Submitted:
29 November 2023
Posted:
30 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Methodology
2.1. Data Collection
2.2. Specimen Sampling
2.3. Testing and Identification
2.4. Determining Hospital Associated Transmission
2.5. Statistical Analysis
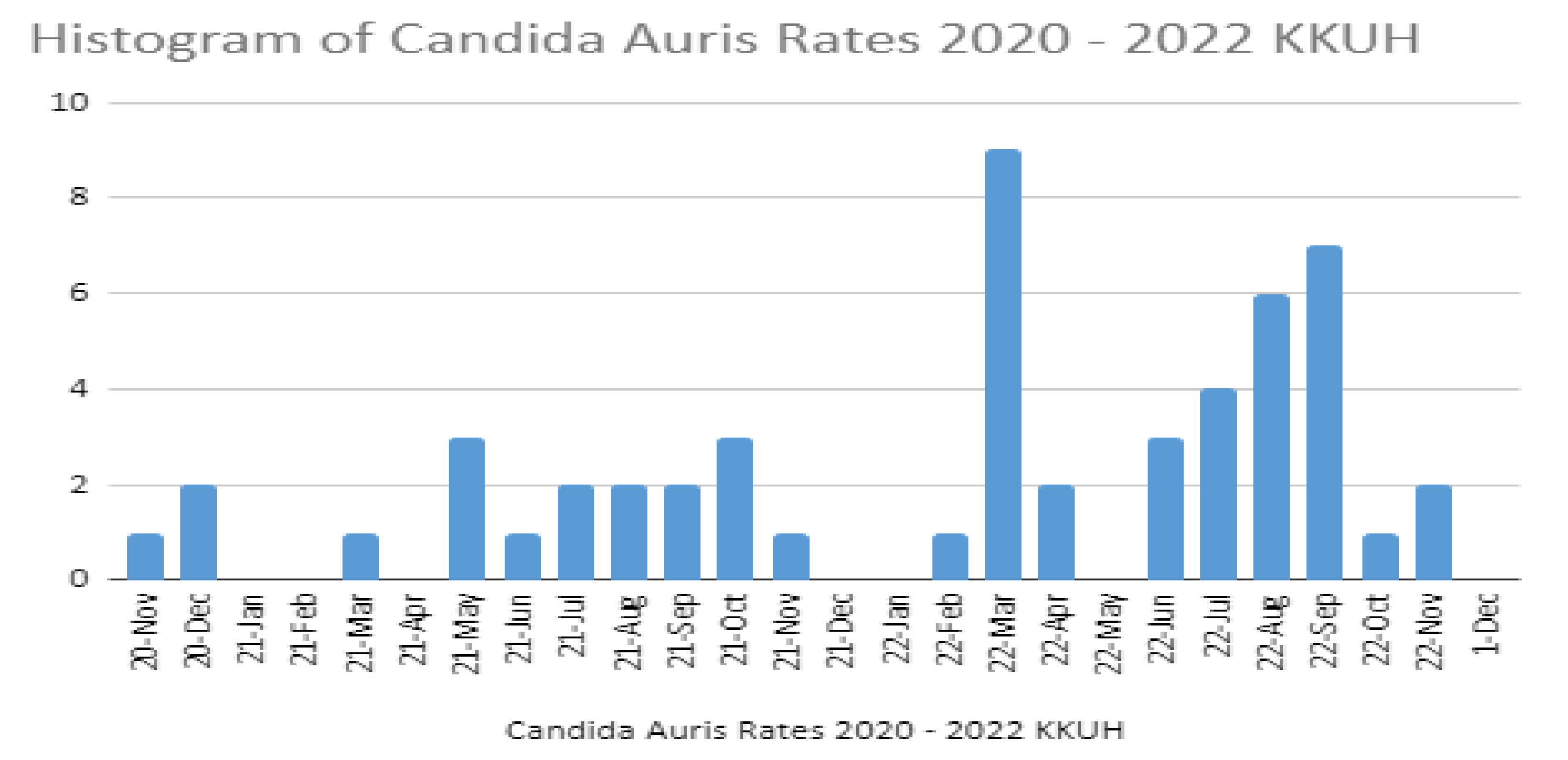
3. Results
3.1. Occurrence by Age
3.2. Occurrence by Patient Characteristics
3.3. Occurrence as Hospital- versus Community-Acquired Infection
3.4. Occurrence as Infection versus Colonization
3.5. Occurrence as Clinical versus Surveillance Samples
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- S. Vallabhaneni et al., “Investigation of the First Seven Reported Cases of Candida auris, a Globally Emerging Invasive, Multidrug-Resistant Fungus—United States, 13–August 2016,” American Journal of Transplantation, vol. 17, no. 1. Blackwell Publishing Ltd, pp. 296–299, Jan. 01, 2017. 20 May. [CrossRef]
- K. Satoh, K. Makimura, Y. Hasumi, Y. Nishiyama, K. Uchida, and H. Yamaguchi, “Candida auris sp. nov., a novel ascomycetous yeast isolated from the external ear canal of an inpatient in a Japanese hospital,” Microbiol Immunol, vol. 53, no. 1, pp. 41–44, Jan. 2009. [CrossRef]
- S. R. Lockhart et al., “Simultaneous emergence of multidrug-resistant candida auris on 3 continents confirmed by whole-genome sequencing and epidemiological analyses,” Clinical Infectious Diseases, vol. 64, no. 2, pp. 134–140, 2017. [CrossRef]
- M. Biswal et al., “Controlling a possible outbreak of Candida auris infection: lessons learnt from multiple interventions,” Journal of Hospital Infection, vol. 97, no. 4, pp. 363–370, Dec. 2017. [CrossRef]
- M. A. Sayeed, J. Farooqi, K. Jabeen, S. Awan, and S. F. Mahmood, “Clinical spectrum and factors impacting outcome of Candida auris: A single center study from Pakistan,” BMC Infect Dis, vol. 19, no. 1, p. 384, Dec. 2019. [CrossRef]
- R. E. Magobo, C. Corcoran, S. Seetharam, and N. P. Govender, “Candida auris –Associated Candidemia, South Africa,” Emerg Infect Dis, vol. 20, no. 7, pp. 1250–1251, Jul. 2014. [CrossRef]
- D. A. Solomon, A. K. Nyerere, A. Kanyua, and C. W. Ngugi, “Prevalence, Species Distribution and Antifungal Susceptibility Profile of <i>Candida</i> Species Isolated from Bloodstream of Critical Care Unit Patients in a Tertiary Care Hospital in Kenya,” Open J Med Microbiol, vol. 11, no. 01, 2021. [CrossRef]
- H. F. Garcia-Jeldes et al., “Prevalence of Candida auris in Canadian acute care hospitals among at-risk patients, 2018,” Antimicrob Resist Infect Control, vol. 9, no. 1, 2020. [CrossRef]
- Sanyaolu et al., “Candida auris : An Overview of the Emerging Drug-Resistant Fungal Infection,” Infect Chemother, vol. 54, no. 2, p. 236, 2022. [CrossRef]
- Abdalhamid, R. Almaghrabi, S. Althawadi, and A. Omrani, “First report of Candida auris infections from Saudi Arabia,” Journal of Infection and Public Health, vol. 11, no. 4. Elsevier Ltd, pp. 598–599, Jul. 01, 2018. [CrossRef]
- R. Kaki, “Risk factors and mortality of the newly emerging Candida auris in a university hospital in Saudi Arabia,” Mycology, pp. 1–8, Jun. 2023. [CrossRef]
- M. B. Alashqar, L. Alabdan, M. Khan, A. H. Almakadma, and S. Almustanyir, “A Case Report of a Candida auris Infection in Saudi Arabia,” Cureus, May 2021. 20 May. [CrossRef]
- R. Sabino, C. Veríssimo, Á. A. Pereira, and F. Antunes, “Candida auris, an agent of hospital-associated outbreaks: Which challenging issues do we need to have in mind?,” Microorganisms, vol. 8, no. 2. 2020. [CrossRef]
- H. Du, J. Bing, T. Hu, C. L. Ennis, C. J. Nobile, and G. Huang, “Candida auris: Epidemiology, biology, antifungal resistance, and virulence,” PLoS Pathog, vol. 16, no. 10, 2020. [CrossRef]
- R. S. Almaghrabi et al., “Molecular characterisation and clinical outcomes of Candida auris infection: Single-centre experience in Saudi Arabia,” Mycoses, vol. 63, no. 5, pp. 452–460, May 2020. [CrossRef]
- S. S. Ilan and Tanis C. Dingle, “Candida auris,” CMAJ, vol. 191, no. E865, Aug. 2019, Accessed: Jul. 02, 2023. [Online]. Available: https://www.cmaj.ca/content/cmaj/191/31/E865.full.pdf.
- J. de Cássia Orlandi Sardi, D. R. Silva, M. J. Soares Mendes-Giannini, and P. L. Rosalen, “Candida auris: Epidemiology, risk factors, virulence, resistance, and therapeutic options,” Microbial Pathogenesis, vol. 125. Academic Press, pp. 116–121, Dec. 01, 2018. [CrossRef]
- K. Vinayagamoorthy, K. C. Pentapati, and H. Prakash, “Prevalence, risk factors, treatment and outcome of multidrug resistance Candida auris infections in Coronavirus disease (COVID-19) patients: A systematic review,” Mycoses, vol. 65, no. 6. 2022. [CrossRef]
- S. Mahmoudi, K. Ahmadikia, M. Kord, A. Ahmadi, and S. Khodavaisy, “Candida auris, an emerging fungal pathogen,” Journal of Mazandaran University of Medical Sciences, vol. 29, no. 172, 2019. [CrossRef]
- M. Kordalewska and D. S. Perlin, “Identification of drug resistant candida auris,” Frontiers in Microbiology, vol. 10, no. AUG. Frontiers Media S.A., 2019. [CrossRef]
- M. G. Frías-De-león et al., “Antifungal resistance in Candida auris: Molecular determinants,” Antibiotics, vol. 9, no. 9. 2020. [CrossRef]
- Narayanan, P. Selvakumar, R. Siddharthan, and K. Sanyal, “ClaID: a Rapid Method of Clade-Level Identification of the Multidrug Resistant Human Fungal Pathogen Candida auris,” Microbiol Spectr, vol. 10, no. 2, Apr. 2022. [CrossRef]
- N. A. Chow, T. de Groot, H. Badali, M. Abastabar, T. M. Chiller, and J. F. Meis, “Potential Fifth Clade of Candida auris, Iran, 2018,” Emerg Infect Dis, vol. 25, no. 9, pp. 1780–1781, Sep. 2019. [CrossRef]
- D. G. De Luca et al., “Four genomic clades of Candida auris identified in Canada, 2012–2019,” Med Mycol, vol. 60, no. 1, Jan. 2022. [CrossRef]
- S. Tsay, A. Kallen, B. R. Jackson, T. M. Chiller, and S. Vallabhaneni, “Approach to the Investigation and Management of Patients with Candida auris, an Emerging Multidrug-Resistant Yeast,” Clinical Infectious Diseases, vol. 66, no. 2, pp. 306–311, Jan. 2018. [CrossRef]
- Cortegiani, G. Misseri, T. Fasciana, A. Giammanco, A. Giarratano, and A. Chowdhary, “Epidemiology, clinical characteristics, resistance, and treatment of infections by Candida auris,” Journal of Intensive Care, vol. 6, no. 1. BioMed Central Ltd., p. 69, Oct. 29, 2018. [CrossRef]
- H. Du, J. Bing, T. Hu, C. L. Ennis, C. J. Nobile, and G. Huang, “Candida auris: Epidemiology, biology, antifungal resistance, and virulence,” PLoS Pathog, vol. 16, no. 10, p. e1008921, Oct. 2020. [CrossRef]
- W. Alfouzan, R. Dhar, A. Albarrag, and H. Al-Abdely, “The emerging pathogen Candida auris: A focus on the Middle-Eastern countries,” Journal of Infection and Public Health, vol. 12, no. 4. Elsevier Ltd, pp. 451–459, Jul. 01, 2019. [CrossRef]
- M. Snayd, F. Dias, R. W. Ryan, D. Clout, and D. B. Banach, “Misidentification of Candida auris by RapID yeast plus, a commercial, biochemical enzyme-based manual rapid identification system,” Journal of Clinical Microbiology, vol. 56, no. 5. 2018. [CrossRef]
- R. M. Welsh et al., “Survival, Persistence, and Isolation of the Emerging Multidrug-Resistant Pathogenic Yeast Candida auris on a Plastic Health Care Surface,” J Clin Microbiol, vol. 55, no. 10, p. 2996, Oct. 2017. [CrossRef]
- W. Alfouzan, R. Dhar, A. Albarrag, and H. Al-Abdely, “The emerging pathogen Candida auris: A focus on the Middle-Eastern countries,” Journal of Infection and Public Health, vol. 12, no. 4. Elsevier Ltd, pp. 451–459, Jul. 01, 2019. [CrossRef]
- S. Chatterjee, S. V. Alampalli, R. K. Nageshan, S. T. Chettiar, S. Joshi, and U. S. Tatu, “Draft genome of a commonly misdiagnosed multidrug resistant pathogen Candida auris,” BMC Genomics, vol. 16, no. 1, 2015. [CrossRef]
- “Antifungal Susceptibility Testing and Interpretation | Candida auris | Fungal Diseases | CDC.” Accessed: May 31, 2023. [Online]. Available: https://www.cdc.gov/fungal/candida-auris/c-auris-antifungal.html. 31 May.
- S. Tsay, A. Kallen, B. R. Jackson, T. M. Chiller, and S. Vallabhaneni, “Approach to the Investigation and Management of Patients with Candida auris, an Emerging Multidrug-Resistant Yeast,” Clinical Infectious Diseases, vol. 66, no. 2, pp. 306–311, Jan. 2018. [CrossRef]
- D. H. Caceres et al., “Candida auris: A review of recommendations for detection and control in healthcare settings,” Journal of Fungi, vol. 5, no. 4. MDPI AG, Dec. 01, 2019. [CrossRef]
- T. S. N. Ku, C. J. Walraven, and S. A. Lee, “Candida auris: Disinfectants and Implications for Infection Control,” Front Microbiol, vol. 9, Apr. 2018. [CrossRef]
- P. Arora et al., “Environmental Isolation of Candida auris from the Coastal Wetlands of Andaman Islands, India,” mBio, vol. 12, no. 2, Apr. 2021. [CrossRef]
- F. Visan et al., “ First Candida auris Outbreak Experience in a Tertiary-Care General Hospital in Qatar, 2019 ,” Infect Control Hosp Epidemiol, vol. 41, no. S1, 2020. [CrossRef]
- M. Theut et al., “The first two cases of Candida auris in Denmark,” Ugeskr Laeger, vol. 184, no. 16, 2022.
- S. Schelenz et al., “First hospital outbreak of the globally emerging Candida auris in a European hospital.,” Antimicrob Resist Infect Control, vol. 5, no. 1, p. 35, Oct. 2016. [CrossRef]
- J. O. KIM, K. H. ST. JOHN, and S. E. COFFIN, “Epidemiology and Infection Prevention and Control,” in Pediatric Infectious Diseases, 2008. [CrossRef]
- S. Hu et al., “Retrospective Analysis of the Clinical Characteristics of Candida auris Infection Worldwide From 2009 to 2020,” Front Microbiol, vol. 12, 2021. [CrossRef]
- CDC, Ncezid, and DHQP, “Identifying Healthcare-associated Infections (HAI) for NHSN Surveillance,” 2023, Accessed: Jun. 01, 2023. [Online]. Available: https://www.cdc.gov/nhsn/pdfs/pscmanual/2psc_identifyinghais_nhsncurrent.pdf.
- S. Ahmad and W. Alfouzan, “Candida auris: Epidemiology, diagnosis, pathogenesis, antifungal susceptibility, and infection control measures to combat the spread of infections in healthcare facilities,” Microorganisms, vol. 9, no. 4. 2021. [CrossRef]
- W. Alfouzan et al., “Molecular epidemiology of candida auris outbreak in a major secondary-care hospital in Kuwait,” Journal of Fungi, vol. 6, no. 4, 2020. [CrossRef]
- Al Maani et al., “Ongoing challenges with healthcare-associated candida auris outbreaks in Oman,” Journal of Fungi, vol. 5, no. 4, 2019. [CrossRef]
- J. Mohsin et al., “A Cluster of Candida auris Blood Stream Infections in a Tertiary Care Hospital in Oman from 2016 to 2019.,” Antibiotics (Basel), vol. 9, no. 10, pp. 1–11, Sep. 2020. [CrossRef]
- J. V. Mulet Bayona et al., “Characteristics and management of candidaemia episodes in an established candida auris outbreak,” Antibiotics, vol. 9, no. 9, 2020. [CrossRef]
- H. Villanueva-Lozano et al., “Outbreak of Candida auris infection in a COVID-19 hospital in Mexico,” Clinical Microbiology and Infection, vol. 27, no. 5. 2021. [CrossRef]
- P. S. Shastri, S. A. Shankarnarayan, J. Oberoi, S. M. Rudramurthy, C. Wattal, and A. Chakrabarti, “Candida auris candidaemia in an intensive care unit – Prospective observational study to evaluate epidemiology, risk factors, and outcome,” J Crit Care, vol. 57, 2020. [CrossRef]
- K. Arensman et al., “Clinical Outcomes of Patients Treated for Candida auris Infections in a Multisite Health System, Illinois, USA,” Emerg Infect Dis, vol. 26, no. 5, 2020.
- C. Prestel et al., “ Candida auris Outbreak in a COVID-19 Specialty Care Unit — Florida, July–August 2020 ,” MMWR Morb Mortal Wkly Rep, vol. 70, no. 2, 2021. 20 August. [CrossRef]
- K. Etienne et al., “Epidemiology and Whole Genome Sequence Typing of Globally Emerging, Multidrug-Resistant Candida auris,” Open Forum Infect Dis, vol. 3, no. suppl_1, Dec. 2016. [CrossRef]
- B. Abdalhamid, R. Almaghrabi, S. Althawadi, and A. Omrani, “First report of Candida auris infections from Saudi Arabia,” Journal of Infection and Public Health, vol. 11, no. 4. Elsevier Ltd, pp. 598–599, Jul. 01, 2018. [CrossRef]
- Elsawy, K. Alquthami, N. Alkhutani, D. Marwan, and A. Abbas, “The second confirmed case of Candida auris from Saudi Arabia,” Journal of Infection and Public Health, vol. 12, no. 6. Elsevier Ltd, pp. 907–908, Nov. 01, 2019. [CrossRef]
- R. AlJindan, D. M. AlEraky, N. Mahmoud, S. AbdulAzeez, and J. F. Borgio, “Emergence of multi-drug resistance Candida auris in Saudi Arabia,” Jun. 2020. [CrossRef]
- M. M. Alshamrani et al., “Management of Candida auris outbreak in a tertiary-care setting in Saudi Arabia,” Infect Control Hosp Epidemiol, 2020. [CrossRef]
- Alatoom et al., “Persistent candidemia despite appropriate fungal therapy: First case of Candida auris from the United Arab Emirates,” International Journal of Infectious Diseases, vol. 70, pp. 36–37, May 2018. [CrossRef]
- “WHO Director-General’s opening remarks at the media briefing on COVID-19 - 11 March 2020.” Accessed: Jul. 03, 2023. [Online]. Available: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020.
- H. Najeeb et al., “The Menace of Candida auris Epidemic Amidst the COVID-19 Pandemic: A Systematic Review,” Diseases, vol. 10, no. 3, p. 58, Aug. 2022. [CrossRef]
- D. Lin et al., “Co-infections of SARS-CoV-2 with multiple common respiratory pathogens in infected patients,” Sci China Life Sci, vol. 63, no. 4, pp. 606–609, Apr. 2020. [CrossRef]
- Chowdhary, B. Tarai, A. Singh, and A. Sharma, “Multidrug-resistant candida auris infections in critically Ill Coronavirus disease patients, India, April–July 2020,” Emerg Infect Dis, vol. 26, no. 11, pp. 2694–2696, Nov. 2020. [CrossRef]
- Chowdhary and A. Sharma, “The lurking scourge of multidrug resistant Candida auris in times of COVID-19 pandemic,” J Glob Antimicrob Resist, vol. 22, pp. 175–176, Sep. 2020. [CrossRef]
- N. Pandya et al., “International Multicentre Study of Candida auris Infections,” Journal of Fungi, vol. 7, no. 10, p. 878, Oct. 2021. [CrossRef]
- K. Southwick et al., “A description of the first Candida auris-colonized individuals in New York State, 2016-2017,” Am J Infect Control, vol. 50, no. 3, pp. 358–360, Mar. 2022. [CrossRef]
- Al-Rashdi, A. Al-Maani, A. Al-Wahaibi, A. Alqayoudhi, A. Al-Jardani, and S. Al-Abri, “Characteristics, risk factors, and survival analysis of candida auris cases: Results of one-year national surveillance data from oman,” Journal of Fungi, vol. 7, no. 1, 2021. [CrossRef]
- E. van Schalkwyk et al., “Epidemiologic Shift in Candidemia Driven by Candida auris, South Africa, 2016–20171,” Emerg Infect Dis, vol. 25, no. 9, pp. 1698–1707, Sep. 2019. [CrossRef]
- X. Wang et al., “The first isolate of Candida auris in China: Clinical and biological aspects article,” Emerg Microbes Infect, vol. 7, no. 1, 2018. [CrossRef]
- M. V. Moreno et al., “First isolation de Candida auris in Chile,” Revista Chilena de Infectologia, vol. 36, no. 6, 2019. [CrossRef]
- F. Allaw et al., “First candida auris outbreak during a covid-19 pandemic in a tertiary-care center in Lebanon,” Pathogens, vol. 10, no. 2, 2021. [CrossRef]
- E. J. Zasowski et al., “ 289. International Validation of a Methicillin-Resistant Staphylococcus aureus (MRSA) Risk Assessment Tool for Acute Bacterial Skin and Skin Structure Infections (ABSSSI) ,” Open Forum Infect Dis, vol. 5, no. suppl_1, 2018. [CrossRef]
- T. Helfand, C. A. Conran, J. Xu, and W. J. Catalona, “A multiparametric approach to improve upon existing prostate cancer screening and biopsy recommendations,” Curr Opin Urol, vol. 27, no. 5, pp. 475–480, Sep. 2017. [CrossRef]
| Bundle Element/Risk Factor | Score |
|---|---|
|
3 |
|
1 |
|
1 |
|
1 |
|
1 |
|
1 |
|
1 |
|
1 |
| N | % | |
| Age (years) | ||
| ≤20 | 2 | 3.8 |
| 21-30 | 4 | 7.5 |
| 31-40 | 3 | 5.7 |
| 41-50 | 2 | 3.8 |
| 51-60 | 13 | 24.5 |
| 61-70 | 14 | 26.4 |
| ≥71 | 15 | 28.3 |
| Gender | ||
| Male | 33 | 62.3 |
| Female | 20 | 37.7 |
| Specimen | ||
| Urine | 16 | 30.2 |
| Axilla | 6 | 11.3 |
| Thigh | 5 | 9.4 |
| Anus | 5 | 9.4 |
| Arm | 4 | 7.5 |
| Swab | 3 | 5.7 |
| Penis | 3 | 5.7 |
| Hip | 3 | 5.7 |
| Nose | 2 | 3.8 |
| Buttock | 2 | 3.8 |
| Leg | 2 | 3.8 |
| Neck | 2 | 3.8 |
| Nasal | 2 | 3.8 |
| Tissue | 1 | 1.9 |
| Wound | 1 | 1.9 |
| Nail | 1 | 1.9 |
| Rectal | 1 | 1.9 |
| Blood | 1 | 1.9 |
| Foot | 1 | 1.9 |
| N | % | 95% CI of rate | |
|---|---|---|---|
| Comorbidities | 44 | 83 | 60.3 to 111.5 |
| Admission to other hospital | 27 | 50.9 | 33.6 to 74.1 |
| Admission to high-risk unit | 19 | 35.8 | 21.6 to 56 |
| Wounds | 18 | 34.0 | 20.1 to 53.7 |
| Devices | 17 | 32.1 | 18.7 to 51.4 |
| Antimicrobials | 12 | 22.6 | 11.7 to 39.6 |
| ASC | 11 | 20.8 | 10.4 to 37.1 |
| Surgeries | 7 | 13.2 | 5.3 to 27.2 |
| MDRO | 1 | 1.9 | 0.0 to 10.5 |
| Outside KSA | 0 | 0.0 | --- |
| Contact of MDRO | 0 | 0.0 | --- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





