1. Introduction
Cancer is one of the leading causes of death in the world, as shown by the 2022 cancer statistics, predicting 1,918,030 new cases of cancer and 609,360 related deaths per year [
1].
The classic therapeutic options when approaching a cancer patient are chemotherapy, radiotherapy and surgery. The choice of approach depends on several characteristics, such as the cancer stage and location or patient’s fitness, which is compromised by the disease itself, worsening with each treatment intervention, that frequently persist in the long term [
2]. These treatments can reduce cancer recurrence and mortality, but have important side effects, that can lead to severe complications and to the risk of death from other diseases [
3].
Radiotherapy has been for a long time an extremely important tool against cancer, offering a possible cure, symptoms relief and extended survival. Nonetheless, it is linked to important side effects. When a patient is exposed to radiotherapy, not only the tumoral cells are targeted, but also the normal tissue around the tumor is also damaged [
4].
As for chemotherapy, many pharmacological classes can be used in the treatment of cancer, all with potential side effects such as autoimmune-like disorders and fatal adverse events caused by the reactivation of cellular immunity [
5].
There have been great efforts in diverse scientific fields to limit the above mentioned problems, by exploring alternatives which prevent the toxicity and side effects associated with the conventional therapies when approaching a patient with cancer. Overall, most of these new approaches are still object of intense research, such as the exploitation of surface modified inorganic nanoparticles to fight cancer [
6,
7,
8].
However, they have proved to have substantial limitations. The main disadvantages of chemotherapy and radiotherapy have been the lack of specificity, which causes the drug delivery in the targeted site at inadequate concentrations, and also high toxicity to the healthy and surrounding cells, tissues, and organs, leading to the development of drug resistance during the treatment [
9]. The scientific community leans on the use of nanotechnology, as a strategy with great potential to overcome these challenges [
10], specifically by enhancing the drug delivery into target sites, increasing efficacy and reducing side effects [
11]. In this regard, the huge specific surface areas conferred by nanoparticles give them useful properties, such as the ability for biofunctionalization and a huge interface to mediate processes involving the nanoparticles and the surrounding tissues [
12]. Nowadays, a variety of products involving the synthesis of nanoparticles or its use are in development and nanomedicine is becoming an attractive field for research thanks to its potential efficacy and requirement of small amounts with better utility for drugs that are rare or expensive [
13]. Therefore, the use of nanoparticles in this context might also contribute to enhance, stimulate or improve the effectiveness of drug treatment at more affordable costs [
14,
15].
Herein, we briefly review the application of fine divided systems termed nanoparticles, which are characterized by dimensions typically between 1-100 nm and show properties strongly dependent on size and surface [
16,
17]. As such, non-conventional particulate systems that have been explored for cancer therapies are mostly surface functionalized inorganic nanomaterials typically obtained as stable colloidal nanoparticles [
6,
18,
19]. However, other types of nanoparticles have been investigated for a longer time in the context of cancer therapies, such as diverse polymeric nanoparticles and liposomes; the latter are well-known drug carriers [
20,
21].
Considering the diverse types of colloidal nanoparticles available, their systematic classification is challenging, and several attempts have been implemented. Hence, nanoparticles can be classified according to their shape, average size, chemical nature, and preparation method, among other criteria [
22,
23]. The classification of nanomaterials proposed by Miernicki et al. goes further in the parametric assessment by considering also their applicability and safety, besides the morphological characteristics [
24]. A number of applications mediated by surface phenomena take advantage of the high ratios of surface area per volume that characterize nanoparticles. For example, specific surface area and surface functionalization are important aspects to take into consideration in the application of nanoparticles for drug delivery. An increased surface area available implies an increased amount of anti-cancer agents that can be attached, making them more efficient candidates for drug-delivery vectors [
9]. Due to their nanometric size, nanoparticles are able to cross pores which contributes to more effective treatments for neurological conditions and brain cancer due to their ability to go through the blood-brain barrier [
25,
26].
According to Fraga et al. [
27], there are many statements in favor of nanosized therapeutic development and one of them is the nanoparticle’s ability to overcome solubility and stability problems of anticancer drugs. Since the bioavailability is restricted by water solubility and it hampers the development of early agents of drugs, the delivery and consumption of a poorly soluble drug can be increased by an encapsulation of the compound within a hydrophilic nanocarrier [
13]. Another way of protecting anticancer compounds from excretion or decomposition requires the encapsulation of antineoplastic agents in nanocarriers or pairing perishable compounds with synthetic ones [
13]. Also, nanotechnology can improve drug penetration and redirection, or selectively redirect compounds to cancer cells through its physicochemical properties. For redirection of antitumor drugs, active and passive targeting schemes are used.
Additionally, nanocarriers are made to expel their cargo at the beginning, so it results in a stimuli-sensitive nanomedicine treatment. For example, a medicine that is pH-independent, like doxorubicin, can be catenated to pH-sensitive nanoparticles to increase the cellular uptake and intracellular release of the medicine [
28]. Eventually, the endurance of tumors is attenuated against antitumor medicines through guided nanomedicine treatments. Generally, non-specificity is decreased by targeted input and multidrug resistance/adenosine triphosphate (MDR/ATP) outflow pump-driven excretion. Therefore, the circulation time of a drug can be held by nanomedicine, helping the release of stimuli-responsive medicines to intervene endocytic input of the drug [
13].
2. Methodology
This article was written based on a bibliographic survey that was performed by consulting relevant online scientific communication platforms. A search was made on those platforms to find the major contents related to the theme of this review: the advantages and disadvantages of using nanotechnology in cancer treatments, the nanoparticle’s toxicity, the innovations in the treatment of cancer, and the concerns about unknown potential long-term effects.
The main keywords used to make the bibliographic research were: “nanotechnology”, “cancer treatment”, “drug delivery”, “nanoparticles”, and “nanomedicine”.
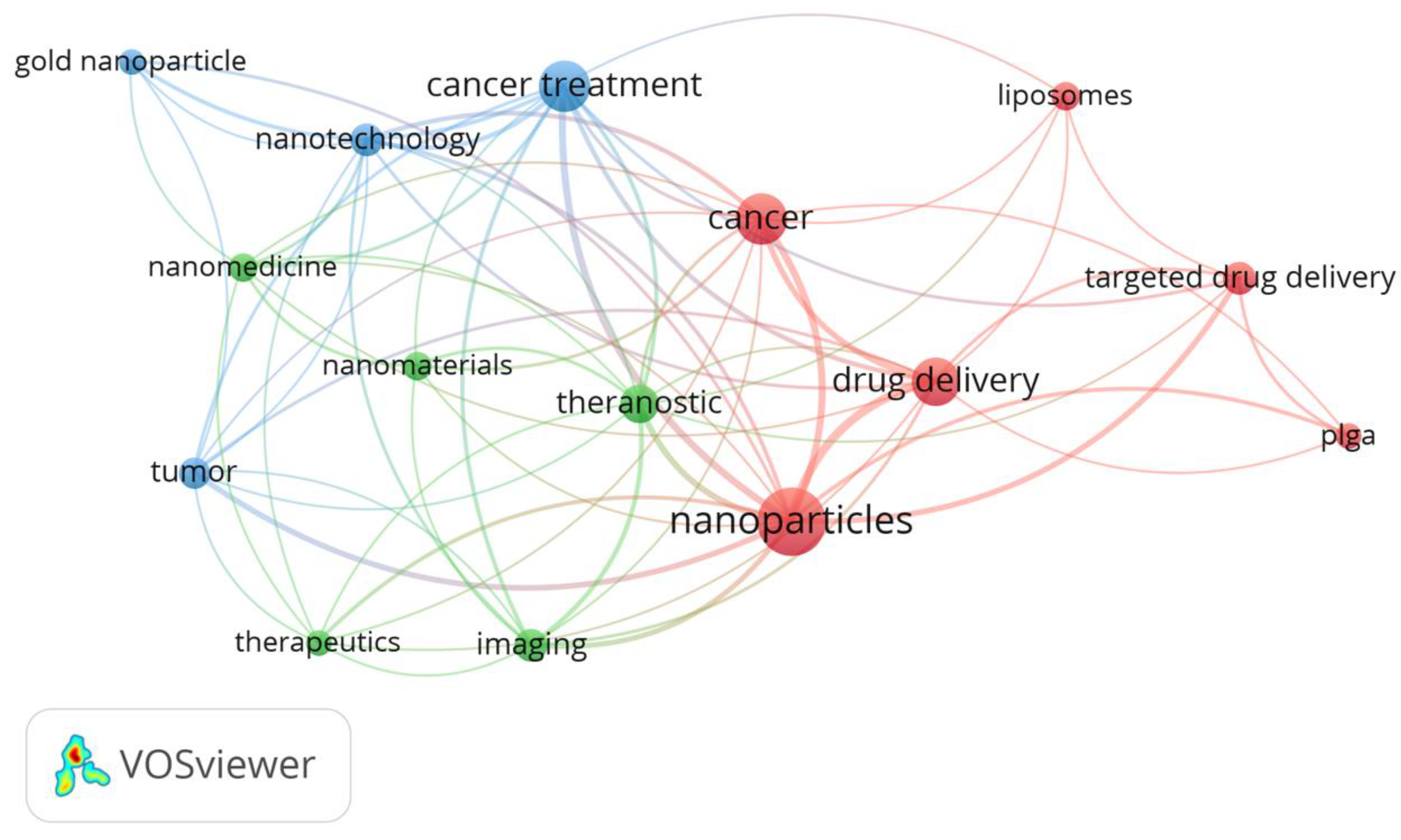
Figure 1 represents a cloud obtained from the keywords that appeared in all the sources that were used in this research, and which occurred at least five times in different articles. It can be observed that the most frequent keywords used were nanoparticles, cancer, cancer treatment, and drug delivery, which is in accordance with the main subject of this review.
The analysis with the VOSviewer software selected 15 keywords, grouped in three clusters with 66 links and a total link strength of 160. In
Table 1 the clusters are shown and the score for each one is calculated as the average publication year of the documents in which a keyword or a term occurs.
The literature review focused on the critical analysis and presentation of information related to the application of nanotechnology in cancer treatments, from diagnosis and imaging to the mechanisms of drug delivery. Furthermore, selected bibliography was also considered for supporting aspects that have been reviewed in the above context.
3. Tumor microenvironment and vasculature
It is known that the tumor microenvironment acts as a barrier to avoid drug delivery due to its poor vasculature, high interstitial fluid pressure, and dense extracellular matrix. Therefore, it is important to understand the tumor’s structure and specific aspects to reach an efficient drug delivery capable to fight it [
29].
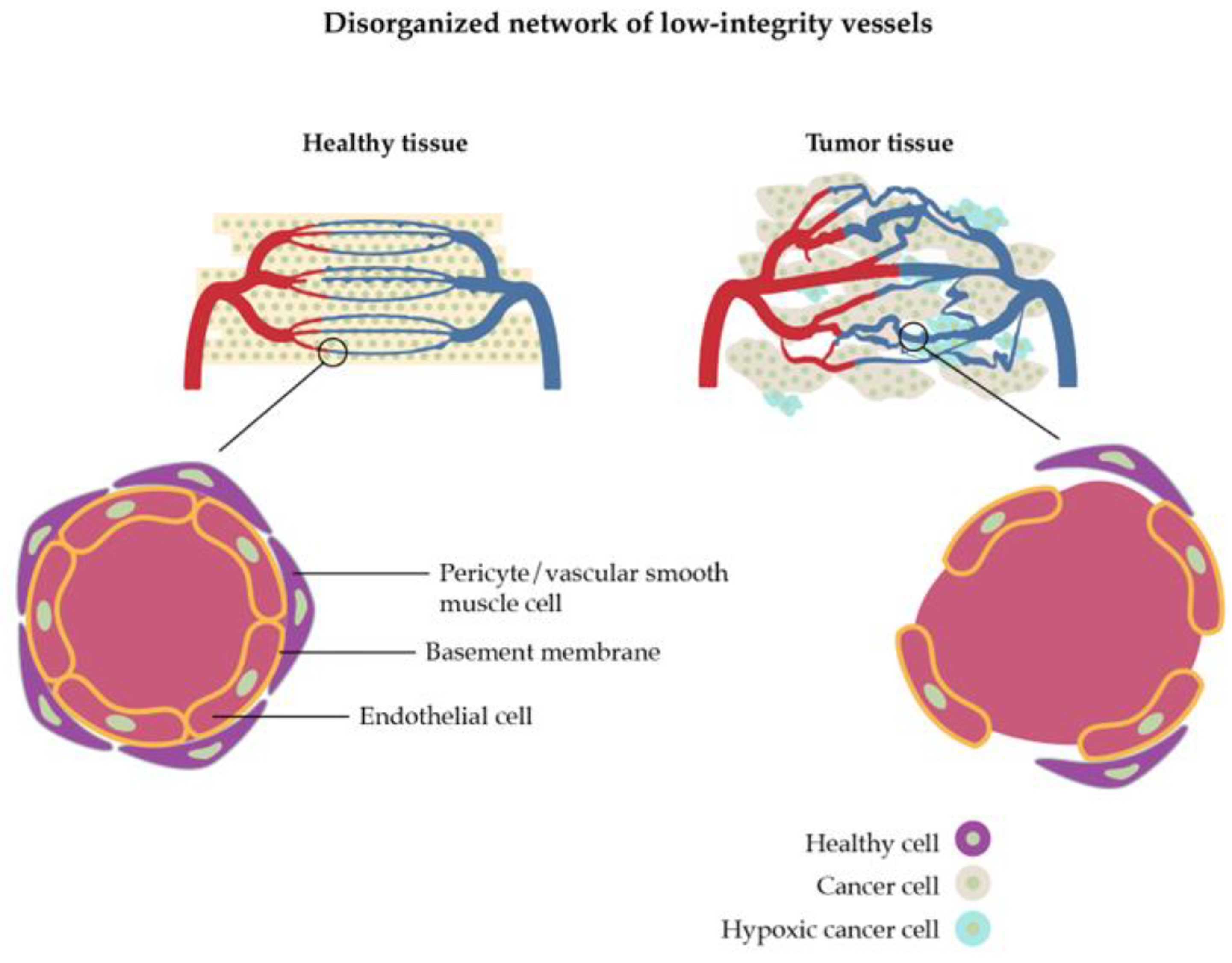
The vasculature of a tumor is characterized by extra production of angiogenic factors which means that new blood vessels originate from already-existing blood vessel structures, resulting in convoluted and leaky vessels (
Figure 2) [
30]. This process can come as an advantage but also shows limitations for nanoparticle drug delivery. The growth of new vascular vessels increases the enhanced permeability and retention (EPR) effect which allows nanoparticles to be discharged from vessels and accumulate inside the tumor [
31]. Nevertheless, it can also happen with blood components, blocking the overflow of nanoparticles. Furthermore, some areas inside the tumor have a lack of perfusion which creates an acidic and hypoxic environment leading to the advancement of the tumor, increasing its resistance, and making the drug delivery more difficult [
29,
32].
The high interstitial fluid pressure, which is caused by abnormal blood flow and impaired venous and lymphatic drainage [
34], is the reason why the extracellular matrix of a tumor is so dense. Like vasculature, it can increase the EPR effect of nanoparticles, but it can also result in the limitation of fluid transfer, blocking its penetration through the tumor. Solid stress, which means the disorderly proliferation of tumor cells, is another reason that difficult the drug delivery: it weakens the immune response as it expands the cancer cells’ invasion [
29].
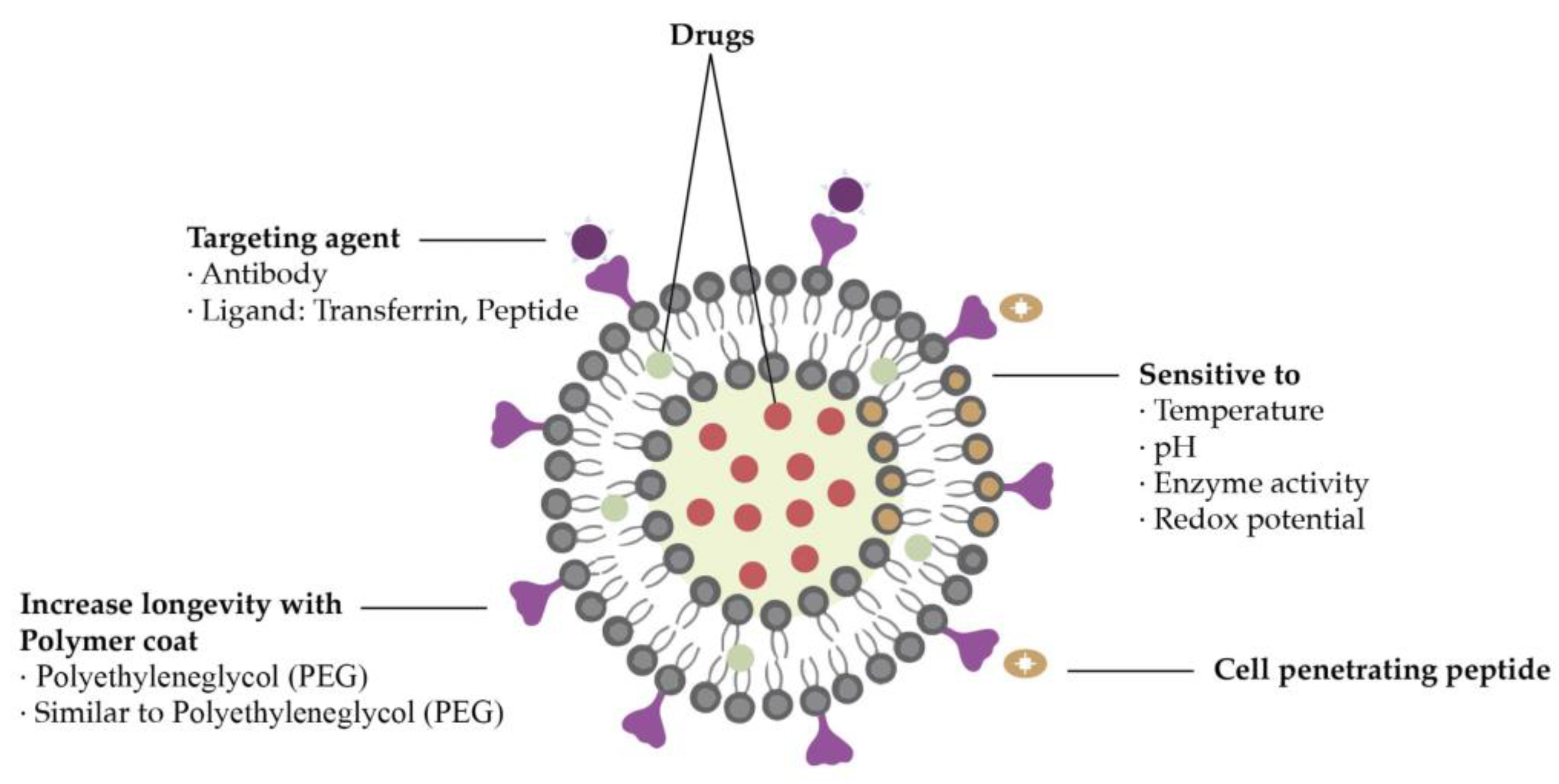
4. General requirements for nanoparticles in drug delivery
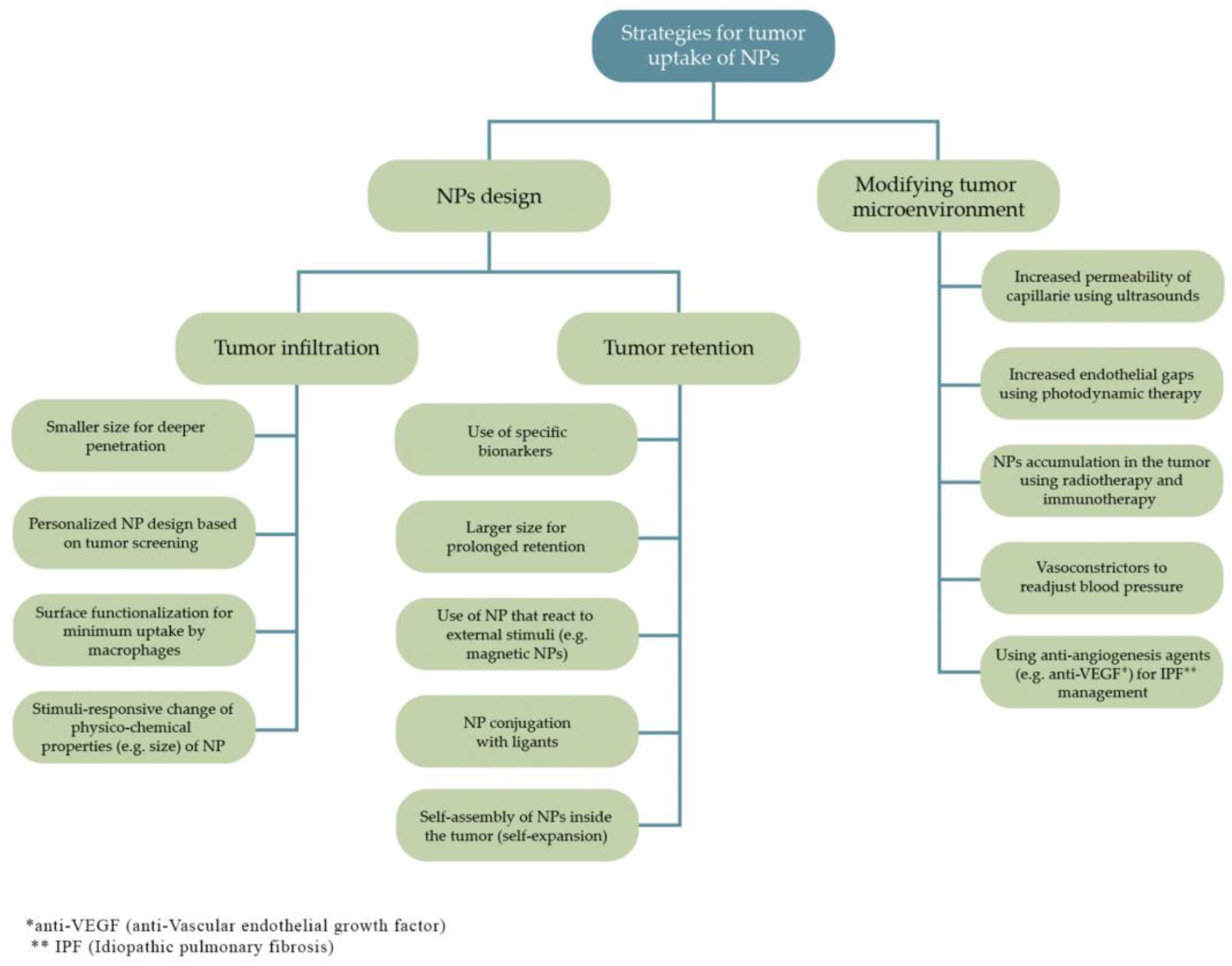
Nanoparticles must have some properties to attain an effective system for cancer treatment such as being biocompatible, of high bioavailability, and stable under physiological conditions. Furthermore, they must be able to target only the tumor cells without deteriorating surrounding healthy cells and need to release the load as soon as they reach the target site. All these features can be affected by the physicochemical properties of the nanoparticles employed as drug delivery vectors (
Figure 3) [
35,
36].
The nanoparticle’s size distribution has a major effect on their performance in cancer therapies. Due to the tumor’s leaky vasculature, the size of nanoparticles can be adapted to be small enough to penetrate the tumor and big enough to prevent extravasation from normal blood vessels, preventing agglomeration in unwished parts of the body [
36]. Nevertheless, different organs have different size uptake specifications. Therefore, many types of research have been made to define the adequate size of nanoparticles used in cancer therapy, showing that smaller-sized particles (< 50 nm) present better anti-tumoral efficiency rates than larger-sized particles [
38].
Another important feature when it comes to nanoparticle design for cancer treatment is its shape, since it influences fluid dynamics, among other effects [
31]. The shape of a nanocarrier can control the interaction between cell membrane and nanoparticles. It is also noted that the particle’s shape influences whether the nanoparticles are taken up by the reticuloendothelial system [
31].
Surface chemistry comprises a rather complex set of processes that includes namely surface charge, porosity, defects and chemical groups alterations, among other aspects. Numerous system features, for example, surface interactions, degradation and agglomeration rates, and cellular uptake are influenced by surface chemistry. Some studies imply for instance that a positively charged surface raises the chances of cellular uptake [
35,
39]. However, different types of cancer or different stages of the same cancer type can require different surface properties [
31], as shown in
Figure 4.
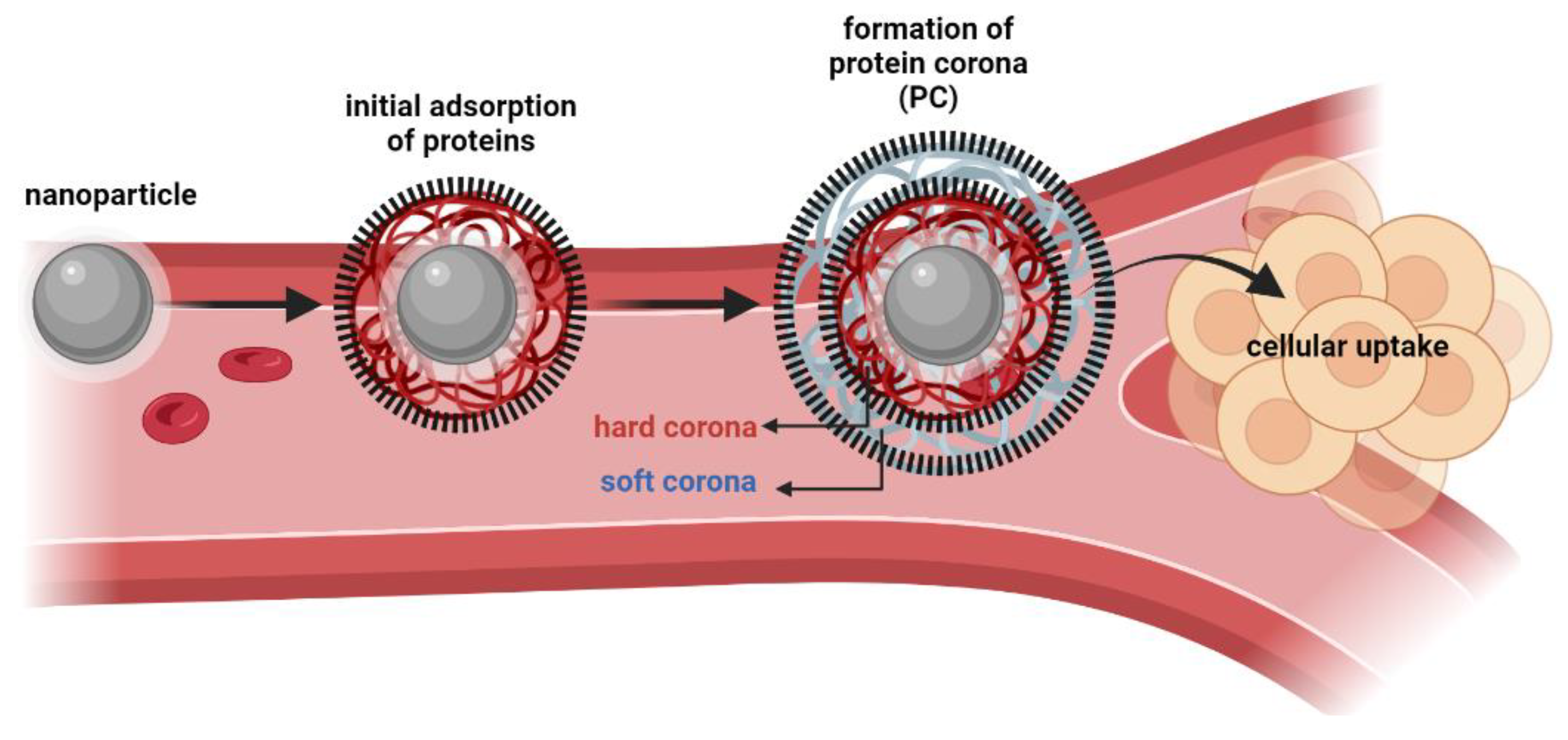
In biological environments, the surface of a nanoparticle is rapidly coated with a layer of biomolecules, which due to its high protein content is commonly known as “protein corona” (PC), [
40,
41,
42,
43]. The characteristics of PC (as molecular properties and composition) play a key role in governing the cellular uptake, biocompatibility, distribution, and circulation lifetime of nanoparticles [
40,
44]. Two of the major factors influencing protein adsorption are the structural stability of proteins (namely their conformational changes) and the hydrophilic/hydrophobic properties of the surface of nanoparticles. With the dynamic behavior of proteins in physiological media, the layers of PC could be divided into hard corona (inner layer, with tightly bound proteins) and soft corona (weakly bound proteins, rapidly exchanged with free proteins from the media), as illustrated in
Figure 5 [
45]. Several techniques have been employed to separate, identify and quantify the composition of PC’s layers, such as dialysis, centrifugation, gel filtration, size-exclusion chromatography and high-pressure liquid chromatography (HPLC), coupled to spectroscopic methods and bioinformatics predictions [
46,
47]. An in-depth understanding of the corona-mediated functionalities can be explored for the development of anticancer strategies. For instance, five distinct types of human cancers (lung, glioblastoma, meningioma, myeloma and pancreatic cancers) have already been identified and discriminated by Caracciolo et al. by using liposome-based nanoparticles with three distinct surface properties, the authors developed a successful detection platform based on a PC sensor array [
48]. Still, it is worth mention that PC might also negatively affect the delivery fate of nanoparticles for tumor targeting, particularly if their main component are dysopsonins [
49] or if the components of PC promote unfavorable steric effect that hamper the interactions with cell membranes (and consequently, decrease cellular uptake) [
49,
50,
51,
52]. As so, due to the high complexity and heterogeneity of PC, future research is required in this field to allow the development of safe and effective nanoparticle-based applications.
5. Nanoparticles in cancer therapies and clinical diagnosis
Recently, many accomplishments have been achieved in the field of nanomedicine regarding drug delivery systems. Among them, an abundant number of nanoparticle types have been developed to be used in cancer therapy due to their unique properties [
53].
A nanovector is generally defined as a functionalized nanoparticle that can carry and deliver anticancer drugs or detection agents. Nanovectors have been classified into three different classes: first-, second- and third-generation systems (
Figure 6) [
10].
As an example of a first-generation nanovector, there is albumin-bound paclitaxel [
54]. Paclitaxel can be used in breast cancer treatments and its solubility problem is solved by using Cremophor EL. However, first-generation nanovectors are not able to target any specific biomolecule in a tumor cell [
10].
The second-generation is an evolution of first-generation nanovectors and these are able to target a specific biomolecule in a tumor cell, which means it has the active targeting capability. Examples of nanovectors from this generation are the antibody-targeted nanoparticles [
54], such as mAb-conjugated liposomes [
10]. The nanovectors of the second-generation have an improved biodistribution and present a reduced toxicity level when compared to the first-generation [
10].
The third-generation nanovectors, such as the nanoshuttle, are multistage agents and can handle more complex functions [
54]. According to Chatterjeee and Kumar, this generation represents the next generation of the first wave nanotherapeutics that are specially equipped to introduce biological barriers to improve the drug delivery to the tumor site [
10].
The sections below provide a summary of important nanoparticles that have been used in drug delivery and other clinical applications for fighting cancer. There are no attempts to provide a detailed description of the selected nanoparticles but rather an indication of their potential in the context approached in this review.
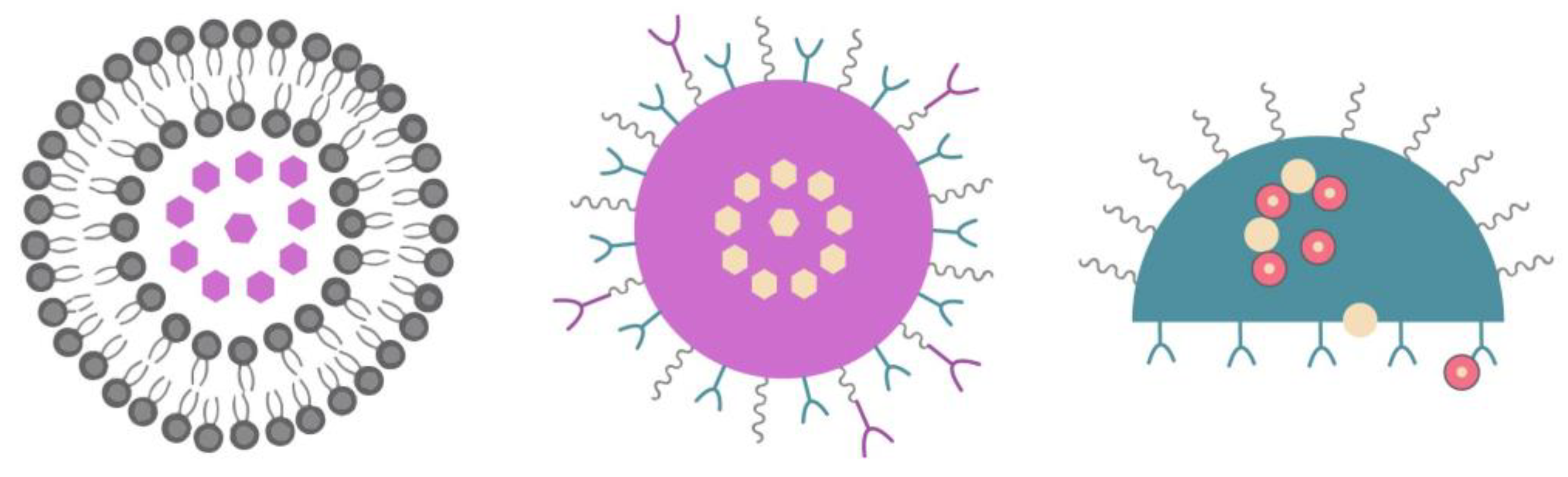
5.1. Liposomes
Liposomes, which are made up of nontoxic and biocompatible lipid bilayers, can act as pharmaceutical carriers [
20]. Their core is aqueous, its head is hydrophilic, and the tails are hydrophobic which means they are being oriented away from the intercellular fluid. The conventional nanoparticle size is up to 100 nm and liposomes fluctuate between 90 and 150 nm. Liposomes are used to deliver the drug to the outer membrane of targeting tumor cells and meanwhile the fatty layer protects the enclosed drug [
55]. This mechanism can decrease the effect of drug toxicity on healthy cells and increase the efficacy [
10].
Liposomes can be synthesized from cholesterol and phospholipids and they have one particular property, which is the amphipathic nature, that enables them to bind to both hydrophilic and hydrophobic compounds [
31]. In other words, they can encapsulate water-soluble drugs in their core and nonpolar compounds in their bilayer membrane simultaneously [
31]. Liposomes have other advantageous properties, like biocompatibility and biodegradability, and they do not present toxicity or immunogenicity [
56,
57]. Moreover, Food and Drug Administration (FDA) has already approved drug delivery systems based on liposomes like MyocetTM [
58].
Zhang et al. developed a lyophilized system based on liposomes and paclitaxel applicable for cancer therapy [
14]. It has an encapsulation efficiency of over 90% and physical and chemical stability for 12 months while the particles have about 150 nm average size. When the system was diluted, the size remained the same, and the drug was encapsulated.
Zhao et al. focused on a pH-responsive liposome-containing system for glioma tumor cells [
59]. As the system is made up of a tumor-specific pH-sensitive peptide and liposomes, it responds to the acidic pH of gliomas and releases the drug. The same occurs when doxorubicin is used.
Theragnostic systems based on liposomes have been studied to be used in imaging and drug delivery, as shown in
Figure 7 [
10]. Ren et al. designed a system in which a pharmaceutically active component was encapsulated and its biodistribution was imaged in real-time by magnetic resonance imaging (MRI) [
60]. The system was compared to a commercially available MRI contrast agent called Omniscan® and the first showed not only better results but a longer circulation time
in vivo. Furthermore, liposomes enable both polar and nonpolar chemotherapeutic drugs entrapment providing synergetic therapy with sustained release and substantially lower toxicity.
5.2. Polymeric nanoparticles
Polymeric nanoparticles have been considered efficient carriers for prolonged drug delivery systems. In the 1990s, the synthesis of polymeric nanoparticles using polylactic acid (PLA) and poly lactic-co-glycolic acid (PLGA) was explored and reported as “long-circulating” [
61]. Since then, the interest in polymeric nanoparticles and their use in cancer therapy has increased. These nanoparticles are considered very versatile because they can be manipulated to be either biodegradable or non-biodegradable, either synthetic or derived from natural sources [
62,
63]. Biodegradable polymers have the advantage that they can break down into monomers that can be simply eliminated by the body’s natural metabolic pathways [
31].
Natural polymers such as polyhydroxyalkanoates (PHAs), as well as synthetic polymers like PLGA have been studied for targeted drug delivery applications paired with anti-cancer agents like paclitaxel [
64,
65], doxorubicin [
66,
67], and cisplatin [
68,
69]. These studies were tested
in vivo and there are some that have been used in preclinical trials on mice [
70].
A conjugation between folic acid and PLGA nanoparticles with chitosan as the vehicle was tested for the treatment of prostate cancer [
71]. The compound was loaded with bicalutamide and tested
in vitro. In comparative studies, unfunctionalized PLGA nanoparticles were also synthetized and exposed to the same circumstances. It was observed that the functionalized nanoparticles showed improved efficiency than the unfunctionalized nanoparticles, because of their altered surface and specific targeted delivery [
71]. Folic acid coupled with poly(3-hydroxybutyrate-co-3-hydroxyoctanoate) and loaded with doxorubicin presented a drug encapsulation performance of above 80% [
67].
Furthermore, in vitro assays exhibited a release profile of the anti-cancer drug of approximately 50% in the first five days, and
in vivo assays showed that the system displayed enhanced therapeutic efficiency in limiting the tumor growth when compared to controls [
67]. Additionally, PLGA nanoparticles loaded with methotrexate-transferrin conjugates and coated with Polysorbate 80, a water-soluble surfactant, were investigated as vehicles for brain cancer treatment. Polysorbate 80 is known to enhance the nanoparticles loaded blood-brain barrier (BBB) cross. According to Jain and al., the continuous delivery of methotrexate-transferrin conjugates was attained by virtue of the over-expressed transferrin receptors on the surface of tumor cells, and the results of both
in vivo and
in vitro assays highlighted the efficiency of the conjugated system when compared to controls [
72].
Among the polymeric nanoparticles, dendrimers stand as a unique class of macromolecules with narrow molecular weight distribution, comprising an almost monodispersed nanosystem for target drug delivery. They are composed of a hyperbranched polymeric mantle, a central core, and corona and it has numerous branches that can carry a variety of drugs [
73]. The molecular size of dendrimers of a certain family is very often identified by its generation, which increases as the molecular weight of the dendrimer increases. The particle size and shape of dendrimers can be adjusted via chemical synthesis, thus providing branched macromolecules with diverse chemical groups that can be explored for target applications. This is of uttermost relevance for drug delivery because the loading of guest species (e.g. drug molecules) depends on the nature and number of chemical groups in the branched architecture. Due to its single surface, dendrimers have made a great contribution to the design of nanosystems but cytotoxicity has been a critical issue in these systems; the toxicity of these nanocarriers has been related namely to surface terminal groups [
74]. The most valuable ability of these nanoparticles is the active and passive tumor targeting.
5.3. Quantum dots
Quantum dots are semiconducting nanocrystals whose charge carriers are confined in the three dimensions, thus showing quantum size effects in their optical properties [
16,
17]. These inorganic nanoparticles have been prepared by a variety of chemical methods, however those relying on colloidal synthesis offer several advantages for nanomedicines such as their easy biofunctionalization namely for bioimaging diagnosis. Among the biomarkers used for these purposes, quantum dots stand for their size-dependent photoluminescence, narrow and tunable emission bands, photostability and pronounced Stokes shift. Furthermore, the observation of size-tuned photoluminescence in quantum dots under irradiation using a single light source, makes these particles suitable for multiplexing methods of analysis. Seminal research on colloidal quantum dots involved mainly the synthesis of Cd containing materials using hot injection methods, whose surfaces could be subsequently modified with biomolecules. Currently, alternatives to toxic Cd containing quantum dots are available and have been the subject of interest for bioimaging, such as ZnS coated InP quantum dots or other types of fluorescent nanoparticles, including silica nanocomposites [
75,
76].
The present imaging techniques available such as X-ray scan, MRI, and computer tomography have serious limitations when it comes to cancer diagnosis and the main limitation is that those techniques cannot recognize small numbers of malignant cells in primary or in metastatic sites [
77]. Because quantum dots have improved signal brightness, synchronous excitation of multiple fluorescence colors, and size-tunable light emission, they have been explored as biofunctionalized labels for cancer imaging [
10]. However, besides the requirements for cytotoxicity assessment, quantum dots still pose challenges concerning their use in bioimaging, such as the observation of tissue autofluorescence and photon scattering.
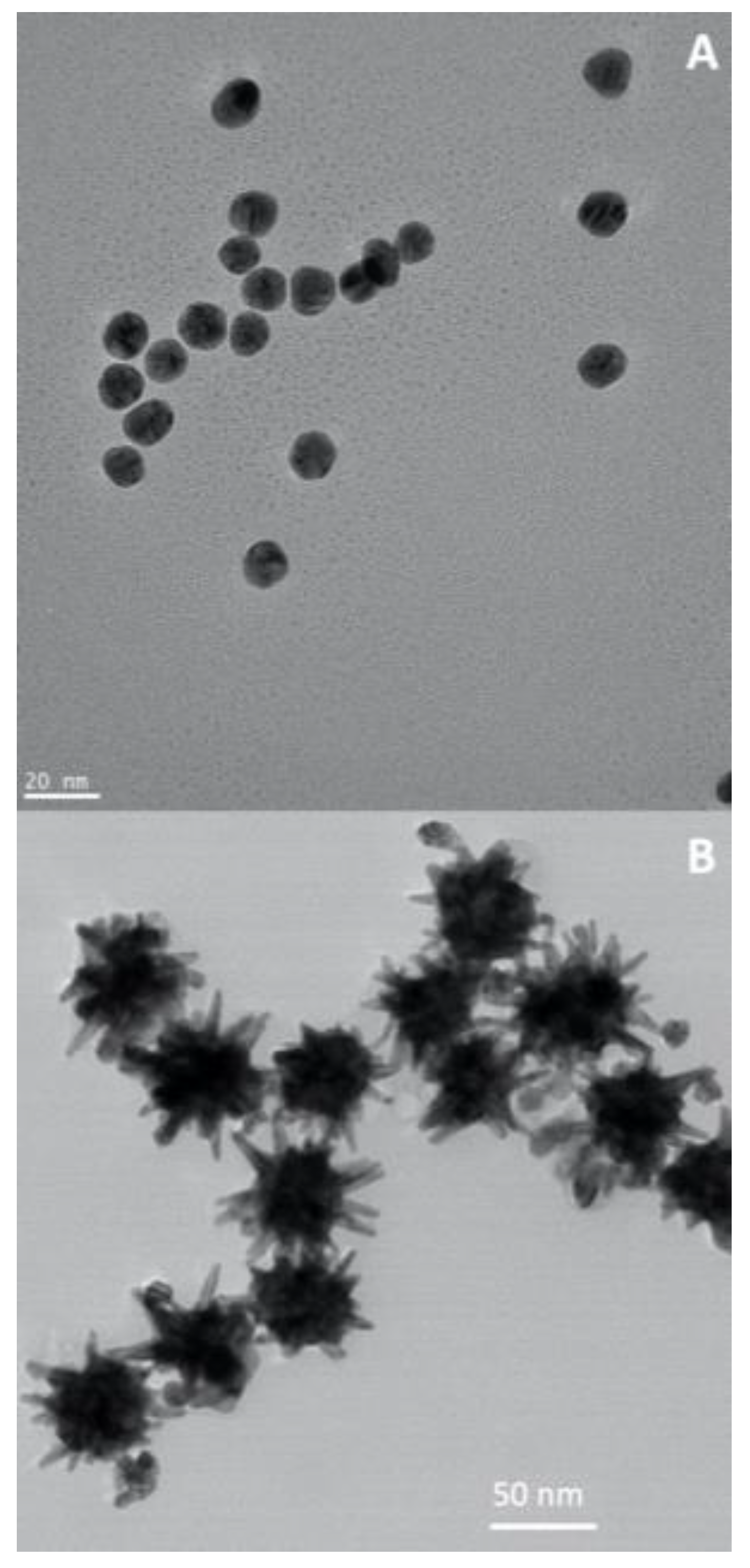
5.4. Gold nanoparticles
Throughout history, gold has consistently held its place as one of the most prized metals on Earth. Gold nanoparticles have been widely investigated for cancer therapies, due to their high chemical stability, well-established synthetic and surface modification methods, shape and size tunability and biocompatibility [
78,
79,
80,
81]. In addition, gold nanoparticles show strong absorption in the visible (VIS), due to localized surface plasmon resonances (LSPR): this means that in the presence of light (an oscillating electromagnetic field), the free electrons from these plasmonic nanoparticles will oscillate and resonate at a particular frequency of light [
82]. In fact, gold nanospheres(
Figure 8A) absorb light up to 10
5 times stronger than most efficient light-absorbing dye molecules [
83], which is a clear advantage in comparison to the conventional drugs. The LSPR oscillation can decay by non-radiative processes and convert energy to heat, which turns gold nanoparticles particularly important for plasmonic photothermal therapy (PPTT) applications [
84]. Furthermore, anisotropic gold nanostructures (e.g. gold nanorods or gold nanostars,
Figure 8B) can be synthesized to show resonances in the near-infrared windows (650-950 nm; 1000-1700 nm), a spectral range that allows maximum depth of penetration of incident light in a tissue. Hence, a gold nanorod show two LSPR bands, associated to two dipole oscillations along its axis, the transverse and longitudinal modes. The latter originates strong absorption in the NIR, whose exact location can be adjusted by controlling the particle’s aspect ratio during the synthesis. The ability for controlling the plasmonic behavior of gold nanoparticles via chemical and surface modification methods, turns these nanosystems of great relevance in a number of cancer therapies, including PPTT and surface enhanced Raman scattering (SERS) bioimaging [
18,
19,
85,
86,
87,
88].
Considering the capability to thermally destroy the cancerous cells, the photothermal heating capacity, and ease in surface functionalization, gold nanoparticles stand out for their application in multiple cancer therapies. According to Lungu et al., hyperthermia, a common approach in terms of cancer treatment, consists in heating up to 40 °C the tumor site using microwaves and radio waves as heat generators [
31]. Nonetheless, gold nanoparticles can be used as heat sources showing many advantages over conventional hyperthermia, such as the ability to affect only the adjacent sites, without damaging healthy cells, leading to efficient targeted action [
89,
90]. The gold nanoparticles start heating up the adjacent locations when an external radiofrequency electric field acts upon them. However, radiofrequency hyperthermia has some serious inconveniences, such as high levels of pain for the patient [
89].
It has been reported that gold nanoparticles generate local dose augmentation at the cancerous location by virtue of their properties, such as strong optical absorption in the LSPR region. Furthermore, a system consisting of gold nanoparticles and organic molecules, such as bovine serum albumin (BSA), results in a higher agglomeration of nanoparticles in the tumor site [
31]. Also, this system exhibits better features, such as uniform dimensions, ease in synthesis, and stability under physiological conditions [
31]. According to Chen et al., both
in vitro and
in vivo assays using BSA-modified gold nanoparticles showed auspicious results, such as inhibition of cloning formation, cancerous cell death, and did not present destructive consequences on healthy tissues and cells [
74].
The basis of using gold nanoparticles in cancer radiotherapy is to inject them into the tumor location, then the external X-ray source will act upon them, and it will produce radicals that will damage the cancerous cells and promote their death [
31]. When it comes to radiotherapy, assays were made by injecting gold nanoparticles in mice with the EMT-6 cancerous cell line. The mice were exposed to X-ray therapy and the survival rate considerably increased compared to mice that were subjected to conventional treatment, such as irradiation [
31].
As mentioned above, colloidal Au nanoparticles can be synthesized with distinct particle size distributions and specific particle’s shape, such as nanospheres and anisotropic particles (
Figure 8). As such, the optical behavior of such colloids can be judiciously tuned by controlling their morphological characteristics. Besides, the surfaces of such nanoparticles can be functionalized envisaging specific bioapplications. Hence, Au nanoparticles coated with cysteamine and thioglucose were synthesized by Kong et al. and applied to healthy and cancerous breast cell lines [
91] It was reported that the gold nanoparticles coated with glucose were internalized by the tumor cells, while the ones coated with cysteamine were essentially disposed on the surface. The assays showed that the number of internalized functionalized nanoparticles was substantially higher than the unfunctionalized ones. Nonetheless, when the irradiation acted upon the nanoparticles, it was noticed that the cytotoxic effect of the functionalized nanoparticles was considerably higher than the one arising from the unfunctionalized ones.
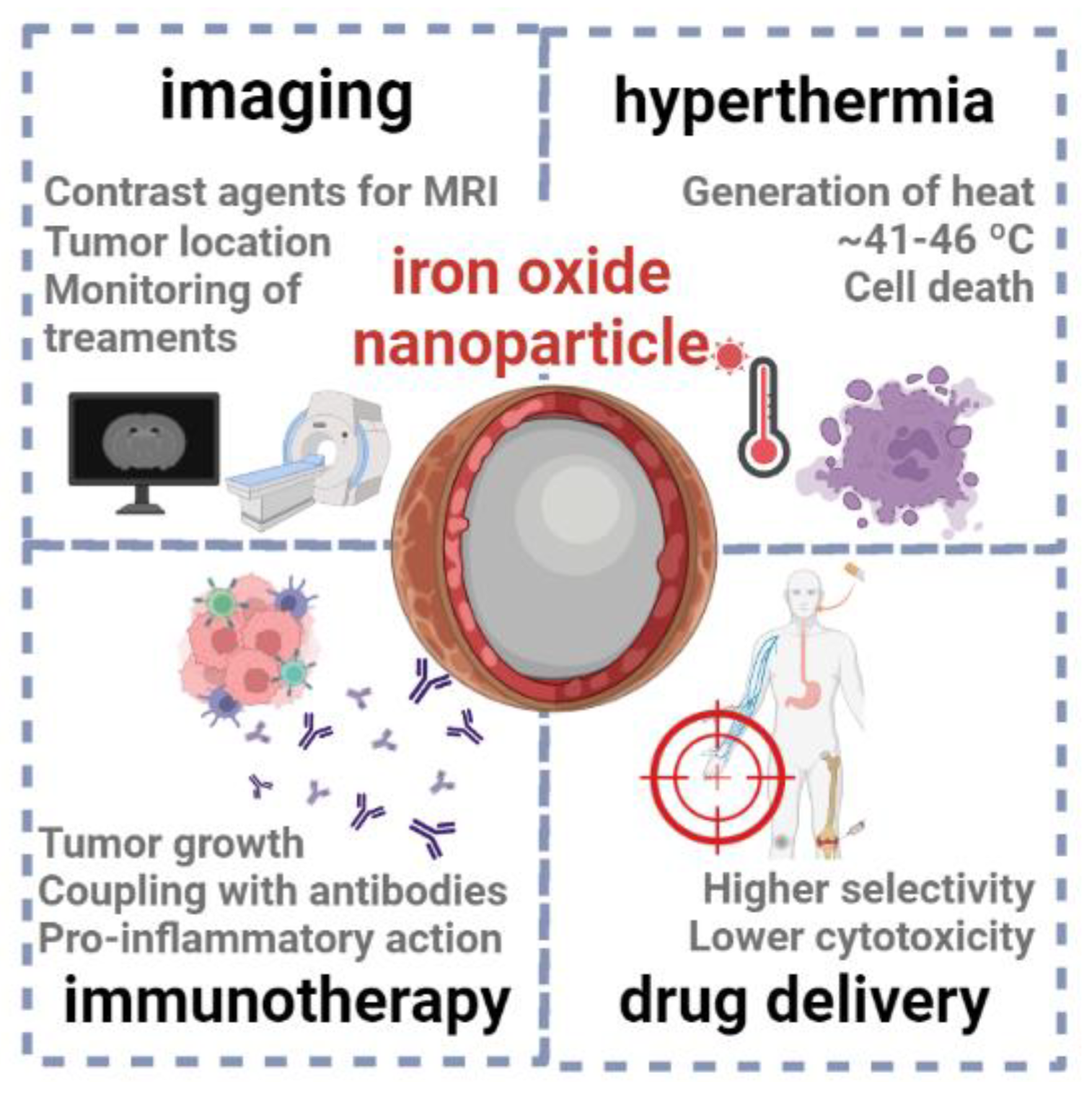
5.5. Iron oxide nanoparticles
Iron oxide nanoparticles, namely of magnetite (Fe
3O
4) and maghemite (γ-Fe
2O
3), have garnered significant attention in cancer therapies (
Figure 9), due to their unique properties, such as small size, high surface-to-volume ratio and magnetic properties (which differ from their bulk counterparts) [
92,
93]. Often, the surface of these magnetic nanoparticles is coated with a material that increases the biocompatibility and stability in physiological media, such as a polysaccharide or smaller carbohydrates, endowing the final material with a hard-core/soft-shell structure [
94].
One of the primary applications of these nanoparticles is their use as contrast agents for MRI scans (such as Ferumoxsil, Lumirem® or Gastro MARK®), enabling accurate tumor localization, staging and monitoring of treatment response [
95]. For example, Han et al. developed multifunctional iron oxide nanoparticles with a carbon-based shell, whose magnetic and fluorescence properties allowed the detection and imaging of cancer cells [
96]. To provide an optimal balance of sensitivity and selectivity, MRI-based approaches can be combined with other imaging techniques, such as computerized tomography (CT), which relies on the application of X-rays to generate two-dimensional images of the body. Within this context, Deng et al. [
97] reported the synthesis of radiolabeled superparamagnetic iron oxide nanoparticles functionalized with a small peptide, as selective dual-modality agents for imaging of breast cancer.
Another well-documented application is the use of magnetic iron oxide nanoparticles in hyperthermia therapy, where these systems are exposed to alternating magnetic fields (typically ranging between ~100-300 kHz) and generate heat, mostly via magnetic hysteresis loss [
94,
98]. Due to hyperthermia, the temperature of cancer tissues might be raised up to 41-46 ºC, triggering various paths as necrosis, apoptosis, protein denaturation and immune system reactions [
99]. Still, a major challenge of this strategy is to assure true tumoral tissue specificity, without damaging surrounding healthy tissues. Currently, several iron oxide nanoparticles have been approved for use in hyperthermia-based cancer therapy, such as NanoTherm® and ThermoDox [
94]®.
It has been described that that this class of nanoparticles can stimulate pro-inflammatory immune cell phenotypes, facilitating the recognition of tumors to enhance cancer therapies [
94,
100]. For example, Korangath et al. [
101] recently reported the coupling of amine-functionalized starch-coated ferrite nanoparticles with a monoclonal antibody (HER2/neu), which has been clinically approved in therapies for breast cancer. After exposing cancer cells to these nanoparticles, the authors observed an infiltration of T cell populations (part of the immune system) into tumors, followed by tumor growth suppression. Similarly, the exposure of cancer cells to ferumoxytol [
102], an example of a FDA-approved iron oxide nanoparticle, triggers an inflammatory response that leads to the prevention of metastases.
The functionalization of iron oxide nanoparticles with targeting ligands, antibodies or peptides might enhance their selectivity towards cancer cell receptors or markers, facilitating targeted delivery of drug molecules (as doxorubicin and paclitaxel) [
103,
104] and short ribonucleotides (e.g. miRNAs, siRNAs) [
105,
106].
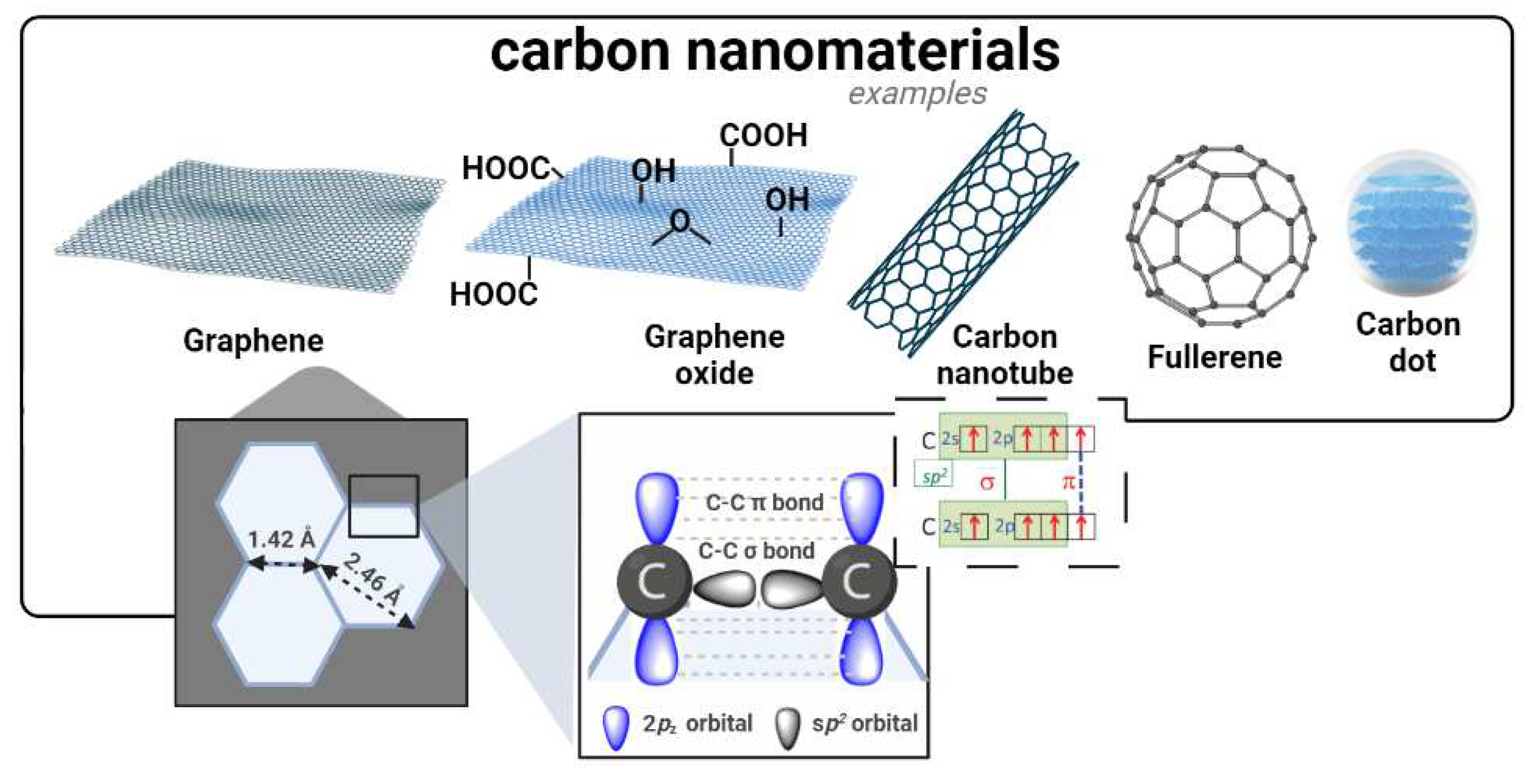
5.6. Carbon nanomaterials
Carbon nanostructures are an important class of materials in the field of cancer therapies, including, for example, graphene-based structures, carbon nanotubes (CNTs), fullerenes and carbon dots (CDs) [
107], as illustrated in
Figure 10. These carbonaceous structures have found applications as drug carriers, photoactive and diagnostic agents in several cancer theragnostics [
107,
108]. A significant advantage of these systems lies in their large surface area-to-volume ratios, which allows for enhanced loading and delivery of anticancer drugs towards the target cells, thus minimizing off-target effects. Moreover, because of their easy functionalization possibilities, the surface of these nanomaterials can be tailored to achieve different types of interactions (covalent and/or non-covalent) with drug molecules and assure their controlled release to tumor sites [
107].
The two-dimensional nature of graphene-based nanomaterials and their
sp2 hybridization endows them with a unique honeycomb lattice structure to act as nanovehicles of anticancer drugs [
109,
110]. Within this context, oxidized derivatives of graphene, such as graphene oxide (GO) play a key role due to its higher dispersibility in physiological media and ability for chemical functionalization [
111,
112]. For example, Zhang et al [
113] loaded doxorubicin and camptothecin (CPT) onto GO, to simultaneously explore the cytotoxic effect arising from DNA intercalation and topoisomerase inhibition in MCF-7 breast cancer cells. Moreover, due to their strong absorbance in the NIR region, it has been reported [
114] that graphene derivatives can be stimulated by light to produce hyperthermia [
115]. Additionally, these nanomaterials can aid typical photodynamic therapy due to their ability to carry multiple PS that generate reactive oxygen species (ROS) under light irradiation.
CNTs assume special relevance in cancer treatment and diagnosis, namely when chemically-functionalized with biocompatible molecules that increase their inner stability in physiological media. For example, Oh et al. [
116] developed a delivery systems that carried doxorubicin with PEGylated single-wall CNTs (SWNTs), which showed interest in chemotherapy and in combined NIR-irradiated PTT against human breast cancer cells [
117]. Wen et al. [
118] followed a similar rationale to load another anticancer drug (Sor) and EGFR onto multi-wall CNTs (MWNTs): the results showed that such nanocomposite could decrease tumor growth in liver cancer cells, mostly by apoptosis. While attempting to target mitochondria, Yoong et al. [
119] functionalized multi-wall CNTs (MWNTs) with fluorescent rhodamine molecules to encapsulate a chemo-potentiator 3-bromopyruvate (BP) and platinum prodrug; the as-developed system led to mitochondrial malfunction, causing apoptosis of cancer cells.
The unique geometry and molecular topology of fullerene C
60 consists of a round cage-type structure bearing 60 carbon atoms arranged in 12 pentagons and 20 hexagons [
120]. Other fullerenes exist with other number of C atoms arranged in fused rings of five to seven atoms or with the surfaces functionalized with a variety of chemical groups. The abundant π-π conjugation of these nanomaterials endow them with important optical and thermodynamic properties, suitable for its use as a photosensitizing agent in PDT, hyperthermia, imaging and photoacoustic-assisted theragnostics [
121].
As a more recent member of this carbonaceous nanomaterial family, fluorescent CDs have been acquiring increasing importance in cancer therapies, namely in bioimaging [
122,
123]. Targeted staining of specific cancer cells using CDs typically relies on the attachment of special ligands, such as transferrin, folic acid and hyaluronic acid [
124,
125,
126]. These materials can also be used as delivery systems [
127,
128].
Despite these encouraging breakthroughs, the biocompatibility of carbon nanomaterials remains challenging: surface functionalization, modification, and encapsulation strategies have been employed to enhance biocompatibility, biodegradability, and control immune responses [
129]. For example, CNTs have raised nanotoxicological concerns which prompt the necessity of more studies, namely associated to surface functionalization and biological impact. Hence, rigorous preclinical and clinical studies are still required to evaluate the safety and efficacy of carbon nanomaterial-based cancer therapies.
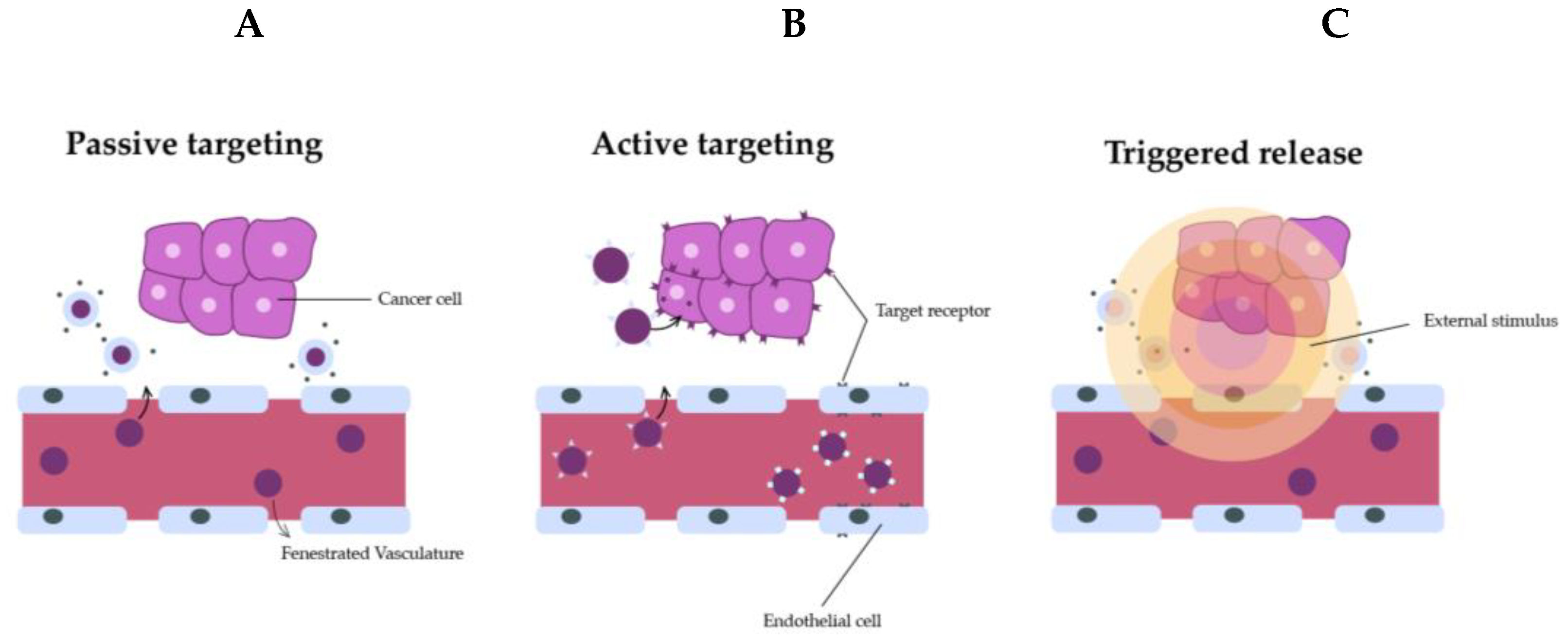
6. Passive and active targeting
6.1. Passive targeting
Nanocarrier-based cancer therapies are mostly passively targeted first-generation nanomedicines. This generation relies on manipulating pharmacokinetics and biodistribution by regulating physicochemical properties [
130]. The pathophysiological properties of cancer and its environment have been used for inactive targeting, especially where the accumulation of nanomedicine in tumor cells is promoted by the EPR effect. Thus, nanomedicine treatments from passive targeting into neoplasms can occur by diffusion and convection without the attachment of a special substance to the nanocarrier surface. In spite of that, it is known that EPR effect-based passive targeting is inefficient to control cytotoxic drug side effects. The drug delivery can be negatively affected through passive targeting due to the cancer heterogeneity and its stroma, and the consequence is a reduced or a nulled transport of the components into neoplasms [
131]. This is the reason why researchers preferably focus on the standardization of neoplasm vasculature before starting cancer treatment Also, the extracellular matrix restricts drug penetration [
132], and the accumulation of nanocarriers in former organs is not avoided by passive targeting [
133]. Therefore, a next generation based on drug delivery with active directing transmitters nanocarriers having stimuli-reactive properties was developed, resulting in improved directing and enhanced efficiency potential [
134] (
Figure 11).
6.2. Active targeting
According to Bazak et al., a high-affinity material annexes to the carrier surface area so the ligand can selectively bind to the target cell receptor [
135]. Many ligand ranges (like carbohydrates and folic acid, or macromolecules, like proteins, oligonucleotides, and aptamers) have been used with this intention. The preferred ligand binds to a targeted cell while it minimizes binding to healthy cells [
13] (
Figure 11).
7. Cancer theragnostics
Theragnostics is a term used to describe systems that can diagnose, provide target drug delivery, and track the effects of the treatment. The main objective of bringing all these aspects together is to improve the chances of cure while minimizing the risks and the costs [
31]. Thus, the intention behind designing this kind of agent is to develop a nano-sized system with a two-fold function. It is necessary to consider all the steps of this process, such as the method chosen or the materials that will be used in the preparation of the particles, and their removal from the body [
137].
Different challenges have been acknowledged for the purpose of reaching an effective theragnostic system. Zhao et al. and Bae et al. highlighted that two main characteristics must be considered in the biomarker that is used for imaging and treatment: it must be exceedingly expressed in the cancerous cells and absent in healthy cells [
32,
138]. Furthermore, it is required that the ligand must be highly reproducible for
in vivo testing purposes, and it is crucial that the nanoparticles used in the theragnostics agents are biocompatible, biodegradable, show a high loading capacity, and when it reaches the cancerous site, presents a controlled release profile of the therapeutic elements [
31].
The mentioned specifications are critical for the therapeutic part of a theragnostic system, but the diagnosis part mainly represents imaging requirements. The nanoparticles should be capable of producing a constant and clear imaging signal in view to effectively monitor the targeted drug delivery as well as the response [
139,
140].
8. Conclusions
Nanomedicines brought a new perspective to cancer therapy mainly because of unique properties explored at the nanometric scale and the high bioavailability at the site of action. Additionally, they can reduce the toxic effect of the drug once incorporated into targeted drug delivery systems. Furthermore, nanomedicines are considered economical, since they save costs by reducing the amounts of necessary drugs, in comparison to standard treatments.
Liposomes, polymeric and inorganic nanoparticles, among others, have been tested as potential candidates for cancer treatments and, in certain scenarios, they have been already implemented in clinical context. These structures present relevant features for application in cancer therapies, like size and surface dependent properties, versatility in their synthesis and surface modification, diverse functionalities, and can be modified to improve the required biocompatibility. The use of nanotechnology in cancer therapy has produced great improvements so far but there are still many challenges ahead. While colloidal nanoparticles hold great potential in various fields such as drug delivery, imaging, and diagnostics, research on the nanotoxicology aspects must be carefully considered. Size, surface characteristics, composition, and concentration are critical factors that can influence the toxicity of such colloids. Further studies focusing on their potential long-term effects in different biological systems are necessary to ensure their safe use as nanomedicines.
Although there are still current challenges when it comes to using nanoparticles as therapeutics, it is expected that much progress will be achieved in the near-future not only in cancer treatments, but also in other fields of medicine.
Author Contributions
Conceptualization, L.C.-L. and M.S.; methodology, L.C.-L., M.S, A.R.L., and B.E.; formal analysis, L.C.-L., M.S. and A.R.M.; investigation, L.C.-L., M.S., A.R.L., A.R.M. and B.E.; resources, L.C.-L., A.R.L., M.E., M.S., A.R.M. and B.E.; writing—original draft preparation, M.S., A.R.L. and A.R.M..; writing—review and editing, L.C.-L., T.T. and B.E.; supervision, L.C.-L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Funds through the FCT - Foundation for Science and Technology, I.P., within the scope of the projects Ref. UIDB/00681/2020 (CERNAS), L.C.-L. and B.E. A.R.M. and T.T. thank the project CICECO-Aveiro Institute of Materials, UIDB/50011/2020, UIDP/50011/2020 & LA/P/0006/2020, financed by national funds through the FCT/MCTES (PIDDAC).
Data Availability Statement
We encourage all authors of articles published in MDPI journals to share their research data. In this section, please provide details regarding where data supporting reported results can be found, including links to publicly archived datasets analyzed or generated during the study. Where no new data were created, or where data is unavailable due to privacy or ethical restrictions, a statement is still required. Suggested Data Availability Statements are available in section “MDPI Research Data Policies” at
https://www.mdpi.com/ethics.
Acknowledgments
In this section, you can acknowledge any support given which is not covered by the author contribution or funding sections. This may include administrative and technical support, or donations in kind (e.g., materials used for experiments). Graphical content was partially designed using BioRender.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kim, S.-D.; Kwag, E.-B.; Yang, M.-X.; Yoo, H.-S. Efficacy and Safety of Ginger on the Side Effects of Chemotherapy in Breast Cancer Patients: Systematic Review and Meta-Analysis. International Journal of Molecular Sciences 2022, 23, 11267. [Google Scholar] [CrossRef] [PubMed]
- Reijneveld, E.A.; Bor, P.; Dronkers, J.J.; Argudo, N.; Ruurda, J.P.; Veenhof, C. Impact of Curative Treatment on the Physical Fitness of Patients with Esophageal Cancer: A Systematic Review and Meta-Analysis. European Journal of Surgical Oncology 2022, 48, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Kerr, A.J.; Dodwell, D.; McGale, P.; Holt, F.; Duane, F.; Mannu, G.; Darby, S.C.; Taylor, C.W. Adjuvant and Neoadjuvant Breast Cancer Treatments: A Systematic Review of Their Effects on Mortality. Cancer treatment reviews 2022, 102375. [Google Scholar] [CrossRef]
- Chen, Y.H.; Molenaar, D.; Uyl-de Groot, C.A.; van Vulpen, M.; Blommestein, H.M. Medical Resource Use and Medical Costs for Radiotherapy-Related Adverse Effects: A Systematic Review. Cancers 2022, 14, 2444. [Google Scholar] [CrossRef] [PubMed]
- Adverse Events of Immune Checkpoint Inhibitors Therapy for Urologic Cancer Patients in Clinical Trials: A Collaborative Systematic Review and Meta-Analysis - ScienceDirect Available online:. Available online: https://www.sciencedirect.com/science/article/pii/S0302283822000653 (accessed on 25 January 2023).
- Lohse, S.E.; Murphy, C.J. Applications of Colloidal Inorganic Nanoparticles: From Medicine to Energy. Journal of the American Chemical Society 2012, 134, 15607–15620. [Google Scholar] [CrossRef] [PubMed]
- Germain, M.; Caputo, F.; Metcalfe, S.; Tosi, G.; Spring, K.; Åslund, A.K.; Pottier, A.; Schiffelers, R.; Ceccaldi, A.; Schmid, R. Delivering the Power of Nanomedicine to Patients Today. Journal of Controlled Release 2020, 326, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Valdivieso, J.; Girotti, A.; Schneider, J.; Arias, F.J. Advanced Nanomedicine and Cancer: Challenges and Opportunities in Clinical Translation. International Journal of Pharmaceutics 2021, 599, 120438. [Google Scholar] [CrossRef]
- Narayana, A. Applications of Nanotechnology in Cancer: A Literature Review of Imaging and Treatment. J Nucl Med Radiat Ther 2014, 05. [Google Scholar] [CrossRef]
- Chatterjee, P.; Kumar, S. Current Developments in Nanotechnology for Cancer Treatment. Materials Today: Proceedings 2022, 48, 1754–1758. [Google Scholar] [CrossRef]
- Kola, I.; Landis, J. Can the Pharmaceutical Industry Reduce Attrition Rates? Nat Rev Drug Discov 2004, 3, 711–716. [Google Scholar] [CrossRef]
- Krown, S.E.; Northfelt, D.W.; Osoba, D.; Stewart, J.S. Use of Liposomal Anthracyclines in Kaposi’s Sarcoma. Seminars in Oncology 2004, 31, 36–52. [Google Scholar] [CrossRef] [PubMed]
- Rasool, M.; Malik, A.; Waquar, S.; Arooj, M.; Zahid, S.; Asif, M.; Shaheen, S.; Hussain, A.; Ullah, H.; Gan, S.H. New Challenges in the Use of Nanomedicine in Cancer Therapy. Bioengineered 2022, 13, 759–773. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Hu, C.; Ran, W.; Meng, J.; Yin, Q.; Li, Y. Recent Progress in Light-Triggered Nanotheranostics for Cancer Treatment. Theranostics 2016, 6, 948. [Google Scholar] [CrossRef] [PubMed]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Le Breton, A.; Préat, V. PLGA-Based Nanoparticles: An Overview of Biomedical Applications. Journal of controlled release 2012, 161, 505–522. [Google Scholar] [CrossRef] [PubMed]
- Schmid, G. Nanoparticles: From Theory to Application; John Wiley & Sons, 2011.
- Trindade, T.; Thomas, P.J. Defining and Using Very Small Crystals. 2013. [CrossRef]
- Daniel-da-Silva, A.L.; Trindade, T. Surface Chemistry of Colloidal Nanocrystals; Royal Society of Chemistry, 2021. [Google Scholar]
- Pereira, S.O.; Barros-Timmons, A.; Trindade, T. Biofunctionalisation of Colloidal Gold Nanoparticles via Polyelectrolytes Assemblies. Colloid and Polymer Science 2014, 292, 33–50. [Google Scholar] [CrossRef]
- Al-Jamal, W.; Kostarelos, K. Liposomes: From a Clinically Established Drug Delivery System to a Nanoparticle Platform for Theranostic Nanomedicine. Accounts of chemical research 2011, 44, 1094–1104. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Sheng, Z.; Hu, D.; Yan, F.; Zhu, M.; Gao, G.; Wang, P.; Liu, X.; Wang, X.; Zheng, H. Highly Penetrative Liposome Nanomedicine Generated by a Biomimetic Strategy for Enhanced Cancer Chemotherapy. Biomater. Sci. 2018, 6, 1546–1555. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.-M.J.; Aryal, S.; Zhang, L. Nanoparticle-Assisted Combination Therapies for Effective Cancer Treatment. Therapeutic Delivery 2010, 1, 323–334. [Google Scholar] [CrossRef]
- Rao, J.P.; Geckeler, K.E. Polymer Nanoparticles: Preparation Techniques and Size-Control Parameters. Progress in Polymer Science 2011, 36, 887–913. [Google Scholar] [CrossRef]
- Miernicki, M.; Hofmann, T.; Eisenberger, I.; von der Kammer, F.; Praetorius, A. Legal and Practical Challenges in Classifying Nanomaterials According to Regulatory Definitions. Nature nanotechnology 2019, 14, 208–216. [Google Scholar] [CrossRef]
- Omidifar, N.; Nili-Ahmadabadi, A.; Nakhostin-Ansari, A.; Lankarani, K.B.; Moghadami, M.; Mousavi, S.M.; Hashemi, S.A.; Gholami, A.; Shokripour, M.; Ebrahimi, Z. The Modulatory Potential of Herbal Antioxidants against Oxidative Stress and Heavy Metal Pollution: Plants against Environmental Oxidative Stress. Environ Sci Pollut Res 2021, 28, 61908–61918. [Google Scholar] [CrossRef] [PubMed]
- Omrani, M.M.; Ansari, M.; Kiaie, N. Therapeutic Effect of Stem Cells and Nano-Biomaterials on Alzheimer’s Disease. 2016, 8.
- Fraga, M.F.; Ballestar, E.; Paz, M.F.; Ropero, S.; Setien, F.; Ballestar, M.L.; Heine-Suñer, D.; Cigudosa, J.C.; Urioste, M.; Benitez, J. Epigenetic Differences Arise during the Lifetime of Monozygotic Twins. Proceedings of the National Academy of Sciences 2005, 102, 10604–10609. [Google Scholar] [CrossRef] [PubMed]
- Maitland, M.L.; Schilsky, R.L. Clinical Trials in the Era of Personalized Oncology. CA: A Cancer Journal for Clinicians 2011, 61, 365–381. [Google Scholar] [CrossRef] [PubMed]
- Gao, H. Shaping Tumor Microenvironment for Improving Nanoparticle Delivery. Current drug metabolism 2016, 17, 731–736. [Google Scholar] [CrossRef] [PubMed]
- Schaaf, M.B.; Garg, A.D.; Agostinis, P. Defining the Role of the Tumor Vasculature in Antitumor Immunity and Immunotherapy. Cell death & disease 2018, 9, 1–14. [Google Scholar] [CrossRef]
- Lungu, I.I.; Grumezescu, A.M.; Volceanov, A.; Andronescu, E. Nanobiomaterials Used in Cancer Therapy: An up-to-Date Overview. Molecules 2019, 24, 3547. [Google Scholar] [CrossRef] [PubMed]
- Bae, K.H.; Chung, H.J.; Park, T.G. Nanomaterials for Cancer Therapy and Imaging. Molecules and cells 2011, 31, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Lungu, I.I.; Grumezescu, A.M.; Volceanov, A.; Andronescu, E. Nanobiomaterials Used in Cancer Therapy: An Up-To-Date Overview. Molecules 2019, 24, 3547. [Google Scholar] [CrossRef]
- Kim, K.Y. Nanotechnology Platforms and Physiological Challenges for Cancer Therapeutics. Nanomedicine: Nanotechnology, Biology and Medicine 2007, 3, 103–110. [Google Scholar] [CrossRef]
- Chakraborty, S.; Dhakshinamurthy, G.S.; Misra, S.K. Tailoring of Physicochemical Properties of Nanocarriers for Effective Anti-Cancer Applications. Journal of Biomedical Materials Research Part A 2017, 105, 2906–2928. [Google Scholar] [CrossRef]
- Raju, G.S.R.; Benton, L.; Pavitra, E.; Su Yu, J. Multifunctional Nanoparticles: Recent Progress in Cancer Therapeutics. Chemical Communications 2015, 51, 13248–13259. [Google Scholar] [CrossRef] [PubMed]
- Hauert, S.; Bhatia, S.N. Mechanisms of Cooperation in Cancer Nanomedicine: Towards Systems Nanotechnology. Trends in Biotechnology 2014, 32, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Gabrielson, N.P.; Uckun, F.M.; Fan, T.M.; Cheng, J. Size-Dependent Tumor Penetration and in Vivo Efficacy of Monodisperse Drug–Silica Nanoconjugates. Molecular pharmaceutics 2013, 10, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Tran, S.; DeGiovanni, P.-J.; Piel, B.; Rai, P. Cancer Nanomedicine: A Review of Recent Success in Drug Delivery. Clinical and translational medicine 2017, 6, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, M.; Landry, M.P.; Moore, A.; Coreas, R. The Protein Corona from Nanomedicine to Environmental Science. Nature Reviews Materials 2023, 1–17. [Google Scholar] [CrossRef] [PubMed]
- González-García, L.E.; MacGregor, M.N.; Visalakshan, R.M.; Lazarian, A.; Cavallaro, A.A.; Morsbach, S.; Mierczynska-Vasilev, A.; Mailänder, V.; Landfester, K.; Vasilev, K. Nanoparticles Surface Chemistry Influence on Protein Corona Composition and Inflammatory Responses. Nanomaterials 2022, 12, 682. [Google Scholar] [CrossRef] [PubMed]
- Bashiri, G.; Padilla, M.S.; Swingle, K.L.; Shepherd, S.J.; Mitchell, M.J.; Wang, K. Nanoparticle Protein Corona: From Structure and Function to Therapeutic Targeting. Lab Chip 2023, 23, 1432–1466. [Google Scholar] [CrossRef]
- Kopac, T. Protein Corona, Understanding the Nanoparticle–Protein Interactions and Future Perspectives: A Critical Review. International Journal of Biological Macromolecules 2021, 169, 290–301. [Google Scholar] [CrossRef]
- Miceli, E.; Kar, M.; Calderón, M. Interactions of Organic Nanoparticles with Proteins in Physiological Conditions. J. Mater. Chem. B 2017, 5, 4393–4405. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, W. The Janus of Protein Corona on Nanoparticles for Tumor Targeting, Immunotherapy and Diagnosis. Journal of Controlled Release 2022, 345, 832–850. [Google Scholar] [CrossRef]
- Breznica, P.; Koliqi, R.; Daka, A. A Review of the Current Understanding of Nanoparticles Protein Corona Composition. Med Pharm Rep 2020, 93, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Capriotti, A.L.; Caracciolo, G.; Cavaliere, C.; Colapicchioni, V.; Piovesana, S.; Pozzi, D.; Laganà, A. Analytical Methods for Characterizing the Nanoparticle–Protein Corona. Chromatographia 2014, 77, 755–769. [Google Scholar] [CrossRef]
- Caracciolo, G.; Safavi-Sohi, R.; Malekzadeh, R.; Poustchi, H.; Vasighi, M.; Chiozzi, R.Z.; Capriotti, A.L.; Laganà, A.; Hajipour, M.; Domenico, M.D.; et al. Disease-Specific Protein Corona Sensor Arrays May Have Disease Detection Capacity. Nanoscale Horiz. 2019, 4, 1063–1076. [Google Scholar] [CrossRef]
- Lu, X.; Xu, P.; Ding, H.-M.; Yu, Y.-S.; Huo, D.; Ma, Y.-Q. Tailoring the Component of Protein Corona via Simple Chemistry. Nat Commun 2019, 10, 4520. [Google Scholar] [CrossRef] [PubMed]
- Farshbaf, M.; Valizadeh, H.; Panahi, Y.; Fatahi, Y.; Chen, M.; Zarebkohan, A.; Gao, H. The Impact of Protein Corona on the Biological Behavior of Targeting Nanomedicines. International Journal of Pharmaceutics 2022, 121458. [Google Scholar] [CrossRef] [PubMed]
- Lazarovits, J.; Chen, Y.Y.; Sykes, E.A.; Chan, W.C. Nanoparticle–Blood Interactions: The Implications on Solid Tumour Targeting. Chemical Communications 2015, 51, 2756–2767. [Google Scholar] [CrossRef] [PubMed]
- Tomak, A.; Cesmeli, S.; Hanoglu, B.D.; Winkler, D.; Oksel Karakus, C. Nanoparticle-Protein Corona Complex: Understanding Multiple Interactions between Environmental Factors, Corona Formation, and Biological Activity. Nanotoxicology 2021, 15, 1331–1357. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, L.; Yang, T.; Wu, H. Stimuli-Responsive Polymeric Micelles for Drug Delivery and Cancer Therapy. International journal of nanomedicine 2018, 13, 2921. [Google Scholar] [CrossRef]
- Godin, B.P.; Driessen, W.H.; Proneth, B.; Lee, S.-Y.; Srinivasan, S.; Rumbaut, R.; Arap, W.; Pasqualini, R.; Ferrari, M.; Decuzzi, P. 2 - An Integrated Approach for the Rational Design of Nanovectors for Biomedical Imaging and Therapy. In Advances in Genetics; Tissue-Specific Vascular Endothelial Signals and Vector Targeting, Part B; Pasqualini, R., Ed.; Academic Press, 2010; Vol. 69, pp. 31–64. [Google Scholar]
- Saraf, S.; Jain, A.; Tiwari, A.; Verma, A.; Panda, P.K.; Jain, S.K. Advances in Liposomal Drug Delivery to Cancer: An Overview. Journal of Drug Delivery Science and Technology 2020, 56, 101549. [Google Scholar] [CrossRef]
- Olusanya, T.O.B.; Haj Ahmad, R.R.; Ibegbu, D.M.; Smith, J.R.; Elkordy, A.A. Liposomal Drug Delivery Systems and Anticancer Drugs. Molecules 2018, 23, 907. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, L.; Wu, H. Nanomaterials for Cancer Therapies. Nanotechnology Reviews 2017, 6, 473–496. [Google Scholar] [CrossRef]
- Mross, K.; Niemann, B.; Massing, U.; Drevs, J.; Unger, C.; Bhamra, R.; Swenson, C.E. Pharmacokinetics of Liposomal Doxorubicin (TLC-D99; Myocet) in Patients with Solid Tumors: An Open-Label, Single-Dose Study. Cancer Chemother Pharmacol 2004, 54, 514–524. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Ren, W.; Zhong, T.; Zhang, S.; Huang, D.; Guo, Y.; Yao, X.; Wang, C.; Zhang, W.-Q.; Zhang, X. Tumor-Specific pH-Responsive Peptide-Modified pH-Sensitive Liposomes Containing Doxorubicin for Enhancing Glioma Targeting and Anti-Tumor Activity. Journal of controlled release 2016, 222, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Chen, S.; Li, H.; Zhang, Z.; Zhong, J.; Liu, M.; Zhou, X. MRI-Guided Liposomes for Targeted Tandem Chemotherapy and Therapeutic Response Prediction. Acta Biomaterialia 2016, 35, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Gref, R.; Minamitake, Y.; Peracchia, M.T.; Trubetskoy, V.; Torchilin, V.; Langer, R. Biodegradable Long-Circulating Polymeric Nanospheres. Science 1994, 263, 1600–1603. [Google Scholar] [CrossRef] [PubMed]
- Alexis, F.; Pridgen, E.M.; Langer, R.; Farokhzad, O.C. Nanoparticle Technologies for Cancer Therapy. In Drug Delivery; Schäfer-Korting, M., Ed.; Handbook of Experimental Pharmacology; Springer: Berlin, Heidelberg, 2010; ISBN 978-3-642-00477-3. [Google Scholar]
- Parveen, S.; Sahoo, S.K. Polymeric Nanoparticles for Cancer Therapy. Journal of Drug Targeting 2008, 16, 108–123. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Yang, Y.; Ling, Y.; Huang, Y.; Li, T.; Li, X. Improved Therapeutic Effect of Folate-Decorated PLGA–PEG Nanoparticles for Endometrial Carcinoma. Bioorganic & Medicinal Chemistry 2011, 19, 4057–4066. [Google Scholar] [CrossRef]
- Vilos, C.; Morales, F.A.; Solar, P.A.; Herrera, N.S.; Gonzalez-Nilo, F.D.; Aguayo, D.A.; Mendoza, H.L.; Comer, J.; Bravo, M.L.; Gonzalez, P.A.; et al. Paclitaxel-PHBV Nanoparticles and Their Toxicity to Endometrial and Primary Ovarian Cancer Cells. Biomaterials 2013, 34, 4098–4108. [Google Scholar] [CrossRef]
- Chittasupho, C.; Xie, S.-X.; Baoum, A.; Yakovleva, T.; Siahaan, T.J.; Berkland, C.J. ICAM-1 Targeting of Doxorubicin-Loaded PLGA Nanoparticles to Lung Epithelial Cells. European Journal of Pharmaceutical Sciences 2009, 37, 141–150. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, L.; Dong, Y.; Zhang, X.; Lin, J.; Chen, Z. Folate-Mediated Poly(3-Hydroxybutyrate-Co-3-Hydroxyoctanoate) Nanoparticles for Targeting Drug Delivery. European Journal of Pharmaceutics and Biopharmaceutics 2010, 76, 10–16. [Google Scholar] [CrossRef]
- Dhar, S.; Gu, F.X.; Langer, R.; Farokhzad, O.C.; Lippard, S.J. Targeted Delivery of Cisplatin to Prostate Cancer Cells by Aptamer Functionalized Pt(IV) Prodrug-PLGA–PEG Nanoparticles. Proceedings of the National Academy of Sciences 2008, 105, 17356–17361. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; Ullah, N.; Choi, M.H.; Kim, M.O.; Yoon, S.C. Amorphous Amphiphilic P(3HV-Co-4HB)-b-mPEG Block Copolymer Synthesized from Bacterial Copolyester via Melt Transesterification: Nanoparticle Preparation, Cisplatin-Loading for Cancer Therapy and in Vitro Evaluation. European Journal of Pharmaceutics and Biopharmaceutics 2012, 80, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Masood, F. Polymeric Nanoparticles for Targeted Drug Delivery System for Cancer Therapy. Materials Science and Engineering: C 2016, 60, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Dhas, N.L.; Ige, P.P.; Kudarha, R.R. Design, Optimization and in-Vitro Study of Folic Acid Conjugated-Chitosan Functionalized PLGA Nanoparticle for Delivery of Bicalutamide in Prostate Cancer. Powder Technology 2015, 283, 234–245. [Google Scholar] [CrossRef]
- Jain, A.; Jain, A.; Garg, N.K.; Tyagi, R.K.; Singh, B.; Katare, O.P.; Webster, T.J.; Soni, V. Surface Engineered Polymeric Nanocarriers Mediate the Delivery of Transferrin–Methotrexate Conjugates for an Improved Understanding of Brain Cancer. Acta biomaterialia 2015, 24, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Zelikin, A.N.; Chandrawati, R. Progress and Promise of Nitric Oxide-Releasing Platforms. Advanced Science 2018, 5, 1701043. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-T.; Neerman, M.F.; Parrish, A.R.; Simanek, E.E. Cytotoxicity, Hemolysis, and Acute in Vivo Toxicity of Dendrimers Based on Melamine, Candidate Vehicles for Drug Delivery. Journal of the American Chemical Society 2004, 126, 10044–10048. [Google Scholar] [CrossRef] [PubMed]
- Dirheimer, L.; Pons, T.; Marchal, F.; Bezdetnaya, L. Quantum Dots Mediated Imaging and Phototherapy in Cancer Spheroid Models: State of the Art and Perspectives. Pharmaceutics 2022, 14, 2136. [Google Scholar] [CrossRef]
- Granadeiro, C.M.; Ferreira, R.A.; Soares-Santos, P.C.; Carlos, L.D.; Trindade, T.; Nogueira, H.I. Lanthanopolyoxotungstates in Silica Nanoparticles: Multi-Wavelength Photoluminescent Core/Shell Materials. Journal of Materials Chemistry 2010, 20, 3313–3318. [Google Scholar] [CrossRef]
- Peng, C.-W.; Li, Y. Application of Quantum Dots-Based Biotechnology in Cancer Diagnosis: Current Status and Future Perspectives. Journal of Nanomaterials 2010, 2010, e676839. [Google Scholar] [CrossRef]
- António, M.; Nogueira, J.; Vitorino, R.; Daniel-da-Silva, A.L. Functionalized Gold Nanoparticles for the Detection of C-Reactive Protein. Nanomaterials 2018, 8, 200. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P.; Han, G.; De, M.; Kim, C.K.; Rotello, V.M. Gold Nanoparticles in Delivery Applications. Advanced drug delivery reviews 2008, 60, 1307–1315. [Google Scholar] [CrossRef] [PubMed]
- Shukla, R.; Bansal, V.; Chaudhary, M.; Basu, A.; Bhonde, R.R.; Sastry, M. Biocompatibility of Gold Nanoparticles and Their Endocytotic Fate inside the Cellular Compartment: A Microscopic Overview. Langmuir 2005, 21, 10644–10654. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Gao, X.; Liu, D.; Chen, X. Gold Nanoparticles for in Vitro Diagnostics. Chemical reviews 2015, 115, 10575–10636. [Google Scholar] [CrossRef] [PubMed]
- Amendola, V.; Pilot, R.; Frasconi, M.; Maragò, O.M.; Iatì, M.A. Surface Plasmon Resonance in Gold Nanoparticles: A Review. J. Phys.: Condens. Matter 2017, 29, 203002. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.K.; Lee, K.S.; El-Sayed, I.H.; El-Sayed, M.A. Calculated Absorption and Scattering Properties of Gold Nanoparticles of Different Size, Shape, and Composition: Applications in Biological Imaging and Biomedicine. J. Phys. Chem. B 2006, 110, 7238–7248. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.R.K.; Wu, Y.; El-Sayed, M.A. Gold-Nanoparticle-Assisted Plasmonic Photothermal Therapy Advances Toward Clinical Application. J. Phys. Chem. C 2019, 123, 15375–15393. [Google Scholar] [CrossRef]
- Lane, L.A.; Xue, R.; Nie, S. Emergence of Two Near-Infrared Windows for in Vivo and Intraoperative SERS. Current Opinion in Chemical Biology 2018, 45, 95–103. [Google Scholar] [CrossRef]
- Liao, S.; Yue, W.; Cai, S.; Tang, Q.; Lu, W.; Huang, L.; Qi, T.; Liao, J. Improvement of Gold Nanorods in Photothermal Therapy: Recent Progress and Perspective. Front. Pharmacol. 2021, 12, 664123. [Google Scholar] [CrossRef]
- Gomes, M.C.; Chen, J.; Cunha, A.; Trindade, T.; Zheng, G.; Tomé, J.P.C. Complex Cellular Environments Imaged by SERS Nanoprobes Using Sugars as an All-in-One Vector. J. Mater. Chem. B 2021, 9, 9285–9294. [Google Scholar] [CrossRef]
- Kesharwani, P.; Ma, R.; Sang, L.; Fatima, M.; Sheikh, A.; Abourehab, M.A.S.; Gupta, N.; Chen, Z.-S.; Zhou, Y. Gold Nanoparticles and Gold Nanorods in the Landscape of Cancer Therapy. Mol Cancer 2023, 22, 98. [Google Scholar] [CrossRef] [PubMed]
- Abadeer, N.S.; Murphy, C.J. Recent Progress in Cancer Thermal Therapy Using Gold Nanoparticles. J. Phys. Chem. C 2016, 120, 4691–4716. [Google Scholar] [CrossRef]
- Sztandera, K.; Gorzkiewicz, M.; Klajnert-Maculewicz, B. Gold Nanoparticles in Cancer Treatment. Mol. Pharmaceutics 2019, 16, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Kong, T.; Zeng, J.; Wang, X.; Yang, X.; Yang, J.; McQuarrie, S.; McEwan, A.; Roa, W.; Chen, J.; Xing, J.Z. Enhancement of Radiation Cytotoxicity in Breast-Cancer Cells by Localized Attachment of Gold Nanoparticles. small 2008, 4, 1537–1543. [Google Scholar] [CrossRef] [PubMed]
- Nabavinia, M.; Beltran-Huarac, J. Recent Progress in Iron Oxide Nanoparticles as Therapeutic Magnetic Agents for Cancer Treatment and Tissue Engineering. ACS Appl. Bio Mater. 2020, 3, 8172–8187. [Google Scholar] [CrossRef] [PubMed]
- Hosseinkazemi, H.; Samani, S.; O’Neill, A.; Soezi, M.; Moghoofei, M.; Azhdari, M.H.; Aavani, F.; Nazbar, A.; Keshel, S.H.; Doroudian, M. Applications of Iron Oxide Nanoparticles against Breast Cancer. Journal of Nanomaterials 2022, 2022, 1–12. [Google Scholar] [CrossRef]
- Soetaert, F.; Korangath, P.; Serantes, D.; Fiering, S.; Ivkov, R. Cancer Therapy with Iron Oxide Nanoparticles: Agents of Thermal and Immune Therapies. Adv Drug Deliv Rev 2020, 163–164, 65–83. [Google Scholar] [CrossRef]
- Geppert, M.; Himly, M. Iron Oxide Nanoparticles in Bioimaging – An Immune Perspective. Frontiers in Immunology 2021, 12. [Google Scholar] [CrossRef]
- Han, C.; Zhang, A.; Kong, Y.; Yu, N.; Xie, T.; Dou, B.; Li, K.; Wang, Y.; Li, J.; Xu, K. Multifunctional Iron Oxide-Carbon Hybrid Nanoparticles for Targeted Fluorescent/MR Dual-Modal Imaging and Detection of Breast Cancer Cells. Analytica Chimica Acta 2019, 1067, 115–128. [Google Scholar] [CrossRef]
- Deng, S.; Zhang, W.; Zhang, B.; Hong, R.; Chen, Q.; Dong, J.; Chen, Y.; Chen, Z.; Wu, Y. Radiolabeled Cyclic Arginine-Glycine-Aspartic (RGD)-Conjugated Iron Oxide Nanoparticles as Single-Photon Emission Computed Tomography (SPECT) and Magnetic Resonance Imaging (MRI) Dual-Modality Agents for Imaging of Breast Cancer. J Nanopart Res 2015, 17, 19. [Google Scholar] [CrossRef]
- Dennis, C.L.; Ivkov, R. Physics of Heat Generation Using Magnetic Nanoparticles for Hyperthermia. International Journal of Hyperthermia 2013, 29, 715–729. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Salvioni, L.; Malatesta, M.; Vurro, F.; Mannucci, S.; Gerosa, M.; Antonietta Rizzuto, M.; Tullio, C.; Degrassi, A.; Colombo, M.; et al. Colloidal Polymer-Coated Zn-Doped Iron Oxide Nanoparticles with High Relaxivity and Specific Absorption Rate for Efficient Magnetic Resonance Imaging and Magnetic Hyperthermia. Journal of Colloid and Interface Science 2020, 579, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, C.S.; Alves, É.A.R.; de Melo, C.P.; Corrêa-Oliveira, R.; Calzavara-Silva, C.E. Immunotherapy for Cancer: Effects of Iron Oxide Nanoparticles on Polarization of Tumor-Associated Macrophages. Nanomedicine (Lond) 2021, 16, 2633–2650. [Google Scholar] [CrossRef] [PubMed]
- Korangath, P.; Barnett, J.D.; Sharma, A.; Henderson, E.T.; Stewart, J.; Yu, S.-H.; Kandala, S.K.; Yang, C.-T.; Caserto, J.S.; Hedayati, M.; et al. Nanoparticle Interactions with Immune Cells Dominate Tumor Retention and Induce T Cell–Mediated Tumor Suppression in Models of Breast Cancer. Science Advances 2020, 6, eaay1601. [Google Scholar] [CrossRef] [PubMed]
- Zanganeh, S.; Hutter, G.; Spitler, R.; Lenkov, O.; Mahmoudi, M.; Shaw, A.; Pajarinen, J.S.; Nejadnik, H.; Goodman, S.; Moseley, M.; et al. Iron Oxide Nanoparticles Inhibit Tumour Growth by Inducing Pro-Inflammatory Macrophage Polarization in Tumour Tissues. Nat Nanotechnol 2016, 11, 986–994. [Google Scholar] [CrossRef] [PubMed]
- Norouzi, M.; Yathindranath, V.; Thliveris, J.A.; Kopec, B.M.; Siahaan, T.J.; Miller, D.W. Doxorubicin-Loaded Iron Oxide Nanoparticles for Glioblastoma Therapy: A Combinational Approach for Enhanced Delivery of Nanoparticles. Sci Rep 2020, 10, 11292. [Google Scholar] [CrossRef] [PubMed]
- Al-Obaidy, R.; Haider, A.J.; Al-Musawi, S.; Arsad, N. Targeted Delivery of Paclitaxel Drug Using Polymer-Coated Magnetic Nanoparticles for Fibrosarcoma Therapy: In Vitro and in Vivo Studies. Sci Rep 2023, 13, 3180. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.; Yang, F.-S.; Sivasankaran, V.P.; Lo, Y.-L.; Wu, Y.-T.; Chang, C.-Y.; Chiu, C.-C.; Liao, Z.-X.; Wang, L.-F. Comparing the Variants of Iron Oxide Nanoparticle-Mediated Delivery of miRNA34a for Efficiency in Silencing of PD-L1 Genes in Cancer Cells. Pharmaceutics 2023, 15, 215. [Google Scholar] [CrossRef]
- Yang, Z.; Duan, J.; Wang, J.; Liu, Q.; Shang, R.; Yang, X.; Lu, P.; Xia, C.; Wang, L.; Dou, K. Superparamagnetic Iron Oxide Nanoparticles Modified with Polyethylenimine and Galactose for siRNA Targeted Delivery in Hepatocellular Carcinoma Therapy. Int J Nanomedicine 2018, 13, 1851–1865. [Google Scholar] [CrossRef]
- Hosnedlova, B.; Kepinska, M.; Fernandez, C.; Peng, Q.; Ruttkay-Nedecky, B.; Milnerowicz, H.; Kizek, R. Carbon Nanomaterials for Targeted Cancer Therapy Drugs: A Critical Review. The Chemical Record 2019, 19, 502–522. [Google Scholar] [CrossRef]
- Tang, L.; Li, J.; Pan, T.; Yin, Y.; Mei, Y.; Xiao, Q.; Wang, R.; Yan, Z.; Wang, W. Versatile Carbon Nanoplatforms for Cancer Treatment and Diagnosis: Strategies, Applications and Future Perspectives. Theranostics 2022, 12, 2290–2321. [Google Scholar] [CrossRef] [PubMed]
- Roy, H.; Bhanja, S.; Panigrahy, U.P.; Theendra, V.K. Chapter 4 - Graphene-Based Nanovehicles for Drug Delivery. In Characterization and Biology of Nanomaterials for Drug Delivery; Micro and Nano Technologies; Mohapatra, S.S., Ranjan, S., Dasgupta, N., Mishra, R.K., Thomas, S., Eds.; Elsevier, 2019; pp. 77–111. ISBN 978-0-12-814031-4. [Google Scholar]
- Jampilek, J.; Kralova, K. Advances in Drug Delivery Nanosystems Using Graphene-Based Materials and Carbon Nanotubes. Materials (Basel) 2021, 14, 1059. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.C.; Lee, S.; Lalwani, G.; Suhrland, C.; Chowdhury, S.M.; Sitharaman, B. Graphene-Based Platforms for Cancer Therapeutics. Ther Deliv 2016, 7, 101–116. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, A.R.; Neves, M.G.P.M.S.; Trindade, T. Functionalization of Graphene Oxide with Porphyrins: Synthetic Routes and Biological Applications. ChemPlusChem 2020, 85, 1857–1880. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xia, J.; Zhao, Q.; Liu, L.; Zhang, Z. Functional Graphene Oxide as a Nanocarrier for Controlled Loading and Targeted Delivery of Mixed Anticancer Drugs. Small 2010, 6, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Abdelsayed, V.; Moussa, S.; Hassan, H.M.; Aluri, H.S.; Collinson, M.M.; El-Shall, M.S. Photothermal Deoxygenation of Graphite Oxide with Laser Excitation in Solution and Graphene-Aided Increase in Water Temperature. J. Phys. Chem. Lett. 2010, 1, 2804–2809. [Google Scholar] [CrossRef]
- Lu, X.; Yang, L.; Yang, Z. Photothermal Sensing of Nano-Devices Made of Graphene Materials. Sensors (Basel) 2020, 20, 3671. [Google Scholar] [CrossRef]
- Tang, L.; Xiao, Q.; Mei, Y.; He, S.; Zhang, Z.; Wang, R.; Wang, W. Insights on Functionalized Carbon Nanotubes for Cancer Theranostics. Journal of Nanobiotechnology 2021, 19, 423. [Google Scholar] [CrossRef]
- Oh, Y.; Jin, J.-O.; Oh, J. Photothermal-Triggered Control of Sub-Cellular Drug Accumulation Using Doxorubicin-Loaded Single-Walled Carbon Nanotubes for the Effective Killing of Human Breast Cancer Cells. Nanotechnology 2017, 28, 125101. [Google Scholar] [CrossRef]
- Wen, Z.; Feng, Y.; Hu, Y.; Lian, L.; Huang, H.; Guo, L.; Chen, S.; Yang, Q.; Zhang, M.; Wan, L.; et al. Multiwalled Carbon Nanotubes Co-Delivering Sorafenib and Epidermal Growth Factor Receptor siRNA Enhanced Tumor-Suppressing Effect on Liver Cancer. Aging (Albany NY) 2021, 13, 1872–1882. [Google Scholar] [CrossRef]
- Yoong, S.L.; Wong, B.S.; Zhou, Q.L.; Chin, C.F.; Li, J.; Venkatesan, T.; Ho, H.K.; Yu, V.; Ang, W.H.; Pastorin, G. Enhanced Cytotoxicity to Cancer Cells by Mitochondria-Targeting MWCNTs Containing Platinum(IV) Prodrug of Cisplatin. Biomaterials 2014, 35, 748–759. [Google Scholar] [CrossRef] [PubMed]
- Acquah, S.F.; Penkova, A.V.; Markelov, D.A.; Semisalova, A.S.; Leonhardt, B.E.; Magi, J.M. The Beautiful Molecule: 30 Years of C60 and Its Derivatives. ECS Journal of Solid State Science and Technology 2017, 6, M3155. [Google Scholar] [CrossRef]
- Chen, Z.; Ma, L.; Liu, Y.; Chen, C. Applications of Functionalized Fullerenes in Tumor Theranostics. Theranostics 2012, 2, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Han, X.; Li, S.; Al-Youbi, A.O.; Bashammakh, A.S.; El-Shahawi, M.S.; Leblanc, R.M. Carbon Dots: Biomacromolecule Interaction, Bioimaging and Nanomedicine. Coordination Chemistry Reviews 2017, 343, 256–277. [Google Scholar] [CrossRef]
- Jia, Q.; Zhao, Z.; Liang, K.; Nan, F.; Li, Y.; Wang, J.; Ge, J.; Wang, P. Recent Advances and Prospects of Carbon Dots in Cancer Nanotheranostics. Mater. Chem. Front. 2020, 4, 449–471. [Google Scholar] [CrossRef]
- Goh, E.J.; Kim, K.S.; Kim, Y.R.; Jung, H.S.; Beack, S.; Kong, W.H.; Scarcelli, G.; Yun, S.H.; Hahn, S.K. Bioimaging of Hyaluronic Acid Derivatives Using Nanosized Carbon Dots. Biomacromolecules 2012, 13, 2554–2561. [Google Scholar] [CrossRef] [PubMed]
- Lei, D.; Yang, W.; Gong, Y.; Jing, J.; Nie, H.; Yu, B.; Zhang, X. Non-Covalent Decoration of Carbon Dots with Folic Acid via a Polymer-Assisted Strategy for Fast and Targeted Cancer Cell Fluorescence Imaging. Sensors and Actuators B: Chemical 2016, 230, 714–720. [Google Scholar] [CrossRef]
- Li, Q.; Ohulchanskyy, T.Y.; Liu, R.; Koynov, K.; Wu, D.; Best, A.; Kumar, R.; Bonoiu, A.; Prasad, P.N. Photoluminescent Carbon Dots as Biocompatible Nanoprobes for Targeting Cancer Cells in Vitro. J. Phys. Chem. C 2010, 114, 12062–12068. [Google Scholar] [CrossRef]
- Calabrese, G.; De Luca, G.; Nocito, G.; Rizzo, M.G.; Lombardo, S.P.; Chisari, G.; Forte, S.; Sciuto, E.L.; Conoci, S. Carbon Dots: An Innovative Tool for Drug Delivery in Brain Tumors. Int J Mol Sci 2021, 22, 11783. [Google Scholar] [CrossRef]
- Zeng, Q.; Shao, D.; He, X.; Ren, Z.; Ji, W.; Shan, C.; Qu, S.; Li, J.; Chen, L.; Li, Q. Carbon Dots as a Trackable Drug Delivery Carrier for Localized Cancer Therapy in Vivo. J. Mater. Chem. B 2016, 4, 5119–5126. [Google Scholar] [CrossRef]
- Liu, W.; Speranza, G. Functionalization of Carbon Nanomaterials for Biomedical Applications. C 2019, 5, 72. [Google Scholar] [CrossRef]
- Landen, C.N.; Kinch, M.S.; Sood, A.K. EphA2 as a Target for Ovarian Cancer Therapy. Expert Opinion on Therapeutic Targets 2005, 9, 1179–1187. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tian, S.; Petros, R.A.; Napier, M.E.; DeSimone, J.M. The Complex Role of Multivalency in Nanoparticles Targeting the Transferrin Receptor for Cancer Therapies. J. Am. Chem. Soc. 2010, 132, 11306–11313. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.M.; Chess, R.B. Effect of Pegylation on Pharmaceuticals. Nat Rev Drug Discov 2003, 2, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Valencia, S.; Vargas, X.; Rios, L.; Restrepo, G.; Marín, J.M. Sol–Gel and Low-Temperature Solvothermal Synthesis of Photoactive Nano-Titanium Dioxide. Journal of Photochemistry and Photobiology A: Chemistry 2013, 251, 175–181. [Google Scholar] [CrossRef]
- Gref, R.; Lück, M.; Quellec, P.; Marchand, M.; Dellacherie, E.; Harnisch, S.; Blunk, T.; Müller, R.H. ‘Stealth’ Corona-Core Nanoparticles Surface Modified by Polyethylene Glycol (PEG): Influences of the Corona (PEG Chain Length and Surface Density) and of the Core Composition on Phagocytic Uptake and Plasma Protein Adsorption. Colloids and Surfaces B: Biointerfaces 2000, 18, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Bazak, R.; Houri, M.; El Achy, S.; Kamel, S.; Refaat, T. Cancer Active Targeting by Nanoparticles: A Comprehensive Review of Literature. Journal of cancer research and clinical oncology 2015, 141, 769–784. [Google Scholar] [CrossRef] [PubMed]
- Tran, S.; DeGiovanni, P.-J.; Piel, B.; Rai, P. Cancer Nanomedicine: A Review of Recent Success in Drug Delivery. Clin Trans Med 2017, 6, 44. [Google Scholar] [CrossRef]
- Ahmed, N.; Fessi, H.; Elaissari, A. Theranostic Applications of Nanoparticles in Cancer. Drug discovery today 2012, 17, 928–934. [Google Scholar] [CrossRef]
- Zhao, C.-Y.; Cheng, R.; Yang, Z.; Tian, Z.-M. Nanotechnology for Cancer Therapy Based on Chemotherapy. Molecules 2018, 23, 826. [Google Scholar] [CrossRef]
- Figueiredo, P.; Bauleth-Ramos, T.; Hirvonen, J.; Sarmento, B.; Santos, H.A. Chapter 1 - The Emerging Role of Multifunctional Theranostic Materials in Cancer Nanomedicine. In Handbook of Nanomaterials for Cancer Theranostics; Conde, J., Ed.; Elsevier, 2018; pp. 1–31 ISBN 978-0-12-813339-2.
- Zhu, L.; Yang, L.; Zhou, Z. Nanomaterials in Cancer Theranostics. In Bioactivity of Engineered Nanoparticles; Yan, B., Zhou, H., Gardea-Torresdey, J.L., Eds.; Nanomedicine and Nanotoxicology; Springer: Singapore, 2017; ISBN 978-981-10-5864-6. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).