Submitted:
07 October 2023
Posted:
08 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
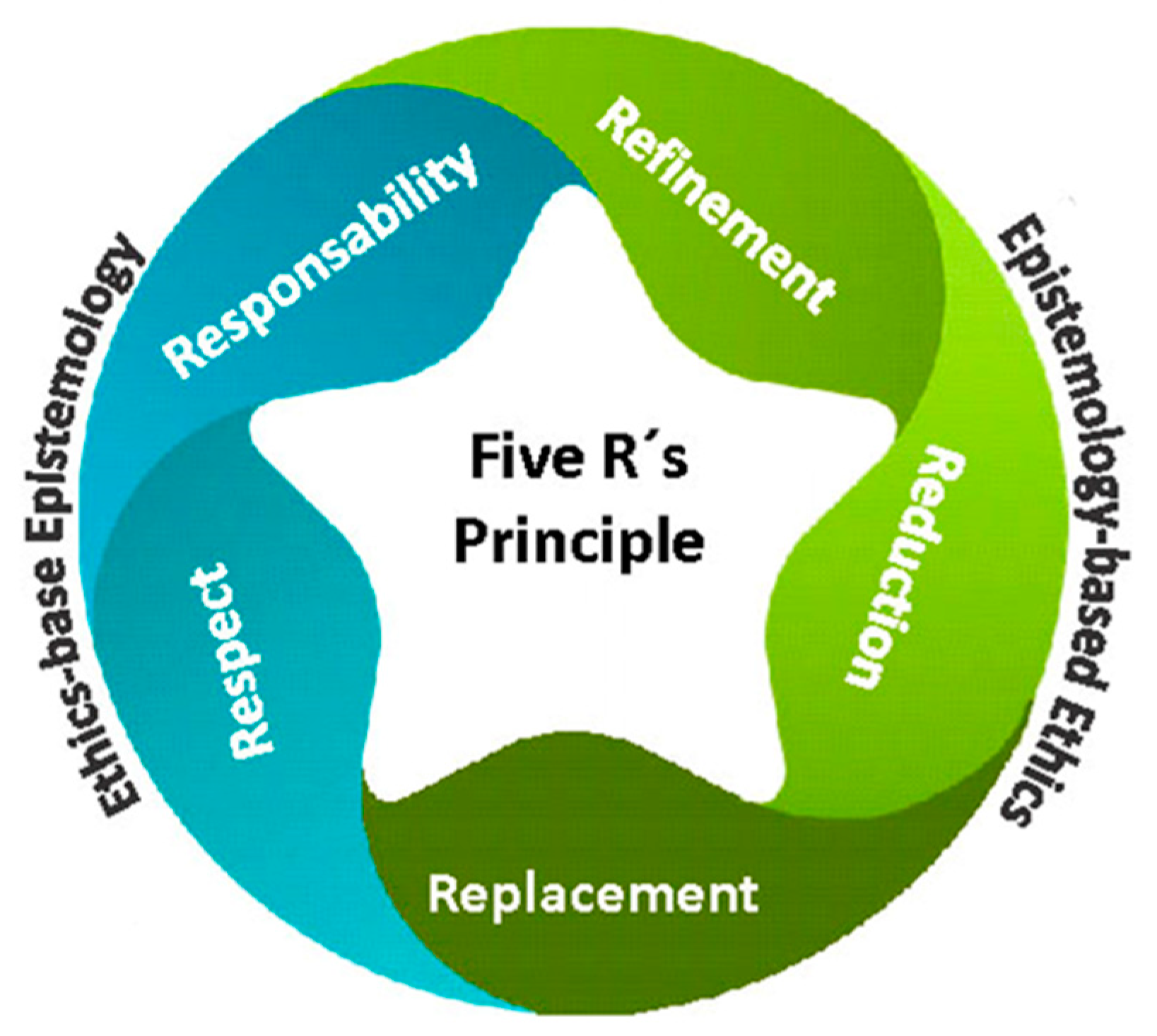
2. Ethical concerns and the 5R principle
3. Ethical approach in Echinoderms
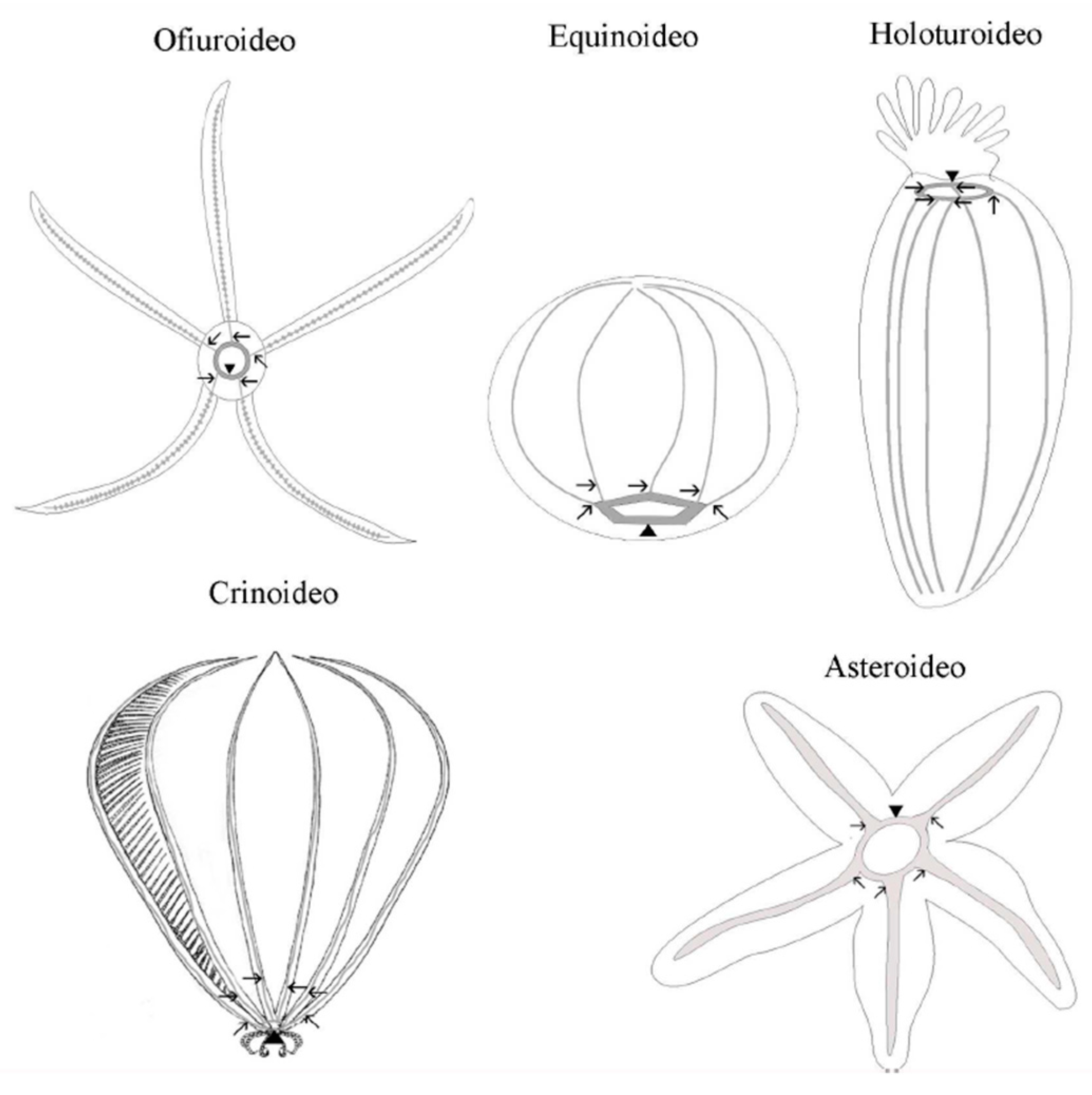
3.1. Phylum Echinodermata
3.2. Historical use of echinoderms
3.3. Echinoderms as models
3.3. Echinoderms nervous system
3.3. Pain and echinoderms
4. Echinoderms welfare
- Each and every significant aspect must be addressed in order for the assessment to be comprehensive;
- The criteria must not be redundant or irrelevant;
- Each criterion must be independent of the others.
- All stakeholders must agree on the criteria, and they must have a practical basis
- The criteria, as well as their application, should be transparent and simple to understand.
- The number of criteria should be limited to 12 at most.
- Tube feet adhesion: Echinoderms move by using their tube feet, which are frequently adhesive to the bottom or walls of the aquarium in captivity. A detached or loosely attached echinoderm indicates that the individual is not in good health.
- Echinoderms have a distinct righting behavior because they are most vulnerable with their oral face above [153,154] (Percy, 1973; Himmelman, 1984). The speed at which this behavior is carried out can be used to gauge its physiological activity, health, and overall condition. This indicator must first be established for the species, and size and sex must be evaluated before establishing a normal time of righting for each species. After you have set a time, you can use it as a stress indicator.
- Echinoderms use their spines / Pedicelaria / Arms for a variety of functions (feeding, moving, defense, eating), so their response to stimuli is a good indicator of their health. An echinoderm that is nonresponsive or responds slowly indicates that the individual is not in good health.
- Feeding behavior: like any other animal’s, is an indicator of health. Echinoderms feeding and defecating indicate a healthy individual.
- Epidermis appearance: A healthy echinoderm has a shiny, non-interrumped epidermis. In contrast, the presence of reddish or blackish coloration, as well as inflammation and mucus, indicates the presence of an infection.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kellert, S. R. Values and perceptions of invertebrates. Conservation Biology 1993, 7, 845–855. [Google Scholar] [CrossRef]
- Schmidt-Rhaesa, & schwartz 2016. Nematomorpha from the Philippines, with description of two new species. Zootaxa, 4158(2), 246-260.
- Pollo, S., & Vitale, A. (2019). Invertebrates and humans: science, ethics, and policy. In C. Carere & J. Mather (Eds.), The Welfare of Invertebrate Animals (pp. 7–22). Springer.
- Crespi-Abril, A. C., & Rubilar, T. Ética e invertebrados: análisis de los casos de los cefalópodos y equinodermos. Revista Latinoamericana de Estudios Críticos Animales 2018, 8, 210–232.
- Horvath, K., Angeletti, D., Nascetti, G., Carere, C. Invertebrate welfare: an overlooked issue. Annali dell’Istituto superiore di sanità 2013, 49, 9–17.
- Mather, J. A. An invertebrate perspective on pain. Animal Sentience 2016, 1, 12. [Google Scholar] [CrossRef]
- Carere, C., & Mather, J. (2019). The Welfare of Invertebrate Animals. Springer. [CrossRef]
- Crespi-Abril, A. C., & Rubilar, T. Moving forward in the ethical consideration of invertebrates in experimentation: Beyond the Three R’s Principle. Revista de Biología Tropical 2021, 69(Suppl. 1), 346–357. [CrossRef]
- Mather, J.A. Why (and how) personalities in invertebrates? Current Zoology 2012, 58, 566. [Google Scholar] [CrossRef]
- Schmidt-Rhaesa, A., Harzsch, S., & Purschke, G. (2015). Structure and Evolution of Invertebrate Nervous Systems. Oxford University Press.
- Mikhalevich, I., & Powell, R. Minds without spines: evolutionarily inclusive animal ethics. Animal Sentience 2020, 29, 1–25.
- Ellwood, R.W. Pain and suffering in invertebrates? Institute for Laboratory Animal Research 2011, 52, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Guariglia, O., & Vidiella, G. (2011). Breviario de ética. Buenos Aires: Edhasa.
- Edelman, D. B., & Seth, A. K. Animal Consciousness: A Synthetic Approach. Trends in Neurosciences 2009, 32, 476–484.
- Chandroo, K. P., Duncan, I. J. H., & Moccia, R. D. Can fish suffer?: Perspectives on Sentience, Pain, Fear, and Stress. Applied Animal Behavior Science 2004, 86, 225–250.
- Griffin, D. R., & Speck, G. B. New Evidence of Animal Consciousness. Animal Cognition 2004, 7, 5–18.
- Ellwood, R. W. Pain and suffering in invertebrates? Institute for Laboratory Animal Research 2011, 52, 175–184. [Google Scholar] [CrossRef]
- Lewbart, G. A. , & Mosley, C. Clinical Anesthesia and Analgesia in Invertebrates. Journal of Exotic Pet Medicine 2012, 21, 59–70. [Google Scholar]
- Birch, J (2017) Animal Sentience and the Precautionary Principle. Animal Sentience, 16(1). [CrossRef]
- Birch, J., Burn, C., Schnell, A., Browning, H., y Crump, A. (2021). Review of the Evidence of Sentience in Cephalopod Molluscs and Decapod Crustaceans. London: London School of Economics and Politics.
- Rubilar, C. T., & Crespi-Abril, A. C. Does Echinoderm research deserve an ethical consideration? International Journal of Tropical Biology 2017, 65, S11–S22.
- Russell, W. M. S., & Burch, R. L. (1959). The Principles of Humane Experimental Technique. London: Methuen.
- Tomasik, B. (2014). Suffering in Animals vs. humans, essays on reducing suffering. Retrieved from http://reducing-suffering.org/suffering-in-animals-vs-humans/.
- Blattner, C. E. (2019). Rethinking the 3Rs: From Whitewashing to Rights. In K. Hermann y K. Jayne (Eds.), Animal Experimentation: Working Towards a Paradigm Change (pp. 168-193). Amsterdam: Brill.
- Taylor, K., & Rego, L. EU statistics on animal experiments for 2014. ALTEX 2016, 33, 465–468.
- Johnson, J., & Degeling, C. Animals-as-Patients: Improving the Practice of Animal Experimentation. Between the Species 2012, 15, 43–58.
- Brusca, R. C., Moore, W., & Schuster, M. (2016). Invertebrates. Massachusetts: Sinauer Associated, Inc, Publishers.
- Telford, M. J., Lowe, C. J., Cameron, C. B., Ortega-Martinez, O., Aronowicz, J., Oliveri, P., & Copley, R. R. Phylogenomic analysis of echinoderm class relationships supports Asterozoa. Proceedings of the Royal Society of London B: Biological Sciences 2014, 281, 20140479.
- Smith, A. B. Echinoderms (Other Than Echinoids). In R. Cocks & I. Plimer (Eds.), Encyclopedia of Geology (pp. 334–341). Elsevier, Oxford.
- Arnone, M.I., Byrne, M., Martinez, P. (2015). Echinodermata. In: Wanninger, A. (eds) Evolutionary Developmental Biology of Invertebrates 6. Springer, Vienna. [CrossRef]
- Amemiya, C. T., Miyake, T., & Rast, J. P. Echinoderms. Current biology 2005, 15, R944–R946.
- Hess, H., Messing, C., & Ausich, W. I. (2011). Treatise on Invertebrate Paleontology. University of Kansas Paleontological Institute.
- Chen, J. Overview of sea cucumber farming and sea ranching practices in China. SPC beche-de-mer Information Bulletin 2003, 18, 18–23. [Google Scholar]
- Slater, M., & Chen, J. Sea cucumber biology and ecology. Echinoderm Aquaculture 2015, 47–55.
- Lawrence, J. M. (Ed.). (2013). Sea Urchins: Biology and Ecology. Academic Press.
- Sun, J., & Chiang, F. S. Use and exploitation of sea urchins. Echinoderm aquaculture 2015, 25–45.
- López-Pérez, Andrés, Rebeca Granja-Fernández, Cuauhtémoc Aparicio-Cid, Ronald C. Zepeta-Vilchis, Ana M. Torres-Huerta, Francisco Benítez-Villalobos, Daniel A. López-López, Carlos Cruz-Antonio, and Omar Valencia-Méndez. “Corales pétreos, equinodermos y peces asociados a comunidades y arrecifes coralinos del Parque Nacional Huatulco, Pacífico sur mexicano.” Revista mexicana de biodiversidad 85, no. 4 (2014): 1145-1159.
- Luna Salguero, B and Bonilla, H. “Estructura comunitaria y trófica de las estrellas de mar (Echinodermata: Asteroidea) en arrecifes rocosos de Loreto, Golfo de California, México.” Hidrobiológica 20, no. 2 (2010): 127-134.
- Lawrence, J. M. (2007). Sea urchin roe cuisine. In Developments in Aquaculture and Fisheries Science (Vol. 37, pp. 521–523). Elsevier.
- Brown, N., & Eddy, S. (2015). Echinoderm Aquaculture. New Jersey: Wiley-Blackwell.
- Lamarck, J. B. (1809). Philosophie zoologique, ou, Exposition des considérations relative à l’histoire naturelle des animaux. Pzaris.
- De Tornos, L. (1839). Compendio de Historia Natural Dividido en los Tres Ramos de Mineralogía, Botánica y Zoología. Madrid, Imprenta Salvador Albert.
- Salacroux, A. P. G. (1840). Nuevos Elementos de Historia Natural. Madrid, Imprenta de Verges.
- Frey, H., & Luckart, R. (1847). Zootomie. Voss, Germany: Leipzig. Agassiz, A. (1881). Report on the Echinoidea dredged by H.M.S. Challenger during the Years 1873-1876. ZOOLOGY Part IX Challenger Reports.
- Agassiz, A. Report on the scientific results of the voyage of the H.M.S. Challenger during the years 1873–76, report on the Echinoidea. Challenger Rep 1881, 3, 1–321. [Google Scholar]
- Théel, H. (1882). Report on the Holothurioidea, dredged by H.M.S. Challenger during the Years 1873-1876. ZOOLOGY Part IX Challenger Reports.
- Agassiz, A. Notice of Calamocrinus diomedeae, a new stalked Crinoid from the Galapagos, dredged by the U. S. Fish Commission Steamer ‘Albatross’. Bulletin of the Museum of Comparative Zoology 1890, 20, 165–167. [Google Scholar]
- Clark, H. L. (1902). Papers from the Hopkins Stanford Galapagos Expedition. Proceeding of the Washington Academy of Science, IV, 521-531.
- De Morgan, W. The Echinoderms collected by the “Huxley” from the North Side of the Bay of Biscay in August, 1906. Journal of the Marine Biological Association of the United Kingdom 1913, 9, 530–541. [Google Scholar] [CrossRef]
- Dufossé, A. (1847). Observations sur le developpement des oursins. In Annales des Sciences Naturelles (Vol. 7, pp. 44–52).
- Derbès, A. A. Observations sur le Méchanisme et les Phenomènes qui Accompagnent la Formation de l’Embryon chez l’Oursin Comestible. Ann Sci Nat Zool 1847, 8, 80–98. [Google Scholar]
- Briggs, E., & Wessel, G. M. In the beginning… animal fertilization and sea urchin development. Developmental biology 2006, 300, 15–26.
- Hertwig, R. (1876). Zur Histologie der Radiolarien: Untersuchungen über den Bau und die Entwicklung der Sphaerozoiden und Thalassicolliden. W. Englemann.
- Boveri, T. Die Polarität von Oocyte, Ei und Larve des Strongylocentrotus lividus. Zoologische Jahrbücher. Abteilung für Anatomie und Ontogenie der Tiere 1901, 14, 630–653. [Google Scholar]
- Dolmatov, I. Y. (1988). Structure of the aquapharyngeal complex in the holothurian Eupentacta fraudatrix under the normal conditions and during regeneration. Canadian Science Dissertation. Vladivostok: Institute of Marine Biology, Far East Division of the USSR Academy of Sciences.
- Briggs, E., & Wessel, G. M. In the beginning… animal fertilization and sea urchin development. Developmental biology 2006, 300, 15–26.
- McClay, D. R. Evolutionary crossroads in developmental biology: sea urchins. Development 2011, 138, 2639–2648. [Google Scholar] [CrossRef]
- Garner, S., Zysk, I., Byrne, G., Kramer, M., Moller, M. Taylor, V., & Burke, R. D. Neurogenesis in sea urchin embryos and the diversity of deuterostome neurogenic mechanisms. Development 2016, 143, 286–297.
- Metchnikoff, I. (1893). Lectures on the Comparative Pathology of Inflammation, Delivered at the Pasteur Institute in 1891. London.
- Pinsino, A., Thorndyke, M. C., & Matranga, V. Coelomocytes and post-traumatic response in the common sea Star Asterias rubens. Cell Stress & Chaperones 2007, 12, 331–341.
- Furukawa, R. , Funabashi, H., Matsumoto, M., & Kaneko, H. Starfish ApDOCK protein essentially functions in larval defense system operated by mesenchyme cells. Immunology and Cell Biology 2012, 90, 955–965. [Google Scholar]
- Ho, E. C. H. (2016). A simple animal model for characterizing gene regulatory control of an immune response. University of Toronto (Canada).
- Mashanov, V. S., Zueva, O. R., & Garcia-Arraras, J. E. Radial glial cells play a key role in echinoderm neural regeneration. BMC Biology 2013, 11, 49.
- Mashanov, V., & Zueva, O. Radial glia in echinoderms. Developmental neurobiology 2019, 79, 396–405.
- Ben Khadra, Y., Said, K., Thorndyke, M., & Martinez, P. Homeobox genes expressed during echinoderm arm regeneration. Biochemical genetics 2014, 52, 166–180.
- Díaz-Balzac, C. A., & García-Arrarás, J. E. (2018). Echinoderm nervous system. In Oxford Research Encyclopedia of Neuroscience.
- Dolmatov, I. Y. Molecular aspects of regeneration mechanisms in holothurians. Genes 2021, 12, 250. [Google Scholar] [CrossRef]
- Medina-Feliciano, J. G., & García-Arrarás, J. E. Regeneration in echinoderms: Molecular advancements. Frontiers in Cell and Developmental Biology 2021, 9, 768641.
- Mashanov, V. S., Ademiluyi, S., Machado, D. J., Reid, R., & Janies, D. (2023) Echinoderm Radial Glia in Adult Cell Renewal, Indeterminate Growth, and Regeneration. Frontiers in Neural Circuits, 17, 1258370. [CrossRef]
- Candia-Carnevali, M. D., Bonasoro, F., Lucca, E., & Thorndyke, M. C. Pattern of cell proliferation in the early stages of arm regeneration in the feather star Antedon mediterranea. The Journal of Experimental Zoology 1995, 272, 464–474.
- Dolmatov, I. Y., & Ginanova, T. T. Muscle Regeneration in Holothurians. Microscopy Research and Technique 2001, 55, 452–463.
- Candelaria, A. G., Murray, M., File, S. K., & García-Arrarás, J. E. Contribution of mesenterial muscle dedifferentiation to intestine regeneration in the sea cucumber Holothuria glaberrima. Cell and Tissue Research 2006, 325, 55–65.
- San Miguel-Ruiz, J. E., & García-Arrarás, J. E. Common cellular events occur during wound healing and organ regeneration in the sea cucumber Holothuria glaberrima. BioMed Central Developmental Biology 2007, 7, 115–124.
- Mashanov, V. S., Zueva, O. R., & Heinzeller, T. Regeneration of the radial nerve cord in a holothrian: A promising new model system for studying post-traumatic recovery in the adult nervous system. Tissue & Cell 2008, 40, 351–372.
- Carnevali, M. D. C., & Burighel, P. (2010). Regeneration in echinoderms and ascidians. eLS.
- Mashanov, V. S., Zueva, O. R., & García-Arrarás, J. E. Transcriptomic changes during regeneration of the central nervous system in an echinoderm. BMC genomics 2014, 15, 1–21.
- Mashanov, V. S., Zueva, O. R., & García-Arrarás, J. E. Expression of pluripotency factors in echinoderm regeneration. Cell and tissue research 2015, 359, 521–536.
- Mashanov, V. S., Zueva, O. R., & García-Arrarás, J. E. Inhibition of cell proliferation does not slow down echinoderm neural regeneration. Frontiers in zoology 2017, 14, 1–9.
- Ferrario, C., Khadra, Y. B., Czarkwiani, A., Zakrzewski, A., Martinez, P., Colombo, G.,... & Sugni, M. Fundamental aspects of arm repair phase in two echinoderm models. Developmental biology 2018, 433, 297–309.
- Dolmatov, I. Y. Variability of regeneration mechanisms in echinoderms. Russian Journal of Marine Biology 2020, 46, 391–404. [Google Scholar] [CrossRef]
- Byrne, M. The Link between Autotomy and CNS Regeneration: Echinoderms as Non-Model Species for Regenerative Biology. BioEssays 2020, 42, 1900219. [Google Scholar] [CrossRef]
- Dolmatov, I. Y., Kalacheva, N. V., Mekhova, E. S., & Frolova, L. T. Autotomy and regeneration of the visceral mass in feather stars. Zoomorphology 2020, 139, 171–187.
- Medina-Feliciano, J. G., & García-Arrarás, J. E. Regeneration in echinoderms: Molecular advancements. Frontiers in Cell and Developmental Biology 2021, 9, 768641.
- Magalhães, F., Andrade, C., Simões, B., Brigham, F., Valente, R., Martinez, P., ... & Coelho, A. V. (2023). Regeneration of Starfish Radial Nerve Cord restores animal mobility and unveils a new coelomocyte population. Cell and Tissue Research, 1-16.
- Eisen, A. (1995). A Holistic Approach to Teaching a Laboratory Using Sea Urchin. Development as an Example System. In C. A. Goldman (Ed.), Tested Studies for Laboratory Teaching (pp. 25-32). Atlanta, GA: Proceedings of the 16th Conference of the Association for Biology Laboratory Education.
- Burke, R. D., et al. A genomic view of the sea urchin nervous system. Developmental biology 2006, 300, 434–460. [CrossRef]
- Mashanov, V. Mashanov, V., Zueva, O., Rubilar, T., Epherra, L., & García-Arrarás, J. E. (2016). Echinodermata. In A. Schmidt-Rhaesa, S. Harzsch, & G. Purschke (Eds.), Structure and Evolution of Invertebrate Nervous Systems (pp. 665–688). Oxford: Oxford University Press.
- Cobb, J. L. S. (1987). Neurobiology of the Echinodermata. In Nervous Systems in Invertebrates (pp. 483-525). Boston, MA: Springer US.
- Heinzeller, T. and Welsch, U. (2001). The echinoderm nervous system and its phylogenetic interpretation. In G. Roth and M. Wullimann, eds. Brain Evolution and Cognition, pp. 41–75. Wiley-Spektrum, New York.
- Hyman, L. (1955). The Invertebrates. IV. Echinodermata. The Coelomate Bilateria. McGraw-Hill Book Co., New York.
- Heinzeller, T. and Welsch, U. (1994). Crinoidea. In F. Harrison and F.-S. Chia, eds. Microscopic Anatomy of Invertebrates. Vol. 14. Echinodermata. pp. 1–148. Wiley-Liss, New York.
- Arnone, M.I. , Byrne, M., Martinez, P. (2015). Echinodermata. In: Wanninger, A. (eds) Evolutionary Developmental Biology of Invertebrates 6. Springer, Vienna. [CrossRef]
- Mashanov, V. S., Zueva, O. R., & Garcia-Arraras, J. E. Organization of glial cells in the adult sea cucumber central nervous system. Glia 2010, 58, 1581–1593.
- Kriegstein, A. and Alvarez-Buylla, A. The glial nature of embryonic and adult neural stem cells. Annual Reviews in Neurosciences 2009, 32, 149–184. [Google Scholar] [CrossRef]
- Malatesta, P., Appolloni, I., and Calzolari, F. Radial glia and neural stem cells. Cell & Tissue Research 2008, 331, 165–178.
- Tanaka, E.M. and Ferretti, P. Considering the evolution of regeneration in the central nervous system. Nature Reviews in Neuroscience 2009, 10, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Zamora, A.J. The ependymal and glial configuration in the spinal cord of urodeles. Anatomy and Embryology (Berlin) 1978, 154, 67–82. [Google Scholar] [CrossRef]
- Binyon, J., & Hasler, B. Electrophysiology of the starfish radial nerve cord. Comparative Biochemistry and Physiology 1970, 32, 747–753.
- Millott, N. , & Okumura, H. The electrical activity of the radial nerve in Diadema antillarum Philippi and certain other echinoids. Journal of Experimental Biology 1968, 48, 279–287. [Google Scholar]
- Cobb, J. (1995). The nervous systems of Echinodermata: recent results and new approaches. In O. Breidbach and W. Kutsch, eds. The Nervous Systems of Invertebrates: An Evolutionary and Comparative Approach, pp. 407–424. Birkhhäuser Verlag, Basel.
- Burke, R.D., Angerer, L.M., Elphick, M.R., et al. A genomic view of the sea urchin nervous system. Developmental Biology 2006, 300, 434–460. [CrossRef]
- Mashanov, V. , Zueva, O., Heinzeller, T., and Dolmatov, I. Ultrastructure of the circumoral nerve ring and the radial nerve cords in holothurians (Echinodermata). Zoomorphology 2006, 125, 27–38. [Google Scholar] [CrossRef]
- De Bremaeker, N., Deheyn, D., Thorndyke, M.C., Baguet, F., and Mallefet, J. Localization of S1- and S2-like immunoreactivity in the nervous system of the brittle star Amphipholis squamata (Delle Chiaje 1828). Proceedings of the Royal Society of London B Biological Sciences 1997, 264, 667–674. [CrossRef] [PubMed]
- Cobb, J.L. and Stubbs, T.R. The giant neurone system in Ophiuroids. I. The general morphology of the radial nerve cords and circumoral nerve ring. Cell & Tissue Research 1981, 219, 197–207. [Google Scholar]
- Devlin, C.L. The pharmacology of gamma-aminobutyric acid and acetylcholine receptors at the echinoderm neuromuscular junction. Journal of Experimental Biology 2001, 204, 887–896. [Google Scholar] [CrossRef]
- Florey, E. and Cahill, M.A. Cholinergic motor control of sea urchin tube feet: evidence for chemical transmission without synapses. Journal of Experimental Biology 1980, 88, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Heinzeller, t. and Welsch, U. (1994). crinoidea. in f. Harrison and f.-S. chia, eds. Microscopic Anatomy of Invertebrates. vol. 14. Echinodermata. pp. 1–148. Wiley-liss, new york.
- Ryberg, E. The localization of cholinesterases and non-specific esterases in the Echinopluteus. Zoologica Scripta 1974, 2, 163–170. [Google Scholar] [CrossRef]
- Newman, S.J. and Thorndyke, M.C. (1994). Localisation of gamma aminobutyric acid (GABA)-like immunoreactivity in the echinoderm Asterias rubens. Cell & Tissue Research.
- Wilkie, I.C., Barbaglio, A., Maclaren, W.M., and Carnevali, M.D.C. Physiological and immunocytochemical evidence that glutamatergic neurotransmission is involved in the activation of arm autotomy in the featherstar Antedon mediterranea (Echinodermata: Crinoidea). Journal of Experimental Biology 2010, 213, 2104–2115.
- Wilkie, I.C., Barbaglio, A., and Carnevali, M.D.C. The elusive role of L-glutamate as an echinoderm neurotransmitter: evidence for its involvement in the control of crinoid arm muscles. Zoology 2013, 116, 1–8. [CrossRef]
- Candia Carnevali, M., Bonasoro, F., Thorndyke, M.C., and Patruno, M. (2001). Role of the nervous system in echinoderm regeneration. In M. Barker, ed. Echinoderms 2000, pp. 5–20. Balkema, Rotterdam.
- Inoue, M., Tamori, M., and Motokawa, T. Innervation of holothurian body wall muscle: inhibitory effects and localization of 5-HT. Zoological Science 2002, 19, 1217–1222. [CrossRef]
- Sugni, M., Ferreri, F., Bonasoro, F., Candia Carnevali, M., and Wilkie, I.C. (2004). New evidence for serotonergic control of regenerative processes in crinoids. In T. Heinzeller and J. Nebelsick, eds. Echinoderms: München, pp. 141–146. Taylor & Francis Group, London.
- Cottrell, G.A. and Pentreath, V.W. Localization of catecholamines in the nervous system of a starfish, Asterias rubens, and of a brittlestar, Ophiothrix fragilis. Comparative and General Pharmacology 1970, 1, 73–81. [Google Scholar] [CrossRef]
- Díaz-Balzac, C.A., Mejías, W., Jiménez, L.B., and García-Arrarás, J.E. The catecholaminergic nerve plexus of Holothuroidea. Zoomorphology 2010, 129, 99–109.
- Howell, E., Lancaster, A., Besh, J., Richardson, B., Gomez, E., Harnew-Spradley, S., & Shelley, C. The dopamine receptor antagonist haloperidol disrupts behavioral responses of sea urchins and sea stars. Journal of Experimental Biology 2023, 226.
- Hoekstra, L.A., Moroz, L.L., and Heyland, A. Novel insights into the echinoderm nervous system from histaminergic and FMRFaminergic-like cells in the sea cucumber Leptosynapta clarki. PloS One 2012, 7, e44220.
- Kotsiuba, E.P. and Kotsiuba, A.E. NADPH-diaphorase localization in the radial nerve cords of the starfish Patiria pectinifera. Tsitologiia 2004, 46, 346–351. [Google Scholar] [PubMed]
- Martinez, A., Riveros-Moreno, V., Polak, J.M., Moncada, S., and Sesma, P. Nitric oxide (NO) synthase immunoreactivity in the starfish Marthasterias glacialis. Cell & Tissue Research 1994, 275, 599–603.
- Elphick, M.R., and Melarange, R. Nitric oxide function in an echinoderm. Biological Bulletin 1998, 194, 260–266. [CrossRef] [PubMed]
- Elphick, M.R. and Melarange, R. Neural control of muscle relaxation in echinoderms. Journal of Experimental Biology 2001, 204, 875–885. [Google Scholar] [CrossRef]
- Beer, A. J., Moss, C., & Thorndyke, M. Development of serotonin-like and SALMFamide-like immunoreactivity in the nervous system of the sea urchin Psammechinus miliaris. Biological Bulletin 2001, 200, 268–280.
- Elphick, M. R., Achhala, S., & Martynyuk, N. The evolution and diversity of SALMFamide neuropeptides. PLoS One 2013, 8, e59076.
- Elphick, M. R., Kemenes, G., Staras, K., & O’Shea, M. Behavioral role for nitric oxide in chemosensory activation of feeding in a mollusc. Journal of Neuroscience 1995, 15, 7653–7664.
- Kaplan , H.M. 1969 . Anesthesia in invertebrates . Fed. Proc. , 28 : 1557 – 1569.
- Wilkie, I. C. Nervously mediated change in the mechanical properties of a brittlestar ligament. Marine Behaviour and Physiology 1978, 5, 289–306. [Google Scholar] [CrossRef]
- O’Neill, P. L. Structure and mechanics of starfish body wall. J. Exp. Biol. 1989, 147, 53–89. [Google Scholar] [CrossRef]
- O’Neill, P. L. Torsion in the asteroid ray. J. Morph. 1990, 203, 141–149. [Google Scholar] [CrossRef]
- O’Neill, P. L. The effect of anaesthesia on spontaneous contraction of the body wall musculature in the asteroid Coscinasterias calamaria. Marine Behaviour and Physiology 1994, 24, 137–150. [Google Scholar] [CrossRef]
- Motokawa, T., & Wainwright, S. A. Stiffness of starfish arm and involvement of catch connective tissue in the stiffness change. Comp. Biochem. Physiol. 1991, 100A, 393–397.
- Wilkie, I. C. Nervously mediated change in the mechanical properties of the cirral ligaments of a crinoid. Marine Behaviour and Physiology 1983, 9, 229–248. [Google Scholar] [CrossRef]
- Wilkie, I. C. Variable tensility of the oral arm plate ligaments of the brittlestar Ophiura ophiura (Echinodermata: Ophiuroidea). Journal of Zoology 1992, 228, 5–26. [Google Scholar] [CrossRef]
- Byrne, M. The mechanical properties of the autotomy tissues of the holothurian Eupentacta quinquesemita and the effects of certain physico-chemical agents. J. exp. Biol. 1985, 117, 69–86. [Google Scholar] [CrossRef]
- Byrne, M. Induction of evisceration in the holothurian Eupentacta quinquesemita and the evidence for the existence of an endogenous evisceration factor. J. exp. Biol. 1986, 120, 25–40. [Google Scholar] [CrossRef]
- Wilkie, I. C., Emson, R. H., & Mladenov, P. V. Morphological and mechanical aspects of fission in Ophiocomella ophiactoides (Echinodermata, Ophiuroidea). Zoomorphology 1984, 104, 310–332.
- Wilkie, I. C., Griffiths, G. V. R., & Glennie, S. F. (1990). Morphological and physiological aspects of the autotomy plane in the aboral integument of Asterias rubens L. (Echinodermata). In: Echinoderm Research. C. De Ridder, P. Dubois, M-C. Lahaye and M. Jangoux (Eds.), A.A. Balkema, Rotterdam, pp. 301–313.
- MacDonald, A. G., & Wann, K. T. (1978). Physiological Aspects of Anaesthetics and Inert Gases. Academic Press.
- Jolly, D. W., Mawdsley-Thomas, L. E., & Bucke, D. Anaesthesia of fish. Vet. Record 1972, 91, 424–426.
- Fraser, D. (2008a). Understanding animal welfare. Acta Veterinaria Scandinavica, 50(1), 1–7. [CrossRef]
- Fraser, D. (2008b). Understanding Animal Welfare: The Science in its Cultural Context. Wiley-Blackwell Publishing.
- Fraser, D., Weary, D. M., Pajor, E. A., & Milligan, B. N. A scientific conception of animal welfare that reflects ethical concerns. Animal Welfare 1997, 6, 187–205.
- Lassen, J., Sandøe, P., & Forkman, B. Happy pigs are dirty!–conflicting perspectives on animal welfare. Livestock Science 2006, 103, 221–230. [CrossRef]
- Mason, G. J., & Mendl, M. (1993). Why is there no simple way of measuring animal welfare? Animal Welfare, 2, 301–319.
- Mellor, D., Patterson-Kane, E., & Stafford, K. J. (2009). The Sciences of Animal Welfare. John Wiley & Sons.
- Sandøe, P., & Simonsen, H. B. (1992). Assessing animal welfare: where does science end and philosophy begin? Animal Welfare, 1(4), 257–267.
- Hemsworth, P. H., Mellor, D. J., Cronin, G. M., & Tilbrook, A. J. Scientific assessment of animal welfare. New Zealand Veterinary Journal 2015, 63, 24–30. [CrossRef]
- Bovenkerk, B., & Verweij, M. (2016). Between individualistic animal ethics and holistic environmental ethics blurring the boundaries. In B. Bovenkerk & J. Keulartz (Eds.), Animal Ethics in the Age of Humans (pp. 369–385). Springer. [CrossRef]
- Botreau, R., Veissier, I., Butterworth, A., Bracke, M. B., & Keeling, L. J. Definition of criteria for overall assessment of animal welfare. Animal Welfare 2007, 16, 225–228.
- Mellor, D. (2015a). Enhancing Animal Welfare by Creating Opportunities for Positive Affective Engagement. New Zealand Veterinary Journal, 63, 3–8.
- Mellor, D. (2015b). Positive Animal Welfare States and Encouraging Environment-Focused and Animal-to-Animal Interactive Behaviours. New Zealand Veterinary Journal, 63, 9–16.
- Lahvis, G. Animal Welfare: Make Animal Models More Meaningful. Nature 2017, 543, 623. [Google Scholar] [CrossRef] [PubMed]
- Percy, J. A. (1973). Thermal Adaptation in the Boreo-Arctic Echinoid Strongylocentrotus droebachiensis (O. F. Müller, 1776). II. Seasonal Acclimatization and Urchin Activity. Physiology and Zoology, 46, 129–138.
- Himmelman, J. H., Guderley, H., Vignault, G., Drouin, G., & Wells, P. G. Response of the sea urchin, Strongylocentrotus droebachiensis, to reduced salinities: importance of size, acclimation, and interpopulation differences. Canadian journal of zoology 1984, 62, 1015–1021.

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




