Submitted:
08 October 2023
Posted:
09 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
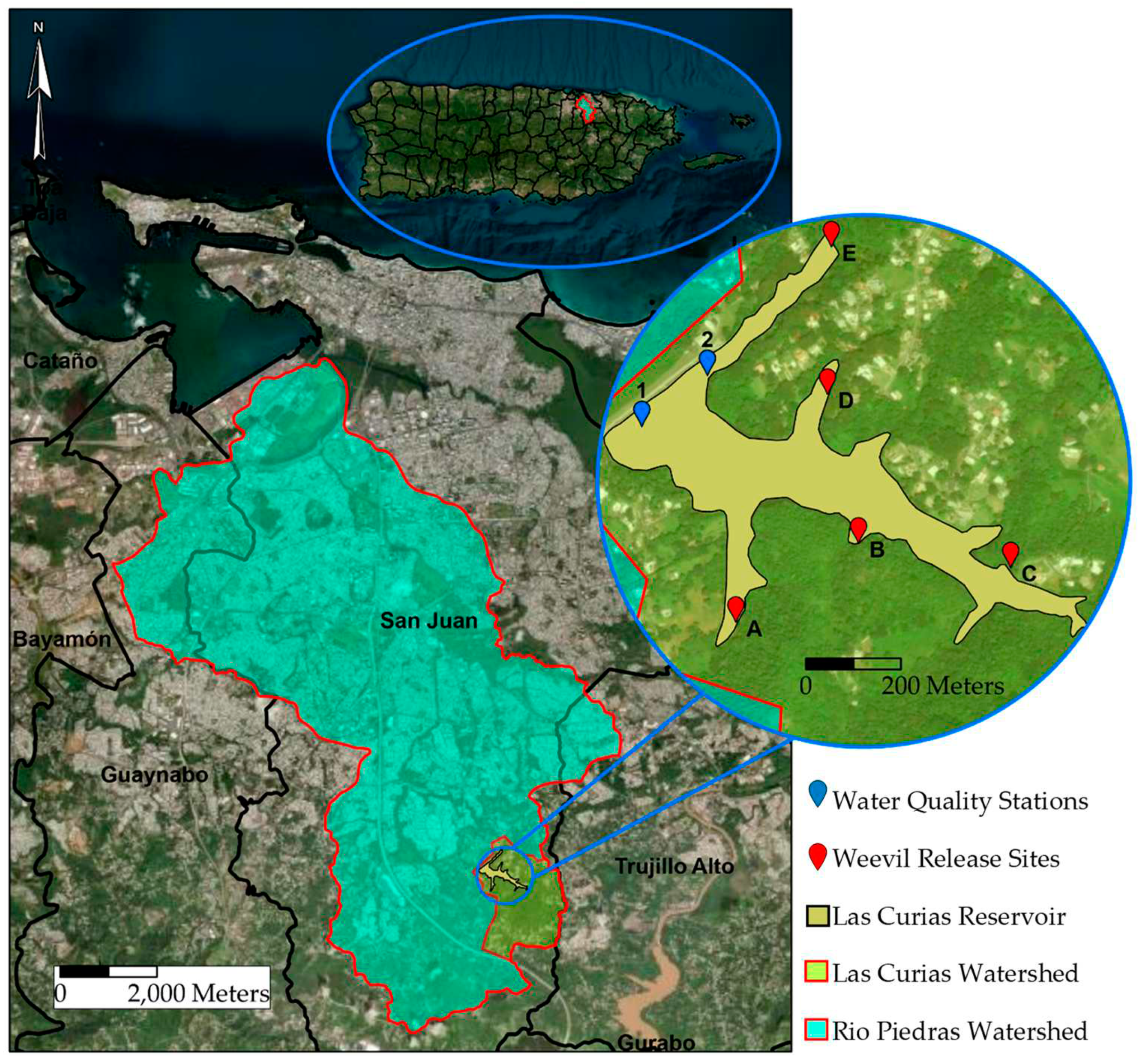
2.1. Site description
2.2. Salvinia performance
2.3. Weevil introduction and densities
2.4. Water quality
2.5. Statistical analysis
3. Results
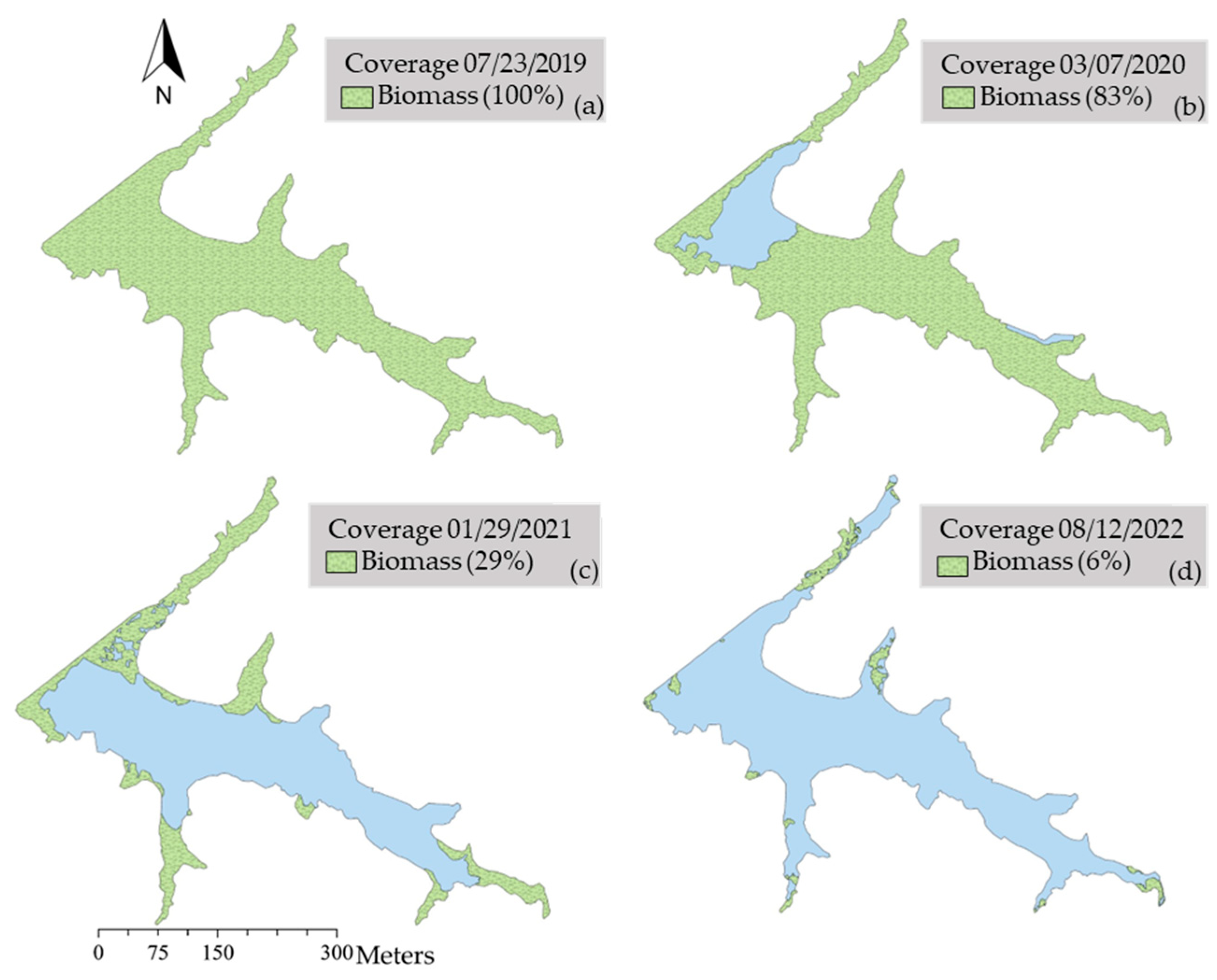
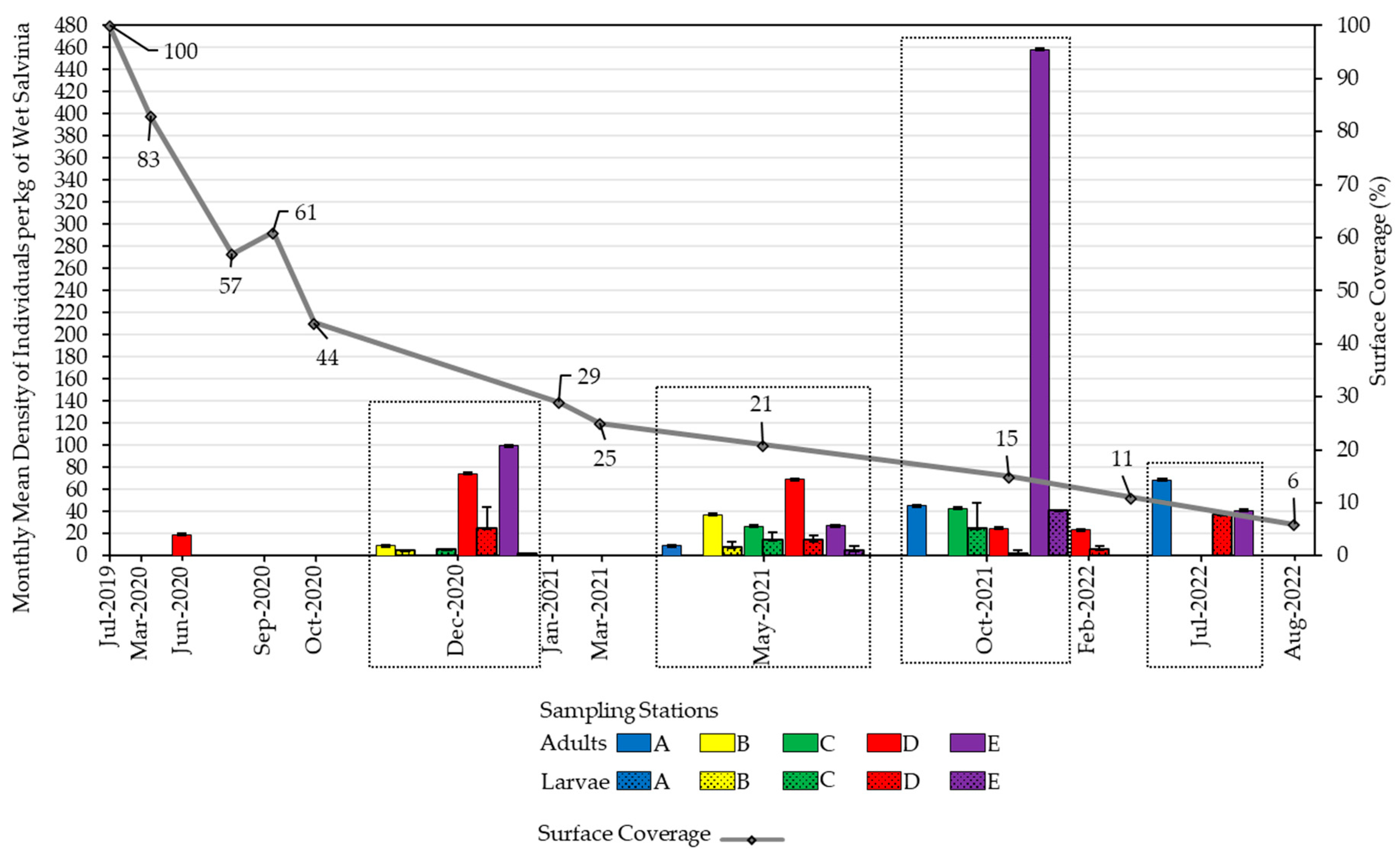
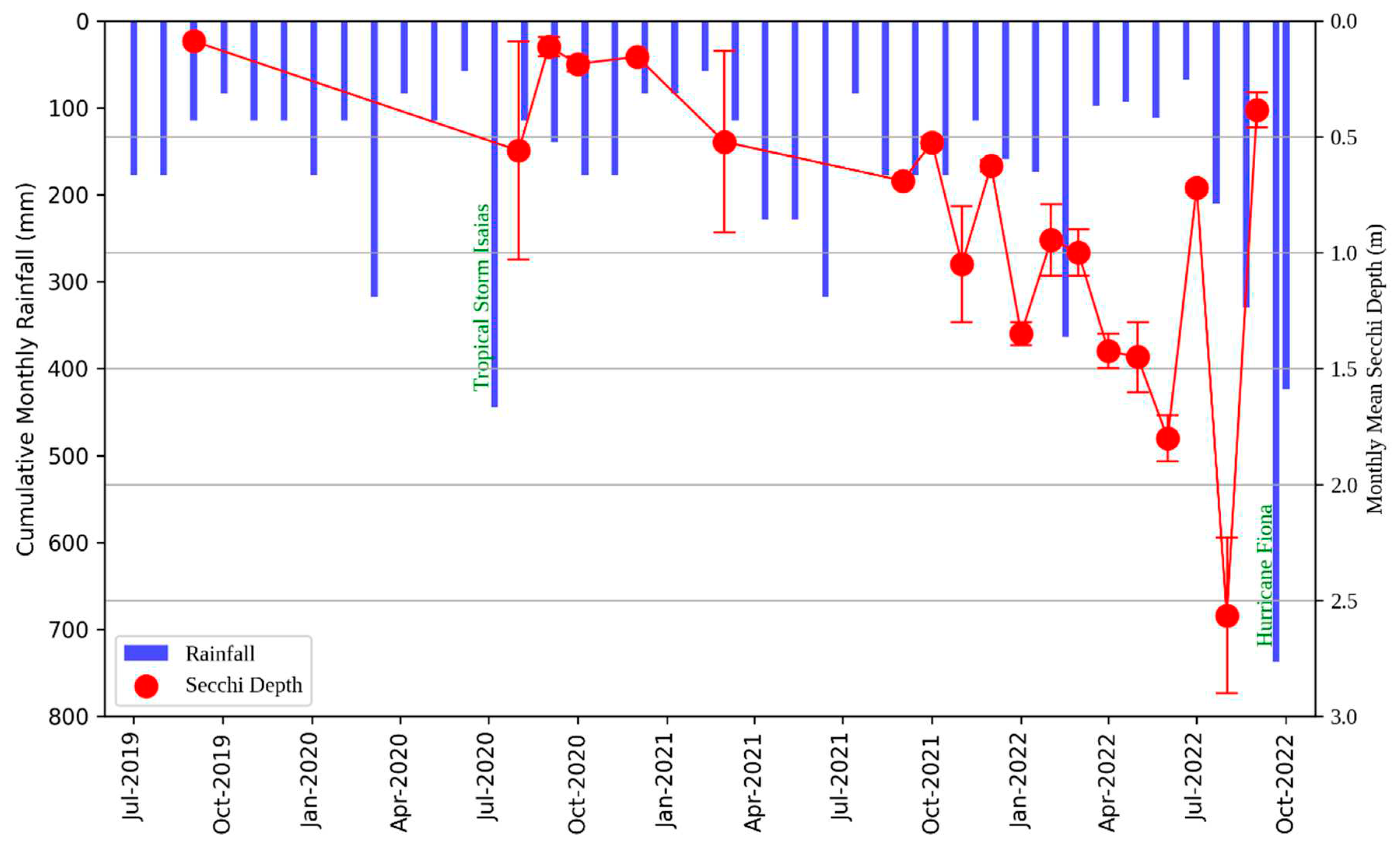
3.1. Salvinia performance
3.2. Weevil performance
3.3. Water quality
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johnson, P.T.; Olden, J.D.; Zanden, M.J.V. Dam invaders: impoundments facilitate biological invasions into freshwaters. Front. Ecol. Environ. 2008, 6, 357–363. [Google Scholar] [CrossRef]
- Havel, J.E.; Lee, C.E.; Vander Zanden, M.J. Do Reservoirs Facilitate Invasions into Landscapes? BioScience 2005, 55(6), 518–525. [Google Scholar] [CrossRef]
- Havel, J.E.; Kovalenko, K.E.; Thomaz, S.M.; Amalfitano, S.; Kats, L.B. Aquatic invasive species: challenges for the future. Hydrobiologia 2015, 750, 147–170. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Fitzgerald, D.G.; Mayer, C.M.; Rudstam, L.G.; Mills, E.L. Alteration of Ecosystem Function by Zebra Mussels in Oneida Lake: Impacts on Submerged Macrophytes. Ecosystems 2006, 9, 1017–1028. [Google Scholar] [CrossRef]
- Tasker, A. V. USDA Demonstration Project: Giant Salvinia (Toledo Bend Reservoir and Surrounding Areas in Louisiana and Eastern Texas. Environmental Assessment, 01); U.S. Department of Agriculture, 2001. 20 March.
- Jayan, P. R.; Sathyanathan, N. Aquatic Weed Classification, Environmental Effects and the Management Technologies for Its Effective Control in Kerala, India. International Journal of Agricultural and Biological Engineering 2012, 5(1), 76–91. [Google Scholar]
- Mitchell, D.S. The Kariba Weed: Salvinia Molesta. Brittonia 1972, 24(3), 228–231. [Google Scholar]
- Luque, G.M.; Bellard, C.; Bertelsmeier, C.; Bonnaud, E.; Genovesi, P.; Simberloff, D.; Courchamp, F. The 100th of the world’s worst invasive alien species. Biol. Invasions 2013, 16, 981–985. [Google Scholar] [CrossRef]
- Díaz, R. ; Johnson, S; Mudge, C; Russell, A. The Biology and Ecology of the Salvinia Weevil: A Biological Control Agent for the Management of Giant Salvinia.; 3474; Louisiana State University Agricultural Center, 2015.
- Forno, I.; Harley, K. The occurrence of Salvinia molesta in Brazil. Aquat. Bot. 1979, 6, 185–187. [Google Scholar] [CrossRef]
- McFarland, D. G.; Nelson, L. S.; Grodowitz, M. J.; Smart, R. M.; Owens, C. S. Salvinia Molesta D. S. Mitchell (Giant Salvinia) in the United States: A Review of Species Ecology and Approaches to Management.; ERDC/EL SR-04-2; U.S. Army Engineer Research and Development Center, Environmental Laboratory; Lewisville Aquatic Ecosystem Research Facility, 2004; p 40.
- Julien, M.H.; Hill, M.P.; Tipping, P.W. Salvinia Molesta DS Mitchell (Salviniaceae). In Weed biological control with arthropods in the tropics.; Muniappan, R., Ed.; Cambridge University Press: Cambridge, 2009. [Google Scholar]
- Finlayson, C. Growth rates of Salvinia molesta in Lake Moondarra, Mount Isa, Australia. Aquat. Bot. 1984, 18, 257–262. [Google Scholar] [CrossRef]
- Room, P.M.; Thomas, P.A. Nitrogen, Phosphorus and Potassium in Salvinia Molesta Mitchell in the Field: Effects of Weather, Insect Damage, Fertilizers and Age. Aquatic Botany 1986, 1, 213–232. [Google Scholar] [CrossRef]
- Motitsoe, S.N.; Coetzee, J.A.; Hill, J.M.; Hill, M.P. Biological Control of Salvinia molesta (D.S. Mitchell) Drives Aquatic Ecosystem Recovery. Diversity 2020, 12, 204. [Google Scholar] [CrossRef]
- Wahl, C.; Kaller, M.; Diaz, R. Invasion of floating fern alters freshwater macroinvertebrate community structure with implications for bottom-up processes. Hydrobiologia 2021, 848, 2523–2537. [Google Scholar] [CrossRef]
- Woodley, S.E.; Wahl, C. F.; Tryforos, A.; Díaz, R. Biological Control of Invasive Floating Fern Leads to Rapid Recovery of Ecological Functions in Coastal Freshwater Wetlands in Louisiana. Journal of Aquatic Plant Management 2023, 61. [Google Scholar]
- Thayer, D.D.; Pfingsten, I.A.; Jacono, C.C.; Richerson, M.M.; Howard, V. Salvinia Molesta Mitchell: U.S. Geological Survey, Nonindigenous Aquatic Species Database.; U.S. Geological Survey (USGS): Gainesville, FL, 2018. [Google Scholar]
- Pasch, R. J.; Penny, A. B.; Berg, R. National Hurricane Center Tropical Cyclone Report: Hurricane Maria (AL152017), 16-; National Oceanic And Atmospheric Administration and the National Weather Service, 2023; pp 1–48. https://www.nhc.noaa.gov/data/tcr/AL152017_Maria.pdf. 30 September.
- Wahl, C.; Diaz, R.; Ortiz-Zayas, J. Assessing Salvinia molesta impact on environmental conditions in an urban lake: case study of Lago Las Curias, Puerto Rico. Aquat. Invasions 2020, 15, 562–577. [Google Scholar] [CrossRef]
- Calder, A.A.; Sands, D.P.A. A NEW BRAZILIAN CYRTOBAGOUS HUSTACHE (COLEOPTERA: CURCULIONIDAE) INTRODUCED INTO AUSTRALIA TO CONTROL SALVINIA. Aust. J. Èntomol. 1985, 24, 57–64. [Google Scholar] [CrossRef]
- Coetzee, J.A.; Hill, M.P. Salvinia molesta D. Mitch. (Salviniaceae): impact and control. CAB Rev. Perspect. Agric. Veter- Sci. Nutr. Nat. Resour. [CrossRef]
- Tetra Tech. Las Curias Reservoir Lake Watershed Characterization.; Fairfax, VA, 2018; p 22.
- U.S. Census Bureau. American Community Survey 5-Year Estimates. Retrieved from Census Reporter Profile Page for Cupey Barrio, San Juan Municipio, PR; U.S. Census Bureau. 2021. Available online: https://censusreporter.org/profiles/06000US7212722847-cupey-barrio-san-juan-municipio-pr/ (accessed on 3 March 2023).
- Department of Natural and Environmental Resources (DRNA). Puerto Rico Water Resources Inventory.; Water Plan Office, Puerto Rico: Department of Natural and Environmental Resources (DRNA), 2004. [Google Scholar]
- Lugo, A. E.; Ramos, O.; Rodriguez, C. The Río Piedras Watershed and Its Surrounding Environment.; US Department of Agriculture, Forest Service, International Institute of Tropical Forestry, 2011; p 46.
- ArcGIS Pro, 2020. https://www.esri.com/arcgis-blog/products/arcgis-pro/announcements/whats-new-in-arcgis-pro-2-5/.
- ArcGIS Pro, 2022.
- Drone2Map, 2021. https://www.esri.com/en-us/arcgis/products/arcgis-drone2map/overview?rmedium=www_esri_com_EtoF&rsource=https%3A%2F%2Fwww.esri.com%2Fen-us%2Farcgis%2Fproducts%2Fdrone2map%2Foverview.
- Ireland, P.; Knutson, A.; Gregory, L. Rearing the Salvinia Weevil in Outdoor Tanks at Caddo Lake, Texas. In A guide to mass rearing the salvinia weevil for biological control of giant salvinia.; Knutson, A., Nachtrieb, J., Eds.; Texas A&M AgriLife Extension Service, Special Publication ESP-475, 2012; pp 25–36.
- Wahl, C.F.; Diaz, R. Winter and spring conditions determine the production of the salvinia weevil mass rearing programme. Biocontrol Sci. Technol. 2020, 30, 569–580. [Google Scholar] [CrossRef]
- Nachtrieb, J.G. Rearing the Salvinia Weevil for Biological Control of Giant Salvinia at the U.S. Army Corps of Engineers Lewisville Aquatic Ecosystem Research Facility. In A guide to mass rearing the Salvinia weevil for biological control of giant Salvinia.; Knutson, A., Nachtrieb, J., Eds.; Texas A&M AgriLife Extension Service, Special Publication, 2012; pp 13–24.
- R Core Team. R: A Language and Environment for Statistical Computing., 2023.
- R Core Team. R, 2023. https://www.r-project.org/.
- Python, 2023. https://www.python.org/downloads/release/python-31012/.
- Crooks, J.A. Characterizing ecosystem-level consequences of biological invasions: the role of ecosystem engineers. Oikos 2002, 97, 153–166. [Google Scholar] [CrossRef]
- Bozec, Y.; Alvarez-Filip, L.; Mumby, P.J. The dynamics of architectural complexity on coral reefs under climate change. Glob. Chang. Biol. 2014, 21, 223–235. [Google Scholar] [CrossRef]
- Linders, T.E.W.; Schaffner, U.; Eschen, R.; Abebe, A.; Choge, S.K.; Nigatu, L.; Mbaabu, P.R.; Shiferaw, H.; Allan, E. Direct and indirect effects of invasive species: Biodiversity loss is a major mechanism by which an invasive tree affects ecosystem functioning. J. Ecol. 2019, 107, 2660–2672. [Google Scholar] [CrossRef]
- Jones, H.P.; Schmitz, O.J. Rapid Recovery of Damaged Ecosystems. PLOS ONE 2009, 4, e5653. [Google Scholar] [CrossRef]
- Tanentzap, A.J.; Burrows, L.E.; Lee, W.G.; Nugent, G.; Maxwell, J.M.; Coomes, D.A. Landscape-level vegetation recovery from herbivory: progress after four decades of invasive red deer control. J. Appl. Ecol. 2009, 46, 1064–1072. [Google Scholar] [CrossRef]
- Prior, K.M.; Adams, D.C.; Klepzig, K.D.; Hulcr, J. When does invasive species removal lead to ecological recovery? Implications for management success. Biol. Invasions 2018, 20, 267–283. [Google Scholar] [CrossRef]
- Martin, G.; Coetzee, J.; Weyl, P.; Parkinson, M.; Hill, M. Biological control of Salvinia molesta in South Africa revisited. Biol. Control. 2018, 125, 74–80. [Google Scholar] [CrossRef]
- Pieterse, A. H.; Kettunen, M.; Diouf, S.; Ndao, I.; Sarr, K.; Tarvainen, A.; Hellsten, S. Effective Biological Control of Salvinia Molesta in the Senegal River by Means of the Weevil Cyrtobagous Salviniae. AMBIO: A Journal of the Human Environment 2003, 32 (7), 458–462.
- Sullivan, P.R.; Postle, L.A.; Julien, M. Biological control of Salvinia molesta by Cyrtobagous salviniae in temperate Australia. Biol. Control. 2011, 57, 222–228. [Google Scholar] [CrossRef]
- Room, P.M.; Harley, K.L.S.; Forno, T.W.; Sands, D.P.A. Successful Biological Control of the Floating Weed Salvinia. Nature 1981, 294, 78–80. [Google Scholar] [CrossRef]
- Tipping, P.W.; Martin, M.R.; Center, T.D.; Davern, T.M. Suppression of Salvinia molesta Mitchell in Texas and Louisiana by Cyrtobagous salviniae Calder and Sands. Aquat. Bot. 2008, 88, 196–202. [Google Scholar] [CrossRef]
- Forno, I.W.; Sands, D.P.A.; Sexton, W. Distribution, biology and host specificity of Cyrtobagous singularis Hustache (Coleoptera: Curculionidae) for the biological control of Salvinia molesta. Bull. Èntomol. Res. 1983, 73, 85–95. [Google Scholar] [CrossRef]
- Micinski, S.; Fitzpatrick, B.J.; Ferro, M.L.; Johnson, S.J.; Johnson, B.; Williams, S. Flight Activity of Cyrtobagous Salvinia Calder and Sands in Louisiana. Southwestern Entomologist 2016, 41(2), 313–320. [Google Scholar] [CrossRef]
- Sands, D.P.A.; Schotz, M.; Bourne, A.S. THE FEEDING CHARACTERISTICS AND DEVELOPMENT OF LARVAE OF A SALVINIA WEEVIL CYRTOBAGOUS SP. Èntomol. Exp. et Appl. 1983, 34, 291–296. [Google Scholar] [CrossRef]
- Wahl, C.; Diaz, R.; Kaller, M. Optimising Berlese funnel extraction for population estimates of adult Cyrtobagous salviniae from Salvinia molesta. Biocontrol Sci. Technol. 2022, 32, 1–16. [Google Scholar] [CrossRef]
- Masifwa, WF.; Okello, W.; Ochieng, H.; Ganda, E. Phosphorus Release from Decomposing Water Hyacinth and Effects of Decomposition on Water Quality. Uganda Journal of Agricultural Sciences 2004, 9, 389–395. [Google Scholar]
- Wu, Y.; Wen, Y.; Zhou, J.; Wu, Y. Phosphorus release from lake sediments: Effects of pH, temperature and dissolved oxygen. KSCE J. Civ. Eng. 2013, 18, 323–329. [Google Scholar] [CrossRef]
- Cary, P.R.; Weerts, P.G. Growth of Salvinia molesta as affected by water temperature and nutrition. III. Nitrogen-phosphorus interactions and effect of pH. Aquat. Bot. 1984, 19, 171–182. [Google Scholar] [CrossRef]
- Hupfer, M.; Lewandowski, J. Oxygen Controls the Phosphorus Release from Lake Sediments–a Long-lasting Paradigm in Limnology. International Review of Hydrobiology 2008, 93 (4–5), 415–432. [CrossRef]
- Sondergaard, M.; Jensen, P.J.; Jeppesen, E. Retention and Internal Loading of Phosphorus in Shallow, Eutrophic Lakes. Sci. World J. 2001, 1, 427–442. [Google Scholar] [CrossRef] [PubMed]
- Frodge, J.D.; Thomas, G.L.; Pauley, G.B. Sediment Phosphorus Loading Beneath Dense Canopies of Aquatic Macrophytes. Lake Reserv. Manag. 1991, 7, 61–71. [Google Scholar] [CrossRef]
- Hutchinson, G. E. A Treatise on Limnology: Geography, Physics and Chemistry.; Wiley, 1957.
- Buffle, J.; Stumm, W. General Chemistry of Aquatic Systems. In Chemical and Biological Regulation of Aquatic Systems; Buffle, J., De Vitre, R.R., Eds.; CRC, 1994; pp 1–42.
- Bianchini, I.; da Cunha-Santino, M.B. CH4 and CO2 from Decomposition of Salvinia auriculata Aublet, a Macrophyte with High Invasive Potential. Wetlands 2016, 36, 557–564. [Google Scholar] [CrossRef]
- O'Sullivan, C.; Rounsefell, B.; Grinham, A.; Clarke, W.; Udy, J. Anaerobic digestion of harvested aquatic weeds: water hyacinth (Eichhornia crassipes), cabomba (Cabomba Caroliniana) and salvinia (Salvinia molesta). Ecol. Eng. 2010, 36, 1459–1468. [Google Scholar] [CrossRef]
- Grasset, C.; Mendonça, R.; Saucedo, G.V.; Bastviken, D.; Roland, F.; Sobek, S. Large but variable methane production in anoxic freshwater sediment upon addition of allochthonous and autochthonous organic matter. Limnol. Oceanogr. 2018, 63, 1488–1501. [Google Scholar] [CrossRef]
- Quiñones-Márquez, F. Limnology of Lago Loiza, Puerto Rico; U.S. Geological Survey (USGS), 1980; Vol. 79.
- Lewis Jr, W. M. The Thermal Regime of Lake Lanao (Philippines) and Its Theoretical Implications for Tropical Lakes 1. Limnology and Oceanography 1973, 18(2), 200–217. [Google Scholar] [CrossRef]
- Lewis Jr, W. M. Temperature, Heat, and Mixing in Lake Valencia, Venezuela. Limnology and Oceanography 1983, 28(2), 273–286. [Google Scholar] [CrossRef]
- MacIntyre, S.; Melack, J.M. Vertical and Horizontal Transport in Lakes: Linking Littoral, Benthic, and Pelagic Habitats. J. North Am. Benthol. Soc. 1995, 14, 599–615. [Google Scholar] [CrossRef]
- Crowe, S.A.; O’neill, A.H.; Katsev, S.; Hehanussa, P.; Haffner, G.D.; Sundby, B.; Mucci, A.; Fowle, D.A. The biogeochemistry of tropical lakes: A case study from Lake Matano, Indonesia. Limnol. Oceanogr. 2008, 53, 319–331. [Google Scholar] [CrossRef]
- Fan, J.; Morris, G. L. Reservoir Sedimentation Handbook: Design and Management of Dams, Reservoirs, and Watersheds for Sustainable Use; McGraw-Hill, 1998.
- Briese, D.T. Classical Biological Control. In Australian weed management systems; 2000; Vol. 2, pp 161-192.

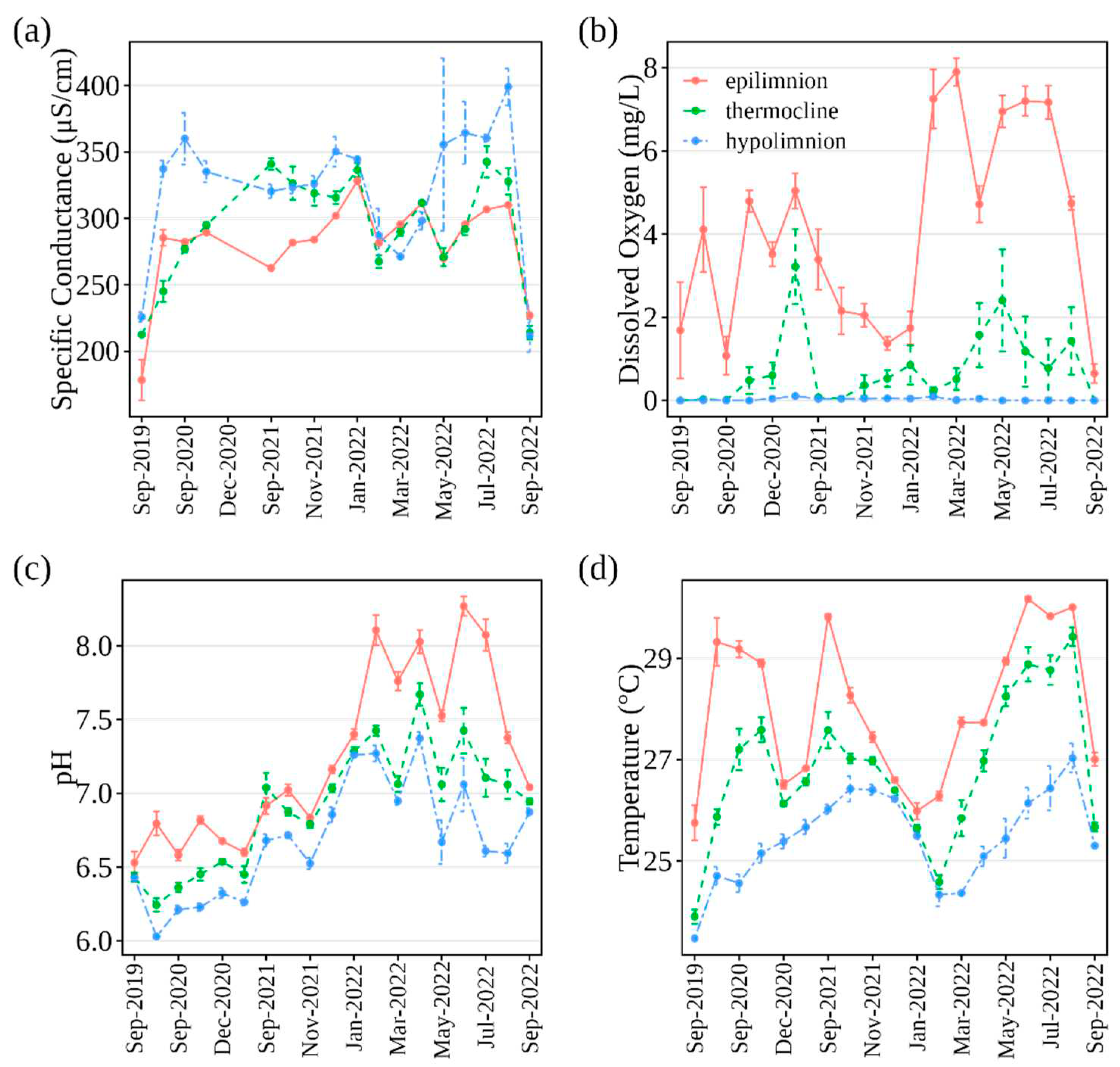
| Depth Zone | Year | Water Cspecific conductance (μS/cm) | Dissolved Oxygen (mg/L) | pH (s.u.) | Temperature (°C) | ||||
|---|---|---|---|---|---|---|---|---|---|
| N | Mean (± SE) | N | Mean (± SE) | N | Mean (± SE) | N | Mean (± SE) | ||
| Epilimnion | 2019 | 6 | 178.36 (± 15.35)a | 6 | 1.69 (± 1.15)a | 6 | 6.53 (±0.07)a | 6 | 25.75 (±0.24)a |
| 2020 | 18 | 285.72 (± 1.98)b | 24 | 3.37 (± 0.40)a | 24 | 6.71 (±0.02)a | 24 | 28.47 (±0.26)b | |
| 2021 | 24 | 282.60 (± 2.93)b | 30 | 2.80 (±0.31)a | 30 | 6.90 (±0.03)a | 30 | 27.79 (±0.22)b | |
| 2022 | 54 | 291.79 (± 3.85)b | 54 | 5.36 (±0.36)b | 54 | 7.73 (±0.05)b | 54 | 28.18 (±0.21)b | |
| Thermocline | 2019 | 6 | 212.55 (± 0.37)b | 6 | 0.00 (±0.00) | 6 | 6.43 (±0.03)a | 6 | 23.90 (±0.13)a |
| 2020 | 18 | 272.42 (± 5.67)b | 24 | 0.25 (±0.11) | 24 | 6.39 (±0.02)a | 24 | 26.69 (±0.19)b | |
| 2021 | 24 | 325.51 (± 4.45)c | 30 | 0.84 (±0.28) | 30 | 6.83 (±0.04)b | 30 | 27.09 (±0.24)b | |
| 2022 | 53 | 295.25 (± 5.73)b | 53 | 0.96 (±0.22) | 53 | 7.22 (±0.04)c | 53 | 23.47 (±0.01)a | |
| Hypolimnion | 2019 | 17 | 225.85 (± 3.76)a | 17 | 0.00 (±0.00) | 17 | 6.42 (±0.01)a | 17 | 23.47 (±0.01)a |
| 2020 | 27 | 344.33 (± 7.28)b | 36 | 0.13 (±0.00) | 36 | 6.18 (±0.02)a | 36 | 24.92 (±0.10)b | |
| 2021 | 17 | 331.03 (± 4.80)b | 24 | 0.06 (±0.01) | 24 | 6.57 (±0.04)ab | 24 | 26.07 (±0.08)c | |
| 2022 | 37 | 322.09 (± 11.83)b | 37 | 0.02 (±0.01) | 37 | 6.96 (±0.05)b | 37 | 25.46 (±0.17)bc | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




