Submitted:
04 October 2023
Posted:
06 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
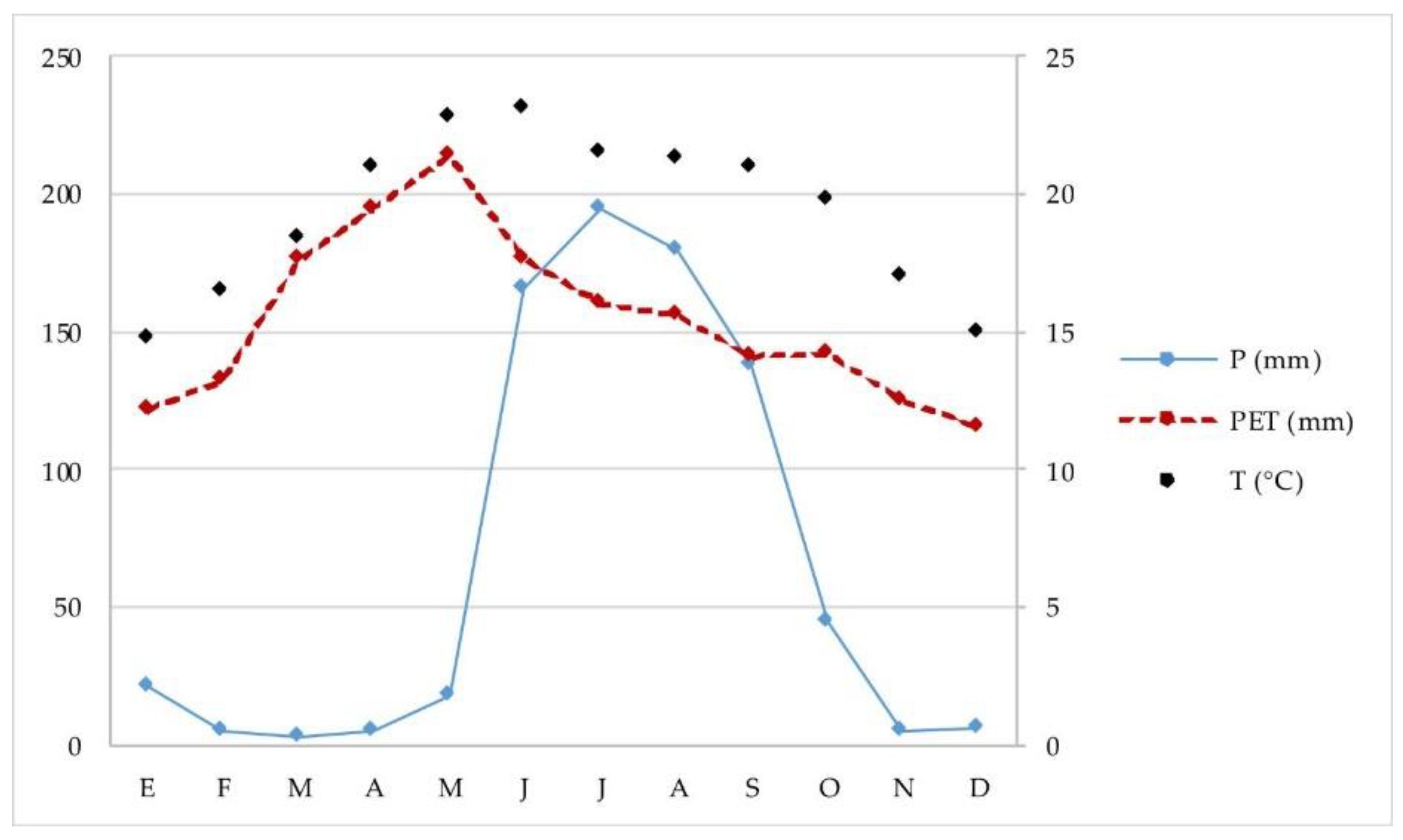
2.1. Research Area
2.2. Methodology
2.2.1. Characterization and Classification of Experimental Soils
2.2.2. Biological Material
2.2.3. Fertilization
2.2.4. Experimental Design
2.2.5. Experiment Setup
2.2.6. Variables Evaluated
- The AS was determined in the soils before applying the treatments and at the end of the experiment (potting soil); the AS was determined by wet sieving and air-drying method (2 to 0.5 mm) [41].
- Ps was determined by the Bray and Kurtz method 1; Pp by colorimetry with nitro-vanadomolybdate; and Ns in soil and plant were obtained by the Kjeldahl method [38], both at the end of the experiment.
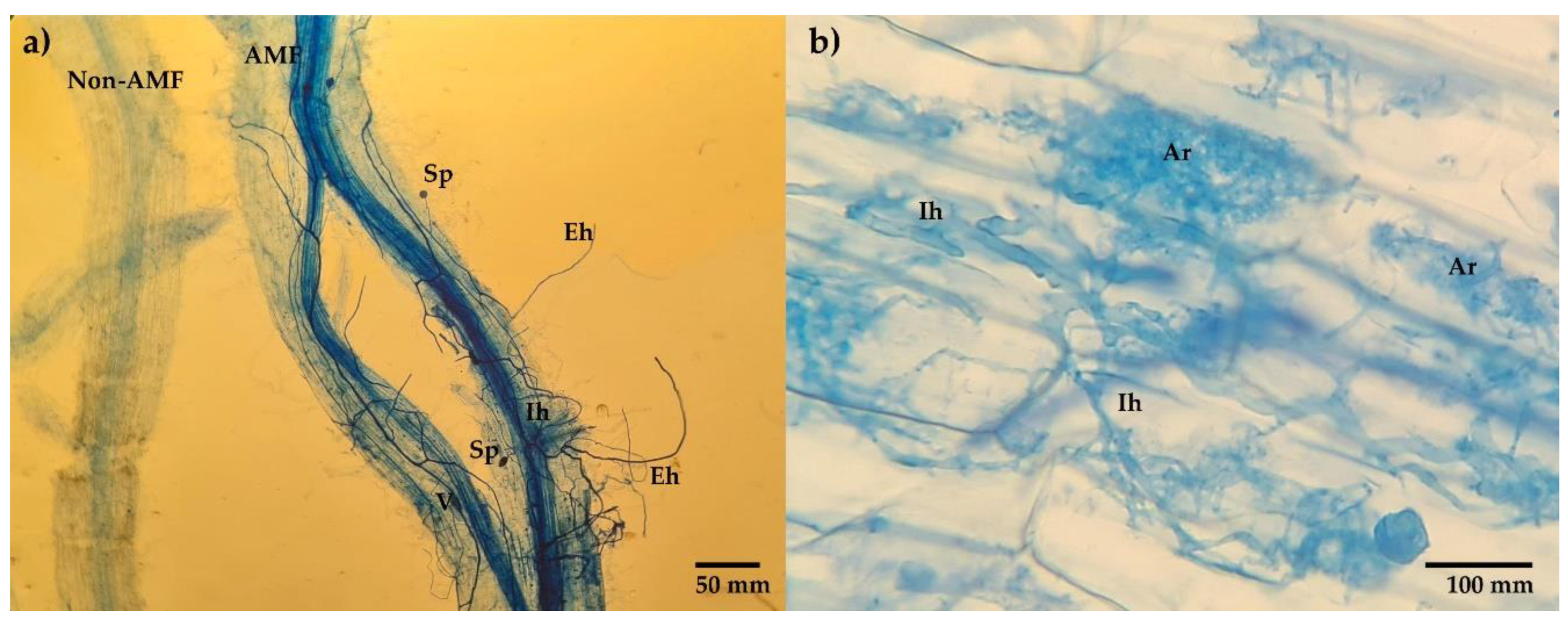
- Co by AMF was obtained by differential staining technique with trypan blue [48] at the end of the experiment.
- Sp was obtained in 100 g of soil by direct counting of AMF spores extracted from the study soils, using the wet sieving method [42] at the beginning and the end of the experiment.
- The HP was measured with a flexometer, the measurement was taken from the soil surface to the highest part of the plant; while, the plant diameter was measured with a vernier at 20 cm from the soil surface, before harvesting.
2.2.7. Statistical Analysis
3. Results
3.1. Soils under Study
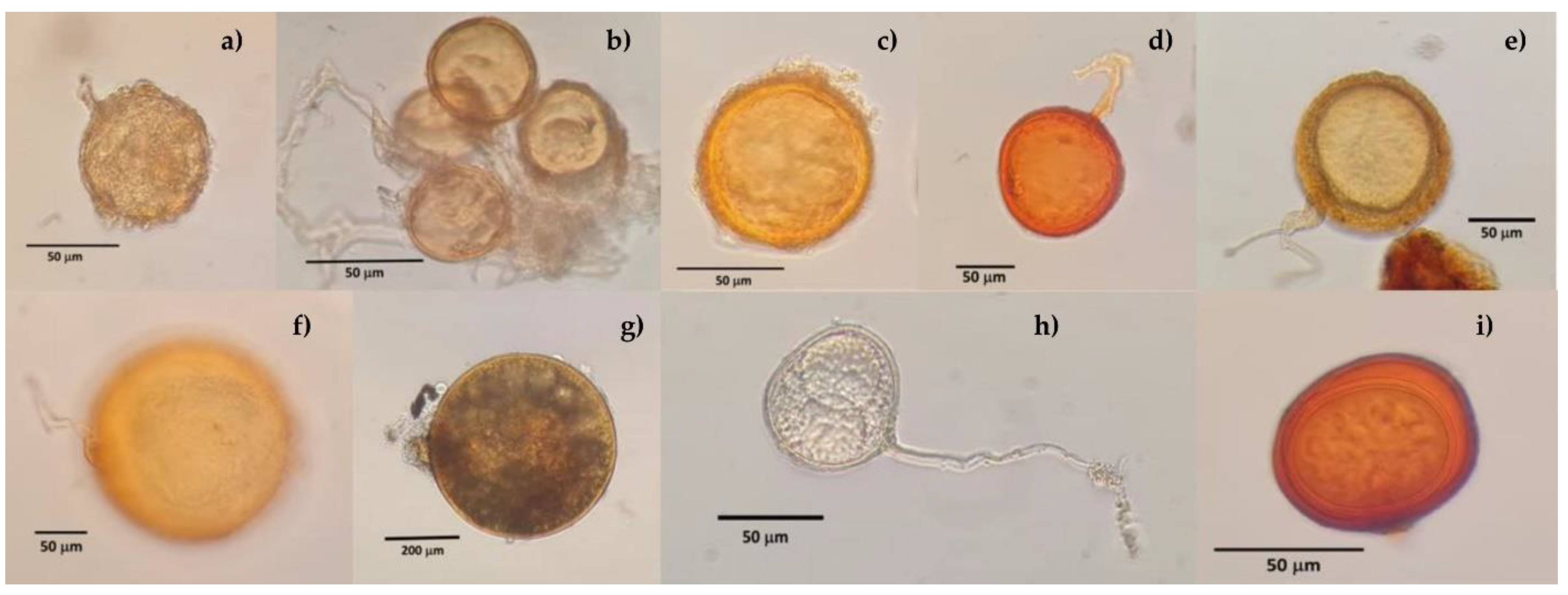
3.2. Mycorrhizal Fungi in Soils
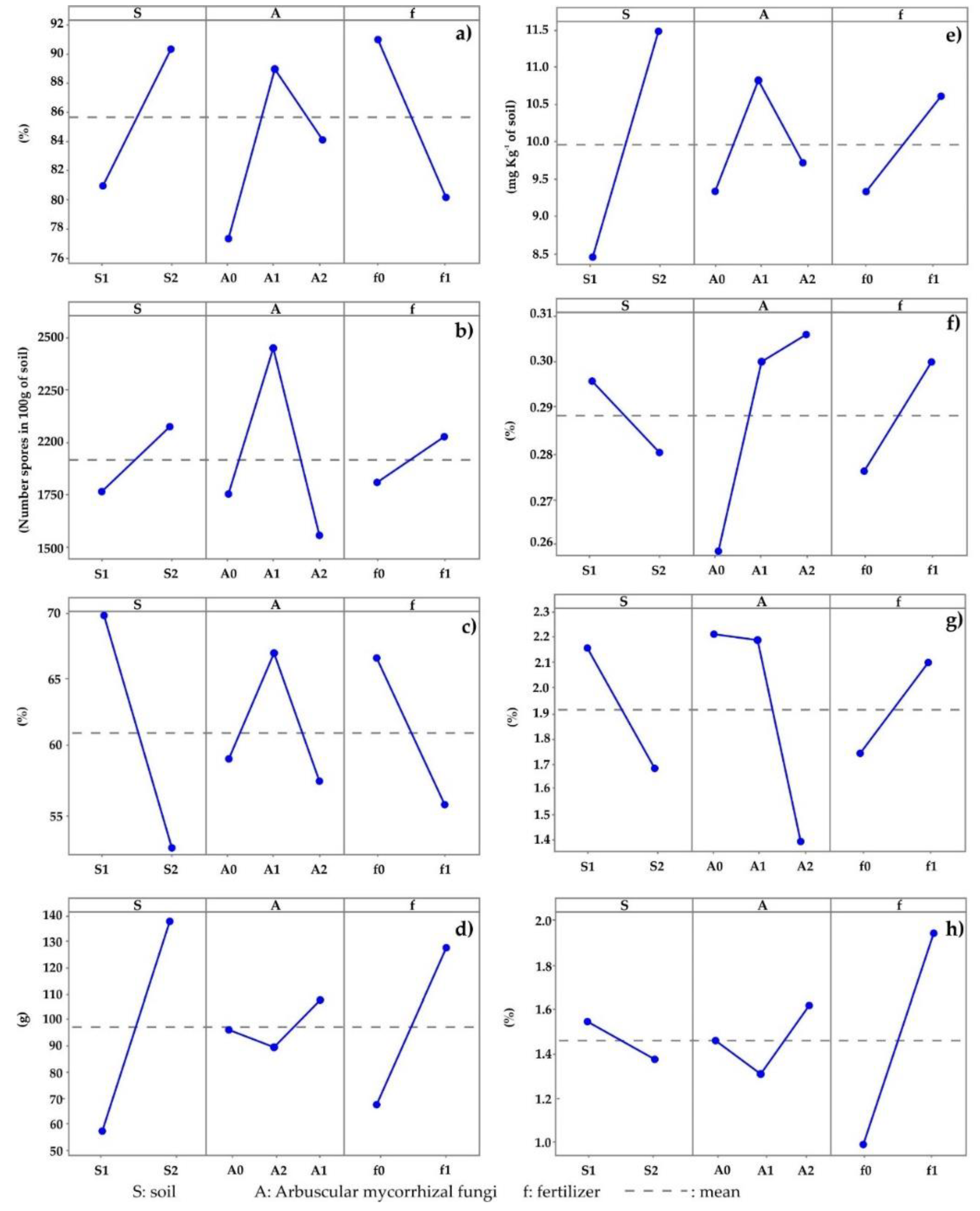
3.3. Aggregates Stability
| Factor/ | Soil | AMF | Fertilizer | Interactions | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | F2 | P | F | P | F | P | S*A | S*f | f*A | S*A*f |
| Sp 1 | 674.00 | 0.000 | 2111.49 | 0.000 | 344.62 | 0.000 | * | |||
| Co | 23.38 | 0.000 | 18.15 | 0.000 | 30.48 | 0.000 | * | |||
| AS | 573.96 | 0.000 | 68.95 | 0.000 | 243.23 | 0.000 | * | |||
| Ps | 190442.69 | 0.000 | 15917.47 | 0.000 | 33829.45 | 0.000 | * | |||
| Ns | 4.10 | 0.048 | 15.19 | 0.000 | 9.13 | 0.004 | * | |||
| Pp | 6.74 | 0.012 | 9.76 | 0.000 | 3.90 | 0.054 | * | |||
| Np | 50.17 | 0.000 | 59.44 | 0.000 | 1719.79 | 0.000 | * | * | ||
| Yd | 519.48 | 0.000 | 9.04 | 0.000 | 290.80 | 0.000 | * | |||
| Variable | Co | Sp | AS | AP | DT | Ps | Ns | Pp | Np | |
|---|---|---|---|---|---|---|---|---|---|---|
| Sp |
r p |
0.237 0.069 |
||||||||
| AS |
r p |
0.015 0.075 |
0.537 0.050 |
|||||||
| AP |
r p |
- 0.215 0.099 |
0.061 0.644 |
- 0.482 0.000 |
||||||
| DT |
r p |
- 0.270 0.037 |
- 0.222 0.644 |
0.654 0.000 |
- 0.242 0.062 |
|||||
| Ps |
r p |
0.253 0.050 |
0.048 0.714 |
-0.485 0.000 |
0.290 0.024 |
- 0.594 0.000 |
||||
| Ns |
r p |
0.154 0.241 |
- 0.212 0.105 |
- 0.075 0.570 |
0.138 0.293 |
0.084 0.525 |
0.339 0.009 |
|||
| Pp |
r p |
- 0.232 0.075 |
- 0.107 0.414 |
0.023 0.861 |
0.239 0.065 |
0.148 0.260 |
0.301 0.019 |
0.201 0.123 |
||
| Np |
r p |
- 0.448 0.000 |
- 0.086 0.515 |
- 0.371 0.003 |
0.657 0.000 |
- 0.008 0.954 |
0.170 0.193 |
0.148 0.258 |
0.131 0.317 |
|
| Yd |
r p |
0.101 0.444 |
0.433 0.049 |
-0.820 0.051 |
0.516 0.000 |
0.394 0.000 |
0.515 0.000 |
0.159 0.224 |
- 0.141 0.282 |
0.392 0.002 |
3.4. Yield
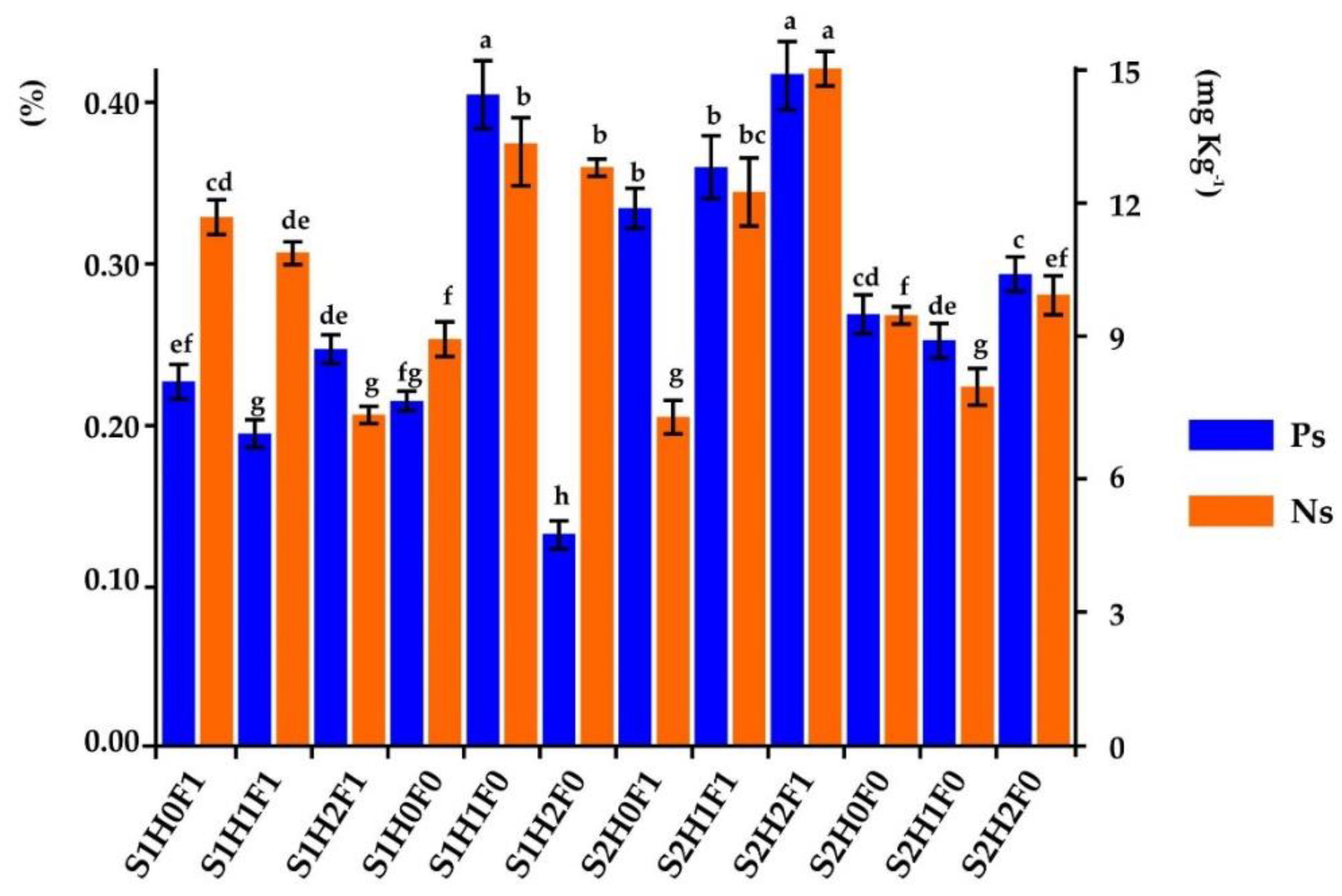
4. Discussion
4.1. Substrate Characteristics
4.2. Colonization and Sporulation
4.3. Aggregates Stability
4.4. Yield
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Zhao, R.; Guo, W.; Bi, N.; Guo, J.; Wang, L.; Zhao, J.; Zhang, J. Arbuscular mycorrhizal fungi affect the growth, nutrient uptake and water status of maize (Zea mays L.) grown in two types of coal mine spoils under drought stress. Appl. Soil Ecol. 2015, 88, 41–49. [CrossRef]
- Diagne, N.; Ngom, M.; Djighaly, P.I.; Fall, D.; Hocher, V.; Svistoonoff, S. Roles of arbuscular mycorrhizal fungi on plant growth and performance: Importance in biotic and abiotic stressed regulation. Diversity 2020, 12, 370. [CrossRef]
- Mathur, S.; Jajoo, A. Arbuscular mycorrhizal fungi protect maize plants from high temperature stress by regulating photosystem II heterogeneity. Ind. Crops Prod. 2020, 143, 111934. [CrossRef]
- Zhang, S.; Meng, L.; Hou, J.; Liu, X.; Ogundeji, A.O.; Cheng, Z.; Yin, T.; Clarke, N.; Hu, B.; Li, S. Maize/soybean intercropping improves stability of soil aggregates driven by arbuscular mycorrhizal fungi in a black soil of northeast China. Plant Soil 2022, 481(1-2), 63-82.
- Hartmann, A.; Schmid, M.; van Tuinen, D.; Berg, G. Plant-driven selection of microbes. Plant Soil 2009, 321, 235–257. [CrossRef]
- Yu, Z.; Zhao, X.; Su, L.; Yan, K.; Li, B.; He, Y.; Zhan, F. Effect of an arbuscular mycorrhizal fungus on maize growth and cadmium migration in a sand column. Ecotoxicol. Environ. Saf. 2021, 225, 112782. [CrossRef]
- Lu, X.; Lu, X.; Liao, Y. Effect of tillage treatment on the diversity of soil arbuscular mycorrhizal fungal and soil aggregate-associated carbon content. Front. Microbiol. 2018, 9, 2986. [CrossRef]
- Moebius-Clune, D.J.; Moebius-Clune, B.N.; van Es, H.M.; Pawlowska, T.E. Arbuscular mycorrhizal fungi associated with a single agronomic plant host across the landscape: community differentiation along a soil textural gradient. Soil Biol. Biochem. 2013, 64, 191–199. [CrossRef]
- Davison, J.; Moora, M.; Semchenko, M.; Adenan, S.B.; Ahmed, T.; Akhmetzhanova, A.A.; ...; Öpik, M. Temperature and pH define the realised niche space of arbuscular mycorrhizal fungi. New Phytol. 2021, 231, 2, 763-776. [CrossRef]
- Xu, J.; Liu, S.; Song, S.; Guo, H.; Tang, J.; Yong, J.W.H.; Ma, Y.; Chen, X. Arbuscular mycorrhizal fungi influence decomposition and the associated soil microbial community under different soil phosphorus availability. Soil Biol. Biochem. 2018, 120, 181–190. [CrossRef]
- Sanchez-Lizarraga, A.L.; Arenas-Montaño, V.; Marino-Marmolejo, E.N.; Dendooven, L.; Velazquez-Fernandez, J.B.; Davila-Vazquez, G.; Rodriguez-Campos, J.; Hernández-Cuevas, L.; Contreras-Ramos, S.M. Vinasse irrigation: effects on soil fertility and arbuscular mycorrhizal fungi population. J. Soils Sediments 2018, 18, 3256–3270. [CrossRef]
- Oehl, F.; Laczko, E.; Bogenrieder, A.; Stahr, K.; Bösch, R.; van der Heijden, M.; Sieverding, E. Soil type and land use intensity determine the composition of arbuscular mycorrhizal fungal communities. Soil Biol. Biochem. 2010, 42, 5, 724-738. http://doi:10.1016/j.soilbio.2010.01.006.
- Horn, S.; Hempel, S.; Verbruggen, E.; Rillig, M.C.; Caruso, T. Linking the community structure of arbuscular mycorrhizal fungi and plants: a story of interdependence. ISME Journal 2017, 11, 1400–1411. [CrossRef]
- Hoeksema, J.D.; Chaudhary, V.B.; Gehring, C.A.; Johnson, N.C.; Karst, J.:; Koide, R.T.; Pringle, A.; Zabinski, C.; Bever, J.D.; Moore, J.C. A meta-analysis of context-dependency in plant response to inoculation with mycorrhizal fungi. Ecol. Lett. 2010, 13, 394–407. http://doi,org/10.1111/j.1461-0248.2009.01430.x.
- Schappe, T.; Albornoz, F.E.; Turner, B.L.; Neat, A.; Condit, R.; Jones, F.A. The role of soil chemistry and plant neighbourhoods in structuring fungal communities in three Panamanian rainforests. J. Ecol. 2017, 105, 569–579. [CrossRef]
- Bueno, C.G.; Gerz, M.; Moora, M.; León, D.; Gomez-García, D.; García de León, D.; Fonte, X.; Al-Quraishy, S.; Hozzein, W.N., Zobel, M. Distribution of plant mycorrhizal traits along an elevational gradient does not fully mirror the latitudinal gradient. Mycorrhiza, 2021, 31, 141–159. [CrossRef]
- Koorem, K.; Tulva, I.; Davison, J.; Jairus, T.; Opik, M.; Vasar, M.; Zobel, M.; Moora, M. Arbuscular mycorrhizal fungal communities in forest plant roots are simultaneously shaped by host characteristics and canopy-mediated light availability. Plant Soil 2017, 410, 259–271. [CrossRef]
- Carrillo-Aguilar, D.G.; Hernández-Ortega, H.A.; Franco-Ramírez, A.; Vallejo-Jiménez, B.; Guzmán-González, S.; Manzo-Sánchez, G.; Sánchez-Rangel, J.C. Influencia de las propiedades edáficas en la abundancia de esporas y colonización de hongos micorrízicos arbusculares en banano en dos temporadas del año. Scientia Fungorum 2021, 51, e1306. [CrossRef]
- Stark, S.; Kytöviita, M.M. Evidence of antagonistic interactions between rhizosphere microorganisms and mycorrhizal fungi associated with birch (Betula pubescens). Acta Oecol. 2005, 28, 2, 149-155.
- Welc, M.; Ravnskov, S.; Kieliszewsk-Rokicka, B.; Larsen, J. Suppression of other soil microorganisms by mycelium of arbuscular mycorrhizal fungi in root-free soil. Soil Biol. Biochem. 2010, 42, 1534–1540. [CrossRef]
- Ishaq, L., Tae, A.A., Airthur, M.A.; Bako, P.O. Effect of single and mixed inoculation of arbuscular mycorrhizal fungi and phosphorus fertilizer application on corn growth in calcareous soil. Biodiversitas 2021, 22, 4, 1920-1926. [CrossRef]
- Palacios-Zambrano, J.J.; García-García, V.S. Influence of dosages of biofertilizers composed of mycorrhizae and diazotrophs on the corn productivity. ESPOCH Congresses: The Ecuadorian Journal of S.T.E.A.M. 2021, 1, 2, 953-961. [CrossRef]
- Sundram, S.; Meon, S.; Seman, I.A.; Othman, R. Symbiotic interaction of endophytic bacteria with arbuscular mycorrhizal fungi and its antagonistic effect on Ganoderma boninense. J. Microbiol. 2011, 49, 4, 551-557. http://doi.org/10.1007/s12275-011-0489-3.
- El-Sharkawy, E.E.; Abdelrazik, E. Biocontrol of Fusarium root rot in squash using mycorrhizal fungi and antagonistic microorganisms. Egypt. J. Biol. Pest Control, 2022, 32, 1, 13. [CrossRef]
- Okiobé, S.T.; Abossolo-Angue, M.; Bougnom, B.P.; Onana, B.; Nwaga, D. Improvement of arbuscular mycorrhizal fungi inoculum production by nutrient solution concentration and soil texture variation. Int. J. Agron. Agric. Res. 2015, 6, 5, 7-20.
- Adeyemi, N.O.; Atayese, M.O.; Olubode, A.A. Identification and relative abundance of native arbuscular mycorrhizal fungi associated with oil-seed crops and maize (Zea mays L.) in derived savannah of Nigeria. Acta fytotechn. Zootechn. 2019, 22, 3, 84–89.
- Sukmawati, S.; Adnyana, A.; Suprapta, D.N.; Proborini, M.; Soni, P.; Adinurani, P.G. Multiplication arbuscular mycorrhizal fungi in corn (Zea mays L.) with pots culture at greenhouse. In E3S Web Conf. 2021, 226, 00044. [CrossRef]
- Jiménez-Ortiz, M.M.; Gómez-Álvarez, R.; Oliva-Hernández, J.; Granados-Zurita, L.; Pat-Fernández, J.M.; Aranda-Ibáñez, E.M. Influencia del estiércol composteado y micorriza arbuscular sobre la composición química del suelo y el rendimiento productivo de maíz forrajero (Zea mays L.). Nova scientia 2019, 11, 23. [CrossRef]
- Thapa, B.; Mowrer, J. Effects of carbon amendments, tillage and cover cropping on arbuscular mycorrhizal fungi association and root architecture in corn and cotton crop sequence. Agronomy 2022, 12, 2185. [CrossRef]
- Soudzilovskaia, N.A.; Stijn Vaessen, S.; Barcelo, M.; He, J.; Rahimlou, S.; Abarenkov, K.; Brundrett, M.C.; Gomes, S.I.F.; Merckx, V.; Tedersoo, L. FungalRoot: global online database of plant mycorrhizal associations. New Phytologist 2020, 227, 955–966. https://ddoi.org/10.1111/nph.16569.
- Ureta, C.; González, E.J.; Espinosa, A.; Trueba, A.; Piñeyro-Nelson, A.; Álvarez-Buylla, E.R. Maize yield in Mexico under climate change. Agric. Syst. 2020, 177, 102697. [CrossRef]
- Cervantes-Gámez, R.G.; Peñuelas-Rubio, O.; Araujo-Benard, N.; Alicia Fierro- Coronado, R.A.; Dora Trejo-Aguilar, D.; Maldonado-Mendoza, I.E.; Cordero-Ramírez, J.D. Diversidad de hongos micorrízicos arbusculares asociados a plantas voluntarias de maíz en suelos de transición: ecosistema natural - uso agrícola. Scientia Fungorum 2021, 51, e1330. https://doi.10.33885/sf.2021.51.1330.
- Xu, P.; Liang, L.Z.; Dong, X.Y.; Shen, R.F. Effect of arbuscular mycorrhizal fungi on aggregate stability of a clay soil inoculating with two different host plants. Acta Agric. Scand. B — Soil Plant Sci. 2015, 65, 1, 23-29. https://doi:10.1080/09064710.2014.960887.
- Weil, R.R.; Brady, N.C. The Nature and Properties of Soils. 15th edition. Pearson Education Limited. London, England, 2017; 1104 p.
- García, E. Modificaciones al sistema de clasificación climática de Köppen. Instituto de Geografía-Universidad Nacional Autónoma de México. México, DF. México, 2004; 98 p.
- SMN (Servicio Meteorológico Nacional). Normales climatológicas Periodos 1951-2010 y 1981-2000. Servicio Meteorológico Nacional, México, 2015. Disponible en http://smn.cna.gob.mx (Consultation: 13 january 2023).
- Gobierno del estado de Jalisco. Actualización del atlas municipal de riesgos por amenazas naturales y antrópicas en el municipio de Tlajomulco de Zúñiga, Jalisco. Mapa geológico. https://tlajomulco.gob.mx/ProteccionCivil/1.1.7%20MAPA%20GEOL%C3%93GICO.pdf, 2018. (Consultation: 20 june 2023).
- IIEG-Jalisco (Instituto de Información Estadística y Geografía de Jalisco). Recursos Cartográficos/Suelos, Vegetación y Uso del suelo. https://iieg.gob.mx/contenido/Municipios/TlajomulcodeZuniga.pdf 2018. (Consultation: 18 june 2023).
- Schoeneberger, P.J.; Wysocki, D.A.; Benham, E.C.; Soil Survey Staff. 2012. Field book for describing and sampling soils, Version 3.0. Natural Resources Conservation Service, National Soil Survey Center, Lincoln, NE.
- SEMARNAT (Secretaria del Medio Ambiente y Recursos Naturales). Norma Oficial Mexicana que establece las Especificaciones de Fertilidad, Salinidad y Clasificación de Suelos. Estudios, Muestreos y Análisis (NOM-021-RECNAT-2000). Diario Oficial de la Federación 31 de diciembre 2002. México, DF. 85 p.
- SSS (Soil Survey Staff). Keys to Soil Taxonomy, 13th edition. USDA Natural Resources Conservation Service. Washington, D.C., United States of America, 2022; 325 p.
- Gerdemann, J.W.; Nicolson, T.H. Spores of mycorrhizal Endogone species extracted from soil by wet sieving and decanting. Trans. Br. Mycol. Soc. 1963, 46, 2, 235-244.
- INVAM. The International Collection of Vesicular Arbuscular Mycorrhizal Fungi. University of Kansas. https://invam.ku.edu 2023 (Consultation: 25 june 2023).
- Glomeromycota. Agricultural University of Szczecin. http://www.zor.zut.edu.pl/Glomeromycota/Introduction.html 2023. (Consultation: 18 june 2023).
- Glomeromycota PHYLOGENY. Symplanta. https://www.amf-phylogeny.com 2023. (Consultation: 18 june 2023).
- Redecker, D.; Schüβler, A.; Stockinger, H.; Stürmer, S.; Morton, J.B.; Walker, C. An evidence-based consensus for the classification or arbuscular mycorrhizal fungi (Glomeromycota). Mycorrhiza 2013, 23, 515–531. [CrossRef]
- SADER (Secretaría de Agricultura y Desarrollo Rural). Guía para el uso de fertilizantes en el Estado de Jalisco. https://www.gob.mx/cms/uploads/attachment/file/829014/Triptico_Jalisco.pdf 2021. (Consiltation: june 2022).
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 1, 158-IN18.
- Minitab Inc. Minitab® State College. Minitab Inc. Pennsylvania, EEUU, 2013. https://www.minitab.com/es-mx/ (Consultion: 15 january 2021).
- Southard, R.J.; Driese, S.G.; Nordt, L.C. Vertisols. In: Huang, P.M.; Li, Y.; Sumner, M.E. (Ed). Handbook of soli science. 2nd edition. Vol. 1: Properties and processes. CRC Press (Taylor & Francis), Boca Raton, FL. EEUU, 2012; pp. 33.82–33.97.
- Nordt, L.C.; Collins, M.E.; Monger, H.C.; Fanning, G.S. Entisols. In: Huang, P.M.; Li, Y.; Sumner, M.E. (Ed). Handbook of soli science. 2nd edition. Vol. 1: Properties and processes. CRC Press (Taylor & Francis), Boca Raton, FL. EEUU, 2012; pp. 33.49–33.63.
- López-Acevedo, R., M.; Poch, C., R.M.; Porta, C., J. Edafología: uso y protección de suelos. Cuarta ed. Mundi-Prensa Libros, México, 2019; 624 p.
- Jamiołkowska, A.; Księżniak, A.; Gałązka, A.; Hetman, B.; Kopacki, M.; Skwaryło-Bednarz, B. Impact of abiotic factors on development of the community of arbuscular mycorrhizal fungi in the soil: a Review. Int. Agrophys. 2018, 32, 133–140. [CrossRef]
- Getachew, A.; Chilot, Y.; Teklu, E. Soil Acidity Management. Ethiopian Institute of Agricultural Research (EIAR). Addis Ababa, Ethiopia, 2019; 56 p.
- Kome, G.K.; Enang, R.K.; Tabi, F.O.; Yerima, B.P.K. Influence of Clay Minerals on Some Soil Fertility Attributes: A Review. Open J. Soil Sci. 2019, 9, 155–188. [CrossRef]
- Gosling, P.; Proctor, M.; Jones, J.; Bending, G.D. Distribution and diversity of Paraglomus spp. in tilled agricultural soils. Mycorrhiza 2013, 24, 1–11. [CrossRef]
- Carballar-Hernández, S.; Hernández-Cuevas, L.V.; Montaño, N.M.; Larsen, J.; Ferrera-Cerrato, R.; Taboada-Gaytán, O.R.; Montiel-González, A.M.; Alarcón, A. Native communities of arbuscular mycorrhizal fungi associated with Capsicum annuum L. respond to soil properties and agronomic management under field conditions. Agric. Ecosyst. Environ. 2017, 245, 43–51. [CrossRef]
- Rouphael, Y.; Franken, P.; Schneider, C.; Schwarz, D.; Giovannetti, M.; Agnolucci, M.; De Pascale, S.; Bonini, P.; Colla, G. Arbuscular mycorrhizal fungi act as biostimulants in horticultural crops. Sci. Hortic. 2015, 196, 91–108. [CrossRef]
- Payró de la Cruz, E.; Salgado-García, S.; De los Santos-López, G.; Castelán-Estrada, M.; Córdova-Sánchez, S.; Gómez-Leyva, J.F.; Hernández-Cuevas, L. Diversity of VAM in soils used to cultivate sugarcane (Saccharum spp.) in Tabasco, Mexico. Agro Productividad 2021, 14, 11. [CrossRef]
- Jansa, J.; Erb, A.; Oberholzer, H.R.; Šmilauer, P.; Egli, S. Soil and geography are more important determinants of indigenous arbuscular mycorrhizal communities than management practices in Swiss agricultural soils. Mol. Ecol. 2014, 23, 8, 2118-2135. [CrossRef]
- Polo-Marcial, M.H.; Lara-Pérez, L.A.; Goto, B.T.; Margarito-Vista, X.; Andrade-Torres, A. Glomeromycota in Mexico, a country with very high richness. Sydowia, 2021, 74, 33–63. [CrossRef]
- Bahadur, A.; Batool, A.; Nasir, F.; Jiang, S.; Mingsen, Q.; Zhang, Q.; Pan, J.; Liu, Y.; Feng, H. Mechanistic insights into arbuscular mycorrhizal fungi-mediated drought stress tolerance in plants. Int. J. Mol. Sci. 2019, 20, 17, 4199. [CrossRef]
- Sanclemente Reyes, O.E.; Sánchez de Prager, M.; Prager-Mosquera, M. Prácticas agroecológicas, micorrización y productividad del intercultivo maíz-soya (Zea mays L.-Glycine max L.). Idesia (Arica), 2018, 36, 2, 217-224.
- Njeru, E.; Avio, L.; Sbrana, C.; Turrini, A.; Bocci, G.; Bàrberi, P.; Giovannetti, M. First evidence for a major cover crop effect on arbuscular mycorrhizal fungi and organic maize growth. ASDt, 2014, 34, 4, 841-848. [CrossRef]
- Liu, W.; Shanshan Jiang, S.; Zhang, Y.; Yue, S.; Christie, P.; Murray, P.J.; Li, X.; Zhang, J. Spatiotemporal changes in arbuscular mycorrhizal fungal communities under different nitrogen inputs over a 5-year period in intensive agricultural ecosystems on the North China Plain. FEMS Microbiol Ecol. 2014, 90, 436–453. [CrossRef]
- Mena-Echevarría, A.M.; Portugal, V.O.; Suárez, K.F.; Mompié, E.J.; Flores, R.S. Influencia de la inoculación con Glomus hoi-like y un conglomerado de especies de HMA en el crecimiento de plantas de sorgo sometidas o no a estrés hídrico. Cultivos Tropicales, 2011, 32, 1, 11-17. https://www.redalyc.org/pdf/1932/193222352002.pdf.
- Bender, S.F.; Schlaeppi, K.; Held, A.; Van der Heijden, M.G.A. Establishment success and crop growth effects of an arbuscular mycorrhizal fungus inoculated into Swiss corn fields. Agric. Ecosyst. Environ. 2019, 273, 13–24. [CrossRef]
- Wilson, J.M. Competition for infection between vesicular-arbuscular mycorrhizal fungi. New Phytol. 1984, 97, 427–435.
- Ghezzehei, T.A. Soil Structure. In: Huang, P.M.; Li, Y.; Sumner, M.E. (Ed). Handbook of soli science. 2nd edition. Vol. 1: Properties and processes. CRC Press (Taylor & Francis), Boca Raton, FL. EEUU, 2012; pp. 2.1–2.17.
- Behrends-Kraemer, F.; Morrás, H.; Fernández, P.L.; Duval, M.; Galantini, J.; Garibaldi, L. Influence of edaphic and management factors on soils aggregates stability under no-tillage in Mollisols and Vertisols of the Pampa Region, Argentina. Soil Tillage Res. 2021, 209, 104901. [CrossRef]
- Qin, H.; Niu, L.; Wu, Q.; Chen, J.; Li, Y.; Liang, C.; Xu, Q.; Fuhrmann, J.J.; Shen, Y. Bamboo forest expansion increases soil organic carbon through its effect on soil arbuscular mycorrhizal fungal community and abundance. Plant Soil 2017, 420, 407–421. [CrossRef]
- Liang, T.; Shi, X.; Guo, T.; Peng, S. Arbuscular mycorrhizal fungus mediate changes in mycorrhizosphere soil aggregates. Agric. Sci. 2015, 6, 1455–1463. [CrossRef]
- Syamsiyah, J.; Herawati, M., A. The potential of arbuscular mycorrhizal fungi application on aggregrate stability in alfisol soil. In IOP Conference Series: Environ. Earth Sci. 2018, 142, 012045. [CrossRef]
- Morris, E.K.; Morris, D.J.P.; Vogt, S.; Gleber, S.C.; Bigalke, M.: Wilcke, W.; Rillig, M.C. Visualizing the dynamics of soil aggregation as affected by arbuscular mycorrhizal fungi. ISME J. 2019, 13, 1639–1646. [CrossRef]
- Heller, P.; Carrara, J.E. Multiplex qPCR assays to distinguish individual species of arbuscular mycorrhizal fungi from roots and soil. Mycorrhiza 2022, 32, 2,155–164. [CrossRef]
- Li, J.; Wang, Q.; Wang, Y.; Zhang, Q.; Luo, J.; Jiang, X.; Liu, W.; Zhao, X. Effects of arbuscular mycorrhizal fungi on improvement of degraded landscape soil in an ionized rare earth mining area, subtropical China. Soil Sci. Soc. Am. J. 2022, 86, 2, 275-292. [CrossRef]
- Koziol. L.; Bever, J.D. The missing link in grassland restoration: arbuscular mycorrhizal fungi inoculation increases plant diversity and accelerates succession. J. Appl. Ecol, 2017, 54, 1301–1309. [CrossRef]
- Barbosa, M.V.; Pedroso, D.F.; Curi, N.; Carbone, C.M.A. Do different arbuscular mycorrhizal fungi affect the formation and stability of soil aggregates. Cienc. Agrotecnologia 2019, 43, e003519. [CrossRef]
- Leifheit, E.F., Veresoglu, S.D., Lehmann, A., Morris, E.K., Rilling, M.C. Multiple factors influence the role of arbuscular mycorrhizal fungi in soil aggregation-a meta-analysis. Plant Soil 2014, 374, 523–537. [CrossRef]
- Cozzolino, V.; Di Meo, V.; Piccolo, A. Impact of arbuscular mycorrhizal fungi applications on maize production and soil phosphorus availability. J. Geochem. Explor. 2013, 129, 40–44. [CrossRef]
- Moreira-Salgado, F.H.; Sousa-Moreira, F.M.; Siqueira, J.O.; Barbosa, R.H.; Paulino, H.B.; Carbone-Carneiro, M.A. Arbuscular mycorrhizal fungi and colonization stimulant in cotton and maize. Ciência Rural 2017, 06, e20151535. [CrossRef]
- Wahid, F.; Fahad, S.; Danish, S.; Adnan, M.; Yue, Z.; Saud, S.; Siddiqui, M.H.; Brtnicky, M.; Hammerschmiedt, T.; Datta, R. Sustainable management with mycorrhizae and phosphate solubilizing bacteria for enhanced phosphorus uptake in calcareous soils. Agriculture 2020, 10, 334. [CrossRef]
- Saboor, A.; Ali, M.A.; Danish, S.; Ahmed, N.; Fahad, S.; Datta, R.; Ansari, M.J.; Nasif, O.; Habib ur Rahman, M.; Glick, B.R. Effect of arbuscular mycorrhizal fungi on the physiological functioning of maize under zinc-deficient soils. Sci. Rep. 2021, 11, 18468. [CrossRef]
- Mena-Echevarría, A.; Fernández, K.; Olalde, V.; Serrato, R. Diferencias en la respuesta del maíz (Zea mays L.) a la inoculación con Glomus cubense (Y. Rodr. & Dalpé) y con un conglomerado de especies de hongos micorrízicos arbusculares (HMA). Cult. Trop. 2013, 34, 12–15.
- Colina-Navarrete, E.; Paredes-Acosta, E.; Gutiérrez-Mora, X.; Vera-Suarez, M. Efecto de fertilización nitrogenada en maíz (Zea mays L.) sobre poblaciones de hongos micorrízicos, en Babahoyo. J. Sci. Res. 2020, 5, 135–155. [CrossRef]
- Fall, A.F.; Nakabonge, G.; Ssekandi, J.; Founoune-Mboup. H.; Apori, S.O.; Ndiaye, A.; Badji, A.; Ngom, K. Roles of arbuscular mycorrhizal fungi on soil fertility: Contribution in the improvement of physical, chemical, and biological properties of the soil. Front. Fungal Biol. 2022, 3, 723892. [CrossRef]
| Soil | Hs1 | Deep (cm) |
OC (%) |
pH | EC dS m-1 |
S (%) |
L (%) |
R (%) |
Bd (g cm-3) |
CEC (cmol(+) Kg-1) |
PBS (%) |
P2O5 (mg Kg-1) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | Ap | 0-20 | 1.09 | 4.40 | 0.89 | 3.26 | 46.98 | 49.76 | 1.34 | 13.45 | 34.80 | 93.04 |
| C1 | 20-45 | 1.03 | 4.35 | 0.52 | 1.80 | 48.12 | 50.08 | 1.36 | 11.22 | 19.32 | 52.93 | |
| C2 | 45-72 | 0.59 | 6.31 | 0.47 | 1.10 | 59.86 | 39.04 | 1.53 | 12.10 | 38.83 | 6.42 | |
| C3 | >72 | 0.92 | 7.63 | 0.60 | 3.54 | 38.24 | 58.22 | 1.47 | 17.46 | 21.35 | 3.21 | |
| S2 | Ap | 0-16 | 1.64 | 6.87 | 0.98 | 44.60 | 32.04 | 23.36 | 1.34 | 15.41 | 57.12 | 140.40 |
| C1 | 16-45 | 0.83 | 5.62 | 0.16 | 40.28 | 36.36 | 23.36 | 1.32 | 16.51 | 60.30 | 116.15 | |
| C2 | 45-65 | 0.46 | 6.28 | 0.33 | 59.72 | 23.32 | 16.96 | 1.15 | 9.10 | 30.41 | 16.40 | |
| C3 | 65-82 | 0.75 | 6.80 | 0.34 | 65.52 | 17.48 | 20.00 | 1.11 | 11.40 | 41.73 | 6.12 | |
| C4 | 82-95 | 0.48 | 6.61 | 0.38 | 58.64 | 21.68 | 19.48 | 1.29 | 11.20 | 40.79 | 12.43 | |
| C5 | >95 | 0.35 | 6.80 | 0.25 | 40.84 | 32.76 | 26.40 | 1.36 | 15.12 | 55.80 | 10.68 |
| AMF | Typic Dystrustert | Typic Ustifluvent |
|---|---|---|
| Rhizophagus aggregatus (N.C. Schenck & G.S.Sm.) C.Walker | X | |
| Funneliformis geosporum (T.H. Nicolson & Gerd.) C. Walker & A. Schluessler | X | X |
| Paraglomus occultum (C.Walker) J.B.Morton & D.Redecker | X | X |
| Diversispora aurantia (Błaszk., Blanke, Renker & Buscot) C. Walker & A. Schüßler | X | X |
| Diversispora trimurales (Koske y Halvorson) C. Walker & A. Schüßler | X | |
| Gigaspora candida Bhattacharjee, Mukerji, J.P. Tewari & Skoropad | X | |
| Gigaspora gigantean (T.H.Nicolson & Gerd.) Gerd. & Trappe | X | |
| Acaulospora mellea Spain & N.C.Schenck | X | |
| Septoglomus sp. | X |
| Treat 1 | N | Sp | Co | AS | Yd | PH | SD | Pp | Np |
|---|---|---|---|---|---|---|---|---|---|
| S1A0f0 | 5 | 866.00 h2 | 70.66 cd | 63.29 de | 56.90 b | 2.40 abcd | 3.10 a | 3.03 a | 1.06 d |
| S1A1f0 | 5 | 2000.00 d | 97.33 a | 79.76 a | 27.50 b | 2.16 cd | 2.78 b | 3.00 a | 1.08 d |
| S1A2f0 | 5 | 1570.00 f | 96.67 a | 72.58 b | 36.00 b | 2.16 cd | 3.38 a | 0.76 d | 1.06 d |
| S2A0f0 | 5 | 2284.00 c | 96.67 a | 62.93 de | 68.20 b | 2.34 bcd | 1.92 c | 1.44 bcd | 1.00 d |
| S2A1f0 | 5 | 3153.00 a | 90.67 ab | 67.86 bcd | 76.09 b | 1.95 d | 1.92 c | 1.50 abcd | 0.60 e |
| S2A2f0 | 5 | 1760.00 e | 94.00 ab | 50.35 f | 74.60 b | 2.07 d | 1.84 c | 0.91 cd | 1.13 d |
| S1A0f1 | 5 | 1492.00 f | 59.32 d | 62.27 e | 55.40 b | 2.51 abcd | 2.78 b | 2.55 ab | 2.08 b |
| S1A1f1 | 5 | 2742.00 b | 83.33 abc | 68.52 bc | 73.50 b | 2.78 abc | 3.10 ab | 2.16 abcd | 1.73 c |
| S1A2f1 | 5 | 1926.00 d | 77.99 bc | 64.89 cde | 61.50 b | 2.83 ab | 2.68 b | 1.42 bcd | 2.43 a |
| S2A0f1 | 5 | 2342.00 c | 82.67 abc | 46.21 f | 176.70 a | 3.05 a | 1.76 c | 1.66 abcd | 1.82 c |
| S2A1f1 | 5 | 1914.00 d | 84.64 abc | 50.51 f | 151.30 a | 3.08 a | 1.82 c | 2.28 abcd | 1.94 bc |
| S2A2f1 | 5 | 1760.00 e | 93.33 ab | 40.45 g | 186.90 a | 3.02 a | 1.88 c | 2.32 abc | 1.94 bc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





