chen, Y.; Lischinski, D.; Cohen-Or, D.; et al. Do-conv: Depthwise over-parameterized convolutional layer. IEEE Transactions on Image Processing 2022, 31, 3726–3736. [Google Scholar] [CrossRef]
- Wang, S. Cerebral micro-bleeding identification based on a nine-layer convolutional neural network with stochastic pooling. Concurrency and Computation: Practice and Experience 2020, 31, e5130. [Google Scholar] [CrossRef]
- Zafar, A.; Aamir, M.; Nawi, N.M.; Arshad, A.; Riaz, S.; Alruban, A.; et al. A comparison of pooling methods for convolutional neural networks. Applied Sciences 2022, 12, 8643. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, H.; Meng, Z.; Koniusz, P.; King, I. Graph-adaptive rectified linear unit for graph neural networks. in Proceedings of the ACM Web Conference 2022, 2022, 1331–1339. [Google Scholar]
- Sharma, A.; Singh, S.; Ratna, S. Graph Neural Network Operators: a Review. Multimedia Tools and Applications 2023, 1–24. [Google Scholar] [CrossRef]
- Zheng, T.; Wang, Q.; Shen, Y.; Lin, X. Gradient rectified parameter unit of the fully connected layer in convolutional neural networks. Knowledge-Based Systems 2022, 248, 108797. [Google Scholar] [CrossRef]
- Fan, X.; Feng, X.; Dong, Y.; Hou, H. COVID-19 CT image recognition algorithm based on transformer and CNN. Displays 2022, 72, 102150. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Shi, Y.; Lin, R.; Qiao, W.; Ba, W. A novel oil pipeline leakage detection method based on the sparrow search algorithm and CNN. Measurement 2022, 204, 112122. [Google Scholar] [CrossRef]
- Tufail, A.B.; Ullah, I.; Rehman, A.U.; Khan, R.A.; Khan, M.A.; Ma, Y.-K.; et al. On disharmony in batch normalization and dropout methods for early categorization of Alzheimer’s disease. Sustainability 2022, 14, 14695. [Google Scholar] [CrossRef]
- Bonakdarpour, B.; Takarabe, C. Brain Networks, Clinical Manifestations, and Neuroimaging of Cognitive Disorders: The Role of Computed Tomography (CT), Magnetic Resonance Imaging (MRI), Positron Emission Tomography (PET), and Other Advanced Neuroimaging Tests. Clinics in Geriatric Medicine 2023, 39, 45–65. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Guo, Q.; Zhang, Y.; Zheng, J.; Yang, Y.; Du, X.; et al. Application of Deep Learning for Prediction of Alzheimer’s Disease in PET/MR Imaging. Bioengineering 2023, 10, 1120. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-T.T.; Rosa-Neto, P.; Gauthier, S. Advanced brain imaging for the diagnosis of Alzheimer disease. Current Opinion in Neurology 2023, 36, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Fathi, S.; Ahmadi, M.; Dehnad, A. Early diagnosis of Alzheimer’s disease based on deep learning: A systematic review. Computers in biology and medicine 2022, 146, 105634. [Google Scholar] [CrossRef]
- Aytaç, U.C.; Güneş, A.; Ajlouni, N. A novel adaptive momentum method for medical image classification using convolutional neural network. BMC Medical Imaging 2022, 22, 1–12. [Google Scholar] [CrossRef]
- Wang, S. Pathological Brain Detection by a Novel Image Feature—Fractional Fourier Entropy. Entropy 2015, 17, 8278–8296. [Google Scholar] [CrossRef]
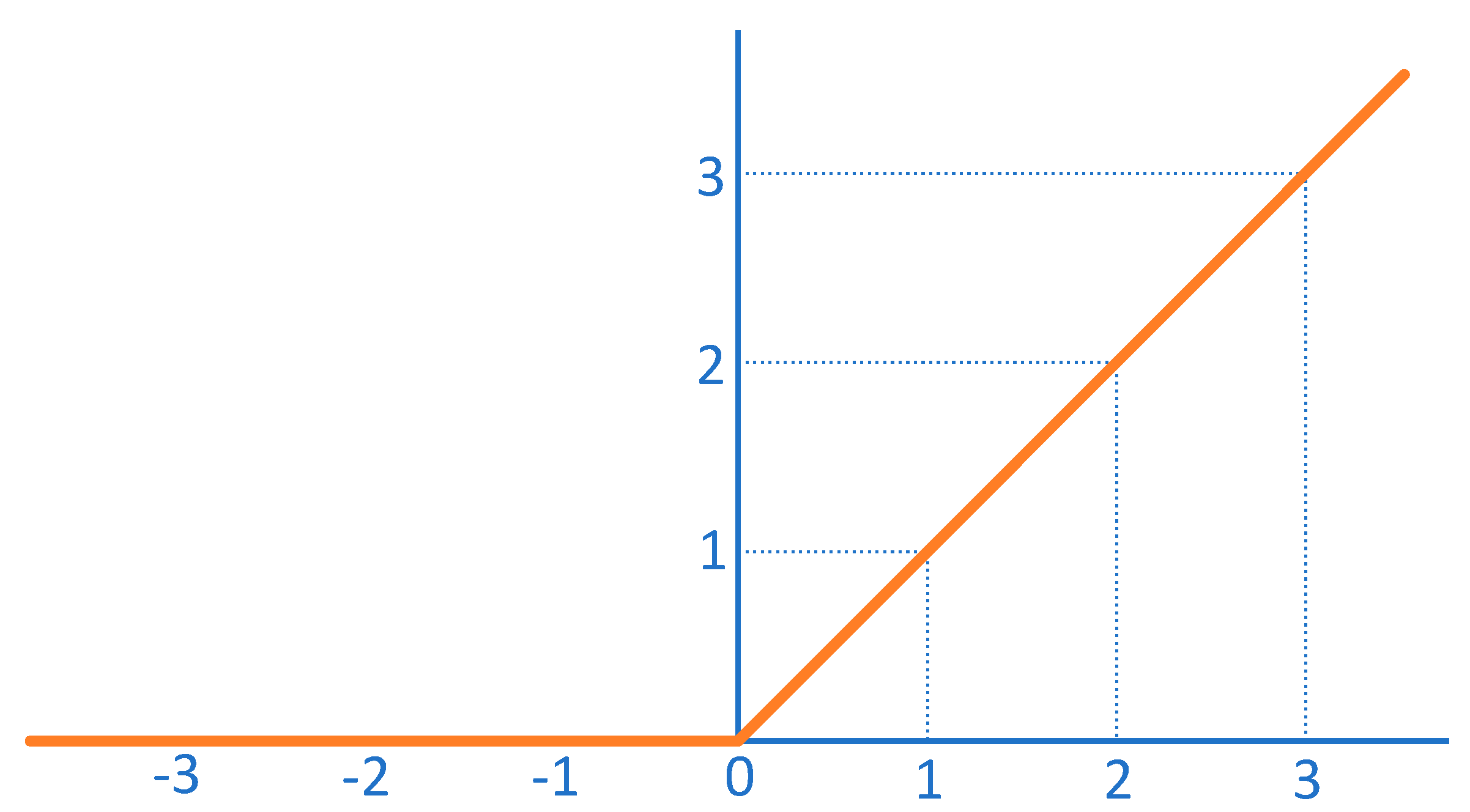
| Type of biomarkers for AD | Biomarker testing |
|---|---|
| Biomarkers in Cerebrospinal Fluid (CSF) | Two key biomarkers in CSF are elevated levels of tau protein and decreased levels of beta-amyloid. Increased tau levels are indicative of neurodegeneration, while reduced beta-amyloid levels suggest amyloid plaque buildup in the brain, which is a hallmark of AD. |
| Neuroimaging Biomarkers | PET scans using radiotracers like PiB can detect amyloid plaques, while FDG PET scans can assess brain metabolism. Structural MRI can reveal changes in brain volume and atrophy associated with AD. Functional MRI can assess brain connectivity and network disruptions. |
| Blood-Based Biomarkers | Plasma Aβ42 reflects changes in brain amyloid, and the Aβ42/Aβ40 ratio is thought to predict Aβ protein pathology deposition in people at risk for AD, and can be used as a prescreening method for AD in people with subjective cognitive decline and mild cognitive impairment. Elevated plasma Tau concentrations in patients with AD can help in the diagnosis of AD. |
| Genetic Biomarkers | The genetic risk of Alzheimer’s disease can be assessed by testing the APOE and MTHFR genes. Mutations in pathogenic AD genes (APP, PSEN1 or PSEN2) can increase the certainty of clinical diagnosis of AD dementia. |
| Stage of AD | Common symptom |
|---|---|
| Asymptomatic stage | Amyloidosis occurs only in brain cells, without significant cognitive decline or mental behavioural abnormalities, and the process often lasts from ten to twenty years. |
| Mild cognitive impairment | Subjective cognition continues to decline from the previous level, and objective testing confirms the presence of cognitive impairment or psycho-behavioural changes. However, the patient can carry out activities of daily living independently. The patient’s main manifestation is memory loss, and there may also be emotional apathy. |
| Mild dementia | The patient is unable to perform labour and work independently, and the main symptoms are severe memory loss and loss of time orientation. |
| Moderate dementia | Extensive impact on daily life, basic functions partially impaired, patients unable to live independently, often needing assistance, disorientation of the location. |
| Severe dementia | It has a serious impact on daily life, and the patient is completely dependent on others for basic activities, including self-care. The main symptoms are aphasia, dysfunction, incontinence, and so on. |
| Risk factor | Introduction |
|---|---|
| Hypertension | Hypertension is one of the most common chronic diseases in modern times and is an important risk factor for cardiovascular disease, and current research suggests that high blood pressure (either elevated systolic or diastolic) in midlife (between the ages of 40 and 60) increases the risk of developing Alzheimer’s disease. |
| High cholesterol | Cholesterol cannot penetrate the blood-brain barrier, but hypercholesterolaemia is associated with an increased risk of Alzheimer’s disease and vascular cognitive impairment. |
| Diabetes | The age of onset of diabetes is significantly associated with the risk of developing dementia later in life, with the earlier the age of onset the higher the risk of dementia. |
| Obesity | When a person gains weight, activity and blood flow to all areas of the brain decrease. Being overweight or obese severely affects brain activity and can increase the risk of Alzheimer’s disease as well as many other mental and cognitive disorders. |
| Risk factor | Manifestation |
|---|---|
| Age | Age is the biggest risk factor for Alzheimer’s disease. Studies have shown that the incidence of Alzheimer’s disease increases by a factor of one for every 5 to 10 years of age over the age of 65, on average. And if you carry Alzheimer’s disease risk genes such as APOEε4, the likelihood of developing the disease is even greater with age. |
| Family history | Familial Alzheimer’s disease is autosomal dominant, which means that if a parent has familial Alzheimer’s disease, the causative agent must be passed on to the offspring, and the incidence of the disease in the offspring carrying the causative gene is almost 100 per cent, whereas in those not carrying the causative gene, the offspring will not have the disease. |
| Gene | Studies have shown that APOE genes play an important role in the development of late-onset Alzheimer’s disease and sporadic Alzheimer’s disease, with the APOε4 allele being the best-known for people over the age of 65. |
| Traumatic brain injury | Traumatic Brain Injury (TBI) often leads to changes in brain structure and function, as well as cognitive problems such as memory deficits, impaired social functioning, and decision-making difficulties. Mild TBI (also known as concussion) is a known risk factor for Alzheimer’s disease. |
| The key component of CNNs | Features | Functionalities |
|---|---|---|
| Convolutional layers | A convolutional layer is a layer in which the output is obtained by performing a convolutional operation on the input by means of a convolutional kernel (also known as a filter). | Convolutional layers can efficiently extract local features from the input and are therefore widely used in fields such as image recognition and computer vision. |
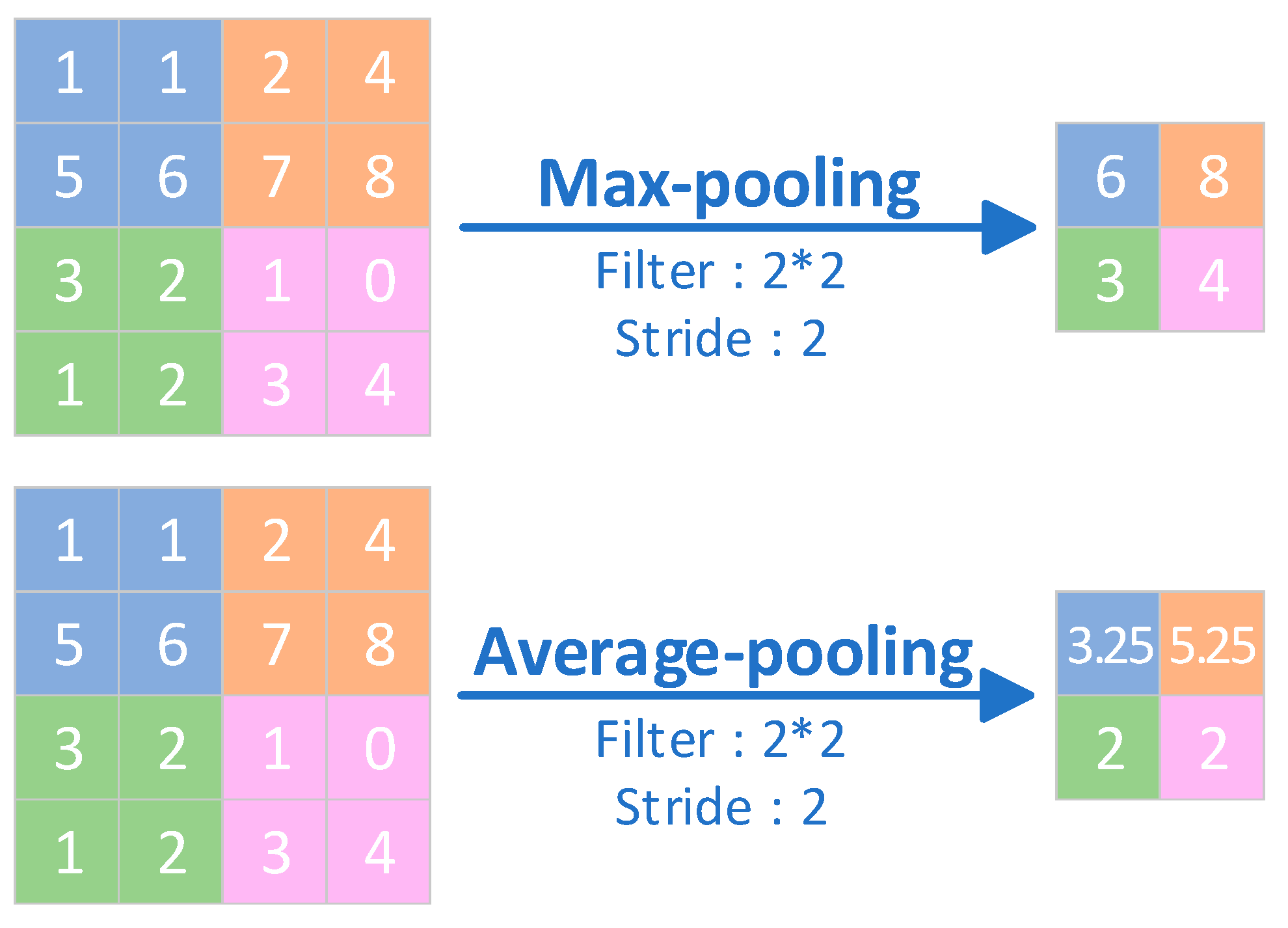
| Pooling layers | The pooling layer is the layer where the output is obtained by performing a downsampling operation on the input. | The pooling layer reduces the size of the inputs and reduces computational complexity, while providing a degree of translation invariance that helps to improve the generalisation of the model. |
| Fully-connected layers | The fully connected layer is the layer that connects all neurons of the input to all neurons of the output. | Fully-connected layers can make full use of all the information in the input, but they are also prone to overfitting, and therefore require attention to techniques such as regularisation during training. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




