Submitted:
30 September 2023
Posted:
02 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Structure and Lifecycle of Polyomaviruses: JCPyV and MuPyV
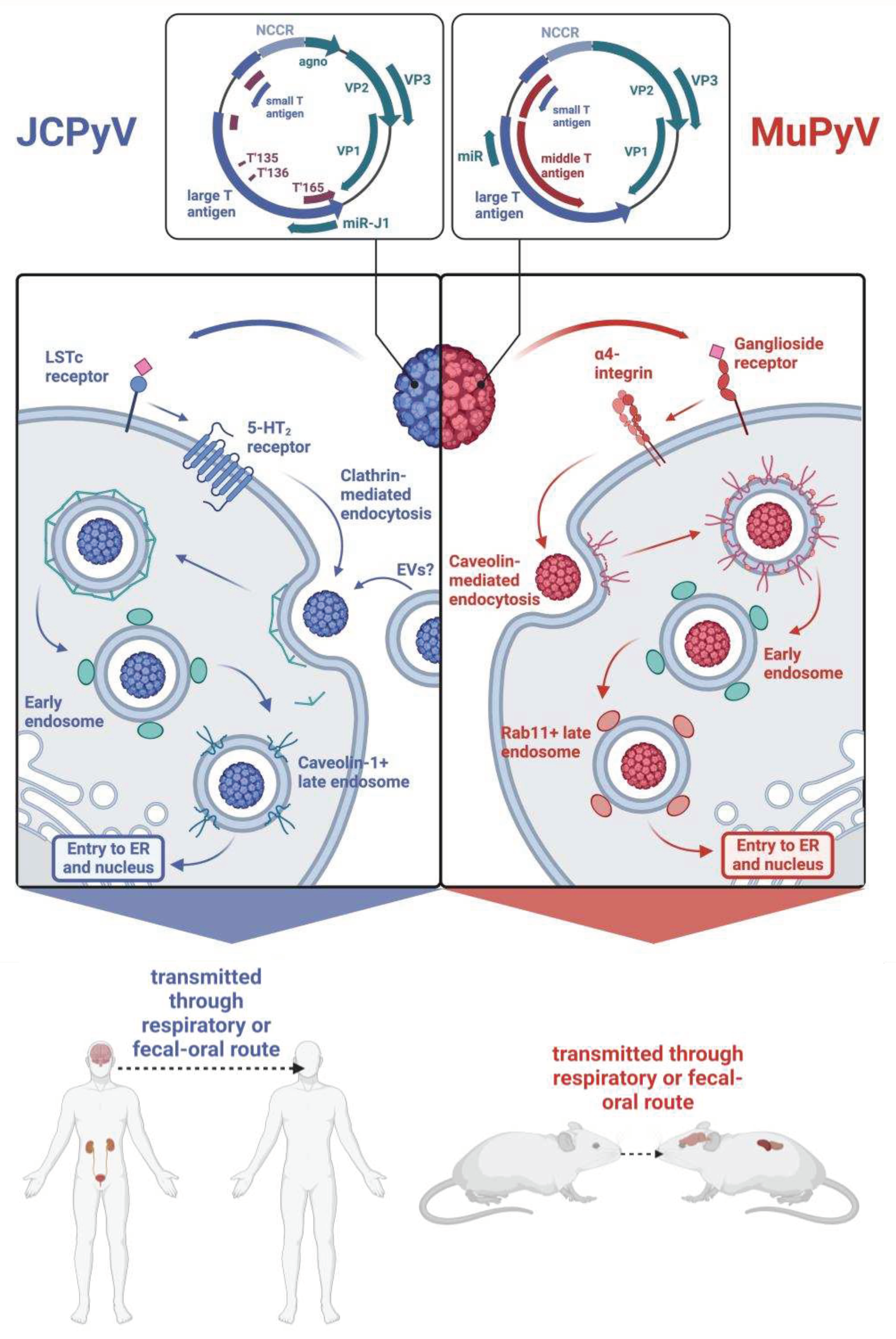
3. How Is JCPyV Transmitted?
3.1. Polyomaviruses in Peripheral Tissues
4. How Do Polyomaviruses Maintain Long-Term Persistence?
5. How Does Polyomavirus Travel from the Periphery to the Brain?
6. How Does Polyomavirus Invade the Brain?
6.1. Via the Blood-Brain Barrier?
6.2. Via the ChP-CSF-Ependyma Barriers?
6.3. Via the Meninges?
7. What Brain Cells Are Infected by Neurotrophic Polyomaviruses, and What Pathologies Does This Infection Cause?
7.1. Oligodendrocytes
7.2. Astrocytes
7.3. Glial Progenitor Cells
7.4. ChP Epithelium and Ependyma
7.5. Leptomeningeal Cells
7.6. Neurons
8. What Is the Innate Immune Response to Polyomavirus?
8.1. Sensing, Reporting, and Alarming Innate Immunity
8.2. The Innate Immune Enzyme Family APOBEC3: A Driver of Polyomavirus VP1 Mutations?
9. What Is the Adaptive Immune Response to Polyomavirus Infection?
9.1. CD8+ T Cells
9.2. CD4+ T Cells
9.3. B Cells
10. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-HT2 | 5-hydroxytryptamine 2 |
| AIDS | Acquired imunodeficiency syndrome |
| APOBEC3 | Apolipoprotein B mRNA-editing catalytic polypeptide-like 3 |
| BBB | Blood-brain barrier |
| BCSFB | Blood-CSF-brain barrier |
| BK-VAN | BKPyV-associated nephropathy |
| BKPyV | BK polyomavirus |
| BMDC | Bone marrow-derived dendritic cells |
| bTrm | brain-resident memory |
| C/EBPβ | CAAT/enhancer binding protein beta |
| cGAMP | Cyclic GMP-AMP |
| cGAS | Cyclic GMP-AMP synthase |
| ChP | Choroid plexus epithelium |
| CNS | Central nervous system |
| COVID-19 | Coronavirus disease 2019 |
| CSF | Cerebrospinal fluid |
| ERAD | ER-associated degradation pathway |
| ESRD | End stage renal disease |
| EV | Extracellular vesicle |
| GFAP | Glial fibrillary acidic protein |
| Iba1 | Ionized calcium binding adaptor molecule 1 |
| IFN | Interferon |
| IFN-α | Interferon-α |
| IFN-β | Interferon-β |
| IFN-γ | Interferon-γ |
| IFNAR | Interferon-α/β receptor |
| IL | Interleukin |
| IRF3 | Interferon regulatory factor 3 |
| ISG | Interferon-stimulated gene |
| JAK | Janus kinase |
| JC-PVAN | JCPyV-associated nephropathy |
| JCPyV | JC polyomavirus |
| LIP | Liver inhibitory protein |
| LSTc | Lactoseries tetrasaccharide c |
| LTAg | Large T antigen |
| mAB | Monoclonal antibody |
| MDA5 | Melanoma differentiation-associated protein 5 |
| MHC | Major histocompatibility complex |
| miRNA | microRNA |
| MS | Multiple sclerosis |
| MTAg | Middle T antigen |
| MuPyV | Mouse polyomavirus |
| NCCR | Non-coding control region |
| NK | Natural killer |
| NPC | Neural progenitor cells |
| PD-1 | Programmed cell death protein 1 |
| PD-L1 | Programmed cell death ligand 1 |
| PML | Progressive multifocal leukoencephalopathy |
| PRR | Pattern recognition receptor |
| RB | Retinoblastoma-associated protein |
| RIG-I | Retinoic-acid inducible gene I |
| STAg | Small T antigen |
| STAT | Signal transducers and activators of transcription |
| STING | Stimulatory of interferon genes |
| SV40 | Simian virus 40 |
| TLR | Toll-like receptor |
| Trm | Resident memory |
| VLP | Virus-like particle |
| WT | Wild-type |
References
- Atkinson, A.L.; Atwood, W.J. Fifty Years of JC Polyomavirus: A Brief Overview and Remaining Questions. Viruses 2020, 12, 969. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.S.; Koralnik, I.J. Progressive Multifocal Leukoencephalopathy and Other Disorders Caused by JC Virus: Clinical Features and Pathogenesis. Lancet Neurol 2010, 9, 425–437. [Google Scholar] [CrossRef] [PubMed]
- Cortese, I.; Muranski, P.; Enose-Akahata, Y.; Ha, S.-K.; Smith, B.; Monaco, M.; Ryschkewitsch, C.; Major, E.O.; Ohayon, J.; Schindler, M.K.; et al. Pembrolizumab Treatment for Progressive Multifocal Leukoencephalopathy. N Engl J Med 2019, 380, 1597–1605. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, H.H.; Kardas, P.; Kranz, D.; Leboeuf, C. The Human JC Polyomavirus (JCPyV): Virological Background and Clinical Implications. APMIS 2013, 121, 685–727. [Google Scholar] [CrossRef] [PubMed]
- Laine, H.K.; Waterboer, T.; Syrjänen, K.; Grenman, S.; Louvanto, K.; Syrjänen, S. Seroprevalence of Polyomaviruses BK and JC in Finnish Women and Their Spouses Followed-up for Three Years. Sci Rep 2023, 13, 879. [Google Scholar] [CrossRef] [PubMed]
- Kamminga, S.; van der Meijden, E.; Feltkamp, M.C.W.; Zaaijer, H.L. Seroprevalence of Fourteen Human Polyomaviruses Determined in Blood Donors. PLoS One 2018, 13, e0206273. [Google Scholar] [CrossRef]
- Zheng, H.-Y.; Kitamura, T.; Takasaka, T.; Chen, Q.; Yogo, Y. Unambiguous Identification of JC Polyomavirus Strains Transmitted from Parents to Children. Arch Virol 2004, 149, 261–273. [Google Scholar] [CrossRef]
- Ijaz, R.; Shahzad, N.; Farhan Ul Haque, M. Detection of BK and JC Polyomaviruses in Sewage Water of the Urban Areas of Lahore, Pakistan. Biologia (Bratisl) 2023, 1–8. [Google Scholar] [CrossRef]
- Prezioso, C.; Ciotti, M.; Obregon, F.; Ambroselli, D.; Rodio, D.M.; Cudillo, L.; Gaziev, J.; Mele, A.; Nardi, A.; Favalli, C.; et al. Polyomaviruses Shedding in Stool of Patients with Hematological Disorders: Detection Analysis and Study of the Non-Coding Control Region’s Genetic Variability. Med Microbiol Immunol 2019, 208, 845–854. [Google Scholar] [CrossRef]
- Bofill-Mas, S.; Pina, S.; Girones, R. Documenting the Epidemiologic Patterns of Polyomaviruses in Human Populations by Studying Their Presence in Urban Sewage. Appl Environ Microbiol 2000, 66, 238–245. [Google Scholar] [CrossRef]
- Kato, A.; Kitamura, T.; Takasaka, T.; Tominaga, T.; Ishikawa, A.; Zheng, H.-Y.; Yogo, Y. Detection of the Archetypal Regulatory Region of JC Virus from the Tonsil Tissue of Patients with Tonsillitis and Tonsilar Hypertrophy. J Neurovirol 2004, 10, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, F.; Carbone, D.; Mugavero, R.; Palmieri, A.; Lauritano, D.; Baggi, L.; Nardone, M.; Martinelli, M.; Carinci, F. Human Polyomavirus in Tonsillar Microbiota of an Afghan Population Group. J Biol Regul Homeost Agents 2018, 32, 185–190. [Google Scholar] [PubMed]
- Ayers, K.N.; Carey, S.N.; Lukacher, A.E. Understanding Polyomavirus CNS Disease - a Perspective from Mouse Models. FEBS J 2022, 289, 5744–5761. [Google Scholar] [CrossRef] [PubMed]
- Carroll, J.; Dey, D.; Kreisman, L.; Velupillai, P.; Dahl, J.; Telford, S.; Bronson, R.; Benjamin, T. Receptor-Binding and Oncogenic Properties of Polyoma Viruses Isolated from Feral Mice. PLoS Pathog 2007, 3, e179. [Google Scholar] [CrossRef] [PubMed]
- Swanson, P.A.; Lukacher, A.E.; Szomolanyi-Tsuda, E. Immunity to Polyomavirus Infection: The Polyomavirus-Mouse Model. Semin Cancer Biol 2009, 19, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Drake, D.R.; Lukacher, A.E. Beta 2-Microglobulin Knockout Mice Are Highly Susceptible to Polyoma Virus Tumorigenesis. Virology 1998, 252, 275–284. [Google Scholar] [CrossRef]
- Frisque, R.J.; Bream, G.L.; Cannella, M.T. Human Polyomavirus JC Virus Genome. J Virol 1984, 51, 458–469. [Google Scholar] [CrossRef] [PubMed]
- Carmichael, G.G. Gene Regulation and Quality Control in Murine Polyomavirus Infection. Viruses 2016, 8, 284. [Google Scholar] [CrossRef]
- Trowbridge, P.W.; Frisque, R.J. Identification of Three New JC Virus Proteins Generated by Alternative Splicing of the Early Viral mRNA. J Neurovirol 1995, 1, 195–206. [Google Scholar] [CrossRef]
- Seo, G.J.; Fink, L.H.L.; O’Hara, B.; Atwood, W.J.; Sullivan, C.S. Evolutionarily Conserved Function of a Viral microRNA. J Virol 2008, 82, 9823–9828. [Google Scholar] [CrossRef]
- Lauver, M.D.; Lukacher, A.E. JCPyV VP1 Mutations in Progressive MultifocalLeukoencephalopathy: Altering Tropismor Mediating Immune Evasion? Viruses 2020, 12. [Google Scholar] [CrossRef] [PubMed]
- Marshall, L.J.; Moore, L.D.; Mirsky, M.M.; Major, E.O. JC Virus Promoter/Enhancers Contain TATA Box-Associated Spi-B-Binding Sites That Support Early Viral Gene Expression in Primary Astrocytes. J Gen Virol 2012, 93, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Yogo, Y.; Kitamura, T.; Sugimoto, C.; Ueki, T.; Aso, Y.; Hara, K.; Taguchi, F. Isolation of a Possible Archetypal JC Virus DNA Sequence from Nonimmunocompromised Individuals. J Virol 1990, 64, 3139–3143. [Google Scholar] [CrossRef] [PubMed]
- Daniel, A.M.; Swenson, J.J.; Mayreddy, R.P.; Khalili, K.; Frisque, R.J. Sequences within the Early and Late Promoters of Archetype JC Virus Restrict Viral DNA Replication and Infectivity. Virology 1996, 216, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Ciardi, M.R.; Zingaropoli, M.A.; Iannetta, M.; Prezioso, C.; Perri, V.; Pasculli, P.; Lichtner, M.; d’Ettorre, G.; Altieri, M.; Conte, A.; et al. JCPyV NCCR Analysis in PML Patients with Different Risk Factors: Exploring Common Rearrangements as Essential Changes for Neuropathogenesis. Virol J 2020, 17, 23. [Google Scholar] [CrossRef] [PubMed]
- Gosert, R.; Kardas, P.; Major, E.O.; Hirsch, H.H. Rearranged JC Virus Noncoding Control Regions Found in Progressive Multifocal Leukoencephalopathy Patient Samples Increase Virus Early Gene Expression and Replication Rate. J Virol 2010, 84, 10448–10456. [Google Scholar] [CrossRef]
- Treisman, R.; Novak, U.; Favaloro, J.; Kamen, R. Transformation of Rat Cells by an Altered Polyoma Virus Genome Expressing Only the Middle-T Protein. Nature 1981, 292, 595–600. [Google Scholar] [CrossRef]
- Dilworth, S.M. Polyoma Virus Middle T Antigen and Its Role in Identifying Cancer-Related Molecules. Nat Rev Cancer 2002, 2, 951–956. [Google Scholar] [CrossRef]
- Ströh, L.J.; Maginnis, M.S.; Blaum, B.S.; Nelson, C.D.S.; Neu, U.; Gee, G.V.; O’Hara, B.A.; Motamedi, N.; DiMaio, D.; Atwood, W.J.; et al. The Greater Affinity of JC Polyomavirus Capsid for A2,6-Linked Lactoseries Tetrasaccharide c than for Other Sialylated Glycans Is a Major Determinant of Infectivity. J Virol 2015, 89, 6364–6375. [Google Scholar] [CrossRef]
- Neu, U.; Maginnis, M.S.; Palma, A.S.; Ströh, L.J.; Nelson, C.D.S.; Feizi, T.; Atwood, W.J.; Stehle, T. Structure-Function Analysis of the Human JC Polyomavirus Establishes the LSTc Pentasaccharide as a Functional Receptor Motif. Cell Host Microbe 2010, 8, 309–319. [Google Scholar] [CrossRef]
- Maginnis, M.S.; Haley, S.A.; Gee, G.V.; Atwood, W.J. Role of N-Linked Glycosylation of the 5-HT2A Receptor in JC Virus Infection. J Virol 2010, 84, 9677–9684. [Google Scholar] [CrossRef] [PubMed]
- Tsai, B.; Gilbert, J.M.; Stehle, T.; Lencer, W.; Benjamin, T.L.; Rapoport, T.A. Gangliosides Are Receptors for Murine Polyoma Virus and SV40. EMBO J 2003, 22, 4346–4355. [Google Scholar] [CrossRef] [PubMed]
- Caruso, M.; Belloni, L.; Sthandier, O.; Amati, P.; Garcia, M.-I. A4β1 Integrin Acts as a Cell Receptor for Murine Polyomavirus at the Postattachment Level. J Virol 2003, 77, 3913–3921. [Google Scholar] [CrossRef] [PubMed]
- Vajn, K.; Viljetić, B.; Degmečić, I.V.; Schnaar, R.L.; Heffer, M. Differential Distribution of Major Brain Gangliosides in the Adult Mouse Central Nervous System. PLoS One 2013, 8, e75720. [Google Scholar] [CrossRef] [PubMed]
- Pho, M.T.; Ashok, A.; Atwood, W.J. JC Virus Enters Human Glial Cells by Clathrin-Dependent Receptor-Mediated Endocytosis. J Virol 2000, 74, 2288–2292. [Google Scholar] [CrossRef] [PubMed]
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding Light on the Cell Biology of Extracellular Vesicles. Nat Rev Mol Cell Biol 2018, 19, 213–228. [Google Scholar] [CrossRef]
- O’Hara, B.A.; Morris-Love, J.; Gee, G.V.; Haley, S.A.; Atwood, W.J. JC Virus Infected Choroid Plexus Epithelial Cells Produce Extracellular Vesicles That Infect Glial Cells Independently of the Virus Attachment Receptor. PLoS Pathog 2020, 16, e1008371. [Google Scholar] [CrossRef]
- Liebl, D.; Difato, F.; Horníková, L.; Mannová, P.; Stokrová, J.; Forstová, J. Mouse Polyomavirus Enters Early Endosomes, Requires Their Acidic pH for Productive Infection, and Meets Transferrin Cargo in Rab11-Positive Endosomes. J Virol 2006, 80, 4610–4622. [Google Scholar] [CrossRef]
- Querbes, W.; O’Hara, B.A.; Williams, G.; Atwood, W.J. Invasion of Host Cells by JC Virus Identifies a Novel Role for Caveolae in Endosomal Sorting of Noncaveolar Ligands. J Virol 2006, 80, 9402–9413. [Google Scholar] [CrossRef]
- Kilcher, S.; Mercer, J. DNA Virus Uncoating. Virology 2015, 479–480, 578–590. [Google Scholar] [CrossRef]
- Vilchez, R.A.; Butel, J.S. Emergent Human Pathogen Simian Virus 40 and Its Role in Cancer. Clin Microbiol Rev 2004, 17, 495–508. [Google Scholar] [CrossRef] [PubMed]
- Walczak, C.P.; Tsai, B. A PDI Family Network Acts Distinctly and Coordinately with ERp29 to Facilitate Polyomavirus Infection. J Virol 2011, 85, 2386–2396. [Google Scholar] [CrossRef] [PubMed]
- Kuksin, D.; Norkin, L.C. Disassembly of Simian Virus 40 during Passage through the Endoplasmic Reticulum and in the Cytoplasm. J Virol 2012, 86, 1555–1562. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Tsai, B. A Large and Intact Viral Particle Penetrates the Endoplasmic Reticulum Membrane to Reach the Cytosol. PLoS Pathog 2011, 7, e1002037. [Google Scholar] [CrossRef]
- Gee, G.V.; O’Hara, B.A.; Derdowski, A.; Atwood, W.J. Pseudovirus Mimics Cell Entry and Trafficking of the Human Polyomavirus JCPyV. Virus Res 2013, 178, 10–1016. [Google Scholar] [CrossRef]
- Dupzyk, A.; Tsai, B. How Polyomaviruses Exploit the ERAD Machinery to Cause Infection. Viruses 2016, 8, 242. [Google Scholar] [CrossRef]
- Chen, Z.; Ji, Z.; Ngiow, S.F.; Manne, S.; Cai, Z.; Huang, A.C.; Johnson, J.; Staupe, R.P.; Bengsch, B.; Xu, C.; et al. TCF-1-Centered Transcriptional Network Drives an Effector versus Exhausted CD8 T Cell-Fate Decision. Immunity 2019, 51, 840–855. [Google Scholar] [CrossRef]
- Ramsperger, U.; Stahl, H. Unwinding of Chromatin by the SV40 Large T Antigen DNA Helicase. EMBO J 1995, 14, 3215–3225. [Google Scholar] [CrossRef]
- Bollag, B.; Hofstetter, C.A.; Reviriego-Mendoza, M.M.; Frisque, R.J. JC Virus Small t Antigen Binds Phosphatase PP2A and Rb Family Proteins and Is Required for Efficient Viral DNA Replication Activity. PLoS One 2010, 5, e10606. [Google Scholar] [CrossRef]
- Prins, C.; Frisque, R.J. JC Virus T’ Proteins Encoded by Alternatively Spliced Early mRNAs Enhance T Antigen-Mediated Viral DNA Replication in Human Cells. J Neurovirol 2001, 7, 250–264. [Google Scholar] [CrossRef]
- Saxena, R.; Saribas, S.; Jadiya, P.; Tomar, D.; Kaminski, R.; Elrod, J.W.; Safak, M. Human Neurotropic Polyomavirus, JC Virus, Agnoprotein Targets Mitochondrion and Modulates Its Functions. Virology 2021, 553, 135–153. [Google Scholar] [CrossRef] [PubMed]
- Eash, S.; Tavares, R.; Stopa, E.G.; Robbins, S.H.; Brossay, L.; Atwood, W.J. Differential Distribution of the JC Virus Receptor-Type Sialic Acid in Normal Human Tissues. Am J Pathol 2004, 164, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Hametoja, H.; Hagstrom, J.; Haglund, C.; Back, L.; Makitie, A.; Syrjanen, S. Polyomavirus JCPyV Infrequently Detectable in Adenoid Cystic Carcinoma of the Oral Cavity and the Airways. Virchows Arch 2019, 475, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Abdel Aziz, H.O.; Nakanishi, Y.; Masuda, S.; Saito, H.; Tsuneyama, K.; Takano, Y. Oncogenic Role of JC Virus in Lung Cancer. J Pathol 2007, 212, 306–315. [Google Scholar] [CrossRef]
- Csoma, E.; Bidiga, L.; Mehes, G.; Katona, M.; Gergely, L. Survey of KI, WU, MW, and STL Polyomavirus in Cancerous and Non-Cancerous Lung Tissues. Pathobiology 2018, 85, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Joh, J.; Jenson, A.B.; Moore, G.D.; Rezazedeh, A.; Slone, S.P.; Ghim, S.J.; Kloecker, G.H. Human Papillomavirus (HPV) and Merkel Cell Polyomavirus (MCPyV) in Non Small Cell Lung Cancer. Exp Mol Pathol 2010, 89, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, J.; Waterboer, T.; Pawlita, M.; Michel, A.; Cai, Q.; Zheng, W.; Gao, Y.T.; Lan, Q.; Rothman, N.; Langseth, H.; et al. Serum Biomarkers of Polyomavirus Infection and Risk of Lung Cancer in Never Smokers. Br J Cancer 2016, 115, 1131–1139. [Google Scholar] [CrossRef] [PubMed]
- Lasithiotaki, I.; Antoniou, K.M.; Derdas, S.P.; Sarchianaki, E.; Symvoulakis, E.K.; Psaraki, A.; Spandidos, D.A.; Stathopoulos, E.N.; Siafakas, N.M.; Sourvinos, G. The Presence of Merkel Cell Polyomavirus Is Associated with Deregulated Expression of BRAF and Bcl-2 Genes in Non-Small Cell Lung Cancer. Int J Cancer 2013, 133, 604–611. [Google Scholar] [CrossRef] [PubMed]
- da Silva, A.S.; de Campos, G.M.; Giovanetti, M.; Zucherato, V.S.; Lima, A.R.J.; Santos, E.V.; Haddad, R.; Ciccozzi, M.; Carlos Junior Alcantara, L.; Elias, M.C.; et al. Viral Metagenomics Unveils MW (Malawi) Polyomavirus Infection in Brazilian Pediatric Patients with Acute Respiratory Disease. J Med Virol 2023, 95, e28688. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.-C.; Wang, M.; Chou, M.-C.; Chao, C.-N.; Fang, C.-Y.; Chen, P.-L.; Chang, D.; Shen, C.-H. Gene Therapy for Castration-Resistant Prostate Cancer Cells Using JC Polyomavirus-like Particles Packaged with a PSA Promoter Driven-Suicide Gene. Cancer Gene Ther 2019, 26, 208–215. [Google Scholar] [CrossRef]
- Gottlieb, K.; Villarreal, L.P. The Distribution and Kinetics of Polyomavirus in Lungs of Intranasally Infected Newborn Mice. Virology 2000, 266, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Hernandez, T.; Carnathan, D.G.; Jones, S.; Cork, A.J.; Davies, M.R.; Moyle, P.M.; Toth, I.; Batzloff, M.R.; McCarthy, J.; Nizet, V.; et al. An Experimental Group A Streptococcus Vaccine That Reduces Pharyngitis and Tonsillitis in a Nonhuman Primate Model. mBio 2019, 10, e00693–19. [Google Scholar] [CrossRef] [PubMed]
- Sinagra, E.; Raimondo, D.; Gallo, E.; Stella, M.; Cottone, M.; Rossi, F.; Messina, M.; Spada, M.; Tomasello, G.; Ferrara, G.; et al. JC Virus and Lung Adenocarcinoma: Fact or Myth? Anticancer Res 2017, 37, 3311–3314. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.C.; Xue, H.; Zhang, C.Y. The Oncogenic Roles of JC Polyomavirus in Cancer. Front Oncol 2022, 12, 976577. [Google Scholar] [CrossRef] [PubMed]
- Levican, J.; Levican, A.; Ampuero, M.; Gaggero, A. JC Polyomavirus Circulation in One-Year Surveillance in Wastewater in Santiago, Chile. Infect Genet Evol 2019, 71, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Dias, A.M.; Correia, A.; Pereira, M.S.; Almeida, C.R.; Alves, I.; Pinto, V.; Catarino, T.A.; Mendes, N.; Leander, M.; Oliva-Teles, M.T.; et al. Metabolic Control of T Cell Immune Response through Glycans in Inflammatory Bowel Disease. Proc Natl Acad Sci U S A 2018, 115, E4651–E4660. [Google Scholar] [CrossRef]
- Hewitt, J.; Greening, G.E.; Leonard, M.; Lewis, G.D. Evaluation of Human Adenovirus and Human Polyomavirus as Indicators of Human Sewage Contamination in the Aquatic Environment. Water Res 2013, 47, 6750–6761. [Google Scholar] [CrossRef]
- Chiu, N.C.; Chi, H.; Tai, Y.L.; Peng, C.C.; Tseng, C.Y.; Chen, C.C.; Tan, B.F.; Lin, C.Y. Impact of Wearing Masks, Hand Hygiene, and Social Distancing on Influenza, Enterovirus, and All-Cause Pneumonia During the Coronavirus Pandemic: Retrospective National Epidemiological Surveillance Study. J Med Internet Res 2020, 22, e21257. [Google Scholar] [CrossRef]
- Vigiser, I.; Piura, Y.; Kolb, H.; Shiner, T.; Komarov, I.; Karni, A.; Regev, K. JCV Seroconversion Rate during the SARS COVID-19 Pandemic. Mult Scler Relat Disord 2022, 68, 104244. [Google Scholar] [CrossRef]
- Dwyer, C.; Sharmin, S.; Kalincik, T. Rates of John Cunningham Virus Seroconversion Greatly Reduced in Natalizumab-Treated Patients during COVID-19-Related Lockdowns. Eur J Neurol 2023. [Google Scholar] [CrossRef]
- Esmailzadeh, N.; Ranaee, M.; Alizadeh, A.; Khademian, A.; Saber Amoli, S.; Sadeghi, F. Presence of JC Polyomavirus in Nonneoplastic Inflamed Colon Mucosa and Primary and Metastatic Colorectal Cancer. Gastrointest Tumors 2020, 7, 30–40. [Google Scholar] [CrossRef]
- Uleri, E.; Piu, C.; Caocci, M.; Ibba, G.; Sanges, F.; Pira, G.; Murgia, L.; Barmina, M.; Giannecchini, S.; Porcu, A.; et al. Multiple Signatures of the JC Polyomavirus in Paired Normal and Altered Colorectal Mucosa Indicate a Link with Human Colorectal Cancer, but Not with Cancer Progression. Int J Mol Sci 2019, 20. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.Y.; Chen, S.Y.; Hsiao, B.X.; Huang, H.Y.; Chen, Y.J.; Tung, C.L.; Fang, C.Y. Unusually High Incidence of Polyomavirus JC Infection in the Higher Grade of Colorectal Cancer Tissues in Taiwan. Eur J Med Res 2022, 27, 127. [Google Scholar] [CrossRef] [PubMed]
- Ksiaa, F.; Allous, A.; Ziadi, S.; Mokni, M.; Trimeche, M. Assessment and Biological Significance of JC Polyomavirus in Colorectal Cancer in Tunisia. J BUON 2015, 20, 762–769. [Google Scholar] [PubMed]
- Muhsen, M.A.Z.; Ghaith Sachit, H.; Jaafar Hussein, M.; Mohammed Ali, S.H.; Mohammed Al-Alwany, S.H.; Khalil Hussein, A. Molecular Interplay of John Cunningham Virus with Interleukin 1 Beta in Colorectal Carcinomatous Tissues from a Group of Iraqi Patients. Arch Razi Inst 2022, 77, 2299–2306. [Google Scholar] [CrossRef]
- Rowe, W.P.; Hartley, J.W.; Brodsky, I.; Huebner, R.J.; Law, L.W. Observations on the Spread of Mouse Polyoma Virus Infection. Nature 1958, 182, 1617. [Google Scholar] [CrossRef]
- Burke, J.M.; Bass, C.R.; Kincaid, R.P.; Ulug, E.T.; Sullivan, C.S. The Murine Polyomavirus MicroRNA Locus Is Required To Promote Viruria during the Acute Phase of Infection. J Virol 2018, 92, e02131–17. [Google Scholar] [CrossRef]
- Houff, S.A.; Major, E.O.; Katz, D.A.; Kufta, C.V.; Sever, J.L.; Pittaluga, S.; Roberts, J.R.; Gitt, J.; Saini, N.; Lux, W. Involvement of JC Virus-Infected Mononuclear Cells from the Bone Marrow and Spleen in the Pathogenesis of Progressive Multifocal Leukoencephalopathy. N Engl J Med 1988, 318, 301–305. [Google Scholar] [CrossRef]
- Grinnell, B.W.; Padgett, B.L.; Walker, D.L. Distribution of Nonintegrated DNA from JC Papovavirus in Organs of Patients with Progressive Multifocal Leukoencephalopathy. J Infect Dis 1983, 147, 669–675. [Google Scholar] [CrossRef]
- Tan, C.S.; Dezube, B.J.; Bhargava, P.; Autissier, P.; Wuthrich, C.; Miller, J.; Koralnik, I.J. Detection of JC Virus DNA and Proteins in Bone Marrow of HIV-Positive and HIV-Negative Patients. J Infect Dis 2009, 199, 881–888. [Google Scholar] [CrossRef]
- Prezioso, C.; Ciotti, M.; Brazzini, G.; Piacentini, F.; Passerini, S.; Grimaldi, A.; Landi, D.; Nicoletti, C.G.; Zingaropoli, M.A.; Iannetta, M.; et al. Diagnostic Value of JC Polyomavirus Viruria, Viremia, Serostatus and microRNA Expression in Multiple Sclerosis Patients Undergoing Immunosuppressive Treatment. J Clin Med 2022, 11. [Google Scholar] [CrossRef] [PubMed]
- Dolci, M.; Favero, C.; Bollati, V.; Campo, L.; Cattaneo, A.; Bonzini, M.; Villani, S.; Ticozzi, R.; Ferrante, P.; Delbue, S. Particulate Matter Exposure Increases JC Polyomavirus Replication in the Human Host. Environ Pollut 2018, 241, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Delbue, S.; Franciotta, D.; Giannella, S.; Dolci, M.; Signorini, L.; Ticozzi, R.; D’Alessandro, S.; Campisciano, G.; Comar, M.; Ferrante, P.; et al. Human Polyomaviruses in the Cerebrospinal Fluid of Neurological Patients. Microorganisms 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Boukoum, H.; Nahdi, I.; Sahtout, W.; Skiri, H.; Segondy, M.; Aouni, M. BK and JC Virus Infections in Healthy Patients Compared to Kidney Transplant Recipients in Tunisia. Microb Pathog 2016, 97, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Ye, D.; Zimmermann, T.; Demina, V.; Sotnikov, S.; Ried, C.L.; Rahn, H.; Stapf, M.; Untucht, C.; Rohe, M.; Terstappen, G.C.; et al. Trafficking of JC Virus-like Particles across the Blood-Brain Barrier. Nanoscale Adv 2021, 3, 2488–2500. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Abdulkhalek, S. Kidney Allograft Dysfunction Due to John Cunningham (JC) Virus Nephropathy. Cureus 2022, 14, e32021. [Google Scholar] [CrossRef]
- Hocker, B.; Tabatabai, J.; Schneble, L.; Oh, J.; Thiel, F.; Pape, L.; Rusai, K.; Topaloglu, R.; Kranz, B.; Klaus, G.; et al. JC Polyomavirus Replication and Associated Disease in Pediatric Renal Transplantation: An International CERTAIN Registry Study. Pediatr Nephrol 2018, 33, 2343–2352. [Google Scholar] [CrossRef]
- Seppala, H.M.; Helantera, I.T.; Laine, P.K.S.; Lautenschlager, I.T.; Paulin, L.G.; Jahnukainen, T.J.; Auvinen, P.O.V.; Auvinen, E. Archetype JC Polyomavirus (JCPyV) Prevails in a Rare Case of JCPyV Nephropathy and in Stable Renal Transplant Recipients With JCPyV Viruria. J Infect Dis 2017, 216, 981–989. [Google Scholar] [CrossRef]
- Janphram, C.; Worawichawong, S.; Disthabanchong, S.; Sumethkul, V.; Rotjanapan, P. Absence of JC Polyomavirus (JCPyV) Viremia in Early Post-Transplant JCPyV Nephropathy: A Case Report. Transpl Infect Dis 2017, 19. [Google Scholar] [CrossRef]
- Lautenschlager, I.; Jahnukainen, T.; Kardas, P.; Lohi, J.; Auvinen, E.; Mannonen, L.; Dumoulin, A.; Hirsch, H.H.; Jalanko, H. A Case of Primary JC Polyomavirus Infection-Associated Nephropathy. Am J Transplant 2014, 14, 2887–2892. [Google Scholar] [CrossRef]
- Munker, D.; Veit, T.; Schonermarck, U.; Arnold, P.; Leuschner, G.; Barton, J.; Mummler, C.; Briegel, I.; Mumm, J.N.; Zoller, M.; et al. Polyomavirus Exerts Detrimental Effects on Renal Function in Patients after Lung Transplantation. J Clin Virol 2021, 145, 105029. [Google Scholar] [CrossRef] [PubMed]
- Purighalla, R.; Shapiro, R.; McCauley, J.; Randhawa, P. BK Virus Infection in a Kidney Allograft Diagnosed by Needle Biopsy. Am J Kidney Dis 1995, 26, 671–673. [Google Scholar] [CrossRef] [PubMed]
- Honsova, E.; Lodererova, A.; Havrdova, T.; Voska, L.; Boucek, P. [Recurrent Polyomavirus Infections in Kidney Transplants (BK Virus Nephropathy)]. Cesk Patol 2004, 40, 25–28. [Google Scholar] [PubMed]
- Hirsch, H.H.; Knowles, W.; Dickenmann, M.; Passweg, J.; Klimkait, T.; Mihatsch, M.J.; Steiger, J. Prospective Study of Polyomavirus Type BK Replication and Nephropathy in Renal-Transplant Recipients. N Engl J Med 2002, 347, 488–496. [Google Scholar] [CrossRef]
- Ramos, E.; Drachenberg, C.B.; Wali, R.; Hirsch, H.H. The Decade of Polyomavirus BK-Associated Nephropathy: State of Affairs. Transplantation 2009, 87, 621–630. [Google Scholar] [CrossRef]
- Shen, C.L.; Wu, B.S.; Lien, T.J.; Yang, A.H.; Yang, C.Y. BK Polyomavirus Nephropathy in Kidney Transplantation: Balancing Rejection and Infection. Viruses 2021, 13. [Google Scholar] [CrossRef]
- Gras, J.; Le Flecher, A.; Dupont, A.; Verine, J.; Amara, A.; Delaugerre, C.; Molina, J.M.; Peraldi, M.N. Characteristics, Risk Factors and Outcome of BKV Nephropathy in Kidney Transplant Recipients: A Case-Control Study. BMC Infect Dis 2023, 23, 74. [Google Scholar] [CrossRef]
- Barth, H.; Solis, M.; Lepiller, Q.; Sueur, C.; Soulier, E.; Caillard, S.; Stoll-Keller, F.; Fafi-Kremer, S. 45 Years after the Discovery of Human Polyomaviruses BK and JC: Time to Speed up the Understanding of Associated Diseases and Treatment Approaches. Crit Rev Microbiol 2017, 43, 178–195. [Google Scholar] [CrossRef]
- Broekema, N.M.; Imperiale, M.J. Efficient Propagation of Archetype BK and JC Polyomaviruses. Virology 2012, 422, 235–241. [Google Scholar] [CrossRef]
- Knowles, W.A.; Pipkin, P.; Andrews, N.; Vyse, A.; Minor, P.; Brown, D.W.; Miller, E. Population-Based Study of Antibody to the Human Polyomaviruses BKV and JCV and the Simian Polyomavirus SV40. J Med Virol 2003, 71, 115–123. [Google Scholar] [CrossRef]
- Egli, A.; Infanti, L.; Dumoulin, A.; Buser, A.; Samaridis, J.; Stebler, C.; Gosert, R.; Hirsch, H.H. Prevalence of Polyomavirus BK and JC Infection and Replication in 400 Healthy Blood Donors. J Infect Dis 2009, 199, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, H.H. BK Virus: Opportunity Makes a Pathogen. Clin Infect Dis 2005, 41, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Siguier, M.; Sellier, P.; Bergmann, J.F. BK-Virus Infections: A Literature Review. Med Mal Infect 2012, 42, 181–187. [Google Scholar] [CrossRef]
- Alcendor, D.J. BK Polyomavirus Virus Glomerular Tropism: Implications for Virus Reactivation from Latency and Amplification during Immunosuppression. J Clin Med 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Lamarche, C.; Orio, J.; Collette, S.; Senecal, L.; Hebert, M.J.; Renoult, E.; Tibbles, L.A.; Delisle, J.S. BK Polyomavirus and the Transplanted Kidney: Immunopathology and Therapeutic Approaches. Transplantation 2016, 100, 2276–2287. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Chen, X.; Zhang, H.; Zhao, G.D.; Yang, H.; Qiu, J.; Meng, S.; Wu, P.; Tao, L.; Wang, Q.; et al. Single-Cell Transcriptome Identifies the Renal Cell Type Tropism of Human BK Polyomavirus. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.C.; Wang, J.; Dong, Y.; Mathews, D.V.; Albrecht, J.A.; Breeden, C.P.; Farris, A.B.; Lukacher, A.E.; Ford, M.L.; Newell, K.A.; et al. Alloimmunity But Not Viral Immunity Promotes Allograft Loss in a Mouse Model of Polyomavirus-Associated Allograft Injury. Transplant Direct 2017, 3, e161. [Google Scholar] [CrossRef] [PubMed]
- Maru, S.; Jin, G.; Desai, D.; Amin, S.; Shwetank, null; Lauver, M. D.; Lukacher, A.E. Inhibition of Retrograde Transport Limits Polyomavirus Infection In Vivo. mSphere 2017, 2, e00494–17. [Google Scholar] [CrossRef] [PubMed]
- Frost, E.L.; Kersh, A.E.; Evavold, B.D.; Lukacher, A.E. Cutting Edge: Resident Memory CD8 T Cells Express High-Affinity TCRs. J Immunol 2015, 195, 3520–3524. [Google Scholar] [CrossRef]
- Lauver, M.D.; Jin, G.; Ayers, K.N.; Carey, S.N.; Specht, C.S.; Abendroth, C.S.; Lukacher, A.E. T Cell Deficiency Precipitates Antibody Evasion and Emergence of Neurovirulent Polyomavirus. Elife 2022, 11. [Google Scholar] [CrossRef]
- Shwetank, N.; Abdelsamed, H.A.; Frost, E.L.; Schmitz, H.M.; Mockus, T.E.; Youngblood, B.A.; Lukacher, A.E. Maintenance of PD-1 on Brain-Resident Memory CD8 T Cells Is Antigen Independent. Immunol Cell Biol 2017, 95, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Bonek, R.; Guenter, W.; Jalowinski, R.; Karbicka, A.; Litwin, A.; Maciejowski, M.; Zajdel, R.; Zajdel, K.; Petit, V.; Rejdak, K. JC Virus Seroprevalence and JCVAb Index in Polish Multiple Sclerosis Patients Treated with Immunomodulating or Immunosuppressive Therapies. J Clin Med 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Bonek, R.; Guenter, W.; Jalowinski, R.; Karbicka, A.; Litwin, A.; Maciejowski, M.; Zajdel, R.; Petit, V.; Rejdak, K. JC Virus Seroprevalence and JCVAb Index in Polish Multiple Sclerosis Treatment-Naive Patients. J Clin Med 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Hanaei, S.; Sahraian, M.A.; Mohammadifar, M.; Ramagopalan, S.V.; Ghajarzadeh, M.; Ghajarzadeh, M. Prevalence of Anti-JC Virus Antibody Seropositivity in Patients with Multiple Sclerosis: A Systematic Review and Meta-Analysis. Intervirology 2019, 62, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Rochford, R.; Moreno, J.P.; Peake, M.L.; Villarreal, L.P. Enhancer Dependence of Polyomavirus Persistence in Mouse Kidneys. J Virol 1992, 66, 3287–3297. [Google Scholar] [CrossRef]
- Shwetank, N.; Frost, E.L.; Mockus, T.E.; Ren, H.M.; Toprak, M.; Lauver, M.D.; Netherby-Winslow, C.S.; Jin, G.; Cosby, J.M.; Evavold, B.D.; et al. PD-1 Dynamically Regulates Inflammation and Development of Brain-Resident Memory CD8 T Cells During Persistent Viral Encephalitis. Front Immunol 2019, 10, 783. [Google Scholar] [CrossRef]
- Qin, Q.; Shwetank, *!!! REPLACE !!!*; Frost, E.L.; Maru, S.; Lukacher, A.E. Type I Interferons Regulate the Magnitude and Functionality of Mouse Polyomavirus-Specific CD8 T Cells in a Virus Strain-Dependent Manner. J Virol 2016, 90, 5187–5199. [Google Scholar] [CrossRef]
- Qin, Q.; Lauver, M.; Maru, S.; Lin, E.; Lukacher, A.E. Reducing Persistent Polyomavirus Infection Increases Functionality of Virus-Specific Memory CD8 T Cells. Virology 2017, 502, 198–205. [Google Scholar] [CrossRef]
- Mockus, T.E.; Shwetank, N.; Lauver, M.D.; Ren, H.M.; Netherby, C.S.; Salameh, T.; Kawasawa, Y.I.; Yue, F.; Broach, J.R.; Lukacher, A.E. CD4 T Cells Control Development and Maintenance of Brain-Resident CD8 T Cells during Polyomavirus Infection. PLoS Pathog 2018, 14, e1007365. [Google Scholar] [CrossRef]
- Mockus, T.E.; Netherby-Winslow, C.S.; Atkins, H.M.; Lauver, M.D.; Jin, G.; Ren, H.M.; Lukacher, A.E. CD8 T Cells and STAT1 Signaling Are Essential Codeterminants in Protection from Polyomavirus Encephalopathy. J Virol 2020, 94, e02038–19. [Google Scholar] [CrossRef]
- Boldorini, R.; Caldarelli-Stefano, R.; Monga, G.; Zocchi, M.; Mediati, M.; Tosoni, A.; Ferrante, P. PCR Detection of JC Virus DNA in the Brain Tissue of a 9-Year-Old Child with Pleomorphic Xanthoastrocytoma. J Neurovirol 1998, 4, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Passerini, S.; Prezioso, C.; Prota, A.; Babini, G.; Bargiacchi, L.; Bartolini, D.; Moens, U.; Antonelli, M.; Pietropaolo, V. Detection of Human Neurotropic JCPyV DNA Sequence in Pediatric Anaplastic Xanthoastrocytoma. J Neurovirol 2023, 29, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Passerini, S.; Prezioso, C.; Prota, A.; Babini, G.; Coppola, L.; Lodi, A.; Epifani, A.C.; Sarmati, L.; Andreoni, M.; Moens, U.; et al. Detection Analysis and Study of Genomic Region Variability of JCPyV, BKPyV, MCPyV, HPyV6, HPyV7 and QPyV in the Urine and Plasma of HIV-1-Infected Patients. Viruses 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- Del Valle, L.; Khalili, K. Induction of Brain Tumors by the Archetype Strain of Human Neurotropic JCPyV in a Transgenic Mouse Model. Viruses 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Signorini, L.; Dolci, M.; Favi, E.; Colico, C.; Ferraresso, M.; Ticozzi, R.; Basile, G.; Ferrante, P.; Delbue, S. Viral Genomic Characterization and Replication Pattern of Human Polyomaviruses in Kidney Transplant Recipients. Viruses 2020, 12. [Google Scholar] [CrossRef] [PubMed]
- Scribano, S.; Guerrini, M.; Arvia, R.; Guasti, D.; Nardini, P.; Romagnoli, P.; Giannecchini, S. Archetype JC Polyomavirus DNA Associated with Extracellular Vesicles Circulates in Human Plasma Samples. J Clin Virol 2020, 128, 104435. [Google Scholar] [CrossRef]
- Moens, U.; Prezioso, C.; Pietropaolo, V. Genetic Diversity of the Noncoding Control Region of the Novel Human Polyomaviruses. Viruses 2020, 12. [Google Scholar] [CrossRef]
- Prezioso, C.; Bianchi, M.; Obregon, F.; Ciotti, M.; Sarmati, L.; Andreoni, M.; Palamara, A.T.; Pascarella, S.; Moens, U.; Pietropaolo, V. Structural Analysis of Merkel Cell Polyomavirus (MCPyV) Viral Capsid Protein 1 (VP1) in HIV-1 Infected Individuals. Int J Mol Sci 2020, 21. [Google Scholar] [CrossRef]
- Maginnis, M.S.; Stroh, L.J.; Gee, G.V.; O’Hara, B.A.; Derdowski, A.; Stehle, T.; Atwood, W.J. Progressive Multifocal Leukoencephalopathy-Associated Mutations in the JC Polyomavirus Capsid Disrupt Lactoseries Tetrasaccharide c Binding. mBio 2013, 4, e00247–13. [Google Scholar] [CrossRef]
- Hu, C.; Huang, Y.; Su, J.; Wang, M.; Zhou, Q.; Zhu, B. Detection and Analysis of Variants of JC Polyomavirus in Urine Samples from HIV-1-Infected Patients in China’s Zhejiang Province. J Int Med Res 2018, 46, 1024–1032. [Google Scholar] [CrossRef]
- Karalic, D.; Lazarevic, I.; Banko, A.; Cupic, M.; Jevtovic, D.; Jovanovic, T. Analysis of Variability of Urinary Excreted JC Virus Strains in Patients Infected with HIV and Healthy Donors. J Neurovirol 2018, 24, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Reid, C.E.; Li, H.; Sur, G.; Carmillo, P.; Bushnell, S.; Tizard, R.; McAuliffe, M.; Tonkin, C.; Simon, K.; Goelz, S.; et al. Sequencing and Analysis of JC Virus DNA from Natalizumab-Treated PML Patients. J Infect Dis 2011, 204, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Gorelik, L.; Reid, C.; Testa, M.; Brickelmaier, M.; Bossolasco, S.; Pazzi, A.; Bestetti, A.; Carmillo, P.; Wilson, E.; McAuliffe, M.; et al. Progressive Multifocal Leukoencephalopathy (PML) Development Is Associated with Mutations in JC Virus Capsid Protein VP1 That Change Its Receptor Specificity. J Infect Dis 2011, 204, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Bayliss, J.; Karasoulos, T.; Bowden, S.; Glogowski, I.; McLean, C.A. Immunosuppression Increases Latent Infection of Brain by JC Polyomavirus. Pathology 2011, 43, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Bayliss, J.; Harrison, E.; McLean, C.A. Progressive Multifocal Leukoencephalopathy Development Is Associated with Mutations in JC Virus Capsid Protein VP1 That Change the Receptor Specificity of the Virus. J Infect Dis 2011, 204, 1643–1644. [Google Scholar] [CrossRef]
- Bayliss, J.; Karasoulos, T.; McLean, C.A. Frequency and Large T (LT) Sequence of JC Polyomavirus DNA in Oligodendrocytes, Astrocytes and Granular Cells in Non-PML Brain. Brain Pathol 2012, 22, 329–336. [Google Scholar] [CrossRef]
- Bayliss, J.; Karasoulos, T.; McLean, C.A. Immunosuppression Increases JC Polyomavirus Large T Antigen DNA Load in the Brains of Patients without Progressive Multifocal Leukoencephalopathy. J Infect Dis 2013, 207, 133–136. [Google Scholar] [CrossRef]
- Brassesco, M.S.; Darrigo, L.G., Jr.; Valera, E.T.; Oliveira, R.S.; Yamamoto, Y.A.; de Castro Barros, M.V.; Tone, L.G. Giant-Cell Glioblastoma of Childhood Associated with HIV-1 and JC Virus Coinfection. Childs Nerv Syst 2013, 29, 1387–1390. [Google Scholar] [CrossRef]
- Okamoto, H.; Mineta, T.; Ueda, S.; Nakahara, Y.; Shiraishi, T.; Tamiya, T.; Tabuchi, K. Detection of JC Virus DNA Sequences in Brain Tumors in Pediatric Patients. J Neurosurg 2005, 102, 294–298. [Google Scholar] [CrossRef]
- Rollison, D.E.; Utaipat, U.; Ryschkewitsch, C.; Hou, J.; Goldthwaite, P.; Daniel, R.; Helzlsouer, K.J.; Burger, P.C.; Shah, K.V.; Major, E.O. Investigation of Human Brain Tumors for the Presence of Polyomavirus Genome Sequences by Two Independent Laboratories. Int J Cancer 2005, 113, 769–774. [Google Scholar] [CrossRef]
- Del Valle, L.; Enam, S.; Lara, C.; Ortiz-Hidalgo, C.; Katsetos, C.D.; Khalili, K. Detection of JC Polyomavirus DNA Sequences and Cellular Localization of T-Antigen and Agnoprotein in Oligodendrogliomas. Clin Cancer Res 2002, 8, 3332–3340. [Google Scholar] [PubMed]
- Del Valle, L.; Delbue, S.; Gordon, J.; Enam, S.; Croul, S.; Ferrante, P.; Khalili, K. Expression of JC Virus T-Antigen in a Patient with MS and Glioblastoma Multiforme. Neurology 2002, 58, 895–900. [Google Scholar] [CrossRef] [PubMed]
- Del Valle, L.; Gordon, J.; Enam, S.; Delbue, S.; Croul, S.; Abraham, S.; Radhakrishnan, S.; Assimakopoulou, M.; Katsetos, C.D.; Khalili, K. Expression of Human Neurotropic Polyomavirus JCV Late Gene Product Agnoprotein in Human Medulloblastoma. J Natl Cancer Inst 2002, 94, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Krynska, B.; Otte, J.; Franks, R.; Khalili, K.; Croul, S. Human Ubiquitous JCV(CY) T-Antigen Gene Induces Brain Tumors in Experimental Animals. Oncogene 1999, 18, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Bergsagel, D.J.; Finegold, M.J.; Butel, J.S.; Kupsky, W.J.; Garcea, R.L. DNA Sequences Similar to Those of Simian Virus 40 in Ependymomas and Choroid Plexus Tumors of Childhood. N Engl J Med 1992, 326, 988–993. [Google Scholar] [CrossRef]
- White, F.A., 3rd; Ishaq, M.; Stoner, G.L.; Frisque, R.J. JC Virus DNA Is Present in Many Human Brain Samples from Patients without Progressive Multifocal Leukoencephalopathy. J Virol 1992, 66, 5726–5734. [Google Scholar] [CrossRef]
- Perez-Liz, G.; Del Valle, L.; Gentilella, A.; Croul, S.; Khalili, K. Detection of JC Virus DNA Fragments but Not Proteins in Normal Brain Tissue. Ann Neurol 2008, 64, 379–387. [Google Scholar] [CrossRef]
- White, M.K.; Khalili, K. Pathogenesis of Progressive Multifocal Leukoencephalopathy--Revisited. J Infect Dis 2011, 203, 578–586. [Google Scholar] [CrossRef]
- Pietropaolo, V.; Prezioso, C.; Bagnato, F.; Antonelli, G. John Cunningham Virus: An Overview on Biology and Disease of the Etiological Agent of the Progressive Multifocal Leukoencephalopathy. New Microbiol 2018, 41, 179–186. [Google Scholar]
- O’Hara, B.A.; Gee, G.V.; Atwood, W.J.; Haley, S.A. Susceptibility of Primary Human Choroid Plexus Epithelial Cells and Meningeal Cells to Infection by JC Virus. J Virol 2018, 92, e00105–18. [Google Scholar] [CrossRef]
- Ampie, L.; McGavern, D.B. Immunological Defense of CNS Barriers against Infections. Immunity 2022, 55, 781–799. [Google Scholar] [CrossRef] [PubMed]
- Buckley, M.W.; McGavern, D.B. Immune Dynamics in the CNS and Its Barriers during Homeostasis and Disease. Immunol Rev 2022, 306, 58–75. [Google Scholar] [CrossRef] [PubMed]
- Koralnik, I.J.; Boden, D.; Mai, V.X.; Lord, C.I.; Letvin, N.L. JC Virus DNA Load in Patients with and without Progressive Multifocal Leukoencephalopathy. Neurology 1999, 52, 253–260. [Google Scholar] [CrossRef]
- Dubois, V.; Dutronc, H.; Lafon, M.E.; Poinsot, V.; Pellegrin, J.L.; Ragnaud, J.M.; Ferrer, A.M.; Fleury, H.J. Latency and Reactivation of JC Virus in Peripheral Blood of Human Immunodeficiency Virus Type 1-Infected Patients. J Clin Microbiol 1997, 35, 2288–2292. [Google Scholar] [CrossRef] [PubMed]
- Chapagain, M.L.; Verma, S.; Mercier, F.; Yanagihara, R.; Nerurkar, V.R. Polyomavirus JC Infects Human Brain Microvascular Endothelial Cells Independent of Serotonin Receptor 2A. Virology 2007, 364, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Haley, S.A.; O’Hara, B.A.; Nelson, C.D.; Brittingham, F.L.; Henriksen, K.J.; Stopa, E.G.; Atwood, W.J. Human Polyomavirus Receptor Distribution in Brain Parenchyma Contrasts with Receptor Distribution in Kidney and Choroid Plexus. Am J Pathol 2015, 185, 2246–2258. [Google Scholar] [CrossRef]
- Dorries, K.; Johnson, R.T.; Ter Meulen, V. Detection of Polyoma Virus DNA in PML-Brain Tissue by (in Situ) Hybridization. Journal of General Virology 1979, 42, 49–57. [Google Scholar] [CrossRef]
- RW, von E.; IW, S.; M, P.; B, Z.; M, D.; HV, V. New JC Virus Infection Patterns by in Situ Polymerase Chain Reaction in Brains of Acquired Immunodeficiency Syndrome Patients with Progressive Multifocal Leukoencephalopathy. Journal of NeuroVirology 2004, 10, 1–11. [CrossRef]
- Monaco, M.C.; Atwood, W.J.; Gravell, M.; Tornatore, C.S.; Major, E.O. JC Virus Infection of Hematopoietic Progenitor Cells, Primary B Lymphocytes, and Tonsillar Stromal Cells: Implications for Viral Latency. J Virol 1996, 70, 7004–7012. [Google Scholar] [CrossRef]
- Wei, G.; Liu, C.K.; Atwood, W.J. JC Virus Binds to Primary Human Glial Cells, Tonsillar Stromal Cells, and B-Lymphocytes, but Not to T Lymphocytes. J Neurovirol 2000, 6, 127–136. [Google Scholar] [CrossRef]
- Dubois, V.; Dutronc, H.; Lafon, M.E.; Poinsot, V.; Pellegrin, J.L.; Ragnaud, J.M.; Ferrer, A.M.; Fleury, H.J. Latency and Reactivation of JC Virus in Peripheral Blood of Human Immunodeficiency Virus Type 1-Infected Patients. J Clin Microbiol 1997, 35, 2288–2292. [Google Scholar] [CrossRef] [PubMed]
- Dubois, V.; Lafon, M.E.; Ragnaud, J.M.; Pellegrin, J.L.; Damasio, F.; Baudouin, C.; Michaud, V.; Fleury, H.J. Detection of JC Virus DNA in the Peripheral Blood Leukocytes of HIV-Infected Patients. AIDS 1996, 10, 353–358. [Google Scholar] [CrossRef]
- Dubois, V.; Moret, H.; Lafon, M.E.; Janvresse, C.B.; Dussaix, E.; Icart, J.; Karaterki, A.; Ruffault, A.; Taoufik, Y.; Vignoli, C.; et al. Prevalence of JC Virus Viraemia in HIV-Infected Patients with or without Neurological Disorders: A Prospective Study. J Neurovirol 1998, 4, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Frohman, E.M.; Monaco, M.C.; Remington, G.; Ryschkewitsch, C.; Jensen, P.N.; Johnson, K.; Perkins, M.; Liebner, J.; Greenberg, B.; Monson, N.; et al. JC Virus in CD34+ and CD19+ Cells in Patients with Multiple Sclerosis Treated with Natalizumab. JAMA Neurol 2014, 71, 596–602. [Google Scholar] [CrossRef]
- Tornatore, C.; Berger, J.R.; Houff, S.A.; Curfman, B.; Meyers, K.; Winfield, D.; Major, E.O. Detection of JC Virus DNA in Peripheral Lymphocytes from Patients with and without Progressive Multifocal Leukoencephalopathy. Ann Neurol 1992, 31, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Major, E.O.; Amemiya, K.; Elder, G.; Houff, S.A. Glial Cells of the Human Developing Brain and B Cells of the Immune System Share a Common DNA Binding Factor for Recognition of the Regulatory Sequences of the Human Polyomavirus, JCV. J Neurosci Res 1990, 27, 461–471. [Google Scholar] [CrossRef]
- Atwood, W.J.; Amemiya, K.; Traub, R.; Harms, J.; Major, E.O. Interaction of the Human Polyomavirus, JCV, with Human B-Lymphocytes. Virology 1992, 190, 716–723. [Google Scholar] [CrossRef]
- Chapagain, M.L.; Nerurkar, V.R. Human Polyomavirus JC (JCV) Infection of Human B Lymphocytes: A Possible Mechanism for JCV Transmigration across the Blood-Brain Barrier. J Infect Dis 2010, 202, 184–191. [Google Scholar] [CrossRef]
- Reff, M.E.; Carner, K.; Chambers, K.S.; Chinn, P.C.; Leonard, J.E.; Raab, R.; Newman, R.A.; Hanna, N.; Anderson, D.R. Depletion of B Cells in Vivo by a Chimeric Mouse Human Monoclonal Antibody to CD20. Blood 1994, 83, 435–445. [Google Scholar] [CrossRef]
- Hanif, N.; Anwer, F. StatPearls. In; Treasure Island: StatPearls Publishing: Florida, 2023.
- Administration, U.S.F. and D. List of Licensed Biological Products with Reference Product Exclusivity and Biosimilarity or Interchangeability Evaluations (Purple Book); FDA, 2020.
- Anolik, J.H.; Friedberg, J.W.; Zheng, B.; Barnard, J.; Owen, T.; Cushing, E.; Kelly, J.; Milner, E.C.; Fisher, R.I.; Sanz, I. B Cell Reconstitution after Rituximab Treatment of Lymphoma Recapitulates B Cell Ontogeny. Clin Immunol 2007, 122, 139–145. [Google Scholar] [CrossRef]
- Roll, P.; Palanichamy, A.; Kneitz, C.; Dorner, T.; Tony, H.P. Regeneration of B Cell Subsets after Transient B Cell Depletion Using Anti-CD20 Antibodies in Rheumatoid Arthritis. Arthritis Rheum 2006, 54, 2377–2386. [Google Scholar] [CrossRef] [PubMed]
- Haley, S.A.; O’Hara, B.A.; Nelson, C.D.S.; Brittingham, F.L.P.; Henriksen, K.J.; Stopa, E.G.; Atwood, W.J. Human Polyomavirus Receptor Distribution in Brain Parenchyma Contrasts with Receptor Distribution in Kidney and Choroid Plexus. Am J Pathol 2015, 185, 2246–2258. [Google Scholar] [CrossRef] [PubMed]
- Agnihotri, S.P.; Wuthrich, C.; Dang, X.; Nauen, D.; Karimi, R.; Viscidi, R.; Bord, E.; Batson, S.; Troncoso, J.; Koralnik, I.J. A Fatal Case of JC Virus Meningitis Presenting with Hydrocephalus in a Human Immunodeficiency Virus-Seronegative Patient. Ann Neurol 2014, 76, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Corbridge, S.M.; Rice, R.C.; Bean, L.A.; Wüthrich, C.; Dang, X.; Nicholson, D.A.; Koralnik, I.J. JC Virus Infection of Meningeal and Choroid Plexus Cells in Patients with Progressive Multifocal Leukoencephalopathy. J Neurovirol 2019, 25, 520–524. [Google Scholar] [CrossRef]
- Morris-Love, J.; Gee, G.V.; O’Hara, B.A.; Assetta, B.; Atkinson, A.L.; Dugan, A.S.; Haley, S.A.; Atwood, W.J. JC Polyomavirus Uses Extracellular Vesicles To Infect Target Cells. mBio 2019, 10. [Google Scholar] [CrossRef]
- Giannecchini, S. Evidence of the Mechanism by Which Polyomaviruses Exploit the Extracellular Vesicle Delivery System during Infection. Viruses 2020, 12. [Google Scholar] [CrossRef]
- Berger, J.R.; Aksamit, A.J.; Clifford, D.B.; Davis, L.; Koralnik, I.J.; Sejvar, J.J.; Bartt, R.; Major, E.O.; Nath, A. PML Diagnostic Criteria: Consensus Statement from the AAN Neuroinfectious Disease Section. Neurology 2013, 80, 1430–1438. [Google Scholar] [CrossRef]
- Tornatore, C.; Berger, J.R.; Houff, S.A.; Curfman, B.; Meyers, K.; Winfield, D.; Major, E.O. Detection of JC Virus DNA in Peripheral Lymphocytes from Patients with and without Progressive Multifocal Leukoencephalopathy. Ann Neurol 1992, 31, 454–462. [Google Scholar] [CrossRef]
- Viallard, J.F.; Ellie, E.; Lazaro, E.; Lafon, M.E.; Pellegrin, J.L. JC Virus Meningitis in a Patient with Systemic Lupus Erythematosus. Lupus 2005, 14, 964–966. [Google Scholar] [CrossRef]
- Chiu, C.Y.; Amenta, E.; Chavez, V.; Fukuta, Y.; Hasbun, R. Undetectable JC Virus CSF PCR in Patients with JC Virus-Induced Progressive Multifocal Leukoencephalopathy. J Neurovirol 2023, 29, 94–99. [Google Scholar] [CrossRef]
- Mornese Pinna, S.; Trunfio, M.; Imperiale, D.; Calcagno, A. JC Virus DNA in Cerebrospinal Fluid: Insight into Clinical Significance. Diagn Microbiol Infect Dis 2020, 97, 115017. [Google Scholar] [CrossRef] [PubMed]
- Swinnen, B.; Saegeman, V.; Beuselinck, K.; Wouters, A.; Cypers, G.; Meyfroidt, G.; Schrooten, M. Predictive Value of JC Virus PCR in Cerebrospinal Fluid in the Diagnosis of PML. Diagn Microbiol Infect Dis 2019, 95, 114859. [Google Scholar] [CrossRef] [PubMed]
- Mockus, T.E.; Netherby-Winslow, C.S.; Atkins, H.M.; Lauver, M.D.; Jin, G.; Ren, H.M.; Lukacher, A.E. CD8 T Cells and STAT1 Signaling Are Essential Codeterminants in Protection from Polyomavirus Encephalopathy. J Virol 2020, 94, e02038–19. [Google Scholar] [CrossRef] [PubMed]
- Chalkias, S.; Dang, X.; Bord, E.; Stein, M.C.; Kinkel, R.P.; Sloane, J.A.; Donnelly, M.; Ionete, C.; Houtchens, M.K.; Buckle, G.J.; et al. JC Virus Reactivation during Prolonged Natalizumab Monotherapy for Multiple Sclerosis. Ann Neurol 2014, 75, 925–934. [Google Scholar] [CrossRef] [PubMed]
- Cross, A.H.; Stark, J.L.; Lauber, J.; Ramsbottom, M.J.; Lyons, J.A. Rituximab Reduces B Cells and T Cells in Cerebrospinal Fluid of Multiple Sclerosis Patients. J Neuroimmunol 2006, 180, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Casselli, T.; Divan, A.; Vomhof-DeKrey, E.E.; Tourand, Y.; Pecoraro, H.L.; Brissette, C.A. A Murine Model of Lyme Disease Demonstrates That Borrelia Burgdorferi Colonizes the Dura Mater and Induces Inflammation in the Central Nervous System. PLoS Pathog 2021, 17, e1009256. [Google Scholar] [CrossRef] [PubMed]
- Cugurra, A.; Mamuladze, T.; Rustenhoven, J.; Dykstra, T.; Beroshvili, G.; Greenberg, Z.J.; Baker, W.; Papadopoulos, Z.; Drieu, A.; Blackburn, S.; et al. Skull and Vertebral Bone Marrow Are Myeloid Cell Reservoirs for the Meninges and CNS Parenchyma. Science 2021, 373. [Google Scholar] [CrossRef]
- Brioschi, S.; Wang, W.L.; Peng, V.; Wang, M.; Shchukina, I.; Greenberg, Z.J.; Bando, J.K.; Jaeger, N.; Czepielewski, R.S.; Swain, A.; et al. Heterogeneity of Meningeal B Cells Reveals a Lymphopoietic Niche at the CNS Borders. Science 2021, 373. [Google Scholar] [CrossRef]
- Dang, L.; Dang, X.; Koralnik, I.J.; Todd, P.K. JC Polyomavirus Granule Cell Neuronopathy in a Patient Treated with Rituximab. JAMA Neurol 2014, 71, 487–489. [Google Scholar] [CrossRef]
- Granot, R.; Lawrence, R.; Barnett, M.; Masters, L.; Rodriguez, M.; Theocharous, C.; Pamphlett, R.; Hersch, M. What Lies beneath the Tent? JC-Virus Cerebellar Granule Cell Neuronopathy Complicating Sarcoidosis. J Clin Neurosci 2009, 16, 1091–1092. [Google Scholar] [CrossRef]
- Hecht, J.H.; Glenn, O.A.; Wara, D.W.; Wu, Y.W. JC Virus Granule Cell Neuronopathy in a Child with CD40 Ligand Deficiency. Pediatr Neurol 2007, 36, 186–189. [Google Scholar] [CrossRef] [PubMed]
- Koralnik, I.J.; Wüthrich, C.; Dang, X.; Rottnek, M.; Gurtman, A.; Simpson, D.; Morgello, S. JC Virus Granule Cell Neuronopathy: A Novel Clinical Syndrome Distinct from Progressive Multifocal Leukoencephalopathy. Ann Neurol 2005, 57, 576–580. [Google Scholar] [CrossRef] [PubMed]
- Reoma, L.B.; Trindade, C.J.; Monaco, M.C.; Solis, J.; Montojo, M.G.; Vu, P.; Johnson, K.; Beck, E.; Nair, G.; Khan, O.I.; et al. Fatal Encephalopathy with Wild-Type JC Virus and Ruxolitinib Therapy. Ann Neurol 2019, 86, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Wüthrich, C.; Dang, X.; Westmoreland, S.; McKay, J.; Maheshwari, A.; Anderson, M.P.; Ropper, A.H.; Viscidi, R.P.; Koralnik, I.J. Fulminant JC Virus Encephalopathy with Productive Infection of Cortical Pyramidal Neurons. Ann Neurol 2009, 65, 742–748. [Google Scholar] [CrossRef]
- ZURHEIN, G.; CHOU, S.M. PARTICLES RESEMBLING PAPOVA VIRUSES IN HUMAN CEREBRAL DEMYELINATING DISEASE. Science 1965, 148, 1477–1479. [Google Scholar] [CrossRef]
- ÅSTRÖM, K.-E.; MANCALL, E.L.; RICHARDSON, E.P., JR. PROGRESSIVE MULTIFOCAL LEUKO-ENCEPHALOPATHY: A HITHERTO UNRECOGNIZED COMPLICATION O CHRONIC LYMPHATIC LEUKÆMIA AND HODGKIN’S DISEASE1. Brain 1958, 81, 93–111. [Google Scholar] [CrossRef] [PubMed]
- Dörries, K.; Johnson, R.T.; ter Meulen, V. Detection of Polyoma Virus DNA in PML-Brain Tissue by (in Situ) Hybridization. J Gen Virol 1979, 42, 49–57. [Google Scholar] [CrossRef]
- Ironside, J.W.; Lewis, F.A.; Blythe, D.; Wakefield, E.A. The Identification of Cells Containing JC Papovavirus DNA in Progressive Multifocal Leukoencephalopathy by Combined in Situ Hybridization and Immunocytochemistry. J Pathol 1989, 157, 291–297. [Google Scholar] [CrossRef]
- McCance, D.J. The Types of Mouse Brain Cells Susceptible to Polyoma Virus Infection in Vitro. J Gen Virol 1984, 65 ( Pt 1) Pt 1, 221–226. [Google Scholar] [CrossRef]
- McCance, D.J.; Sebesteny, A.; Griffin, B.E.; Balkwill, F.; Tilly, R.; Gregson, N.A. A Paralytic Disease in Nude Mice Associated with Polyoma Virus Infection. J Gen Virol 1983, 64 (Pt 1) Pt 1, 57–67. [Google Scholar] [CrossRef]
- Sebesteny, A.; Tilly, R.; Balkwill, F.; Trevan, D. Demyelination and Wasting Associated with Polyomavirus Infection in Nude (Nu/Nu) Mice. Lab Anim 1980, 14, 337–345. [Google Scholar] [CrossRef]
- Nakamichi, K.; Takayama-Ito, M.; Nukuzuma, S.; Kurane, I.; Saijo, M. Long-Term Infection of Adult Mice with Murine Polyomavirus Following Stereotaxic Inoculation into the Brain. Microbiol Immunol 2010, 54, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Sim, F.J.; McClain, C.R.; Schanz, S.J.; Protack, T.L.; Windrem, M.S.; Goldman, S.A. CD140a Identifies a Population of Highly Myelinogenic, Migration-Competent and Efficiently Engrafting Human Oligodendrocyte Progenitor Cells. Nat Biotechnol 2011, 29, 934–941. [Google Scholar] [CrossRef] [PubMed]
- Windrem, M.S.; Schanz, S.J.; Guo, M.; Tian, G.F.; Washco, V.; Stanwood, N.; Rasband, M.; Roy, N.S.; Nedergaard, M.; Havton, L.A.; et al. Neonatal Chimerization with Human Glial Progenitor Cells Can Both Remyelinate and Rescue the Otherwise Lethally Hypomyelinated Shiverer Mouse. Cell Stem Cell 2008, 2, 553–565. [Google Scholar] [CrossRef] [PubMed]
- Kondo, Y.; Windrem, M.S.; Zou, L.; Chandler-Militello, D.; Schanz, S.J.; Auvergne, R.M.; Betstadt, S.J.; Harrington, A.R.; Johnson, M.; Kazarov, A.; et al. Human Glial Chimeric Mice Reveal Astrocytic Dependence of JC Virus Infection. J Clin Invest 2014, 124, 5323–5336. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.S.; Allen, N.J.; Eroglu, C. Astrocytes Control Synapse Formation, Function, and Elimination. Cold Spring Harb Perspect Biol 2015, 7, a020370. [Google Scholar] [CrossRef]
- Molina-Gonzalez, I.; Miron, V.E. Astrocytes in Myelination and Remyelination. Neurosci Lett 2019, 713, 134532. [Google Scholar] [CrossRef]
- Han, R.T.; Kim, R.D.; Molofsky, A.V.; Liddelow, S.A. Astrocyte-Immune Cell Interactions in Physiology and Pathology. Immunity 2021, 54, 211–224. [Google Scholar] [CrossRef]
- Lawrence, J.M.; Schardien, K.; Wigdahl, B.; Nonnemacher, M.R. Roles of Neuropathology-Associated Reactive Astrocytes: A Systematic Review. Acta Neuropathol Commun 2023, 11, 42. [Google Scholar] [CrossRef]
- Haley, S.A.; O’Hara, B.A.; Nelson, C.D.; Brittingham, F.L.; Henriksen, K.J.; Stopa, E.G.; Atwood, W.J. Human Polyomavirus Receptor Distribution in Brain Parenchyma Contrasts with Receptor Distribution in Kidney and Choroid Plexus. Am J Pathol 2015, 185, 2246–2258. [Google Scholar] [CrossRef]
- Wilczek, M.P.; DuShane, J.K.; Armstrong, F.J.; Maginnis, M.S. JC Polyomavirus Infection Reveals Delayed Progression of the Infectious Cycle in Normal Human Astrocytes. J Virol 2020, 94. [Google Scholar] [CrossRef] [PubMed]
- Mázló, M.; Tariska, I. Are Astrocytes Infected in Progressive Multifocal Leukoencephalopathy (PML)? Acta Neuropathol 1982, 56, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Dyson, N.; Bernards, R.; Friend, S.H.; Gooding, L.R.; Hassell, J.A.; Major, E.O.; Pipas, J.M.; Vandyke, T.; Harlow, E. Large T Antigens of Many Polyomaviruses Are Able to Form Complexes with the Retinoblastoma Protein. J Virol 1990, 64, 1353–1356. [Google Scholar] [CrossRef] [PubMed]
- Kao, C.; Huang, J.; Wu, S.Q.; Hauser, P.; Reznikoff, C.A. Role of SV40 T Antigen Binding to pRB and P53 in Multistep Transformation in Vitro of Human Uroepithelial Cells. Carcinogenesis 1993, 14, 2297–2302. [Google Scholar] [CrossRef]
- Reich, N.C.; Levine, A.J. Specific Interaction of the SV40 T Antigen-Cellular P53 Protein Complex with SV40 DNA. Virology 1982, 117, 286–290. [Google Scholar] [CrossRef]
- Tan, T.H.; Wallis, J.; Levine, A.J. Identification of the P53 Protein Domain Involved in Formation of the Simian Virus 40 Large T-Antigen-P53 Protein Complex. J Virol 1986, 59, 574–583. [Google Scholar] [CrossRef]
- Ferenczy, M.W.; Johnson, K.R.; Marshall, L.J.; Monaco, M.C.; Major, E.O. Differentiation of Human Fetal Multipotential Neural Progenitor Cells to Astrocytes Reveals Susceptibility Factors for JC Virus. J Virol 2013, 87, 6221–6231. [Google Scholar] [CrossRef]
- Tretiakova, A.; Krynska, B.; Gordon, J.; Khalili, K. Human Neurotropic JC Virus Early Protein Deregulates Glial Cell Cycle Pathway and Impairs Cell Differentiation. J Neurosci Res 1999, 55, 588–599. [Google Scholar] [CrossRef]
- Sturrock, R.R. Myelination of the Mouse Corpus Callosum. Neuropathol Appl Neurobiol 1980, 6, 415–420. [Google Scholar] [CrossRef]
- Kirby, L.; Jin, J.; Cardona, J.G.; Smith, M.D.; Martin, K.A.; Wang, J.; Strasburger, H.; Herbst, L.; Alexis, M.; Karnell, J.; et al. Oligodendrocyte Precursor Cells Present Antigen and Are Cytotoxic Targets in Inflammatory Demyelination. Nat Commun 2019, 10, 3887. [Google Scholar] [CrossRef]
- Karimy, J.K.; Zhang, J.; Kurland, D.B.; Theriault, B.C.; Duran, D.; Stokum, J.A.; Furey, C.G.; Zhou, X.; Mansuri, M.S.; Montejo, J.; et al. Inflammation-Dependent Cerebrospinal Fluid Hypersecretion by the Choroid Plexus Epithelium in Posthemorrhagic Hydrocephalus. Nat Med 2017, 23, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Robert, S.M.; Reeves, B.C.; Kiziltug, E.; Duy, P.Q.; Karimy, J.K.; Mansuri, M.S.; Marlier, A.; Allington, G.; Greenberg, A.B.W.; DeSpenza, T.; et al. The Choroid Plexus Links Innate Immunity to CSF Dysregulation in Hydrocephalus. Cell 2023, 186, 764–785. [Google Scholar] [CrossRef] [PubMed]
- Thompson, D.; Brissette, C.A.; Watt, J.A. The Choroid Plexus and Its Role in the Pathogenesis of Neurological Infections. Fluids Barriers CNS 2022, 19, 75. [Google Scholar] [CrossRef]
- Chakravarty, S.; Herkenham, M. Toll-like Receptor 4 on Nonhematopoietic Cells Sustains CNS Inflammation during Endotoxemia, Independent of Systemic Cytokines. J Neurosci 2005, 25, 1788–1796. [Google Scholar] [CrossRef]
- Laflamme, N.; Rivest, S. Toll-like Receptor 4: The Missing Link of the Cerebral Innate Immune Response Triggered by Circulating Gram-Negative Bacterial Cell Wall Components. FASEB J 2001, 15, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Rivest, S. Molecular Insights on the Cerebral Innate Immune System. Brain Behav Immun 2003, 17, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Yamagata, K.; Krukoff, T.L. Differential Expression of the CD14/TLR4 Complex and Inflammatory Signaling Molecules Following i.c.v. Administration of LPS. Brain Res 2006, 1095, 85–95. [Google Scholar] [CrossRef]
- Quintana, E.; Fernández, A.; Velasco, P.; de Andrés, B.; Liste, I.; Sancho, D.; Gaspar, M.L.; Cano, E. DNGR-1(+) Dendritic Cells Are Located in Meningeal Membrane and Choroid Plexus of the Noninjured Brain. Glia 2015, 63, 2231–2248. [Google Scholar] [CrossRef]
- Wang, A.Z.; Bowman-Kirigin, J.A.; Desai, R.; Kang, L.I.; Patel, P.R.; Patel, B.; Khan, S.M.; Bender, D.; Marlin, M.C.; Liu, J.; et al. Single-Cell Profiling of Human Dura and Meningioma Reveals Cellular Meningeal Landscape and Insights into Meningioma Immune Response. Genome Med 2022, 14, 49. [Google Scholar] [CrossRef]
- Thompson, D.; Sorenson, J.; Greenmyer, J.; Brissette, C.A.; Watt, J.A. The Lyme Disease Bacterium, Borrelia Burgdorferi, Stimulates an Inflammatory Response in Human Choroid Plexus Epithelial Cells. PLoS One 2020, 15, e0234993. [Google Scholar] [CrossRef]
- Figueiredo, C.A.; Steffen, J.; Morton, L.; Arumugam, S.; Liesenfeld, O.; Deli, M.A.; Kröger, A.; Schüler, T.; Dunay, I.R. Immune Response and Pathogen Invasion at the Choroid Plexus in the Onset of Cerebral Toxoplasmosis. J Neuroinflammation 2022, 19, 17. [Google Scholar] [CrossRef] [PubMed]
- Banizs, B.; Pike, M.M.; Millican, C.L.; Ferguson, W.B.; Komlosi, P.; Sheetz, J.; Bell, P.D.; Schwiebert, E.M.; Yoder, B.K. Dysfunctional Cilia Lead to Altered Ependyma and Choroid Plexus Function, and Result in the Formation of Hydrocephalus. Development 2005, 132, 5329–5339. [Google Scholar] [CrossRef] [PubMed]
- Medina-Bolívar, C.; González-Arnay, E.; Talos, F.; González-Gómez, M.; Moll, U.M.; Meyer, G. Cortical Hypoplasia and Ventriculomegaly of P73-Deficient Mice: Developmental and Adult Analysis. J Comp Neurol 2014, 522, 2663–2679. [Google Scholar] [CrossRef] [PubMed]
- Tissir, F.; Qu, Y.; Montcouquiol, M.; Zhou, L.; Komatsu, K.; Shi, D.; Fujimori, T.; Labeau, J.; Tyteca, D.; Courtoy, P.; et al. Lack of Cadherins Celsr2 and Celsr3 Impairs Ependymal Ciliogenesis, Leading to Fatal Hydrocephalus. Nat Neurosci 2010, 13, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Wodarczyk, C.; Rowe, I.; Chiaravalli, M.; Pema, M.; Qian, F.; Boletta, A. A Novel Mouse Model Reveals That Polycystin-1 Deficiency in Ependyma and Choroid Plexus Results in Dysfunctional Cilia and Hydrocephalus. PLoS One 2009, 4, e7137. [Google Scholar] [CrossRef]
- Xia, Y.; Yamagata, K.; Krukoff, T.L. Differential Expression of the CD14/TLR4 Complex and Inflammatory Signaling Molecules Following i.c.v. Administration of LPS. Brain Res 2006, 1095, 85–95. [Google Scholar] [CrossRef]
- Rasmussen, M.K.; Mestre, H.; Nedergaard, M. The Glymphatic Pathway in Neurological Disorders. Lancet Neurol 2018, 17, 1016–1024. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.J.; Hua, J.; Cao, D.; Ho, M.L. Neurofluids and the Glymphatic System: Anatomy, Physiology, and Imaging. Br J Radiol 2023, 20230016. [Google Scholar] [CrossRef] [PubMed]
- Bystritsky, R.J.; Chow, F.C. Infectious Meningitis and Encephalitis. Neurol Clin 2022, 40, 77–91. [Google Scholar] [CrossRef]
- Gundamraj, V.; Hasbun, R. Viral Meningitis and Encephalitis: An Update. Curr Opin Infect Dis 2023, 36, 177–185. [Google Scholar] [CrossRef]
- Ballesta, B.; González, H.; Martín, V.; Ballesta, J.J. Fatal Ruxolitinib-Related JC Virus Meningitis. J Neurovirol 2017, 23, 783–785. [Google Scholar] [CrossRef] [PubMed]
- Consalez, G.G.; Goldowitz, D.; Casoni, F.; Hawkes, R. Origins, Development, and Compartmentation of the Granule Cells of the Cerebellum. Front Neural Circuits 2020, 14, 611841. [Google Scholar] [CrossRef] [PubMed]
- Du Pasquier, R.A.; Corey, S.; Margolin, D.H.; Williams, K.; Pfister, L.A.; De Girolami, U.; Mac Key, J.J.; Wüthrich, C.; Joseph, J.T.; Koralnik, I.J. Productive Infection of Cerebellar Granule Cell Neurons by JC Virus in an HIV+ Individual. Neurology 2003, 61, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Tagliati, M.; Simpson, D.; Morgello, S.; Clifford, D.; Schwartz, R.L.; Berger, J.R. Cerebellar Degeneration Associated with Human Immunodeficiency Virus Infection. Neurology 1998, 50, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Dang, X.; Koralnik, I.J. A Granule Cell Neuron-Associated JC Virus Variant Has a Unique Deletion in the VP1 Gene. J Gen Virol 2006, 87, 2533–2537. [Google Scholar] [CrossRef] [PubMed]
- Dang, X.; Vidal, J.E.; Penalva de Oliveira, A.C.; Simpson, D.M.; Morgello, S.; Hecht, J.H.; Ngo, L.H.; Koralnik, I.J. JC Virus Granule Cell Neuronopathy Is Associated with VP1 C Terminus Mutants. J Gen Virol 2012, 93, 175–183. [Google Scholar] [CrossRef]
- Janovec, V.; Ryabchenko, B.; Skarkova, A.; Pokorna, K.; Rosel, D.; Brabek, J.; Weber, J.; Forstova, J.; Hirsch, I.; Huerfano, S. TLR4-Mediated Recognition of Mouse Polyomavirus Promotes Cancer-Associated Fibroblast-Like Phenotype and Cell Invasiveness. Cancers (Basel) 2021, 13. [Google Scholar] [CrossRef]
- Velupillai, P.; Sung, C.K.; Andrews, E.; Moran, J.; Beier, D.; Kagan, J.; Benjamin, T. Polymorphisms in Toll-like Receptor 4 Underlie Susceptibility to Tumor Induction by the Mouse Polyomavirus. J Virol 2012, 86, 11541–11547. [Google Scholar] [CrossRef]
- Kanse, S.; Khandelwal, M.; Pandey, R.K.; Khokhar, M.; Desai, N.; Kumbhar, B.V. Designing a Multi-Epitope Subunit Vaccine against VP1 Major Coat Protein of JC Polyomavirus. Vaccines (Basel) 2023, 11. [Google Scholar] [CrossRef]
- Shahzad, N.; Shuda, M.; Gheit, T.; Kwun, H.J.; Cornet, I.; Saidj, D.; Zannetti, C.; Hasan, U.; Chang, Y.; Moore, P.S.; et al. The T Antigen Locus of Merkel Cell Polyomavirus Downregulates Human Toll-like Receptor 9 Expression. J Virol 2013, 87, 13009–13019. [Google Scholar] [CrossRef]
- Yaghobi, R.; Khodavandi, A.; Alizadeh, F. Association of TLR4 Polymorphisms and Polyomavirus BK Infection in Liver Transplant Patients. Trop Biomed 2017, 34, 886–894. [Google Scholar] [PubMed]
- Redondo, N.; Rodriguez-Goncer, I.; Parra, P.; Lopez-Medrano, F.; Gonzalez, E.; Hernandez, A.; Trujillo, H.; Ruiz-Merlo, T.; San Juan, R.; Folgueira, M.D.; et al. Genetic Polymorphisms in TLR3, IL10 and CD209 Influence the Risk of BK Polyomavirus Infection after Kidney Transplantation. Sci Rep 2022, 12, 11338. [Google Scholar] [CrossRef] [PubMed]
- Ryabchenko, B.; Soldatova, I.; Sroller, V.; Forstova, J.; Huerfano, S. Immune Sensing of Mouse Polyomavirus DNA by P204 and cGAS DNA Sensors. FEBS J 2021, 288, 5964–5985. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.; Dvorkin, S.; Chiang, J.J.; Potter, R.B.; Gack, M.U. The Small t Antigen of JC Virus Antagonizes RIG-I-Mediated Innate Immunity by Inhibiting TRIM25’s RNA Binding Ability. mBio 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- An, P.; Saenz Robles, M.T.; Duray, A.M.; Cantalupo, P.G.; Pipas, J.M. Human Polyomavirus BKV Infection of Endothelial Cells Results in Interferon Pathway Induction and Persistence. PLoS Pathog 2019, 15, e1007505. [Google Scholar] [CrossRef] [PubMed]
- Heutinck, K.M.; Rowshani, A.T.; Kassies, J.; Claessen, N.; van Donselaar-van der Pant, K.A.; Bemelman, F.J.; Eldering, E.; van Lier, R.A.; Florquin, S.; Ten Berge, I.J.; et al. Viral Double-Stranded RNA Sensors Induce Antiviral, pro-Inflammatory, and pro-Apoptotic Responses in Human Renal Tubular Epithelial Cells. Kidney Int 2012, 82, 664–675. [Google Scholar] [CrossRef] [PubMed]
- Huerfano, S.; Ryabchenko, B.; Forstova, J. Nucleofection of Expression Vectors Induces a Robust Interferon Response and Inhibition of Cell Proliferation. DNA Cell Biol 2013, 32, 467–479. [Google Scholar] [CrossRef]
- Jiang, Z.; Mak, T.W.; Sen, G.; Li, X. Toll-like Receptor 3-Mediated Activation of NF-kappaB and IRF3 Diverges at Toll-IL-1 Receptor Domain-Containing Adapter Inducing IFN-Beta. Proc Natl Acad Sci U S A 2004, 101, 3533–3538. [Google Scholar] [CrossRef]
- Chen, H.C.; Zhan, X.; Tran, K.K.; Shen, H. Selectively Targeting the Toll-like Receptor 9 (TLR9)--IRF 7 Signaling Pathway by Polymer Blend Particles. Biomaterials 2013, 34, 6464–6472. [Google Scholar] [CrossRef]
- May, D.; Bellizzi, A.; Kassa, W.; Cipriaso, J.M.; Caocci, M.; Wollebo, H.S. IFNalpha and Beta Mediated JCPyV Suppression through C/EBPbeta-LIP Isoform. Viruses 2021, 13. [Google Scholar] [CrossRef]
- Zerbe, C.S.; Marciano, B.E.; Katial, R.K.; Santos, C.B.; Adamo, N.; Hsu, A.P.; Hanks, M.E.; Darnell, D.N.; Quezado, M.M.; Frein, C.; et al. Progressive Multifocal Leukoencephalopathy in Primary Immune Deficiencies: Stat1 Gain of Function and Review of the Literature. Clin Infect Dis 2016, 62, 986–994. [Google Scholar] [CrossRef] [PubMed]
- Assetta, B.; De Cecco, M.; O’Hara, B.; Atwood, W.J. JC Polyomavirus Infection of Primary Human Renal Epithelial Cells Is Controlled by a Type I IFN-Induced Response. mBio 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.J.; Lin, E.; Pack, C.D.; Frost, E.L.; Hadley, A.; Swimm, A.I.; Wang, J.; Dong, Y.; Breeden, C.P.; Kalman, D.; et al. Gamma Interferon Controls Mouse Polyomavirus Infection in Vivo. J Virol 2011, 85, 10126–10134. [Google Scholar] [CrossRef] [PubMed]
- Peng, G.; Lei, K.J.; Jin, W.; Greenwell-Wild, T.; Wahl, S.M. Induction of APOBEC3 Family Proteins, a Defensive Maneuver Underlying Interferon-Induced Anti–HIV-1 Activity. J Exp Med 2006, 203, 41–46. [Google Scholar] [CrossRef]
- Okeoma, C.M.; Low, A.; Bailis, W.; Fan, H.Y.; Peterlin, B.M.; Ross, S.R. Induction of APOBEC3 in Vivo Causes Increased Restriction of Retrovirus Infection. J Virol 2009, 83, 3486–3495. [Google Scholar] [CrossRef]
- Sadeghpour, S.; Khodaee, S.; Rahnama, M.; Rahimi, H.; Ebrahimi, D. Human APOBEC3 Variations and Viral Infection. Viruses 2021, 13, 1366. [Google Scholar] [CrossRef]
- Sheehy, A.M.; Gaddis, N.C.; Choi, J.D.; Malim, M.H. Isolation of a Human Gene That Inhibits HIV-1 Infection and Is Suppressed by the Viral Vif Protein. Nature 2002, 418, 646–650. [Google Scholar] [CrossRef]
- Poulain, F.; Lejeune, N.; Willemart, K.; Gillet, N.A. Footprint of the Host Restriction Factors APOBEC3 on the Genome of Human Viruses. PLoS Pathog 2020, 16, e1008718. [Google Scholar] [CrossRef]
- Peretti, A.; Geoghegan, E.M.; Pastrana, D.V.; Smola, S.; Feld, P.; Sauter, M.; Lohse, S.; Ramesh, M.; Lim, E.S.; Wang, D.; et al. Characterization of BK Polyomaviruses from Kidney Transplant Recipients Suggests a Role for APOBEC3 in Driving In-Host Virus Evolution. Cell Host Microbe 2018, 23, 628–635. [Google Scholar] [CrossRef]
- Baker, S.C.; Mason, A.S.; Slip, R.G.; Skinner, K.T.; Macdonald, A.; Masood, O.; Harris, R.S.; Fenton, T.R.; Periyasamy, M.; Ali, S.; et al. Induction of APOBEC3-Mediated Genomic Damage in Urothelium Implicates BK Polyomavirus (BKPyV) as a Hit-and-Run Driver for Bladder Cancer. Oncogene 2022, 41, 2139–2151. [Google Scholar] [CrossRef]
- Jelcic, I.; Jelcic, I.; Kempf, C.; Largey, F.; Planas, R.; Schippling, S.; Budka, H.; Sospedra, M.; Martin, R. Mechanisms of Immune Escape in Central Nervous System Infection with Neurotropic JC Virus Variant. Ann Neurol 2016, 79, 404–418. [Google Scholar] [CrossRef] [PubMed]
- Dunham, S.R.; Schmidt, R.; Clifford, D.B. Treatment of Progressive Multifocal Leukoencephalopathy Using Immune Restoration. Neurotherapeutics 2020, 17, 955–965. [Google Scholar] [CrossRef] [PubMed]
- Gheuens, S.; Bord, E.; Kesari, S.; Simpson, D.M.; Gandhi, R.T.; Clifford, D.B.; Berger, J.R.; Ngo, L.; Koralnik, I.J. Role of CD4+ and CD8+ T-Cell Responses against JC Virus in the Outcome of Patients with Progressive Multifocal Leukoencephalopathy (PML) and PML with Immune Reconstitution Inflammatory Syndrome ▿. J Virol 2011, 85, 7256–7263. [Google Scholar] [CrossRef] [PubMed]
- Du Pasquier, R.A.; Kuroda, M.J.; Zheng, Y.; Jean-Jacques, J.; Letvin, N.L.; Koralnik, I.J. A Prospective Study Demonstrates an Association between JC Virus-Specific Cytotoxic T Lymphocytes and the Early Control of Progressive Multifocal Leukoencephalopathy. Brain 2004, 127, 1970–1978. [Google Scholar] [CrossRef]
- Wüthrich, C.; Kesari, S.; Kim, W.-K.; Williams, K.; Gelman, R.; Elmeric, D.; De Girolami, U.; Joseph, J.T.; Hedley-Whyte, T.; Koralnik, I.J. Characterization of Lymphocytic Infiltrates in Progressive Multifocal Leukoencephalopathy: Co-Localization of CD8(+) T Cells with JCV-Infected Glial Cells. J Neurovirol 2006, 12, 116–128. [Google Scholar] [CrossRef]
- Martin-Blondel, G.; Bauer, J.; Cuvinciuc, V.; Uro-Coste, E.; Debard, A.; Massip, P.; Delisle, M.-B.; Lassmann, H.; Marchou, B.; Mars, L.T.; et al. In Situ Evidence of JC Virus Control by CD8+ T Cells in PML-IRIS during HIV Infection. Neurology 2013, 81, 964–970. [Google Scholar] [CrossRef]
- Ariotti, S.; Hogenbirk, M.A.; Dijkgraaf, F.E.; Visser, L.L.; Hoekstra, M.E.; Song, J.-Y.; Jacobs, H.; Haanen, J.B.; Schumacher, T.N. T Cell Memory. Skin-Resident Memory CD8+ T Cells Trigger a State of Tissue-Wide Pathogen Alert. Science 2014, 346, 101–105. [Google Scholar] [CrossRef]
- Schenkel, J.M.; Masopust, D. Tissue-Resident Memory T Cells. Immunity 2014, 41, 886–897. [Google Scholar] [CrossRef]
- Abdelbary, M.; Hobbs, S.J.; Gibbs, J.S.; Yewdell, J.W.; Nolz, J.C. T Cell Receptor Signaling Strength Establishes the Chemotactic Properties of Effector CD8. Nat Commun 2023, 14, 3928. [Google Scholar] [CrossRef]
- Evrard, M.; Becht, E.; Fonseca, R.; Obers, A.; Park, S.L.; Ghabdan-Zanluqui, N.; Schroeder, J.; Christo, S.N.; Schienstock, D.; Lai, J.; et al. Single-Cell Protein Expression Profiling Resolves Circulating and Resident Memory T Cell Diversity across Tissues and Infection Contexts. Immunity 2023, 56, 1664–1680. [Google Scholar] [CrossRef]
- Wein, A.N.; McMaster, S.R.; Takamura, S.; Dunbar, P.R.; Cartwright, E.K.; Hayward, S.L.; McManus, D.T.; Shimaoka, T.; Ueha, S.; Tsukui, T.; et al. CXCR6 Regulates Localization of Tissue-Resident Memory CD8 T Cells to the Airways. J Exp Med 2019, 216, 2748–2762. [Google Scholar] [CrossRef] [PubMed]
- Heim, T.A.; Lin, Z.; Steele, M.M.; Mudianto, T.; Lund, A.W. CXCR6 Promotes Dermal CD8. bioRxiv 2023. [Google Scholar] [CrossRef]
- Zaid, A.; Hor, J.L.; Christo, S.N.; Groom, J.R.; Heath, W.R.; Mackay, L.K.; Mueller, S.N. Chemokine Receptor-Dependent Control of Skin Tissue-Resident Memory T Cell Formation. J Immunol 2017, 199, 2451–2459. [Google Scholar] [CrossRef] [PubMed]
- Tse, S.W.; Radtke, A.J.; Espinosa, D.A.; Cockburn, I.A.; Zavala, F. The Chemokine Receptor CXCR6 Is Required for the Maintenance of Liver Memory CD8+ T Cells Specific for Infectious Pathogens. J Infect Dis 2014, 210, 1508–1516. [Google Scholar] [CrossRef] [PubMed]
- Rosen, S.F.; Soung, A.L.; Yang, W.; Ai, S.; Kanmogne, M.; Davé, V.A.; Artyomov, M.; Magee, J.A.; Klein, R.S. Single-Cell RNA Transcriptome Analysis of CNS Immune Cells Reveals CXCL16/CXCR6 as Maintenance Factors for Tissue-Resident T Cells That Drive Synapse Elimination. Genome Med 2022, 14, 108. [Google Scholar] [CrossRef]
- Su, W.; Saravia, J.; Risch, I.; Rankin, S.; Guy, C.; Chapman, N.M.; Shi, H.; Sun, Y.; Kc, A.; Li, W.; et al. CXCR6 Orchestrates Brain CD8+ T Cell Residency and Limits Mouse Alzheimer’s Disease Pathology. Nat Immunol 2023, 24, 1735–1747. [Google Scholar] [CrossRef]
- Cibrián, D.; Sánchez-Madrid, F. CD69: From Activation Marker to Metabolic Gatekeeper. Eur J Immunol 2017, 47, 946–953. [Google Scholar] [CrossRef]
- Szabo, P.A.; Miron, M.; Farber, D.L. Location, Location, Location: Tissue Resident Memory T Cells in Mice and Humans. Sci. Immunol. 2019, 4, eaas9673. [Google Scholar] [CrossRef]
- Shwetank, *!!! REPLACE !!!*; Frost, E.L.; Mockus, T.E.; Ren, H.M.; Toprak, M.; Lauver, M.D.; Netherby-Winslow, C.S.; Jin, G.; Cosby, J.M.; Evavold, B.D.; et al. PD-1 Dynamically Regulates Inflammation and Development of Brain-Resident Memory CD8 T Cells During Persistent Viral Encephalitis. Front Immunol 2019, 10, 783. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, K.K.W. Glial Fibrillary Acidic Protein: From Intermediate Filament Assembly and Gliosis to Neurobiomarker. Trends Neurosci 2015, 38, 364–374. [Google Scholar] [CrossRef]
- Ito, D.; Imai, Y.; Ohsawa, K.; Nakajima, K.; Fukuuchi, Y.; Kohsaka, S. Microglia-Specific Localisation of a Novel Calcium Binding Protein, Iba1. Molecular Brain Research 1998, 57, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Pawlitzki, M.; Schneider-Hohendorf, T.; Rolfes, L.; Meuth, S.G.; Wiendl, H.; Schwab, N.; Grauer, O.M. Ineffective Treatment of PML with Pembrolizumab: Exhausted Memory T-Cell Subsets as a Clue? Neurol Neuroimmunol Neuroinflamm 2019, 6, e627. [Google Scholar] [CrossRef] [PubMed]
- Vidya Vijayan, K.K.; Karthigeyan, K.P.; Tripathi, S.P.; Hanna, L.E. Pathophysiology of CD4+ T-Cell Depletion in HIV-1 and HIV-2 Infections. Front. Immunol. 2017, 8, 580. [Google Scholar] [CrossRef] [PubMed]
- Laidlaw, B.J.; Craft, J.E.; Kaech, S.M. The Multifaceted Role of CD4+ T Cells in CD8+ T Cell Memory. Nat Rev Immunol 2016, 16, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Elsaesser, H.; Sauer, K.; Brooks, D.G. IL-21 Is Required to Control Chronic Viral Infection. Science 2009, 324, 1569–1572. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, A.; Kisielow, J.; Schmitz, I.; Freigang, S.; Shamshiev, A.T.; Weber, J.; Marsland, B.J.; Oxenius, A.; Kopf, M. IL-21R on T Cells Is Critical for Sustained Functionality and Control of Chronic Viral Infection. Science 2009, 324, 1576–1580. [Google Scholar] [CrossRef]
- Ren, H.M.; Kolawole, E.M.; Ren, M.; Jin, G.; Netherby-Winslow, C.S.; Wade, Q.; Shwetank, *!!! REPLACE !!!*; Rahman, Z.S.M.; Evavold, B.D.; Lukacher, A.E.C.-T.; et al. IL-21 from High-Affinity CD4 T Cells Drives Differentiation of Brain-Resident CD8 T Cells during Persistent Viral Infection. Sci Immunol 2020, 5. [Google Scholar] [CrossRef]
- Bennett, C.L.; Focosi, D.; Socal, M.P.; Bian, J.C.; Nabhan, C.; Hrushesky, W.J.; Bennett, A.C.; Schoen, M.W.; Berger, J.R.; Armitage, J.O. Progressive Multifocal Leukoencephalopathy in Patients Treated with Rituximab: A 20-Year Review from the Southern Network on Adverse Reactions. The Lancet Haematology 2021, 8, e593–e604. [Google Scholar] [CrossRef]
- Solis, M.; Guffroy, A.; Lersy, F.; Soulier, E.; Gallais, F.; Renaud, M.; Douiri, N.; Argemi, X.; Hansmann, Y.; De Sèze, J.; et al. Inadequate Immune Humoral Response against JC Virus in Progressive Multifocal Leukoencephalopathy Non-Survivors. Viruses 2020, 12, 1380. [Google Scholar] [CrossRef]
- Bertoli, D.; Sottini, A.; Capra, R.; Scarpazza, C.; Bresciani, R.; Notarangelo, L.D.; Imberti, L. Lack of Specific T- and B-Cell Clonal Expansions in Multiple Sclerosis Patients with Progressive Multifocal Leukoencephalopathy. Sci Rep 2019, 9, 16605. [Google Scholar] [CrossRef]
- Lauver, M.D.; Goetschius, D.J.; Netherby-Winslow, C.S.; Ayers, K.N.; Jin, G.; Haas, D.G.; Frost, E.L.; Cho, S.H.; Bator, C.M.; Bywaters, S.M.; et al. Antibody Escape by Polyomavirus Capsid Mutation Facilitates Neurovirulence. Elife 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Jelcic, I.; Combaluzier, B.; Jelcic, I.; Faigle, W.; Senn, L.; Reinhart, B.J.; Ströh, L.; Nitsch, R.M.; Stehle, T.; Sospedra, M.; et al. Broadly Neutralizing Human Monoclonal JC Polyomavirus VP1–Specific Antibodies as Candidate Therapeutics for Progressive Multifocal Leukoencephalopathy. Sci Transl Med 2015, 7, 306ra150. [Google Scholar] [CrossRef] [PubMed]
- Ray, U.; Cinque, P.; Gerevini, S.; Longo, V.; Lazzarin, A.; Schippling, S.; Martin, R.; Buck, C.B.; Pastrana, D.V. JC Polyomavirus Mutants Escape Antibody-Mediated Neutralization. Sci. Transl. Med. 2015, 7. [Google Scholar] [CrossRef] [PubMed]
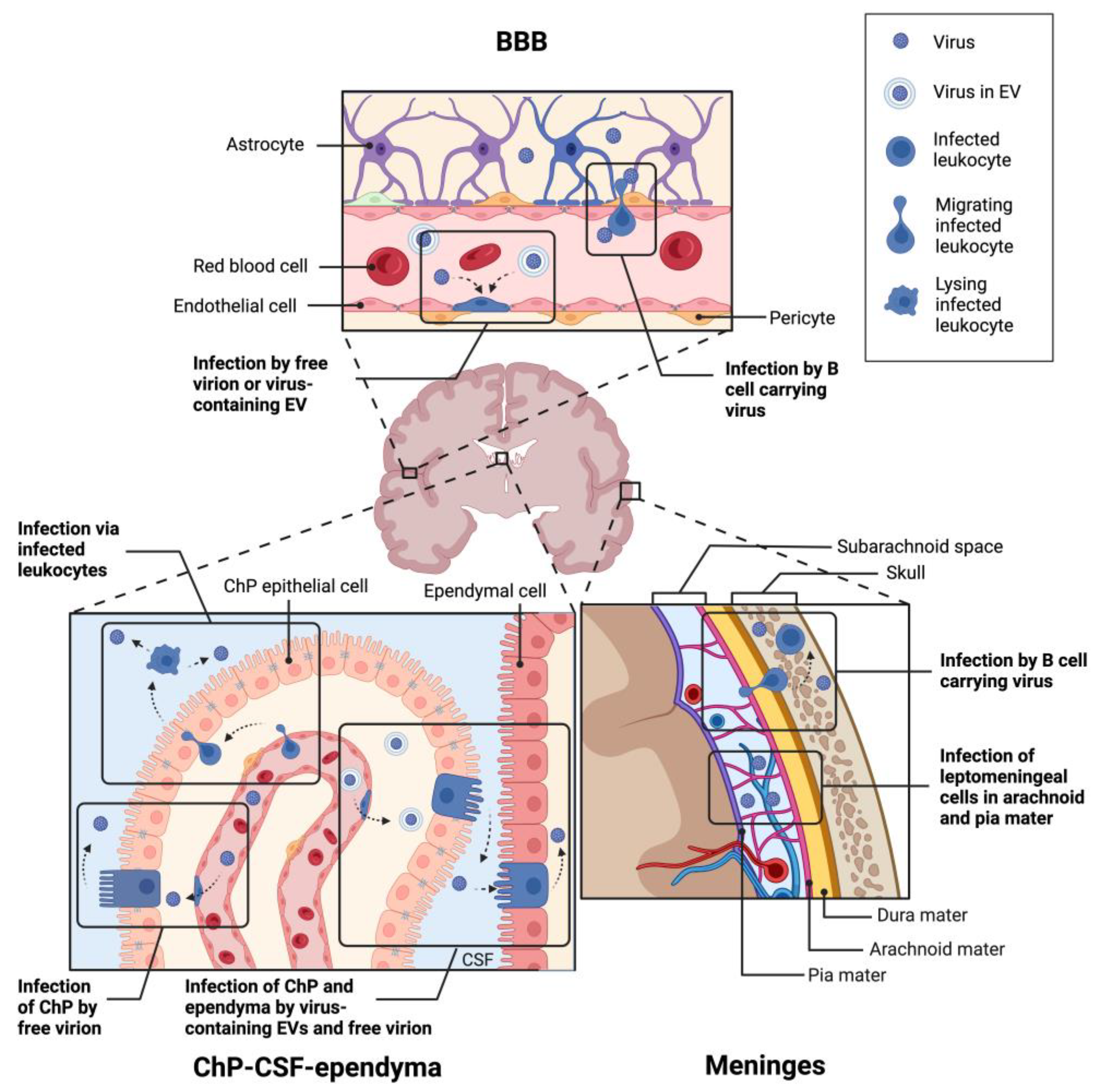
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




