Submitted:
07 November 2023
Posted:
08 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Cell culture
2.2. Treatments
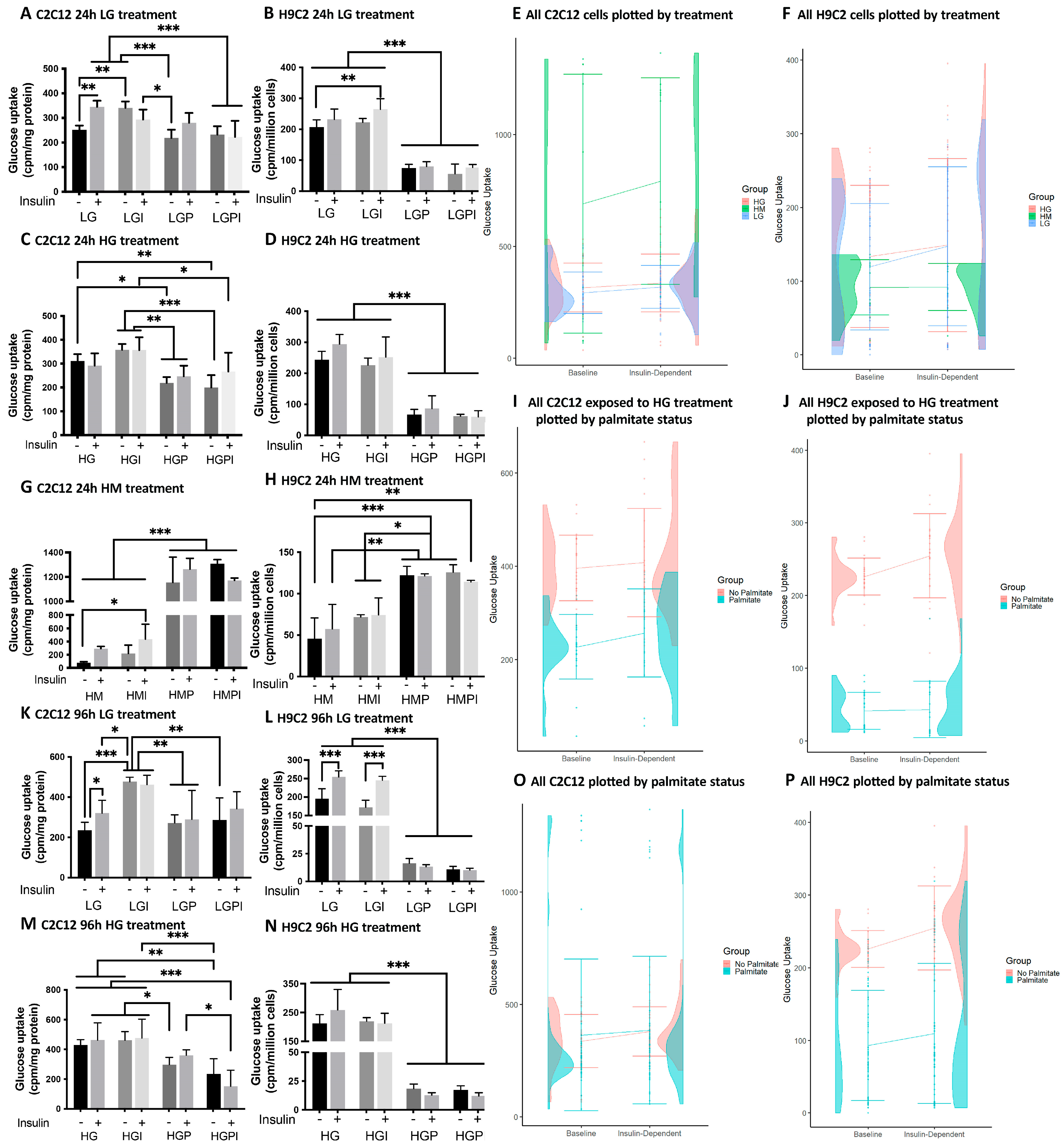
2.3. 3H-2-deoxy-glucose-uptake
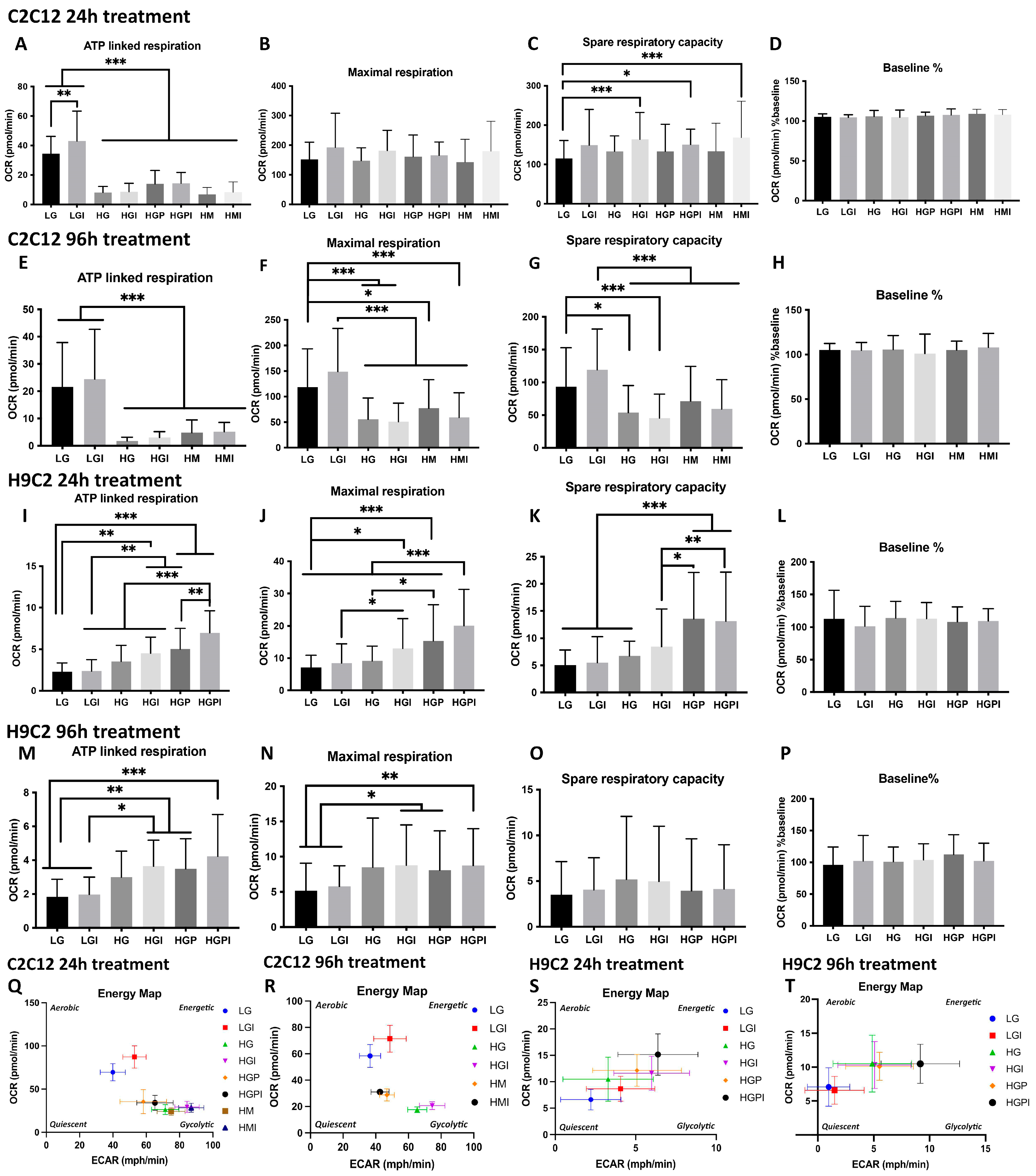
2.4. Mitochondrial function measurements
2.5. Electron microscopy
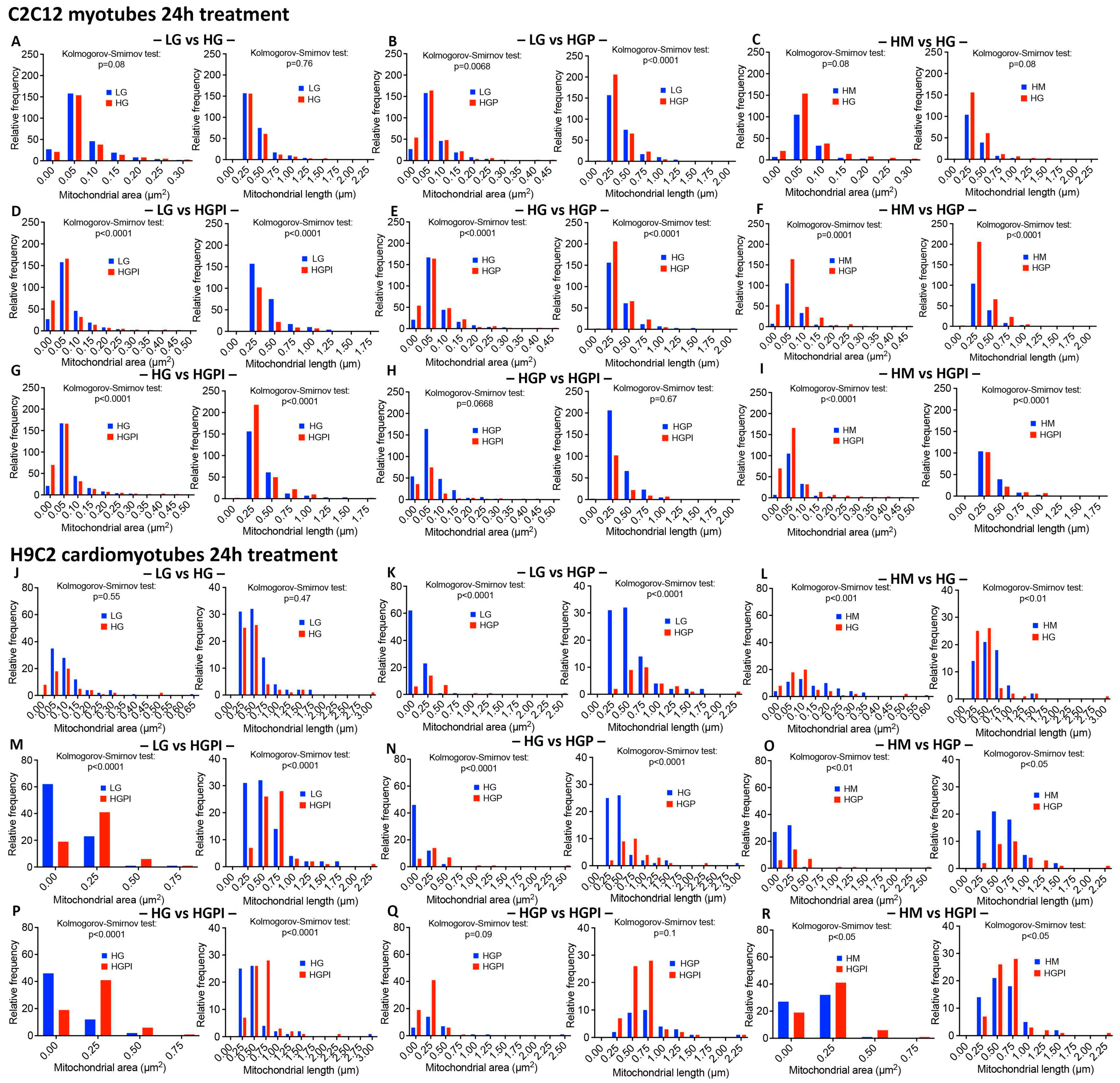
2.6. Morphometric analyses of mitochondria
2.7. Statistical analysis
3. Results
3.1. 3H-2-deoxy-glucose-uptake
3.2. Mitochondrial Function measurements using the Agilent Seahorse XFe 96 Extracellular Flux Analyzer
3.2.1. C2C12 Myotubes
3.2.2. H9C2 Myotubes
3.3. Morphometric analyses of mitochondrial number, density, relative frequency of mitochondria with differing area and length
3.3.1. Electron Microscopy for Mitochondrial appearance.
3.3.2. Mitochondrial number and density
3.3.3. Palmitate but not HG changed relative frequency of mitochondria with respect to area and length
3.3.4. Insulin failed to alter palmitate-induced changes in mitochondrial health
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bommer C, Sagalova V, Heesemann E, et al. (2018) Global Economic Burden of Diabetes in Adults: Projections From 2015 to 2030. Diabetes care 41(5): 963-970. [CrossRef]
- International Diabetes Federation (2019) IDF Diabetes Atlas, 9th edn. Available at: https://www.diabetesatlas.org, Brussels, Belgium.
- Harreiter J, Roden M (2019) [Diabetes mellitus-Definition, classification, diagnosis, screening and prevention (Update 2019)]. Wiener klinische Wochenschrift 131(Suppl 1): 6-15. [CrossRef]
- Centers for Disease Control and Prevention (2020) National Diabetes Statistics Report, 2020. In, Atlanta, GA: , U.S. Dept of Health and Human Services.
- 2016; World Health Organization (2016) Global Report on Diabetes, Geneva, Switzerland.
- Mozaffarian D, Benjamin EJ, Go AS, et al. (2015) Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation 131(4): e29-322. [CrossRef]
- Lilao-Garzón J, Valverde-Tercedor C, Muñoz-Descalzo S, Brito-Casillas Y, Wägner AM (2021) In Vivo and In Vitro Models of Diabetes: A Focus on Pregnancy. In: Islam MS (ed) Diabetes: from Research to Clinical Practice: Volume 4. Springer International Publishing, Cham, pp 553-576.
- Reed MJ, Scribner KA (1999) In-vivo and in-vitro models of type 2 diabetes in pharmaceutical drug discovery. Diabetes, Obesity and Metabolism 1(2): 75-86. [CrossRef]
- Smith AG, Muscat GE (2005) Skeletal muscle and nuclear hormone receptors: implications for cardiovascular and metabolic disease. The international journal of biochemistry & cell biology 37(10): 2047-2063. [CrossRef]
- Tumova J, Andel M, Trnka J (2016) Excess of free fatty acids as a cause of metabolic dysfunction in skeletal muscle. Physiological research 65(2): 193-207. [CrossRef]
- Wong CY, Al-Salami H, Dass CR (2020) C2C12 cell model: its role in understanding of insulin resistance at the molecular level and pharmaceutical development at the preclinical stage. Journal of Pharmacy and Pharmacology 72(12): 1667-1693. [CrossRef]
- Ashrafian H, Frenneaux MP, Opie LH (2007) Metabolic Mechanisms in Heart Failure. Circulation 116(4): 434-448. [CrossRef]
- Stanley WC, Recchia FA, Lopaschuk GD (2005) Myocardial Substrate Metabolism in the Normal and Failing Heart. Physiological Reviews 85(3): 1093-1129. [CrossRef]
- Carpentier AC (2018) Abnormal Myocardial Dietary Fatty Acid Metabolism and Diabetic Cardiomyopathy. The Canadian journal of cardiology 34(5): 605-614. [CrossRef]
- Westermeier F, Navarro-Marquez M, Lopez-Crisosto C, et al. (2015) Defective insulin signaling and mitochondrial dynamics in diabetic cardiomyopathy. Biochimica et biophysica acta 1853(5): 1113-1118. [CrossRef]
- Amaral N, Okonko DO (2015) Metabolic abnormalities of the heart in type II diabetes. Diabetes & vascular disease research 12(4): 239-248. [CrossRef]
- Kimes BW, Brandt BL (1976) Properties of a clonal muscle cell line from rat heart. Experimental cell research 98(2): 367-381. [CrossRef]
- Watkins SJ, Borthwick GM, Arthur HM (2011) The H9C2 cell line and primary neonatal cardiomyocyte cells show similar hypertrophic responses in vitro. In Vitro Cell Dev Biol Anim 47(2): 125-131. [CrossRef]
- Zordoky BN, El-Kadi AO (2007) H9c2 cell line is a valuable in vitro model to study the drug metabolizing enzymes in the heart. Journal of pharmacological and toxicological methods 56(3): 317-322. [CrossRef]
- Chavez JA, Summers SA (2003) Characterizing the effects of saturated fatty acids on insulin signaling and ceramide and diacylglycerol accumulation in 3T3-L1 adipocytes and C2C12 myotubes. Archives of Biochemistry and Biophysics 419(2): 101-109. [CrossRef]
- Krako Jakovljevic N, Pavlovic K, Zujovic T, et al. (2021) In vitro models of insulin resistance: Mitochondrial coupling is differently affected in liver and muscle cells. Mitochondrion 61: 165-173. [CrossRef]
- Regazzetti C, Peraldi P, Grémeaux T, et al. (2009) Hypoxia Decreases Insulin Signaling Pathways in Adipocytes. Diabetes 58(1): 95-103. [CrossRef]
- Sakoda H, Ogihara T, Anai M, et al. (2000) Dexamethasone-induced insulin resistance in 3T3-L1 adipocytes is due to inhibition of glucose transport rather than insulin signal transduction. Diabetes 49(10): 1700-1708. [CrossRef]
- Nelson BA, Robinson KA, Buse MG (2000) High glucose and glucosamine induce insulin resistance via different mechanisms in 3T3-L1 adipocytes. Diabetes 49(6): 981-991.
- Dohl J, Foldi J, Heller J, Gasier HG, Deuster PA, Yu T (2018) Acclimation of C2C12 myoblasts to physiological glucose concentrations for in vitro diabetes research. Life Sciences 211: 238-244. [CrossRef]
- Yang M, Wei D, Mo C, et al. (2013) Saturated fatty acid palmitate-induced insulin resistance is accompanied with myotube loss and the impaired expression of health benefit myokine genes in C2C12 myotubes. Lipids in health and disease 12: 104. [CrossRef]
- Chanon S, Durand C, Vieille-Marchiset A, et al. (2017) Glucose Uptake Measurement and Response to Insulin Stimulation in In Vitro Cultured Human Primary Myotubes. J Vis Exp(124): 55743. [CrossRef]
- Feng CC, Pandey S, Lin CY, et al. (2018) Cardiac apoptosis induced under high glucose condition involves activation of IGF2R signaling in H9c2 cardiomyoblasts and streptozotocin-induced diabetic rat hearts. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie 97: 880-885. [CrossRef]
- Ding W, Chang WG, Guo XC, et al. (2019) Exenatide Protects Against Cardiac Dysfunction by Attenuating Oxidative Stress in the Diabetic Mouse Heart. Frontiers in endocrinology 10: 202. [CrossRef]
- Ha H, Pak Y (2005) Modulation of the caveolin-3 and Akt status in caveolae by insulin resistance in H9c2 cardiomyoblasts. Experimental & molecular medicine 37(3): 169-178. [CrossRef]
- Chang W, Chen L, Hatch GM (2016) Berberine treatment attenuates the palmitate-mediated inhibition of glucose uptake and consumption through increased 1,2,3-triacyl-sn-glycerol synthesis and accumulation in H9c2 cardiomyocytes. Biochimica et Biophysica Acta (BBA)—Molecular and Cell Biology of Lipids 1861(4): 352-362. [CrossRef]
- Eckel RH, Grundy SM, Zimmet PZ (2005) The metabolic syndrome. Lancet (London, England) 365(9468): 1415-1428. [CrossRef]
- Delarue J, Magnan C (2007) Free fatty acids and insulin resistance. Current opinion in clinical nutrition and metabolic care 10(2): 142-148. [CrossRef]
- Katsarou A, Gudbjörnsdottir S, Rawshani A, et al. (2017) Type 1 diabetes mellitus. Nature Reviews Disease Primers 3(1): 17016. [CrossRef]
- American Diabetes Association (2010) Diagnosis and classification of diabetes mellitus. Diabetes care 33 Suppl 1(Suppl 1): S62-S69. [CrossRef]
- World Health Organization Mean fasting blood glucose. Available from https://www.who.int/data/gho/indicator-metadata-registry/imr-details/2380. Accessed 16.02. 2021.
- Zhou B, Lu Y, Hajifathalian K, et al. (2016) Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4·4 million participants. The Lancet 387(10027): 1513-1530. [CrossRef]
- Hsu HC, Chen CY, Lee BC, Chen MF (2016) High-fat diet induces cardiomyocyte apoptosis via the inhibition of autophagy. European journal of nutrition 55(7): 2245-2254. [CrossRef]
- Zou L, Li X, Wu N, Jia P, Liu C, Jia D (2017) Palmitate induces myocardial lipotoxic injury via the endoplasmic reticulum stress-mediated apoptosis pathway. Molecular medicine reports 16(5): 6934-6939. [CrossRef]
- Abdelmagid SA, Clarke SE, Nielsen DE, et al. (2015) Comprehensive profiling of plasma fatty acid concentrations in young healthy Canadian adults. PLoS One 10(2): e0116195. [CrossRef]
- Nawrocki A, Górski J (2004) Effect of plasma free fatty acid concentration on the content and composition of the free fatty acid fraction in rat skeletal muscles. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme 36(9): 601-606. [CrossRef]
- Mangnall D, Bruce C, Fraser RB (1993) Insulin-stimulated glucose uptake in C2C12 myoblasts. Biochemical Society transactions 21(4): 438s. [CrossRef]
- Conejo R, Lorenzo M (2001) Insulin signaling leading to proliferation, survival, and membrane ruffling in C2C12 myoblasts. Journal of Cellular Physiology 187(1): 96-108. [CrossRef]
- Patten V, Chabaesele I, Sishi B, Vuuren D (2017) Cardiomyocyte differentiation: Experience and observations from 2 laboratories. Journal of the South African Heart Association (SA Heart) 14: 96-107. [CrossRef]
- Lopaschuk GD, Jaswal JS (2010) Energy metabolic phenotype of the cardiomyocyte during development, differentiation, and postnatal maturation. Journal of cardiovascular pharmacology 56(2): 130-140. [CrossRef]
- Nobuhara M, Saotome M, Watanabe T, et al. (2013) Mitochondrial dysfunction caused by saturated fatty acid loading induces myocardial insulin-resistance in differentiated H9c2 myocytes: a novel ex vivo myocardial insulin-resistance model. Experimental cell research 319(7): 955-966. [CrossRef]
- Kelley DE, He J, Menshikova EV, Ritov VB (2002) Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes 51(10): 2944-2950.
- Kim JY, Hickner RC, Cortright RL, Dohm GL, Houmard JA (2000) Lipid oxidation is reduced in obese human skeletal muscle. American journal of physiology Endocrinology and metabolism 279(5): E1039-1044. [CrossRef]
- Li P, Oh DY, Bandyopadhyay G, et al. (2015) LTB4 promotes insulin resistance in obese mice by acting on macrophages, hepatocytes and myocytes. Nat Med 21(3): 239-247. [CrossRef]
- Serrage HJ, Joanisse S, Cooper PR, et al. (2019) Differential responses of myoblasts and myotubes to photobiomodulation are associated with mitochondrial number. J Biophotonics 12(6): e201800411. [CrossRef]
- Divakaruni AS, Paradyse A, Ferrick DA, Murphy AN, Jastroch M (2014) Chapter Sixteen—Analysis and Interpretation of Microplate-Based Oxygen Consumption and pH Data. In: Murphy AN, Chan DC (eds) Methods in Enzymology. Vol 547. Academic Press, pp 309-354.
- Patel HH, Zhang S, Murray F, et al. (2007) Increased smooth muscle cell expression of caveolin-1 and caveolae contribute to the pathophysiology of idiopathic pulmonary arterial hypertension. FASEB J 21(11): 2970-2979. [CrossRef]
- Pasqua T, Mahata S, Bandyopadhyay GK, et al. (2016) Impact of Chromogranin A deficiency on catecholamine storage, catecholamine granule morphology and chromaffin cell energy metabolism in vivo. Cell and Tissue Research 363(3): 693-712. [CrossRef]
- Zhang Q, Kong X, Yuan H, Guan H, Li Y, Niu Y (2019) Mangiferin Improved Palmitate-Induced-Insulin Resistance by Promoting Free Fatty Acid Metabolism in HepG2 and C2C12 Cells via PPARα: Mangiferin Improved Insulin Resistance. J Diabetes Res 2019: 2052675. [CrossRef]
- Fealy CE, Mulya A, Axelrod CL, Kirwan JP (2018) Mitochondrial dynamics in skeletal muscle insulin resistance and type 2 diabetes. Transl Res 202: 69-82. [CrossRef]
- McMillin SL, Schmidt DL, Kahn BB, Witczak CA (2017) GLUT4 Is Not Necessary for Overload-Induced Glucose Uptake or Hypertrophic Growth in Mouse Skeletal Muscle. Diabetes 66(6): 1491-1500. [CrossRef]
- Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI (2004) Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. The New England journal of medicine 350(7): 664-671. [CrossRef]
- Morino K, Petersen KF, Dufour S, et al. (2005) Reduced mitochondrial density and increased IRS-1 serine phosphorylation in muscle of insulin-resistant offspring of type 2 diabetic parents. J Clin Invest 115(12): 3587-3593. [CrossRef]
- Befroy DE, Petersen KF, Dufour S, et al. (2007) Impaired Mitochondrial Substrate Oxidation in Muscle of Insulin-Resistant Offspring of Type 2 Diabetic Patients. Diabetes 56(5): 1376-1381. [CrossRef]
- Mailloux RJ, Harper M-E (2010) Glucose regulates enzymatic sources of mitochondrial NADPH in skeletal muscle cells; a novel role for glucose-6-phosphate dehydrogenase. The FASEB Journal 24(7): 2495-2506. [CrossRef]
- Elkalaf M, Anděl M, Trnka J (2013) Low glucose but not galactose enhances oxidative mitochondrial metabolism in C2C12 myoblasts and myotubes. PLoS One 8(8): e70772-e70772. [CrossRef]
- Wang X, McLennan SV, Allen TJ, Tsoutsman T, Semsarian C, Twigg SM (2009) Adverse effects of high glucose and free fatty acid on cardiomyocytes are mediated by connective tissue growth factor. American Journal of Physiology-Cell Physiology 297(6): C1490-C1500. [CrossRef]
- Hickson-Bick DL, Buja LM, McMillin JB (2000) Palmitate-mediated alterations in the fatty acid metabolism of rat neonatal cardiac myocytes. J Mol Cell Cardiol 32(3): 511-519. [CrossRef]
- Nishi H, Higashihara T, Inagi R (2019) Lipotoxicity in Kidney, Heart, and Skeletal Muscle Dysfunction. Nutrients 11(7). [CrossRef]
- Lisa CH, Kieran C (2011) Metabolism, hypoxia and the diabetic heart. Journal of Molecular and Cellular Cardiology 50(4): 598-605. [CrossRef]
- Barsotti A, Giannoni A, Di Napoli P, Emdin M (2009) Energy metabolism in the normal and in the diabetic heart. Current pharmaceutical design 15(8): 836-840. [CrossRef]
- An D, Rodrigues B (2006) Role of changes in cardiac metabolism in development of diabetic cardiomyopathy. American Journal of Physiology-Heart and Circulatory Physiology 291(4): H1489-H1506. [CrossRef]
- Sharma S, Adrogue JV, Golfman L, et al. (2004) Intramyocardial lipid accumulation in the failing human heart resembles the lipotoxic rat heart. The FASEB Journal 18(14): 1692-1700. [CrossRef]
- Zorzano A, Liesa M, Palacín M (2009) Role of mitochondrial dynamics proteins in the pathophysiology of obesity and type 2 diabetes. The international journal of biochemistry & cell biology 41(10): 1846-1854. [CrossRef]
- Chen W, Xiang H, Chen R, et al. (2019) S1PR2 antagonist ameliorate high glucose-induced fission and dysfunction of mitochondria in HRGECs via regulating ROCK1. BMC Nephrology 20(1): 135. [CrossRef]
- Davis MA, Jeffery EH (2002) 4—Organelle Biochemistry and Regulation of Cell Death. In: Haschek WM, Rousseaux CG, Wallig MA (eds) Handbook of Toxicologic Pathology (Second Edition). Academic Press, San Diego, pp 67-81.
- Quehenberger O, Armando AM, Brown AH, et al. (2010) Lipidomics reveals a remarkable diversity of lipids in human plasma1[S]. Journal of Lipid Research 51(11): 3299-3305. [CrossRef]

| Cell type and differentiation | Preincubation | Type of treatment, concentration, duration | Read outs | Reference |
|
3T3-L1 adipocytes |
DMEM 5 mM glucose | Palmitate 0.75 mM 17h Hypoxia 16h Dexamethasone 1 µmol/l 24h High glucose 25 mM 18h |
Inhibition of phosphorylation of insulin receptor and protein kinase B; decrease in insulin dependent glucose uptake Impaired GLUT4 membrane intercalation |
[20] [22] [23] [24] |
|
C2C12 myoblasts |
DMEM 25 mM glucose | Insulin 60 nM 24h Palmitate 0.4 mM 24h |
Inhibition of insulin stimulated activation of Akt/protein kinase B; Swollen mitochondria | [21] |
| DMEM 5 mM glucose | Glucose 15 mM 24h Palmitate 0.25 mM 24h |
Increased apoptosis, increased ROS production | [25] | |
|
C2C12 myotubes |
DMEM 5 mM glucose DMEM not specified |
Palmitate 0.75 mM 17h Palmitate 0.6 mM 24h |
Inhibition of insulin stimulated glycogen synthesis and activation of protein kinase B, diacylglyceride accumulation Reduced Akt phosphorylation, glucose uptake and GLUT4 expression |
[20] [26] |
| Huh7 differentiated hepatocellular carcinoma | DMEM 25 mM glucose | Insulin 60 nM 24h Palmitate 0.4 mM 24h |
Inhibition of insulin stimulated activation of Akt/protein kinase B | [21] |
| Primary human myotubes | DMEM not specified | Palmitate 0.5 mM 48h | Decrease in insulin stimulated glucose uptake | [27] |
|
H9C2 myoblasts |
DMEM 25 mM glucose DMEM 5 mM glucose |
Glucose 33 mM 36h Glucose 40 mM 24h Glucose 25 mM + insulin 100 nM 24h |
Enhanced apoptosis, activation of cardiac hypertrophy proteins Increased ROS production + apoptosis Decrease in insulin stimulated glucose uptake, Inhibition of insulin stimulated activation of Akt |
[28] [29] [30] |
|
H9C2 myotubes |
DMEM not specified | Palmitate 100 µM 24h | Decrease in insulin stimulated glucose uptake | [31] |
| Treatment duration | Conc. For C2C12 | Conc. For H9C2 |
| 24h | 35 µM | 5 µM |
| 48h | 70 µM | 25 µM |
| 72h | 105 µM | 50 µM |
| 96h | 150 µM | 75 µM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





