Submitted:
15 October 2023
Posted:
17 October 2023
You are already at the latest version
Abstract

Keywords:
1. Introduction
2. Materials and Methods
2.1. Plant material and fractions preparation
2.2. Cell culture
2.3. Western blot
2.4. Animals
2.5. Blood pressure measurement
2.6. Organ retrieving
2.7. Biochemical analysis of plasma and liver
2.8. ELISA
2.9. Histopathology
2.10. Statistical analysis
3. Results
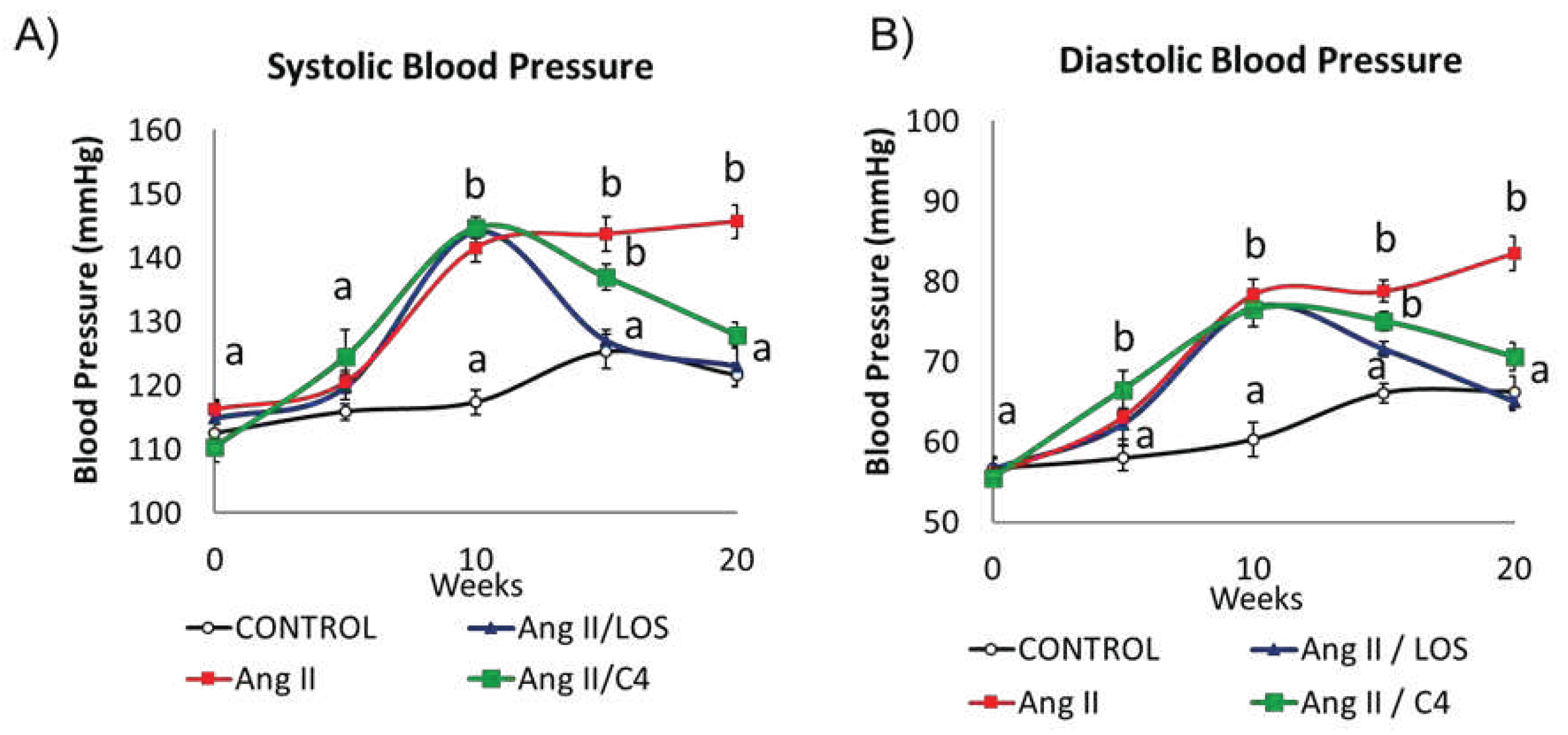
3.1. C4 regulates blood pressure
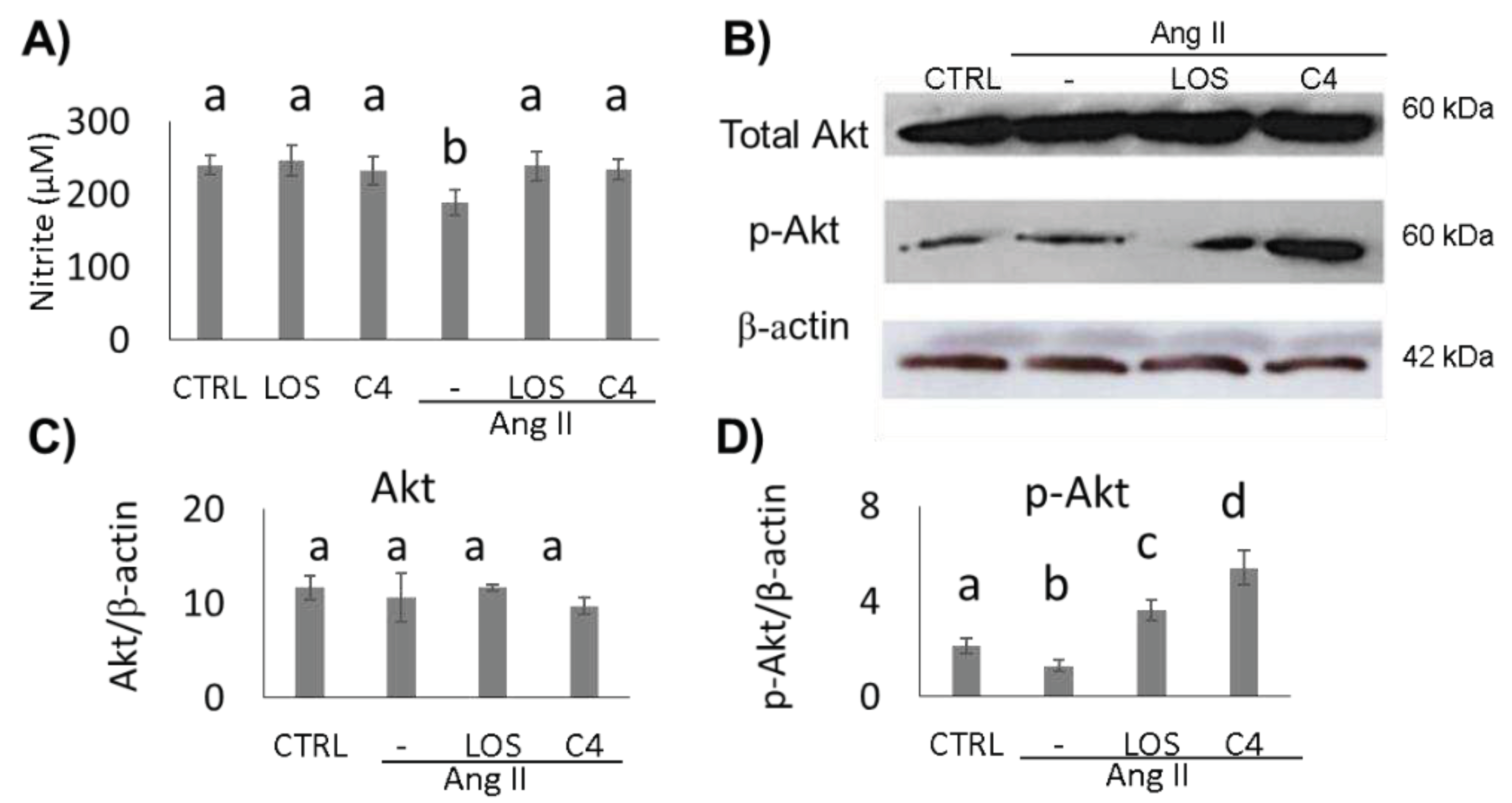
3.2. C4 stimulates Akt phosphorylation and NO production
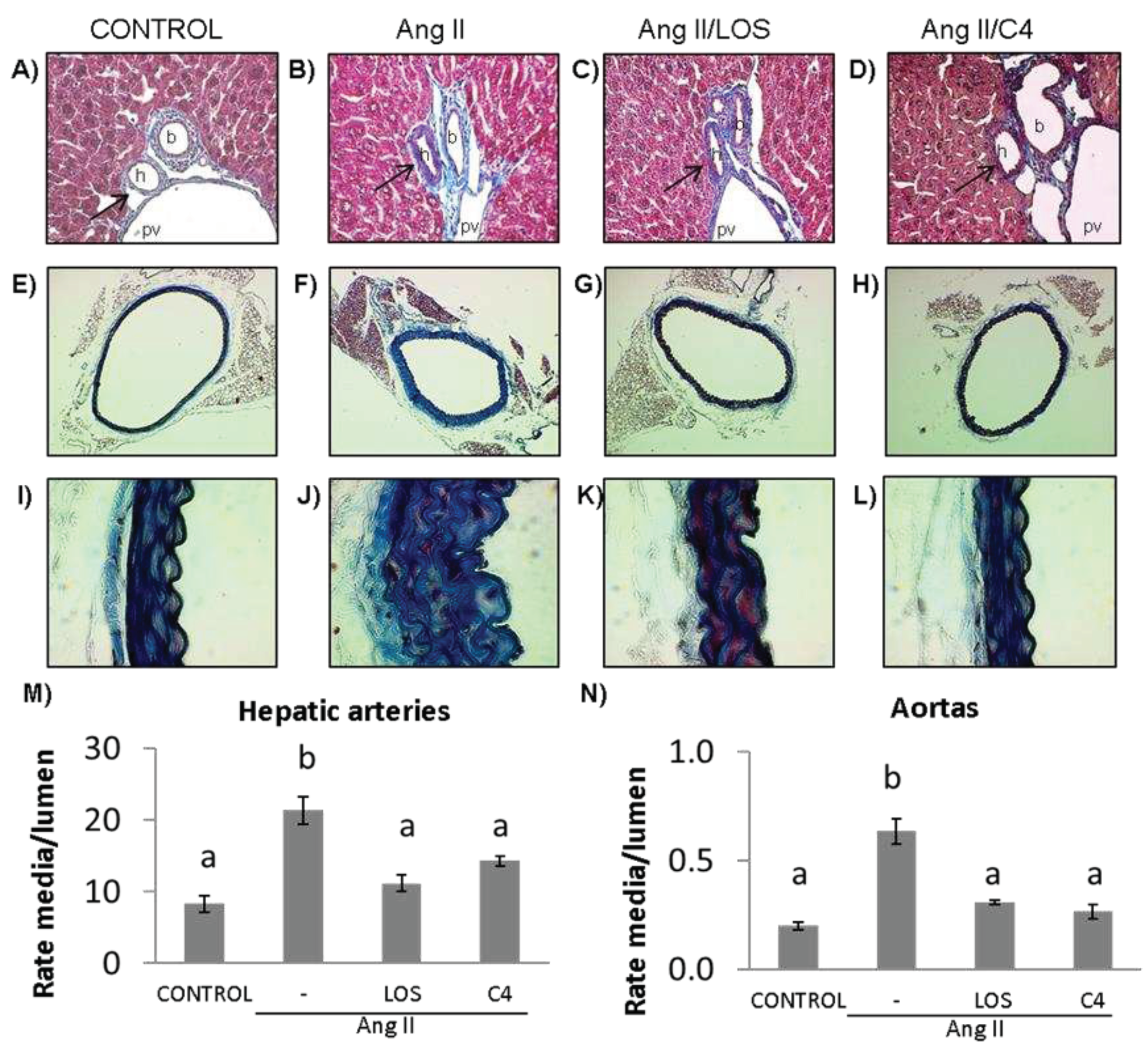
3.3. C4 reverses vascular remodeling
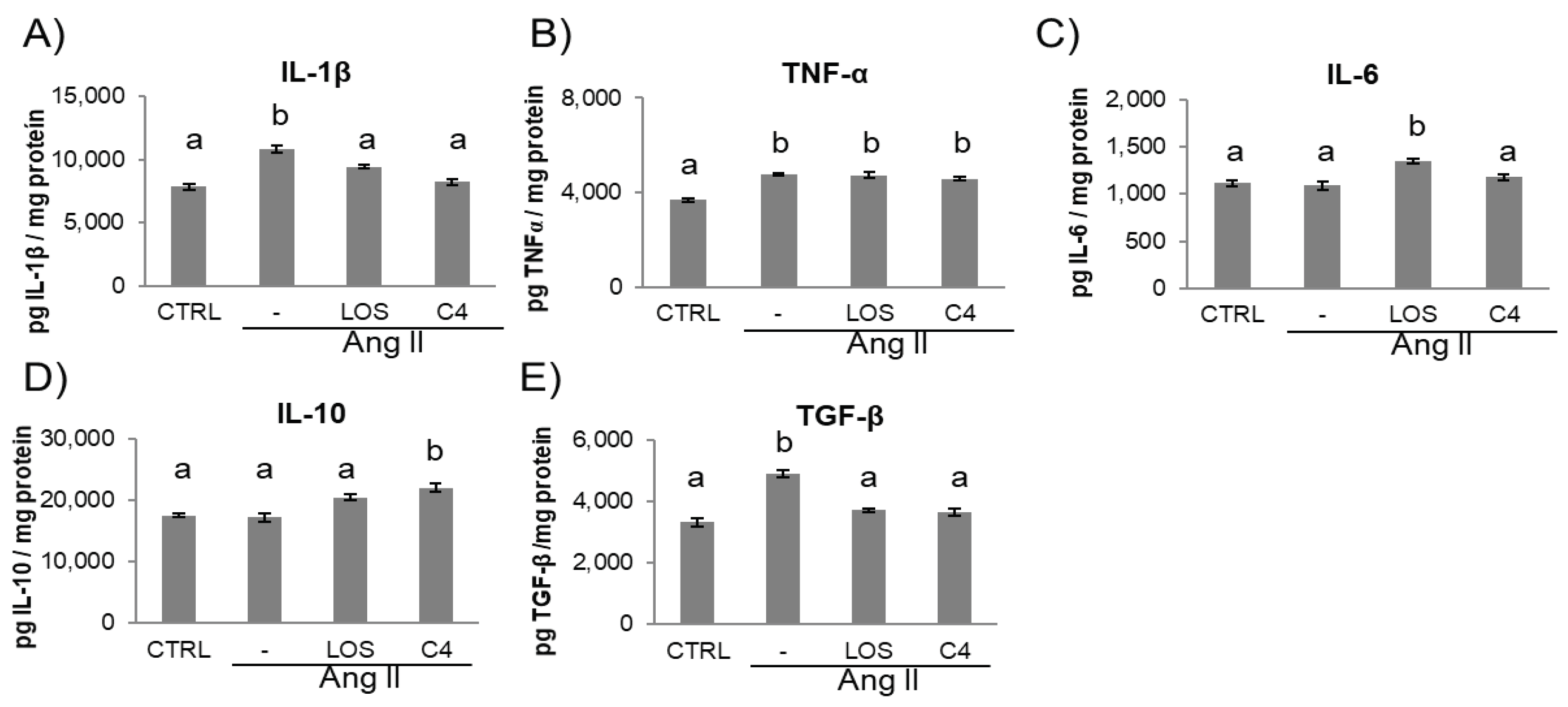
3.4. C4 modifies the inflammatory environment
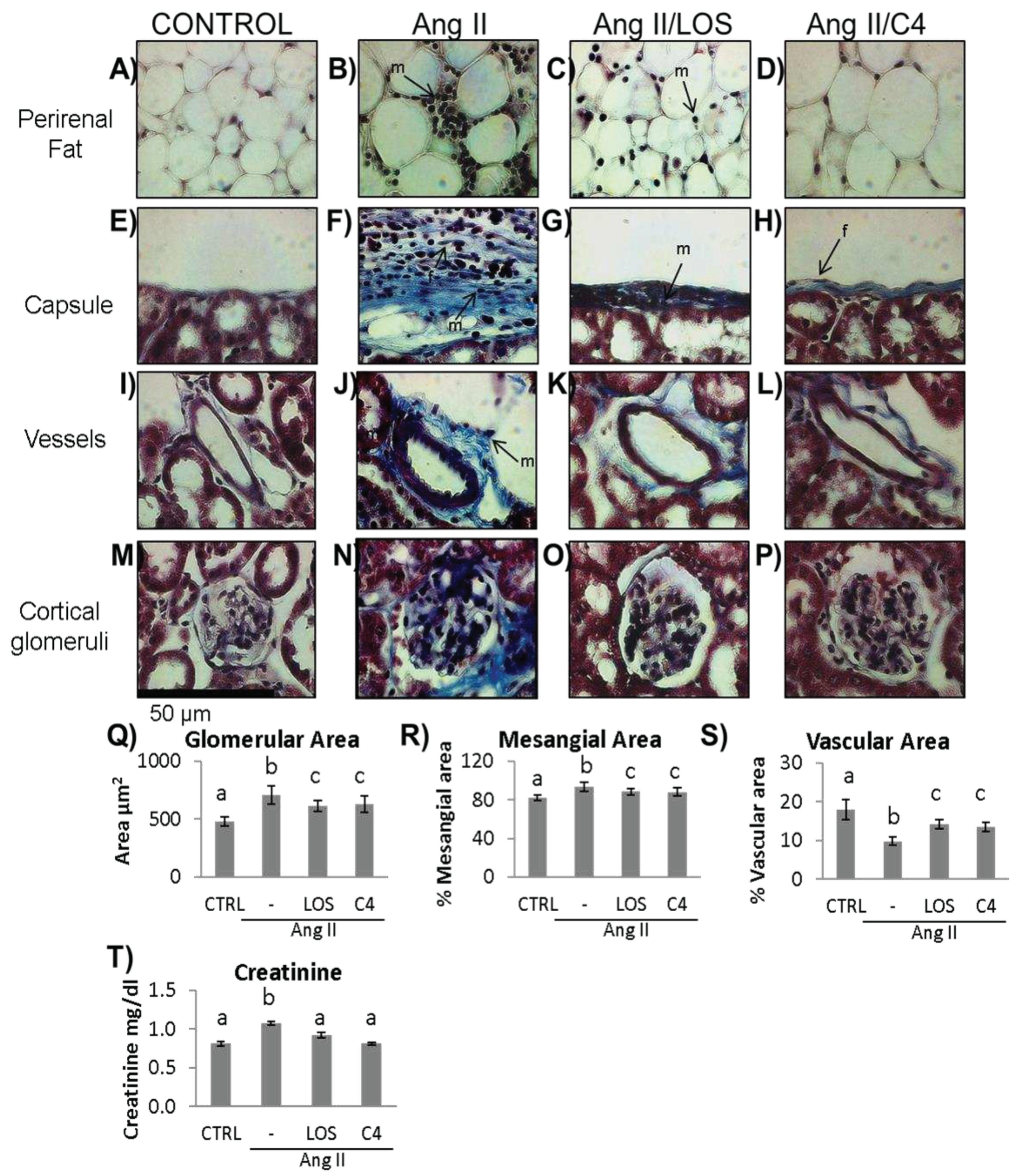
3.5. C4 ameliorates kidney damage
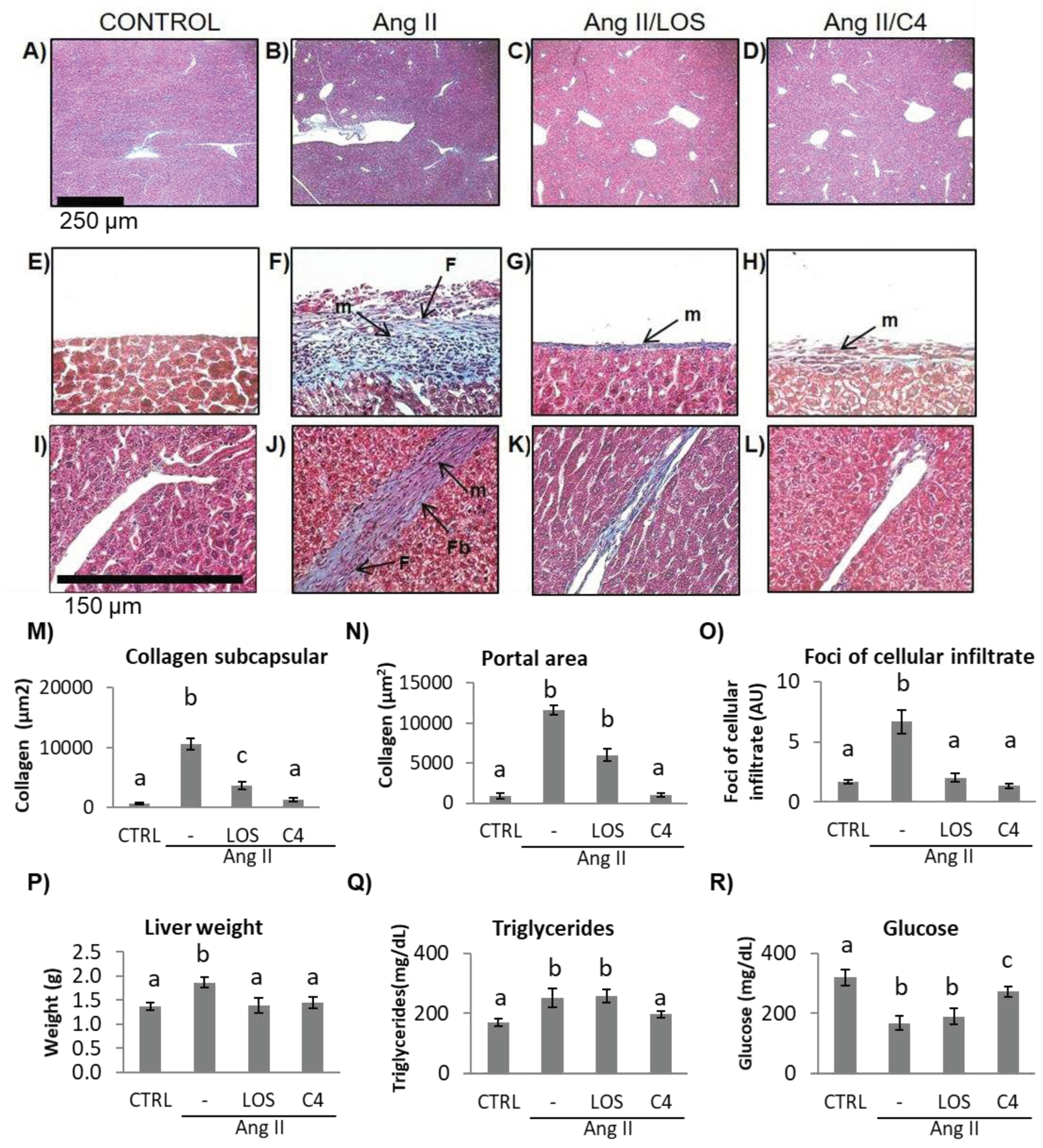
3.6. C4 ameliorates liver damage
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Cyr, A. R. , Huckaby, L. V., Shiva, S. S., & Zuckerbraun, B. S. Nitric Oxide and Endothelial Dysfunction. Crit Care Clin 2020, 36(2), 307–321. [Google Scholar] [CrossRef] [PubMed]
- Kinzenbaw, D. A. , Langmack, L., & Faraci, F. M.. Angiotensin II-induced endothelial dysfunction: Impact of sex, genetic background, and rho kinase. Physiol Rep 2022, 10. [Google Scholar] [CrossRef]
- Gallo, G. , Volpe, M., & Savoia, C. Endothelial Dysfunction in Hypertension: Current Concepts and Clinical Implications. Front Med, 8958. [Google Scholar] [CrossRef]
- Theofilis, P. , Sagris, M., Oikonomou, E., Antonopoulos, A. S., Siasos, G., Tsioufis, C., & Tousoulis, D. Inflammatory Mechanisms Contributing to Endothelial Dysfunction. Biomedicines 2021, 9. [Google Scholar] [CrossRef]
- Kobayashi, N. , Mita, S., Yoshida, K., Honda, T., Kobayashi, T., Hara, K., Nakano, S., Tsubokou, Y., & Matsuoka, H. Celiprolol activates eNOS through the PI3K-Akt pathway and inhibits VCAM-1 Via NF-kappaB induced by oxidative stress. Hypertension 2003, 42, 1004–1013. [Google Scholar] [CrossRef] [PubMed]
- Iaccarino, G. , Ciccarelli, M., Sorriento, D., Cipolletta, E., Cerullo, V., Iovino, G. L., Paudice, A., Elia, A., Santulli, G., Campanile, A., Arcucci, O., Pastore, L., Salvatore, F., Condorelli, G., & Trimarco, B. AKT participates in endothelial dysfunction in hypertension. Circulation 2004, 109, 2587–2593. [Google Scholar] [CrossRef]
- Ding, J. , Yu, M., Jiang, J., Luo, Y., Zhang, Q., Wang, S., Yang, F., Wang, A., Wang, L., Zhuang, M., Wu, S., Zhang, Q., Xia, Y., & Lu, D. Angiotensin II Decreases Endothelial Nitric Oxide Synthase Phosphorylation via AT1R Nox/ROS/PP2A Pathway. Fron Physiol 2020, 11, 566410. [Google Scholar] [CrossRef]
- Medina-Leyte, D. J. , Zepeda-García, O., Domínguez-Pérez, M., González-Garrido, A., Villarreal-Molina, T., & Jacobo-Albavera, L. Endothelial Dysfunction, Inflammation and Coronary Artery Disease: Potential Biomarkers and Promising Therapeutical Approaches. Int J Mol Sci 2021, 22. [Google Scholar] [CrossRef]
- da Silva, G. M. , da Silva, M. C., Nascimento, D. V. G., Lima Silva, E. M., Gouvêa, F. F. F., de França Lopes, L. G., Araújo, A. V., Ferraz Pereira, K. N., & de Queiroz, T. M. Nitric Oxide as a Central Molecule in Hypertension: Focus on the Vasorelaxant Activity of New Nitric Oxide Donors. Biology (Basel) 2021, 10. [Google Scholar] [CrossRef]
- Trejo-Moreno, C. , Jiménez-Ferrer, E., Castro-Martínez, G., Méndez-Martínez, M., Santana, M. A., Arrellín-Rosas, G., Pedraza-Chaverri, J., Medina-Campos, O. N., Hernández-Téllez, B., Ramírez-Pliego, O., Herrera-Ruiz, M., Cervantes-Torres, J., Alvarado-Ojeda, Z. A., Costet-Mejía, A., Fragoso, G., & Rosas-Salgado, G. Characterization of a murine model of endothelial dysfunction induced by chronic intraperitoneal administration of angiotensin II. Sci Rep 2021, 11. [Google Scholar] [CrossRef]
- Mukherjee, P. K. , Nema, N. K., Maity, N., & Sarkar, B. K. Phytochemical and therapeutic potential of cucumber. Fitoterapia 2013, 84, 227–236. [Google Scholar] [CrossRef]
- Kumar, D. , Kumar, S., Singh, J., Narender, Rashmi, Vashistha, B., & Singh, N. Free Radical Scavenging and Analgesic Activities of Cucumis sativus L. Fruit Extract. J Young Pharm. [CrossRef]
- Bernardini, C. , Zannoni, A., Bertocchi, M., Tubon, I., Fernandez, M., & Forni, M. Water/ethanol extract of Cucumis sativus L. fruit attenuates lipopolysaccharide-induced inflammatory response in endothelial cells. BMC Complement Altern Med 2018, 18. [Google Scholar] [CrossRef]
- Trejo-Moreno, C. , Méndez-Martínez, M., Zamilpa, A., Jiménez-Ferrer, E., Perez-Garcia, M. D., Medina-Campos, O. N., Pedraza-Chaverri, J., Santana, M. A., Esquivel-Guadarrama, F. R., Castillo, A., Cervantes-Torres, J., Fragoso, G., & Rosas-Salgado, G. Cucumis sativus Aqueous Fraction Inhibits Angiotensin II-Induced Inflammation and Oxidative Stress In Vitro. Nutrients 2018, 10. [Google Scholar] [CrossRef]
- Liang, X. X. , Wang, R. Y., Guo, Y. Z., Cheng, Z., Lv, D. Y., Luo, M. H., He, A., Luo, S. X., & Xia, Y. Phosphorylation of Akt at Thr308 regulates p-eNOS Ser1177 during physiological conditions. FEBS open bio 2021, 11, 1953–1964. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, K. , Altmann, H. M., Straub, A. C., & Isenberg, J. S. Nitric oxide: what’s new to NO? Am J Physiol Cell Physiol 2017, 312, C254–C262. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L. , Christoph, M., & Hoffmann, G. Effects of Olive Oil on Markers of Inflammation and Endothelial Function-A Systematic Review and Meta-Analysis. Nutrients, 7651. [Google Scholar] [CrossRef]
- Vairappan, B. Endothelial dysfunction in cirrhosis: Role of inflammation and oxidative stress. World J Hepatol 2015, 7, 443–459. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, K. , Hida, M., Hasegawa, M., Matsumoto, T., & Kobayashi, T. Dietary polyphenol morin rescues endothelial dysfunction in a diabetic mouse model by activating the Akt/eNOS pathway. Mol Nutr Food Res 2016, 60, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D. , Chen, J., Zhang, Y., Liao, H. B., Zhang, Z. F., Zhuang, Y., Pan, M. X., Tang, J. C., Liu, R., Lei, Y., Wang, S., Qin, X. P., Feng, Y. G., Chen, Y., & Wan, Q. Glycine confers neuroprotection through PTEN/AKT signal pathway in experimental intracerebral hemorrhage. Biochem Biophys Res Commun 2018, 501, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Tsuji-Tamura, K. , Sato, M., Fujita, M., & Tamura, M. The role of PI3K/Akt/mTOR signaling in dose-dependent biphasic effects of glycine on vascular development. Biochem Biophys Res Commun 2020, 529, 596–602. [Google Scholar] [CrossRef]
- Gómez-Zamudio, J. H. , García-Macedo, R., Lázaro-Suárez, M., Ibarra-Barajas, M., Kumate, J., & Cruz, M. Vascular endothelial function is improved by oral glycine treatment in aged rats. Can J Physiol Pharmacol 2015, 93, 465–473. [Google Scholar] [CrossRef]
- Ruiz-Ramírez, A. , Ortiz-Balderas, E., Cardozo-Saldaña, G., Diaz-Diaz, E., & El-Hafidi, M. Glycine restores glutathione and protects against oxidative stress in vascular tissue from sucrose-fed rats. Clin Sci (Lond) 2014, 126, 19–29. [Google Scholar] [CrossRef]
- Hnia, K. , Gayraud, J., Hugon, G., Ramonatxo, M., De La Porte, S., Matecki, S., & Mornet, D. L-arginine decreases inflammation and modulates the nuclear factor-kappaB/matrix metalloproteinase cascade in mdx muscle fibers. Am J Pathol 2008, 172(6), 1509–1519. [Google Scholar] [CrossRef]
- Ren, W. , Yin, J., Wu, M., Liu, G., Yang, G., Xion, Y., Su, D., Wu, L., Li, T., Chen, S., Duan, J., Yin, Y., & Wu, G. Serum amino acids profile and the beneficial effects of L-arginine or L-glutamine supplementation in dextran sulfate sodium colitis. PloS one 2014, 9. [Google Scholar] [CrossRef]
- Fujiwara, T. , Kanazawa, S., Ichibori, R., Tanigawa, T., Magome, T., Shingaki, K., Miyata, S., Tohyama, M., & Hosokawa, K. L-arginine stimulates fibroblast proliferation through the GPRC6A-ERK1/2 and PI3K/Akt pathway. PloS one 2014, 9. [Google Scholar] [CrossRef]
- Hou, E. , Sun, N., Zhang, F., Zhao, C., Usa, K., Liang, M., & Tian, Z. Malate and Aspartate Increase L-Arginine and Nitric Oxide and Attenuate Hypertension. Cell Rep 2017, 19, 1631–1639. [Google Scholar] [CrossRef]
- Rizk, S. M. , El-Maraghy, S. A., & Nassar, N. N. A novel role for SIRT-1 in L-arginine protection against STZ induced myocardial fibrosis in rats. PloS one 2014, 9. [Google Scholar] [CrossRef]
- Gao, P. , Xu, T. T., Lu, J., Li, L., Xu, J., Hao, D. L., Chen, H. Z., & Liu, D. P. Overexpression of SIRT1 in vascular smooth muscle cells attenuates angiotensin II-induced vascular remodeling and hypertension in mice. J Mol Med (Berl) 2014, 92, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Kim, H. J. , Hart, J., Knatz, N., Hall, M. W., & Wewers, M. D. Janus kinase 3 down-regulates lipopolysaccharide-induced IL-1 beta-converting enzyme activation by autocrine IL-10. J Immunol 2004, 172, 4948–4955. [Google Scholar] [CrossRef]
- Chen, J. , Ma, X., Yang, Y., Dai, Z., Wu, Z., & Wu, G. Glycine enhances expression of adiponectin and IL-10 in 3T3-L1 adipocytes without affecting adipogenesis and lipolysis. Amino acids 2018, 50, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Yu, H. R. , Tsai, C. C., Chang, L. S., Huang, H. C., Cheng, H. H., Wang, J. Y., Sheen, J. M., Kuo, H. C., Hsieh, K. S., Huang, Y. H., Yang, K. D., & Hsu, T. Y. l-Arginine-Dependent Epigenetic Regulation of Interleukin-10, but Not Transforming Growth Factor-β, Production by Neonatal Regulatory T Lymphocytes. Front Immunol 2017, 8, 487. [Google Scholar] [CrossRef] [PubMed]
- Morais, S. R. L. , Brito, V. G. B., Mello, W. G., & Oliveira, S. H. P. l-arginine modulates inflammation and muscle regulatory genes after a single session of resistance exercise in rats. Scand J Med Sci Sports 2018, 28, 425–435. [Google Scholar] [CrossRef]
- Wang, H. D. , Lü, X. X., Lu, D. X., Qi, R. B., Wang, Y. P., Fu, Y. M., & Wang, L. W. Glycine inhibits the LPS-induced increase in cytosolic Ca2+ concentration and TNFalpha production in cardiomyocytes by activating a glycine receptor. Acta Pharmacol Sin 2009, 30, 1107–1114. [Google Scholar] [CrossRef]
- Almanza-Perez, J. C. , Alarcon-Aguilar, F. J., Blancas-Flores, G., Campos-Sepulveda, A. E., Roman-Ramos, R., Garcia-Macedo, R., & Cruz, M. Glycine regulates inflammatory markers modifying the energetic balance through PPAR and UCP-2. Biomed Pharmacother. [CrossRef]
- Amin, F. U. , Shah, S. A., & Kim, M. O. Glycine inhibits ethanol-induced oxidative stress, neuroinflammation and apoptotic neurodegeneration in postnatal rat brain. Neurochem Int. [CrossRef]
- Campbell, J. , Ciesielski, C. J., Hunt, A. E., Horwood, N. J., Beech, J. T., Hayes, L. A., Denys, A., Feldmann, M., Brennan, F. M., & Foxwell, B. M. A novel mechanism for TNF-alpha regulation by p38 MAPK: involvement of NF-kappa B with implications for therapy in rheumatoid arthritis. J Immunol. 2004, 173, 6928–6937. [Google Scholar] [CrossRef]
- Diaz-Ricart, M. , Torramade-Moix, S., Pascual, G., Palomo, M., Moreno-Castaño, A. B., Martinez-Sanchez, J., Vera, M., Cases, A., & Escolar, G. Endothelial Damage, Inflammation and Immunity in Chronic Kidney Disease. Toxins (Basel). [CrossRef]
- Guerrot, D. , & Bellien, J. (2014). Editorial: renal endothelial dysfunction: evolving concepts and perspectives. Cardiovasc Hematol Disord Drug Targets 2014, 14(1), 1–2. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P. W. , Do, Y. S., & Hsueh, W. A. (1993). Angiotensin II causes mesangial cell hypertrophy. Hypertension 1993, 21(1), 29–35. [Google Scholar] [CrossRef] [PubMed]
- Orth, S. R. , Weinreich, T., Bönisch, S., Weih, M., & Ritz, E. Angiotensin II induces hypertrophy and hyperplasia in adult human mesangial cells. Exp Nephrol 1995, 3(1), 23–33. [Google Scholar]
- Ruiz-Ortega, M. , Rupérez, M., Esteban, V., Rodríguez-Vita, J., Sánchez-López, E., Carvajal, G., & Egido, J. Angiotensin II: a key factor in the inflammatory and fibrotic response in kidney diseases. 2006. [Google Scholar] [CrossRef]
- Froh, M. , Thurman, R. G., & Wheeler, M. D. Molecular evidence for a glycine-gated chloride channel in macrophages and leukocytes. Am J Physiol Gastrointest Liver Physiol. [CrossRef]
- Ramprasath, T. , Kumar, P. H., Puhari, S. S., Murugan, P. S., Vasudevan, V., & Selvam, G. S. L-Arginine ameliorates cardiac left ventricular oxidative stress by upregulating eNOS and Nrf2 target genes in alloxan-induced hyperglycemic rats. Biochem Biophys Res Commun 2012, 428(3), 389–394. [Google Scholar] [CrossRef] [PubMed]
- Korkina L., G. Phenylpropanoids as naturally occurring antioxidants: from plant defense to human health. Cell Mol Biol (Noisy-le-grand.
- Oh, C. J. , Kim, J. Y., Min, A. K., Park, K. G., Harris, R. A., Kim, H. J., & Lee, I. K. Sulforaphane attenuates hepatic fibrosis via NF-E2-related factor 2-mediated inhibition of transforming growth factor-β/Smad signaling. Free Radic Biol Med. [CrossRef]
- Choi, H. K. , Pokharel, Y. R., Lim, S. C., Han, H. K., Ryu, C. S., Kim, S. K., Kwak, M. K., & Kang, K. W. Inhibition of liver fibrosis by solubilized coenzyme Q10: Role of Nrf2 activation in inhibiting transforming growth factor-beta1 expression. Toxicol Appl Pharmacol. [CrossRef]
- Choi, B. H. , Kang, K. S., & Kwak, M. K. Effect of redox modulating NRF2 activators on chronic kidney disease. Molecules 2014, 19(8), 12727–12759. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y. , Clark, S. E., Morris, E. M., Thyfault, J. P., Uptergrove, G. M., Whaley-Connell, A. T., Ferrario, C. M., Sowers, J. R., & Ibdah, J. A. Angiotensin II-induced non-alcoholic fatty liver disease is mediated by oxidative stress in transgenic TG(mRen2)27(Ren2) rats. J Hepatol. [CrossRef]
- Alvarado-Ojeda, Z. A. , Coset, Mejia, A., Arrellin, Rosas, G., Jiménez-Ferrer, J. E., Zamilpa, A., Trejo-Moreno, C., Castro, Martínez, G., Méndez, Martínez, M., Cervantes, Torres, J., Báez, Reyes, J. C., Fragoso, G. & Rosas, Salgado, G. Hepatoprotective effect of hydroalcoholic extract from root of Sechium edule (Jacq.) Sw. over hepatic injury induced by chronic application of angiotensin II. Front. Nat. Produc. 2022, 1. [Google Scholar] [CrossRef]
- Wu, Y. , Ma, K. L., Zhang, Y., Wen, Y., Wang, G. H., Hu, Z. B., Liu, L., Lu, J., Chen, P. P., Ruan, X. Z., & Liu, B. C. Lipid disorder and intrahepatic renin-angiotensin system activation synergistically contribute to non-alcoholic fatty liver disease. Liver Int, 1525. [Google Scholar] [CrossRef]
- Edvardsson, U. , Ljungberg, A., & Oscarsson, J. Insulin and oleic acid increase PPARgamma2 expression in cultured mouse hepatocytes. Biochem Biophys Res Commun 2006, 340(1), 111–117. [Google Scholar] [CrossRef]
- Rogue, A. , Anthérieu, S., Vluggens, A., Umbdenstock, T., Claude, N., de la Moureyre-Spire, C., Weaver, R. J., & Guillouzo, A. PPAR agonists reduce steatosis in oleic acid-overloaded HepaRG cells. Toxicol Appl Pharmacol. [CrossRef]
- Schupp, M. , Janke, J., Clasen, R., Unger, T., & Kintscher, U. Angiotensin type 1 receptor blockers induce peroxisome proliferator-activated receptor-gamma activity. Circulation, 2054. [Google Scholar] [CrossRef]
- Yin, M. , Ikejima, K., Arteel, G. E., Seabra, V., Bradford, B. U., Kono, H., Rusyn, I., & Thurman, R. G. Glycine accelerates recovery from alcohol-induced liver injury. J Pharmacol Exp Ther, 1014. [Google Scholar]
- Taha, M. O. , Caricati-Neto, A., Ferreira, R. M., Simões, M.deJ., Monteiro, H. P., & Fagundes, D. J. L-arginine in the ischemic phase protects against liver ischemia-reperfusion injury. Acta Cir Bras. 2012, 27(9), 616–623. [Google Scholar] [CrossRef]
- Kawano, Y. , & Cohen, D. E. (2013). Mechanisms of hepatic triglyceride accumulation in non-alcoholic fatty liver disease. J Gastroenterol. [CrossRef]
- Brasier, A. R. , Jamaluddin, M., Han, Y., Patterson, C., & Runge, M. S. Angiotensin II induces gene transcription through cell-type-dependent effects on the nuclear factor-kappaB (NF-kappaB) transcription factor. Mol Cell Biochem.
- Guo, F. , Chen, X. L., Wang, F., Liang, X., Sun, Y. X., & Wang, Y. J. Role of angiotensin II type 1 receptor in angiotensin II-induced cytokine production in macrophages. J Interferon Cytokine Res. [CrossRef]
- Gressner, A. M. , Weiskirchen, R., Breitkopf, K., & Dooley, S. Roles of TGF-beta in hepatic fibrosis. Front Biosci. [CrossRef]
- Lugo-Baruqui, A. , Muñoz-Valle, J. F., Arévalo-Gallegos, S., & Armendáriz-Borunda, J. Role of angiotensin II in liver fibrosis-induced portal hypertension and therapeutic implications. Hepatol Res. [CrossRef]
- Rivera, C. A. , Bradford, B. U., Hunt, K. J., Adachi, Y., Schrum, L. W., Koop, D. R., Burchardt, E. R., Rippe, R. A., & Thurman, R. G. Attenuation of CCl(4)-induced hepatic fibrosis by GdCl(3) treatment or dietary glycine. Am J Physiol Gastrointest Liver Physiol. [CrossRef]
- Senthilkumar, R. , & Nalini, N. Glycine prevents hepatic fibrosis by preventing the accumulation of collagen in rats with alcoholic liver injury. Pol J Pharmacol. 2004, 56(1), 121–128. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




