Submitted:
12 October 2023
Posted:
13 October 2023
You are already at the latest version
Abstract
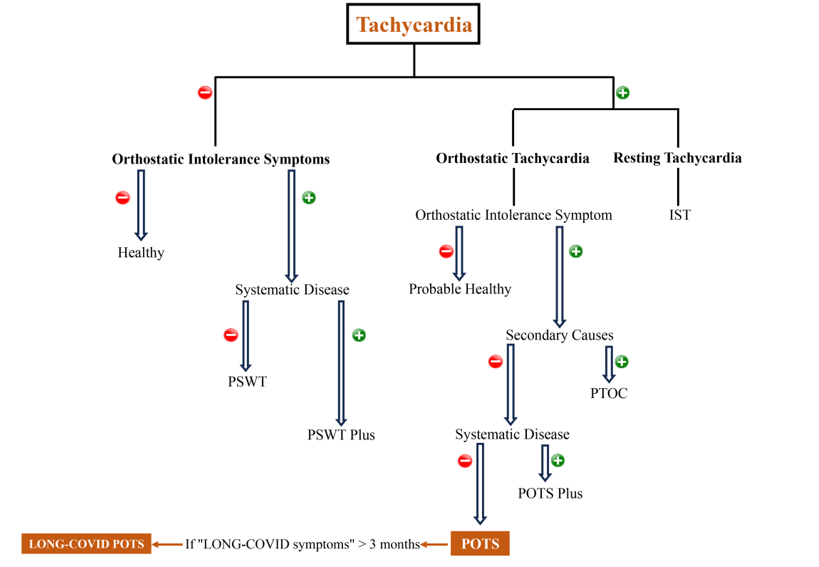
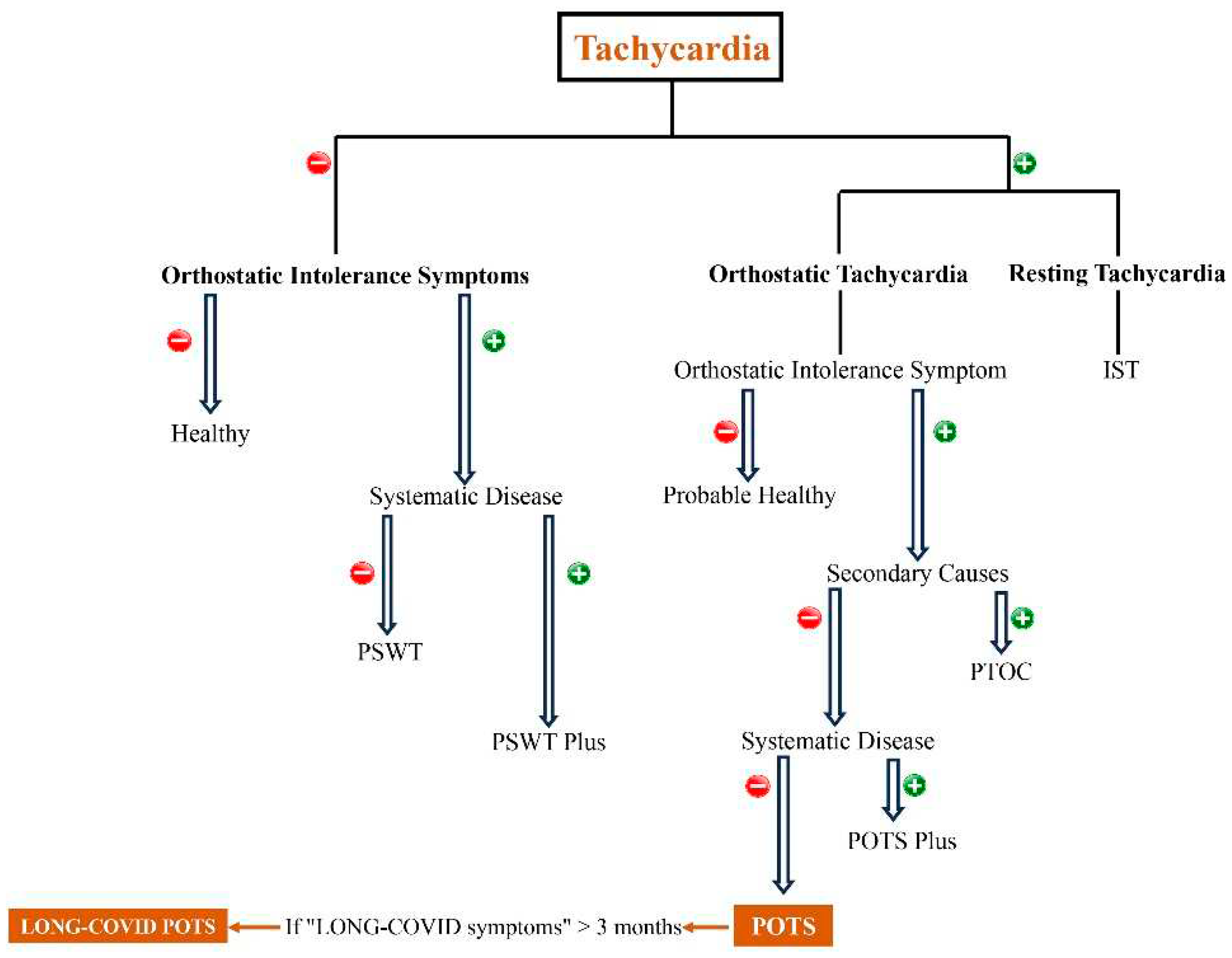
Keywords:
1. Introduction
2. POTS
2.1. What is POTS?
2.2. What is the pathophysiology of POTS?
2.3. POTS diagnosis and management
3. POTS following SARS-CoV-2 infection
3.1. Pathogenesis of LONG-Covid POTS
3.2. Prevalence
3.3. Overview of clinical reports on suspicious post-Covid-POTS
| Author | Region | Number of diagnosed patients | Gender | Time to diagnosis | Manifestations | hospitalization | Management | Outcome | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | ≥ 3 months | < 3 months | Yes | No | ||||||
| Umapathi et al,[62] |
Asia | 4 | 1 | 3 | 0 | 4 | Sweating, constipation, nausea, after-meal abdominal discomfort, tachycardia on standing and during passive 60-degree tilt without blood pressure decreasing | 4 | 0 | Fludrocortisone, sodium tablets, Pyridostigmine | Good (Recovered and symptoms improvement) |
| Rudofker et al,[63] |
America | 3 | 1 | 2 | 2 | 1 | fatigue, lightheadedness, brain fog, and palpitations, syncope, breathlessness, paroxysmal coughing ,increasing resting heart rate of 10 to 15 Beats per minute | 1 | 2 | exercise (rowing/biking) for 2 months |
Good (Recovered) |
| Shouman et al,[64] |
America | 27 | 11 | 16 | some | some | Common symptoms : Lightheadedness, orthostatic headache, syncope, hyperhidrosis, and pain n=11 →Orthostatic intolerance without tachycardia/n=6 →Orthostatic intolerance with orthostatic tachycardia/n= 3 → Overlapping symptoms |
N/A | N/A | Rehabilitation, low dose beta blockers, Anxiolytics Gabapentin and topical lidocaine for neuropathic symptoms in the autonomic neuropathy cases |
Good (Improvement, except for anxyiolytic) |
| Desai et al,[65] |
America | 11 | 2 | 9 | Some | Most (Mean ≈ 40 day) | Palpitations, fatigue, chest discomfort | N/A | N/A | Beta blockers (5), midodrine (1), colchicine (1), ibuprofen (2), lifestyle modifications (2) | Good (all improved except for colchicine) |
| Agnihotri et al,[66] |
America | 1 | 0 | 1 | 1 | 0 | Lightheadedness, palpitations, tingling, hyperhidrosis, tremor, and red discoloration of feet on upright position | 0 | 1 | metoprolol, midodrine, lifestyle change | Good (symptoms improved) |
| Ocher et al,[67] |
America | 1 | 0 | 1 | 0 | 1 | IST | 1 | 0 | Metoprolol,Ivabradine | Poor |
| Parker et al,[68] |
America | 7 | 1 | 6 | 0 | 7 | Palpitations, chest discomfort and tachypnea, GI symptoms | N/A | N/A | supportive therapy; ivabradine, midodrine metoprolol;intravenous immunoglobulin | Not clearly mentioned (2 out of 3 severe patients responded to IV immunoglubolin) |
| O’Sullivan et al,[59] |
Europe | 1 | 0 | 1 | 0 | 1 | Chest tightness, palpitations, breathlessness, fatigue | 0 | 1 | lifestyle change ,ivabradine |
Good (Best symptoms improvement with ivabradine) |
| Blitshteyn et al,[15] |
America | 20 | 6 | 14 | 20 | 0 | Fatigue, postural tachycardia, brain fog, and exercise intolerance | N/A | N/A | All prescribed lifestyle modifications, 16 needed pharmacological drug including beta blockers, fludrocortisone midodrine, ivabradine | Good ( all fully recovered, except three of them which near complete recovered) |
| Jamal et al,[69] |
America | 24 | 4 | 20 | 24 | 0 | tachycardia with subordinary physical activity or positional change, palpitations |
2 | 22 | N/A | N/A |
| Kitsou et al,[70] |
Europe | 1 | 0 | 1 | 1 | 0 | Chest discomfort, exertional dyspnea, palpitations, syncope | 0 | 1 | Metoprolol | Good ( improved after six months) |
| Johansson et al,[71] |
Europe | 3 | 1 | 2 | 1 | 2 | sinus tachycardia; chest discomfort, fatigue, dizziness, headache, fatigue, muscle weakness, sleep disturbance, palpitations, and “brain fog”, concentration issue | 2 | 1 | (A)Lifestyle change, ivabradine (B) lifestyle changes ,propranolol,antihistamines(C) lifestyle changes, pyridostigmine, propranolol antihistamines |
Acceptable(A) Poor(B,C) (A) →Partial recovery (B)→ symptoms remained (C)→ Symptoms Remained |
| Varanasi et al,[61] |
America | 1 | 0 | 1 | 1 | 0 | Fatigue, “brain fog”, breathlessness, exertional dyspnea | 1 | 0 | External counterpulsation | Good |
| Kanjwal et al,[58] |
America | 1 | 0 | 1 | 0 | 1 | headache, dizziness, chest discomfort, and palpitations, fatigue | 0 | 1 | Ivabradine, salt water intake | Acceptable (improved but tachycardia remained) |
| Miglis et al,[30] |
America | 1 | 0 | 1 | 1 | 0 | Tachycardia, chest discomfort, breathlessness, fatigue and exercise intolerance, fever, sleep disturbance | 1 | 0 | Propranolol | Acceptable (partially recovered) |
| Ishibashi et al,[72] |
Asia | 1 | 0 | 1 | 0 | 1 | Fatigue, palpitation, chest discomfort | N/A | N/A | Bisoprolol | Good (marked recovery) |
| Kalia et al,[73] |
America | 1 | 0 | 1 | 1 | 0 | Tachycardia, headaches, pre-syncope | N/A | N/A | Metoprolol | Acceptable (partially recovered) |
| Bosco et al,[74] |
Asia | 1 | 0 | 1 | 1 | 0 | exertional fatigue, memory and concentration impairement, headaches, blurred vision, malaise, | 0 | 1 | Lifestyle changes, exercise therapy | Acceptable (partially recovered) |
4. POTS following COVID-19 vaccination
4.1. Overview of the clinical reports on suspected POTS after Covid-vaccine
| Patient | Gender | Age | PMH | Drug history | Type of vaccine | Vaccine dose | POTS Symptoms onset after vaccination | Manifestations | Management | Outcome | Reference Author |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient 1 |
Female | 37 | seasonal allergy and depression | Vortioxetine | Moderna | First dose | 1 week | lightheadedness, heart racing, weakness, difficulty concentrating, blurry vision, shakiness, vertigo, and clamminess when assuming upright posture, improving symptoms in the supine position, dry eyes and mouth unrelated to medications, heat sensitivity, constipation, numbness and tingling of the feet, legs hands, and occasionally the face. | 5 mg of ivabradine twice a day | "Good" (Improve) |
Eldokla et al., [83] |
| Patient 2 |
Female | 21 | No | No | BioNTech-Pfizer | First dose | 12 days | headache, palpitation, weakness, difficulty thinking, improving symptoms in the supine position, heat sensitivity, sweating, face and extremities numbness and tingling | metoprolol 25 mg, and fludrocortisone 0.2 mg daily | "Good" (Improve) |
Eldokla et al., [83] |
| Patient 3 |
Female | 46 | No | No | BioNTech-Pfizer | First dose | 2 weeks | lightheadedness, nausea, fatigue, poor concentration, palpitations, and brain fog, increase sympathetic activity after the head-up position in spectral Fourier analysis | fludrocortisone and propranolol | "Good" (Improve) |
Eldokla et al., [83] |
| Patient 4 |
Female | 19 | No | No | BioNTech-Pfizer | Second dose | 18 days | dizziness, headache ,nausea, sweating, and fatigue, increase in sympathetic tone after the head-up position in spectral Fourier analysis | salt tablets and propranolol | "Good" (Improve) |
Eldokla et al., [83] |
| Patient 5 |
Female | 17 | No | No | BioNTech–Pfizer | Second dose | 3 weeks | syncope, fatigue, chest tightness, nausea, and heat intolerance, increased sympathetic activity with occasional spikes of vagal tone after the head-up position in spectral Fourier analysis | scopolamine patches for nausea, salt tablets and propranolol | "Good" (Improve) |
Eldokla et al., [83] |
| Patient 6 |
Male | 40 | No | No | Moderna | First dose | one week | Orthostatic palpitation, intermittent headache, fatigue, dyspnea, increasing HR from 72 to 110 in Head-up tilt test | propranolol 20 mg, three times daily for 2 months | "Good" (Improve) |
Park et al., [84] |
| Patient 7 |
Male | 42 | Hypothyroidism, vitamin B-12 deficiency, Left-sided orchiectomy for cryptorchidism | Levothyroxine 125 mg, vitamin B-12 shots | BioNTech–Pfizer | First dose | six days | syncopal episode, sinus tachycardia, intermittent palpitations, anxiety, sleep disturbances, occasional numbness in the lower extremities | lifestyle modifications, such as wearing compression socks and increased sodium intake | "Unchanged" (Existing symptom) |
Reddy et al., [85] |
| Patient 8 |
Male | 13 | No | No | BioNTech–Pfizer | Second dose | Fourteen days | fatigue, headache, and orthostatic intolerance symptoms including lightheadedness and palpitations | salt and fluid intake and exercise,propranolol, midodrine (2 mg, twice daily),droxidopa, (IVIG) 2 g/kg was administered for the myocarditis | "Acceptable" (almost improved) |
Sanada et al.,[82] |
| Patient 9 |
Male | 15 | No | No | BioNTech–Pfizer | Third dose | Two weeks | Presyncope and syncopal episodes, and lightheadedness | fludrocortisone 0.1 mg every eight hours, Ivabradine 2.5 mg every 12 hours, lifestyle modification with respect to increasing sodium and fluid intake, moderate exercise | "Good" (Improve) |
Maharaj et al., [9] |
| Patient 10 | Female | 46 | allergic rhinitis, history of COVID-19 infection | No | BioNTech-Pfizer | First dose | four weeks | lightheadedness, tremors, increasing heart rate, Raynaud’s Phenomenon, fatigue, brain fog, headache, chest discomfort, exercise intolerance, profound fatigue, dizziness, and paresthesia | salt consumption, fluid intake, and 20 mmHG compression stockings, ivabradine 5 mg BID, dietary supplement, | "Acceptable" (all improved except Raynaud’s Phenomenon) |
Hermel et al., [86] |
| Patient 11 | Male | 29 | No | No | Oxford-AstraZeneca | First dose | 4 days | Intermittent paresthesia, palpitations, dizziness, , heart beat racing |
Steroid for 5 weeks without effect, lifestyle modifications |
"Acceptable"(some symptoms improved) | Karimi et al.,[78] |
| Patient 12 | Male | 43 | Sleep disturbance | Naproxen, Lansoprazole, Dihydrocodeine, Melatonin |
Oxford-AstraZeneca | First dose | 6 hours | Dizziness, nausea, weakness, bradykinesia, brain fog, early waking, fatigue, “jelly-legs”, changes to walking gait, |
Hydroxocobalamin IM |
"Good" (Improve) |
Carroll et al.,[87] |
| Patient 13 | Male | 52 | No | No | BioNTech-Pfizer | Second dose | Not specifically mentioned | Feeling stabbing, orthostatic intolerance, syncope, supraventricular tachycardia, tinnitus |
Nadolol, Gabapentin, Amitriptyline, and trazodone (no effect) / plasma exchange for Five time |
"Good" (Improve) |
Schelke et al., [81] |
| Patient 14 | Female | N/M | History of COVID-19 and family history of Sjrogen’s disease |
No | Oxford-AstraZeneca | First dose | 2 hour | Limbs paresthesia, tachycardia, blood pressure fluctuation, intermittent internal tremor, cognitive complaints |
N/A | N/A | Safavi et al., [88] |
| Patient 15 | Female | N/M | Family history of psoriasis |
No | Moderna | Second dose | 2 days | Orthostasis, heart rate and blood pressure fluctuation, nausea, diarrhea, face and limbs paresthesia, internal tremor |
N/A | N/A | Safavi et al., [88] |
| Patient 16 | Female | N/M | Family history of rheumatoid arthritis |
No | BioNTech-Pfizer | Second dose | 10 days | Severe orthostatic Tachycardia, diarrhea, blood pressure fluctuation | N/A | N/A | Safavi et al., [88] |
| Patient 17 | Female | N/M | No | No | Moderna | First dose | 2 days | Intermittent paresthesia, face and limbs numbness, Mild right-hand weakness, episodic positional palpitation |
N/A | N/A | Safavi et al., [88] |
| Patient 18 | Female | N/M | No | No | Moderna | First dose | 6 days | Exercise intolerance, Limbs fasciculation, internal tremor, facial paresthesia, chest discomfort; cognitive disturbance, episodic positional palpitations | N/A | N/A | Safavi et al., [88] |
| Patient 19 | Female | N/M | No | No | Moderna | Second dose | 5 days | Facial Paresthesia and burning sensation, positional Palpitation,dizziness | N/A | N/A | Safavi et al., [88] |
4.2. COVID-19 vaccination-induced autoimmunity
4.2. Common Covid-vaccination side effects and POTS-like symptoms
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mohseni Afshar Z, Babazadeh A, Alizadeh Khatir A, Mohammadnia-Afrouzi M, Javanian M, Vasigala V, et al. Neurological manifestations in COVID-19: an overview. Minerva Pneumologica. 2021:52-8. [CrossRef]
- Afshar ZM, Babazadeh A, Javanian M, Ramezani E, Shemshadi R, Ebrahimpour S. A review of cardiac involvement in COVID-19 infection. Cor et Vasa. 2020;62(6):610-5. [CrossRef]
- Mohseni Afshar Z, Tavakoli Pirzaman A, Liang JJ, Sharma A, Pirzadeh M, Babazadeh A, et al. Do we miss rare adverse events induced by COVID-19 vaccination? Frontiers in medicine. 2022;9:933914.
- Mohseni Afshar Z, Babazadeh A, Janbakhsh A, Afsharian M, Saleki K, Barary M, et al. Vaccine-induced immune thrombotic thrombocytopenia after vaccination against Covid-19: a clinical dilemma for clinicians and patients. Reviews in medical virology. 2022;32(2):e2273. [CrossRef]
- Afshar ZM, Barary M, Babazadeh A, Hosseinzadeh R, Alijanpour A, Miri SR, et al. SARS-CoV-2-related and Covid-19 vaccine-induced thromboembolic events: A comparative review. Reviews in medical virology. 2022;32(4):e2327.
- Benrud-Larson LM, Dewar MS, Sandroni P, Rummans TA, Haythornthwaite JA, Low PA, editors. Quality of life in patients with postural tachycardia syndrome. Mayo Clinic Proceedings; 2002: Elsevier. [CrossRef]
- Bourne KM, Chew DS, Stiles LE, Shaw BH, Shibao CA, Okamoto LE, et al. Postural orthostatic tachycardia syndrome is associated with significant employment and economic loss. Journal of internal medicine. 2021;290(1):203-12. [CrossRef]
- Kesserwani H, Kesserwani HN. Postural orthostatic tachycardia syndrome misdiagnosed as anxiety: A case report with a review of therapy and pathophysiology. Cureus. 2020;12(10). [CrossRef]
- Maharaj N, Swarath S, Seecheran R, Seecheran V, Panday A, Seecheran N, et al. Suspected COVID-19 mRNA Vaccine-Induced Postural Orthostatic Tachycardia Syndrome. Cureus. 2023;15(1). [CrossRef]
- Raj SR, Guzman JC, Harvey P, Richer L, Schondorf R, Seifer C, et al. Canadian Cardiovascular Society position statement on postural orthostatic tachycardia syndrome (POTS) and related disorders of chronic orthostatic intolerance. Canadian Journal of Cardiology. 2020;36(3):357-72.
- Li H, Yu X, Liles C, Khan M, Vanderlinde-Wood M, Galloway A, et al. Autoimmune basis for postural tachycardia syndrome. Journal of the American Heart Association. 2014;3(1):e000755. [CrossRef]
- Raj SR, Biaggioni I, Yamhure PC, Black BK, Paranjape SY, Byrne DW, et al. Renin-aldosterone paradox and perturbed blood volume regulation underlying postural tachycardia syndrome. Circulation. 2005;111(13):1574-82.
- Garland E, Raj S, Black B, Harris P, Robertson D. The hemodynamic and neurohumoral phenotype of postural tachycardia syndrome. Neurology. 2007;69(8):790-8. [CrossRef]
- Fu Q, VanGundy TB, Galbreath MM, Shibata S, Jain M, Hastings JL, et al. Cardiac origins of the postural orthostatic tachycardia syndrome. Journal of the American College of Cardiology. 2010;55(25):2858-68. [CrossRef]
- Blitshteyn S, Whitelaw S. Postural orthostatic tachycardia syndrome (POTS) and other autonomic disorders after COVID-19 infection: a case series of 20 patients. Immunologic research. 2021;69(2):205-11.
- Watari M, Nakane S, Mukaino A, Nakajima M, Mori Y, Maeda Y, et al. Autoimmune postural orthostatic tachycardia syndrome. Annals of Clinical and Translational Neurology. 2018;5(4):486-92. [CrossRef]
- Celletti C, Camerota F, Castori M, Censi F, Gioffrè L, Calcagnini G, et al. Orthostatic intolerance and postural orthostatic tachycardia syndrome in joint hypermobility syndrome/Ehlers-Danlos syndrome, hypermobility type: neurovegetative dysregulation or autonomic failure? BioMed research international. 2017;2017. [CrossRef]
- Mannan H, Pain CM. Sex adjusted standardized prevalence ratios for celiac disease and other autoimmune diseases in patients with postural orthostatic tachycardia syndrome (POTS): A systematic review and meta-analysis. Heliyon. 2023. [CrossRef]
- Lei LY, Chew DS, Sheldon RS, Raj SR. Evaluating and managing postural tachycardia syndrome. Cleve Clin J Med. 2019;86(5):333-44. [CrossRef]
- Shaw B, Stiles L, Bourne K, Green E, Shibao C, Okamoto L, et al. The face of postural tachycardia syndrome–insights from a large cross-sectional online community-based survey. Journal of internal medicine. 2019;286(4):438-48.
- van Halteren AG, Tysma OM, van Etten E, Mathieu C, Roep BO. 1α, 25-Dihydroxyvitamin D3 or analogue treated dendritic cells modulate human autoreactive T cells via the selective induction of apoptosis. Journal of autoimmunity. 2004;23(3):233-9. [CrossRef]
- Arnold AC, Ng J, Raj SR. Postural tachycardia syndrome–diagnosis, physiology, and prognosis. Autonomic Neuroscience. 2018;215:3-11.
- Raj, SR. The postural tachycardia syndrome (POTS): pathophysiology, diagnosis & management. Indian pacing and electrophysiology journal. 2006;6(2):84.
- Vance H, Maslach A, Stoneman E, Harmes K, Ransom A, Seagly K, et al. Addressing post-COVID symptoms: a guide for primary care physicians. The Journal of the American Board of Family Medicine. 2021;34(6):1229-42. [CrossRef]
- Savytskyi IV, Pruc M, Malysz M, Maslyukov A, Szarpak L. Post-COVID-19 postural orthostatic tachycardia syndrome. Cardiology Journal. 2022;29(3):531-2.
- Raj SR, Fedorowski A, Sheldon RS. Diagnosis and management of postural orthostatic tachycardia syndrome. Cmaj. 2022;194(10):E378-E85.
- Grubb, BP. Autonomic Dysfunction as a Consequence of COVID-19 Infection: A New Twist on an Old Problem. American College of Cardiology Foundation Washington DC; 2022. p. 2331-2.
- Fedorowski A, Sutton R. Autonomic dysfunction and postural orthostatic tachycardia syndrome in post-acute COVID-19 syndrome. Nature Reviews Cardiology. 2023;20(5):281-2. [CrossRef]
- Kavi, L. Postural tachycardia syndrome and long COVID: an update. British Journal of General Practice; 2022. p. 8-9. [CrossRef]
- Miglis MG, Prieto T, Shaik R, Muppidi S, Sinn D-I, Jaradeh S. A case report of postural tachycardia syndrome after COVID-19. Clinical Autonomic Research. 2020;30(5):449-51. [CrossRef]
- Johnson JN, Mack KJ, Kuntz NL, Brands CK, Porter CJ, Fischer PR. Postural orthostatic tachycardia syndrome: a clinical review. Pediatric neurology. 2010;42(2):77-85. [CrossRef]
- Junghans-Rutelonis AN, Craner JR, Ale CM, Harbeck-Weber C, Fischer PR, Weiss KE. Youth with chronic pain and postural orthostatic tachycardia syndrome (POTS): treatment mediators of improvement in functional disability. Journal of clinical psychology in medical settings. 2018;25:471-84.
- Kanjwal K, Karabin B, Sheikh M, Elmer L, Kanjwal Y, Saeed B, et al. Pyridostigmine in the treatment of postural orthostatic tachycardia: A single-center experience. Pacing and clinical electrophysiology. 2011;34(6):750-5. [CrossRef]
- Gee ME, Watkins AK, Brown JN, Young EJ. Ivabradine for the treatment of postural orthostatic tachycardia syndrome: a systematic review. American Journal of Cardiovascular Drugs. 2018;18:195-204. [CrossRef]
- Schmidt LL, Karabin BL, Malone AC. Postural orthostatic tachycardia syndrome (POTS): assess, diagnose, and evaluate for POTS treatment (ADEPT). Integrative Medicine International. 2019;4(3-4):142-53. [CrossRef]
- Mehandru S, Merad M. Pathological sequelae of long-haul COVID. Nature immunology. 2022;23(2):194-202. [CrossRef]
- Szabo S, Zayachkivska O, Hussain A, Muller V. What is really ‘Long COVID’? Inflammopharmacology. 2023;31(2):551-7.
- Fernández-de-Las-Peñas, C. Long COVID: current definition. Infection. 2022;50(1):285-6. [CrossRef]
- Raj SR, Arnold AC, Barboi A, Claydon VE, Limberg JK, Lucci V-EM, et al. Long-COVID postural tachycardia syndrome: an American Autonomic Society statement. Clinical Autonomic Research. 2021;31(3):365-8.
- Ramakrishnan RK, Kashour T, Hamid Q, Halwani R, Tleyjeh IM. Unraveling the mystery surrounding post-acute sequelae of COVID-19. Frontiers in immunology. 2021;12:686029. [CrossRef]
- Chertow D, Stein S, Ramelli S, Grazioli A, Chung J, Singh M, et al. SARS-CoV-2 infection and persistence throughout the human body and brain. 2021.
- Parasa S, Desai M, Chandrasekar VT, Patel HK, Kennedy KF, Roesch T, et al. Prevalence of gastrointestinal symptoms and fecal viral shedding in patients with coronavirus disease 2019: a systematic review and meta-analysis. JAMA network open. 2020;3(6):e2011335-e.
- Su Y, Yuan D, Chen DG, Ng RH, Wang K, Choi J, et al. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell. 2022;185(5):881-95.e20. [CrossRef]
- Getts DR, Getts MT, King NJ, Miller SD. Infectious triggers of T cell autoimmunity. The autoimmune diseases: Elsevier; 2014. p. 263-74.
- Cervia C, Zurbuchen Y, Taeschler P, Ballouz T, Menges D, Hasler S, et al. Immunoglobulin signature predicts risk of post-acute COVID-19 syndrome. Nature Communications. 2022;13(1):1-12. [CrossRef]
- Kell DB, Laubscher GJ, Pretorius E. A central role for amyloid fibrin microclots in long COVID/PASC: origins and therapeutic implications. Biochemical Journal. 2022;479(4):537-59. [CrossRef]
- Gaebler C, Wang Z, Lorenzi JC, Muecksch F, Finkin S, Tokuyama M, et al. Evolution of antibody immunity to SARS-CoV-2. Nature. 2021;591(7851):639-44.
- Lee S, Yoon GY, Myoung J, Kim S-J, Ahn D-G. Robust and persistent SARS-CoV-2 infection in the human intestinal brush border expressing cells. Emerging Microbes & Infections. 2020;9(1):2169-79.
- Gamage AM, Tan KS, Chan WO, Lew ZZR, Liu J, Tan CW, et al. Human nasal epithelial cells sustain persistent SARS-CoV-2 infection in vitro, despite eliciting a prolonged antiviral response. Mbio. 2022;13(1):e03436-21.
- Thoms M, Buschauer R, Ameismeier M, Koepke L, Denk T, Hirschenberger M, et al. Structural basis for translational shutdown and immune evasion by the Nsp1 protein of SARS-CoV-2. Science. 2020;369(6508):1249-55.
- Stukalov A, Girault V, Grass V, Karayel O, Bergant V, Urban C, et al. Multilevel proteomics reveals host perturbations by SARS-CoV-2 and SARS-CoV. Nature. 2021;594(7862):246-52.
- Yang B, Fan J, Huang J, Guo E, Fu Y, Liu S, et al. Clinical and molecular characteristics of COVID-19 patients with persistent SARS-CoV-2 infection. Nature Communications. 2021;12(1):3501.
- Moran E, Cook T, Goodman AL, Gupta RK, Jolles S, Menon DK, et al. Persistent SARS-CoV-2 infection: the urgent need for access to treatment and trials. The Lancet Infectious Diseases. 2021;21(10):1345-7.
- Espinosa-Gonzalez AB, Master H, Gall N, Halpin S, Rogers N, Greenhalgh T. Orthostatic tachycardia after COVID-19. Bmj. 2023;380. [CrossRef]
- Gunning III WT, Kvale H, Kramer PM, Karabin BL, Grubb BP. Postural orthostatic tachycardia syndrome is associated with elevated G-protein coupled receptor autoantibodies. Journal of the American Heart Association. 2019;8(18):e013602.
- Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS, et al. Post-acute COVID-19 syndrome. Nature medicine. 2021;27(4):601-15.
- Kwan AC, Ebinger JE, Wei J, Le CN, Oft JR, Zabner R, et al. Apparent risks of postural orthostatic tachycardia syndrome diagnoses after COVID-19 vaccination and SARS-Cov-2 Infection. Nature cardiovascular research. 2022;1(12):1187-94. [CrossRef]
- Kanjwal K, Jamal S, Kichloo A, Grubb BP. New-onset postural orthostatic tachycardia syndrome following coronavirus disease 2019 infection. The Journal of innovations in cardiac rhythm management. 2020;11(11):4302. [CrossRef]
- O'Sullivan JS, Lyne A, Vaughan CJ. COVID-19-induced postural orthostatic tachycardia syndrome treated with ivabradine. BMJ Case Reports CP. 2021;14(6):e243585. [CrossRef]
- Davis HE, McCorkell L, Vogel JM, Topol EJ. Long COVID: major findings, mechanisms and recommendations. Nature Reviews Microbiology. 2023;21(3):133-46.
- Varanasi S, Sathyamoorthy M, Chamakura S, Shah SA. Management of long-COVID postural orthostatic tachycardia syndrome with enhanced external counterpulsation. Cureus. 2021;13(9). [CrossRef]
- Umapathi T, Poh MQ, Fan BE, Li KFC, George J, Tan JY. Acute hyperhidrosis and postural tachycardia in a COVID-19 patient. Clinical Autonomic Research. 2020;30:571-3. [CrossRef]
- Rudofker EW, Parker H, Cornwell III WK. An exercise prescription as a novel management strategy for treatment of long COVID. Case Reports. 2022;4(20):1344-7.
- Shouman K, Vanichkachorn G, Cheshire WP, Suarez MD, Shelly S, Lamotte GJ, et al. Autonomic dysfunction following COVID-19 infection: an early experience. Clinical Autonomic Research. 2021;31:385-94. [CrossRef]
- Desai AD, Boursiquot BC, Moore CJ, Gopinathannair R, Waase MP, Rubin GA, et al. Autonomic dysfunction post–acute COVID-19 infection. HeartRhythm Case Reports. 2022;8(3):143-6. [CrossRef]
- Agnihotri SP, Luis CVS, Kazamel M. Autonomic neuropathy as post-acute sequela of SARS-CoV-2 infection: a case report. Journal of NeuroVirology. 2022;28(1):158-61.
- Ocher RA, Padilla E, Hsu JC, Taub PR. Clinical and laboratory improvement in hyperadrenergic postural orthostatic tachycardia syndrome (POTS) after COVID-19 infection. Case Reports in Cardiology. 2021;2021. [CrossRef]
- Parker WH, Moudgil R, Wilson RG, Tonelli AR, Mayuga KA, Singh TK. COVID-19 and postural tachycardia syndrome: a case series. European Heart Journal-Case Reports. 2021;5(12):ytab325. [CrossRef]
- Jamal SM, Landers DB, Hollenberg SM, Turi ZG, Glotzer TV, Tancredi J, et al. Prospective evaluation of autonomic dysfunction in post-acute sequela of COVID-19. Journal of the American College of Cardiology. 2022;79(23):2325-30. [CrossRef]
- Kitsou V, Blomberg B, Lunde T, Saeed S. Intermittent left bundle branch block with septal flash and postural orthostatic tachycardia syndrome in a young woman with long COVID-19. BMJ Case Reports CP. 2022;15(6):e249608. [CrossRef]
- Johansson M, Ståhlberg M, Runold M, Nygren-Bonnier M, Nilsson J, Olshansky B, et al. Long-haul post–COVID-19 symptoms presenting as a variant of postural orthostatic tachycardia syndrome: the Swedish experience. Case Reports. 2021;3(4):573-80.
- Ishibashi Y, Yoneyama K, Tsuchida T, Akashi YJ. Post-COVID-19 postural orthostatic tachycardia syndrome. Internal Medicine. 2021;60(14):2345-. [CrossRef]
- Kalia R, Kalia R, Musih J, Cubelo M, Popat J. Post-COVID-19 syndrome: a novel diagnosis. Cureus. 2022;14(8).
- Bosco J, Titano R. Severe Post-COVID-19 dysautonomia: a case report. BMC Infectious Diseases. 2022;22(1):1-4. [CrossRef]
- Blitshteyn S, Fedorowski A. The risks of POTS after COVID-19 vaccination and SARS-CoV-2 infection: it’s worth a shot. Nature Cardiovascular Research. 2022;1(12):1119-20.
- Brinth LS, Pors K, Theibel AC, Mehlsen J. Orthostatic intolerance and postural tachycardia syndrome as suspected adverse effects of vaccination against human papilloma virus. Vaccine. 2015;33(22):2602-5. [CrossRef]
- Blitshteyn, S. Postural tachycardia syndrome after vaccination with Gardasil. European Journal Of Neurology:. 2010;17(7):e52. [CrossRef]
- Karimi Galougahi, K. Autonomic dysfunction post-inoculation with ChAdOx1 nCoV-19 vaccine. European Heart Journal-Case Reports. 2021;5(12):ytab472.
- Tsai C-K, Chu H, Cheng C-A. Novel H1N1 influenza vaccine the cause of postural orthostatic tachycardia syndrome followed by cerebral hypoperfusion. Journal of Medical Sciences. 2011;31(2):91-3.
- Blitshteyn S, Brook J. Postural tachycardia syndrome (POTS) with anti-NMDA receptor antibodies after human papillomavirus vaccination. Immunologic research. 2017;65:282-4. [CrossRef]
- Schelke MW, Barcavage S, Lampshire E, Brannagan III TH. Post–COVID-19 vaccine small-fiber neuropathy and tinnitus treated with plasma exchange. Muscle & Nerve. 2022;66(4):E21.
- Sanada Y, Azuma J, Hirano Y, Hasegawa Y, Yamamoto T, Hirano Sr Y. Overlapping myocarditis and postural orthostatic tachycardia syndrome after COVID-19 messenger RNA vaccination: a case report. Cureus. 2022;14(11).
- Eldokla AM, Numan MT. Postural orthostatic tachycardia syndrome after mRNA COVID-19 vaccine. Clinical Autonomic Research. 2022;32(4):307-11. [CrossRef]
- Park J, Kim S, Lee J, An JY. A case of transient POTS following COVID-19 vaccine. Acta Neurologica Belgica. 2022;122(4):1081-3. [CrossRef]
- Reddy S, Reddy S, Arora M. A case of postural orthostatic tachycardia syndrome secondary to the messenger RNA COVID-19 vaccine. Cureus. 2021;13(5).
- Hermel M, Sweeney M, Abud E, Luskin K, Criado JP, Bonakdar R, et al. COVID-19 vaccination might induce postural orthostatic tachycardia syndrome: a case report. Vaccines. 2022;10(7):991. [CrossRef]
- Carroll HA, Millar E, Deans KA. Vitamin B12 and D deficiency as cofactors of COVID-19 vaccine-induced chronic neurological adverse reactions: Two cases and a hypothesis. 2022.
- Safavi F, Gustafson L, Walitt B, Lehky T, Dehbashi S, Wiebold A, et al. Neuropathic symptoms with SARS-CoV-2 vaccination. MedRxiv. 2022.
- Mustafa HI, Garland EM, Biaggioni I, Black BK, Dupont WD, Robertson D, et al. Abnormalities of angiotensin regulation in postural tachycardia syndrome. Heart Rhythm. 2011;8(3):422-8. [CrossRef]
- Rubin, R. Large cohort study finds possible association between postural orthostatic tachycardia syndrome and COVID-19 vaccination but far stronger link with SARS-CoV-2 infection. JAMA. 2023;329(6):454-6.
- Tv P, Tran TT, Hao HT, Hau NTH, Jain N, Reinis A. Postural orthostatic tachycardia syndrome-like symptoms following COVID-19 vaccination: An overview of clinical literature. Human Antibodies. 2023(Preprint):1-9. [CrossRef]
- Im JH, Kim E, Lee E, Seo Y, Lee Y, Jang Y, et al. Adverse events with the Pfizer-BioNTech COVID-19 vaccine among Korean healthcare workers. Yonsei Medical Journal. 2021;62(12):1162.
- Dighriri IM, Alhusayni KM, Mobarki AY, Aljerary IS, Alqurashi KA, Aljuaid FA, et al. Pfizer-BioNTech COVID-19 vaccine (BNT162b2) side effects: a systematic review. Cureus. 2022;14(3).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





