Submitted:
04 October 2023
Posted:
09 October 2023
You are already at the latest version
Abstract
Keywords:
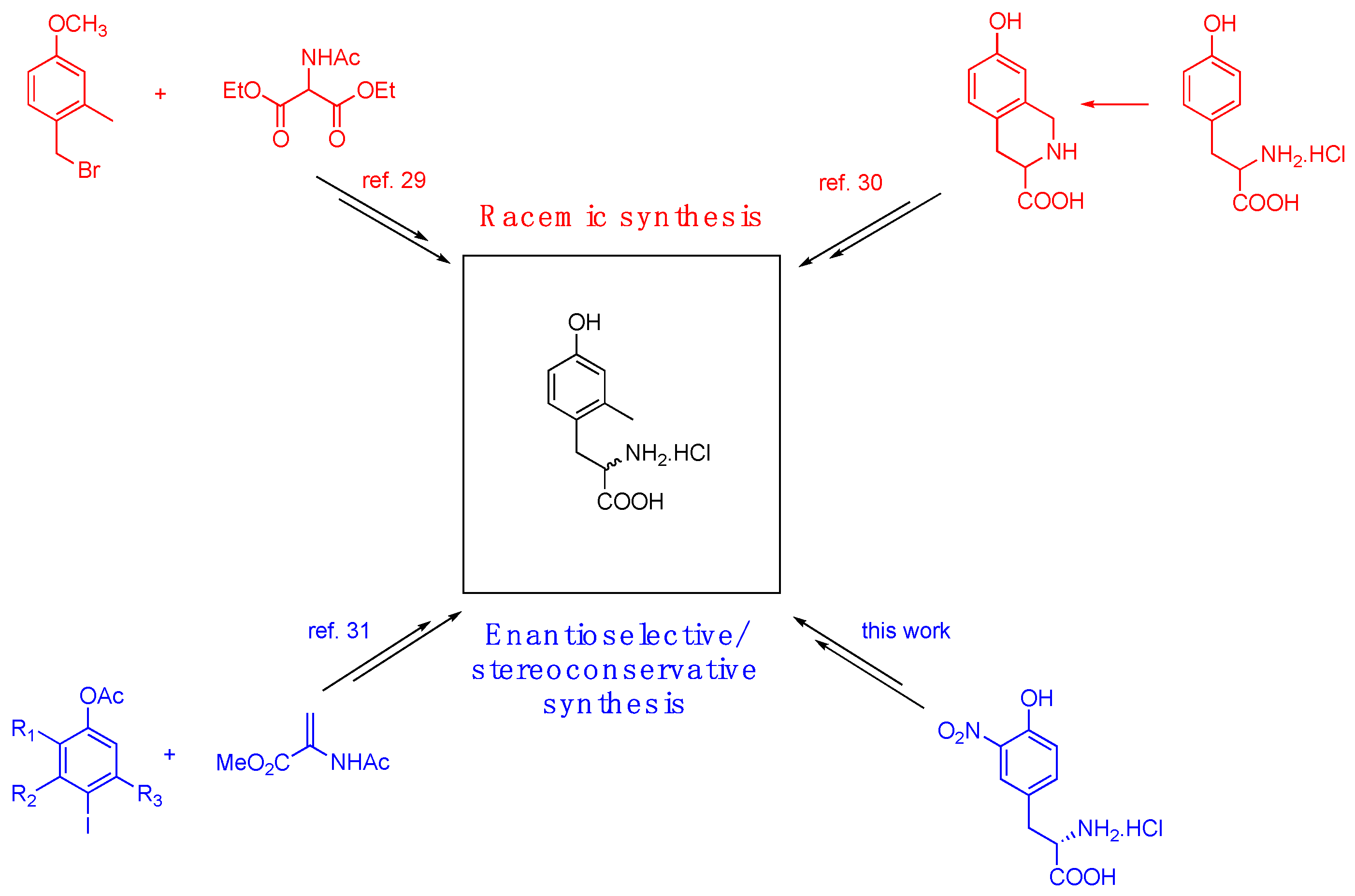
1. Introduction
2. Discussion and Results
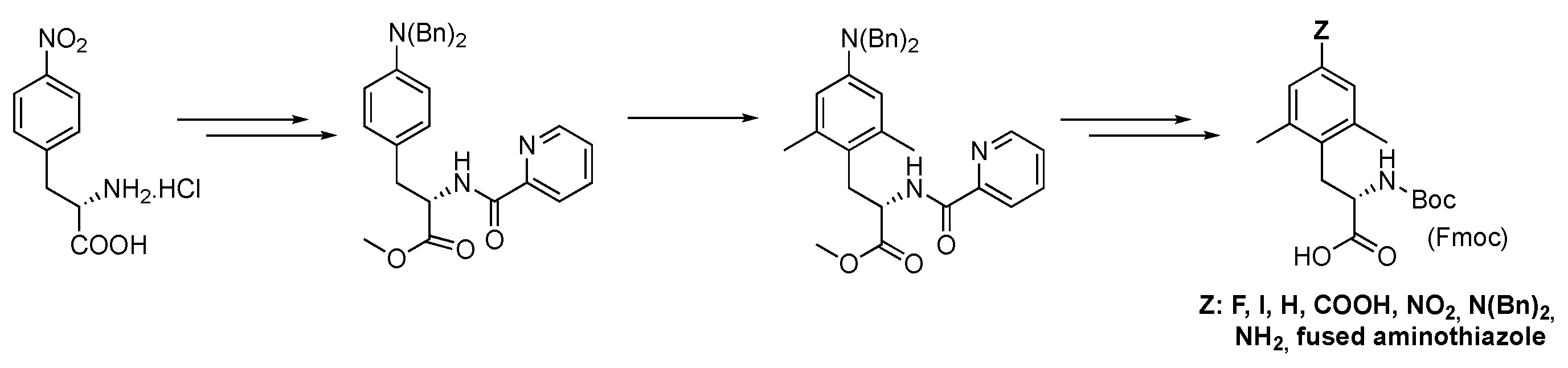
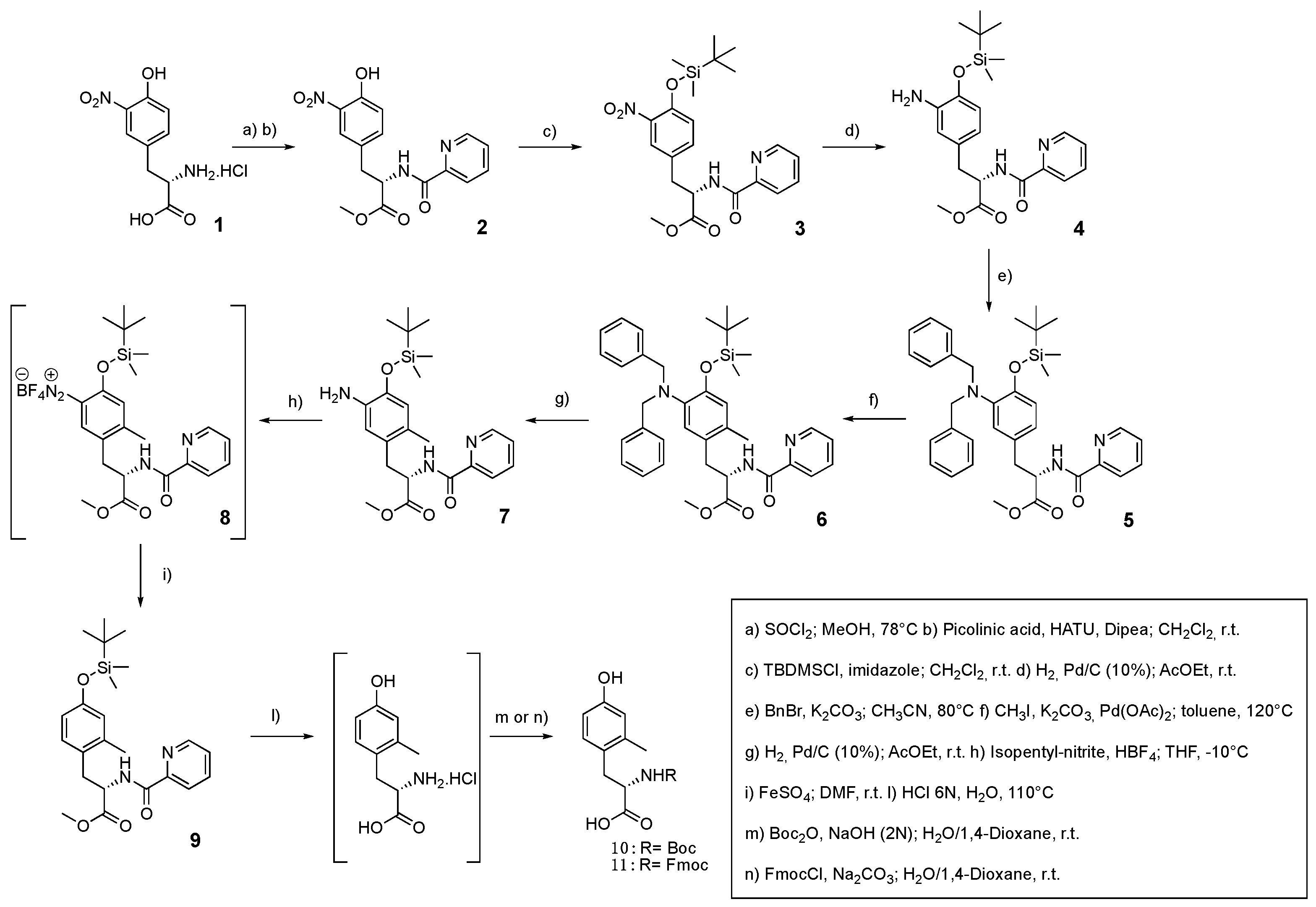
2.1. Chemistry
2.2. Pharmacology
3. Conclusions
4. Material and methods
4.1. Chemistry
4.2. Pharmacology
4.3. Experimental Protocols
4.3.1. Synthesis of methyl (S)-3-(4-hydroxy-3-nitrophenyl)-2-(picolinamido)propanoate (2)
4.3.2. Synthesis of methyl (S)-3-(4-((tert-butyldimethylsilyl)oxy)-3-nitrophenyl)-2-(picolinamido)propanoate (3)
4.3.3. Synthesis of methyl (S)-3-(3-amino-4-((tert-butyldimethylsilyl)oxy)phenyl)-2-(picolinamido)propanoate (4)
4.3.4. Synthesis of methyl (S)-3-(4-((tert-butyldimethylsilyl)oxy)-3-(dibenzylamino)phenyl)-2-(picolinamido)propanoate (5)
4.3.5. Synthesis of methyl (S)-3-(4-((tert-butyldimethylsilyl)oxy)-5-(dibenzylamino)-2-methylphenyl)-2-(picolinamido)propanoate (6)
4.3.6. Synthesis of methyl (S)-3-(5-amino-4-((tert-butyldimethylsilyl)oxy)-2-methylphenyl)-2-(picolinamido)propanoate (7)
4.3.7. Synthesis of (S)-2-((tert-butyldimethylsilyl)oxy)-5-(3-methoxy-3-oxo-2-(picolinamido)propyl)-4-methylbenzenediazonium (8)
4.3.8. Synthesis of methyl (S)-3-(4-((tert-butyldimethylsilyl)oxy)-2-methylphenyl)-2-(picolinamido)propanoate (9)
4.3.9. Synthesis of (S)-2-((tert-butoxycarbonyl)amino)-3-(4-hydroxy-2-methylphenyl)propanoic acid (10)
4.3.10. Synthesis of (S)-2-((((9H-fluoren-9-yl)methoxy)carbonyl)amino)-3-(4-hydroxy-2-methylphenyl)propanoic acid (11)
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dygos, J.H.; Yonan, E.E.; Scaros, M.G.; Goodmonson, O.J.; Getman, D.P.; Periana, R.A.; Beck, G.R. A Convenient Asymmetric Synthesis of the Unnatural Amino Acid 2,6-Dimethyl-L-tyrosine. Synthesis 1992, 8, 741–743. [Google Scholar] [CrossRef]
- Balboni, G.; Marzola, E.; Sasaki, Y.; Ambo, A.; Marczak, E.D.; Lazarus, L.H.; Salvadori, S. Role of 2′,6′-dimethyl-l-tyrosine (Dmt) in some opioid lead compounds. Bioorg. Med. Chem. 2010, 18, 6024–6030. [Google Scholar] [CrossRef] [PubMed]
- Salvadori, S.; Attila, M.; Balboni, G.; Bianchi, C.; Bryant, S.D.; Crescenzi, O.; Guerrini, R.; Picone, D.; Tancredi, T.; Temussi, P.A.; Lazarus, L.H. Delta opioidmimetic antagonists: prototypes for designing a new generation of ultraselective opioid peptides. Mol. Med. 1995, 1, 678–689. [Google Scholar] [CrossRef] [PubMed]
- Bryant, S.D.; Jinsmaa, Y.; Okada, Y.; Lazarus, L.H.; Salvadori, S. Dmt and opioid peptides: a potent alliance. Biopolymers 2003, 71, 86–102. [Google Scholar] [CrossRef]
- Guerrini, R.; Capasso, A.; Sorrentino, L.; Anacardio, R.; Bryant, S.D.; Lazarus, L.H.; Attila, M.; Salvadori, S. Opioid receptor selectivity alteration by single residue replacement: synthesis and activity profile of [Dmt1]deltorphin B. Eur. J.Pharm. 1996, 302, 37–42. [Google Scholar] [CrossRef]
- Schiller, P.W.; Nguyen, T.M.-D.; Berezowska, I.; Dupuis, S.; Weltrowska, G.; Chung, N.N.; Lemieux, C. Synthesis and in vitro opioid activity profiles of DALDA analogues. Eur. J. Med. Chem. 2000, 35, 895–901. [Google Scholar] [CrossRef]
- Mallareddy, J.R.; Borics, A.; Keresztes, A.; Toth, G. Design, Synthesis, Pharmacological Evaluation, and Structure-Activity Study of Novel Endomorphin Analogues with Multiple Structural Modifications. J. Med. Chem.; 2011, 54, 1462–1472. [Google Scholar] [CrossRef]
- Schiller, P.W.; Nguyen, T.M.-D.; Chung, N.N.; Lemieux, C. Dermorphin analogues carrying an increased positive net charge in their "message" domain display extremely high mu opioid receptor selectivity. J. Med. Chem.; 1989, 3, 698–703. [Google Scholar] [CrossRef]
- Schiller, P.W.; Fundytus, M.E.; Merovitz, L.; Weltrowska, G.; Nguyen T., M.-D.; Lemieux, C.; Chung N., N.; Coderre, T.J. J. Med. Chem. 1999, 42, 3520–3526. [CrossRef]
- Molinari, S.; Camarda, V.; Rizzi, A.; Marzola, G.; Salvadori, S.; Marzola, E.; Molinari, P.; McDonald, J.; Ko, M.C.; Lambert, D.G.; Calò, G.; Guerrini, R. [Dmt1]N/OFQ(1–13)-NH2: a potent nociceptin/orphanin FQ and opioid receptor universal agonist. Br. J. Pharmacol. 2013, 168, 151–162. [Google Scholar] [CrossRef]
- Pacifico, S.; Albanese, V.; Illuminati, D.; Marzola, E.; Fabbri, M.; Ferrari, F.; Holanda V. A., D.; Sturaro, C.; Malfacini, D.; Ruzza, C.; Trapella, C.; Preti, D.; Lo Cascio, E.; Arcovito, A.; Della Longa, S.; Marangoni, M.; Fattori, D.; Nassini, R.; Calò, G.; Guerrini, R. Novel Mixed NOP/Opioid Receptor Peptide Agonists. J. Med. Chem. 2021, 64, 6656–6669. [Google Scholar] [CrossRef]
- Ambo, A.; Murase, H.; Niizuma, H. , Ouchi H.; Yamamoto Y.; Sasaki A. Dermorphin and deltorphin heptapeptide analogues: replacement of Phe residue by Dmp greatly improves opioid receptor affinity and selectivity. Bioorg. Med. Chem. Lett. 2002, 12, 879–881. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, Y. , Sasaki A., Ariizumi T.; igari Y.; Sato K.; Kohara H.; Nizuma H.; Ambo A. 2′,6′-Dimethylphenylalanine (Dmp) Can Mimic the N-Terminal Tyr in Opioid Peptides. Biol. Pharm. Bull. 2004, 27, 244–247. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, Y.; Sasaki, A.; Niizuma, H.; Goto, H.; Ambo, A. Endomorphin 2 Analogues Containing Dmp Residue as an Aromatic Amino Acid Surrogate with High μ-Opioid Receptor Affinity and Selectivity. Bioorg. Med. Chem. Lett. 2003, 11, 675–678. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, Y.; Hirabuki, M.; Ambo, A.; Ouchi, H.; Yamamoto, Y. Enkephalin Analogues with 2’,6’-Dimethylphenylalanine Replacing Phenylalanine in Position 4. Bioorg. Med. Chem. Lett.; 2001, 11, 327–329. [Google Scholar] [CrossRef]
- Toll, L.; Bruchas, M.R.; Calo, G.; Cox, B.M.; Zaveri, N.T. Nociceptin/Orphanin FQ Receptor Structure, Signaling, Ligands, Functions, and Interactions with Opioid Systems. Pharmacol. Rev. 2016, 68, 419–457. [Google Scholar] [CrossRef]
- Lin, A.P.; Ko, M.C. The Therapeutic Potential of Nociceptin/Orphanin FQ Receptor Agonists as Analgesics without Abuse Liability. ACS Chem. Neurosci. 2013, 4, 214–224. [Google Scholar] [CrossRef]
- Günther, T.; Dasgupta, P.; Mann, A.; Miess, E.; Kliewer, A.; Fritzwanker, S.; Steinborn, R.; Schulz, S. Targeting multiple opioid receptors - improved analgesics with reduced side effects? Br. J.Pharmacol. 2018, 175, 2857–2868. [Google Scholar] [CrossRef]
- Pacifico, S. , Albanese V., Illuminati D., Fantinati A., Marzola E., Ferrari F., Neto J.A., Sturaro C., Ruzza C., Calò G., Preti D., Guerrini R. Tetrabranched Hetero-Conjugated Peptides as Bifunctional Agonists of the NOP and Mu Opioid Receptors. Bioconj. Chem. 2019, 30, 2444–2451. [Google Scholar] [CrossRef]
- Sasaki, Y.; Ambo, A. 2’, 6’-dimethylphenylalanine: a useful aromatic amino acid surrogate for Tyr or Phe residue in opioid peptides. Int. J. Med. Chem. 2012. [Google Scholar] [CrossRef]
- Soloshonok, V.A.; Tang, X.; Hruby, V.J. Large-scale asymmetric synthesis of novel sterically constrained 2′,6′-dimethyl- and α,2′,6′-trimethyltyrosine and -phenylalanine derivatives via alkylation of chiral equivalents of nucleophilic glycine and alanine. Tetrahedron 2001, 57, 6375–6382. [Google Scholar] [CrossRef]
- Balducci, D.; Contaldi, S.; Lazzari, I.; Porzi, G. A highly efficient stereocontrolled synthesis of (S)-2′,6′-dimethyltyrosine [(S)-DMT] Tetrahedron: Asymmetry 2009, 20, 1398–1401.
- Mollica, A.; Costante, R.; Mirzaie, S.; Carradori, S.; Macedonio, G.; Stefanucci, A.; Novellino, E. Preparation of Constrained Unnatural Aromatic Amino Acids via Unsaturated Diketopiperazine Intermediat. J. Heterocyclic Chem. 2016, 53, 2106–2110. [Google Scholar] [CrossRef]
- Abrash, H.I.; Niemann, C. Steric Hindrance in α-Chymotrypsin-catalyzed Reactions. Biochemistry 1963, 2, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Dygos, J.H.; Yonan, E.E.; Scaros, M.G.; Goodmonson, O.J.; Getman, D.P.; Periana, R.A.; Beck, G.R. A Convenient Asymmetric Synthesis of the Unnatural Amino Acid 2,6-Dimethyl-L-tyrosine. Synthesis 1992, 741–743. [Google Scholar] [CrossRef]
- Praquin, C.F.B.; de Koning, P.D.; Peach, P.J.; Howard, R.M.; Spencer, S.L. Development of an Asymmetric Hydrogenation Route to (S)-N-Boc-2,6-dimethyltyrosine. Org. Process Res. Dev. 2011, 15, 1124–1129. [Google Scholar] [CrossRef]
- Bender, A.M.; Griggs, N.W.; Gao, C.; Trask, T.J.; Traynor, J.R.; Mosberg, H.I. Rapid Synthesis of Boc-2′,6′-dimethyl-l-tyrosine and Derivatives and Incorporation into Opioid Peptidomimetics. ACS Med. Chem. Lett. 2015, 6, 1199–1203. [Google Scholar] [CrossRef]
- Wang, X.; Niu, S.; Xu, L.; Zhang, C.; Meng, L.; Zhang, X.; Ma, D. Pd-Catalyzed Dimethylation of Tyrosine-Derived Picolinamide for Synthesis of (S)-N-Boc-2,6-dimethyltyrosine and Its Analogues. Org. Lett. 2017, 19, 246–249. [Google Scholar] [CrossRef]
- McDonald, I.A.; Nice, P.L.; Jung, M.J.; Sabol, J.S. Syntheses of DL-2-fluoromethy-p-tyrosine and DL-2-difluoromethyl-p-tyrosine as potential inhibitors of tyrosine hydroxylase. Tetrahedron Lett.; 1991, 32, 887–890. [Google Scholar] [CrossRef]
- Li, T.; Fujita, Y.; Tsuda, Y.; Miyazaki, A.; Ambo, A.; Sasaki, Y.; Jinsmaa, Y.; Bryant, S.D.; Lazarus, L.H.; Okada, Y. Unique High-Affinity Synthetic μ-Opioid Receptor Agonists with Central- and Systemic-Mediated Analgesia. J. Med. Chem. 2005, 48, 586–592. [Google Scholar] [CrossRef]
- Majer, P.; Slaninova, J.; Lebl, M. Synthesis of methylated phenylalanines via hydrogenolysis of corresponding 1,2,3,4-tetrahydroisoquinoline-3-carboxylic acids. In. J. Peptide Protein Res. 1994, 43, 62–68. [Google Scholar] [CrossRef]
- Illuminati, D.; Fantinati, A.; De Ventura, T.; Perrone, D.; Sturaro, C.; Albanese, V.; Marzola, E.; Cristofori, V.; Oble, J.; Poli, G.; Trapella, C. Synthesis of 2,6-Dimethyltyrosine-Like Amino Acids through Pinacolinamide-Enabled C–H Dimethylation of 4-Dibenzylamino Phenylalanine. J. Org. Chem. 2022, 87, 2580–2589. [Google Scholar] [CrossRef] [PubMed]
- Guerrini, R.; Calò, G.; Rizzi, A.; Bianchi, C.; Lazarus, L.H.; Salvadori, S.; Temussi, P.A.; Regoli, D. Address and Message Sequences for the Nociceptin Receptor: A Structure−Activity Study of Nociceptin-(1−13)-peptide amide. J. Med. Chem. 1997, 40, 1789–1793. [Google Scholar] [CrossRef] [PubMed]
- Varani, K.; Rizzi, A.; Calò, G.; Bigoni, R.; Toth, G.; Guerrini, R.; Gessi, S.; Salvadori, S.; Borea, P.A.; Regoli, D. Pharmacology of [Tyr1]nociceptin analogs: receptor binding and bioassay studies. Naunyn Schmiedebergs Arch. Pharmacol. 1999, 360, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Coward, P.; Chan, S.D.H.; Wada, H.G.; Humphries, G.M.; Conklin, B.R. Chimeric G proteins allow a high-throughput signaling assay of Gi-coupled receptors. Anal. Biochem. 1999, 270, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Camarda, V.; Fischietti, C.; Anzellotti, N.; Molinari, P.; Ambrosio, C.; Kostenis, E.; Regoli, D.; Trapella, C.; Guerrini, R.; Salvadori, S.; Calò, G. Pharmacological profile of NOP receptors coupled with calcium signaling via the chimeric protein G alpha qi5. Naunyn Schmiedebergs Arch. Pharmacol. 2009, 379, 599–607. [Google Scholar] [CrossRef]
- Fischetti, C.; Camarda, V.; Rizzi, A.; Pelà, M.; Trapella, C.; Guerrini, R.; McDonald, J.; Lambert, D.G.; Salvadori, S.; Regoli, D.; Calo', G. Pharmacological characterization of the nociceptin/orphanin FQ receptor non peptide antagonist Compound 24. Eur. J. Pharmacol. 2009, 614, 50–57. [Google Scholar] [CrossRef]
- Bojnik, E.; Babos, F.; Fischetti, C.; Magyar, A.; Camarda, V.; Borsodi, A.; Bajusz, S.; Calo', G.; Benyhe, S. Comparative biochemical and pharmacological characterization of a novel, NOP receptor selective hexapeptide, Ac-RYYRIR-ol. Brain Res Bull. 2010, 81, 477–483. [Google Scholar] [CrossRef]
- Rizzi, A.; Malfacini, D.; Cerlesi, M.C.; Ruzza, C.; Marzola, E.; Bird, M.F.; Rowbotham, D.J.; Salvadori, S.; Guerrini, R.; Lambert, D.G.; Calo, G. In vitro and in vivo pharmacological characterization of nociceptin/orphanin FQ tetrabranched derivatives. Br. J. Pharmacol. 2014, 171, 4138–4153. [Google Scholar] [CrossRef]
- Guerrini, R.; Marzola, E.; Trapella, C.; Pacifico, S.; Cerlesi, M.C.; Malfacini, D.; Ferrari, F.; Bird, M.F.; Lambert, D.G.; Salvadori, S.; Calò, G. Structure activity studies of nociceptin/orphanin FQ(1–13)-NH2 derivatives modified in position 5. Bioorg. Med. Chem. 2015, 23, 1515–1520. [Google Scholar] [CrossRef]
- Ferrari, F.; Cerlesi, M.C.; Malfacini, D.; Asth, L.; Gavioli, E.C.; Journigan, B.V.; Kamakolanu, U.G.; Meyer, M.E.; Yasuda, D.; Polgar, W.E.; Rizzi, A.; Guerrini, R.; Ruzza, C.; Zaveri, N.T.; Calò, G. In vitro functional characterization of novel nociceptin/orphanin FQ receptor agonists in recombinant and native preparations. Eur. J. Pharmacol. 2016, 793, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Cerlesi, M.C.; Ding, H.; Bird, M.F.; Kiguchi, N.; Ferrari, F.; Malfacini, D.; Rizzi, A.; Ruzza, C.; Lambert, D.G.; Ko, M.C.; Calo, G.; Guerrini, R. Pharmacological studies on the NOP and opioid receptor agonist PWT2-[Dmt1]N/OFQ(1-13). Eur. J. Pharmacol. 2017, 794, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Rizzi, A.; Cerlesi M., C.; Ruzza, C.; Malfacini, D.; Ferrari, F.; Bianco, S.; Costa, T.; Guerrini, R.; Trapella, C.; Calo’, G. Pharmacological characterization of cebranopadol a novel analgesic acting as mixed nociceptin/orphanin FQ and opioid receptor agonist. Pharmacol. Res. Perspect. 2016, 4, e00247. [Google Scholar] [CrossRef]
- Pacifico, S.; Carotenuto, A.; Brancaccio, D.; Novellino, E.; Marzola, E.; Ferrari, F.; Cerlesi, M.C.; Trapella, C.; Preti, D.; Salvadori, S.; Calò, G.; Guerrini, R. Structure- and conformation-activity studies of nociceptin/orphanin FQ receptor dimeric ligands. Sci. Rep. 2017, 7, 45817. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, F.; Malfacini, D.; Journigan B., V.; Bird M., F.; Trapella, C.; Guerrini, R.; Lambert D., G.; Calo’, G.; Zaveri N., T. In vitro pharmacological characterization of a novel unbiased NOP receptor-selective nonpeptide agonist AT-403. Pharmacol. Res. Perspect. 2017, 5, 4–e00333. [Google Scholar] [CrossRef]
- Wang, Y.; Zhuang, Y.; DiBerto, J.F.; Zhou, X.E.; Schmitz, G.P.; Yuan, Q.; Jain, M.K.; Liu, W.; Melcher, K.; Jiang, Y.; Roth, B.L.; Xu, H.E. Structures of the entire human opioid receptor family. Cell. 2023, 186, 413–427. [Google Scholar] [CrossRef]

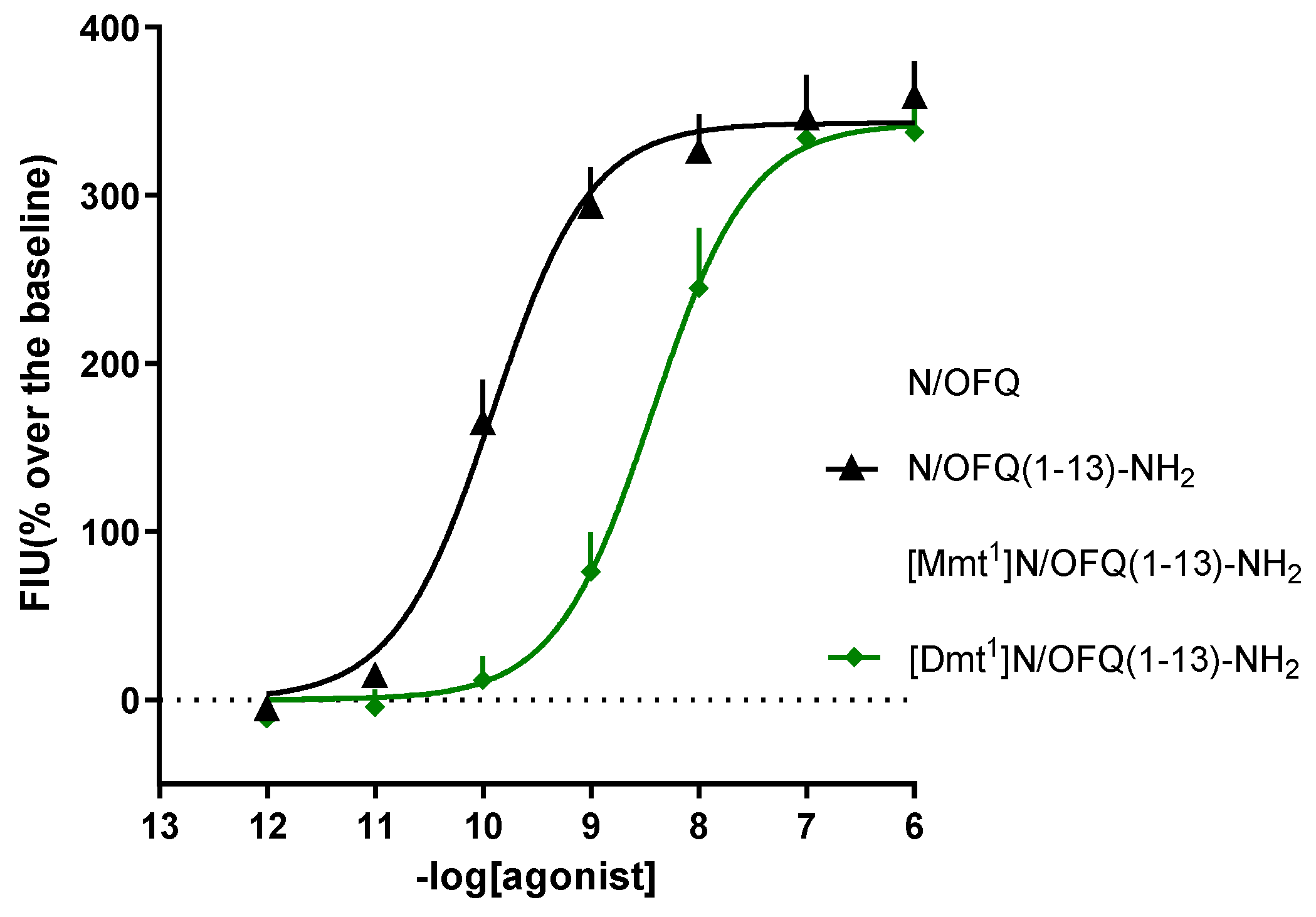
| Calcium mobilization in CHONOP + Gαqi5 cells | ||
|---|---|---|
| pEC50 (CL95%) | Emax + S.E.M. | |
| N/OFQ | 9.56 (9.02 – 10.09) |
335 + 33 |
| N/OFQ(1-13)-NH2 | 9.82 (9.45 – 10.18) |
358 + 22 |
| [Mmt1]N/OFQ(1-13)-NH2 | 9.47 (8.92 – 10.01) |
302 + 39 |
| [Dmt1]N/OFQ(1-13)-NH2 | 8.35 (7.94 – 8.77) |
337 + 26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





