Submitted:
15 October 2023
Posted:
16 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
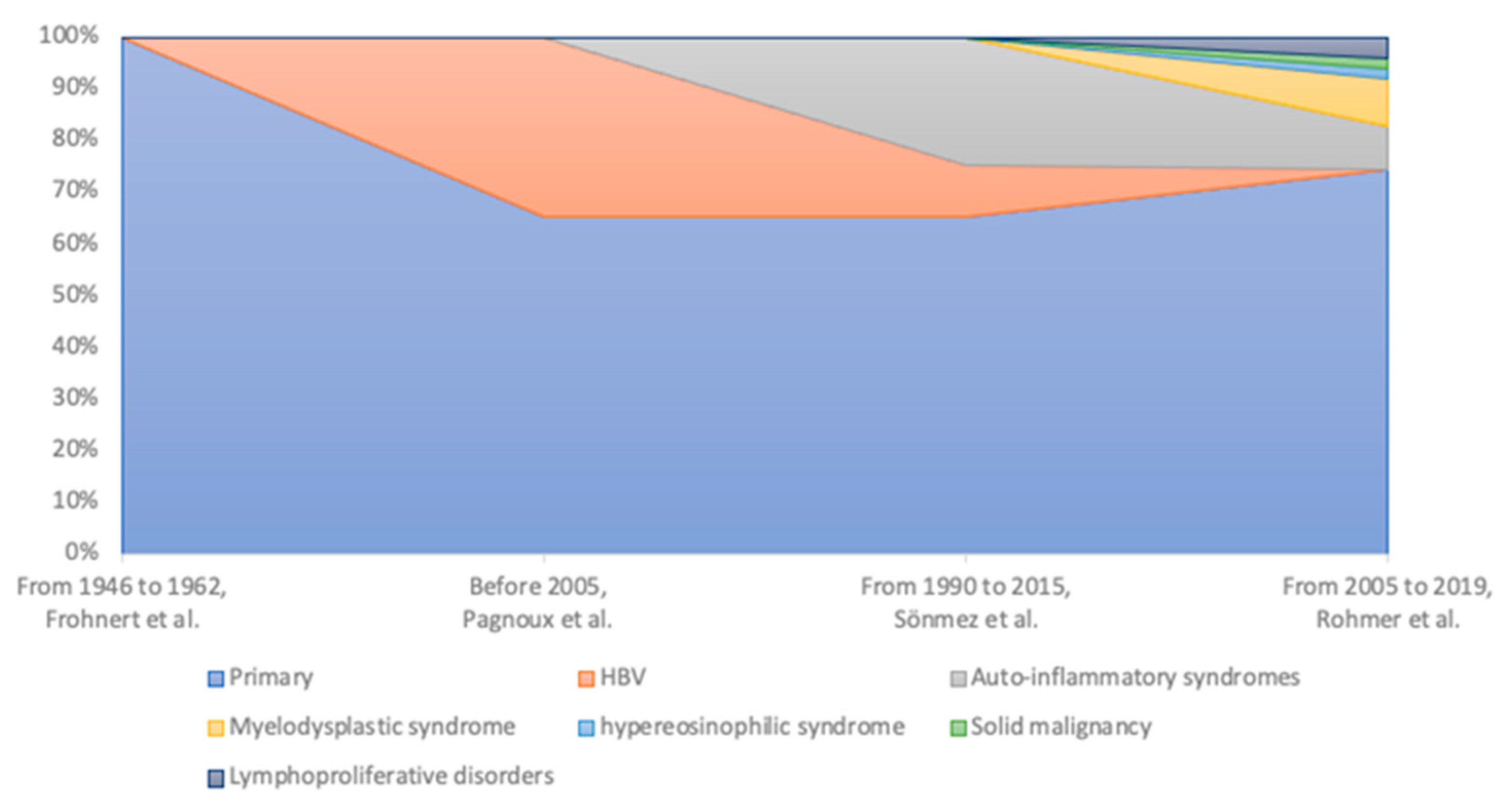
2. Epidemiology
3. Physiopathology
4. Clinical manifestations
4.1. General symptoms (85-93%)
4.2. Neurologic (59-79%)
4.3. Cutaneous (50-59%)
4.4. Renal (15-75%)
4.5. Gastrointestinal (22 to 38%)
4.6. Genital (15 to 17%)
4.7. Cardiovascular (7 to 78%)
4.8. Other manifestations
5. Treatments
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Stone, J.H. Vasculitis: A Collection of Pearls and Myths. Rheum. Dis. Clin. North Am. 2007, 33, 691–739. [Google Scholar] [CrossRef] [PubMed]
- Zeek, P.M. Periarteritis Nodosa: A Critical Review. Am. J. Clin. Pathol. 1952, 22, 777–790. [Google Scholar] [CrossRef] [PubMed]
- Davies, D.J.; E Moran, J.; Niall, J.F.; Ryan, G.B. Segmental necrotising glomerulonephritis with antineutrophil antibody: possible arbovirus aetiology? BMJ 1982, 285, 606–606. [Google Scholar] [CrossRef] [PubMed]
- Karadag, O.; Jayne, D.J. Polyarteritis nodosa revisited: a review of historical approaches, subphenotypes and a research agenda. Clin Exp Rheumatol 2018, 135–142. [Google Scholar]
- Jennette, J.C.; Falk, R.J.; Bacon, P.A.; Basu, N.; Cid, M.C.; Ferrario, F.; Flores-Suarez, L.F.; Gross, W.L.; Guillevin, L.; Hagen, E.C.; et al. 2012 Revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2012, 65, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Guillevin, L.; Mahr, A.; Cohen, P. Les vascularites nécrosantes systémiques : classification et stratégies actuelles de traitement. La Rev. de Médecine Interne 2003, 24, 172–182. [Google Scholar] [CrossRef]
- Rohmer, J.; Trefond, L.; Nguyen, Y.; Durel, C.; Lacout, C.; Maurier, F.; Rouzaud, D.; Cohen, P.; Lazaro, E.; Mekinian, A.; et al. Caractéristiques cliniques et évolution à long-terme des périartérite noueuses systémiques diagnostiquées depuis 2005. La Rev. de Médecine Interne 2020, 41, A33–A34. [Google Scholar] [CrossRef]
- Mahr, A.; Guillevin, L.; Poissonnet, M.; Aymé, S. Prevalences of polyarteritis nodosa, microscopic polyangiitis, Wegener's granulomatosis, and Churg-Strauss syndrome in a French urban multiethnic population in 2000: A capture–recapture estimate. Arthritis Care Res. 2004, 51, 92–99. [Google Scholar] [CrossRef]
- Lane, S.E.; Watts, R.; Scott, D.G.I. Epidemiology of systemic vasculitis. Curr. Rheumatol. Rep. 2005, 7, 270–275. [Google Scholar] [CrossRef]
- Sönmez, H.E.; Armağan, B.; Ayan, G.; Barut, K.; Batu, E.D.; Erden, A.; Ugurlu, S.; Bilginer, Y.; Kasapcopur, O.; Karadag, O.; et al. Polyarteritis nodosa: lessons from 25 years of experience. Clin Exp Rheumatol 2018, 52–56. [Google Scholar]
- Saadoun, D.; Vautier, M.; Cacoub, P. Medium- and Large-Vessel Vasculitis. Circ. 2021, 143, 267–282. [Google Scholar] [CrossRef]
- Pagnoux, C.; Seror, R.; Henegar, C.; Mahr, A.; Cohen, P.; Le Guern, V.; Bienvenu, B.; Mouthon, L.; Guillevin, L. Clinical features and outcomes in 348 patients with polyarteritis nodosa: A systematic retrospective study of patients diagnosed between 1963 and 2005 and entered into the French vasculitis study group database. Arthritis Rheum. 2010, 62, 616–626. [Google Scholar] [CrossRef]
- Rohmer, J.; Nguyen, Y.; Trefond, L.; Agard, C.; Allain, J.S.; Berezne, A.; Charles, P.; Cohen, P.; Gondran, G.; Groh, M.; et al. Clinical features and long-term outcomes of patients with systemic polyarteritis nodosa diagnosed since 2005: Data from 196 patients. J. Autoimmun. 2023, 139, 103093. [Google Scholar] [CrossRef] [PubMed]
- De Virgilio, A.; Greco, A.; Magliulo, G.; Gallo, A.; Ruoppolo, G.; Conte, M.; Martellucci, S.; de Vincentiis, M. Polyarteritis nodosa: A contemporary overview. Autoimmun. Rev. 2016, 15, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Kobayashi, S.; Ogishima, D.; Aoki, Y.; Sonoue, H.; Abe, H.; Fukumura, Y.; Nobukawa, B.; Kumasaka, T.; Mori, S.; et al. Isolated necrotizing arteritis (localized polyarteritis nodosa): examination of the histological process and disease entity based on the histological classification of stage and histological differences from polyarteritis nodosa. Cardiovasc. Pathol. 2007, 16, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Lie, J.T. Systemic and isolated vasculitis. A rational approach to classification and pathologic diagnosis. Pathol Annu 1989, 25–114. [Google Scholar]
- Kallenberg, C.G.; Brouwer, E.; Weening, J.J.; Tervaert, J.W.C. Anti-neutrophil cytoplasmic antibodies: Current diagnostic and pathophysiological potential. Kidney Int. 1994, 46, 1–15. [Google Scholar] [CrossRef] [PubMed]
- E Grau, G.; Roux-Lombard, P.; Gysler, C.; Lambert, C.; Lambert, P.H.; Dayer, J.M.; Guillevin, L. Serum cytokine changes in systemic vasculitis. Immunology 1989, 68, 196–8. [Google Scholar] [PubMed]
- Cid, M.; Grau, J.M.; Casademont, J.; Campo, E.; Coll-Vinent, B.; López-Soto, A.; Ingelmo, M.; Urbano-Márquez, A. Immunohistochemical characterization of inflammatory cells and immunologic activation markers in muscle and nerve biopsy specimens from patients with systemic polyarteritis nodosa. Arthritis Rheum. 1994, 37, 1055–1061. [Google Scholar] [CrossRef] [PubMed]
- Guillevin, L.; Ronco, P.; Verroust, P. Circulating immune complexes in systemic necrotizing vasculitis of the polyarteritis nodosa group. Comparison of HBV-related polyarteritis nodosa and churg strauss angiitis. J. Autoimmun. 1990, 3, 789–792. [Google Scholar] [CrossRef]
- Prince, A.; Trepo, C. ROLE OF IMMUNE COMPLEXES INVOLVING SH ANTIGEN IN PATHOGENESIS OF CHRONIC ACTIVE HEPATITIS AND POLYARTERITIS NODOSA. Lancet 1971, 297, 1309–1312. [Google Scholar] [CrossRef] [PubMed]
- Trepo, C.G.; Zuckerman, A.J.; Bird, R.C.; Prince, A.M. The role of circulating hepatitis B antigen/antibody immune complexes in the pathogenesis of vascular and hepatic manifestations in polyarteritis nodosa. J. Clin. Pathol. 1974, 27, 863–868. [Google Scholar] [CrossRef] [PubMed]
- Fye, K.H.; Becker, M.J.; Theofilopoulos, A.N.; Moutsopoulos, H.; Feldman, J.-L.; Talal, N. Immune complexes in hepatitis B antigen-associated periarteritis nodosa. Am. J. Med. 1977, 62, 783–791. [Google Scholar] [CrossRef]
- Carson, C.W.; Conn, D.L.; Czaja, A.J.; Wright, T.L.; E Brecher, M. Frequency and significance of antibodies to hepatitis C virus in polyarteritis nodosa. J Rheumatol 1993, 20, 304–9. [Google Scholar] [PubMed]
- Saadoun, D.; Terrier, B.; Semoun, O.; Sene, D.; Maisonobe, T.; Musset, L.; Amoura, Z.; Rigon, M.R.; Cacoub, P. Hepatitis C virus–associated polyarteritis nodosa. Arthritis Care Res. 2010, 63, 427–435. [Google Scholar] [CrossRef]
- Cacoub, P.; Maisonobe, T.; Thibault, V.; Gatel, A.; Servan, J.; Musset, L.; Piette, J.C. Systemic vasculitis in patients with hepatitis C. J Rheumatol 2001, 28, 109–18. [Google Scholar]
- Patel, N.; Patel, N.; Khan, T.; Patel, N.; Espinoza, L.R. HIV Infection and Clinical Spectrum of Associated Vasculitides. Curr. Rheumatol. Rep. 2011, 13, 506–512. [Google Scholar] [CrossRef]
- Leruez-Ville, M.; Laugé, A.; Morinet, F.; Guillevin, L.; Dény, P. Polyarteritis riodosa and parvovirus B19. Lancet 1994, 344, 263–264. [Google Scholar] [CrossRef]
- Gherardi, R.; Belec, L.; Mhiri, C.; Gray, F.; Lescs, M.; Sobel, A.; Guillevin, L.; Wechsler, J. The spectrum of vasculitis in human immunodeficiency virus–infected patients. a clinicopathologic evaluation. Arthritis Rheum. 1993, 36, 1164–1174. [Google Scholar] [CrossRef]
- Viguier, M.; Guillevin, L.; Laroche, L. Treatment of Parvovirus B19–Associated Polyarteritis Nodosa with Intravenous Immune Globulin. New Engl. J. Med. 2001, 344, 1481–1482. [Google Scholar] [CrossRef]
- Finkel, T.; Leung, D.; Harbeck, R.; Gelfand, E.; Török, T.; Zaki, S.; Anderson, L.; Ferguson, P.; Saulsbury, F.; Durigon, E.; et al. Chronic parvovirus B19 infection and systemic necrotising vasculitis: opportunistic infection or aetiological agent? Lancet 1994, 343, 1255–1258. [Google Scholar] [CrossRef]
- Ramadan, S.M.; Kasfiki, E.V.; Kelly, C.W.; Ali, I. An interesting case of small vessel pathology following coronavirus infection. BMJ Case Rep. 2020, 13, e237407. [Google Scholar] [CrossRef]
- Vacchi, C.; Meschiari, M.; Milic, J.; Marietta, M.; Tonelli, R.; Alfano, G.; Volpi, S.; Faltoni, M.; Franceschi, G.; Ciusa, G.; et al. COVID-19-associated vasculitis and thrombotic complications: from pathological findings to multidisciplinary discussion. Rheumatology 2020, 59, e147–e150. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Hsu, H.; Chen, Y. Cutaneous polyarteritis nodosa following ChAdOx1 nCoV-19 vaccination. Int. J. Dermatol. 2022, 61, 630–631. [Google Scholar] [CrossRef] [PubMed]
- Ohkubo, Y.; Ohmura, S.-I.; Ishihara, R.; Miyamoto, T. Possible case of polyarteritis nodosa with epididymitis following COVID-19 vaccination: A case report and review of the literature. Mod. Rheumatol. Case Rep. 2022, 7, 172–176. [Google Scholar] [CrossRef]
- Ohmura, S.-I.; Ohkubo, Y.; Ishihara, R.; Otsuki, Y.; Miyamoto, T. Medium-vessel Vasculitis Presenting with Myalgia Following COVID-19 Moderna Vaccination. Intern. Med. 2022, 61, 3453–3457. [Google Scholar] [CrossRef]
- Kermani, T.A.; Ham, E.K.; Camilleri, M.J.; Warrington, K.J. Polyarteritis Nodosa-like Vasculitis in Association with Minocycline Use: A Single-Center Case Series. Semin. Arthritis Rheum. 2012, 42, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, M.T.; West, S.G. Polyarteritis nodosa in hairy cell leukemia: treatment with interferon-alpha. J Rheumatol 1994, 21, 1150–2. [Google Scholar] [PubMed]
- Roupie, A.L.; Guedon, A.; Terrier, B.; Lahuna, C.; Jachiet, V.; Regent, A.; de Boysson, H.; Carrat, F.; Seguier, J.; Terriou, L.; et al. Vasculitis associated with myelodysplastic syndrome and chronic myelomonocytic leukemia: French multicenter case-control study. Semin. Arthritis Rheum. 2020, 50, 879–884. [Google Scholar] [CrossRef] [PubMed]
- Veitch, D.; Tsai, T.; Watson, S.; Joshua, F. Paraneoplastic polyarteritis nodosa with cerebral masses: case report and literature review. Int. J. Rheum. Dis. 2014, 17, 805–809. [Google Scholar] [CrossRef]
- Hamidou, M.A.; Boumalassa, A.; Larroche, C.; El Kouri, D.; Blétry, O.; Grolleau, J.-Y. Systemic medium-sized vessel vasculitis associated with chronic myelomonocytic leukemia. Semin. Arthritis Rheum. 2001, 31, 119–126. [Google Scholar] [CrossRef]
- Fain, O.; Hamidou, M.; Cacoub, P.; Godeau, B.; Wechsler, B.; ParIès, J.; Stirnemann, J.; Morin, A.; Gatfosse, M.; Hanslik, T.; et al. Vasculitides associated with malignancies: Analysis of sixty patients. Arthritis Care Res. 2007, 57, 1473–1480. [Google Scholar] [CrossRef]
- Komrokji, R.S.; Kulasekararaj, A.; Al Ali, N.H.; Kordasti, S.; Bart-Smith, E.; Craig, B.M.; Padron, E.; Zhang, L.; Lancet, J.E.; Pinilla-Ibarz, J.; et al. Autoimmune diseases and myelodysplastic syndromes. Am. J. Hematol. 2016, 91, E280–E283. [Google Scholar] [CrossRef] [PubMed]
- Saif, M.W.; Hopkins, J.L.; Gore, S.D. Autoimmune Phenomena in Patients with Myelodysplastic Syndromes and Chronic Myelomonocytic Leukemia. Leuk. Lymphoma 2002, 43, 2083–2092. [Google Scholar] [CrossRef] [PubMed]
- Balbir-Gurman, A.; Nahir, A.M.; Braun-Moscovici, Y. Vasculitis in siblings with familial Mediterranean fever: a report of three cases and review of the literature. Clin. Rheumatol. 2006, 26, 1183–1185. [Google Scholar] [CrossRef]
- Ozen, S.; Ben-Chetrit, E.; Bakkaloglu, A.; Gur, H.; Tinaztepe, K.; Calguneri, M.; Turgan, C.; Turkmen, A.; Akpolat, I.; Danaci, M.; et al. Polyarteritis nodosa in patients with Familial Mediterranean Fever (FMF): A concomitant disease or a feature of FMF? Semin. Arthritis Rheum. 2001, 30, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Tunca, M.; Akar, S.; Onen, F.; Ozdogan, H.; Kasapcopur, O.; Yalcinkaya, F.; Tutar, E.; Ozen, S.; Topaloglu, R.; Yilmaz, E.; et al. Familial Mediterranean Fever (FMF) in Turkey. Medicine 2005, 84, 1–11. [Google Scholar] [CrossRef]
- Ozen, S. The changing face of polyarteritis nodosa and necrotizing vasculitis. Nat. Rev. Rheumatol. 2017, 13, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Yalçınkaya, F.; Özçakar, Z.B.; Kasapçopur, O.; Öztürk, A.; Akar, N.; Bakkaloğlu, A.; Arısoy, N.; Ekı̇m, M.; Özen, S. Prevalence of the MEFV Gene Mutations in Childhood Polyarteritis Nodosa. J. Pediatr. 2007, 151, 675–678. [Google Scholar] [CrossRef] [PubMed]
- Balbir-Gurman, A.; Nahir, A.M.; Braun-Moscovici, Y. Vasculitis in siblings with familial Mediterranean fever: a report of three cases and review of the literature. Clin. Rheumatol. 2006, 26, 1183–1185. [Google Scholar] [CrossRef]
- Ozen, S.; Ben-Chetrit, E.; Bakkaloglu, A.; Gur, H.; Tinaztepe, K.; Calguneri, M.; Turgan, C.; Turkmen, A.; Akpolat, I.; Danaci, M.; et al. Polyarteritis nodosa in patients with Familial Mediterranean Fever (FMF): A concomitant disease or a feature of FMF? Semin. Arthritis Rheum. 2001, 30, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Tunca, M.; Akar, S.; Onen, F.; Ozdogan, H.; Kasapcopur, O.; Yalcinkaya, F.; Tutar, E.; Ozen, S.; Topaloglu, R.; Yilmaz, E.; et al. Familial Mediterranean Fever (FMF) in Turkey. Medicine 2005, 84, 1–11. [Google Scholar] [CrossRef]
- Liu, Y.; Jesus, A.A.; Marrero, B.; Yang, D.; Ramsey, S.E.; Montealegre Sanchez, G.A.; Tenbrock, K.; Wittkowski, H.; Jones, O.Y.; Kuehn, H.S.; et al. Activated STING in a Vascular and Pulmonary Syndrome. N. Engl. J. Med. 2014, 371, 507–518. [Google Scholar] [CrossRef]
- Caratsch, L.; Schnider, C.; Moi, L.; Theodoropoulou, K.; Candotti, F.; Hofer, M. Déficit en adénosine désaminase 2 : une maladie aux présentations multiples. Rev Med Suisse. [CrossRef]
- Elkan, P.N.; Pierce, S.B.; Segel, R.; Walsh, T.; Barash, J.; Padeh, S.; Zlotogorski, A.; Berkun, Y.; Press, J.J.; Mukamel, M.; et al. Mutant Adenosine Deaminase 2 in a Polyarteritis Nodosa Vasculopathy. New Engl. J. Med. 2014, 370, 921–931. [Google Scholar] [CrossRef]
- Zhou, Q.; Yang, D.; Ombrello, A.K.; Zavialov, A.V.; Toro, C.; Zavialov, A.V.; Stone, D.L.; Chae, J.J.; Rosenzweig, S.D.; Bishop, K.; et al. Early-Onset Stroke and Vasculopathy Associated with Mutations in ADA2. New Engl. J. Med. 2014, 370, 911–920. [Google Scholar] [CrossRef]
- Kaljas, Y.; Liu, C.; Skaldin, M.; Wu, C.; Zhou, Q.; Lu, Y.; Aksentijevich, I.; Zavialov, A. Human adenosine deaminases ADA1 and ADA2 bind to different subsets of immune cells. Cell Mol. Life Sci. 2016, 74, 555–570. [Google Scholar] [CrossRef]
- Zavialov, A.V.; Gracia, E.; Glaichenhaus, N.; Franco, R.; Zavialov, A.V.; Lauvau, G. Human adenosine deaminase 2 induces differentiation of monocytes into macrophages and stimulates proliferation of T helper cells and macrophages. J. Leukoc. Biol. 2010, 88, 279–290. [Google Scholar] [CrossRef]
- Zakine, E.; Rodrigues, F.; Papageorgiou, L.; Georgin-Lavialle, S.; Mekinian, A.; Terrier, B.; Kosmider, O.; Hirsch, P.; Jachiet, M.; Bouaziz, J.; et al. Caractéristiques cliniques et histologiques des manifestations cutanées du syndrome VEXAS : une étude rétrospective centralisée de 59 cas. La Rev. de Médecine Interne 2022, 43, A350–A351. [Google Scholar] [CrossRef]
- Beck, D.B.; Ferrada, M.A.; Sikora, K.A.; Ombrello, A.K.; Collins, J.C.; Pei, W.; Balanda, N.; Ross, D.L.; Cardona, D.O.; Wu, Z.; et al. Somatic Mutations in UBA1 and Severe Adult-Onset Autoinflammatory Disease. New Engl. J. Med. 2020, 383, 2628–2638. [Google Scholar] [CrossRef] [PubMed]
- Tervaert, J.W.C.; Kallenberg, C. NEUROLOGIC MANIFESTATIONS OF SYSTEMIC VASCULITIDES. Rheum. Dis. Clin. North Am. 1993, 19, 913–940. [Google Scholar] [CrossRef]
- Pagnoux, C.; Seror, R.; Henegar, C.; Mahr, A.; Cohen, P.; Le Guern, V.; Bienvenu, B.; Mouthon, L.; Guillevin, L. Clinical features and outcomes in 348 patients with polyarteritis nodosa: A systematic retrospective study of patients diagnosed between 1963 and 2005 and entered into the French vasculitis study group database. Arthritis Rheum. 2010, 62, 616–626. [Google Scholar] [CrossRef] [PubMed]
- Neves, F.S.; Lin, K. Bilateral Foot Drop in Polyarteritis Nodosa. New Engl. J. Med. 2012, 367, e9. [Google Scholar] [CrossRef]
- de Boysson, H.; Guillevin, L. Polyarteritis Nodosa Neurologic Manifestations. Neurol. Clin. 2019, 37, 345–357. [Google Scholar] [CrossRef]
- Provenzale, J.M.; Allen, N.B. Neuroradiologic findings in polyarteritis nodosa. AJNR Am J Neuroradiol 1996, 17, 1119–1126. [Google Scholar] [PubMed]
- Morgan, A.J.; A Schwartz, R. Cutaneous polyarteritis nodosa: a comprehensive review. Int. J. Dermatol. 2010, 49, 750–756. [Google Scholar] [CrossRef]
- Stewart, M.; Lo, A.; Shojania, K.; Au, S.; Seidman, M.A.; Dutz, J.P.; Chan, J. Cutaneous polyarteritis nodosa diagnosis and treatment: A retrospective case series. J. Am. Acad. Dermatol. 2022, 87, 1370–1373. [Google Scholar] [CrossRef] [PubMed]
- Sunderkötter, C.H.; Zelger, B.; Chen, K.; Requena, L.; Piette, W.; Carlson, J.A.; Dutz, J.; Lamprecht, P.; Mahr, A.; Aberer, E.; et al. Nomenclature of Cutaneous Vasculitis. Arthritis Rheumatol. 2018, 70, 171–184. [Google Scholar] [CrossRef]
- El-Reshaid, K.; Kapoor, M.M.; El-Reshaid, W.; Madda, J.P.; Varro, J. The spectrum of renal disease associated with microscopic polyangiitis and classic polyarteritis nodosa in Kuwait. Nephrol. Dial. Transplant. 1997, 12, 1874–1882. [Google Scholar] [CrossRef]
- Maritati, F.; Iannuzzella, F.; Pavia, M.P.; Pasquali, S.; Vaglio, A. Kidney involvement in medium- and large-vessel vasculitis. J. Nephrol. 2016, 29, 495–505. [Google Scholar] [CrossRef]
- Pourafshar, N.; Sobel, E.; Segal, M. A case of isolated renal involvement of polyarteritis nodosa successfully treated with steroid monotherapy. BMJ Case Rep. 2016, 2016. [Google Scholar] [CrossRef]
- Kaur, J.S.; Goldberg, J.P.; Schrier, R.W. Acute Renal Failure Following Arteriography in a Patient With Polyarteritis Nodosa. JAMA 1982, 247, 833–834. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, S.; Yamamoto, Y.; Saita, M.; Hisanaga, S.; Morita, S.; Tanaka, K.; Sumiyoshi, A.; Koono, M. [Renal failure associated with polyarteritis nodosa]. Nihon Jinzo Gakkai Shi 1990, 32, 739–44. [Google Scholar] [PubMed]
- Launay, D.; Michon-Pasturel, U.; Boumbar, Y.; Dubrulle, F.; Bouroz-Joly, J.; Hachulla, E.; Lemaitre, L.; Devulder, B. Hématome périrénal spontané bilatéral: une complication rare de la panartérite noueuse. La Rev. de Médecine Interne 1998, 19, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Nandwani, G.M.; Musker, M.P.; Chaplin, B.J.; El Madhoun, I.; Akbani, H. Spontaneous perirenal haemorrhage in polyarteritis nodosa. J Coll Physicians Surg--Pak JCPSP 2013, 23, 445–7. [Google Scholar]
- Miyagawa, T.; Iwata, Y.; Oshima, M.; Ogura, H.; Sato, K.; Nakagawa, S.; Yamamura, Y.; Kitajima, S.; Toyama, T.; Hara, A.; et al. Polyarteritis nodosa with perirenal hematoma due to the rupture of a renal artery aneurysm. CEN Case Rep. 2021, 10, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Allen, A.W.; Waybill, P.N.; Singh, H.; Brown, D.B. Polyarteritis Nodosa Presenting as Spontaneous Perirenal Hemorrhage: Angiographic Diagnosis and Treatment with Microcoil Embolization. J. Vasc. Interv. Radiol. 1999, 10, 1361–1363. [Google Scholar] [CrossRef]
- Levine, S.M.; Hellmann, D.B.; Stone, J.H. Gastrointestinal involvement in polyarteritis nodosa (1986–2000):: Presentation and outcomes in 24 patients. Am. J. Med. 2002, 112, 386–391. [Google Scholar] [CrossRef]
- Castelhano, R.; Win, K.M.; Carty, S. Celiac artery aneurysm causing an acute abdomen. BMJ Case Rep. 2021, 14, e240533. [Google Scholar] [CrossRef]
- Gendreau, S.; Porcher, R.; Thoreau, B.; Paule, R.; Maurier, F.; Goulenok, T.; Frumholtz, L.; Bernigaud, C.; Ingen-Housz-Oro, S.; Mekinian, A.; et al. Characteristics and risk factors for poor outcome in patients with systemic vasculitis involving the gastrointestinal tract. Semin. Arthritis Rheum. 2021, 51, 436–441. [Google Scholar] [CrossRef]
- Waisayarat, J.; Niyasom, C.; Vilaiyuk, S.; Molagool, S. Polyarteritis Nodosa with Cytomegalovirus Enteritis and Jejunoileal Perforation: Report of a Case with a Literature Review. Vasc. Heal. Risk Manag. 2022, ume 18, 595–601. [Google Scholar] [CrossRef]
- Dillard, B.M.; Black, W.C. Polyarteritis nodosa of the gallbladder and bile ducts. Am Surg 1970, 36, 423–7. [Google Scholar] [PubMed]
- Ito, M.; Sano, K.; Inaba, H.; Hotchi, M. Localized necrotizing arteritis. A report of two cases involving the gallbladder and pancreas. Arch Pathol Lab Med 1991, 115, 780–3. [Google Scholar]
- Empen, K.; Jung, M.-C.; Engelhardt, D.; Sackmann, M. Successful treatment of acute liver failure due to polyarteritis nodosa. Am. J. Med. 2002, 113, 349–351. [Google Scholar] [CrossRef] [PubMed]
- Herskowitz, M.M.; Flyer, M.A.; Sclafani, S.J.A. Percutaneous transhepatic coil embolization of a ruptured intrahepatic aneurysm in polyarteritis nodosa. Cardiovasc. Interv. Radiol. 1993, 16, 254–256. [Google Scholar] [CrossRef] [PubMed]
- Parent, B.A.; Cho, S.W.; Buck, D.G.; Nalesnik, M.A.; Gamblin, T.C. Spontaneous Rupture of Hepatic Artery Aneurysm Associated with Polyarteritis Nodosa. Am. Surg. 2010, 76, 1416–1419. [Google Scholar] [CrossRef] [PubMed]
- Roberto, M.; Meytes, V.; Liu, S. Ruptured hepatic aneurysm as first presenting symptom of polyarteritis nodosa. Oxf. Med Case Rep. 2018, 2018, 64–67. [Google Scholar] [CrossRef]
- Stambo, G.W.; Guiney, M.J.; Cannella, X.F.; Germain, B.F. Coil embolization of multiple hepatic artery aneurysms in a patient with undiagnosed polyarteritis nodosa. J. Vasc. Surg. 2004, 39, 1122–1124. [Google Scholar] [CrossRef]
- Guillevin, L.; Lhote, F.; Gallais, V.; Jarrousse, B.; Royer, I.; Gayraud, M.; Benichou, J. Gastrointestinal tract involvement in polyarteritis nodosa and Churg-Strauss syndrome. Ann Med Interne (Paris) 1995, 146, 260–7. [Google Scholar]
- Cacoub, P.; Guillevin, L.; Godeau, P. [Causes of death in systemic vasculitis of polyarteritis nodosa. Analysis of a series of 165 patients].. 1988, 139, 381–90. [Google Scholar]
- Teichman, J.M.; Mattrey, R.F.; Demby, A.M.; Schmidt, J.D. Polyarteritis Nodosa Presenting as Acute Orchitis: A Case Report and Review of the Literature. J. Urol. 1993, 149, 1139–1140. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Matsubara, S.; Yagi, K.; Kinoshita, K.; Fukunaga, T.; Yamamoto, A.; Uno, M. Intra-abdominal hemorrhage due to segmental arterial mediolysis of an ovarian artery pseudoaneurysm and concomitant aneurysmal subarachnoid hemorrhage: illustrative case. J. Neurosurgery: Case Lessons 2022, 4. [Google Scholar] [CrossRef]
- Kastner, D.; Gaffney, M.; Tak, T. Polyarteritis nodosa and myocardial infarction. Can J Cardiol 2000, 16, 515–8. [Google Scholar] [PubMed]
- Bae, Y.D.; Choi, H.J.; Lee, J.C.; Park, J.J.; Lee, Y.J.; Lee, E.B.; Song, Y.W. Clinical Features of Polyarteritis Nodosa in Korea. J. Korean Med Sci. 2006, 21, 591–595. [Google Scholar] [CrossRef]
- Schrader, M.L.; Hochman, J.S.; Bulkley, B.H. The heart in polyarteritis nodosa: A clinicopathologic study. Am. Hear. J. 1985, 109, 1353–1359. [Google Scholar] [CrossRef]
- Blétry, O.; Godeau, P.; Charpentier, G.; Guillevin, L.; Herreman, G. [Cardiac manifestations of periarteritis nodosa. Incidence of non-hypertensive cardiomyopathy].. 1980, 73, 1027–36. [Google Scholar]
- Lai, J.; Zhao, L.; Zhong, H.; Zhou, J.; Guo, X.; Xu, D.; Tian, X.; Zhang, S.; Zeng, X. Characteristics and Outcomes of Coronary Artery Involvement in Polyarteritis Nodosa. Can. J. Cardiol. 2020, 37, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, D.P.; Moyssakis, I.; Votteas, V.E. Polyarteritis nodosa and hypertrophic obstructive cardiomyopathy. A true association? Clin. Rheumatol. 2004, 23, 57–58. [Google Scholar] [CrossRef]
- Schafigh, M.; Bakhtiary, F.; Kolck, U.W.; Zimmer, S.; Greschus, S.; Silaschi, M. Coronary Artery Aneurysm Rupture in a Patient With Polyarteritis Nodosa. JACC: Case Rep. 2022, 4, 1522–1528. [Google Scholar] [CrossRef]
- Reimold, E.W.; Weinberg, A.G.; Fink, C.W.; Battles, N.D. Polyarteritis in Children. Arch. Pediatr. Adolesc. Med. 1976, 130, 534–541. [Google Scholar] [CrossRef]
- Holt, S.; Jackson, P. Ruptured coronary aneurysm and valvulitis in an infant with polyarthritis nodosa. J. Pathol. 1975, 117, 83–87. [Google Scholar] [CrossRef]
- Iino, T.; Eguchi, K.; Sakai, M.; Nagataki, S.; Ishijima, M.; Toriyama, K. Polyarteritis nodosa with aortic dissection: necrotizing vasculitis of the vasa vasorum. J Rheumatol 1992, 19, 1632–6. [Google Scholar] [PubMed]
- Shukla, A.; Aggarwal, A. Polyarteritis nodosa presenting as peripheral vascular disease and acute limb ischemia. J. Postgrad. Med. 2017, 63, 47–49. [Google Scholar] [CrossRef] [PubMed]
- Holscher, C.M.; Stonko, D.P.; Weaver, M.L.; Reifsnyder, T. Successful bilateral popliteal-plantar bypasses for polyarteritis nodosa induced ischemia. J. Vasc. Surg. Cases Innov. Tech. 2020, 7, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Zahoor, S.; Siddique, S.; Mahboob, H.M. An unusual presentation of polyarteritis nodosa: A case report. Reumatol Clin 2022, 18, 124–126. [Google Scholar] [CrossRef] [PubMed]
- Akova, Y.A.; Jabbur, N.S.; Foster, C.S. Ocular Presentation of Polyarteritis Nodosa. Ophthalmology 1993, 100, 1775–1781. [Google Scholar] [CrossRef] [PubMed]
- Miloslavsky, E.; Unizony, S. The Heart in Vasculitis. Rheum. Dis. Clin. North Am. 2014, 40, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.T.; Kerrison, J.B.; Miller, N.R.; Goldberg, M.F. CHOROIDAL INFARCTION, ANTERIOR ISCHEMIC OPTIC NEUROPATHY, AND CENTRAL RETINAL ARTERY OCCLUSION FROM POLYARTERITIS NODOSA. Retina 2001, 21, 348–351. [Google Scholar] [CrossRef]
- Matsumoto, T.; Homma, S.; Okada, M.; Kuwabara, N.; Kira, S.; Hoshi, T.; Uekusa, T.; Saiki, S. The lung in polyarteritis nodosa: A pathologic study of 10 cases. Hum. Pathol. 1993, 24, 717–724. [Google Scholar] [CrossRef]
- Fort, J.G.; Griffin, R.; Tahmoush, A.; Abruzzo, J.L. Muscle involvement in polyarteritis nodosa: report of a patient presenting clinically as polymyositis and review of the literature. J Rheumatol 1994, 21, 945–8. [Google Scholar]
- Georgin-Lavialle, S.; Terrier, B.; Guedon, A.; Heiblig, M.; Comont, T.; Lazaro, E.; Lacombe, V.; Terriou, L.; Ardois, S.; Bouaziz, J.; et al. Further characterization of clinical and laboratory features in VEXAS syndrome: large-scale analysis of a multicentre case series of 116 French patients*. Br. J. Dermatol. 2021, 186, 564–574. [Google Scholar] [CrossRef]
- Grambow-Velilla, J.; Braun, T.; Pop, G.; Louzoun, A.; Soussan, M. Aortitis PET Imaging in VEXAS Syndrome. Clin. Nucl. Med. 2022, 48, e67–e68. [Google Scholar] [CrossRef]
- Watanabe, R.; Kiji, M.; Hashimoto, M. Vasculitis associated with VEXAS syndrome: A literature review. Front. Med. 2022, 9, 983939. [Google Scholar] [CrossRef]
- Meyts, I.; Aksentijevich, I. Deficiency of Adenosine Deaminase 2 (DADA2): Updates on the Phenotype, Genetics, Pathogenesis, and Treatment. J. Clin. Immunol. 2018, 38, 569–578. [Google Scholar] [CrossRef]
- Fayand, A.; Sarrabay, G.; Belot, A.; Hentgen, V.; Kone-Paut, I.; Grateau, G.; Melki, I.; Georgin-Lavialle, S. Les multiples facettes du déficit en ADA2, vascularite, maladie auto-inflammatoire et immunodéficit : mise au point à partir des 135 cas de la littérature. La Rev. de Médecine Interne 2018, 39, 297–306. [Google Scholar] [CrossRef]
- Chung, S.A.; Gorelik, M.; Langford, C.A.; Maz, M.; Abril, A.; Guyatt, G.; Archer, A.M.; Conn, D.L.; Full, K.A.; Grayson, P.C.; et al. 2021 American College of Rheumatology/Vasculitis Foundation Guideline for the Management of Polyarteritis Nodosa. Arthritis Rheumatol. 2021, 73, 1384–1393. [Google Scholar] [CrossRef] [PubMed]
- Terrier, B.; Darbon, R.; Durel, C.-A.; Hachulla, E.; Karras, A.; Maillard, H.; Papo, T.; Puechal, X.; Pugnet, G.; Quemeneur, T.; et al. French recommendations for the management of systemic necrotizing vasculitides (polyarteritis nodosa and ANCA-associated vasculitides). Orphanet J. Rare Dis. 2020, 15, 1–44. [Google Scholar] [CrossRef] [PubMed]
- Ribi, C.; Cohen, P.; Pagnoux, C.; Mahr, A.; Arène, J.; Puéchal, X.; Carli, P.; Kyndt, X.; Le Hello, C.; Letellier, P.; et al. Treatment of polyarteritis nodosa and microscopic polyangiitis without poor-prognosis factors: A prospective randomized study of one hundred twenty-four patients. Arthritis Rheum. 2010, 62, 1186–1197. [Google Scholar] [CrossRef]
- Samson, M.; Puéchal, X.; Mouthon, L.; Devilliers, H.; Cohen, P.; Bienvenu, B.; Ly, K.H.; Bruet, A.; Gilson, B.; Ruivard, M.; et al. Microscopic polyangiitis and non-HBV polyarteritis nodosa with poor-prognosis factors: 10-year results of the prospective CHUSPAN trial. Clin Exp Rheumatol 2017, 176–184. [Google Scholar]
- Hadjadj, J.; Canzian, A.; Karadag, O.; Contis, A.; Maurier, F.; Sanges, S.; Sartorelli, S.; Denis, L.; de Moreuil, C.; Durel, C.-A.; et al. Use of biologics to treat relapsing and/or refractory polyarteritis nodosa: data from a European collaborative study. Rheumatology 2022, 62, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Guillevin, L.; Fain, O.; Lhote, F.; Jarrousse, B.; Huong, D.L.T.; Bussel, A.; Leon, A. Lack of Superiority of Steroids Plus Plasma Exchange to Steroids Alone in the Treatment of Polyarteritis Nodosa and Churg-Strauss Syndrome. Arthritis Rheum. 1992, 35, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Machet, L.; Vincent, O.; Machet, M.C.; Barruet, K.; Vaillant, L.; Lorette, G. [Cutaneous periarteritis nodosa resistant to combined corticosteroids and immunosuppressive agents. Efficacy of treatment with intravenous immunoglobulins]. Ann Dermatol Venereol 1995, 122, 769–72. [Google Scholar] [PubMed]
- Seri, Y.; Shoda, H.; Hanata, N.; Nagafuchi, Y.; Sumitomo, S.; Fujio, K.; Yamamoto, K. A case of refractory polyarteritis nodosa successfully treated with rituximab. Mod. Rheumatol. 2015, 27, 696–698. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, E.; Cressend, T.; Duffau, P.; Grenouillet-Delacre, M.; Rouanet-Larivière, M.; Vital, A.; Longy-Boursier, M.; Mercié, P. Rituximab Efficacy during a Refractory Polyarteritis Nodosa Flare. Case Rep. Med. 2009, 2009, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Al-Homood, I.A.; Aljahlan, M.A. Successful use of combined corticosteroids and rituximab in a patient with refractory cutaneous polyarteritis nodosa. J. Dermatol. Dermatol. Surg. 2017, 21, 24–26. [Google Scholar] [CrossRef]
- Boistault, M.; Corbeto, M.L.; Quartier, P.; Arcobé, L.B.; Durall, A.C.; Aeschlimann, F.A. A young girl with severe polyarteritis nodosa successfully treated with tocilizumab: a case report. Pediatr. Rheumatol. 2021, 19, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Mekinian, A.; Grignano, E.; Braun, T.; Decaux, O.; Liozon, E.; Costedoat-Chalumeau, N.; Kahn, J.-E.; Hamidou, M.; Park, S.; Puéchal, X.; et al. Systemic inflammatory and autoimmune manifestations associated with myelodysplastic syndromes and chronic myelomonocytic leukaemia: a French multicentre retrospective study. Rheumatology 2015, 55, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.A.; Gorelik, M.; Langford, C.A.; Maz, M.; Abril, A.; Guyatt, G.; Archer, A.M.; Conn, D.L.; Full, K.A.; Grayson, P.C.; et al. 2021 American College of Rheumatology/Vasculitis Foundation Guideline for the Management of Polyarteritis Nodosa. Arthritis Rheumatol. 2021, 73, 1384–1393. [Google Scholar] [CrossRef]
- Boyadzhieva, Z.; Ruffer, N.; Kötter, I.; Krusche, M. How to treat VEXAS syndrome: a systematic review on effectiveness and safety of current treatment strategies. Rheumatology 2023, 62, 3518–3525. [Google Scholar] [CrossRef]
- Diarra A, Duployez N, Fournier E, Preudhomme C, Coiteux V, Magro L, Quesnel B, Heiblig M, Sujobert P, Barraco F, Balsat M, Scanvion Q, Hachulla E, Launay D, Yakoub-Agha I, Terriou L. Successful allogeneic hematopoietic stem cell transplantation in patients with VEXAS syndrome: a 2-center experience. Blood Adv. 2022 Feb 8;6(3):998-1003. [CrossRef]
- Comont, T.; Heiblig, M.; Rivière, E.; Terriou, L.; Rossignol, J.; Bouscary, D.; Rieu, V.; Le Guenno, G.; Mathian, A.; Aouba, A.; et al. Azacitidine for patients with Vacuoles, E1 Enzyme, X-linked, Autoinflammatory, Somatic syndrome (VEXAS) and myelodysplastic syndrome: data from the French VEXAS registry. Br. J. Haematol. 2021, 196, 969–974. [Google Scholar] [CrossRef]
| Characteristics |
Pagnoux et al. (1963 to 2005) |
Sönmez et al (1990 to 2015) |
Rohmer et al. (2005 to 2019) |
Georgin- Lavialle et al. (VEXAS) |
Meyts et al. (ADA2) |
|---|---|---|---|---|---|
|
General symptoms Fever Loss of weight Myalgia |
93.1 63.8 69.5 58.6 |
53.7 53.7 46.2 |
85 54 50 50 |
95.7 64.6 54.5 |
50 |
|
All cutaneous Nodules Purpura Livedo Panniculitis |
49.7 17.2 22.1 16.7 |
67.2 17.9 |
59 7.5 |
83.6 12.9 |
75 14 50 |
|
Renal Hematuria Proteinuria Hypertension |
50.6 15.2 21.6 34.8 |
47.7 41.7 |
20 | 9.5 |
21 |
| Orchitis | 17 | 14.9 | 16 | 4 | |
|
Neurologic PNP Mononeuritis CNS |
79.0 74.1 70.7 4.6 |
43.2 | 59 |
5.2 2.6 |
9 53 |
|
Digestive Abdominal pain Bleeding Perforation |
37.9 35.6 3.4 4.3 |
22.3 37.3 |
28 | 13.8 8.6 0.9 0.9 |
33 12 2 |
|
Cardiovascular Pericarditis Distal necrosis Thrombo-embolism |
22.4 5.5 6.3 |
13.5 |
39 |
4.3 35.3 |
22 |
|
Ophtalmic Retinal vasculitis |
8.6 4.3 |
40.5 | |||
|
Pulmonary Cough Lung infiltrate Pleural effusion |
5.7 3.4 3.4 |
2.9 | 8 | 49.1 40.5 9.5 |
|
| Chondritis | 36.2 | ||||
|
Arthralgia Arthritis |
58.2 17.9 |
28.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





