1. Introduction
Xanthomonas citri subsp.
citri (
X. citri) is a phytopathogenic Gram-negative bacterium and the causal agent of citrus canker, a severe disease that affects all economically important citrus varieties worldwide [
1], causing significant economic losses. Gram-negative bacteria possess a tight peptidoglycan (PG) layer located in the periplasmic space between their outer and inner membranes [
2]. This polymer is composed of glycan strands of β-1,4-glycosidic bond-linked N-acetylglucosamine (GlcNAc) and N-acetylmuramic acid (MurNAc) disaccharides, which are cross-linked by short peptides and play an essential role in the preservation and maintenance of cell shape and cell integrity [
3]. Two main classes of peptidoglycan-lytic enzymes are responsible for the PG's assembly: the glycosidases that cleave the glycan backbone and the amidases (or peptidases) that cleave the peptide sidechain [
4]
The
X. citri strain 306 (GenBank AE008923.1) possesses nine proteins sharing the peptidase M23 domain, and four of them are hypothetical proteins with no function described so far (
Supplementary Table S1). One of these proteins, XAC0024 (GenBank AAM34916.1), is a homolog of EnvC in
E. coli, a protein widely distributed in bacteria [
5]. EnvC is highly conserved within Gram-negative bacteria and functions as part of the septal ring apparatus [
6]. In
E. coli, EnvC is a periplasmic peptidase that plays a role in septal peptidoglycan splitting and daughter cell separation [
7]. Deletion of the E. coli
envC gene, like other genes encoding LytM domain hydrolases such as
nlpD,
ygeR, and
uebA, leads to the formation of long cell chains, suggesting a defect in cell separation [
7]. It was demonstrated that EnvC controls cell separation by activating PG-degrading amidases AmiA and AmiB [
8]. Homologs of
envC have been shown to perform similar functions in multiple bacterial species and are essential for the pathogenicity of several animal bacterial pathogens, including
Vibrio cholera, enterohemorrhagic
E. coli, and
Fusobacterium nucleatum [
9,
10,
11]. The
envC homolog of
Pseudomonas aeruginosa was identified to be functionally redundant to
nlpD, as deletion of both led to the formation of long cell chains and enhanced sensitivity to high temperature and antimicrobial compounds [
12].
The role of LytM factors and PG amidases in pathogenicity and cell division was recently characterized in
X. campestris pv.
campestris [
13]. Deficiency in cell separation was observed in either
nlpD or
envC deletion strains; however, the deletion of the single gene
nlpD had a significant effect on virulence and induction of the hypersensitive response in non-host plants, while deletion of
envC did not significantly affect host interactions [
13].
In the present study, we characterized the envC homolog (XAC0024) of X. citri strain 306. We found that envC is essential for virulence but did not completely compromise the ability of X. citri in triggering weak symptoms in a susceptible host genotype. Also, X. citri ΔenvC displayed an altered cell shape compared to the wild-type strain. Similar to what was observed for other Gram-negative bacteria, X. citri envC gene seems to play a role in daughter cell separation. Moreover, the subcellular localization of X. citri EnvC protein linked to mCherry fluorophore (EnvC-mCherry) is consistent with the protein occupying the periplasmic region.
2. Materials and Methods
2.1. Bacterial strains, plasmids, and culture condition
The bacterial strains and plasmids used in the present work are listed in
Table 1.
Escherichia coli (
E. coli) strains DH10B, SM10ʎpir, and HST08 – Stellar used for cloning were cultivated at 37℃ in LB/LB-agar medium [
14]. Growth in liquid medium was at 250 rpm (shaker) for 14-16 h and growth in solid medium was in a bacteriological incubator for 14-16 h.
Xanthomonas citri subsp.
citri (
X. citri) 306 strain were cultivated at 30℃ in NYG-rich medium (3 g/L yeast extract, 5 g/L peptone, 20 g/L glycerol, pH 7.0), NB medium (“Nutrient Broth”: 3 g/L meat extract, 5 g/L peptone), or on NB-agar plates (NB medium containing 15 g/L agar), supplemented with L-arabinose (0.05% w/v), starch (0.2% w/v), and sucrose (5% w/v) when required. Growth in liquid medium was at 250 rpm (shaker) and growth in solid medium was in a bacteriological incubator for 48 h. The antibiotics carbenicillin and kanamycin or ampicillin and spectinomycin were used when required, at the concentration of 50 μg/mL and 100 μg/mL, respectively.
2.2. Mutant construction
2.2.1. Partial deletion of XAC0024 nucleotide sequence
The
X. citri strain containing the disrupted XAC0024 gene (mutant Δ
envC) was obtained through site-directed mutagenesis using the overlap extension approach in the polymerase chain reaction [
18]. To construct the XAC0024 mutant,
X. citri genomic DNA was used as a template in two-step PCR reaction. In the first step, the primers A(F) and B(R), and C(F) and D(R) (
Supplementary Table S2) were used in two separate reactions to generate fragments AB and CD, respectively, which were then linked through a double-joint PCR. The PCR reaction, in a final volume of 20 µL, contained 26.5 ng of DNA, 0.2 mM of each dNTP, 1U of Phusion High Fidelity DNA Polymerase (New England Biolabs), 0.5 μM of each primer, and 3% of DMSO for primer pairs A-B and 5% of DMSO for primer pairs C-D. The PCR conditions were as follows: 98°C for 30s, 35 cycles of 98°C for 10s, 69°C for 30s, and 72°C for 30s, with a final extension at 72°C for 10 min, in a Veriti® 96-Well Thermal Cycler (Applied Biosystems). The PCR products were purified using the Kit Wizard® SV Gel and PCR Clean-Up System (Promega) and quantitated using the NanoDrop® ND-1000 spectrophotometer (Thermo Fisher Scientific). The size of the amplified fragments AB and CD was confirmed by agarose gel electrophoresis (
Supplementary Figure S1). The double-joint PCR step was performed using fragments AB and CD as templates and the primers A(F) and D(R) to generate the A-D fragment, using 3% DMSO and the same PCR conditions described before, except that the final volume was 50 µL. The amplified product was subjected to 0.7% agarose gel electrophoresis, and the band with the expected size was recovered from the gel using the Kit Wizard® SV Gel and PCR Clean-Up System (Promega). The purified DNA fragment was quantitated in a NanoDrop® ND-1000 spectrophotometer (Thermo Fisher Scientific) and submitted to a PCR reaction to add a 3'-A overhang to the ends of the AD fragment using a final concentration of 0.2 mM dATP and 1 U of Platinum® Taq DNA Polymerase Recombinant (Invitrogen) in a final volume of 50 µL in a 10-min reaction at 72
oC in a GeneAmp® PCR System 9700 (Applied Biosystems).
2.2.2. Deletion vector construction
The PCR-amplified AD fragment containing 3'-A overhangs was ligated into plasmid pGEM
®-T Easy (Promega) using T4 DNA ligase according to the manufacturer's instructions. The ligation reaction contained 50 ng of plasmid, 118 ng of insert, in a final volume of 10 µL. Then, an aliquot of 2 µL of the ligation reaction was used to transform 50 µL of chemically competent
E. coli DH10B cells (Invitrogen) following the protocol described by [
14]. The recombinant bacteria carrying the plasmid DNA harbouring the A-D fragment were selected by plating onto agar plates containing solid LB medium, 100 µg/mL of ampicillin, 0.1 mM of IPTG (Isopropyl-β-D-1-thiolgalactopyranoside) and 0.0032% of Xgal (5-bromo-4-chloro-3-indolyl-β-D-galactoside). After incubation at 37
0C for 16 h, two white colonies were picked and their recombinant plasmid was isolated using the Wizard® Plus SV Minipreps DNA Purification System (Promega) following the manufacturer's instructions. The presence of the A-D fragment was checked by PCR using the vector primers M13/pUC F-20 and M13/pUC R-48 (
Table S1), and the sequence was confirmed by sequencing on an ABI 3730xl DNA analyzer (Applied Biosystems) using the same vector primers. Next, the recombinant plasmid was digested with
ApaI and
SalI restriction enzymes (New England Biolabs), and the A-D fragment was recovered from an agarose gel as described before. Subsequently, it was ligated into the suicide pOK1 plasmid previously digested with the same enzymes. The ligation reaction was used to transform chemically competent
E. coli SM10 pir cells to the protocol described by [
14]. The recombinant bacteria carrying the recombinant pOK1 plasmid were selected by plating them onto agar plates containing solid LB medium and 100 µg/mL of spectinomycin. The pOK1 plasmid DNA was purified using the kit Wizard® Plus SV Minipreps DNA Purification System (Promega), and the presence of A-D fragment was confirmed by PCR using A(F) and D(R) primers.
2.2.3. Mutant obtention
The pOK1 suicide vector carrying the A-D sequence from XAC0024 was used to delete the bases 370 to 962 of the XAC0024 gene (1236 bp) by integrating the suicide vector into chromosomal DNA via double-crossover homologous recombination. Electrocompetent
X. citri 306 wild-type (wt) cells were transformed with recombinant pOK1 vector as described by [
19]. The screening of the deletion mutant followed the methodology described by [
20], using NB-agar medium with the addition of spectinomycin antibiotic. Since pOK1 vector has the
SacB gene, positive selection for the loss of the vector was achieved by growth on sucrose. Colonies that grew in sucrose but not in the presence of spectinomycin were selected, and their genomic DNA was extracted using the Wizard® Genomic DNA Purification Kit (Promega), according to the manufacturer's instructions. The deletion was confirmed by PCR reaction using 50 ng of mutant and
X. citri 306 wt genomic DNAs, GoTaq® DNA Polymerase, primers A(F) and D(R). The amplicons were visualized in a 1% agarose gel, and the ones with the expected length were then sequenced, and the confirmed mutant was named
X. citri Δ
envC (Δ
envC).
2.3. Mutant complementation
For the complementation of the Δ
envC mutant, a fragment of 2236-bp comprising the genome region from 25382 to 27617 bases, containing 1236-bp of the ORF XAC0024 plus 500 bp upstream of the 5’ end and 500 bp downstream of the 3’ end, was amplified using the primers 0024_500_IF_F / 0024_500_IF_R (
Supplementary Table S2) and Q5 High-Fidelity DNA Polymerase (New England Biolabs). The PCR-amplified fragment was ligated into the
XhoI site of pMAJIIc plasmid [
17] using the
In-Fusion HD Cloning Kit (Takara Bio USA, Inc.) as recommended by the manufacturer. The complementation plasmid was confirmed by DNA sequencing (primer XAC0024_mcherry_F –
Supplementary Table S2) and used to transform the
X. citri Δ
envC strain to produce
X. citri Δ
envC pMAJIIc-
envC (X. citri Δ
envC amy:pMAJIIc-
envC).
2.4. Subcellular localization
2.4.1. Vector construction
For the construction of the plasmid pMAJIIc-
envC that enables the subcellular localization of proteins fused to mCherry fluorescent protein, the
X. citri envC gene (XAC0024) sequence was PCR amplified with the primers 0024_IF_F and 0024_IF_R (
Supplementary Table S2) using
X. citri 306 genomic DNA as a template, and ligated into the
XhoI site of pMAJIIc plasmid [
17] using the
In-Fusion HD Cloning Kit (Takara Bio USA, Inc.) as recommended by the manufacturer. The plasmid construction was confirmed by DNA sequencing (primers pGCD21-F and XAC0024_mcherry-F –
Supplementary Table S2) and used to transform electrocompetent
X. citri 306 strain cells to produce the
X. citri pMAJIIc-
envC (
X.citri amy::pMAJIIc-
envC) strain.
2.4.2. Fluorescence Microscopy
Starting cultures of
X.citri wt and
X. citri pMAJIIc-
envC were prepared by cultivating bacteria in 5.0 mL of NB medium for approximately 16 hours at 30℃ and 200 rpm. The cultures were then diluted to an OD 600 nm of 0.1 using fresh NB medium for a final volume of 5.0 mL and subsequently cultivated under the same conditions until an OD 600 nm of 0.3 was reached. At this point, arabinose was added to the medium to a final concentration of 0.05% and the cultures were kept at 30
oC and 200 rpm. After a minimum of two hours of induction, drops of 5 µL of cell cultures were placed on agarose-covered microscope slides for direct microscope observation [
21]. For chromosome visualization,
X.citri wt, Δ
envC and Δ
envC pMAJIIc-
envC cells were cultivated under the same conditions described above and stained with DAPI using the protocol described by [
22]. Bacteria were visualized using an Olympus BX61 microscope equipped with a monochromatic OrcaFlash2.8 camera (Hamamatsu, Japan) and TxRed and DAPI filters. Data collection and analysis were performed with the software CellSens Version 11 (Olympus). Statistical analyses were conducted using GraphPad Prism version 6.
2.5. Pathogenicity and bacterial viability analyses
Pathogenicity tests were conducted in triplicate using Rangpur lime (Citrus limonia) as the plant host. Bacterial cultures (X. citri wt, ΔenvC and ΔenvC pMAJIIc-envC) were adjusted to 108 CFU/mL (OD 600 nm of 0.3) using sterile 0.9% NaCl solution, and then inoculated on the abaxial surface of leaves using a needleless syringe. The negative control consisted of inoculating with sterile 0.9% NaCl solution. Inoculated plants were kept in a controlled environmental plant laboratory equipped with an HEPA filter to maintain air particle purity. The conditions were set at 28–30℃, 55% humidity, and a 12-hour light cycle and the plants were observed for up to 30 days to monitor the appearance of citrus canker symptoms. Photos were taken at the 3rd, 5th, 7th, 10th, 12th, and 15th DAI (days after inoculation).
2.6. Growth curves
X. citri wt, Δ
envC and Δ
envC pMAJIIc-
envC were initially cultivated in NB medium for 16 h at 30℃ and 200 rpm. For the
in vitro growth curves, cultures were subsequently diluted in fresh NB medium to an OD 600nm of 0.1 in a final volume of 1.5 mL (OD 600 nm of 0.3 corresponds to 10
8 CFU/mL). Cell cultures were then distributed in the wells of a 24-wells microtiter plate and incubated in a microtiter plate reader (Synergy H1N1; BioTek) at 30℃ with constant agitation (200 rpm) and the OD at 600 nm was measured every 30 min for 72 hours [
23]. For the
in planta growth curves, bacterial cultures of
X. citri wt and Δ
envC in NB medium were adjusted to 10
6 CFU/mL with ultrapure water and infiltrated into the abaxial surface of leaves of Rangpur lime (
Citrus limonia) using a needleless syringe. The inoculated plants were kept in the controlled environment plant laboratory described before. Samples for colony counting were obtained by collecting 1 cm
2 leaf discs from the inoculation point at 0, 1, 3, 6, 10, 15, 20 and 25 DAI, and the leaf discs were macerated in 1.0 mL of 1x PBS buffer (NaCl 8 g/L, KCl 0.2 g/L, Na
2HPO
4 1.44 g/L and KH
2PO
4 0.24 g/L, pH 7.4) in sterile 1.5 mL Eppendorf tubes with a plastic sterile pestle. After a serial dilution by factors of 10, ranging from 10
−1 to 10
−6, three aliquots of 50 µL for each dilution were plated on NB-agar medium plates for colony counting.
2.7. Phylogenetic analyses and protein modeling
A single gene alignment based on the XAC0024 gene sequence and the recovered sequences from different species of
Xanthomonas (
Table S3) were performed using ClustalW [
24]. For probabilistic analyses, the best evolutionary model was determined using jModelTest performed on the CIPRES resource [
25]. Maximum Likelihood analyses (ML) were conducted with RAxML version 8.0.24 [
26] and the branch support was assessed using bootstrap analysis [
27] with 1000 replicates. The cladogram was drawn using MEGA X software [
28].
For protein modeling, the 'Threading ASSEmbly Refinement' (I-TASSER) program [
29] was utilized to access and compare the 3-D structures of the proteins XAC0024 from
X. citri 306 and the protein EnvC from
E. coli. The protein sequence of XAC0024 was retrieved from the
Xanthomonas sp. database [
30], and the protein sequence of EnvC from
E. coli was retrieved from NCBI [
31].
3. Results
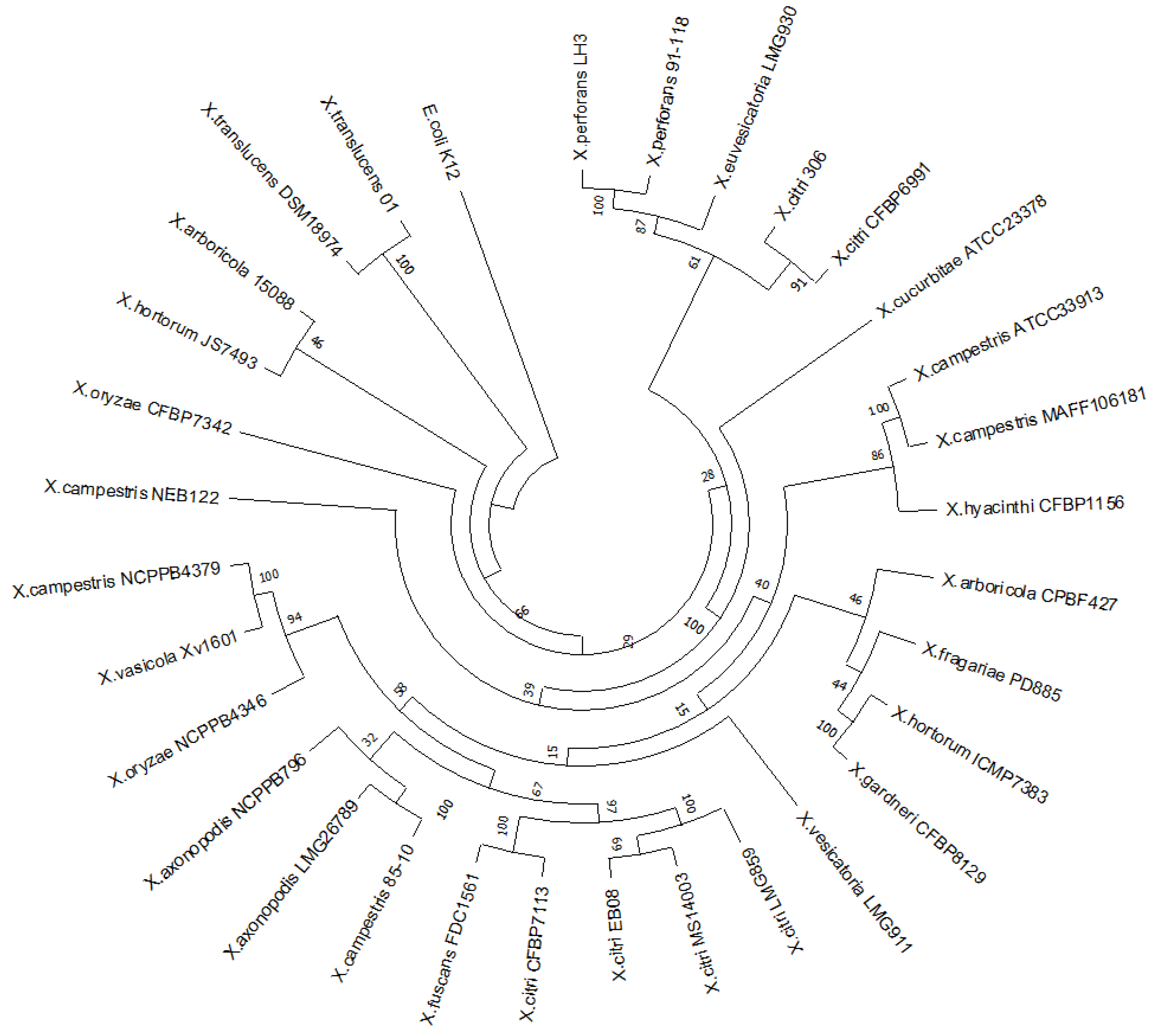
3.1. X. citri encodes an EnvC homolog that is conserved in many bacteria
The protein XAC0024 is highly conserved in other sequenced
Xanthomonas species, as shown by the Maximum Likelihood tree (
Figure 1). Although the single gene used in the reconstruction does not reflect the known phylogeny for the
Xanthomonas genus, based on the core genome alignment [
32], it was possible to recover some expected clusters. The
X. citri and
X. fuscans species showed a close relationship and, together with
X. axonopodis and
X. euvesicatoria (
X. campestris 85-10), formed the clade “
X. axonopodis” [
33]. The clade constituted by the species
X. arboricola,
X. fragariae, X. hortorum and
X. gardneri was consistent with the clade A topology in [
32], having
X. hortorum and
X. gardneri species forming a tight cluster [
34].
The nucleotide sequence comparison between XAC0024 from
X. citri 306 and the recently published homolog XCC0022 from
X. campestris pv.
campestris str. ATCC 33913, showed an identity of 85% (
Figure S2). Further analysis of the protein sequences revealed that XAC0024 and XCC0022 shared an identity of 89%, a coverage of 98%, and a similarity of 93% (
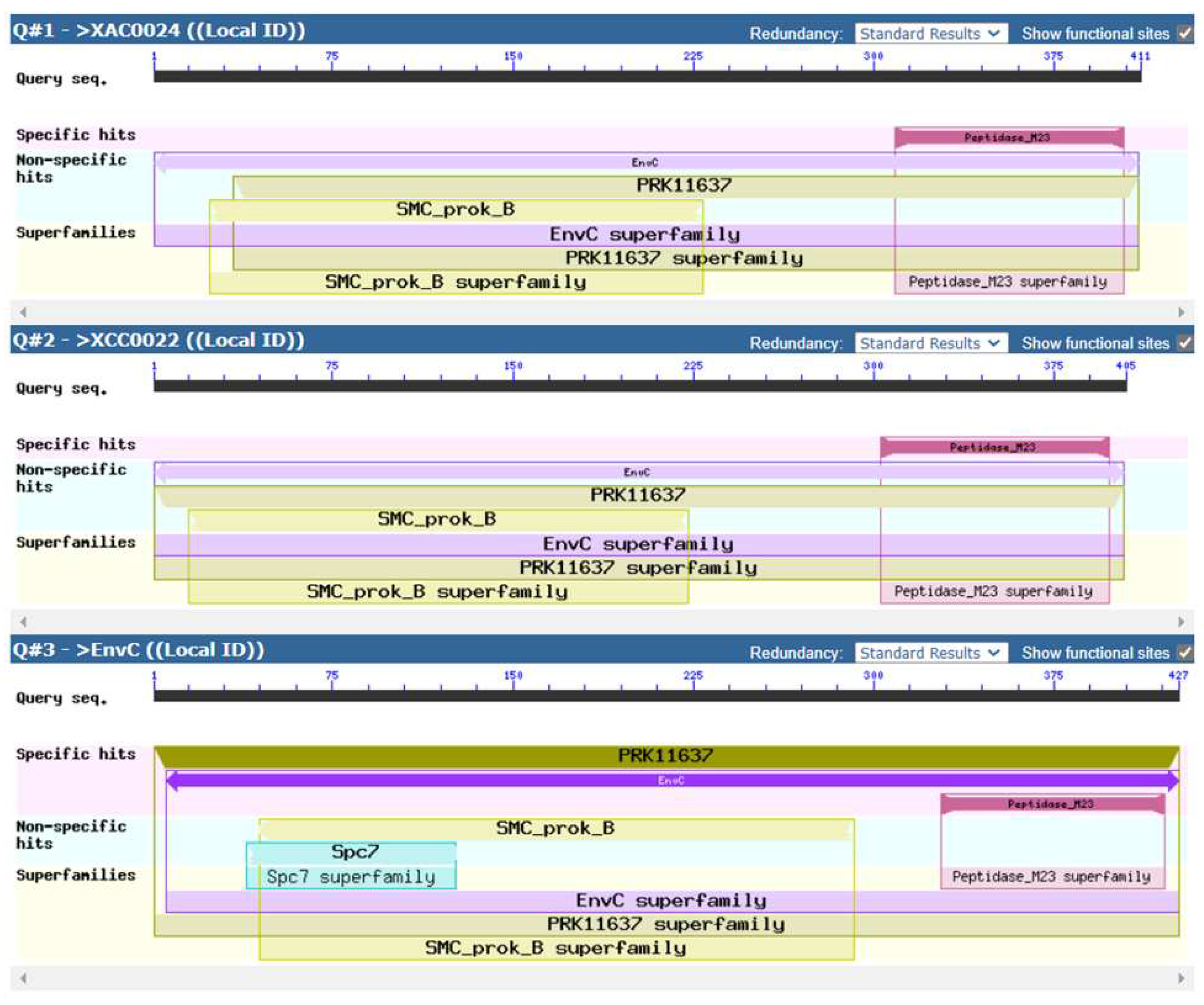
Figure S3). These findings indicate a considerable level of sequence conservation between the two proteins. To explore the shared conserved domains among these proteins, we utilized the NCBI Batch Web CD-Search tool.
Figure 2 displays the visualization of the identified conserved domains between the three protein sequences from
X. citri 306 (XAC0024),
X. campestris (XCC0022), and
E. coli (EnvC) (See
Supplementary Figure S4 for a detailed visualization of the nine ORFs of
X. citri sharing the M23 domain).
In order to reinforce the similarity between the
Xanthomonas strains sequences used for the phylogeny reconstruction, we utilized the NCBI Batch Web CD-Search tool, which showed the conserved domain peptidase M23 being shared by 16 representative
Xanthomonas species (
Supplementary Figure S5). This observation suggests a potential conserved function for this domain among homologs [
35].
As the XAC0024 protein's 3-D structure has not been determined and is unavailable in the Protein Data Bank (PDB), we employed its protein sequence as a query for generating a graphical 3-D molecular model using the I-TASSER server, which was then compared with the known structure of EnvC from
E. coli. The comparison between the EnvC protein from
E. coli and XAC0024 from
X. citri revealed 33% identity and a similarity of 52%, and almost identical 3-D structures (
Figure 3 and
Supplementary Figure S6). EnvC protein from
E. coli was shown to be slightly larger than its homolog XAC0024 from
X. citri.
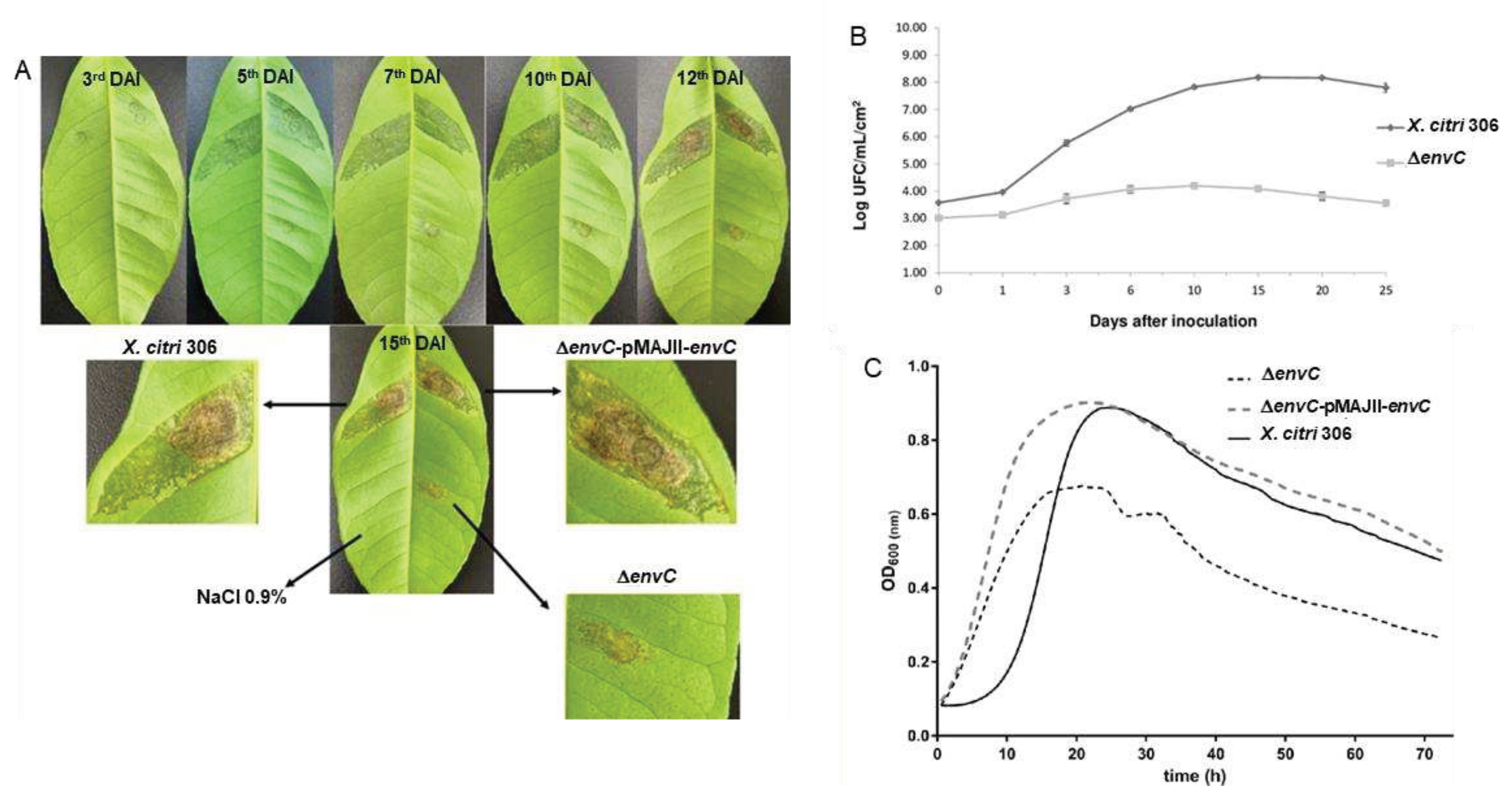
3.2. Disruption of envC affects X. citri virulence
To investigate the role of
envC, we produced an
envC deletion mutant (Δ
envC) and assessed its virulence and viability in comparison with the wild-type isolate
X. citri 306. These analyses involved assessing
in planta symptomatology and conducting both
in planta and
in vitro growth curves. To determine the contribution of EnvC to virulence, Rangpur lime leaves were inoculated with
X. citri wt, Δ
envC, and Δ
envC complemented strain (Δ
envC pMAJIIc
-envC) and monitored for 15 days for development of citrus canker symptoms. The chronological development of symptoms showed hypertrophy/hyperplasia, followed by water soaking, and brownish necrosis lesions at the late stage of infection, typical of citrus canker disease, in both
X. citri wt and Δ
envC pMAJIIc
-envC strains. However, the mutant Δ
envC exhibited a delay in the induction of citrus canker symptoms and produced less severe lesions. These appeared to be concentrated close to the point of inoculation (
Figure 4A).
Furthermore, we investigated the contribution of
envC to host colonization. The population of
X. citri wt reached its maximal level around 10 days after inoculation, increasing its population by approximately 5,000-fold (
Figure 4B). In contrast, the population of Δ
envC did not show significant change
in planta, increasing its population less than 10-fold (
Figure 4B). This indicates that Δ
envC was unable to multiply within the plant mesophyll. Subsequently, we examined whether
envC is essential for cell viability and multiplication by monitoring its growth in NB medium. The results revealed that Δ
envC displayed an altered growth pattern when compared to
X. citri wt (
Figure 4C). Initially, the mutant displayed an accelerated growth rate; however, Δ
envC reached a plateau at a significantly lower population than
X. citri wt and Δ
envC pMAJIIc
-envC strains. These results demonstrate that EnvC is required for the virulence of
X. citri and host colonization, and its disruption affects cell viability and multiplication.
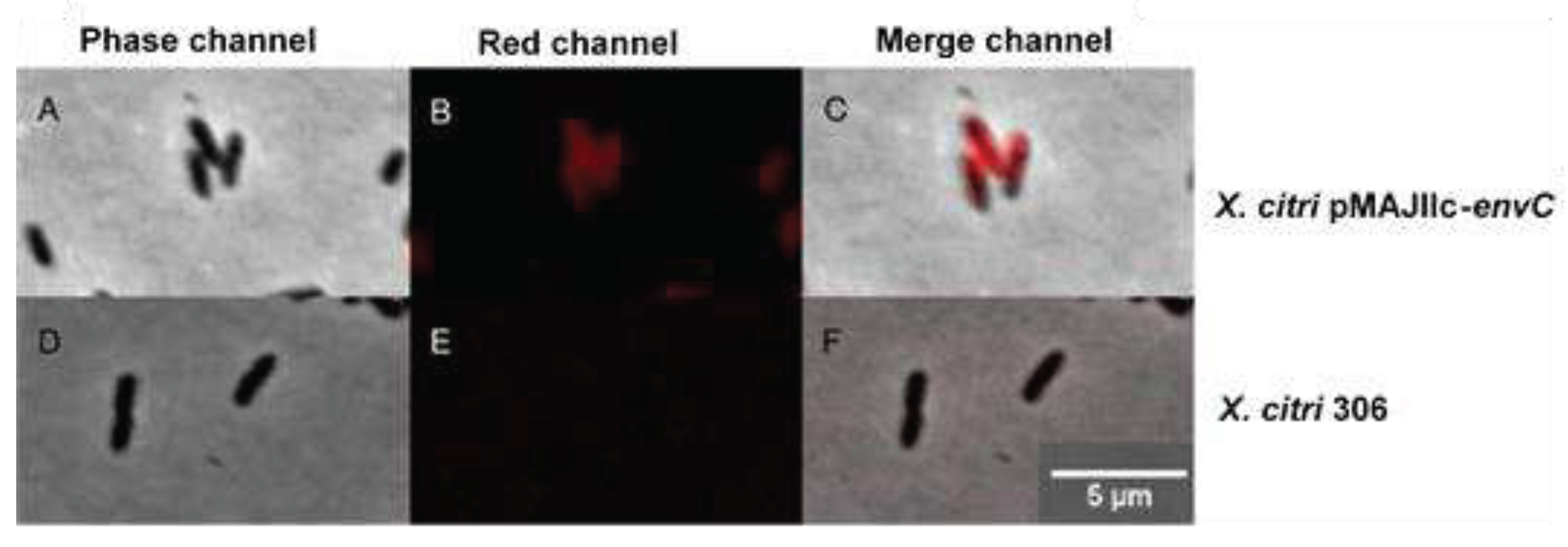
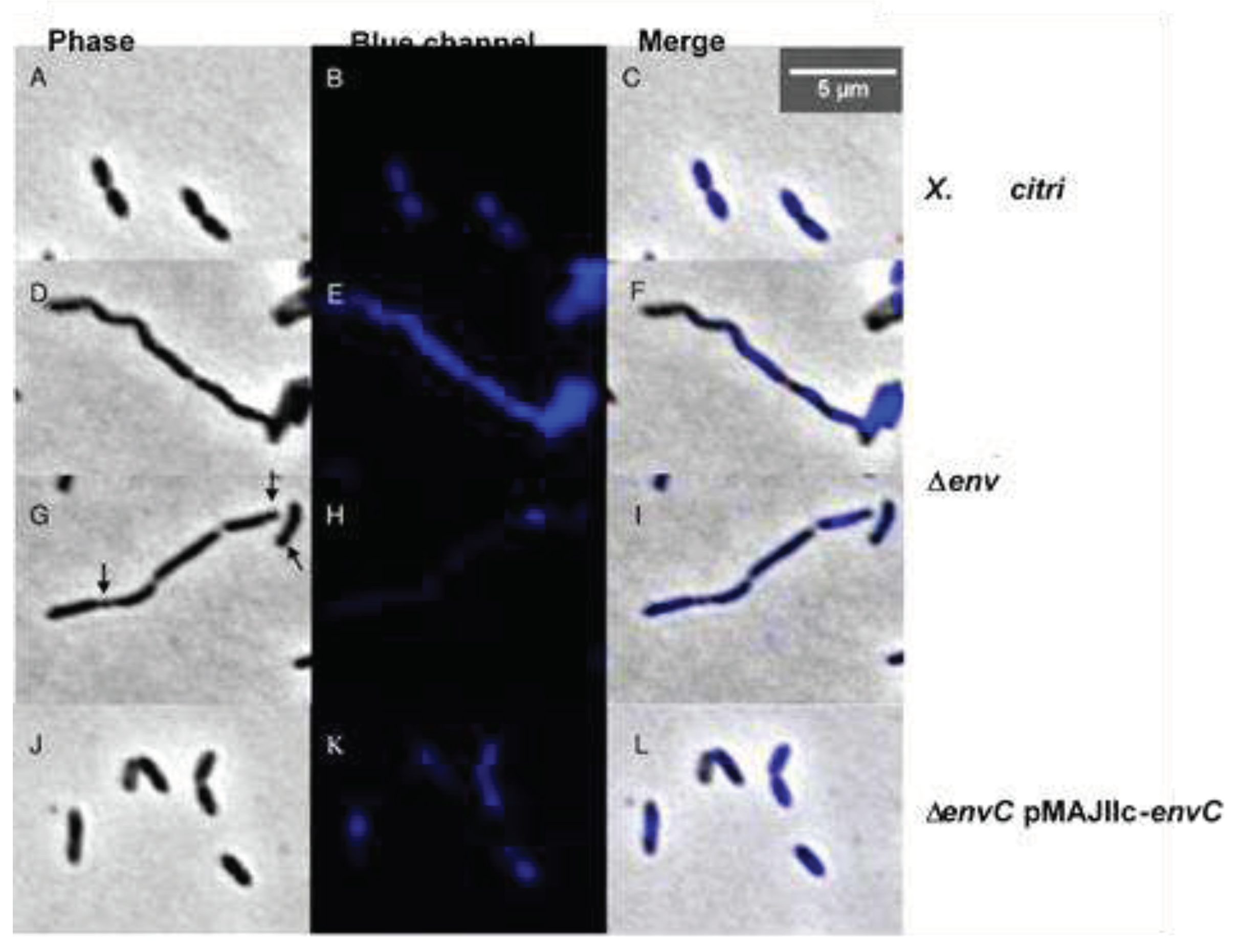
3.3. EnvC is required for X. citri daughter cell separation
To determine the subcellular localization of EnvC encoded by
X. citri, we expressed a version of the protein as an mCherry fusion (EnvC-mCherry) (
Figure 5).
X. citri EnvC-mCherry expressing cells (pMAJIIc-
envC) exhibited a strong fluorescence signal primarily concentrated around the edges of the cells, while the cytoplasm remained non-fluorescent (
Figure 5B,C). This phenotype suggests that
X. citri EnvC occupies the periplasmic region of the cells. Importantly, the wild-type
X. citri strain used as a control showed no fluorescence emission (
Figure 5E,F).
Next, we investigated the possible roles of EnvC in cell division and chromosome segregation by examining DAPI-stained
X. citri wild-type (wt), Δ
envC, and Δ
envC pMAJIIc-
envC cells (
Figure 6). The
X. citri Δ
envC cells exhibited abnormally shaped rods, somewhat curved (
Figure 6 D,F). Many cells were organized in long-chained structures, displaying clear division constrictions, which occasionally gave rise to minicells (
Figure 6G,I; arrows). Although Δ
envC mutants appeared competent in the initial stages of the division process, they exhibited evident and detectable late-division/separation defects.
For the cells that were not part of a chain, thus displaying an overall normal shape, we observed a significant difference in their average cell length compared to
X. citri wild-type and Δ
envC pMAJIIc
-envC strains (
Table 2). To quantitatively evaluate this, we measured 400 individual cells from each culture:
X. citri wild-type, Δ
envC, and Δ
envC pMAJIIc
-envC.
X. citri wild-type had an average cell length of 1.18 ± 0.22 µm, while Δ
envC pMAJIIc
-envC had an average cell length of 1.22 ± 0.29 µm. In contrast,
X. citri Δ
envC mutants exhibited an average cell length of 1.89 ± 0.38 µm. Additionally, we scored the percentages of observed abnormalities for
X. citri Δ
envC mutants (
Table 2), where filamented chains comprised approximately 55.5% of the cells, while minicells accounted for 5.25% (n= 400).
Chromosome organization was visualized using 4’,6-diamidino-2-phenylindole (DAPI)-staining (
Figure 6). Cultures of Δ
envC mutant exhibited a continuous distribution of chromosomal mass spanning through the elongated cells (
Figure 6 E,F,H,I). This continuous distribution in some cells possibly hindered septal closure. In contrast, both
X. citri and Δ
envC pMAJIIc
-envC strains displayed a bilobed chromosome organization pattern with the expected normal distribution, indicating successful complementation of the mutant (
Figure 6 B,C,K,L). (
Figure 6B,C,K,L). In all strains, a strong signal of nucleoid accumulation in the middle or pole of the cells could also be observed (
Figure 6B,H,K), consistent with previous observations in the
E. coli wt strain [
36] and
X. citri 306 wt strain [
17].
4. Discussion
The EnvC protein encoded by
Xanthomonas citri has a predicted M23 peptidase domain, which is part of a superfamily of metallopeptidase, characterized by the presence of zinc in its active enzyme site [
37]. This enzyme family includes the Nlpd from
E. coli, a LytM factor responsible, as well as EnvC, for activating N-acetylmuramoyl-L-alanine amidases (AmiA, AmiB and AmiC). These amidases play a crucial role in daughter cell separation, and the inactivation of the genes that encode them results in long cell chains formation [
38,
7,
8].
In this study, we demonstrated the
envC mutant of
Xanthomonas citri strain displayed a defect in cell separation, accompanied by distinct changes in cell morphology. Cultures of Δ
envC mutant exhibited elongated rods, long-chained cells and minicells, which indicated impaired daughter cell separation. These findings are consistent with results previously reported in
X. campestris strains lacking
nlpD,
envC or
amiC1 [
13], which also exhibited severe defects in daughter cell separation. The physiological roles of peptidoglycan hydrolases, including EnvC, are still not fully understood, as the loss of an individual enzyme has little effect on growth and division, suggesting functional overlap between numerous hydrolases [
2]. To shed light on EnvC's specific role, a collection of
E. coli mutants lacking individual LytM factors (EnvC, NlpD, YgeR and YebA), as well as all possible combinations of them, were previously analyzed [
7]. Among these mutants, only those with
envC deletion failed to separate normally, further confirming the critical role of EnvC in proper cell separation.
Our observations of chromosome segregation errors in the X. citri ΔenvC mutant suggest that they are linked to the delayed division induced by the absence of EnvC. Interestingly, complementation of the mutant with X. citri ΔenvC pMAJIIc-envC restored a normal phenotype, with no detectable morphological discrepancies compared to the wild-type strain. Although further investigation is warranted, these results indicate that the ΔenvC mutant indeed experiences a late-division/separation defect, possibly leading to longer cell compartments and allowing the chromosomal mass to span across.
The X. citri mutant lacking envC exhibited a delay in citrus canker symptomatology compared with the wt and complemented strains. This delay in symptom development demonstrated that the deletion of EnvC had a significant impact on the bacteria's virulence, as evidenced by its reduced ability to induce symptoms when inoculated into citrus leaves, which are susceptible hosts. The complemented strain, ΔenvC pMAJIIc-envC, fully restored virulence, confirming that the phenotype observed in the ΔenvC mutant was specifically due to the absence of EnvC and not a result of a polar mutation.
Interestingly,
Xanthomonas campestris strains lacking either
nlpD or
amiC1 almost completely lost its virulence, but the mutant lacking
envC showed levels of virulence compared to the wt strain [
13]. This indicates that the contribution of EnvC to virulence is species-specific and that EnvC may be directly or indirectly involved in specific virulence pathways that differ between
Xanthomonas strains. The species-specific role of EnvC in virulence highlights the complex and intricate nature of bacterial pathogenesis, where different strains of the same genus may employ distinct mechanisms to establish disease. Further investigations into the specific pathways influenced by EnvC in different
Xanthomonas strains could provide valuable insights into the molecular basis of pathogenicity and potential targets for disease control strategies.
According to the amino acid sequence analysis performed by SignalP 5.0 server [
39], EnvC from
Xanthomonas citri,
E. coli, and
Xanthomonas campestris possesses a signal peptide with specific cleavage sites (
Supplementary Figure S7). The cleavage site for EnvC in
Xanthomonas citri is between amino acids 20 and 21 (
Figure S7A), while for
E. coli, it is between amino acids 42 and 43, and for
Xanthomonas campestris, it is between amino acids 14 and 15 (
Figure S7B,C). The presence of the signal peptide, which can serve as a membrane anchor, suggests that EnvC proteins are likely to be transported to the periplasmic region, a common localization for proteins in many pathogenic bacteria, often facilitated by tat/sec systems in gram-negative bacteria [40, 41, 42, 43, 44, 45, 46, 47]. The localization pattern observed here for EnvC-mCherry fusion protein in
Xanthomonas citri and
E. coli [
7] are consistent with the fusion protein occupying the periplasmic region of the bacterium [
17]. This subcellular localization supports the notion that EnvC functions in the periplasm, where it may play a crucial role in late-division and daughter cell separation processes, as supported by similar findings in other bacterial models [6, 7, 12, 46].
Taken together, our results, as well as similar results from many other bacterial models, support the notion that EnvC has a function in late-division and daughter cell separation [6, 7, 12, 46]. However, the mechanism by which X. citri EnvC operates on daughter cell separation, considering its periplasmic location, and how and if this protein interacts with other hydrolases are intriguing questions that warrant further exploration and investigation.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Figure S1: Deletion of the central portion of gene XAC0024 from X. citri by double-joint PCR; Figure S2: Nucleotide sequence alignment between XAC0024 from Xanthomonas citri and its homologue XCC0022 from Xanthomonas campestris; Figure S3: Protein sequence alignment between XAC0024 from Xanthomonas citri and its homologue XCC0022 from Xanthomonas campestris; Figure S4: Domain multiple sequence alignment of the nine ORFs of X. citri sharing the M23 domain; Figure S5: Domain multiple sequence alignment of 16 representative species within the Xanthomonas species used in the Maximum Likelihood phylogeny; Figure S6: Protein sequence alignment between XAC0024 from Xanthomonas citri and EnvC from E. coli; Table S1: Proteins of Xanthomonas citri subsp. citri 306 strain sharing the Peptidase M23 Domain according to an in-silico search using the IMG ‘find function’ tool; Table S2: Primers used in this study; Table S3: ;
Author Contributions
Conceptualization, Michelle M. Pena, Henrique Ferreira and Jesus A. Ferro; Formal analysis, Michelle M. Pena, Thaisa Z. Martins and Doron Teper; Funding acquisition, Nian Wang, Maria Inês T. Ferro and Jesus A. Ferro; Investigation, Michelle M. Pena, Thaisa Z. Martins, Doron Teper, Caio Zamuner, Helen Penha and Henrique Ferreira; Methodology, Michelle M. Pena, Thaisa Z. Martins, Henrique Ferreira and Jesus A. Ferro; Project administration, Michelle M. Pena and Jesus A. Ferro; Resources, Nian Wang and Maria Inês T. Ferro; Supervision, Henrique Ferreira, Nian Wang and Jesus A. Ferro; Validation, Michelle M. Pena and Jesus A. Ferro; Visualization, Michelle M. Pena and Jesus A. Ferro; Writing – original draft, Michelle M. Pena; Writing – review & editing, Michelle M. Pena, Doron Teper, Henrique Ferreira, Nian Wang and Jesus A. Ferro All authors have read and agreed to the published version of the manuscript.
Funding
This work is part of the Ph.D. thesis of M. M. Pena and master’s dissertation of T. Z. Martins and was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code 001 and by a fellowship grant from Conselho Nacional de Desenvolvimento Científico e Tecnológico – Brasil (CNPq) to JAF (Grant No. 312089/2019-8). M.I.T.F. is a recipient of a CNPq productivity fellowship (XXXX).
Data Availability Statement
Not applicable.
Acknowledgments
We thank Citrus Research and Education Center from the University of Florida for allowed to use their microscope facilities. We also thank Naiara Zancanari for the support with analyzes and the Sequencing Facility of the Center for Biological Resources and Genomic Biology (CREBIO) from the University of São Paulo State (UNESP) at Jaboticabal Campus, Brazil.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- DAS, A.K. Citrus Canker - A review. J. Appl. Hortic. 2003, 5, 52–60. [Google Scholar] [CrossRef]
- Vollmer, W.; Blanot, D.; de Pedro, M. A. Peptidoglycan structure and architecture. FEMS Microbiol. Rev. 2008, 32, 149–167. [Google Scholar] [CrossRef] [PubMed]
- Silhavy, T.J.; Kahne, D.; Walker, S. The bacterial cell envelope. Cold Spring Harb. Perspect. Biol. 2010, 2, a000414. [Google Scholar] [CrossRef] [PubMed]
- Frirdich, E.; Gaynor, E.C. Peptidoglycan hydrolases, bacterial shape, and pathogenesis. Curr. Opin. Microbiol. 2013, 16, 767–778. [Google Scholar] [CrossRef] [PubMed]
- Firczuk, M.; Bochtler, M. Folds and activities of peptidoglycan amidases. FEMS Microbiol. Rev. 2007, 31, 676–691. [Google Scholar] [CrossRef] [PubMed]
- Bernhardt, T.G.; de Boer, P.A. The Escherichia coli amidase AmiC is a periplasmic septal ring component exported via the twin-arginine transport pathway. Mol. Microbiol. 2003, 5, 1171–1182. [Google Scholar] [CrossRef]
- Uehara, T.; Dinh, T.; Bernhardt, T.G. LytM-domain factors are required for daughter cell separation and rapid ampicillin-induced lysis in Escherichia coli. J. Bacteriol. 2009, 191, 5094–5107. [Google Scholar] [CrossRef]
- Uehara, T.; Parzych, K.R.; Dinh, T.; Bernhardt, T.G. Daughter cell separation is controlled by cytokinetic ring-activated cell wall hydrolysis. EMBO J. 2010, 29, 1412–1422. [Google Scholar] [CrossRef]
- Wu, C.; Al Mamun, A.A.M.; Luong, T.T.; Hu, B.; Gu, J.; Lee, J.H.; D'Amore, M.; Das, A.; Ton-That, H. Forward Genetic Dissection of Biofilm Development by Fusobacterium nucleatum: Novel Functions of Cell Division Proteins FtsX and EnvC. mBio, 2018, 9, e00360–18. [Google Scholar] [CrossRef]
- Möll, A.; Dörr, T.; Alvarez, L.; Chao, M.C.; Davis, B.M.; Cava, F.; Waldor, M.K. Cell separation in Vibrio cholerae is mediated by a single amidase whose action is modulated by two nonredundant activators. J. Bacteriol. 2014, 196, 3937–3948. [Google Scholar] [CrossRef] [PubMed]
- Warr, A.R.; Hubbard, T.P.; Munera, D.; Blondel, C.; Wiesch, P.A.; Abel., S.; Wang, X.; Davis, B.M.; Waldor, M.K. Transposon-insertion sequencing screens unveil requirements for EHEC growth and intestinal colonization. PLoS Pathog. 2019, 15, e1007652. [Google Scholar] [CrossRef]
- Yakhnina, A.A.; Mcmanus, H.R.; Bernhardt, T.G. The cell wall amidase AmiB is essential for Pseudomonas aeruginosa cell division, drug resistance and viability. Mol. Microbiol. 2015, 97, 957–973. [Google Scholar] [CrossRef]
- Yang, L.C.; Gan, Y.L.; Yang, L.Y.; Jiang, B.L.; Tang, J.L. Peptidoglycan hydrolysis mediated by the amidase AmiC and its LytM activator NlpD is critical for cell separation and virulence in the phytopathogen Xanthomonas campestris. Mol. Plant Pathol. 2018, 19, 1705–1718. [Google Scholar] [CrossRef]
- Sambrook, J.; Fritsch, E.R.; Maniatis, T. Molecular Cloning: A Laboratory Manual, 2nd ed.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 1989; Volume 1. [Google Scholar]
- da Silva, A.C.; Ferro, J.A.; Reinach, F.C.; Farah, C.S.; Furlan, L.R.; Quaggio, R.B.; Monteiro-Vitorello, C.B.; Van Sluys, M.A.; Almeida, N.F.; Alves, L.M.; et al. Comparison of the genomes of two Xanthomonas pathogens with differing host specificities. Nature 2002, 417, 459–463. [Google Scholar] [CrossRef]
- Huguet, E.; Hahn, K.; Wengelnik, K.; Bonas, U. hpaA mutants of Xanthomonas campestris pv. vesicatoria are affected in pathogenicity but retain the ability to induce host-specific hypersensitive reaction. Mol. Microbiol. 1998, 29, 1379–1390. [Google Scholar] [CrossRef]
- Pena, M.M.; Teper, D.; Ferreira, H.; Wang, N.; Sato, K.U.; Ferro, M.I.T.; Ferro, J.A. mCherry fusions enable the subcellular localization of periplasmic and cytoplasmic proteins in Xanthomonas sp. PLoS ONE 2020, 15, e0236185. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, H.-J.; Shin, M.-K.; Ryu, W.-S. Versatile PCR-mediated insertion or deletion mutagenesis. BioTechniques 2004, 36, 398–400. [Google Scholar] [CrossRef] [PubMed]
- do Amaral, A.M.; Toledo, C.P.; Baptista, J.C.; Machado, M.A. Transformation of Xanthomonas axonopodis pv. citri by electroporation. Fitopatol. Bras. 2005, 30, 292–294. [Google Scholar] [CrossRef]
- Kaniga, K.; Delor, I.; Cornelis, G.R. A wide-host-range suicide vector for improving reverse genetics in Gram-negative bacteria: inactivation of the blaA gene of Yersinia enterocolitica. Gene 1991, 109, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Martins, P.M.M.; Lau, I.F.; Bacci, Jr.M.; Belasque, J.; Do Amaral, A.M.; Taboga, S. R.; Ferreira, H. Subcellular localization of proteins labeled with GFP in Xanthomonas citri ssp. citri: targeting the division septum. FEMS Microbiol. Lett. 2010, 310, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Morão, L.G.; Polaquini, C.R.; Kopacz, M.; Torrezan, G.S.; Ayusso, G.M.; Dilarri, G.; Cavalca, L.B.; Zielinska, A.; Scheffers, D.-J.; Regasini, L.O.; Ferreira, H. A simplified curcumin targets the membrane of Bacillus subtilis. MicrobiologyOpen 2019, 8, e683. [Google Scholar] [CrossRef] [PubMed]
- LACERDA, L.A.; CAVALCA, L.B.; MARTINS, P.M.M.; GOVONE, J.S.; BACCI, JR.M.; FERREIRA, H. Protein depletion using the arabinose promoter in Xanthomonas citri subsp. citri. Plasmid 2017, 90, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, positions-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. Creating the CIPRES Science Gateway for Inference of Large Phylogenetic Trees. Proceedings of the Gateway Computing Environments Workshop 2010, 14, 1–8. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML Version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Felsenstein, J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- KUMAR, S.; STECHER, G.; LI, M.; KNYAZ, C.; TAMURA, K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Yang, J.; Yan, R.; Roy, A.; Xu, D.; Poisson, J.; Zhang, Y. The I-TASSER Suite: Protein structure and function prediction. Nat. Methods 2015, 12, 7–8. [Google Scholar] [CrossRef] [PubMed]
- The Xanthomonas sp. Portal – INRA. Available online: https://iant.toulouse.inra.fr/bacteria/annotation/cgi/xansp.cgi (accessed on 24 July 2023).
- The National Center for Biotechnology Information – NCBI. Available online: https://www.ncbi.nlm.nih.gov/search/all/?term=WP_053884339 (accessed on 24 July 2023).
- Timilsina, S.; Potnis, N.; Newberry, E.A.; et al. Xanthomonas diversity, virulence and plant–pathogen interactions. Nat. Rev. Microbiol. 2020, 18, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-R, L.M.; Grajales, A.; Arrieta-Ortiz, M.L.; Salazar, C.; Restrepo, S.; Bernal, A. Genomes-based phylogeny of the genus Xanthomonas. BMC Microbiol. 2012, 12, 43. [Google Scholar] [CrossRef] [PubMed]
- Timilsina, S.; Kara, S.; Jacques, M.A.; Potnis, N.; Minsavage, G.V.; Vallad, G.E.; Jones, J.B.; Fischer-Le Saux, M. Reclassification of Xanthomonas gardneri (ex Šutič 1957) Jones et al. 2006 as a later heterotypic synonym of Xanthomonas cynarae Trébaol et al. 2000 and description of X. cynarae pv. cynarae and X. cynarae pv. gardneri based on whole genome analyses. Int. J. Syst. Evol. Microbiol. 2019, 69, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Altenhoff, A.M.; Studer, R.A.; Robinson-Rechavi, M.; Dessimoz, C. Resolving the ortholog conjecture: orthologs tend to be weakly, but significantly, more similar in function than paralogs. PLoS Comput. Biol. 2012, 8, e1002514. [Google Scholar] [CrossRef]
- Freiesleben, U.V.; Krekling, M.A.; Hansen, F.G.; Lobner-Olesen, A. The eclipse period of Escherichia coli. The EMBO Journal 2000, 19, 6240–6248. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Lahti, J.M.; Air, G.M.; Burrows, P.D.; Cooper, M.D. Molecular cloning of the murine BP-1/6C3 antigen: a member of the zinc-dependent metallopeptidase family. Proc. Natl. Acad. Sci. U.S.A. 1990, 87, 993–997. [Google Scholar] [CrossRef]
- Heidrich, C.; Templin, M.F.; Ursinus, A.; Merdanovic, M.; Berger, J.; SCHWARZ, H.; de Pedro, M.A.; Holtje, J.V. Involvement of N-acetylmuramyl-L-alanine amidases in cell separation and antibiotic-induced autolysis of Escherichia coli. Mol. Microbiol. 2001, 41, 167–178. [Google Scholar] [CrossRef]
- Armenteros, J.J.A.; Tsirigos, K.D.; Sonderby, C.K.; Peterson, T.N.; Winther, O.; Brunak, S.; Heijne, G.V.; Nielsen, H. SignalP 5.0 improves signal peptide predictions using deep neural networks. Nat. Biotechnol. 2019, 37, 420–423. [Google Scholar] [CrossRef]
- Brunings, A.M.; Gabriel, D.W. Xanthomonas citri: breaking the surface. Mol. Plant Pathol. 2003, 4, 141–157. [Google Scholar] [CrossRef] [PubMed]
- Jha, G.; Rajeshwari, R.; Sonti, R.V. Bacterial Type Two Secretion System Secreted Proteins: Double-Edged Swords for Plant Pathogens. Mol. Plant Microbe Interact. 2005, 18, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Büttner, D.; Bonas, U. Regulation and secretion of Xanthomonas virulence factors. FEMS Microbiol. Rev. 2010, 34, 107–133. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.R.; Facincani, A.P.; Ferreira, R.M.; Moreira, L.M.; DE Oliveira, J.C. F.; Ferro, J.A.; Ferro, M.I.T.; Meneghini, R.; Gozzo, F.C. Proteome of the phytopathogen Xanthomonas citri subsp. citri: a global expression profile. Proteome Sci. 2010, 8, 55. [Google Scholar] [CrossRef] [PubMed]
- Ryan, R.P.; Vorhölter, F.J.; Potnis, N.; Jones, J.B.; Sluys, M.-A.V.; Bogdanove, A.J.; Dow, J.M. Pathogenomics of Xanthomonas: understanding bacterium–plant interactions. Nat. Rev. Microbiol. 2011, 9, 344–355. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Wang, N. The ColR/ColS two-component system plays multiple roles in the pathogenicity of the citrus canker pathogen Xanthomonas citri subsp. citri. J. Bacteriol. 2011, 193, 1590–1599. [Google Scholar] [CrossRef] [PubMed]
- Zimaro, T.; Thomas, L.; Marondedze, C.; Sgro, G.G.; Garofalo, C.G.; Ficarra, F.A.; Gehring, C.; Ottado, J.; Gottig, N. The type III protein secretion system contributes to Xanthomonas citri subsp. citri biofilm formation. BMC Microbiol. 2014, 14, 96. [Google Scholar] [CrossRef]
- Zhou, X.; Hu, X.; Li, J.; Wang, N. A Novel Periplasmic Protein, VrpA, Contributes to Efficient Protein Secretion by the Type III Secretion System in Xanthomonas spp. Mol. Plant Microbe Interact. 2015, 28, 143–153. [Google Scholar] [CrossRef]
Figure 1.
Maximum Likelihood phylogeny based on the nucleotide sequences of 31 strains representing the Xanthomonas genus.
Figure 1.
Maximum Likelihood phylogeny based on the nucleotide sequences of 31 strains representing the Xanthomonas genus.
Figure 2.
Domain multiple sequence alignment of XAC0024 from X. citri 306, XCC0022 from Xanthomonas campestris and EnvC from E. coli (NC_000913). The protein sequences were uploaded from the NCBI Batch Web CD-Search tool.
Figure 2.
Domain multiple sequence alignment of XAC0024 from X. citri 306, XCC0022 from Xanthomonas campestris and EnvC from E. coli (NC_000913). The protein sequences were uploaded from the NCBI Batch Web CD-Search tool.
Figure 3.
3-D molecular model of A: EnvC protein (X. citri) and B: EnvC protein (E. coli) based on the predicted structure performed by I-TASSER server.
Figure 3.
3-D molecular model of A: EnvC protein (X. citri) and B: EnvC protein (E. coli) based on the predicted structure performed by I-TASSER server.
Figure 4.
Pathogenicity assay and growth curves for X. citri 306, ΔenvC and ΔenvC - pMAJIIc-envC. A. Rangpur lime leaves were infiltrated with cells suspensions of the indicated X. citri strains. Pictures were taken on the 3rd, 5th, 7th, 10th, 12th and 15th days after inoculation (DAI). B: in planta growth curve. Leaves of Rangpur lime were infiltrated with cell suspensions of the indicated X. citri strains and bacterial populations were quantified at 0, 1, 3, 6, 10, 15, 20 and 25 DAI. C: in vitro growth curve. X. citri 306, ΔenvC and ΔenvC - pMAJIIc-envC were cultivated in NB medium and OD600nm reading were taken every 30 min for 72 hours. All the experiments were done in triplicate.
Figure 4.
Pathogenicity assay and growth curves for X. citri 306, ΔenvC and ΔenvC - pMAJIIc-envC. A. Rangpur lime leaves were infiltrated with cells suspensions of the indicated X. citri strains. Pictures were taken on the 3rd, 5th, 7th, 10th, 12th and 15th days after inoculation (DAI). B: in planta growth curve. Leaves of Rangpur lime were infiltrated with cell suspensions of the indicated X. citri strains and bacterial populations were quantified at 0, 1, 3, 6, 10, 15, 20 and 25 DAI. C: in vitro growth curve. X. citri 306, ΔenvC and ΔenvC - pMAJIIc-envC were cultivated in NB medium and OD600nm reading were taken every 30 min for 72 hours. All the experiments were done in triplicate.
Figure 5.
Subcellular localization of EnvC-mCherry in X. citri. X. citri strain expressing EnvC-mCherry fusions were cultivated until OD600nm of 0.3, and subsequently induced with 0.05% arabinose for 2 hours prior to microscope observation. Panels show the phase contrast (left), TxRed channels (middle) and the overlay, respectively for A-C: X. citri pMAJIIc-envC, D-F: X. citri 306. Magnification 100X; scale bar 5 µm.
Figure 5.
Subcellular localization of EnvC-mCherry in X. citri. X. citri strain expressing EnvC-mCherry fusions were cultivated until OD600nm of 0.3, and subsequently induced with 0.05% arabinose for 2 hours prior to microscope observation. Panels show the phase contrast (left), TxRed channels (middle) and the overlay, respectively for A-C: X. citri pMAJIIc-envC, D-F: X. citri 306. Magnification 100X; scale bar 5 µm.
Figure 6.
Cell morphology and nucleoid distribution analyses of X. citri 306, ΔenvC and ΔenvC pMAJIIc-envC strains. Panels show the phase contrast (left), DAPI channels (middle) and the overlay, respectively for A-C: X. citri 306, D-I: ΔenvC, H-L: ΔenvC - pMAJIIc-envC. Arrows indicates the minicell position. Magnification 100X; scale bar 5 µm.
Figure 6.
Cell morphology and nucleoid distribution analyses of X. citri 306, ΔenvC and ΔenvC pMAJIIc-envC strains. Panels show the phase contrast (left), DAPI channels (middle) and the overlay, respectively for A-C: X. citri 306, D-I: ΔenvC, H-L: ΔenvC - pMAJIIc-envC. Arrows indicates the minicell position. Magnification 100X; scale bar 5 µm.
Table 1.
List of strains and plasmids used in this work.
Table 1.
List of strains and plasmids used in this work.
| Strains |
Characteristics |
References |
|
X. citri 306 |
Xanthomonas citri subsp. citri strain 306 (wild-type strain) |
IBSBF 1594; [15] |
| ΔenvC
|
X. citri envC deletion mutant(deletion of genomic bases 26156 to 26748) |
This work |
|
E. coli DH10B |
Cloning strain |
Invitrogen, Waltham, MA, USA |
|
E. coli SM10ʎpir |
Cloning strain |
Laboratory Stock |
|
E. coli HST08 |
Cloning strain |
Takara Bio USA, Inc. Mountain View, CA, USA |
| Plasmids |
Characteristics |
References |
| pGEM® -T easy |
Cloning vector; ApR
|
Promega |
| pOK1 |
sacB-sacR; SpR
|
[16] |
| pMAJIIc |
Derivative of pGCD21; mCherry expression vector; ApR; NeoR/KmR; araC-para; integrative vector in X. citri; |
[17] (GenBank MT119765) |
| |
|
|
Table 2.
Morphological analysis of X. citri strains according to cell length.
Table 2.
Morphological analysis of X. citri strains according to cell length.
| |
Cell length µm |
Filaments % |
Minicells % |
|
X. citri 306 |
1.18 ± 0.22a
|
0 |
0 |
| ΔenvC
|
1.89 ± 0.38b
|
55.5 |
5.25 |
| ΔenvC pMAJIIc-envC
|
1.22 ± 0.29a
|
0 |
0 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).