1. Introduction
During cooking, many volatile organic compounds (VOCs), chemicals that easily evaporate at room temperature, are continuously produced, and released in air. Some of these molecules are fundamental in food characterization, in particular because they contribute to both flavour and odour formation [
1]. If on the one hand VOCs play a vital role in the aroma and taste perception of food products, contributing to their overall sensory quality, on the other hand some of these compounds are human carcinogen, others are suspect under study. For this reason, research is focused on reducing harmful VOC emissions, using various methods to reach this goal.
The production of VOCs in food is a complex process influenced by various factors, such as food composition, processing conditions, and microbial activity. The primary process regarding food cooking is Maillard reaction: a complex chemical reaction that occurs between amino acids and reducing sugars, usually at elevated temperatures. The reactants condensate to form a glycosylamine, which rearrange to form Amadori compound, if the sugar is aldose or Heyns compound in the case of ketose one. Then, these compounds react with other molecules such as amines and hydrogen sulfide to produce various VOCs compounding food flavour and odour [
2]. The Strecker degradation is relevant in the subset of final Maillard reactions. In fact, α-amminoacid can react with dicarbonyl molecule to form an aminoketone, which acts as precursor of various heterocyclic compounds, such as thiophene, pyrrole and furan (Shahidi et al., 2014). The last major VOC production mechanism during cooking is lipid oxidation, a radical reaction catalysed via enzymatic or non-enzymatic pathway in the case of autoxidation, regarding mainly unsaturated fatty acids, which decompose to stable end-products, such as aldehydes, ketones, alcohols. A common example of this reaction is the oxidation of linoleic acid, which thanks to singlet oxygen generates hydroperoxide. This intermediate can undergo cleavage to obtain aldehydes, such as hexanal [
4].
Maillard reaction and lipid oxidation by-products can react among them to lead to new complex heterocyclic molecules. Henderson and Nawar (1981) noticed the reaction between 2-4 decadienal, produced by cleavage of 9-hydroperoxide of linoleic acid, and Maillard intermediates of valine, which produces 2-pentylpyridine. Volatile released by this cross-reaction contributes slightly to odour, due to weak odour intensity and high perception threshold.
It is already known that the cooking process produces several types of VOCs among which several can be of concern for environment and long-term health as formaldehyde, methanol, acetaldehyde, acetone and acetic acid [
1,
6]. Two typical methods of VOC degradation are active charcoal (AC) and photocatalysis (PC). However, the two methods have some drawbacks, such as high energy consumption and environmental incompatibility [
7].
1.1. Activated carbon (AC) adsorption
Various works have already demonstrated the possibility to reduce VOC amounts using activated charcoal. Adsorption is the phenomena of gases or solutes being absorbed onto the surface of a solid or liquid support. Adsorbents come in a wide variety of forms, including zeolite, polymeric resins, alumina, and silica structures.
Activated carbon, produced by carbonization and activation, is the most used adsorbent material because of its great surface area, chemical stability, especially towards acid and alkali. Because of their hardness and stability, nutshells such as those from coconut and walnut are frequently employed as precursors. Additionally, it has a natural porosity that makes activation simple [
8]. Then, to improve surface area and pore volume, the coal undergoes a process of physical or chemical activation. Thus, AC possesses improved VOC adsorption capacity, greater surface area, specific pore size and surface chemical functional groups [
9].
There are many limitations on the use of AC as adsorbent; firstly, if the adsorption occurs at elevated temperatures, AC can ignite, or its porous structure can collapse [
8]. Secondly, when humidity is above 50%, competitive water adsorption will form a layer, thus making the surface become hydrophilic, switching class of catchable VOCs. Furthermore, trapped water can displace adsorbed VOCs and react with them or form a two-phase solution with partition equilibrium. Chemisorption and irreversible sorption can also occur, especially at high VOC concentrations and high moisture. All previously mentioned issues may, in the worst-case scenario, result in in reduced adsorption capacity [
10,
11].
1.2. Photocatalytic oxidation (PC)
Recently, intense research on the improvement of metal oxides bulk or nanoparticles as photocatalysts has been carried out. All investigated compounds have semiconductor metal properties, such as particular electronic structure, flexibility, high photocatalytic activity and adsorption capacity. They show also great chemical stability against acid and alkali [
12].
The process involves four steps: 1) UV source or visible light causes electronic promotion, forming a couple electron-hole (e
-/h
+); 2) Adsorption of VOC compounds; 3) Redox reactions charged to H
2O and O
2, which lead to the release of reactive oxygen species (ROS), such as hydroxyl radical (∙OH) and superoxide radical anion (∙O
2-), both strong oxidants; 4) Degradation of VOC due to ROS oxidation, which converts organic compounds into carbon dioxide and water in several steps [
13].
PC can occur at room temperature, but it oxidizes low concentrations of volatile compounds and has low durability due to coke fouling. For this reason, thermocatalysis is recommended to avoid catalyst poisoning and improve degradation rate [
14]. Between these materials, TiO
2 shows nontoxicity, high chemical and thermal stability, strong oxidizing power, best biocompatibility and the greater catalytic activity in relation to defects [
15]. Titania is a semiconductor with a band gap of 3.2 eV, corresponding to the wavelength of 390 nm, which requires UV irradiation to achieve electrons excitation. The broad gap between the valence and conduction bands, as in TiO2, is a drawback of photocatalytic materials. Another problem with employing semiconductors is the recombination between electron and hole photogenerated, which reduces catalytic activity. The most common method to avoid these problems is doping the semiconductor with noble metals, such as Ag and Au, and because of this, titania shows strict band gap and an increased exciton lifetime [
16].
A major limitation to PC is the formation of reaction intermediates. In fact, some oxidation steps of complex VOCs can lead to unwanted intermediates, such as the most common ones: formaldehyde, acetone, benzaldehyde, ethanol and benzyl alcohol, or other toxic molecules. Particularly when used in situations where there are large quantities of VOCs, these by-products can saturate the active sites of the catalyst, which leads to catalyst deactivation and poison the user.
1.3. Synergy adsorption-photocatalysis
A possible way to avoid both drawbacks of the aforementioned methods is to combine adsorption and photocatalysis in carbon-based nanocomposites, as activated carbon coupled with nano-TiO
2 (TiO
2/AC) or activated carbon fibres (TiO
2/ACF). Due to the lack of polar surface functional groups in AC and ACF, only nonpolar and weakly polar substances, such as toluene and formaldehyde, could be removed. A possible advantage of carbon-based nanocomposite paired to photocatalysis is the prevention of the generation of intermediates, which are immediately captured by charcoal, and inactivation of catalyst. In addition, due to its high adsorption potential and fast charge transfer, activated carbon holds VOC molecules in proximity of the active sites, and it promotes the generation of radical ROS [
7]. It would also be conceivable to modify the carbon surface to bring about chemical alterations that increase the interaction between VOCs and the ACF surface.
On the other hand, AC-based nanocomposites have the benefit of in situ regeneration, but it is highly dependent on the size of the micropores, which, when left unaltered, reach dimensions of 2 nm, making it impossible for larger molecules to be adsorbed and leading to the failure of the AC synergy/PC. By modifying the material through acid treatment or water vapour gasification, the ratio of mesopore to micropore can be increased, and as a result, the treated material also exhibits improved mechanical strength [
17]. As cited above, separation between holes and electrons is crucial for system operativity.
Temperature is another key variable in adsorption-photocatalysis. Heterogeneous adsorption is an exothermic process and therefore low temperature helps VOC sorption. Diffusion, instead, is endothermic, and therefore decreasing temperature hinders diffusion of the adsorbed compounds in the internal porosity.
In conclusion, an interesting challenge, which to the best of our knowledge, has not been addressed within the literature, is the evaluation of the behaviour of AC/PC technology related to a complex mixture of VOCs, focusing on the emissions generated during cooking. In such scenario, it would be worth investigating the compensation of the defects present in each method when used alone, and if VOCs with low adsorption rate in AC are oxidized by PC. Furthermore, it would be important to evaluate the intermediate compounds, and their possible interference with air cleaning process.
The gold standard of VOC analysis is gas chromatography coupled to mass spectrometry which provides remarkable compound separation and identification, but it is slow and time consuming. At the contrary, proton transfer reaction - mass spectrometry (PTR-MS) allows VOCs detection with higher time resolution, in the order of tens of seconds / minutes for quadrupole mass analysers and a split second for time-of-flight mass analysers. It is based on the chemical ionization of VOCs by primary ions, typically H
3O
+, which have the peculiar property of not reacting with the mayor components of air (N
2 and O
2), but can react with VOCs having higher proton affinity than water. PTR-MS exhibits great sensitivity, with detection limits reaching parts per trillion by volume (ppbv), and allows real-time VOC monitoring. On the other hand, PTR-MS separates protonated VOCs only on the basis of their m/z values and this causes a difficult qualification of the compounds. In comparison, GC-MS enables a better identification and quantification of complex mixtures due to chromatographic resolution. However, it is time consuming because typical analysis time is about one hour per sample. Another advantage of PTR-MS is the absence of sample pre-treatment and analyte pre-concentration steps, at the contrary of GC-MS. VOC monitoring with GC systems typically needs sample trapping [
10]. Then, a desorption procedure must be applied to inject the analytes into GC-MS system. Two common techniques are solvent desorption and thermal desorption. Thermal desorption needs both preconcentration of VOCs, usually carried out with cryofocusing, and maintenance of the cold chain until analysis [
18]. A major problem of this method is the presence of water in matrix, which can be transferred into column to cause serious problem and therefore must be removed. For this reasons, GC-MS requires several pre-treatment operations and the analysis must be performed with particular caution.
This work aims to compare the performance between adsorption, photocatalysis and combined systems in abatement of complex VOC matrices produced by cooking three different types of hamburgers: meat, greens, and fish. An objective of this research is to determine whether the synergy between adsorption and photocatalysis is useful in the removal of VOCs from indoor air for residential usage. Climatic chambers were used to imitate domestic kitchens. The air purification system that was employed for this project was created in view of possible applications in fume hoods in the future. PTR-MS was employed to monitor VOCs in real-time thus providing time-resolved data on VOC emissions and abatement. Several studies have already demonstrated the possibility of PTR-MS on VOC monitoring and quantification, and in this work, we also aim to show the potential of this technique to evaluate the performance of air cleaning systems in the abatement of complex VOC mixtures, such as the ones produced upon cooking.
2. Results and Discussion
2.1. Principal Component Analysis
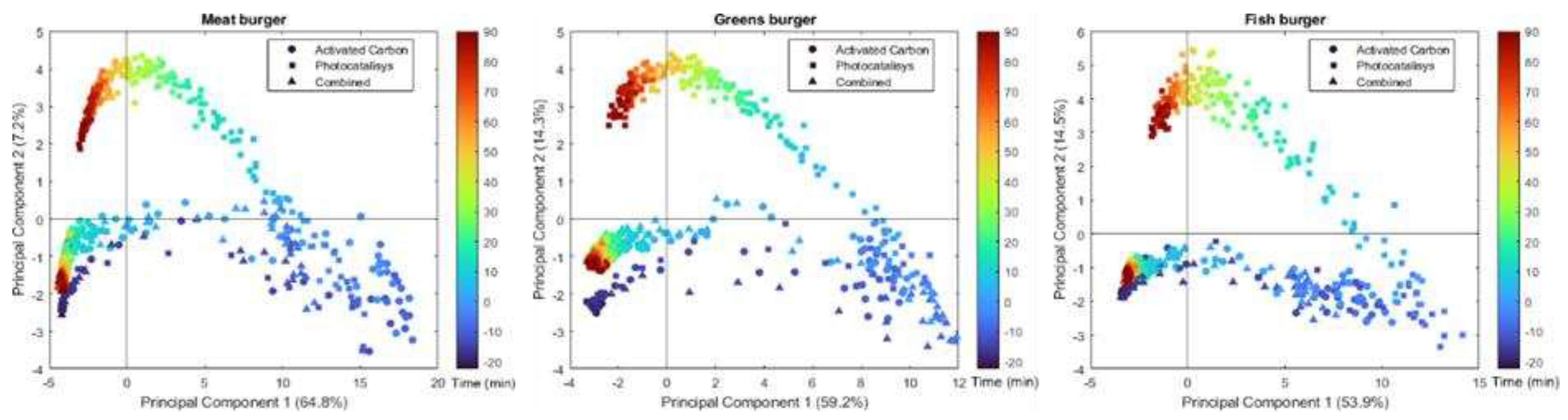
To offer a comprehensive view of the global dataset, we conducted separate PCAs for each type of burger, representing all three abatement systems together on a single plot (
Figure 1). The scores show interesting trends: upon cooking the VOCs in the chamber change dramatically, while when a purifying system is turned on, the VOC composition on the chamber either almost goes back close to the starting point (in the case of “Activated Carbon” and “Combined”) or moves away (in the case of “Photocatalysis”). It is worth noticing that in no cases the original VOC configuration seems completely re-established after 90 minutes of activity of the purifying system. This is particularly evident in the case of vegetal burgers (
Figure 1, central panel). Anyway, the synergetic setup seems to be able to compensate for the negative aspects of photocatalysis. In fact, the results of “Activated Carbon” and “Combined” are rather similar.
2.2. Time trends
The trend of the five compounds that showed highest concentration was specifically reported as well as the “Total VOC” concentration, measured as the sum of all spectral signals. The selected compounds were acetic acid (protonated ion signal at m/z = 61), acetaldehyde (m/z = 45), formaldehyde (m/z = 31), methanol (m/z = 33), acetone (m/z = 59). m/z = 43 has been discarded since it is a non-specific fragment, and therefore difficult to attribute to a single compound. Evidence of these VOC detection with PTR-MS are reported by Cappellin et al. (2012b) with regards to methanol, acetaldehyde and acetone, and by Ni et al. (2020) with regards to acetic acid and formaldehyde. Simultaneous measurement of formaldehyde, acetaldehyde and methanol amount have been carried out by Stucchi et al. (2018), who demonstrated not only the possibility of multi-VOC monitoring, but also that photocatalysis on titania powder is suitable for volatile degradation. This work is also interesting because they study photodegradation of 17 different VOCs, which is similar to complex gaseous matrix we want to investigate.
A first comparison of concentration vs. time plot can be done on emissions: until time=0, the abatement system is turned off to achieve air homogeneity. Confirmation of data repeatability is given by the similar trend for each type of burger despite the method used. Instead, it can be noticed that cooking different types of patties leads to great differences of initial VOC profile, probably because of different composition of burgers, such as carbohydrates and fatty acids.
2.2.1. Formaldehyde
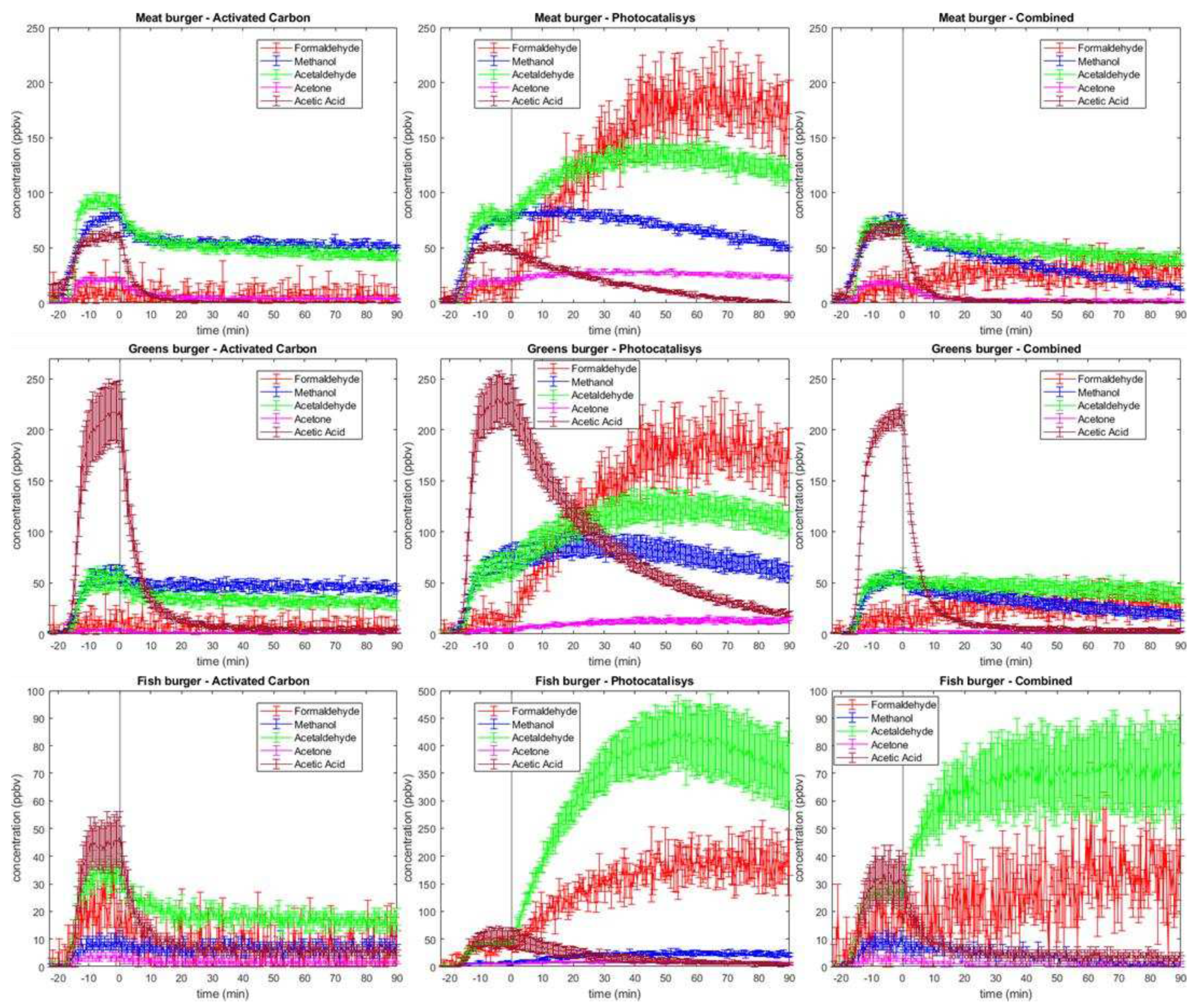
In all types of burgers, formaldehyde (m/z = 31, indicated by the red line) is produced at a low concentration, stabilizing at a few ppb before the purification system is turned on (
Figure 2). Over time, when using activated carbons as adsorbents, the amount remains almost constant. Conversely, when operating with photocatalysis, formaldehyde increases to 200 ppb in all of three burger types, presumably due to its formation as an oxidation reaction intermediate, such as in methanol oxidation [
21]. In the combined system, however, the concentration trend returns to being almost a plateau at ca. 25 ppbv, indicating a great compensation from activated carbon. ACGIH suggest threshold limit values as time weighted over 8h (TLV-TWA), short time exposure (TLV-STEL), or ceiling limit (TLV-C). For formaldehyde, which is confirmed human carcinogen, they report TLV-STEL of 300 ppb and TWA of 100 ppb. Therefore, the use of photocatalytic oxidation filter can configure a health risk due to great production during the time. In “Activated Carbon” and in “Combined”, even if there is a stabilization at higher values than the previous system, both shows values lower than the ACGIH’s suggested values. Thus, indicatively the risk is low with using this filter as purifying system.
2.2.2. Methanol
Methanol (m/z = 33) is detected before the activation of purification system, in different amounts depending on burger composition, since it is readily produced upon cooking. The highest production is found in the case of meat burger (around 80 ppbv) followed by vegetable burger (50 ppbv) and fish burger (10 ppbv).
In meat burgers, we report that activated carbon reaches site saturation in a short time. As expected, methanol concentration does not decrease upon activated carbon filter activation. Despite the low abatement rate, the photocatalysis approach has a better profile and seems partially capable of degrading this VOC after an initial plateau. Combined system has the best trend and shows monotone decrease after switching on, with higher degradation rate. Adsorption on activated carbon in vegetal burgers keeps the methanol amount constant at 50 ppb. In all cases for “Activated Carbon” and “Photocatalysis” at the end of 90 min of air purifier activation, methanol concentration is still high, while the “Combined” method is more effective in the case of fish burger for which
Figure 2 displays complete air purification from methanol in 90 minutes. In the case of meat burger, which produced higher methanol concentration upon cooking, the “Combined” method is still the most effective but would require longer than 90 min for a complete air cleansing. Methanol is not classifiable as human carcinogen, ACGIH suggest a TLV-TWA of 200000 ppb, which is far away from our values (90 ppb ca. peak). Thus, we can assume a low-risk exposure.
2.2.3. Acetaldehyde
Acetaldehyde (m/z=45) has the most various behaviour among the considered VOCs. It is produced upon cooking meat burgers (ca. 80 ppbv), vegetable burgers (50 ppbv), fish burgers (30 ppbv). A possible explanation of this behaviour could be the presence of reactions that occur during the cooking process, as Maillard one and Strecker degradation of protein. The Maillard reaction is a reaction that happens during cooking and involves a free ammino group of amino acid and a carbonyl one of carbohydrate. The final products of this process are various classes of molecules compounding the aliment flavour. Another crucial step in flavour generation is Strecker degradation, which covert also a crucial step in Maillard’s reaction. α-amminoacid reacts with dicarbonyl molecule to form an aminoketone, which act as precursor of heterocyclic compounds responsible for food flavour and odour [
2]. Principal end-product of Strecker degradation is bonded aldehyde, and amminoacid that are known to lead to acetaldehyde production are mainly glycine and alanine. Previous works [
22,
23] have highlighted how acetaldehyde can be released by foods with contain high protein, fatty acids with high degree of unsaturation and high content of monosaccharides and disaccharides, as fructose and sucrose.
A very different behaviour is observed between the outcome of the air cleaning by the three devices on the three different types of burgers.
The first anomaly that can be noticed compared to the other volatiles is in photocatalysis techinque applied to fish burger: after the conditioning of sampling space with cooked burger volatilome and the ignition of TiO2 UVA LED, occurs a great production of acetaldehyde, with a peak of almost 450 ppbv. These are the highest concentration values detected during the experiments. Therefore, the “Photocatalysis” air cleaning system produced a high amount of acetaldehyde as byproduct which pollutes the chamber.
In activated carbon filter, applied to fish burger cooking emissions, the acetaldehyde amount is small and constant during time, likely due to saturation of coal porosity. As it emerges from the combination with photocatalysis, the introduction of carbon as adsorbent material allows to limit negative effects of photodegradation, keeping its value stable over time. In the experiments using meat burger, upon activation of the air cleaning system, acetaldehyde follows the trend of fish burger measures, but with smaller concentration values, which is why adsorption with coal in combined system permits to mitigate the influence of photocatalysis. In the case of vegetable burger, no increase in the acetaldehyde is detected when using the “Combined” system, while, as expected, there is small increase when using the “Photocatalysis” system.
Interferences on the acetaldehyde signal from other molecules are expected to be minor. CO₂ could have an interfering signal, which could bring to overestimate acetaldehyde amount. According to Cappellin et al. (2019), since CO₂ does not react at collision rate with H
3O
+, it is ionized very inefficiently compared to acetaldehyde. In fact, the sensibility towards CO₂ is about 0.0001 cps/ppbv, while for acetaldehyde it is 10 cps/ppbv [
24]. Therefore, even if all VOCs (<1 ppmv) were converted to CO₂ by photodegradation, the maximum values of cps would be <0.1, which correspond to an interference of <0.01 ppbv on the acetaldehyde signal. This value compared to ca. 450 ppbv of detected acetaldehyde would be negligible. Another contribution to m/z=45 could come from ionization of butyraldehyde (m/z=73) for loss of carbonyl group (m/z=28) in ionization and fragmentation process [
25], but the phenomenon is appreciable only in fish cases, because in the others butyraldehyde is not detected over the background. In addition, it causes a negligible effect since m/z=73 is minor compared to m/z=45, and the expected fragmentation is 1:1. A similar argument on complex aldehyde in general allows us to assume that these compounds are negligible in acetaldehyde quantification. Acetaldehyde is reported by ACGIH as suspected human carcinogen, and for this reason they assume as TLV-C 25000 ppb. Then, our most intense value is ca. 450 ppb and this indicate a low-risk situation despite the great production of this VOC released by photocatalytic oxidation.
2.2.4. Acetone
It can be noticed that acetone (m/z = 59) is present in almost every sample around baseline, except for meat measurement, where there is a little production of this VOC upon cooking. The “Photocatalysis” method does not decrease its concentration. However, similarly to previous cases, coupling with activated coal restores its values around few ppb. ACGIH, having regard of its classification as “not classifiable as Human Carcinogen”, suggest for acetone TLV of 250000 ppb (TWA) and 500000 ppb (STEL). Therefore, with a highest value of 25 ppb, exposure risk seems to be negligible.
2.2.5. Acetic acid
Acetic acid (m/z = 61) is another of the most intense VOCs generated upon cooking, and a possible source of its production, as the other compounds, can be traced back to Maillard reaction [
26]. Another source could be the oxidation of acetaldehyde, although its contribution to the overall quantity is relatively minor. Greens burger produces the highest ethanoic acid amount upon cooking, but on par of other burgers, after the switching on of the purifying system, the concentration tends quickly to zero. “Combined” system has a higher slope compared to separated technique. Thus, synergetic coupling seems to be more efficient for acetic acid. Comparison with TLV (15000 ppb in STEL and 10000 ppb TWA) suggest that even in greens burgers, which show the peak value of ca. 250 ppb, it is configured a low risk for health.
2.3. Total Volatile Organic Compounds (TVOCs)
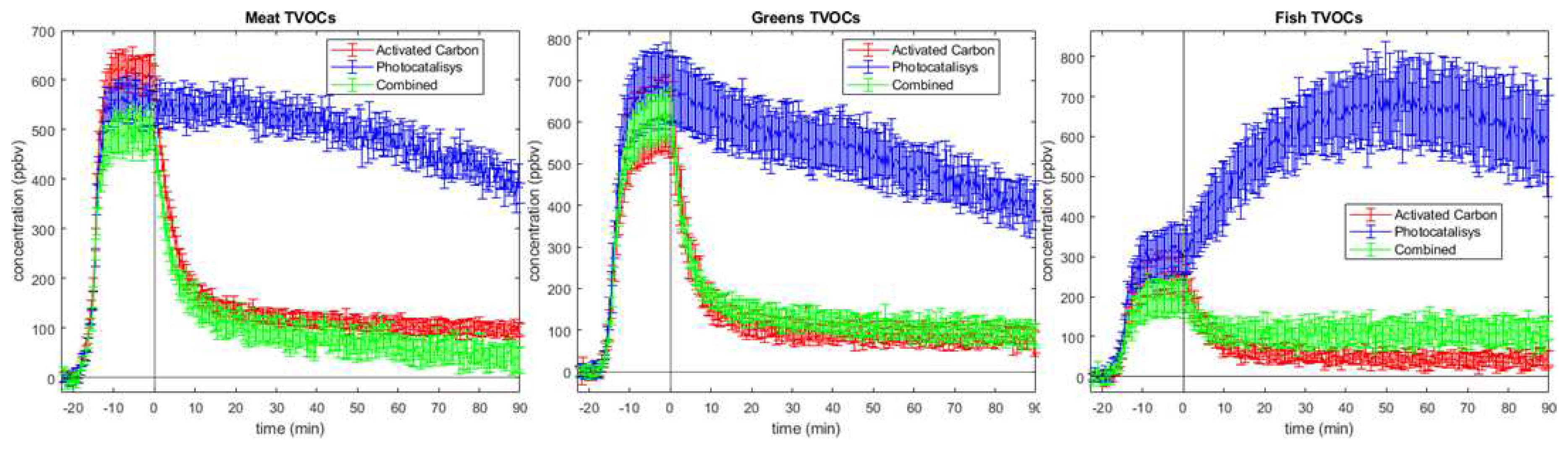
Some other conclusions can be elaborated on the total VOC (TVOC) plot (
Figure 3). First of all, they confirm the initial statement based on PCA plots. In fact, all three burger types show similar trends. After switching on the abatement system, Activated Carbon and Combined have similar TVOC values for most sampling time, except for meat burger, for which the synergy between AC and PC is better than using only coal. For both meat and greens, using the photocatalysis method, a reduction of total VOC amount over the time is displayed, although the decrease rate is lower than for the other two techniques. On the contrary, the same procedure with fish patties leads to a great increase of volatile concentration, mainly dependant on acetaldehyde production by photocatalysis. Looking at volatile production during the cooking phase, it can be noticed that there are different values of TVOC concentrations. This can be the effect of different chemical composition of patties rich in carbohydrates, proteins and lipids. These plots suggest that synergetic coupling yields better results in meat burger, leading TVOCs concentration at 90 minutes under the values obtained from activated carbon measurements, meanwhile for greens burgers there is not a significant difference. Regarding fish patties, photocatalysis exhibits lower filtering performance due to high intermediates emissions (formaldehyde and acetaldehyde,
Figure 2) and causes the combined setup to be slightly less effective than pure activated carbon alone due to stabilization of formaldehdye and acetaldehyde values at ca. 70 ppbv and 25 ppb.
4. Materials and Methods
All filters used as air purifier were manufactured by Elica S.p.A. The photocatalytic system is composed of 2 tiles of titan dioxide, with 4 LED UVA each (peak at 367 nm). It has TiO2 loading weight of 2.5 ± 0.5 g, LED power of 20.8W and average irradiance of 28 mW cm-2.
The adsorption material, instead, is ceramic-reinforced activated carbon composite; a single filter is composed of 4 honeycombs, with cell density of 676.
Measurements have been performed in an 8 m3 polyethylene (PE) chamber and, before cooking experiments, the sampling ambient has been conditioned with purified air. The pollutant abatement system (activated carbon, photocatalysis or both) were installed in the middle of the chamber, beside a hotplate. Meat hamburgers used to monitor the VOC emissions were made of adult bovine (produced by CEM SOC.COOP Cesena, Italy). Instead, greens burgers in use are “Fior dì natura ® “(Eurospin S.p.A.). Lastly, fish burgers, based on rainbow trout (Astro ®) were purchased from a local supermarket. In each measurement, after conditioning the chamber, a burger was placed in a pan and cooked on a hot plate with a set power of 1000 W for 5 minutes. The plate was then switched off, the air in the chamber was given 15 minutes to homogenise, and then the purification system was set on for 90 minutes. This value of sampling time has been chosen because it is comparable with medium working time of extracting hood for home use during cooking. Each step could be triggered remotely by an operator, so that it was not necessary to enter the chamber during the whole experiment.
The instrument used for VOC measurement is a PTR-MS (Ionicon Analytik GmbH), equipped with a quadrupole detector, directly linked to the chamber via Teflon PTFE tubing (1/8”). The sampling flow was set to 40 sccm. The method used to collect data involves a full scan from 20 m/z to 250 m/z, and a scanning time of 200 ms for each mass. For every combination of hamburger type – purification system, we performed three replicates. Every measurement has been run in compliance with the rules on measurement of efficiency of photocatalytic devices used for the elimination of VOCs in indoor air (UNI EN 16846-1:2017) and on performance measure of air cleaners (IEC 63086-1:2020). Calibration curves with pure standard injected into the chamber were constructed for selected VOCs (acetaldehyde, formaldehyde, acetone), while for the other compounds reaction kinetics were used to predict response factors as explained in Cappellin et al. (2012).
The instrumental response of PTR-MS is an intensity measured in [cps or counts per second] that has been translated into concentration (ppbv or parts per billion by volume) using calibration lines. For each replicate, we subtracted the background, computed using the mean of the first seven time points of each measurement Then, for each time point the mean of the replicate signals was calculated, and its associated uncertainty, evaluated as standard error of mean. In-house routines written in MATLAB® (R2023a) were employed to perform Principal Component Analysis (PCA) for data exploration. Before applying the calculation function of PCA, the dataset of three methods, already averaged, has been centred and normalized on standard deviation. Every column having null standard deviation, which correspond to masses that cannot be detected with PTR-MS, has been considered as irrelevant, and therefore excluded from the PCA.
5. Conclusions
Firstly, we managed to apply Direct Injection Mass Spectrometry for the real-time assessing of the efficiency of major indoor air treatment methods. In particular, PTR-MS seems to be a promising technology on VOC monitoring even for complex matrices, such as cooking emissions. We performed analysis on three complex matrixes, which are representative of important aliments macro category or relevant situation for homemade cooking. Our data suggest that formaldehyde in Activated carbon and Combined cleaning processes has low intensity and its amount seems to be low-risk, according to TLV values suggested by ACGIH, while a criticality is displayed when using Photocatalysis. Acetone is present upon cooking, although at small relative concentration, and all air purification systems manage to degrade it. On the contrary, acetaldehyde seems to have low compatibility with AC/TiO2, such as in the case of formaldehyde, for whom the two methods alone seem to have poor efficiency. Especially for photocatalysis, this can be caused by its production as intermediates of oxidation of complex VOCs.
Methanol trend suggests differences between the three methods: in activated carbons, probably due to saturation, its value is constant, while hybrid system manages to slowly degrade methanol. Photocatalysis seems to have particular behaviour: initially the concentration grows a little, and then drops over time. Thus, this technique seems to have poor performance, and the situation get enhanced in the case of acetaldehyde. In both instances, the starting peak is lower than the active coals one, due to initial adsorption also on the photocatalytic system. Also acetone follows the behaviour of acetaldehyde. Acetic acid, finally, seems to have good compatibility with separated systems. Every trend distinguishable from noise seems to stabilize in time, probably due to saturation of active sites of activated carbons; in fact, the two methods have similar plots. Another interpretation could be the contemporaneous production of the compounds as intermediates of oxidation reactions of complex VOCs.
Technique performance is highly dependent on burger composition, and in function of desired VOC abatement, there are pros and cons for each method. Using as a reference the TLV-TWA (8h) , TLV-STEL (15 min) or TLV-C (maximum) defined by ACGIH as exposure values of workers at chemicals without adverse effect, the five selected VOCs have in all measurement lower values than the threshold, except for photocatalysis system in formaldehyde cleansing. This means that generally and with the previous exception, the three purification systems, even if uncapable completely of wiping out the VOCs, the maximum concentration and the residual are not hazardous for human health. In conclusion, with the stated criticalities, all three techniques are promising in indoor air cleaning polluted by cooking-emitted VOCs.
Author Contributions
Conceptualization, L.C. and F.B.; Data Curation, D.Z. and M.S; Formal analysis, D.Z.; Methodology and Experimental, M.S.; Resources, L.C; F.B.; O.A.; G.B.; and R.V.; Supervision, L.C. and F.B.; Visualization, D.Z.; Writing original draft, D.Z.; Writing review and editing, M.S., L.C. and F.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data available on request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ni, Q.; Khomenko, I.; Gallo, L.; Biasioli, F.; Bittante, G. Rapid Profiling of the Volatilome of Cooked Meat by PTR-ToF-MS: Characterization of Chicken, Turkey, Pork, Veal and Beef Meat. Foods 2020, 9, 1776. [Google Scholar] [CrossRef]
- Bleicher, J.; Ebner, E.E.; Bak, K.H. Formation and Analysis of Volatile and Odor Compounds in Meat—A Review. Molecules 2022, 27, 6703. [Google Scholar] [CrossRef]
- Shahidi, F.; Samaranayaka, A.G.P.; Pegg, R.B. Malliard Reaction and Browning. Encyclopedia of Meat Sciences 2014, 1, 391–403. [Google Scholar]
- Shahidi, F.; Hossain, A. Role of Lipids in Food Flavor Generation. Molecules 2022, 27, 5014. [Google Scholar] [CrossRef]
- Henderson, S.K.; Nawar, W.W. Thermal Interaction of Linoleic Acid and Its Esters with Valine. J Am Oil Chem Soc 1981, 58, 632–635. [Google Scholar] [CrossRef]
- Mansur, A.R.; Lee, H.J.; Choi, H.; Lim, T.; Yoo, M.; Jang, H.W.; Nam, T.G. Comparison of Two Commercial Solid-phase Microextraction Fibers for the Headspace Analysis of Volatile Compounds in Different Pork and Beef Cuts. J Food Process Preserv 2018, 42, jfpp–13746. [Google Scholar] [CrossRef]
- Zou, W.; Gao, B.; Ok, Y.S.; Dong, L. Integrated Adsorption and Photocatalytic Degradation of Volatile Organic Compounds (VOCs) Using Carbon-Based Nanocomposites: A Critical Review. Chemosphere 2019, 218, 845–859. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, L.; Yang, Z.; Wang, P.; Yan, Y.; Ran, J. Adsorption Materials for Volatile Organic Compounds (VOCs) and the Key Factors for VOCs Adsorption Process: A Review. Sep Purif Technol 2020, 235, 116213. [Google Scholar] [CrossRef]
- Pui, W.K.; Yusoff, R.; Aroua, M.K. A Review on Activated Carbon Adsorption for Volatile Organic Compounds (VOCs). Reviews in Chemical Engineering 2019, 35, 649–668. [Google Scholar] [CrossRef]
- Harper, M. Sorbent Trapping of Volatile Organic Compounds from Air. J Chromatogr A 2000, 885, 129–151. [Google Scholar] [CrossRef] [PubMed]
- Jo, W.K.; Yang, C.H. Granular-Activated Carbon Adsorption Followed by Annular-Type Photocatalytic System for Control of Indoor Aromatic Compounds. Sep Purif Technol 2009, 66, 438–442. [Google Scholar] [CrossRef]
- Almaie, S.; Vatanpour, V.; Rasoulifard, M.H.; Koyuncu, I. Volatile Organic Compounds (VOCs) Removal by Photocatalysts: A Review. Chemosphere 2022, 306, 135655. [Google Scholar] [CrossRef] [PubMed]
- Koe, W.S.; Lee, J.W.; Chong, W.C.; Pang, Y.L.; Sim, L.C. An Overview of Photocatalytic Degradation: Photocatalysts, Mechanisms, and Development of Photocatalytic Membrane. Environmental Science and Pollution Research 2020, 27, 2522–2565. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wen, M.; Li, G.; An, T. Recent Advances in VOC Elimination by Catalytic Oxidation Technology onto Various Nanoparticles Catalysts: A Critical Review. Appl Catal B 2021, 281, 119447. [Google Scholar] [CrossRef]
- Hanif, Md.A.; Shin, H.; Chun, D.; Kim, H.G.; Kwac, L.K.; Kim, Y.S. Photocatalytic VOCs Degradation Efficiency of Polypropylene Membranes by Incorporation of TiO2 Nanoparticles. Membranes (Basel) 2022, 13, 50. [Google Scholar] [CrossRef]
- Mishra, A.; Mehta, A.; Kainth, S.; Basu, S. Effect of Different Plasmonic Metals on Photocatalytic Degradation of Volatile Organic Compounds (VOCs) by Bentonite/M-TiO2 Nanocomposites under UV/Visible Light. Appl Clay Sci 2018, 153, 144–153. [Google Scholar] [CrossRef]
- Ouzzine, M.; Romero-Anaya, A.J.; Lillo-Ródenas, M.A.; Linares-Solano, A. Spherical Activated Carbon as an Enhanced Support for TiO2/AC Photocatalysts. Carbon N Y 2014, 67, 104–118. [Google Scholar] [CrossRef]
- Oliver, K.D.; Adams, J.R.; Daughtrey, E.H.; McClenny, W.A.; Yoong, M.J.; Pardee, M.A.; Almasi, E.B.; Kirshen, N.A. Technique for Monitoring Toxic VOCs in Air: Sorbent Preconcentration, Closed-Cycle Cooler Cryofocusing, and GC/MS Analysis. Environ Sci Technol 1996, 30, 1939–1945. [Google Scholar] [CrossRef]
- Cappellin, L.; Karl, T.; Probst, M.; Ismailova, O.; Winkler, P.M.; Soukoulis, C.; Aprea, E.; Märk, T.D.; Gasperi, F.; Biasioli, F. On Quantitative Determination of Volatile Organic Compound Concentrations Using Proton Transfer Reaction Time-of-Flight Mass Spectrometry. Environ Sci Technol 2012, 46, 2283–2290. [Google Scholar] [CrossRef]
- Stucchi, M.; Galli, F.; Bianchi, C.L.; Pirola, C.; Boffito, D.C.; Biasioli, F.; Capucci, V. Simultaneous Photodegradation of VOC Mixture by TiO2 Powders. Chemosphere 2018, 193, 198–206. [Google Scholar] [CrossRef]
- Sirivallop, A.; Escobedo, S.; Areerob, T.; de Lasa, H.; Chiarakorn, S. Photocatalytic Conversion of Organic Pollutants in Air: Quantum Yields Using a Silver/Nitrogen/TiO2 Mesoporous Semiconductor under Visible Light. Catalysts 2021, 11, 529. [Google Scholar] [CrossRef]
- Atamaleki, A.; Motesaddi Zarandi, S.; Massoudinejad, M.; Hesam, G.; Naimi, N.; Esrafili, A.; Fakhri, Y.; Mousavi Khaneghah, A. Emission of Aldehydes from Different Cooking Processes: A Review Study. Air Qual Atmos Health 2022, 15, 1183–1204. [Google Scholar] [CrossRef]
- Jung, H.J.; Kim, S.H.; Yoo, K.C.; Lee, J.H. Changes in Acetaldehyde and Formaldehyde Contents in Foods Depending on the Typical Home Cooking Methods. J Hazard Mater 2021, 414, 125475. [Google Scholar] [CrossRef] [PubMed]
- Cappellin, L.; Loreto, F.; Biasioli, F.; Pastore, P.; McKinney, K. A Mechanism for Biogenic Production and Emission of MEK from MVK Decoupled from Isoprene Biosynthesis. Atmos Chem Phys 2019, 19, 3125–3135. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, Q.; Lu, B.; Sun, S.; Zhang, S.; Zhang, J. Rapid Determination of Six Low Molecular Carbonyl Compounds in Tobacco Smoke by the APCI-MS/MS Coupled to Data Mining. J Anal Methods Chem 2017, 2017, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sohail, A.; Al-Dalali, S.; Wang, J.; Xie, J.; Shakoor, A.; Asimi, S.; Shah, H.; Patil, P. Aroma Compounds Identified in Cooked Meat: A Review. Food Research International 2022, 157, 111385. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).