Submitted:
23 October 2023
Posted:
23 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and discussion
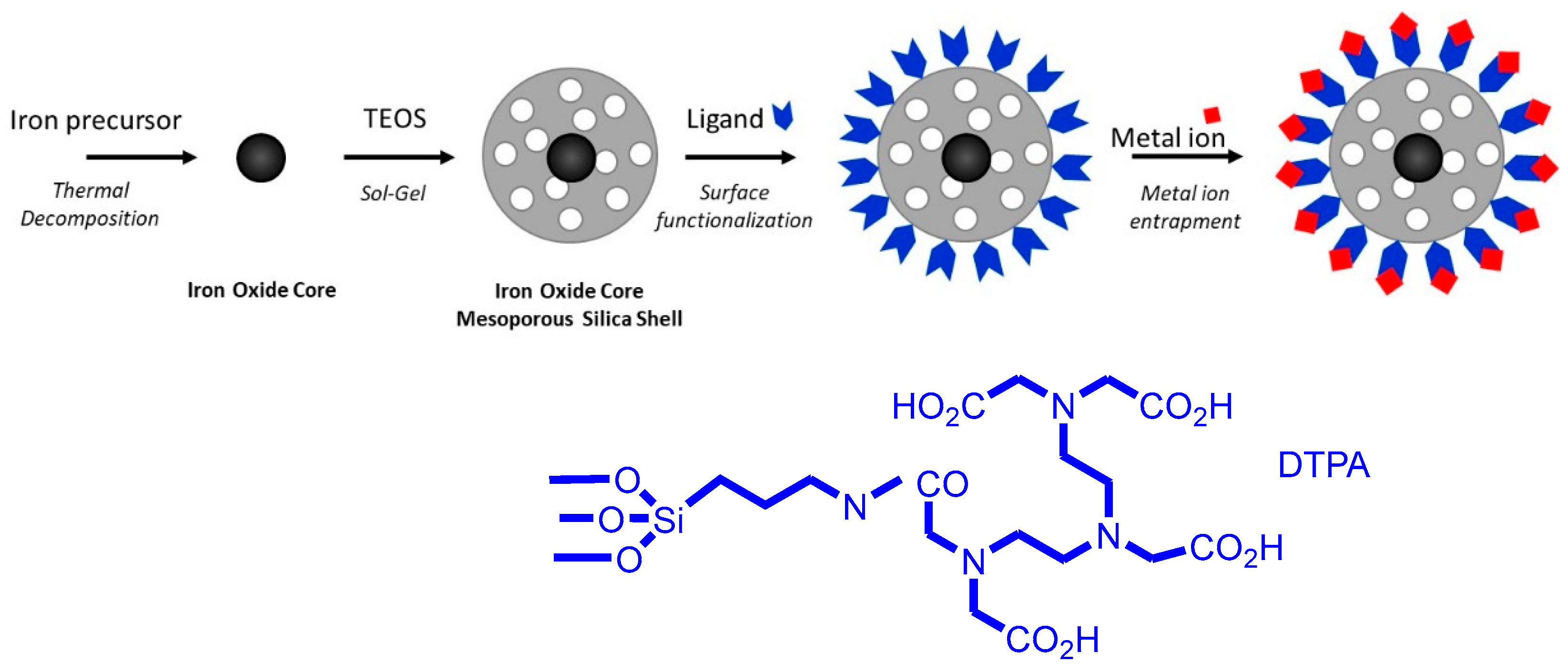
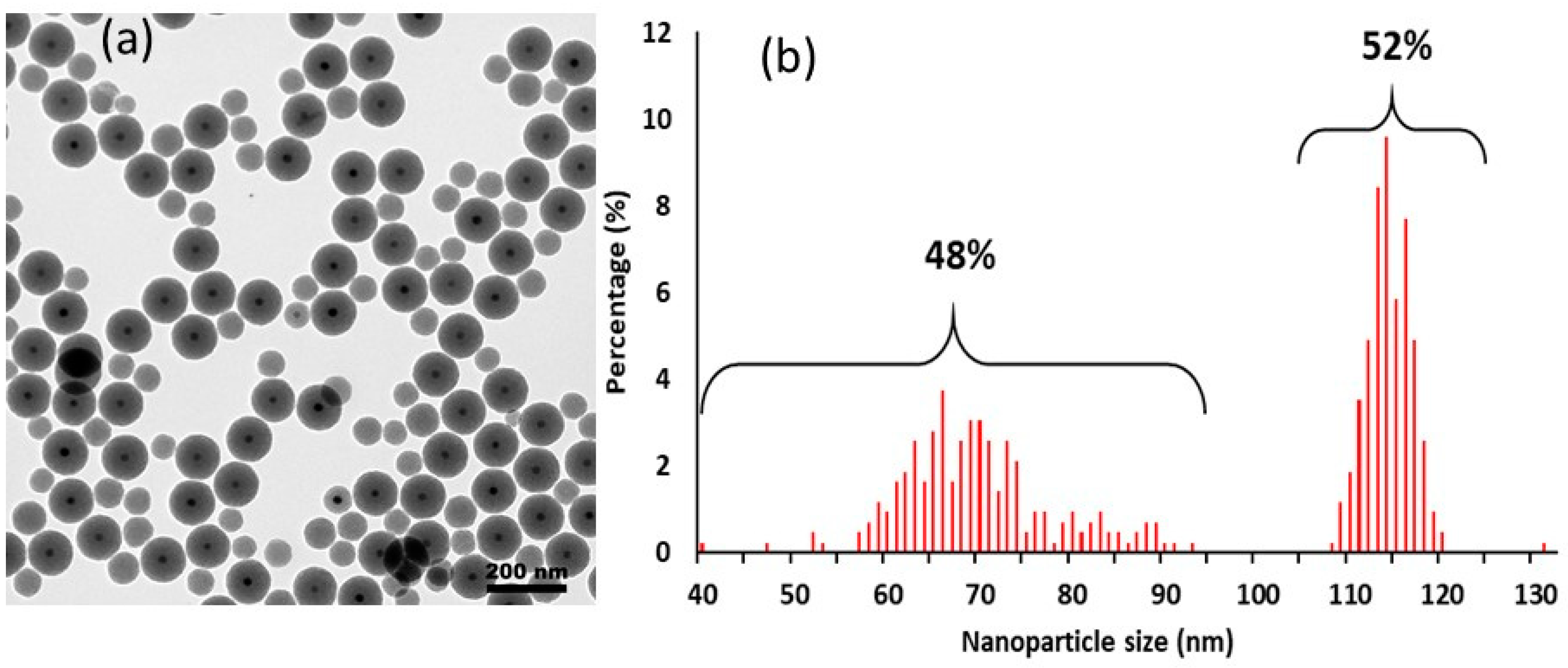
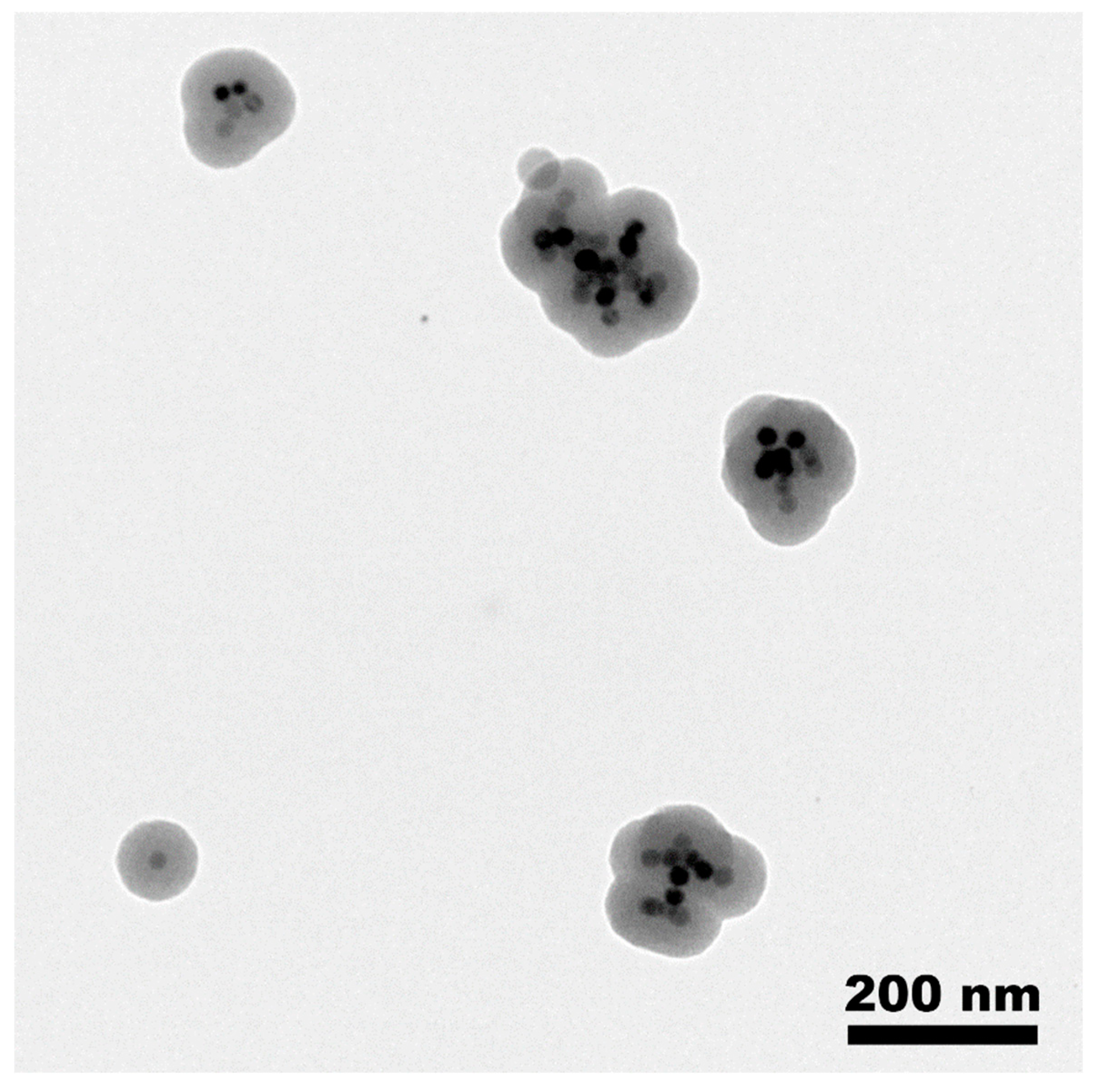
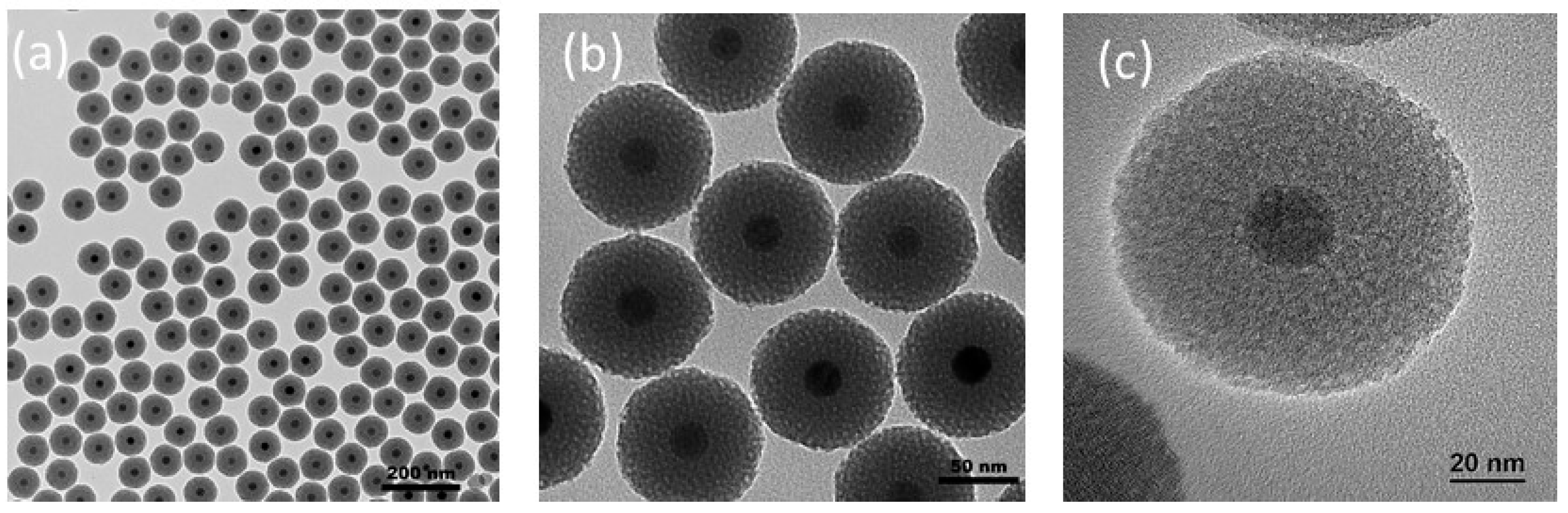
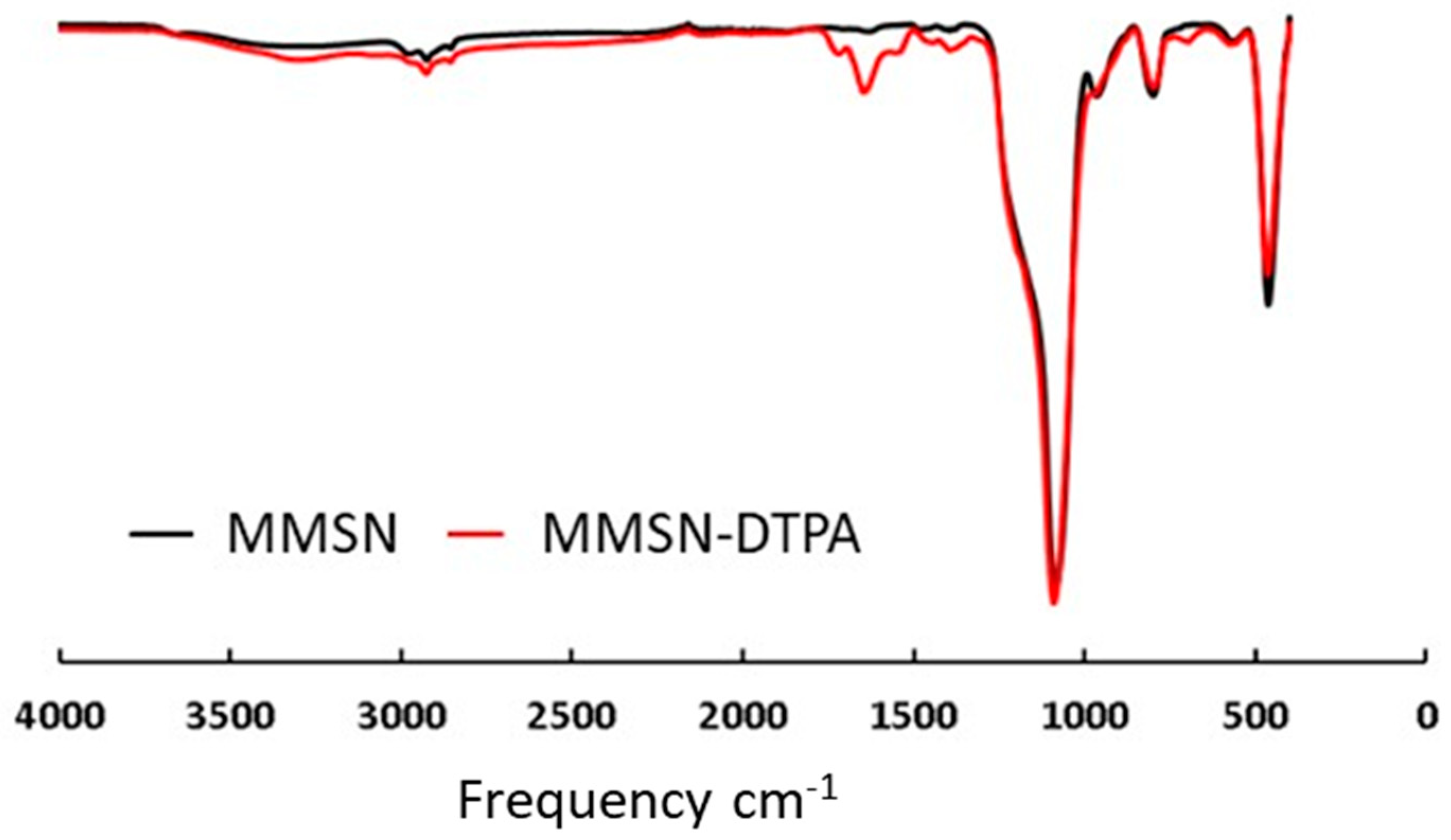
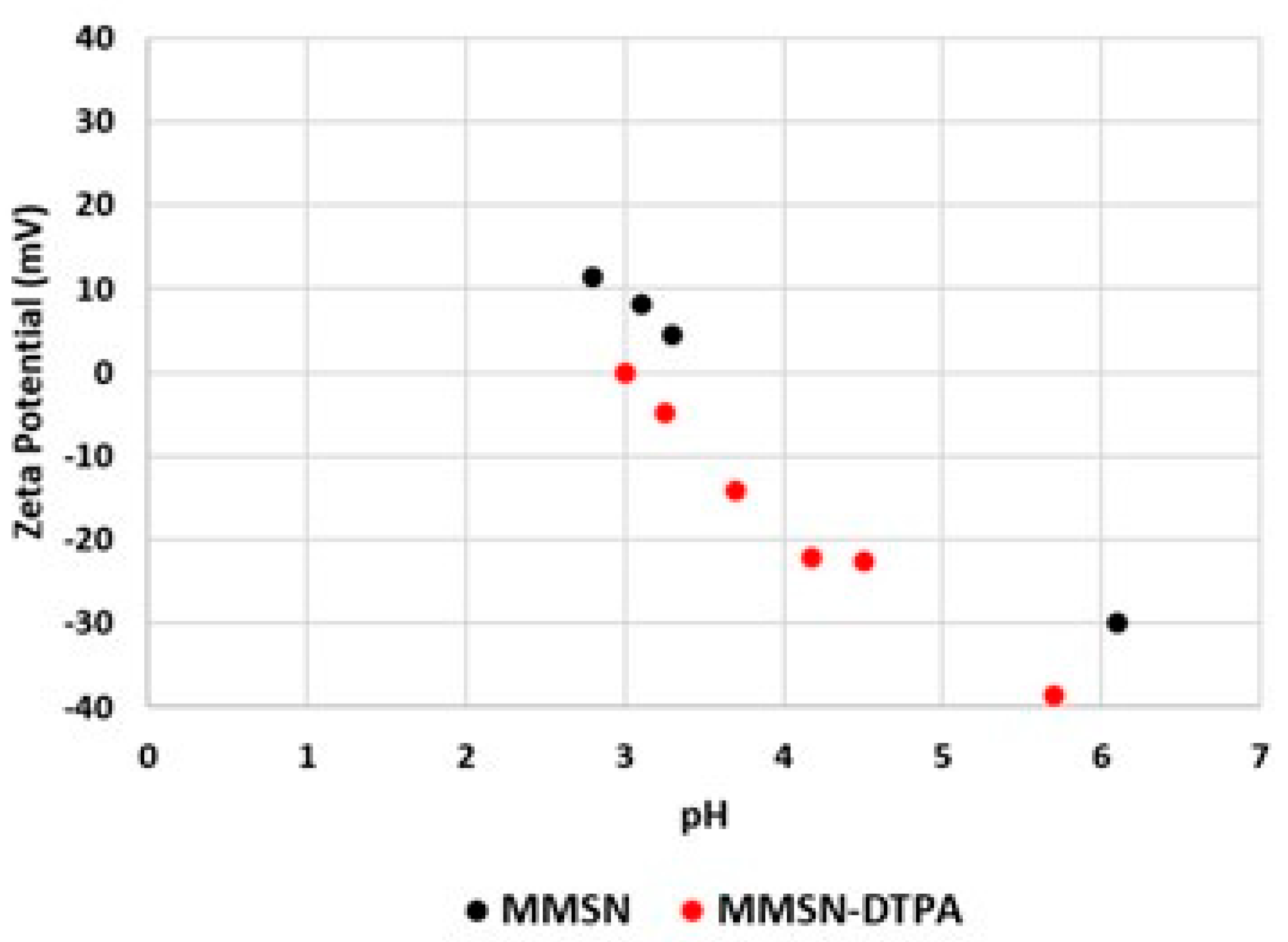
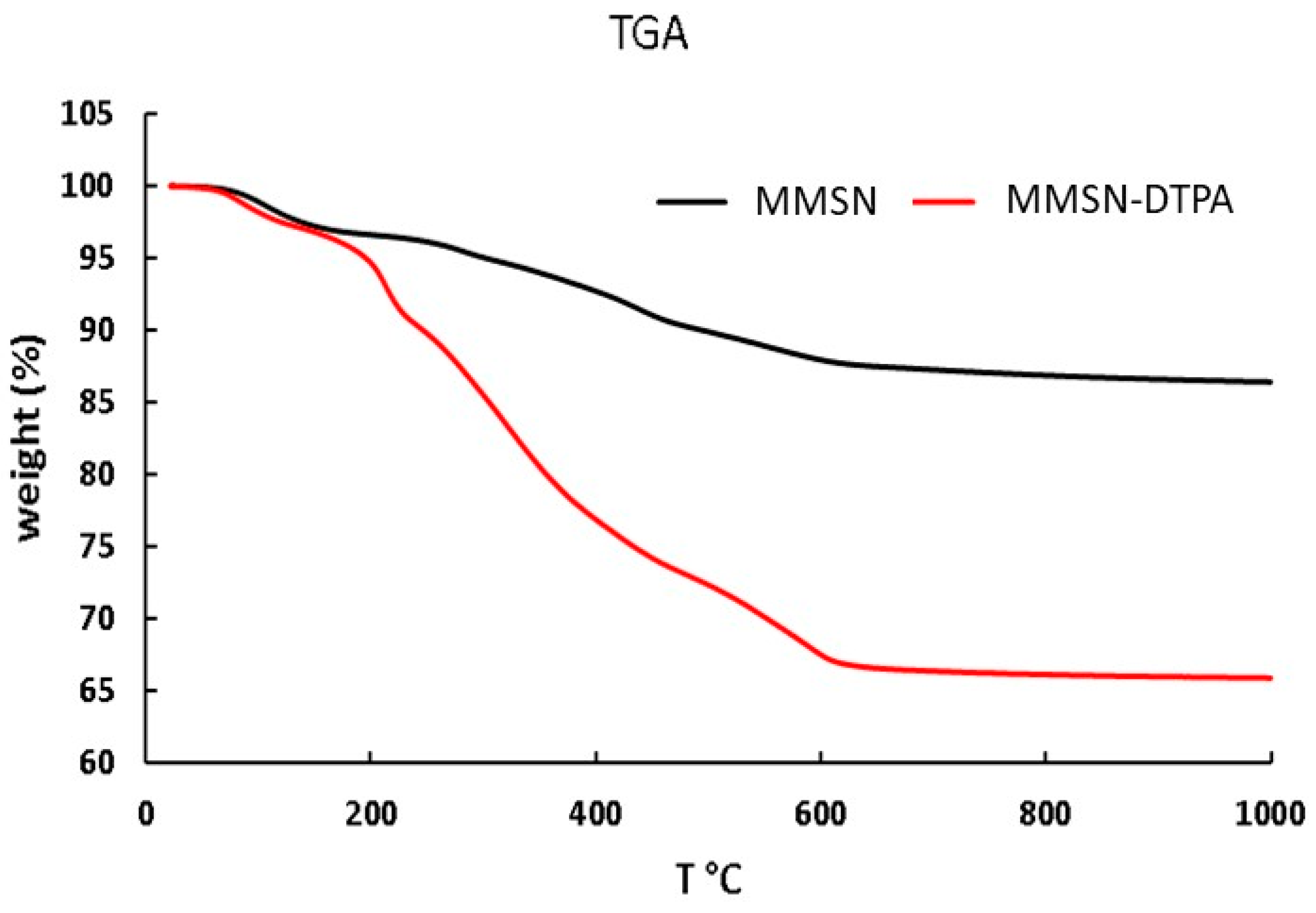
2.1. Preparation of MMSN and MMSN-DTPA
2.2. Adsorption of heavy metals using MMSN and MMSN-DTPA
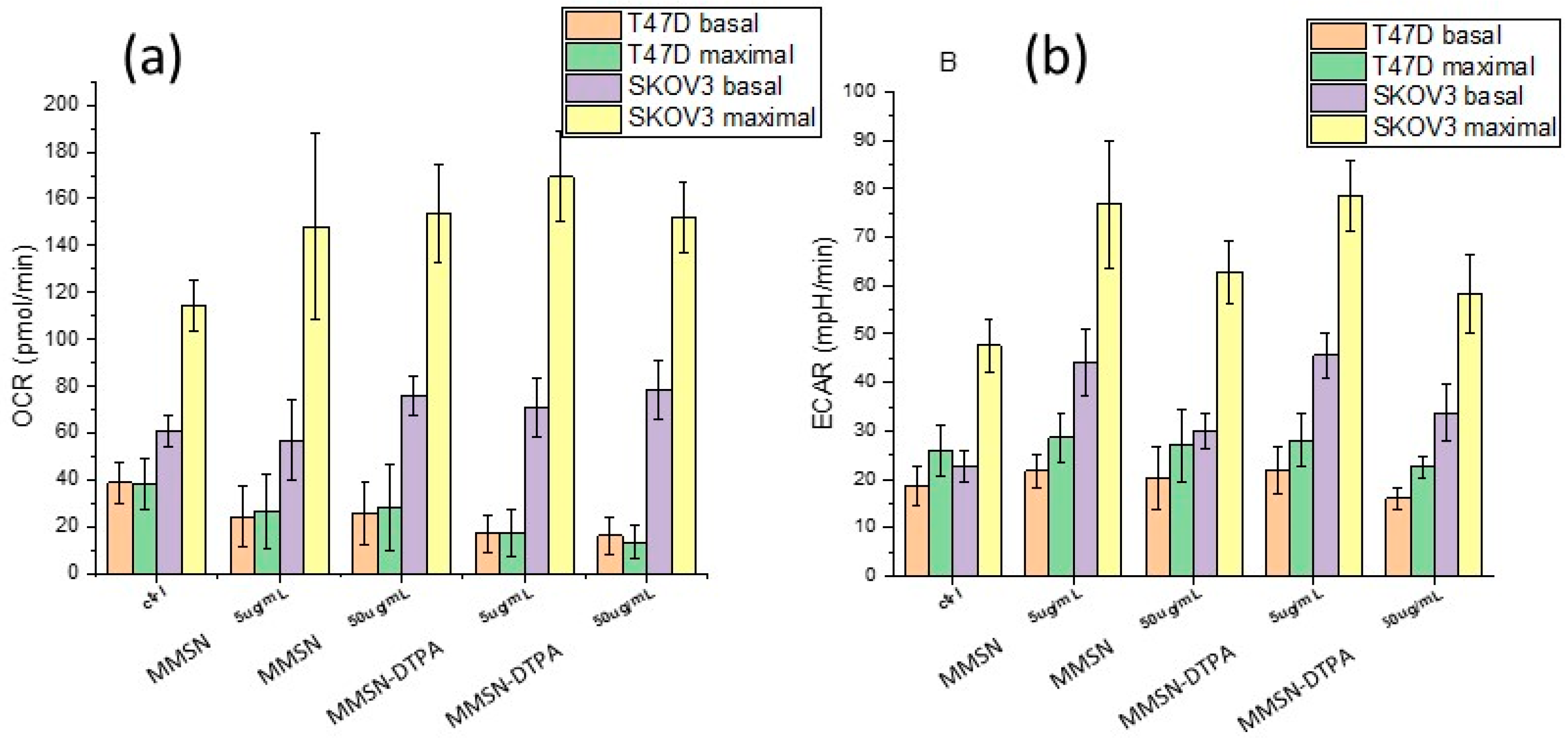
2.3. Nanosafety
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Sample Availability
References
- Ménard, M.; Meyer, F.; Affolter-Zbaraszczuk, C.; Rabineau, M.; Adam, A.; Ramirez, P.D.; Bégin-Colin, S.; Mertz, D. Design of hybrid protein-coated magnetic core-mesoporous silica shell nanocomposites for MRI and drug release assessed in a 3D tumor cell model. Nanotechnology 2019, 30, 174001. [CrossRef]
- Gao, Q.; Xie, W.; Wang, Y.; Wang, D.; Guo, Z.; Gao, F.; Zhao, L.; Cai, Q. A theranostic nanocomposite system based on radial mesoporous silica hybridized with Fe3O4 nanoparticles for targeted magnetic field responsive chemotherapy of breast cancer. RSC Adv. 2018, 8, 4321-4328. [CrossRef]
- Das, R.K.; Pramanik, A.; Majhi, M.; Mohapatra, S. Magnetic Mesoporous Silica Gated with Doped Carbon Dot for Site-Specific Drug Delivery, Fluorescence, and MR Imaging. Langmuir 2018, 34, 5253-5262. [CrossRef]
- Nasirzadeh, K.; Nazarian, S.; Hayat, S.M.G. Inorganic nanomaterials: a brief overview of the applications and developments in sensing and drug delivery. J. Appl. Biotechnol. Rep. 2016, 3, 395-402.
- Wang, Y.; Zhou, B.; Wu, S.; Wang, K.; He, X. Colorimetric detection of hydrogen peroxide and glucose using the magnetic mesoporous silica nanoparticles. Talanta 2015, 134, 712-717. [CrossRef]
- Wang, Y.; Li, B.; Zhang, L.; Li, P.; Wang, L.; Zhang, J. Multifunctional Magnetic Mesoporous Silica Nanocomposites with Improved Sensing Performance and Effective Removal Ability toward Hg(II). Langmuir 2012, 28, 1657-1662. [CrossRef]
- Dib, S.; Boufatit, M.; Chelouaou, S.; Sadi-Hassaine, F.; Croissant, J.; Long, J.; Raehm, L.; Charnay, C.; Durand, J.O. Versatile heavy metals removal via magnetic mesoporous nanocontainers. RSC Adv. 2014, 4, 24838-24841. [CrossRef]
- Li, J.; Gong, A.; Li, F.; Qiu, L.; Zhang, W.; Gao, G.; Liu, Y.; Li, J. Synthesis and characterization of magnetic mesoporous Fe3O4@mSiO2–DODGA nanoparticles for adsorption of 16 rare earth elements. RSC Adv. 2018, 8, 39149-39161. [CrossRef]
- Mahdi, K.; Hamed, M.; Masoumeh, A.; Parham, S.-Z.; Michael, R.H. Smart mesoporous silica nanoparticles for controlled-release drug delivery. Nanotechnology Reviews 2016, 5, 195-207.
- Knezevic, N.Z.; Gadjanski, I.; Durand, J.-O. Magnetic nanoarchitectures for cancer sensing, imaging and therapy. J. Mater. Chem. B 2019, 7, 9-23. [CrossRef]
- Liu, S.; Yu, B.; Wang, S.; Shen, Y.; Cong, H. Preparation, surface functionalization and application of Fe3O4 magnetic nanoparticles. Adv. Colloid Interface Sci. 2020, 281, 102165. [CrossRef]
- Bagheri, E.; Ansari, L.; Abnous, K.; Taghdisi, S.M.; Naserifar, M.; Ramezani, M.; Alibolandi, M. Silica -magnetic inorganic hybrid nanomaterials as versatile sensing platform. Nanomedicine Journal 2020, 7, 183-193. [CrossRef]
- Gao, F. An Overview of Surface-Functionalized Magnetic Nanoparticles: Preparation and Application for Wastewater Treatment. ChemistrySelect 2019, 4, 6805-6811. [CrossRef]
- Clemons, T.D.; Kerr, R.H.; Joos, A. Multifunctional magnetic nanoparticles: design, synthesis, and biomedical applications. In Comprehensive Nanoscience and Nanotechnology (2nd Edition), Elsevier Ltd.: 2019; Vol. 3, pp. 193-210.
- Albinali, K.E.; Zagho, M.M.; Deng, Y.; Elzatahry, A.A. A perspective on magnetic core-shell carriers for responsive and targeted drug delivery systems. Int. J. Nanomed. 2019, 14, 1707-1723. [CrossRef]
- Garcia, R.S.; Stafford, S.; Gun'ko, Y.K. Recent progress in synthesis and functionalization of multimodal fluorescent-magnetic nanoparticles for biological applications. Appl. Sci. 2018, 8, 172/171-172/123. [CrossRef]
- Zhao, T.; Nguyen, N.-T.; Xie, Y.; Sun, X.; Li, Q.; Li, X. Inorganic Nanocrystals Functionalized Mesoporous Silica Nanoparticles: Fabrication and Enhanced Bio-applications. Frontiers in Chemistry 2017, 5. [CrossRef]
- Ling, D.; Lee, N.; Hyeon, T. Chemical Synthesis and Assembly of Uniformly Sized Iron Oxide Nanoparticles for Medical Applications. Acc. Chem. Res. 2015, 48, 1276-1285. [CrossRef]
- Knezevic, N.Z.; Ruiz-Hernandez, E.; Hennink, W.E.; Vallet-Regi, M. Magnetic mesoporous silica-based core/shell nanoparticles for biomedical applications. RSC Adv. 2013, 3, 9584-9593.
- Liong, M.; Lu, J.; Kovochich, M.; Xia, T.; Ruehm, S.G.; Nel, A.E.; Tamanoi, F.; Zink, J.I. Multifunctional Inorganic Nanoparticles for Imaging, Targeting, and Drug Delivery. ACS Nano 2008, 2, 889-896.
- Kim, J.; Kim, H.S.; Lee, N.; Kim, T.; Kim, H.; Yu, T.; Song, I.C.; Moon, W.K.; Hyeon, T. Multifunctional Uniform Nanoparticles Composed of a Magnetite Nanocrystal Core and a Mesoporous Silica Shell for Magnetic Resonance and Fluorescence Imaging and for Drug Delivery. Angew. Chem. Int. Ed. 2008, 47, 8438-8441. [CrossRef]
- Nyalosaso, J.L.; Rascol, E.; Pisani, C.; Dorandeu, C.; Dumail, X.; Maynadier, M.; Gary-Bobo, M.; Kee Him, J.L.; Bron, P.; Garcia, M., et al. Synthesis, decoration, and cellular effects of magnetic mesoporous silica nanoparticles. RSC Adv. 2016, 6, 57275-57283. [CrossRef]
- Fan, H.; Leve, E.; Gabaldon, J.; Wright, A.; Haddad, R.E.; Brinker, C.J. Ordered Two- and Three-Dimensional Arrays Self-Assembled from Water-Soluble Nanocrystal–Micelles. Adv. Mater. 2005, 17, 2587-2590.
- Seisenbaeva, G.A.; Ali, L.M.A.; Vardanyan, A.; Gary-Bobo, M.; Budnyak, T.M.; Kessler, V.G.; Durand, J.-O. Mesoporous silica adsorbents modified with amino polycarboxylate ligands – functional characteristics, health and environmental effects. Journal of Hazardous Materials 2021, 406, 124698.
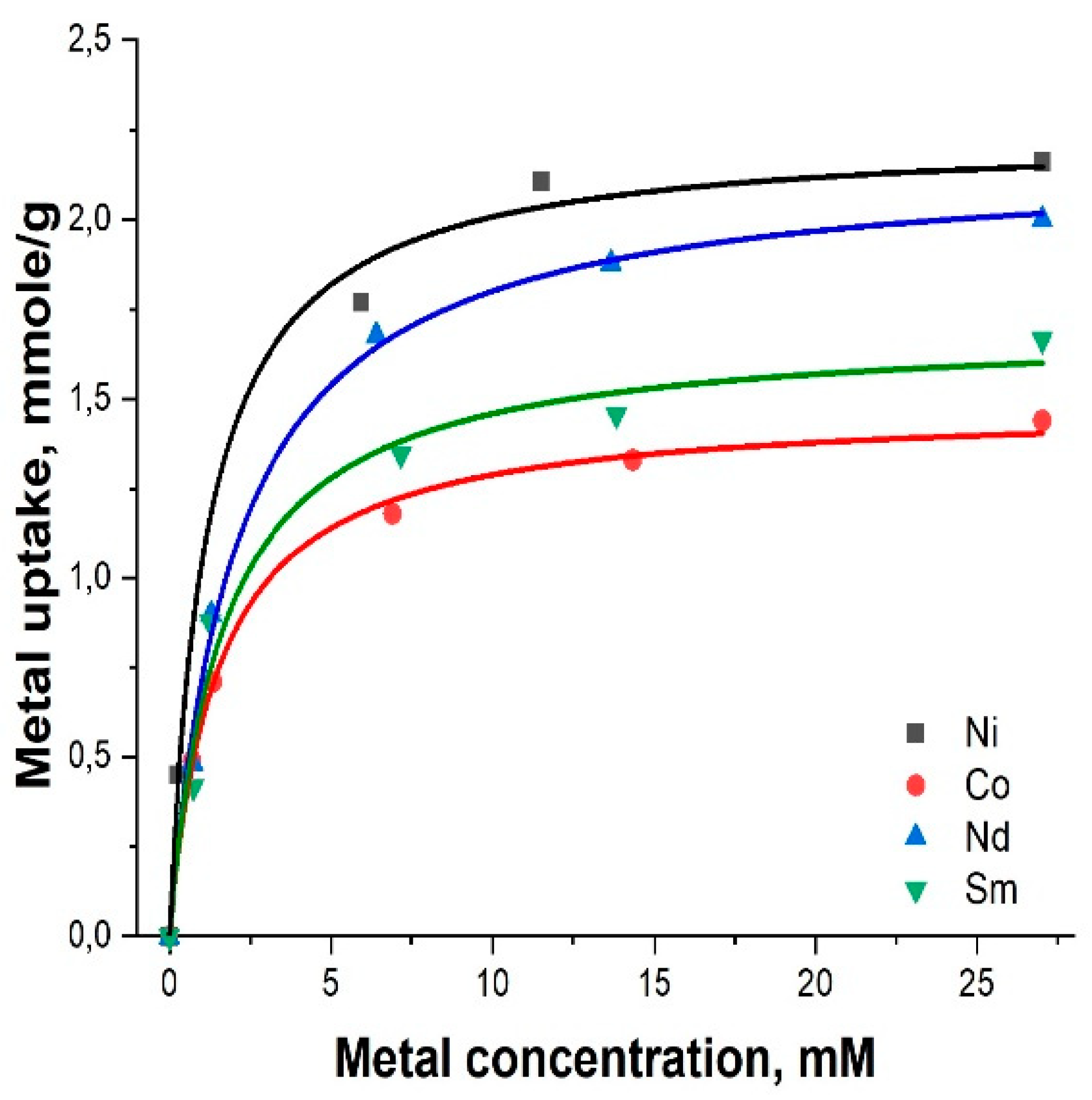
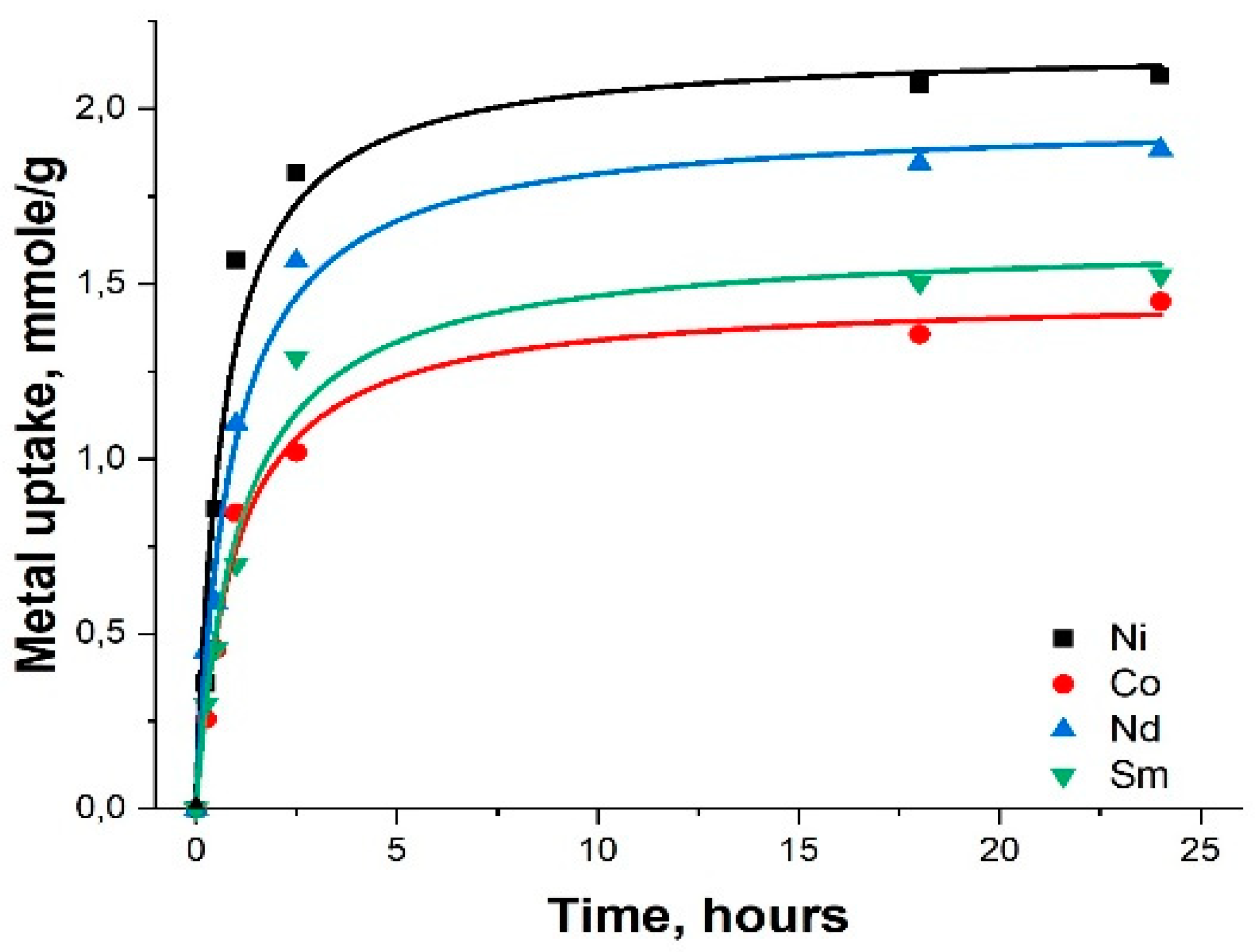
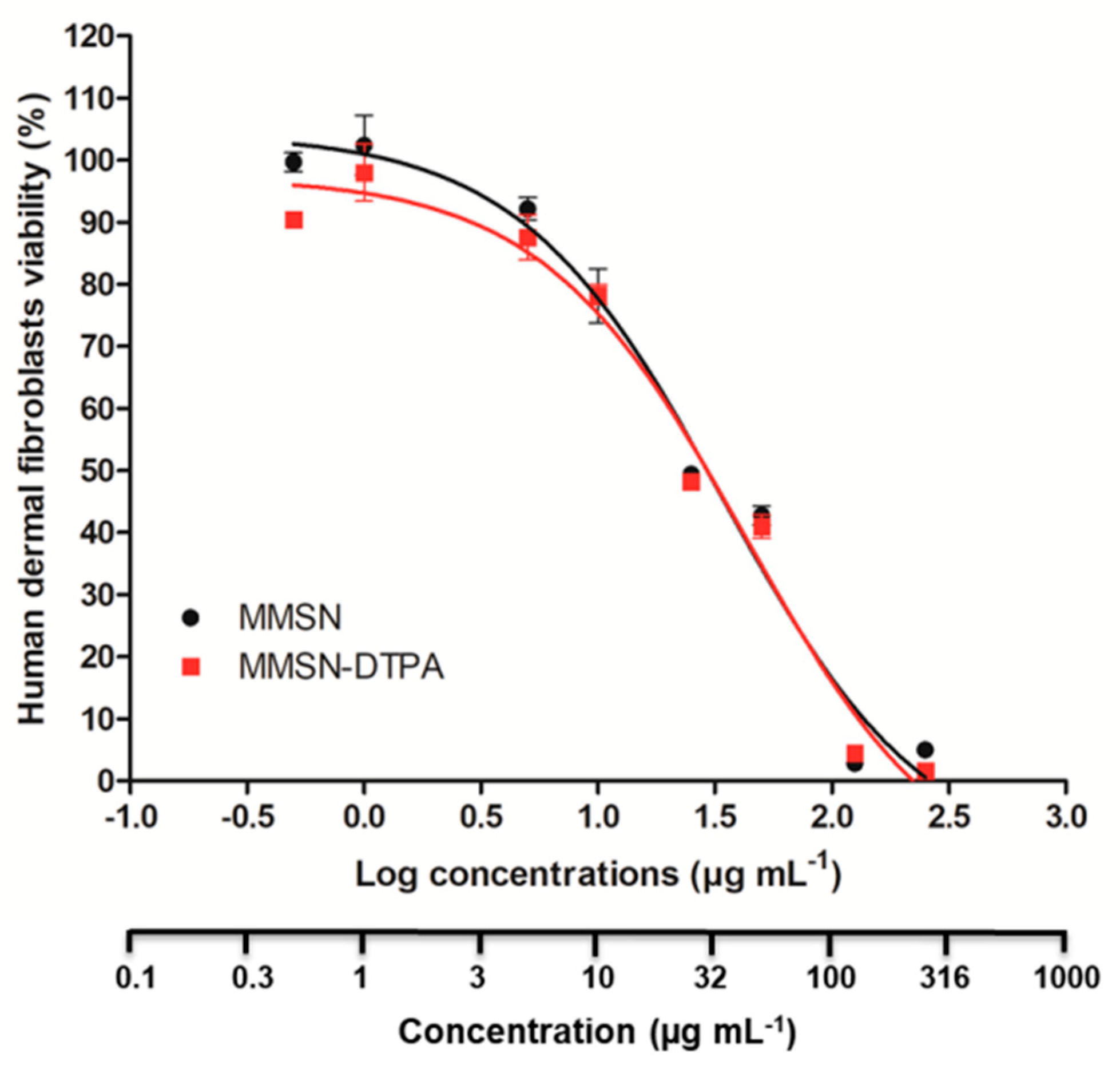
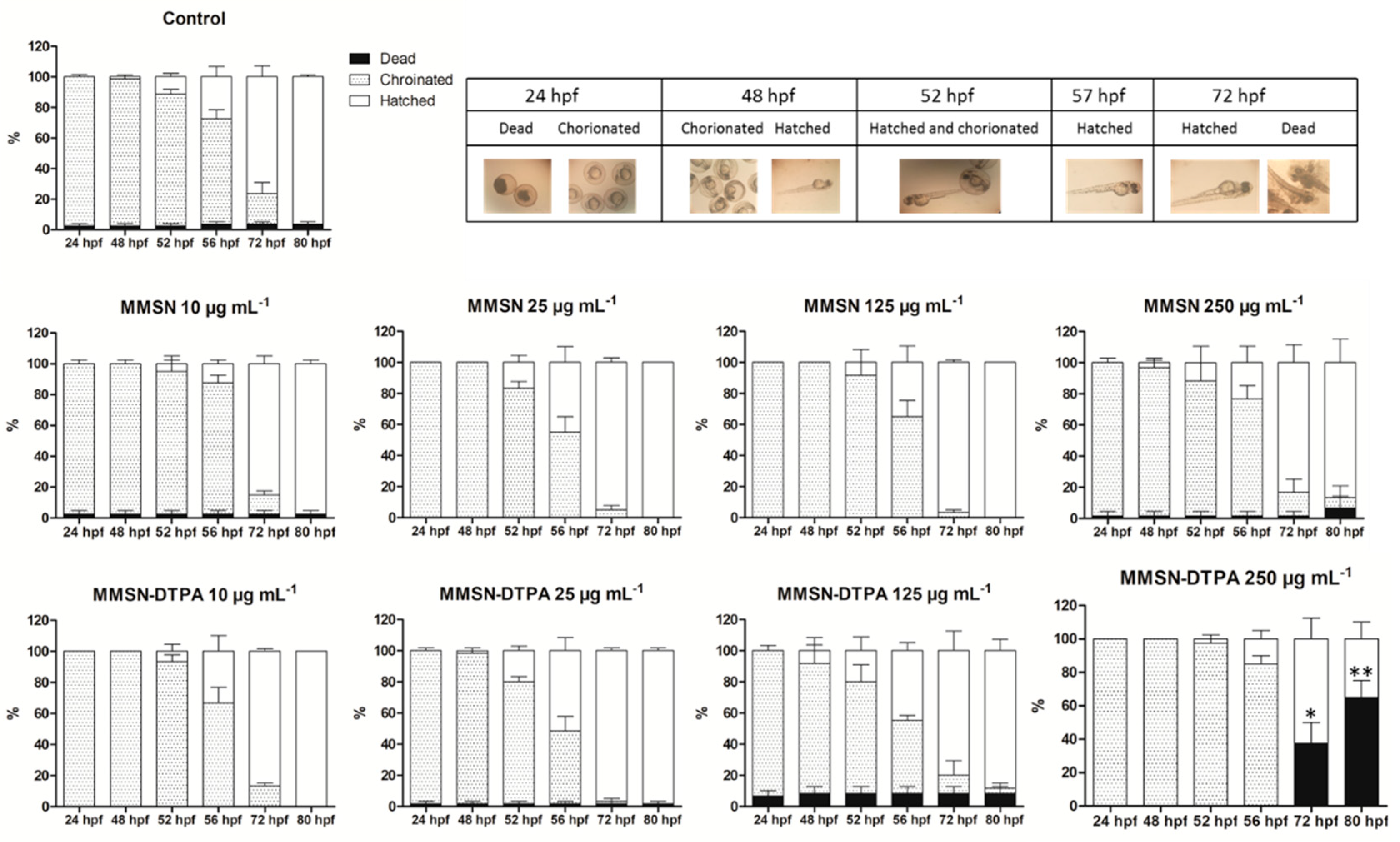
| Sample | Ni | Co | Nd | Sm |
|---|---|---|---|---|
| MMSN | 1.00 | 1.00 | 0.88 | 0.85 |
| MMSN-DTPA | 2.16 | 1.44 | 2.00 | 1.66 |
| Sample | Ni:Nd | Co:Sm | Ni:Co |
|---|---|---|---|
| MMSN | 1:1 | ||
| MMSN-DTPA | 12:1 | 1:2.4 | 9.4:1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





