Introduction
Tuberculosis (TB) is a chronic infectious disease that is mainly caused by
M. tuberculosis. According to the World Health Organization, 10.6 million new TB cases were reported worldwide in 2021, and this is 4.5% more than the 10.1 million new cases reported in 2020[
1]. In recent years, the spread of multidrug-resistant
M. tuberculosis strains has made TB an even bigger threat and challenge to global public health, and has made the development of a more effective vaccine an urgent need [
2]. As of September 2022, there were 16 TB vaccine candidates in clinical trials, and they are classified as recombinant Bacillus Calmette-Guérin (BCG) vaccines,
M. tuberculosis live attenuated vaccines, protein/adjuvant vaccines, virus vector vaccines, and inactivated
M. tuberculosis vaccines [
3]. The components of the vaccines are closely related to their immune strategies, and currently, there are two promising strategies for the development of TB vaccines: ① modified recombinant BCG vaccines, which are mainly administered to infants and is a replacement for BCG; ② subunit protein vaccines, which are mainly administered to adults to enhance the protective effect of BCG after primary immunization [
4].
As the only vaccine that can prevent TB at present, BCG has been in use since 1921, with a global vaccination rate of more than 90% in infants [
5]. It can prevent tuberculous meningitis, miliary tuberculosis, and other serious forms of tuberculosis, and plays an important role in the prevention of TB in infants in countries with a high TB burden [
6]. However, the protective effect of BCG decreases from adolescence, and it has almost no protective effect on adults [7, 8]. Further, the effectiveness of revaccination of adults with BCG is controversial [7, 8]. The protective effect of BCG in adults may be reduced because BCG loses some important immunodominant antigen genes during the long-term passage of the strain. These deletion fragments are referred to as the region of differences (RD) (in comparison to
M. tuberculosis) and include
esxB and
esxA, among other fragments [
9]. The construction of recombinant BCG vaccines is based on molecular biology technology and involves knocking out the original gene or inducing overexpression of an immunodominant antigen gene based on the parental BCG gene [
10]. This strategy overcomes the deficiency in the immune effect and duration of effect of the original BCG (thus extending its protective effect on adults), while retaining the advantages of BCG in terms of stimulating immune protection (especially in children) [
10]. One such recombinant vaccine is the VPM1002 vaccine, in which the
urease C gene is deleted and transferred into the
Hly gene of
Listeria monocytogenes to reduce the pH of phagocytic lysosomes. This improves the phagocytosing and antigen cross-presenting abilities of antigen-presenting cells. VPM1002 was proved to induce stronger immune response and protection against
M. tuberculosis and was expected to be a viable alternative to BCG [
11]. As a result, the construction of safe and effective recombinant BCG vaccines has become a hot topic in the research on TB vaccines.
The immune efficacy of BCG, which is a live attenuated vaccine, is mainly dependent on its effective components and its ability to replicate and persist in the body [
12]. Another reason for the reduced protective effect of BCG could be pre-sensitization to non-tuberculosis mycobacterium and cross-immunization with BCG, which may inhibit BCG replication in humans and lead to reduced colonization ability [
12]. Unlike BCG and other live vaccines, subunit protein vaccines are non-replicative, and their immune effect is mainly dependent on the antigenic component and adjuvant and is not affected by pre-sensitization to
M. tuberculosis and non-tuberculosis mycobacterium [
13]. The antigen component is clear and safe, and is especially suitable for people with HIV infection [
14]. Since newborns in most countries are vaccinated with BCG after birth, combining the subunit vaccine with BCG can expand the antigen spectrum and induce different types of immune responses, while prolonging the protective effect of BCG and improving the body’s ability to resist TB infection. At present, most TB subunit vaccines are in the clinical phase of research. For example, MVA85A, H4/IC31, and H56/IC31 have been designed for use in adults who have been vaccinated with BCG [15-17]. However, there is still no booster vaccine approved for use. It is speculated that the main limitation of current booster shots may be the cross-immune reaction between the components of the booster vaccine and BCG, as this inhibits the growth of BCG and reduces its immune effect. To avoid the effect of the proteins of the booster immunization on BCG colonization and excessive immune response, the target antigen protein of the booster needs to have high specificity for
M. tuberculosis and produce no or little cross-reactivity with BCG [
18]. Although vaccines with two different components of recombinant BCG and subunit proteins have become the main research focus of TB vaccines in recent years, there are few comparative studies on the different immune strategies. Therefore, it is of great significance to clarify the differences in the immunogenicity of these immunization strategies and the level of protection they offer.
At present, the immunodominant antigens of TB mainly include early secretory antigens, virulence-associated antigens, cell membrane antigens, growth phase antigens, and dormant phase antigens, which are potential targets for TB vaccine development [
3]. Culture filtrate proteins (CFPs) are secreted proteins present in the supernatant of
M. tuberculosis culture that can be recognized by T cells and stimulate the production of protective cytokines such as IFN-γ [
19]. For example, the RD region proteins ESAT-6 and CFP-10 were widely used in early research on TB vaccines [
20]. In addition, the PPE18 protein, which belongs to the PE/PPE gene family, is encoded by the
Rv1196 gene, and is enriched with the N-terminal proline (P) and glutamate (E) sequence, has been shown to stimulate rapid proliferation of human T cells in
in vitro experiments and can be used as a potential anti-TB vaccine component [
21]. Further, the
Rv0934 gene encodes phosphate-specific transport substrate binding protein-1 (PstS1), which is involved in the active transport of inorganic phosphate across the membrane and can induce activation of mouse CD8
+ T cells and produce Th1 and Th17 immune-protective responses [
22]. Among these, PPE18 and PstS1 are expressed at low levels in BCG.
Due to the complex composition of
M. tuberculosis, the immunogenicity of individual antigens is low and it is difficult to provide sufficient immune protection with a single antigen. Therefore, combining multiple immunodominant antigens to construct multi-component vaccines is a popular trend in TB vaccine development. In this study, we selected four immunodominant antigens, namely, ESAT-6, CFP-10, nPPE18 (epitope-enriched antigen), and nPstS1 (epitope-enriched antigen), which had been validated in our previous study[
23], to construct recombinant BCG and subunit proteins. First, we induced expression of the fusion protein EPCP009 containing ESAT-6, CFP-10, nPPE18, and nPstS1, and used DDA/poly:IC as an adjuvant to create the EPCP009/DP subunit vaccine. In addition, the genes of the four immunodominant antigens were concatenated and introduced into the pMV361 plasmid, and the recombinant BCG vaccine rBCG-EPCP009 was constructed using the BCG Chinese strain as the parent. With Balb/c mice as a model, we examined the short- and long-term immune responses and immune memory levels,
in vitro mycobacterial growth inhibition ability, and safety after immunization with the BCG, rBCG-EPCP009, BCG prime-EPCP009/DP booster, and EPCP009/DP vaccines. We explore the optimal vaccine components and immunization strategies for constructing a novel and effective TB vaccine and provide a theoretical basis for the study of prime-boost immunization strategies.
Materials and methods
Bacterial strains and culture conditions
M. tuberculosis H37Rv, M. bovis BCG China, and rBCG strains were cultured on Difco ™ Middlebrook 7H9 (BD, USA) or Difco ™ Middlebrook 7H10 agar (BD, USA) supplemented with 10% oleic-albumin-dextrose-catalase (OADC), 0.5% glycerol, and 0.05% Tween 80. Escherichia coli DH5α and BL21(DE3) were cultured in Luria-Bertani medium or agar and used for cloning and expression. Kanamycin was used at a concentration of 25 μg/mL and ampicillin used at a concentration of 100 μg/mL.
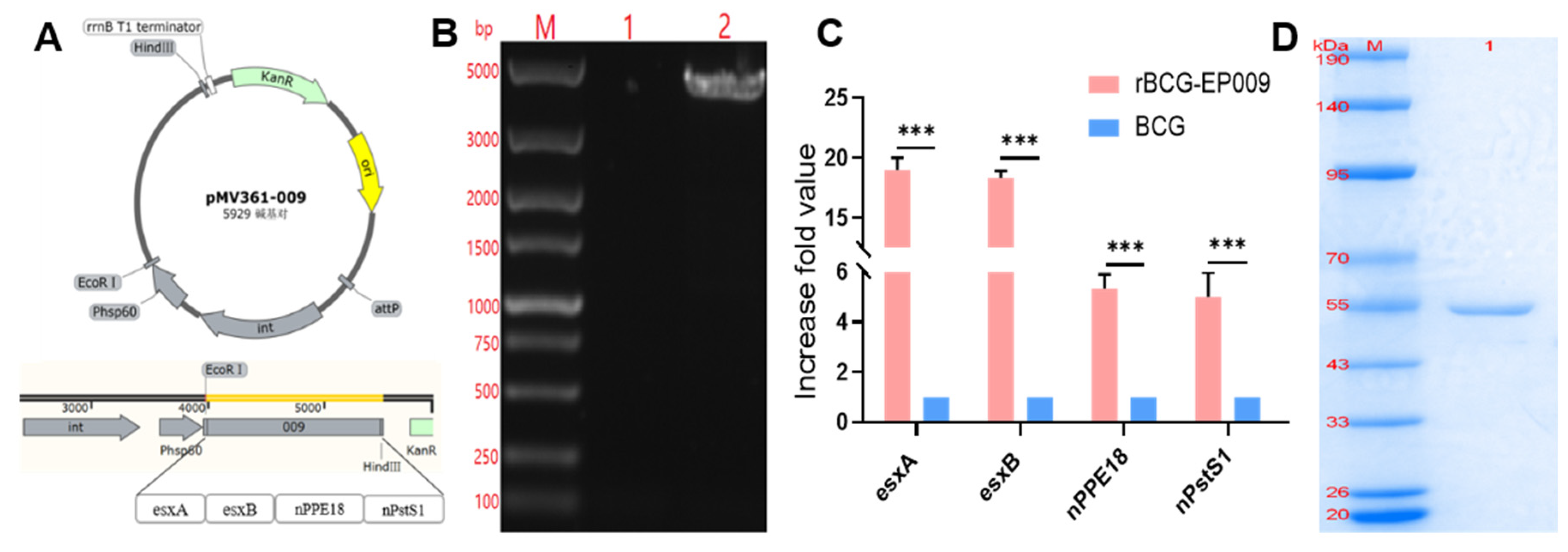
Recombinant rBCG-EPCP009 and the fusion protein EPCP009
The expression plasmid pET43.1a-009 and shuttle plasmid pMV361-009 were constructed by Universal Biosystems Co. Ltd. (Anhui, China). The procedure is briefly described here: EPCP009 (
esxA, esxB, nPPE18, and nPstS1 genes) was attached to the pET43.1a vector and pMV361 vector via a linker. As an integral component of fusion protein recombination, the linker is an amino acid chain that connects two fusion genes and has a certain degree of flexibility that allows the proteins on both sides to perform their independent functions [
24]. Non-polar hydrophobic amino acids, such as glycine (Gly) and serine (Ser), are commonly used. In the design of our fusion protein vaccines, the linker we chose was Gly-Gly-Ser-Gly-Gly, which is encoded by the GGTGGTTCTGGCGGT sequence.
The pMV361-009 plasmid was transformed into the BCG receptor state by electroshock (voltage, 2.5 kV; capacitance, 25 μF, resistance, 1000 Ω) and screened with the 7H10 resistance medium containing 25 μg/mL kanamycin. A monoclonal colony of rBCG-EPCP009 was cultured in 7H9 medium containing 25 μg/mL kanamycin. The integration of pMV361-009 into BCG was verified by PCR, and the amplified product was sent to the TsingKe Biotech Corp. (Beijing, China) for sequencing to verify the successful construction of the rBCG-EPCP009 strain. The expression level of the target gene was verified by extracting mRNA and using it for RT-qPCR. The primers for verifying the plasmid integration of rBCG-EPCP009 and mRNA expression levels of rBCG-EPCP009 are shown in
Table 1.
The transformation of plasmids and the expression and purification of EPCP009 protein are described in previous articles[
25]. Briefly, pET43.1a-009 was transformed into
E. coli BL21 (DE3). A monoclonal colony of BL21 was cultured in Luria-Bertani liquid medium containing 100 μg/mL ampicillin (37°C, 180 rpm). The bacterial cultures were induced with 1 mM isopropyl β-
d-1-thiogalactopyranoside. Then, the proteins were purified from the inclusion bodies by nickel chelate chromatography (GE Healthcare, PA, USA). Endotoxin was removed from the purified proteins using a ToxinEraser™ Endotoxin Removal Kit (GenScript, NJ, USA) and the proteins were filtered through 0.45-μm filters.
Immunization regimens and sample collection
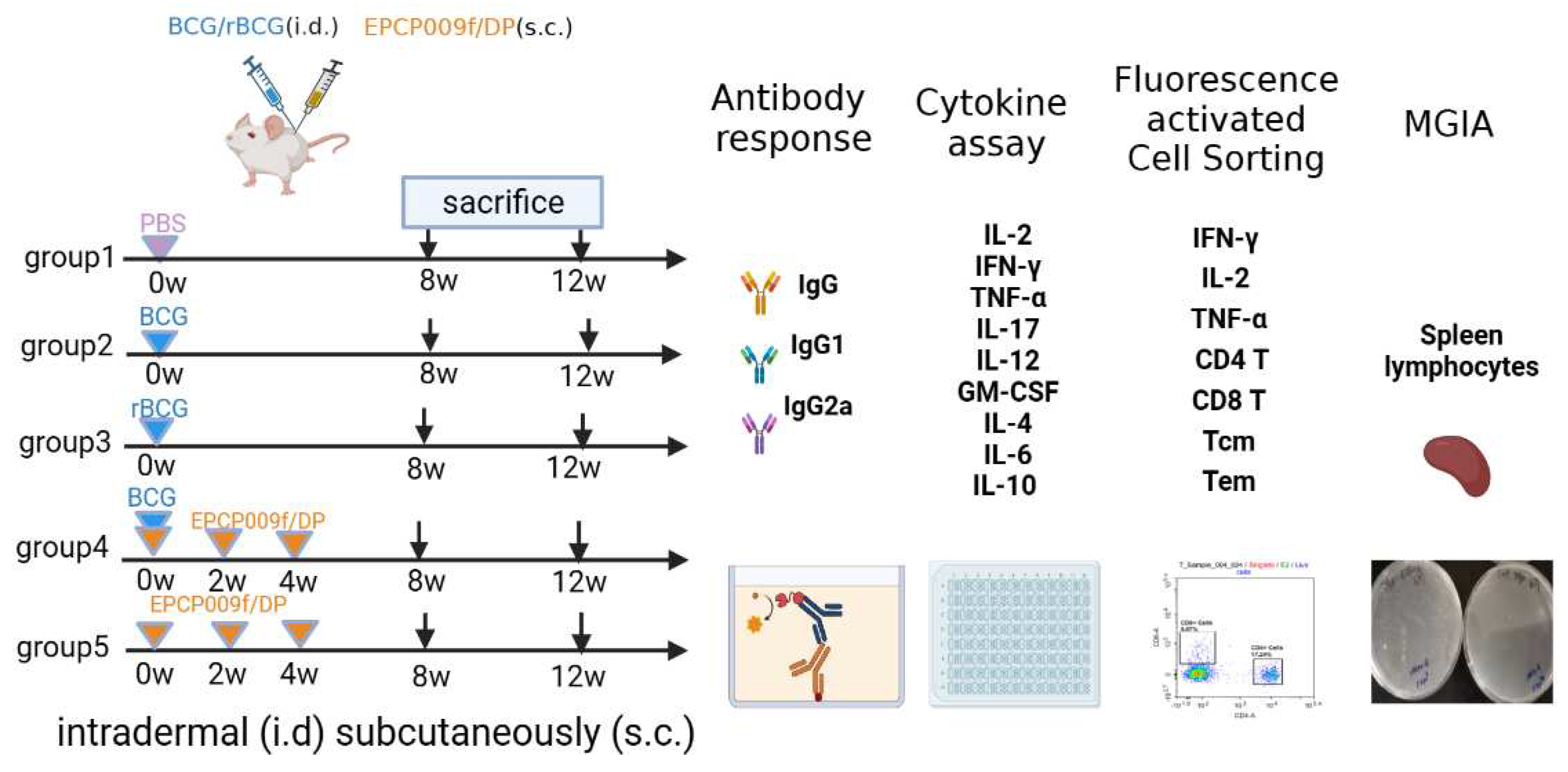
Specific pathogen-free, 4- to 6-week-old female Balb/c mice were obtained from Beijing HFK Bioscience Co. Ltd. (Beijing, China). In order to evaluate the impact of different immunization schemes (shown in
Figure 1), the mice were randomly divided into five groups of six mice each for vaccination. Mice of group 1 (PBS) were immunized with 100 μL of phosphate-buffered saline (PBS, pH 7.4); mice of groups 2 (BCG) and 3 (rBCG-EPCP009) were immunized intradermally with 1 × 10
6 colony-forming units (CFUs) of the BCG/rBCG strain in 100 μL of PBS (pH 7.4); mice of group 4 (BCG+EPCP009) were immunized intradermally with 1 × 10
6 CFUs of the BCG strain at week 0 and immunized subcutaneously three times (at weeks 0, 2, and 4) with
10 μg EPCP009 protein formulated in DP (composed of 250 μg dimethyldioctadecylammonium [DDA]/50 μg poly I:C) in 100 μL of PBS (pH 7.4); mice of group 5 (EPCP009) were immunized subcutaneously three times (at weeks 0, 2, and 4) with
10 μg EPCP009 protein formulated in DP. Eight or twelve weeks from the first immunization, blood samples were collected from the orbits of the mice and they were sacrificed by cervical dislocation. The coagulated blood was centrifuged at 4000 rpm for 10 min, and the serum was stored at -20℃ for antibody titer analysis. Spleen cells were isolated under aseptic conditions by using mouse lymphocyte separation medium (Dakewe, Beijing, China) according to the manufacturer’s instructions.
Mice in each group were immunized with PBS, BCG, rBCG-EPCP009, BCG+EPCP009/DP, or EPCP009/DP, delivered by either the intradermal or the subcutaneous route. Eight or twelve weeks from the first booster, blood samples (from the orbit) and spleen cells were collected to analyze their immunogenicity and in vitro protection ability.
Luminex cytokine test and enzyme-linked immunospot assay
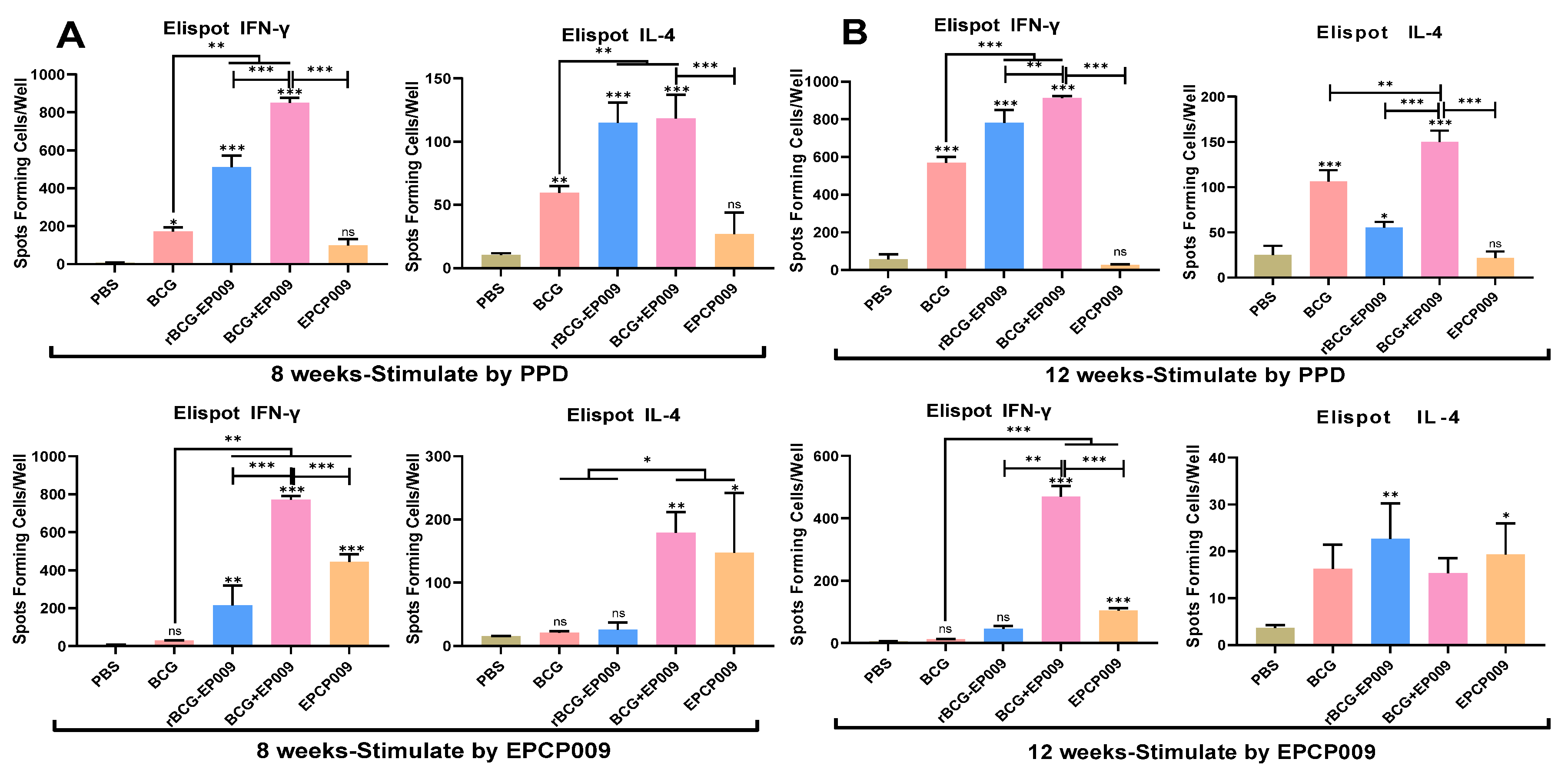
The nine cytokines representing different cellular immune types were detected by Luminex assay, and Th1-type cytokine IFN-γ and Th2-type cytokine IL-4 were detected Enzyme-linked immunospot (ELISpot) method as previously described[
25]. What's different is that cells are stimulated with PPD and EPCP009, respectively. Briefly, the mouse splenocytes were plated in 96-well Luminex Mouse Magnetic Assay (R&D Systems, Minneapolis, MN, USA) culture plates at 2 × 10
5 cells/well and cultured with 10 μg of the PPD or EPCP009 antigen (i.e., 50 μL of a 200 ng/μL solution) for 24 h. Add biotin antibodies and antibodies separately, wash, and then perform testing. In addition, spleen cells detected by Elispot method were stimulated with 2 μg of PPD or EPCP009 protein, respectively.
ELISA
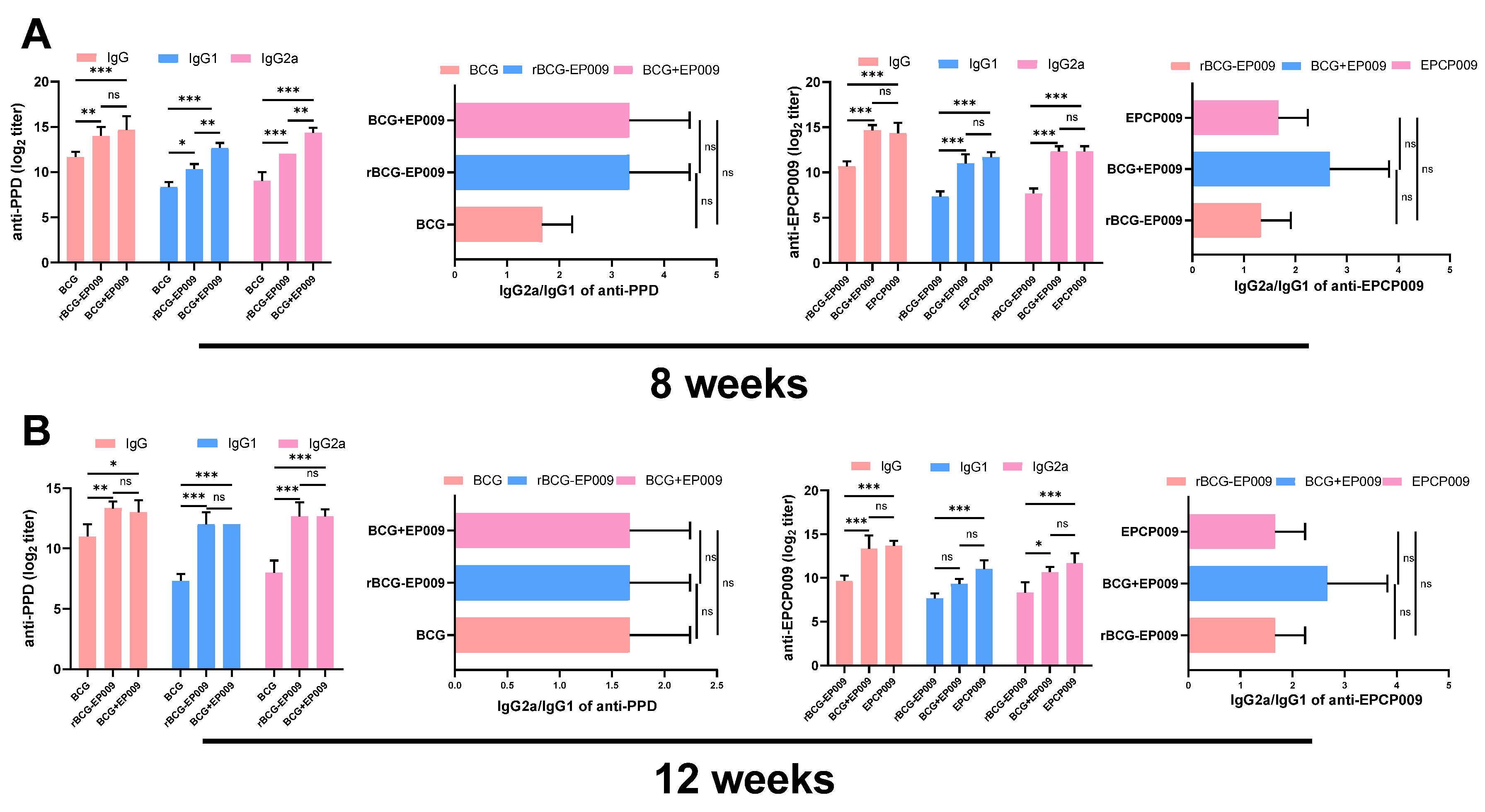
The IgG, IgG1, and IgG2a antibody concentrations in the serum were measured by ELISA[
25]. Briefly, 96-well ELISA plates were coated with 200ng of the PPD or EPCP009 protein and incubated overnight at 4°C, and then washed five times with PBST (PBS with 0.5% Tween 20). The plates were blocked with 3% skimmed milk for 2 h at 37°C and washed five times with PBST. Two-fold dilutions serum was added and incubated for 2 h at 37°C and washed five times, and then added 100 μL/well HRP-conjugated goat anti-mouse IgG, IgG1, or IgG2a (Sigma, St. Louis, MO, USA) diluted to 1:5000 in PBS. The plates were incubated for 1 h at 37°C and washed five times, and 100 μL of tetramethylbenzidine substrate was added for 15 min at 37°C. The reactions were terminated with the addition of 100 μL/well of 2 M H
2SO
4, and absorbance was measured on an ELISA plate reader at a wavelength of 450 nm. Absorbance values of >2.1 (OD450 [experimental group]/OD450 [PBS control]) were considered to indicate the presence of IgG.
Flow cytometry
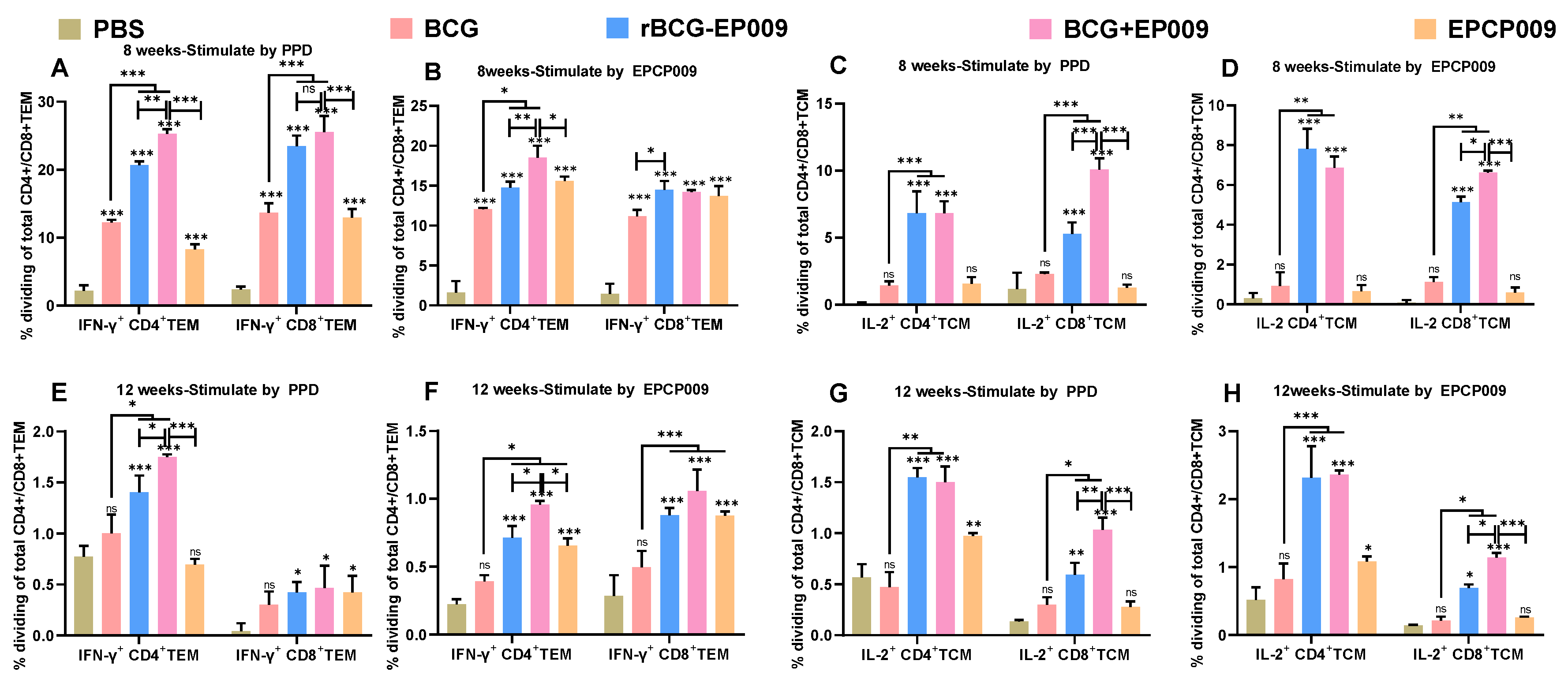
In order to further analyze the immune mechanism of each vaccine, the spleen cells of each group were stimulated with PPD and EPCP009 after 8 and 12 weeks of the initial immunization, and the proportions of CD3+CD4+Th cells and CD3+CD8+CTL cells among T cells were analyzed by flow cytometry combined with intracellular cytokine staining. Following this, the expression of CD62L and CD44 was examined as sorting markers for determining the proportion of effector memory T cells (TEM cells: CD62Llo CD44hi) and central memory T cells (TCM cells: CD62Lhi CD44hi) in the CD4+ or CD8+ cell populations, as well as the proportion of cells secreting IFN-γ among TEM cells and the proportion of cells secreting IL-2 among TCM cells.
The mouse splenocytes were plated in 96-well culture plates containing 100-μL Luria-Bertani culture medium at a density of 2 × 107 cells/well. Then, 2 × 106 cells from the 100-μL cell suspension were added to 96-well culture plates, to which 100 μL of the prepared stimulation mixture was also added to obtain final concentrations of 10 μg/mL of specific antigens, 1 μg/mL of the CD28 antibody, 1 μg/mL of the CD49d antibody, and 1000× dilution of the Brefeldin A (BFA) blocker, and the cultured cells were stimulated for 8 h in a 5% CO2 incubator at 37°C after mixing. Two positive control wells were set up, and the non-specific stimulant Cell Stimulation Cocktail (with BFA) was added to the wells. Lymphocytes were collected from the culture well in a 5-mL flow tube, to which 2 mL of PBS was added and mixed well. The solution was then centrifuged at 300 g for 5 min, and the supernatant was discarded. This solution was mixed well with 1 ml of Ghost Dye™ Violet 510 (for labelling dead cells) prepared by diluting 1000 times with PBS, and incubated for 15 min at room temperature in the dark. Next, 2 mL of flow staining wash was added and mixed well. The solution was centrifuged at 300 g for 5 min, and the supernatant was discarded. The cell pellet was resuspended in 100 μL of flow cytometry washing solution, and fluorescence-labeled antibodies (CD3, CD4, CD8, CD62L, and CD44) (BioLegend, USA) of cell surface antigens were added, mixed well, and incubated for 20 min at room temperature in the dark. Next, 2 mL of flow staining wash was added and mixed well, and the supernatant was discarded after centrifugation at 300 g for 5 min. Cell fixative (0.5 mL) was added to each tube, mixed, and incubated for 20 min at room temperature in the dark. The solution was centrifuged at 300 g for 5 min, and the supernatant was discarded. Following this, 2 mL of cytoplasmic membrane breaking lotion was added, mixed well, and centrifuged at 300 g for 5 min. The supernatant was discarded, and the pellet was mixed with a solution containing fluorescence-labeled antibodies (IL-2, TNF-α, and IFN-γ) (BioLegend, USA) against the cytoplasmic antigens, mixed well, and incubated in the dark at room temperature for 30 min. Then, 2 mL of cytoplasmic membrane-breaking lotion was added to each tube and mixed well. The solution was centrifuged at 300 g for 5 min, and the supernatant was discarded. The cells were resuspended in 0.5 mL of cell stain/wash and subjected to flow cytometry analysis (Cytek NL-CLC3000, USA). The data obtained were analyzed with the FACS Diva 8.0 software.
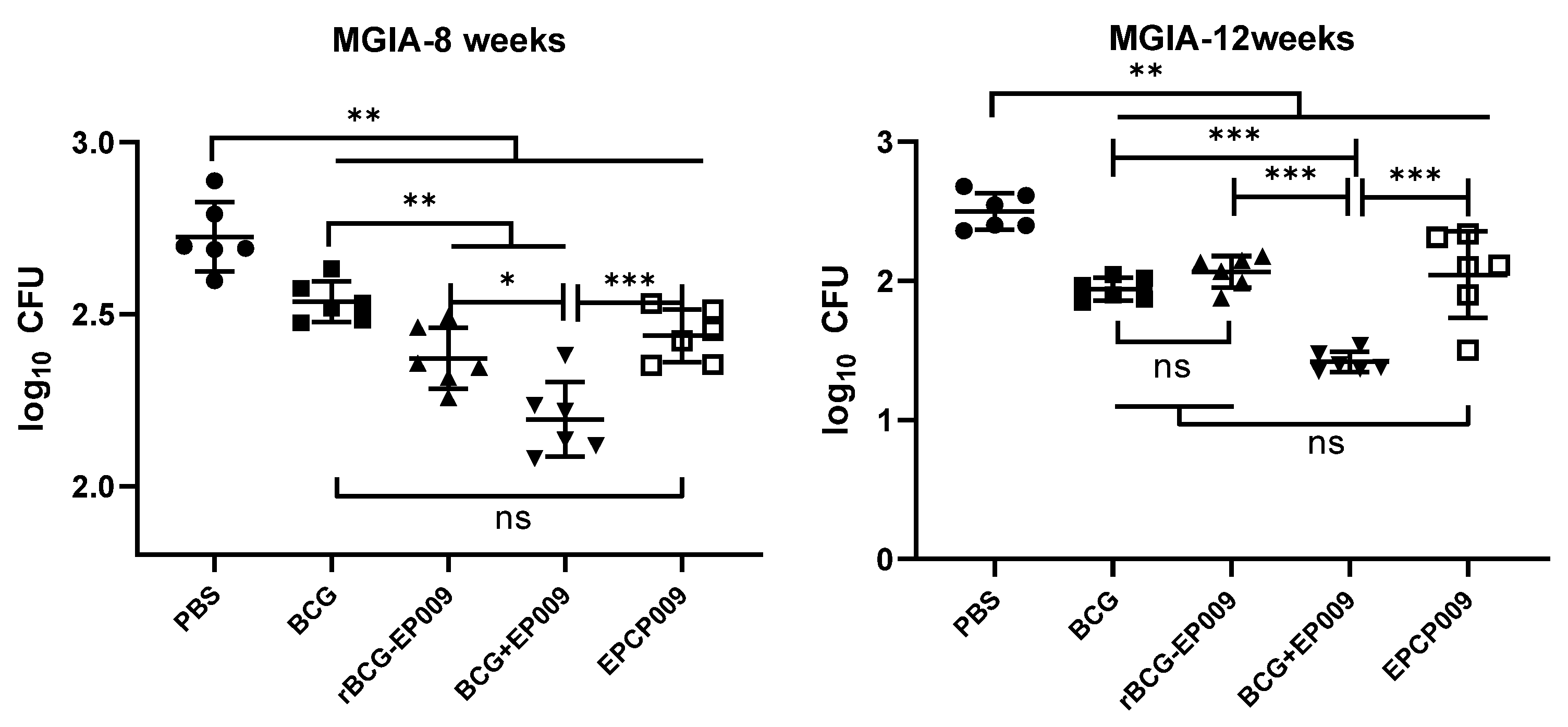
In vitro mycobacterial growth inhibition assay
The mycobacterial growth inhibition assay (MGIA) could evaluate the ability of the splenocytes of mice to inhibit
M. tuberculosis growth under
in vitro conditions and evaluate the protective effect of TB vaccines, which is in line with the 3R principles in animal experiments: reduction, replacement, refinement. The protocol was adapted from a previously published MGIA protocol for mice splenocytes and further optimized in this laboratory[
26]. Briefly, the cells were adjusted to a density of 2 × 10
6 splenocytes per 300 µL of antibiotic-free RPMI 1640 medium containing 10% heat-inactivated fetal bovine serum, 10 mM HEPES, and 2 mM
l-glutamine.
M. tuberculosis H37Rv was diluted to a concentration of 50 CFUs/300 µL. Bacterial aliquots (300 µL) were added to the splenocytes to form a splenocyte–mycobacteria co-culture (600 µL), which was incubated in 24-well plates at 37°C for 4 days. Subsequently, the co-cultures were collected and this was followed by centrifugation at 12,000 rpm for 10 min and the supernatant was discarded after centrifugation. Sterile water (500 µL) was added to each tube and mixed, and 50 μL of the solution was inoculated on 7H10 medium containing 5 μg/mL thiophene 2-carboxylic acid hydrazine (TCH) and 10% OADC supplement and incubated at 37°C for 3 weeks. The number of bacteria was counted, and the data were presented as the log
10CFU value of the total number per sample.
Statistical analysis
The experimental data were expressed as the mean ± SD using GraphPad Prism 8. One-way analysis of variance (ANOVA) with Tukey’s multiple comparison tests were used for comparison of more than two groups. P-values <0.05 were considered to indicate statistical significance.
Discussion
Tuberculosis remains a serious infectious disease that poses a threat to human health. BCG is the only preventive vaccine currently approved for human use. However, while it is effective in preventing severe TB in infants and children, it has severely reduced protection in adolescents and adults [
6]. Currently, 16 novel TB vaccine candidates are in clinical trials worldwide, and recombinant BCG and subunit protein vaccines are two important vaccine strategies. Recombinant BCG is intended for neonatal replacement of BCG, and the subunit protein is mainly used for booster vaccines after primary BCG immunization. Both recombinant BCG and the subunit protein are expected to be useful strategies for the development of new vaccines and for the development of immunization protocols that provide stronger and longer-lasting immunity based on the inherited parental BCG [
27]. In this study, we constructed recombinant rBCG-EPCP009 and subunit protein EPCP009 vaccine candidates based on the multi-component immunodominant antigen EPCP009, and evaluated the short- and long-term immunogenicity and
in vitro protection offered by three immunization strategies, namely, rBCG-EPCP009, BCG+EPCP009, and EPCP009/DP. Our results showed that the BCG prime-EPCP009 booster provided superior and longer-lasting immunogenicity and better
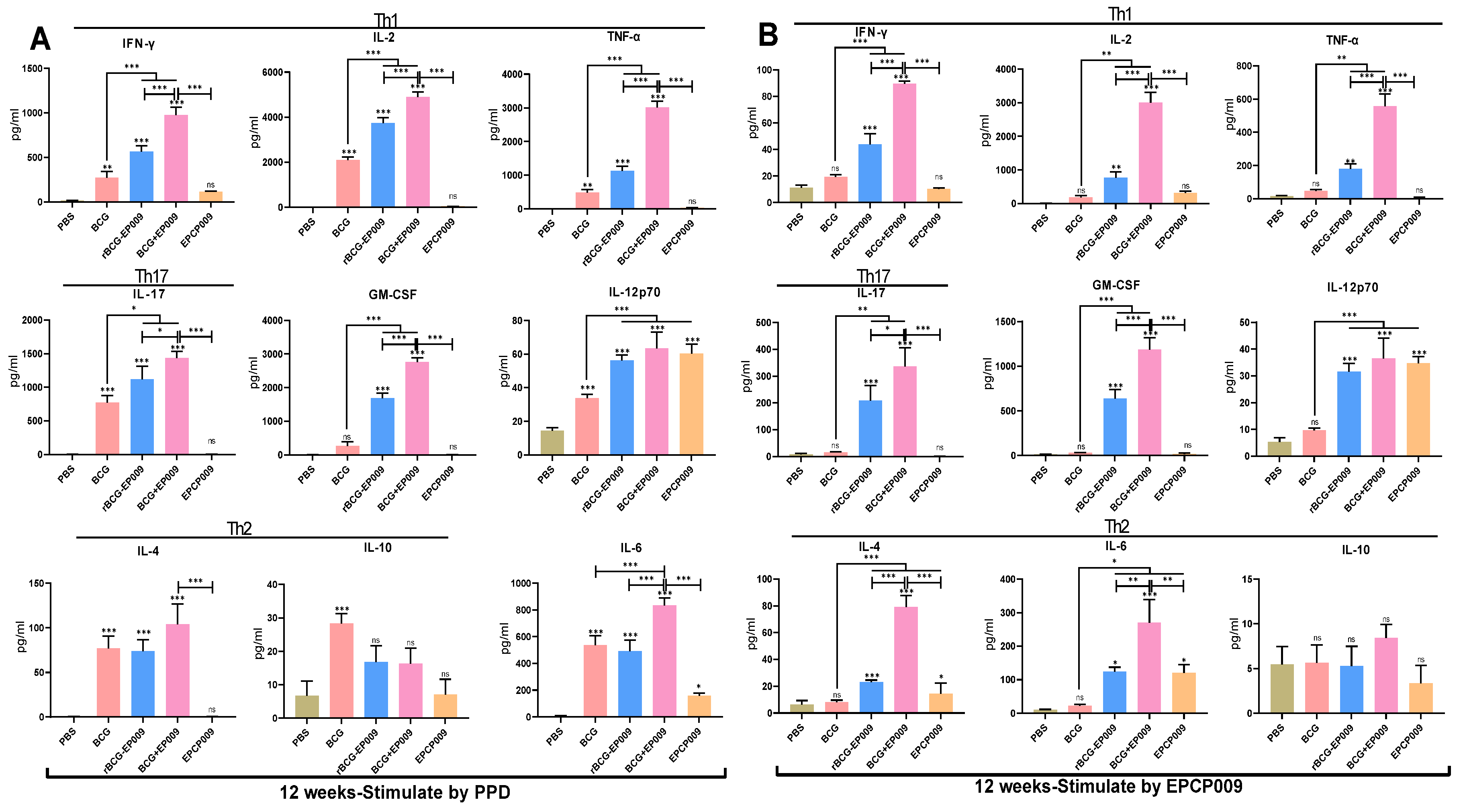
in vitro protection than rBCG-EPCP009 and EPCP009/DP, as reflected in the following results. That is, BCG+EPCP009 induced stronger PPD- and EPCP009-specific Th1-type and Th17-type immune responses based on the levels of the cytokines GM-CSF and IL-12, and the IFN-γ
+CD4
+TEM and IL-2
+CD8
+TCM cell ratios, at both 8 and 12 weeks after immunization, while BCG+EPCP009 induced EPCP009-specific antibodies that were indicative of a bias toward Th1-type immune responses. In addition, immunization with BCG+EPCP009 resulted in stronger
in vitro mycobacterium inhibition by splenocytes and had a longer
in vitro protective effect.
The selection of an appropriate immunodominant antigen is an important factor in determining vaccine efficacy, irrespective of whether the strategy involves the recombinant BCG or subunit vaccine. rBCG30, which expresses the Ag85B antigen, was the first vaccine candidate of this type to enter the clinical trial phase and showed superior immune efficacy to the parent BCG in both mice and guinea pigs. However, the phase I clinical trials conducted in the United States in 2004 showed that the immunogenicity of rBCG30 was lower than that of BCG, and therefore, the findings from human trials were not encouraging [
28]. Subsequently, the strategy for construction of recombinant BCG vaccines changed from overexpression of a single antigen to simultaneous overexpression of multiple immune dominant fusion antigens, in order to further improve the immune effect of recombinant BCG. For example, rBCG::XB, which is in the preclinical phase, is a novel recombinant BCG that expresses both the Ag85B and HspX antigens and induces a stronger antigen-specific Th1-type immune response in mice than BCG [
29]. In addition, rBCG::685A, which expresses both ESAT-6 and Ag85A, also induced higher levels of PPD or r685A-specific IFN-γ in mice [
30]. In this study, based on the ESAT-6 and CFP-10 proteins, the fusion gene EPCP009 was constructed by adding the antigens nPPE18 and nPstS1. Recombinant rBCG-EPCP009 overexpressing the EPCP009 protein was constructed by the pMV361 shuttle plasmid. In addition, the ESAT-6, CFP-10, nPPE18, and nPstS1 genes were arranged in tandem, and the fusion protein EPCP009 and DDA/poly:IC adjuvant were mixed to construct the EPCP009/DP vaccine candidate, which was used as a booster vaccine after initial immunization with BCG. Compared with mice immunized with rBCG-EPCP009, mice immunized with the BCG prime-EPCP009 booster strategy secreted higher levels of PPD- and EPCP009-specific IFN-γ, IL-2, TNF-α, IL-17, GM-CSF, and IL-12 after 8 and 12 weeks of immunization.
In vitro protection assays showed that splenic lymphocytes from BCG+EPCP009-immunized mice showed superior
M. tuberculosis growth inhibition than splenocytes from rBCG-EPCP009-immunized mice at both 8 and 12 weeks. This indicates that although mice from all the immunization groups expressed the fusion protein EPCP009, the immune mechanisms induced differed according to the technology platforms and immunization strategies employed.
Under the stimulation of different antigens, Th0 cells differentiate into different Th1 and Th2 cell subsets, and different subsets of T cells secrete different types of cytokines in response to pathogens [
31]. As an intracellular bacterium,
M. tuberculosis mainly produces IL-12 by inducing its secretion from antigen-presenting cells. These antigen-presenting cells induce Th1 cells to release IFN-γ, IL-2, and TNF-α and activate macrophages and cytotoxic T lymphocytes, which then fulfill their function of
M. tuberculosis clearance [
32]. However, IL-4 induces the polarization of Th0 to the Th2 subset, and the production of IL-4 and IL-10 inhibits the anti-infection process and leads to the dissemination of
M. tuberculosis [
33]. Th17 cells are a unique group of Th cells that mainly secrete IL-17 and GM-CSF, which recruit and activate neutrophils to the site of infection. Th17cells and neutrophils enhance the protective immune response by producing IL-12 and inducing a Th1 response [
34]. IL-6 is a pro-inflammatory cytokine that can enhance the immune response by promoting the proliferation of T cells, B cells, and other immune cells [
35]. According to the latest evidence, the main effector T-cell subsets involved in
M. tuberculosis clearance are Th1 and Th17 cell subsets [
36]. However, certain components of the
M. tuberculosis cell wall, such as ManLAM, can promote IL-10 production and secretion by inhibiting the production of IFN-γ and TNF-α, thereby affecting antigen presentation or inhibiting the migration of effector T cells to the site of
M. tuberculosis infection and resulting in immune escape [
37]. Compared with BCG, the recombinant BCG vaccines rBCG-EPCP009 and BCG+EPCP009 resulted in the secretion of increased levels of PPD- and EPCP009-specific IFN-γ, IL-2, TNF-α, IL-17, GM-CSF, and IL-12 from mouse splenocytes after 8 and 12 weeks of immunization. These results indicate that rBCG-EPCP009 and BCG+EPCP009 induced a protective immune response which was superior to that of BCG. In addition, on comparing the three EPCP009-based immunization strategies, the level of PPD- and EPCP009-specific IFN-γ, IL-2, TNF-α, IL-17, GM-CSF, and IL-6 stimulated by BCG+EPCP009 was found to be significantly higher than that stimulated by rBCG-EPCP009 and EPCP009 after both 8 and 12 weeks of immunization. This result indicated that the combined strategy involving BCG+EPCP009 promoted not only the immune effect of BCG itself but also the immune response to the EPCP009 protein. Thus, BCG and EPCP009 may have induced a stronger immune effect that was brought about by a mutual adjuvant effect.
Memory T cells are mainly divided into TCM cells and TEM cells [
38], and T cells are highly heterogeneous in terms of their immune response. When exposed to antigen stimulation, TCM cells mainly secrete IL-2, rapidly proliferate and differentiate into effector T cells, and produce IFN-γ. TEM cells mainly secretes IFN-γ, which migrates to the site of inflammation and rapidly exerts its immune effects [
39]. It has been shown that IFN-γ
+TEM and IL-2
+TCM cells are biomarkers of vaccine-induced production of anti-
M. tuberculosis antibodies and early and persistent infection, respectively [
40]. The main reason for the decrease in the protective properties of BCG with time may be that its induced protective immune response decreases with time so that it induces a weaker CD8
+ immune response in adolescents and adults [
41]. The results of the present study showed that the proportions of neither IFN-γ
+TEM nor IL-2
+TCM cells were significantly higher in the BCG group compared with the PBS group after 12 weeks of immunization. This may partly explain why the protective effect of BCG is only maintained for 10–15 years and highlights the need for a TB vaccine with a longer immune memory. After 8 or 12 weeks of immunization, the rBCG-EPCP009 and BCG+EPCP009 vaccines induced significantly higher proportions of PPD- or EPCP009-specific IFN-γ
+CD4
+TEM, IL-2
+CD4
+TCM, and IL-2
+CD8
+TCM cells than BCG. This finding indicates that immune memory was significantly enhanced with the rBCG-EPCP009 and BCG+EPCP009 vaccine candidates. In addition, the proportion of antigen-specific IL-2
+CD8
+TCM cells induced by PPD or EPCP009 was significantly higher in the BCG+EPCP009 group than in the rBCG-EPCP009 and EPCP009 groups. When CD8
+TCM cells were exposed to the antigen again, they rapidly proliferated and differentiated and produced IL-2, while promoting the cytotoxic functions of effector CD8
+ T cells, which in turn enhanced their anti-infective effects [42, 43]. Thus, the stronger protective effect of VPM1002 than BCG may be related to its ability to induce high levels of TCM production [
11]. In conclusion, several studies have demonstrated the correlation between the proportions of IFN-γ
+ TEM and IL-2
+ TCM cells and the protective effect of TB vaccines. With regard to the vaccine candidates evaluated in the presents study, BCG+EPCP009 induced higher and longer-lasting levels of IFN-γ
+CD4
+ TEM and IL-2
+CD8
+ TCM cells. Thus, this immunization strategy may provide a longer-lasting immune protective effect.
MGIA is an effective method to comprehensively evaluate the protective effect of TB vaccines
in vitro [26, 44]. Parra et al. used mice as a model to test the inhibitory effect of splenocytes from vaccine-immunized mice on
M. tuberculosis in macrophages, and the results were consistent with the results of challenge protection experiments on animals [
45]. The results of this study showed that after 8 weeks of the initial immunization, splenocytes from the rBCG-EPCP009- and BCG+EPCP009-immunized groups showed stronger
in vitro mycobacterial inhibition than splenocytes from the BCG group; further, EPCP009 group splenocytes showed comparable inhibition to BCG group splenocytes. After 12 weeks of the initial immunization, the BCG+EPCP009 group splenocytes still exhibited better inhibition than the BCG group splenocytes, while the rate of inhibition of the rBCG-EPCP009 and EPCP009 group splenocytes were not significantly different from that of the BCG group splenocytes. This indicates that rBCG-EPCP009 provided better short-term protection than BCG, but did not provide long-term protection. At both 8 and 12 weeks after immunization, BCG+EPCP009 exhibited durable superior bacterial growth inhibition to rBCG-EPCP009 and EPCP009; this may be related to its ability to induce the production of PPD- and EPCP009-specific TNF-α, IFN-γ, IL-12, IL-17, IFN-γ
+CD4
+ TEM cells, and IL-2
+CD8
+ TCM cells.
In recent years, the protective effect of the BCG prime-BCG homologous enhancement strategy has been under debate, and many studies conducted in different countries, such as Japan and Finland, have shown that BCG revaccination does not improve the protective effect of BCG itself and may even cause pathological damage [46, 47]. Currently, the immunization strategy involving primary immunization with BCG and a heterologous boost has become the focus for the development of novel vaccines for TB, and several subunit protein and viral vector vaccines are in the clinical phase of evaluation for their application as booster immunization. The results of this study showed that the BCG+EPCP009 immunization group exhibited a more durable protective immune response and in vitro M. tuberculosis inhibition. Thus, administering the EPCP009 subunit protein vaccine as a booster immunization vaccine for BCG could enhance the immune protective response of the body. Overall, comparison of the different strategies revealed that the BCG prime-EPCP009 protein booster immunization strategy was superior to the rBCG-EPCP009 and EPCP009 strategies and may provide a theoretical basis for future immunization strategies of TB vaccines.