Submitted:
09 October 2023
Posted:
16 October 2023
You are already at the latest version
Abstract
Keywords:
Introduction
Methods
Scaffold Preparation
Density Measurement
hADSC and PRP Preparation
Cell Culture Procedure
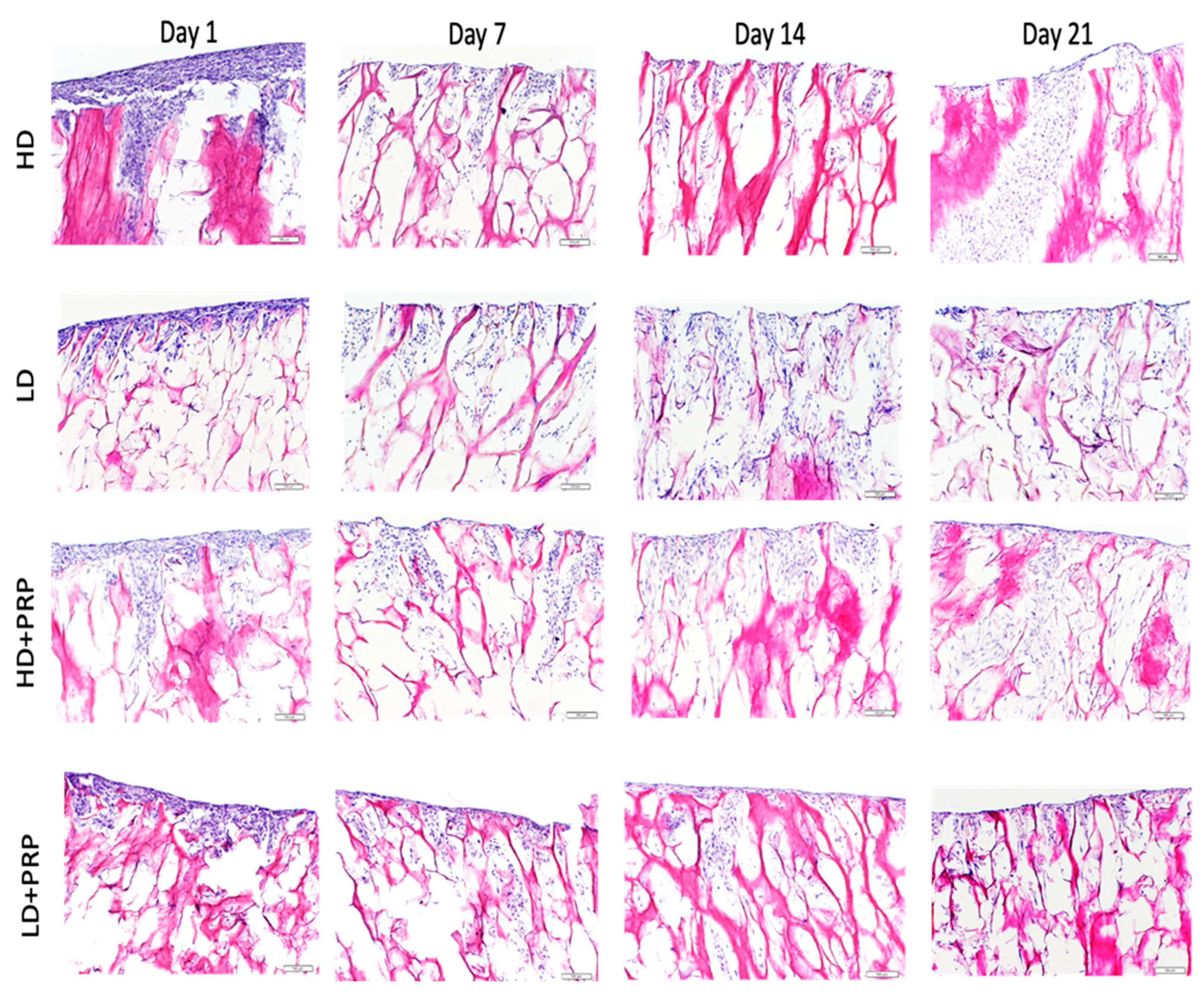
Histological study
Statistical Analysis
Results
Density measurement
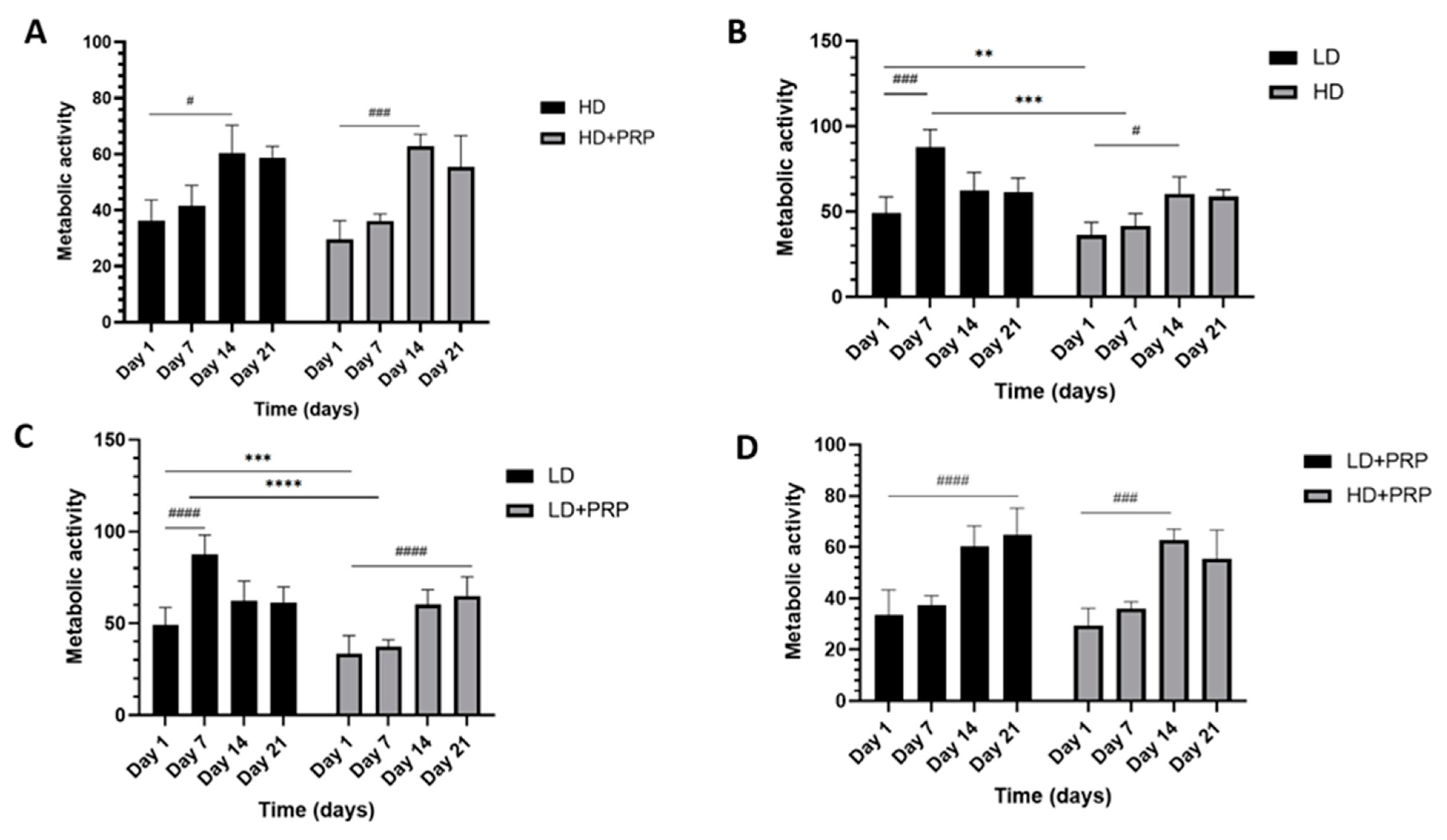
In-vitro cellular proliferation of hADSCs on 3D LD and HD scaffolds
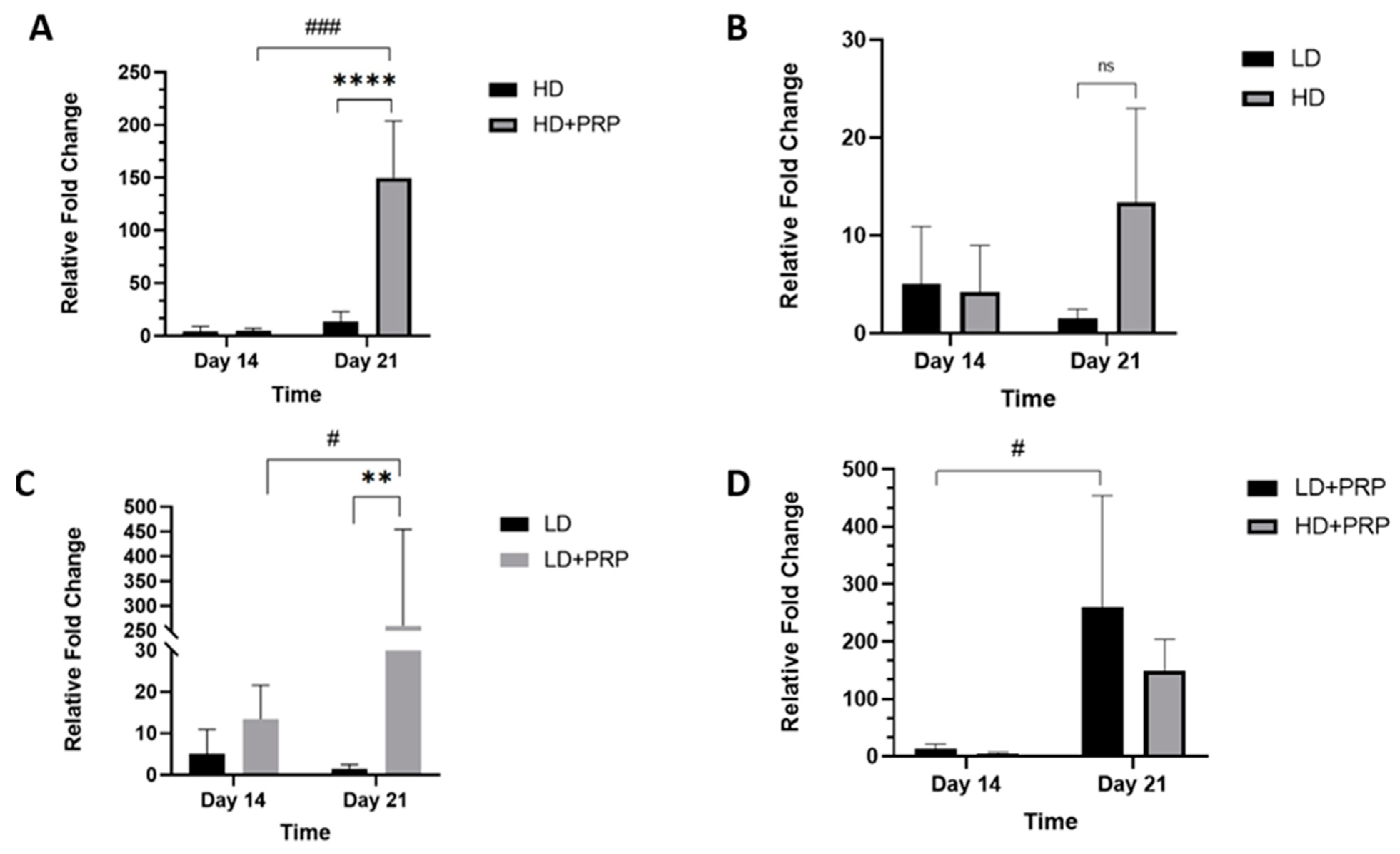
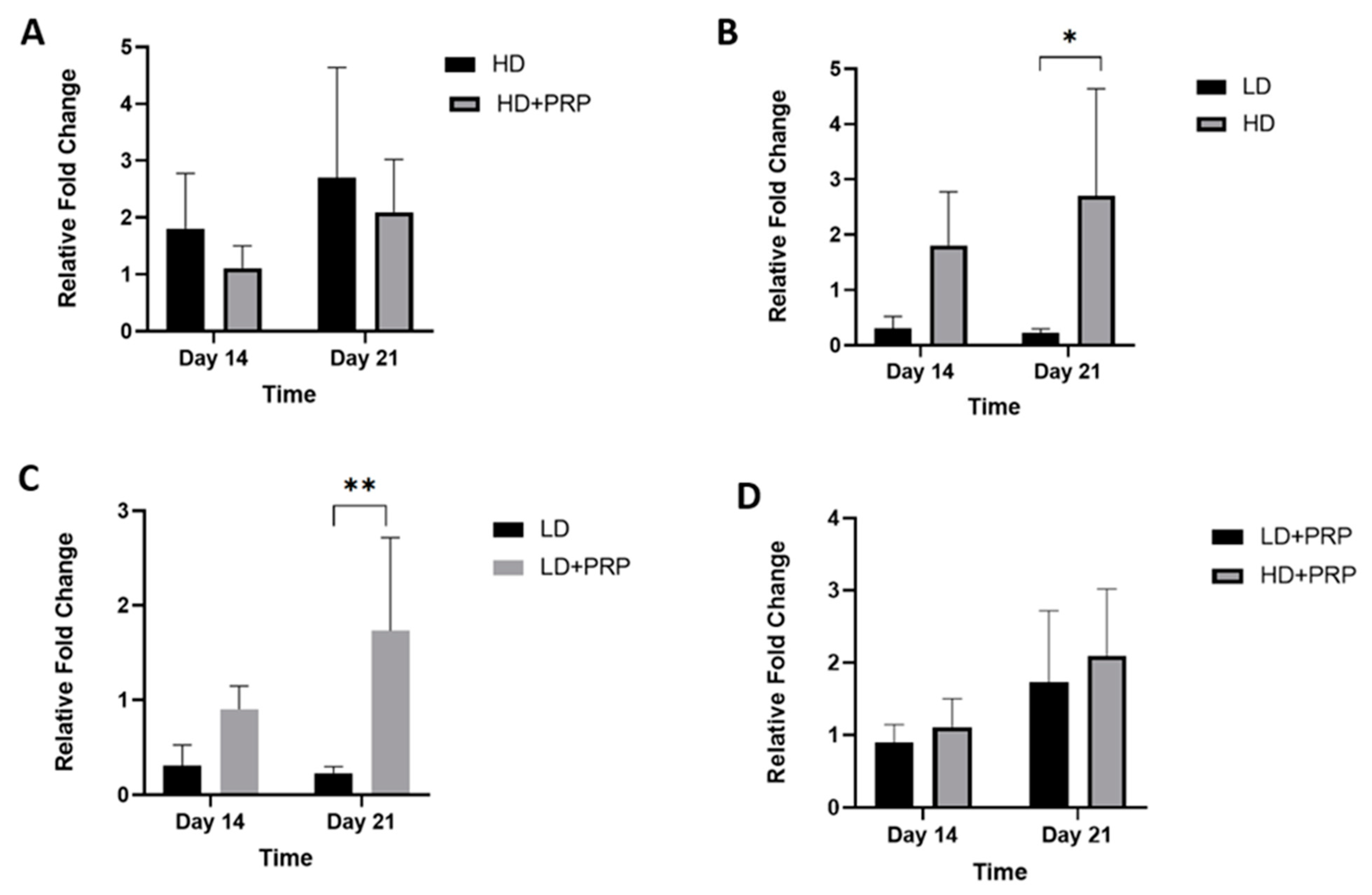
Fibrochondrogenic potential of hADSCs cultured on 3D scaffolds for meniscus tissue repair
In-vitro cell infiltration of hADSCs seeded on 3D LD and HD collagen scaffolds
Discussion
Future research
Limitations of the study
Conclusion
Acknowledgements
References
- Tan GK, Cooper-White JJ. Interactions of meniscal cells with extracellular matrix molecules: towards the generation of tissue engineered menisci. Cell Adh Migr. 2011 May-Jun;5(3):220-6. [CrossRef]
- Eyre DR, Wu JJ. Collagen of fibrocartilage: a distinctive molecular phenotype in bovine meniscus. FEBS Lett. 1983 Jul 25;158(2):265-70. [CrossRef]
- Luvsannyam E, Jain MS, Leitao AR, Maikawa N, Leitao AE. Meniscus Tear: Pathology, Incidence, and Management. Cureus. 2022;14(5): e25121. [CrossRef]
- UCSF Health. Overview Orthopedics: Meniscus Tear. https://www.ucsfhealth.org/conditions/meniscus-tear#:~:text=Meniscus%20tears%20are%20among%20the,shaped%20structures%20made%20of%20cartilage; 2023 Accessed 5 May 2023. 5 May.
- Jacob G, Shimomura K, Krych AJ, Nakamura N. The Meniscus Tear: A Review of Stem Cell Therapies. Cells. 2019;9(1):92. [CrossRef]
- Li, Z, Weng, X. Platelet-rich plasma use in meniscus repair treatment: a systematic review and meta-analysis of clinical studies. J Orthop Surg Res. 2022;17(01). [CrossRef]
- Freymann U, Degrassi L, Krüger J, Metzlaff S, Endres M, Petersen W. Effect of serum and platelet-rich plasma on human early or advanced degenerative meniscus cells. Connect Tissue Res. 2017;58:6,509-519. [CrossRef]
- Özyalvaç O, Tuzuner T, Gurpinar T, Obut A, Acar B, Emre Y. Radiological and functional outcomes of ultrasound-guided PRP injections in intrasubstance meniscal degenerations. J of Orthop Surg. 2019;27:2. [CrossRef]
- Sihvonen R, Paavola M, Malmivaara A, Itälä A, Joukainen A, Kalske J, Nurmi H, Kumm J, Sillanpää N, Kiekara T, Turkiewicz A, Toivonen P, Englund M, Taimela S, Järvinen TLN; FIDELITY (Finnish Degenerative Meniscus Lesion Study) Investigators. Arthroscopic partial meniscectomy for a degenerative meniscus tear: a 5 year follow-up of the placebo-surgery controlled FIDELITY (Finnish Degenerative Meniscus Lesion Study) trial. Br J Sports Med. 2020;54(22):1332-1339.
- Alessio-Mazzola M, Felli L, Trentini R. et al. Efficacy of Autologous Platelet-Rich Plasma Injections for Grade 3 Symptomatic Degenerative Meniscal Lesions: A 1-Year Follow-up Prospective Study. Sports Health. 2021;14:2. [CrossRef]
- Freyman U, Metzlaff S, Kruger J-P. et al. Effect of Human Serum and 2 Different Types of Platelet Concentrates on Human Meniscus Cell Migration, Proliferation, and Matrix Formation. Arthroscopy. 2016;32:6,1106-1116. [CrossRef]
- Rodkey, W.G., et al., Comparison of the collagen meniscus implant with partial meniscectomy: a prospective randomized trial. J Bone Joint Surg Am. 2008. 90(7): p. 1413-1426.
- Zaffagnini, S., et al., Two-year clinical results of lateral collagen meniscus implant: a multicenter study. Arthroscopy: The Journal of Arthroscopic & Related Surgery, 2015. 31(7): p. 1269-1278.
- Li, S.-T., et al., Type I collagen-based template for meniscus regeneration. Tissue engineering and biodegradable equivalents. Scientific and clinical applications, 2002: p. 237-266.
- Stone, K.R., et al., Regeneration of meniscal cartilage with use of a collagen scaffold. Analysis of preliminary data. JBJS, 1997. 79(12): p. 1770.
- Monllau, J.C., et al., Outcome after partial medial meniscus substitution with the collagen meniscal implant at a minimum of 10 years’ follow-up. Arthroscopy: The Journal of Arthroscopic & Related Surgery, 2011. 27(7): p. 933-943.
- Verdonk PC, Demurie A, Almqvist KF, Veys EM, Verbruggen G, Verdonk R. Transplantation of viable meniscal allograft. Survivorship analysis and clinical outcome of one hundred cases. J Bone Joint Surg Am. 2005. 87(4):715-24.
- Verdonk PC, Verstraete KL, Almqvist KF, De Cuyper K, Veys EM, Verbruggen G, Verdonk R. Meniscal allograft transplantation: long-term clinical results with radiological and magnetic resonance imaging correlations. Knee Surg Sports Traumatol Arthrosc. 2006 Aug;14(8):694-706.
- Lee SR, Kim JG, Nam SW. The tips and pitfalls of meniscus allograft transplantation. Knee Surg Relat Res. 2012 Sep;24(3):137-45.
- Pereira, H, et al. Tissue engineering and regenerative medicine strategies in meniscus lesions. Arthroscopy: The journal of arthroscopic & related surgery, 27.12 (2011): 1706-1719. [CrossRef]
- Muran A, Schaffler B, Wong. et al. Effect of increasing hyaluronic acid content in collagen scaffolds on the maintenance of chondrogenic phenotype in chondrocytes and mesenchymal stem cells. JCJP. 2023:100099. [CrossRef]
- Li, ST and Stone KR, Meniscal Augmentation Device (2000), U.S. Patent No. 6,042,610.
- Li ST. Biopolymer-based meniscus implant. 17/625,825. 2022. U.S. Patent Application.
- Harley BA, Kim HD, Zaman MH, Yannas IV, Lauffenburger DA, Gibson LJ. Microarchitecture of three-dimensional scaffolds influences cell migration behavior via junction interactions. Biophys J. 2008; 95(8):4013-24.
- Murphy CM, O’Brien FJ. Understanding the effect of mean pore size on cell activity in collagen-glycosaminoglycan scaffolds. Cell Adh Migr. 2010;4(3):377-81.
- Somo SI, Akar B, Bayrak ES, Larson JC, Appel AA, Mehdizadeh H, Cinar A, Brey EM. Pore Interconnectivity Influences Growth Factor-Mediated Vascularization in Sphere-Templated Hydrogels. Tissue Eng Part C Methods. 2015;21(8):773-85.
- Yang YL, Motte S, Kaufman LJ. Pore size variable type I collagen gels and their interaction with glioma cells. Biomaterials. 2010;31(21):5678-88.
- Buenzli PR, Lanaro M, Wong CS, McLaughlin MP, Allenby MC, Woodruff MA, Simpson MJ. Cell proliferation and migration explain pore bridging dynamics in 3D printed scaffolds of different pore size. Acta Biomater. 2020;114:285-295.
- O’Brien FJ. Biomaterials & scaffolds for tissue engineering. Mater Today. 2011;14 (3):88-95.
- Bružauskaitė I, Bironaitė D, Bagdonas E, Bernotienė E. Scaffolds and cells for tissue regeneration: different scaffold pore sizes-different cell effects. Cytotechnology. 2016 May;68(3):355-69.
- Han Y, Lian M, Wu Q, Qiao Z, Sun B, Dai K. Effect of Pore Size on Cell Behavior Using Melt Electrowritten Scaffolds. Front Bioeng Biotechnol. 2021;9:629270.
- Armingol E, Officer A, Harismendy O, Lewis NE. Deciphering cell-cell interactions and communication from gene expression. Nat Rev Genet. 2021;22(2):71-88.
- Goldstein B, Dembo M. Approximating the effects of diffusion on reversible reactions at the cell surface: ligand-receptor kinetics. Biophys J. 1995;68(4):1222-30.
- Endres RG, Wingreen NS. Accuracy of direct gradient sensing by single cells. Proc Natl Acad Sci USA. 2008;105(41):15749-54.
- Stessuk T, Puzzi MB, Chaim EA, Alves PC, de Paula EV, Forte A, Izumizawa JM, Oliveira CC, Frei F, Ribeiro-Paes JT. Platelet-rich plasma (PRP) and adipose-derived mesenchymal stem cells: stimulatory effects on proliferation and migration of fibroblasts and keratinocytes in vitro. Arch Dermatol Res. 2016;308(7):511-20.
- Akhmanova M, Osidak E, Domogatsky S, Rodin S, Domogatskaya A. Physical, spatial, and molecular aspects of extracellular matrix of in vivo niches and artificial scaffolds relevant to stem cells research. Stem Cells Int. 2015 Aug 16;2015.
- Matsiko A, Levingstone T, O’Brien F, Gleeson J. Addition of hyaluronic acid improves cellular infiltration and promotes early-stage chondrogenesis in a collagen-based scaffold for cartilage tissue engineering. J Mech Behav Biomed Mater. 2012;11:41-52. [CrossRef]
- Ruprecht JC, Waanders T, Rowland, C. et al. Meniscus-Derived Matrix Scaffolds Promote the Integrative Repair of Meniscal Defects. Sci Rep. 2019;9, 8719.
- Oda S, Otsuki S, Kurokawa Y. et al. A new method for meniscus repair using type I collagen scaffold and infrapatellar fat pad. J. Biomater. Appl. 2015;29(10). [CrossRef]
- Li ST, Yuen D, Li PC, Rodkey WG, Stone KR. Collagen as a biomaterial: an application in knee meniscal fibrocartilage regeneration. MRS Online Proceedings Library (OPL). 1993;331:25.
- Warth, R.J. and W.G. Rodkey, Resorbable collagen scaffolds for the treatment of meniscus defects: a systematic review. Arthroscopy: The Journal of Arthroscopic & Related Surgery 2015. 31(5): p. 927-941.
- Korpershoek J, de Windt T, Hagmeijer M, Vonk L, Saris D. Cell-based meniscus repair and regeneration: at the brink of clinical translation? A systematic review of preclinical studies. Orthop J Sports Med. 2017 5(2). [CrossRef]
- Hutchinson I, Rodeo S, Perrone G, Murray M. Can Platelet-Rich Plasma Enhance Anterior Cruciate Ligament and Meniscal Repair. J Knee Surg 2015; 28(01): 019-028. [CrossRef]
- Kwak H, Nam J, Lee J, Kim H, Yoo, J. Meniscal repair in vivo using human chondrocyte-seeded PLGA mesh scaffold pretreated with platelet-rich plasma. J Tissue Eng Regen Med. 2014;(2):471-480. [CrossRef]
- Ishida K, Kuroda R, Miwa, M. et al. The regenerative effects of platelet-rich plasma on meniscal cells in vitro and its in vivo application with biodegradable gelatin hydrogel. Tissue Eng. 2007;13(5):1103-1112. [CrossRef]
- Blough C, Bobba C, DiBartola A. et al. Biologic augmentation during meniscal repair. J. Knee Surg. 2021;36(05):498-506. [CrossRef]
- Migliorini F, Cuozzo F, Cipollaro L, Oliva F, Hildebrand F, Maffulli N. Platelet-rich plasma (PRP) augmentation does not result in more favourable outcomes in arthroscopic meniscal repair: a meta-analysis. JOTR 2022;23(1). [CrossRef]
- Belk JW, Kraeutler MJ, Thon SG, Littlefield CP, Smith JH, McCarty EC. Augmentation of meniscal repair with platelet-rich plasma: A systematic review of comparative studies. Orthop J Sports Med. 2020 Jun 17;8(6):2325967120926145. [CrossRef]
- Kelly, J, Jacobs R. The role of mechanical signals in regulating chondrogenesis and osteogenesis of mesenchymal stem cells. Birth Defects Res C Embryo Today 2010 Mar;90(1):75-85. [CrossRef]
| Primers | Sequences |
|---|---|
| TYPE I COLLAGEN | 5′-ATG CCA TCA AAG TCT TCT GCA A-3′ Forward$$$5′-CTT-GGG-GTT-CTT-GCT-GAT-GTA-C-3′ Reverse |
| AGGRECAN | 5′-CTA-CAT-TGG-TGG-AAG-TGG-TCA-C3′ Forward$$$5′-CCA CTA-GCT-CTC-CCA-CTA-ATG-T-3′ Reverse |
| GAPDH | 5′-ACC-CAG-AAG-ACT-GTG-GAT-GG-3′ Forward$$$5′-GAG-GCA-GGG-ATG-ATG-TTC-TG-3′ Reverse |
| Low Density (LD) | High Density (HD) | |||
|---|---|---|---|---|
| Overall Density (g/cm3) | 0.15±0.01 | 0.19±0.01 | ||
| Partial Density (g/cm3) | Inner rim | Outer rim | Inner rim | Outer rim |
| 0.19±0.01 | 0.13±0.01 | 0.26±0.01 | 0.16±0.01 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





