1. Introduction
Recently, food waste has received a lot of attention due to its negative impact on climate change, food security, and the sustainability of agri-food systems. As stated by the United Nations Food and Agriculture Organization (FAO) reports, 828 million people suffered from hunger in 2021, an increased number compared to previous reports of FAO [
1]. Food waste may derive in a variety of ways, including the removal of fresh produce that deviates from the ideal shape, size, and color during sorting procedures, or the discarding of goods by merchants and customers that are close to or past their "best-before" date. Furthermore, there are frequently significant amounts of edible food leftovers which are
Managing food waste is a significant and complicated issue throughout the food supply chain, from farming to consumption level. Poor harvesting procedures, spoilage, damage, unsuitable storage conditions, and overbuying can all contribute to this problem. As a result, reducing food waste requires a coordinated and integrated approach including multiple stakeholders and actions across the whole food system. The projected loss of food resources after harvesting and before reaching the market is estimated at 14%, valued at
$400 billion per year. Furthermore, according to the UNEPS Food Waste Index Report 2021 [
2], an additional 17% of food, approximately 931 million tonnes, is wasted across three sectors; household, food services and retail. Prioritizing food loss and waste management is therefore crucial for the transition to sustainable agri-food systems that increase the effective use of natural resources, reduce their environmental impact, and maintain food security and nutrition [
3].
Throwing away in landfills or incinerating food waste causes deterioration of quality of land and environment and generates greenhouse gases (GHGs) contributing to an unstable climate [
4]. Apart from that, the European legislation on waste management is evolving the last decades focusing on biowaste valorisation. The Waste Framework Directive (2008/98/EC) promotes the hierarchy of waste management, prioritizing prevention, reuse, recycling, and recovery over disposal, encouraging member states to put in place different waste stream collection systems, including biowaste, while in the meanwhile the Landfill Directive (1999/31/EC) strives to lessen the harmful effects of disposing of waste in landfills, forcing member states to reduce the biodegradable waste going to landfills and promote alternative waste treatment methods. Other steps that the European Union has taken to encourage sustainable biowaste management include the Circular Economy Package (2018) that promotes the separate collection of biowaste and the use of compost and digestate as valuable resources as well as the EU Bioeconomy Strategy that promotes the sustainable use of renewable resources, including biowaste, for various applications, including bioenergy, bioplastics, and bio-based chemicals. Of course, the EU member states often develop their own legislation and regulations to implement EU directives. These national laws may provide even more specific requirements for the separate collection and management of biowaste.
Thus, due to the high organic and nutrient content of biowaste as well as to the European and national legislation, proper strategies for handling this organic waste stream are essential in order to utilize this low-cost and suitable feedstock for producing value-added products through biological processes for resource and energy recovery, including ethanol fermentation, aerobic composting, anaerobic digestion etc.
In view of the above, the aim of this study is to showcase an alternative valorization pathway for source-separated food waste, a substrate rich in carbohydrates, fats, and proteins. This pathway leads to the production of bioethanol. Benefits of bioethanol production from source-separated biowaste include reducing landfill waste, decreasing greenhouse gas emissions (since it is a renewable fuel), and promoting a circular economy by turning waste into a valuable resource. This process may contribute to sustainability goals and reduce the reliance on fossil fuels.
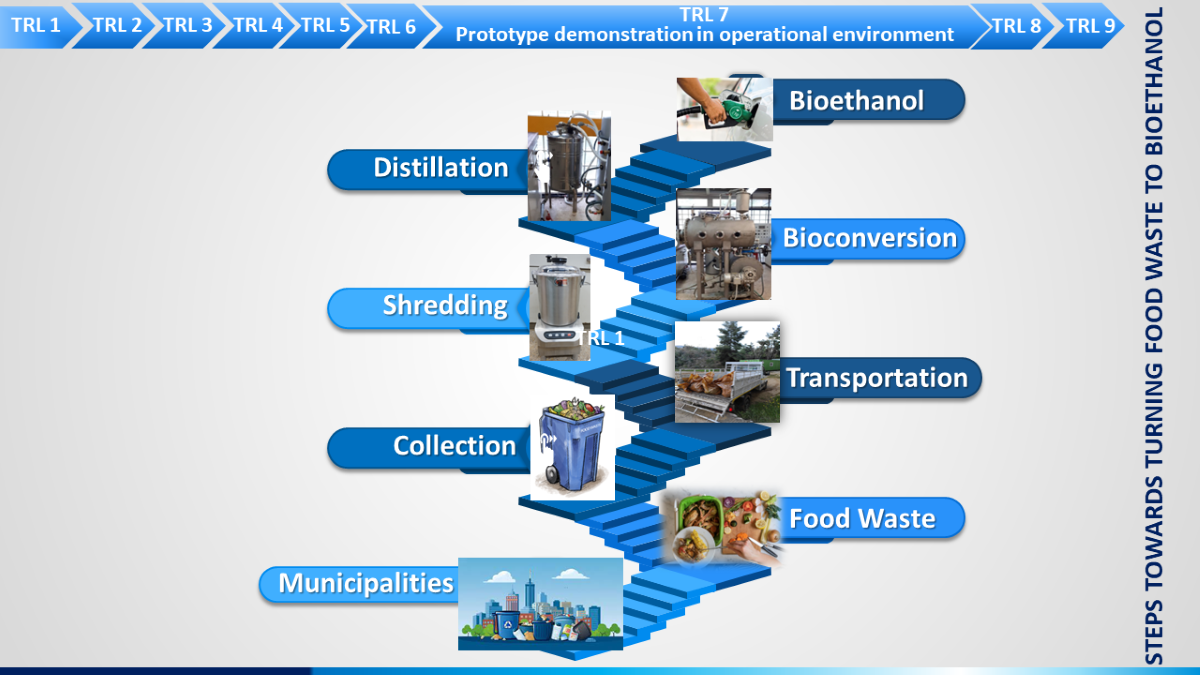
Therefore, the primary goal of the present study was to assess both the feasibility and reproducibility of a process to produce ethanol from raw homogenized feedstock without pre-drying. To the authors’ knowledge, bioethanol production from wet, as received, food waste, without a dehydration pretreatment step is rather novel. Furthermore, very little is reported till now in literature for the production of bioethanol from “real” food waste in pilot scale. Piloting bioethanol production from source-separated biowaste can help boost the Technology Readiness Level (TRL) of this sustainable energy production method. It involves testing and validating the technology at a smaller scale before implementing it on a larger, commercial scale. This helps refine the process, address any technical challenges, and gather data on its feasibility and efficiency, ultimately advancing it to a higher TRL for wider adoption. Piloting is particularly crucial for complex technologies, innovations, or processes, as it helps reduce risks associated with large-scale implementation and ensures that the technology meets the necessary standards and requirements. Hence, the pilot plant shall allow for testing the technology with real biowaste materials, refining the process, and identifying any operational challenges. Data on yields, energy efficiency, and environmental impact will be collected. This stage is crucial for scaling up the technology and further validating its performance under real-world conditions. It also helps in assessing the economics of the process. Successful pilot testing also builds confidence among stakeholders, including investors, regulators, and end-users. It demonstrates that the technology is not just a theoretical concept but a practical solution. Last but not least, pilot testing provides an opportunity to assess the environmental and social impacts of the technology. This information is vital for conducting impact assessments and addressing potential concerns.
2. Materials and Methods
2.1. Materials
The raw material utilised in this study is source-separated food waste, specifically household kitchen waste, from the Municipality of Vari-Voula-Vouliagmeni in Attica, Greece. The biowaste was delivered to the Unit of Environmental Science and Technology (UEST), School of Chemical Engineering, NTUA, for analysis and processing. The wet feedstock, received without a drying stage, presented numerous difficulties in terms of representative sampling and analytical characterization due to its high heterogeneity. The processing of the feedstock was also a significant challenge, which was overcome by using a high-power shredder. To ensure accurate analysis, all raw feedstocks were fully physicochemically characterized in triplicate, with the moisture content being measured first by a moisture analyzer.
Based on the results of bioethanol production trials with dried feedstock [
5] and preliminary lab scale experiments, the enzyme and yeast dosages that were adopted were: 175 μL/g cellulose NS87014, 40μL/g starch SpirizymeXL, and 2% Saccharomyces cerevisiae (d.b.). A mean composition of feedstock was also assumed, namely 10% starch, 15% cellulose, and 1.5% free glucose [
6] since the wet feedstock could not be characterized in time prior to its treatment. The fermentation mode that was applied was Simultaneous Saccharification and Fermentation (SSF) and the solid loading was 10-15% since higher solid loadings induced mechanical problems to the equipment.
2.2. Analytical Methods
For the estimation of total and water soluble solids, hemicellulose, cellulose, and lignin in food waste (raw and pretreated) the National Renewable Energy Laboratory (NREL) procedure was applied [
6,
7,
8]. For total starch determination, the Total Starch (AA/AMG) test kit (e.g., Megazyme, Wicklow, Ireland) was used (AACC Method 76-13.01). The Soxhlet standard method (5520E) was utilized for the quantification of fats and lipids [
9,
10]. Marketable kits (Glucose oxidase-peroxidase method (GOD/PAP), Biosis SA, Athens, Greece; Spectro- quant Volatile Organic Acids Test 1018909 by Merck KGaA Mellipore, Darmstadt, Germany; Ethanol Assay Kit, K-EtOHLQR, Megazymes) were used for the photometric determination of glucose, volatile fatty acids and ethanol in the liquid fraction respectively. All analyses took place in triplicate.
The KERN DAB 100-3 Moisture Analyzer was utilized to determine the moisture content of the raw homogenized feedstock prior to its treatment. This approach was employed to obtain reliable measurements of the dry matter to estimate the solids, enzymes and yeast loadings.
2.3. Experimental Methods
2.3.1. Pretreatment - High-Power Shredding
A high-power shredder was used for the processing of wet source-separated biowaste. The shredder comprised of an 18-liter bowl with 4 low and high-speed rotating cutting knives (
Figure 1), which were able to blend the raw feedstock with water to form a slurry mixture. To ensure efficient blending, approximately 3 liters of water were added to the bowl cutter for every 12-14 kg of fresh biowaste. The residence time required for mixing and homogenization varied depending on the type of biowaste. Typically, each batch of approximately 12-14 kg of wet feedstock was mixed for 5-10 minutes before being sampled for rapid moisture estimation to estimate the dry matter in the feedstock. The solid loading was adjusted to fall within the range of 10-15%, and water was added if necessary. The resulting mixture was then fed into the pilot bioreactor for SSF processing.
2.3.2. Bioconversion pilot plant
The bioconversion experiments were conducted in a pilot plant. This pilot plant is located in NTUA. It includes two horizontal rotating vessels, each with a capacity of 200 liters. These vessels are made of stainless steel, a common material choice for its durability and resistance to corrosion. The rotation and agitation in these vessels are used to facilitate mixing and ensure uniform conditions during the bioconversion process. The two vessels can operate independently, allowing for different operating conditions or different processes to be tested simultaneously if necessary. Since, temperature is a critical parameter in the bioconversion processes, the vessels have a temperature control system that involves water recirculation within their double walls. This system helps maintain a precise and stable temperature inside the vessels. A distillation unit is also part of the pilot plant setup. It is used to recover the ethanol produced during the bioconversion process. The distillation is conducted at a specific temperature (70°C) and under reduced pressure (low vacuum) to separate ethanol efficiently from water. The pilot’s operation is controlled by a Programmable Logic Controller (PLC). The PLC manages and monitors parameters such as temperature, agitation, and distillation [
5]. Overall, this pilot plant serves as a controlled environment for conducting experiments related to bioconversion processes, particularly the production and recovery of ethanol. The use of specialized equipment and automation through a PLC helps ensure accurate and reproducible results, making it valuable for research and development in the field of bioprocessing and bioenergy production.
11 pilot trials were conducted. During the process, samples were taken to monitor the ethanol and glucose levels. Distillation was carried out after 24 h of SSF.
3. Results
3.1. Raw Material
During the course of this study, around 600 kg of fresh and wet organic waste were received in different batches in a 4-month period. Based on the physicochemical characterisation, the mean initial moisture was 82.1 + 4.0 (% w/w wet basis), while the composition of the raw food waste was as follows (% w/w dry basis): ash 8.5 ± 1.5, starch 4.5 ± 2.8, cellulose 9.4 ± 1.1, hemicellulose 8.5 ± 1.9, fats and oils 21.0 ± 7.7, acid soluble lignin 1.0 ± 0.2, acid insoluble residue 9.7 ± 2.1, water soluble solids 39.3 ± 6.4, and free glucose 2.5 ± 2.3. As far heavy metals are concerned, the following concentration were measured in the different batches (mg/kg dry basis): Cr 2.62 ± 0.05, Cu 1.67 ± 3.61, Mn 14.29 ± 5.13, Ni 3.94 ± 2.46, Cd 0.09 ± 0.02, Pb 1.97 ± 0.11 and Zn 25.22 ± 11.23.
According to Barampouti et al. [
11], fats and oils vary from 5.6% in Italy to 24.7% in Turkey. Mean values for lignin are 12.59 ± 4.23%, for cellulose 13.97 ± 3.73%, for hemicellulose 10.22 ± 6.63%, for starch 7.31 ± 3.44 % and for free glucose 10.6 ± 6.0%. In general, it is observed that the average composition of components fall within the range reported in literature. It should also be noted that all heavy metals identified were within the permissible limits. In fact, according to Bozym et al. [
12], cadmium content lower than 0.5 mg/kg is not harmful and the respective toxic limit is 180 mg/kg. Accordingly, copper concentrations over 40 mg/kg may hinder methane production. The zinc levels of biowaste should not be over 100 mg/kg [
13] while the toxic limit for nickel is 10 mg/kg. Finally, in most food residues samples, chromium is usually 1.26-3.42 mg/kg [
12].
Nutrients are essential for yeast metabolism during the fermentation process; therefore, this feedstock appears to have available and adequate amount of nutrients for the healthy yeast growth. This is in accordance with Cekmecelioglu and Uncu [
14] and Tang
et al. [
15], who concluding that nutrient input wasn't necessary throughout fermentation of organic fraction of municipal solid waste, indicating that all the nutrients required for unrestricted yeast growth were present in the hydrolysate.
Also, the complexity of food waste is advantageous for microbial development, therefore a proper and immediate handling was necessary to prevent microbial contamination, as it can affect the bioconversion process in terms of yield [
16]
3.2. Bioconversion Process
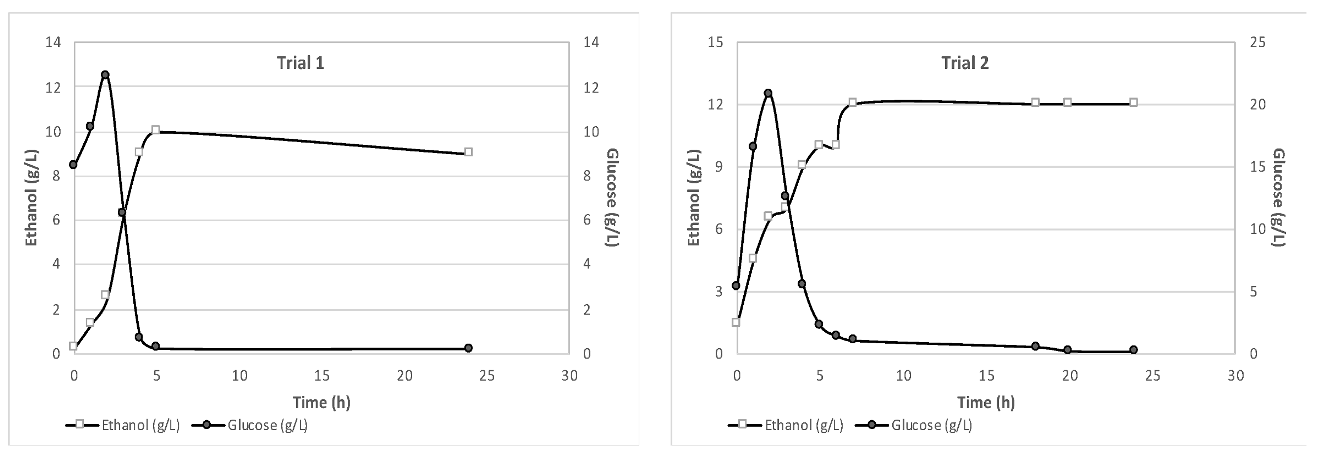
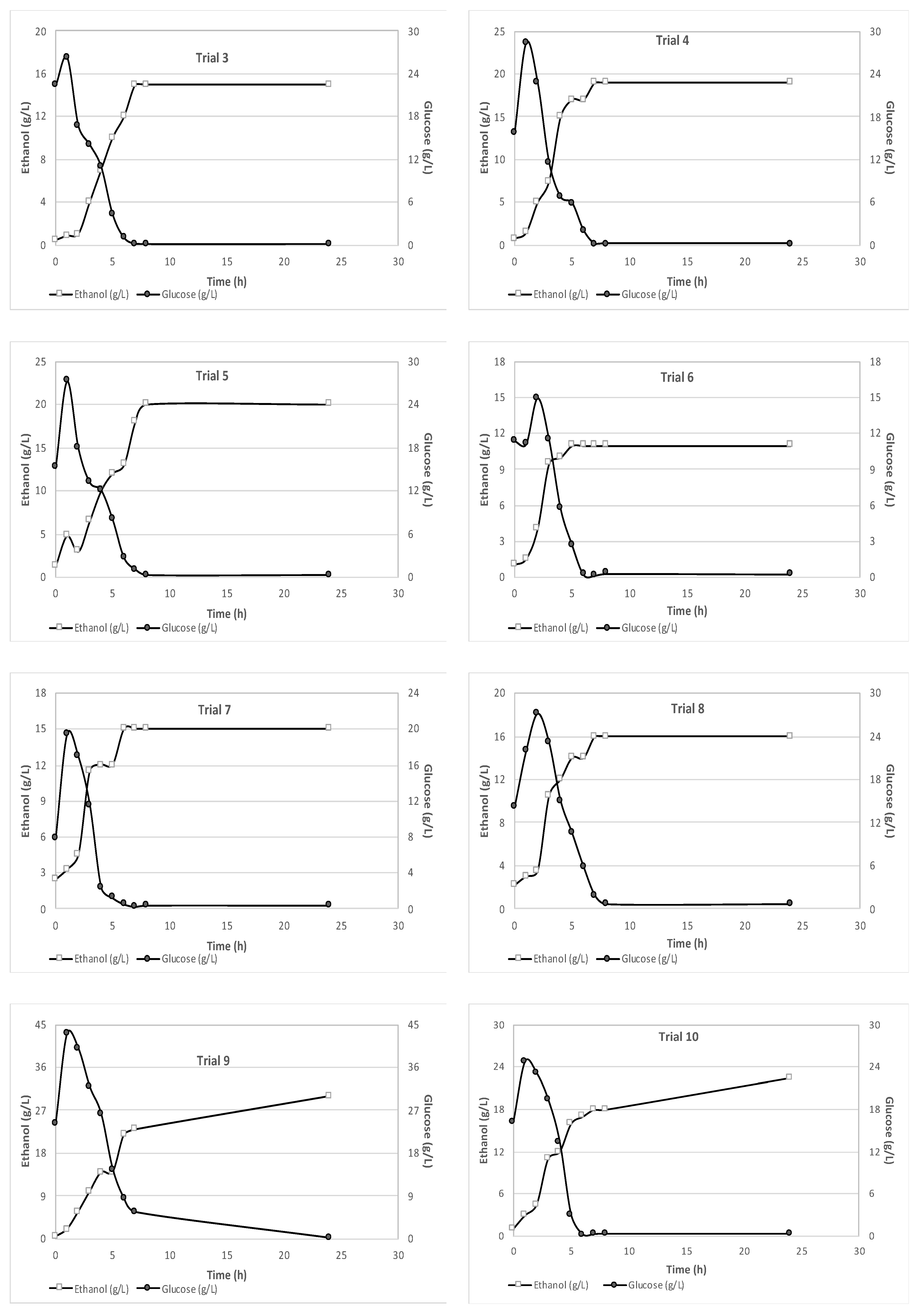
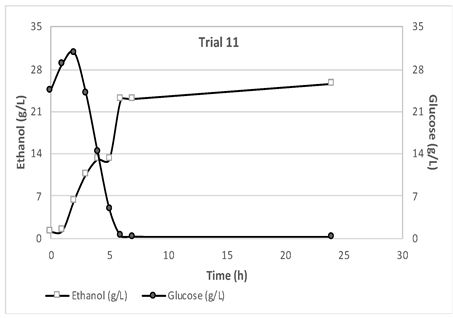
The results obtained from monitoring the glucose and ethanol concentrations during the 24-hour fermentation process are presented in
Figure 2. It is evident from
Figure 2 that after the first 7–8 hours of fermentation, the glucose concentration drops to nearly zero, while the ethanol concentration reaches its maximum value and remains almost stable. For each trial, the maximum ethanol concentration varied, depending on the feedstock characteristics. The shredded wet feedstock used in the experiments presented a free glucose content ranging from 5.24 to 24.21 g/L, that is beneficial for bioethanol production. This can be attributed to a partial breakdown of polysaccharides during the shredding process [
17] as well as sugars from food that were dissolved in the liquid fraction. Therefore, the glucose content in the liquid phase of the mixture was accurately measured and accounted for in the ethanol yield calculations. These results demonstrate the importance of monitoring ethanol and glucose production throughout the SSF process and the need to consider the feedstock characteristics for efficient ethanol production.
The maximum ethanol concentrations achieved in this study, 20 g/L for 10% solid loading and 29 g/L for 15% solid loading, could be considered satisfactory in comparison with the findings of Uncu & Cekmecelioglu [
18]. They reported bioethanol concentrations of as high as 16.96 g/L and 26.01 g/L for solid loadings of 10% and 15% respectively when using kitchen waste as substrate. Additionally, the outcomes of Uçkun Kiran & Liu [
19], who studied bioethanol production from a substrate with initial glucose content of 40 g/L and a solid loading of 15% for 24 hours, indicated an ethanol concentration of, almost, 15 g/L.
In order to investigate the mechanism of biowaste hydrolysis and fermentation, a comprehensive characterization was conducted at the beginning and end of each trial (
Table 1 and
Table 2). Regarding the liquid phase, the pH and the contents of total organic carbon (TOC), total nitrogen (TN), total reducing sugars (TRS), glucose and ethanol were measured (
Table 1). In the solid phase, the total solids (TS), the total organic carbon (TOC), total nitrogen (TN), starch, cellulose, fats and oils, water soluble solids (WSS), hemicellulose, acid soluble lignin (ASL) and acid insoluble residue (AIR) were measured and are presented in
Table 2. By examining the composition of the liquid and solid phase, it was evident that adequate amount of nutrients for the healthy yeast growth was available. Taking into account the composition of the raw material utilised and the produced stillage, the degradation efficiencies were calculated for the structural components of source-separated food waste and are presented in Table.
The Grubbs test is a statistical method used to detect outliers or unusually extreme data points in a dataset. It helps identify values that are significantly different from the rest of the data and can be considered anomalies. In this case, the Grubbs test was applied to the data of
Table 3, and it detected two outliers related to cellulose and starch degradation in trial 6. Excluding the detected outliers from the mean values, the resulting mean degradations of starch and cellulose are 80.7 ± 16.3% and 79.4 ± 10.4% respectively.
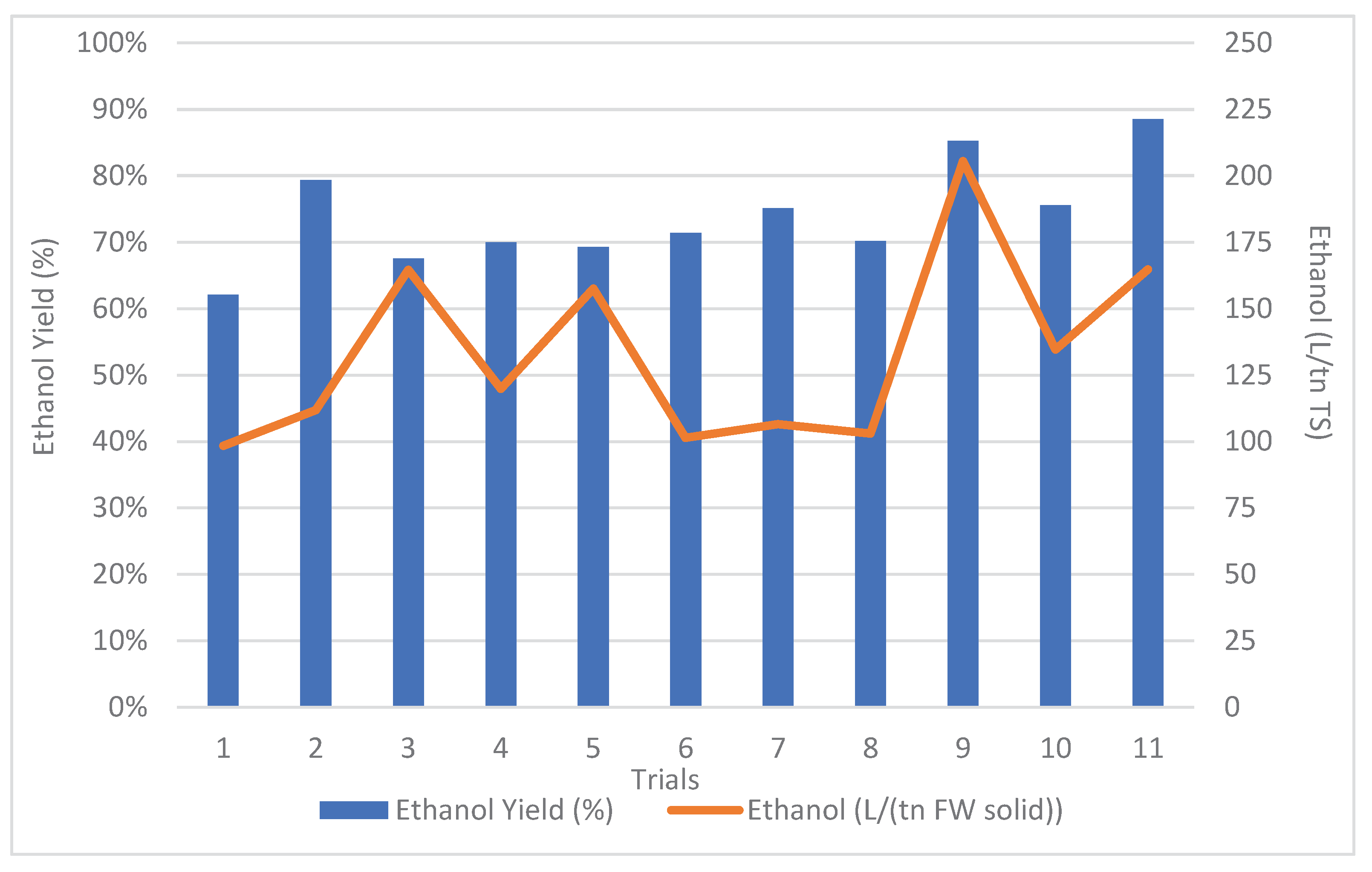
The efficiency of the process, for every trial, was assessed by the ethanol yield and by the ethanol produced per tn of dried total solids are presented in
Figure 3. The term dried feedstock referred to total solids (TS) and is used in order to calculate yields that can be comparable with the results of a previous study [
5] using dry feedstock under similar operational conditions.
The mean ethanol yield was 74.1 ± 6.8% and the results were also very successful and reproducible. It is notable that the polysaccharides degradation verified the observed ethanol yields.
3.3. Energy Consumption
Considering the energy analysis, just the key steps of the process; shredding, SSF process and distillation process were taken into account.
Regarding the shredding process that was used to homogenize the feedstock, the energy consumption was found 0.04 ± 0.01 kWh per kg wet feedstock in average, corresponding to 0.19 ± 0.03 kWh per kg dry feedstock.
The bioconversion process also requires energy for heating and mixing. Two time frames (0-8h and 8-24h) have been established by the fact that the maximal ethanol concentration is usually recorded at the 8th hour of the experiment (
Figure 2). For the time frame 0-8h, the temperature set is 35
oC, the stirring intensity 35% and the stirring rate 40min/h, while for 8-24h 35
oC, 20% and 25min/h respectively.
For the whole bioconversion period (24 hours), the total energy consumption was 1.1 ± 0.2 kWh/kg dry feedstock or 0.3 ± 0.1 kWh/kg wet feedstock.
Regarding distillation, the heating (70oC), stirring (40% stirring rate, 40 min/h), and vacuum pump operation (0.2 kW) accounted for most of the energy usage. The distillation's energy usage was 6.94 kWh/L ethanol.
Overall, the improved process for treating wet source-separated biowaste consumes an average of 16.1 ± 2.9 kWh per L of ethanol produced, or around 2.2 ± 0.4 kWh per kg of dry feedstock, or 0.5 ± 0.1 kWh per kg of wet feedstock as received. The following fractionation of this consumption was estimated: 8.64% for shredding, 48.19% for bioconversion, and 43.17% for distillation.
Conclusively, the processing of wet source-separated biowaste as received towards bioethanol products was rendered feasible with promising yields. Thus, this study successfully showcased the increase of the technology readiness level of the process. This scaling up process demonstrated the bioethanol production in an operational environment and at the same time it helped identify and address practical challenges that would not have been apparent in smaller scale tests.
4. Discussion
Feedstock is a crucial component in any industrial process and plays a significant role in determining the efficiency and output of the process. This study focused on the production of bioethanol from wet, “as received” source-separated biowaste, although most studies include a thermal pretreatment step (e.g. drying) [
11,
20,
21,
22,
23,
24,
25]. Tsafara et al. [
5] studied bioethanol production from dried source-separated biowaste. More specifically in the study of Tsafara [
5], the biowaste originated from the same municipality (Municipality of Vari-Voula-Vouliagmeni, Attica, Greece) with the present study, the bioconversion was carried out in the same pilot plant, the enzymatic formulations (Spirizyme XL, NS87014 by Novozymes) and yeast used were the same, thus a reliable comparison of the results of both studies could be performed. The aim of this evaluation is to compare the performance of wet and dried source-separated biowaste in the process of bioethanol production and evaluate the impact of each type of feedstock on the overall outcome. The comparison shall provide a thorough analysis of the advantages and disadvantages of using wet and dried feedstock and assist to determine the most suitable option for a given process.
The main difference within the feedstocks is that the dried feedstock can be fully characterized prior to the process and the dosages are tailored to the specific composition, while for the wet feedstock an initial assumption of the composition was made. This uncertainty could lead to deviations in the used consumables. The same experimental operational conditions of Tsafara et al. [
5] where applied. The main operational parameter that differed was the solid loading. For the dried feedstock, the applied solid loading was 25%, while for wet feedstock the solid loadings ranged from 10 to 15%, given the limitations of the mechanical equipment. Given the same residence time, the lower solid loading in this study implies that wet shredded feedstock needs larger bioreactors in terms of volume in comparison with the dried feedstock, increasing the CAPEX cost.
Furthermore, Tsafara et al [
5] stated, that the mean ethanol yield achieved with the same bioreactor but with dried feedstock was 86.6 ± 4.9%. It is obvious that the performance of dried feedstock was better than that of wet feedstock (74.1 ± 6.8%%) in terms of ethanol yield. Additionally, it is worth mentioning that the standard deviations using shredded and fresh feedstock are much higher than the respective of dried feedstock. This fact can be attributed to the high heterogeneity of the wet source-separated biowaste. It is evident that the use of dried source-separated biowaste as feedstock offered steady operating conditions and improved process outcomes through the application of the thermal pretreatment. The elevated ethanol yields achieved with dried feedstock (~86%) can be related to the fact that the drying process degrades polysaccharides and lignin to a certain extent, and additionally during this step volatile organic acids, that may act as inhibitors during fermentation, are evaporated.
Additionally, total solids, cellulose, and starch degradation efficiencies were also compared. Total solids and cellulose were found relatively close, while starch degradation was more efficient when dried feedstock was processed, (94.6 ± 2.4%) and this can be attributed to the drying process since it has been proved that hydrothermal pretreatment of starch modifies its internal structure enhancing the enzymatic degradation [
26].
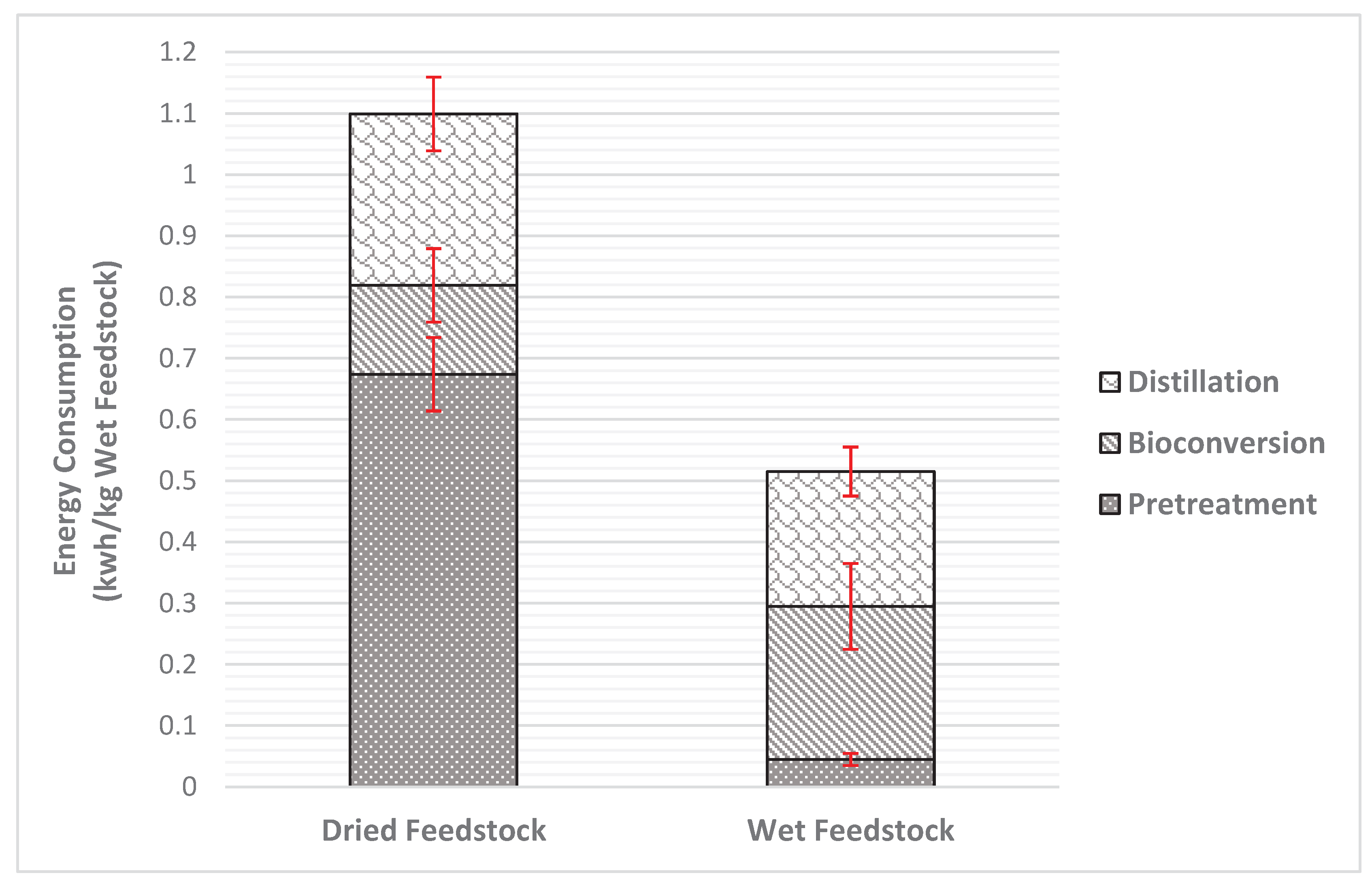
As far as energy consumption is concerned, data derived from Tsafara et al [
5], and from the current study, are presented in
Table 4, along with their distribution in the process stages.
Comparing the energy consumption, it is evident that the wet feedstock is more energy efficient. Regarding the process stages, homogenizing fresh food waste requires almost 15 times lower energy per kg of feedstock used when compared with the drying process. Thermal drying has high energy demands due to the water content of the feedstock. On the other hand, the bioconversion process presented lower energy consumption (0.7 ± 0.4 kWh per kg dry feedstock) nearly half of the calculated energy consumption in this study. This fact is connected tightly to the difference in the solid loading. The same is the case for distillation process. These observations are illustrated in
Figure 4 as well.
5. Conclusions
In this paper, the utilisation of source-separated biowaste towards bioethanol production is presented via the continuous operation (over 10 operating cycles) of a pilot plant with real feedstock – wet separately collected biowaste.
This challenging biowaste stream, wet source-separated kitchen waste, by nature poses a lot of difficulties on the handing and processing. Nevertheless, it was proved that the application of SSF fermentation could be applied with satisfactory ethanol yields but with elevated deviations. In this context, the applied conditions should be tailored and calculated in each case taking into consideration an assumed composition of the treated feedstock and the proposed dosages (175 μL/g cellulose NS87014, 40μL/g starch Spirizyme, and 2% Saccharafication cerevisiae (d.b.)).
Regarding the wet feedstock (as received), a bowl cutter was used for feedstock shredding and it appeared to be an efficient pretreatment for the feedstock without altering its physicochemical composition. From the 11 pilot trials performed with wet feedstock the mean starch and cellulose degradation of the pilot trials amounted up to 80.7 ± 16.3% and 79.4 ± 10.4% respectively, while the bioethanol yield is 74.1 ± 6.8%.
The results obtained are quite encouraging for the process's feasibility. Nevertheless, it is important to consider the energy consumption during the production line, as it constitutes more than 50% in the overall ethanol production cost.
A reliable comparison of the results obtained was performed to a similar study with dried feedstock. It was concluded that the total energy consumption for the production of ethanol with dried feedstock is 26% higher than with wet feedstock. The most energy-intensive stage is drying, followed by distillation. In conclusion, using dried feedstock performs better in terms of ethanol production but the use of wet shredded feedstock also seems favorable due to the low energy consumption despite the slightly lower bioethanol production. To this end, a technoeconomic analysis along with a life cycle analysis is needed in order to reveal the optimum scenario via a multicriteria, integrated assessment.
Piloting bioethanol production from ‘real’ source-separated biowaste advanced the technology along the TRL scale. This approach allowed for iterative improvements, optimization, and addressing challenges along the way, ultimately making it more feasible for large-scale implementation and commercial use in the renewable energy sector. Thus, once the pilot demonstrated consistent and efficient bioethanol production from source-separated biowaste, then it may be able to move towards full-scale commercialization.
Author Contributions
Conceptualization, Mai S. and Barampouti E.M.; methodology, Mai S. and Barampouti E.M..; formal analysis, Mai S. and Barampouti E.M.; investigation, Chatzimaliakas F., Christianides D.; resources, Malamis D.; writing—original draft preparation, Christianides D., Mai S. and Barampouti E.M.; data curation , Mai S. and Barampouti E.M.; writing—review and editing, Mai S. and Barampouti E.M.; visualization, Mai S. and Barampouti E.M.; supervision, Mai S. and Barampouti E.M.; project administration, Malamis D. All authors have read and agreed to the published version of the manuscript.
Funding
This project has received funding from the European Union's Horizon 2020 research and innovation programme under grant agreement No 818308 (WaysTUP!).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable
Conflicts of Interest
The authors declare no conflict of interest.
References
- FAO, IFAD, UNICEF, WFP, and WHO, “FOOD SECURITY AND NUTRITION IN THE WORLD THE STATE OF REPURPOSING FOOD AND AGRICULTURAL POLICIES TO MAKE HEALTHY DIETS MORE AFFORDABLE,” 2022. [CrossRef]
- Hamish Forbes (WRAP), Tom Quested (WRAP), and Clementine O’Connor (UNEP), “UNEP Food Waste Index Report 2021 | UNEP - UN Environment Programme,” 2021. Accessed: Oct. 12, 2023. [Online]. Available: https://www.unep.org/resources/report/unep-food-waste-index-report-2021.
- Rigillo Nicholas (FAO) and Rukikaire Keisha (UNEP), “Tackling food loss and waste: A triple win opportunity,” Sep. 29, 2022. Accessed: Oct. 12, 2023. [Online]. Available: https://www.fao.org/newsroom/detail/FAO-UNEP-agriculture-environment-food-loss-waste-day-2022/en.
- “Food Waste Is a Massive Problem—Here’s Why - FoodPrint.” Accessed: Oct. 12, 2023. [Online]. Available: https://foodprint.org/issues/the-problem-of-food-waste/.
- P. Tsafara et al., “Advanced Bioethanol Production from Source-Separated Bio-waste in Pilot Scale,” Sustainability (Switzerland), vol. 14, no. 19, Oct. 2022. [CrossRef]
- Sluiter, R. Ruiz, C. Scarlata, J. Sluiter, and D. Templeton, “Determination of Extractives in Biomass: Laboratory Analytical Procedure (LAP); Issue Date 7/17/2005,” 2008, Accessed: Oct. 12, 2023. [Online]. Available: http://www.nrel.gov/biomass/analytical_procedures.html.
- Sluiter et al., “Determination of Structural Carbohydrates and Lignin in Biomass: Laboratory Analytical Procedure (LAP) (Revised July 2011),” 2008, Accessed: Oct. 12, 2023. [Online]. Available: http://www.nrel.gov/biomass/analytical_procedures.html.
- Sluiter et al., “Determination of Total Solids in Biomass and Total Dissolved Solids in Liquid Process Samples Laboratory Analytical Procedure (LAP) Issue Date: 3/31/2008,” 2008, Accessed: Oct. 12, 2023. [Online]. Available: www.nrel.gov.
- S. S. Nielsen, “Food Science Text Series Food Analysis Laboratory Manual”, Accessed: Oct. 12, 2023. [Online]. Available: www.springer.com/series/5999.
- G. G. Hewavitharana, D. N. Perera, S. B. Navaratne, and I. Wickramasinghe, “Extraction methods of fat from food samples and preparation of fatty acid methyl esters for gas chromatography: A review,” Arabian Journal of Chemistry, vol. 13, no. 8, pp. 6865–6875, Aug. 2020. [CrossRef]
- E. M. Barampouti, S. Mai, D. Malamis, K. Moustakas, and M. Loizidou, “Liquid biofuels from the organic fraction of municipal solid waste: A review,” Renewable and Sustainable Energy Reviews, vol. 110, pp. 298–314, Aug. 2019. [CrossRef]
- M. Bozym, I. Florczak, P. Zdanowska, J. Wojdalski, and M. Klimkiewicz, “An analysis of metal concentrations in food wastes for biogas production,” Renew Energy, vol. 77, pp. 467–472, May 2015. [CrossRef]
- G. Zając, J. Szyszlak-Bargłowicz, W. Gołębiowski, and M. Szczepanik, “Chemical Characteristics of Biomass Ashes,” Energies 2018, Vol. 11, Page 2885, vol. 11, no. 11, p. 2885, Oct. 2018. [CrossRef]
- D. Cekmecelioglu and O. N. Uncu, “Kinetic modeling of enzymatic hydrolysis of pretreated kitchen wastes for enhancing bioethanol production,” Waste Management, vol. 33, no. 3, pp. 735–739, Mar. 2013. [CrossRef]
- Y. Q. Tang et al., “Ethanol production from kitchen waste using the flocculating yeast Saccharomyces cerevisiae strain KF-7,” Biomass Bioenergy, vol. 32, no. 11, pp. 1037–1045, Nov. 2008. [CrossRef]
- J. O’Connor, B. S. Mickan, K. H. M. Siddique, J. Rinklebe, M. B. Kirkham, and N. S. Bolan, “Physical, chemical, and microbial contaminants in food waste management for soil application: A review,” Environmental Pollution, vol. 300, p. 118860, May 2022. [CrossRef]
- Ma, J. Liu, M. Ye, L. Zou, G. Qian, and Y. Y. Li, “Towards utmost bioenergy conversion efficiency of food waste: Pretreatment, co-digestion, and reactor type,” Renewable and Sustainable Energy Reviews, vol. 90, pp. 700–709, Jul. 2018. [CrossRef]
- N. Uncu and D. Cekmecelioglu, “Cost-effective approach to ethanol production and optimization by response surface methodology,” Waste Management, vol. 31, no. 4, pp. 636–643, Apr. 2011. [CrossRef]
- E. Uçkun Kiran and Y. Liu, “Bioethanol production from mixed food waste by an effective enzymatic pretreatment,” Fuel, vol. 159, pp. 463–469, Nov. 2015. [CrossRef]
- Singh et al., “Production of bioethanol from food waste: Status and perspectives,” Bioresour Technol, vol. 360, p. 127651, Sep. 2022. [CrossRef]
- H. S. Hafid et al., “Over production of fermentable sugar for bioethanol production from carbohydrate-rich Malaysian food waste via sequential acid-enzymatic hydrolysis pretreatment,” 2017. [CrossRef]
- P. Mahmoodi, K. Karimi, and M. J. Taherzadeh, “Hydrothermal processing as pretreatment for efficient production of ethanol and biogas from municipal solid waste,” Bioresour Technol, vol. 261, pp. 166–175, Aug. 2018. [CrossRef]
- H. S. Hafid, N. A. A. Rahman, U. K. M. Shah, A. S. Baharuddin, and A. B. Ariff, “Feasibility of using kitchen waste as future substrate for bioethanol production: A review,” Renewable and Sustainable Energy Reviews, vol. 74, pp. 671–686, Jul. 2017. [CrossRef]
- M. Bibra, D. Samanta, N. K. Sharma, G. Singh, G. R. Johnson, and R. K. Sani, “Food Waste to Bioethanol: Opportunities and Challenges,” Fermentation 2023, Vol. 9, Page 8, vol. 9, no. 1, p. 8, Dec. 2022. [CrossRef]
- R. Estrada-Martínez, E. Favela-Torres, N. O. Soto-Cruz, H. B. Escalona-Buendía, and G. Saucedo-Castañeda, “A Mild Thermal Pre-treatment of the Organic Fraction of Municipal Wastes Allows High Ethanol Production by Direct Solid-state Fermentation,” Biotechnology and Bioprocess Engineering, vol. 24, no. 2, pp. 401–412, Mar. 2019. [CrossRef]
- K. Passadis, D. Christianides, D. Malamis, E. M. Barampouti, and S. Mai, “Valorisation of source-separated food waste to bioethanol: pilot-scale demonstration,” Biomass Convers Biorefin, vol. 12, no. 10, pp. 4599–4609, Oct. 2022. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).