1. Introduction
Oligohydramnios is one of the frequently reported complications of pregnancy [
1]. The prevalence of oligohydramnios in China is approximately 0.42% - 4.16% and the number of cases is on the rise year on year [
2]. Oligohydramnios is one of the major risk factors for adverse pregnancy outcomes. It has been noted in the study [
3] that timely and effective clinical interventions should be offered to pregnant women with oligohydramnios, as failure of which may contribute to intrauterine fetal distress and faecal contamination of amniotic fluid. A foreign literature on this topic [
4] has revealed that the risk of intrauterine fetal distress and the risk of perinatal death are considerably increased when a pregnant woman's amniotic fluid volume is severely inadequate, with serious implications for the safety and health of both mother and infant.
In the previous clinical practice, oligohydramnios was mainly treated by intravenous rehydration and simple water intake, which could increase the volume of amniotic fluid for a short period of time, but once the intervention was discontinued, the amniotic fluid in the pregnant woman would still decline rapidly, which rendered a poor overall outcome of the above-mentioned treatment [
5]. The combination of low molecular heparin sodium and aspirin has been effective in the treatment of pregnant women with oligohydramnios in recent years, but shortcomings in this treatment included the necessity to individualise the dose[
6] and the fact that some studies[
7] concluded that high doses of low molecular heparin sodium and aspirin can affect the coagulation function of the pregnant body, which resulted in a limited acceptance of this method in some patients. According to Chinese medicine, oligohydramnios in pregnant women belongs to the category of "hypoplasticity" and its occurrence is strongly related to the maternal kidney and spleen [
8]. Based on this, some clinical scholars have proposed to treat pregnant women with herbal regimens such as benefiting the kidneys and strengthening the spleen from TCM theory [
9]. In this study, based on the above theory, 114 pregnant women with oligohydramnios in pregnancy admitted to our hospital between May 2019 and March 2022 were selected as the study subjects, and its aim was to observe the effect of the combination of fluid-increasing decoction, low molecular heparin sodium and aspirin in the treatment of oligohydramnios in pregnancy, and the findings were reported as follows.
2. Objects and Methods
2.1. Research object
In this study, 114 pregnant women with oligohydramnios during pregnancy admitted to our hospital from May 2019 to March 2022 were selected as the research objects. The general data of pregnant women including age, gestational age, BMI, and whether they had a first birth were collected. Pregnant women were divided into the observation group and the experimental group by random number table method, with 57 cases in each group. This study was approved by the Ethics Committee of Shiyan maternal and Child Health Hospital, No.9875913.
2.2. Inclusion and exclusion criteria
Inclusion criteria: 1) pregnant women who were diagnosed with oligohydramnios by relevant clinical tests; 2) pregnant women with singleton pregnancies; 3) pregnant women who were examined for the absence of maternal and fetal related diseases; 4) pregnant women and their families who were informed about the study and signed the relevant informed consent forms.
Exclusion criteria: 1) pregnant women with underlying diseases such as hypertension and diabetes; 2) pregnant women with abnormal coagulation and metabolic functions; 3) pregnant women with premature rupture of the placenta, ageing placenta and umbilical cord nodes; 4) pregnant women with infectious diseases; 5) pregnant women with immune diseases; 6) pregnant women with mental illness or communication disorders; 7) pregnant women with severe organ functional diseases; 8) pregnant women with allergies or contraindications to the drugs used in the study.
2.3. Methods
In the observation group, pregnant women received a combination of conventional fluid, low molecular heparin sodium and aspirin: 1000 ml of balanced fluid + 500 ml of glucose injection + 3 g of vitamin C were used for routine rehydration. Low molecular heparin sodium (Laboratoire Bellon, registration number X20000391) was injected subcutaneously (5000 U/d, 1 time/d). Aspirin (Bayer Healthcare Ltd., GMP J20171021) was taken orally (25 mg/dose, 3 times/d). Low molecular heparin sodium was discontinued until 1 d before delivery, and aspirin was discontinued after 36 W of pregnancy.
(2) In the experimental group, pregnant women were treated with fluid-increasing decoction in addition to the treatment given to pregnant women in the observation group: conventional rehydration, low molecular heparin sodium and aspirin were administered in the same way, dosage and frequency as in the observation group. Meanwhile, pregnant women in the experimental group were given fluid-increasing decoction. The formula of fluid-increasing decoction was composed of 10g each of poria, rehmannia, rhizoma chuanxiong, salvia, atractylodes, angelica, peony and medlar, and 15g each of codonopsis and astragalus. The formula was administered by decoction in water, once in the morning and once in the evening. This formula should be taken continuously for 14 d.
2.4. Observation indicators
(1) Treatment outcome: cured: amniotic fluid index (AFI) ≥80 mm on post-treatment review; effective: AFI ≥50 mm but <80 mm on post-treatment review; invalid: AFI <50 mm on post-treatment review.
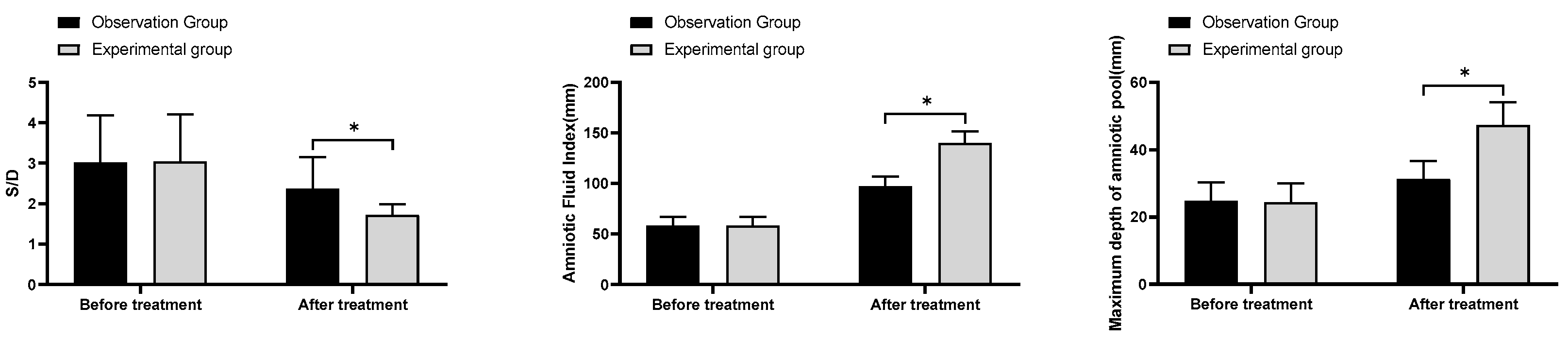
(2) S/D, amniotic fluid index, maximum vertical pocket (MVP): The S/D, amniotic fluid index and MVP of pregnant women were measured and assessed using a Doppler flow analyser before and after treatment.
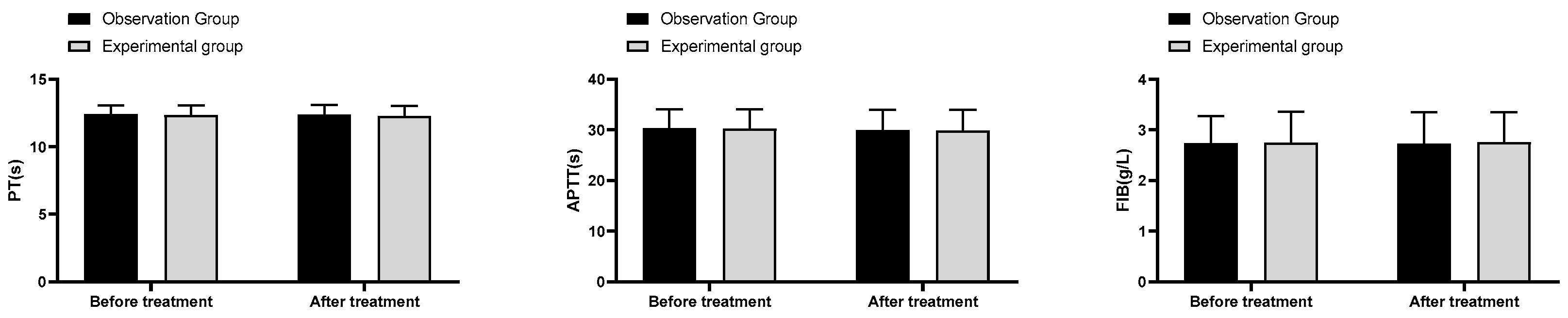
(3) Coagulation indexes: 4ml of fasting venous blood was sampled from pregnant women in the morning before and after treatment, and the blood was routinely centrifuged and sent for examination. The plasma prothrombin time (PT), plasma activated partial thromboplastin time (APTT) and fibrinogen (FIB) levels were assessed using a haemagglutination analyser.
(4) Pregnancy outcome profile: Pregnancy outcomes observed in this study consisted of the rate of oligohydramnios at delivery, rate of preterm delivery, rate of caesarean section, rate of postpartum haemorrhage, neonatal birth weight, rate of preterm delivery, rate of neonatal asphyxia, and rate of amniotic fluid contamination.
(5) Medication safety: The adverse reactions of pregnant women in both groups were recorded. The adverse reactions observed in this study included: subcutaneous haematoma, haemorrhage and thrombocytopenia.
2.5. Statistical methods
The data of this study were collated and analysed using SPSS 22.0. The measurement data were expressed as mean ± standard deviation (±s) and compared using t-test; the count data were presented as number of cases (rate) and tested using x². P < 0.05 indicates statistically significant differences. GraphPad Prism 8 was utilized as the graphing software.
4. Discussion
Oligohydramnios is a condition specific to pregnant women during pregnancy, a condition arising from multiple factors such as fetal malformations of the kidney and urethra [
10]. oligohydramnios is an indicator of intrauterine risk, which might lead to fetal malformation and distress, and is responsible for a marked increase in the rate of caesarean section and perinatal morbidity and mortality, so early detection and active treatment is essential to ensure the safety of mother and child [
11]. The previous Western medical treatments have consisted of water alone, intravenous fluids and low molecular heparin combined with aspirin for anticoagulation [
12]. The premise of water or fluids alone to increase maternal amniotic fluid volume is the presence of adequate uteroplacental perfusion and a good fetal renal response, and the inability of these methods to produce the biological activity of the drug, which leads to some ineffective intervention in amniotic fluid formation and absorption [
13]. Aspirin and low molecular weight heparin sodium are both commonly used anticoagulants in clinical practice. Aspirin is also known clinically as acetylsalicylic acid and the use of this drug can effectively promote the acetylation reaction of platelet cyclooxygenase, thereby preventing the formation of thrombotic conditions [
14]. Low molecular heparin sodium is a substance isolated from heparin which, when combined with antithrombin III, exerts a prominent anticoagulant effect [
15]. In a study [
16], aspirin combined with low molecular weight heparin sodium was found to be effective in the treatment of oligohydramnios in pregnant women, which could effectively improve the hypercoagulable state of the blood and placental perfusion, thereby improving the placental function of the pregnant woman and thus increasing her body amniotic fluid volume. However, domestic and international studies are rare in the treatment of low amniotic fluid with low molecular heparin sodium in combination with aspirin, and the effect of the combination is mostly seen in pre-eclampsia and fetal growth restriction, and some literature [
17] pointed out that the safety of the dosage of low molecular heparin sodium in combination with aspirin treatment remains controversial. Therefore, safe and effective treatment options that are accessible to patients with oligohydramnios have emerged as a pressing challenge for obstetricians and gynaecologists.
According to Chinese medicine, oligohydramnios pregnant women with poor appetite and loose stools, soreness and weakness of the waist and knees, and sluggishness of the pulse are often seen in "fetal restlessness" and "fetal atrophy and failure to grow" [
18]. In this study, the fluid-increasing decoction is composed of several Chinese herbs, including Poria, Salvia and Radix Codonopsis, in which the function of Radix Codonopsis is to benefit the kidneys and strengthen the spleen, generate fluid and nourish the qi; the function of Salvia is to invigorate blood stasis, cool the blood and eliminate carbuncles; the function of Radix Astragali is to generate blood and nourish the qi, benefit the qi and raise the yang; the function of Poria and Atractylodes are to nourish the yin and benefit the qi; the function of Medlar and Radix Rehmannia are to benefit the essence and nourish the spleen, nourish the yin and nourish the blood, calm the foetus and nourish the blood; the function of Radix Angelicae Sinensis is to resolve blood stasis and invigorate the blood, nourish the blood and nourish the yin. The combination of the above-mentioned herbs can be used to tonify the kidneys and calm the foetus, nourish the blood and promote the production of body fluid, invigorate the blood and nourish the yin. Modern pharmacology also expressed that Astragalus saponin and Astragalus polysaccharide, the main components of Astragalus, have good vasodilating effects, which can effectively enhance capillary resistance and inhibit platelet coagulation in pregnant women, thus helping to promote the production and development of blood cells in pregnant women [
20]. At the same time, Astragalus also exhibits certain fetoprotective effects, with its application markedly increasing maternal blood volume, thereby improving maternal blood circulation and consequently fetal circulating blood volume and amniotic fluid volume [
21]. Salvia can effectively reduce maternal plasma osmolality, thereby increasing maternal blood flow and amniotic perfusion, which in turn promotes fetal urine content in the body to increase maternal amniotic fluid volume, a key to fluid-increasing decoction to reduce the risk of fetal malpresentation and improve maternal pregnancy outcomes [
22]. Based on the above theory, the use of fluid-increasing decoction can effectively increase maternal blood volume, thereby improving fetal blood circulation and amniotic fluid volume, and thus reducing the risk of adverse pregnancy outcomes in pregnant women. The aim of this study was to investigate the effect of the combination of fluid-increasing decoction, low molecular heparin sodium and aspirin in the treatment of oligohydramnios during pregnancy.
In this study, the total effective rate of treatment in the experimental group was remarkably better than that in the observation group; the S/D level after treatment in the experimental group was considerably lower than that in the observation group; the amniotic fluid index and MVP after treatment in the experimental group were noticeably higher than those in the observation group. The above results suggested that the combination of fluid-increasing decoction, low molecular heparin sodium and aspirin was effective in treating pregnant women with oligohydramnios during pregnancy, and this combination regimen could effectively enhance the MVP and amniotic fluid index of pregnant women and improve the fetal cord blood flow ratio. Oligohydramnios can seriously affect perinatal prognosis, and increasing maternal amniotic fluid volume at delivery is essential to improve pregnancy outcomes. The results of this study indicated that the rate of oligohydramnios at delivery, cesarean section, neonatal weight and preterm birth were substantially better in the experimental group than in the observation group, which demonstrated that the use of fluid-increasing decoction could further reduce the risk of adverse outcomes in pregnant women. In the final comparison of coagulation indicators and safety of medication, no significant differences were found in the incidence of PT, APTT, FIB and adverse reactions between the two groups before and after treatment (P>0.05). The above results indicated that the addition of fluid-increasing decoction to the combination of low molecular heparin sodium and aspirin did not affect maternal coagulation parameters and the occurrence of adverse reactions.
Deficiencies in this study, such as small sample size and single-centre nature, may lead to some risk of bias in the assessment. Therefore, in future studies, we should improve the deficiencies of this study accordingly in order to further investigate the effects of fluid-increasing decoction in the adjuvant treatment of pregnant women with oligohydramnios during pregnancy.