Submitted:
30 September 2023
Posted:
02 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
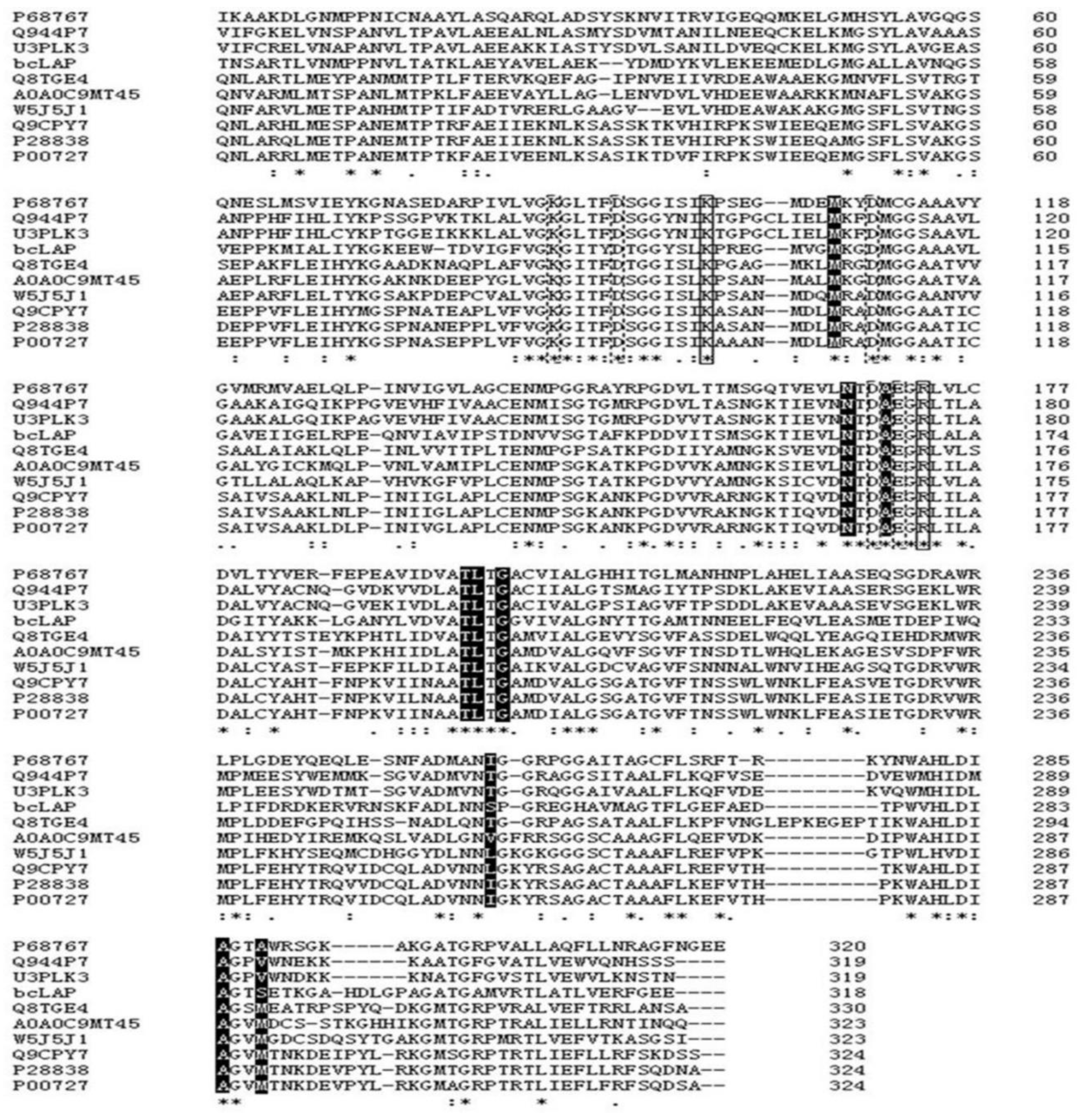
2.1. Primary Structure Analysis of bcLAP
2.2. Expression of bcLAP in E. coli
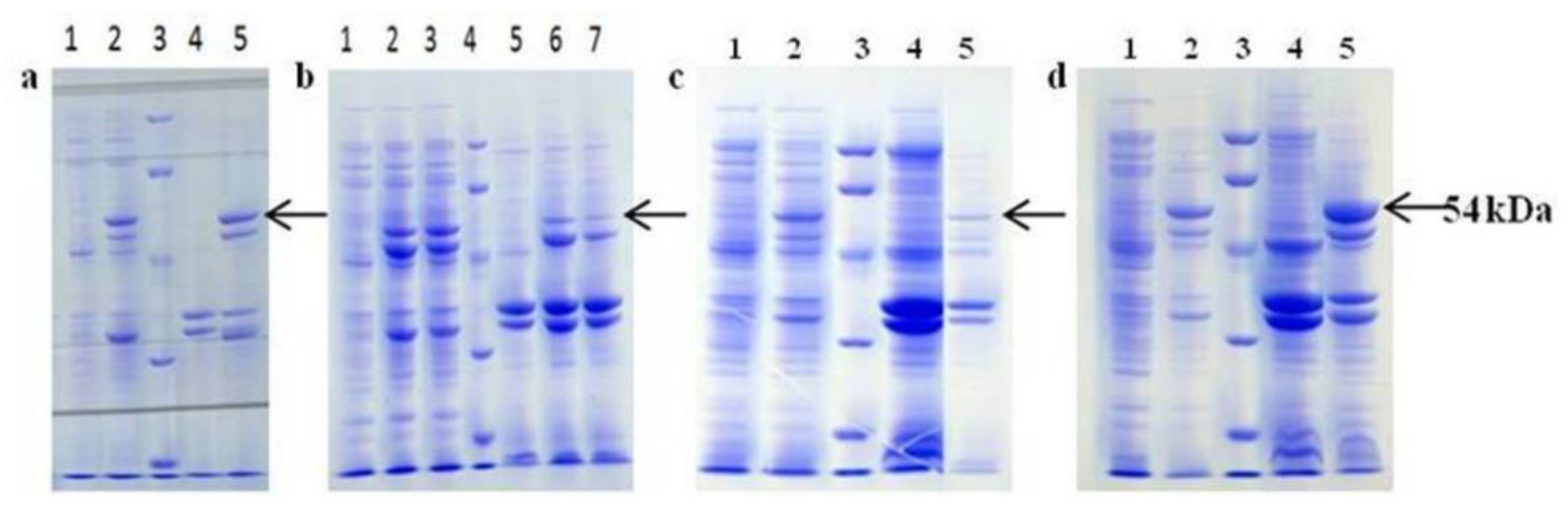
2.2.1. Effects of Different Host-Vector Combinations on bcLAP Expression
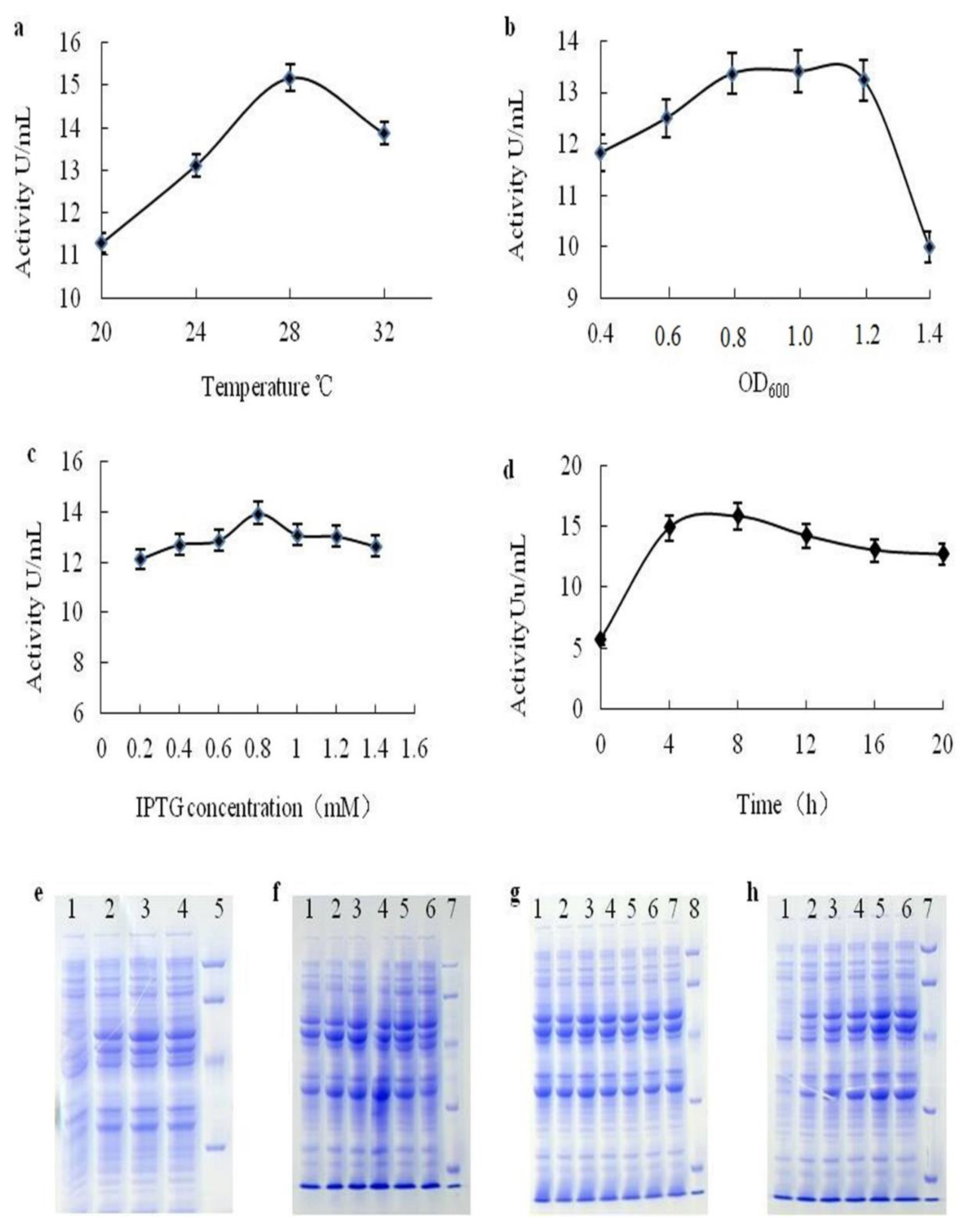
2.2.2. Optimisation of Expression Conditions
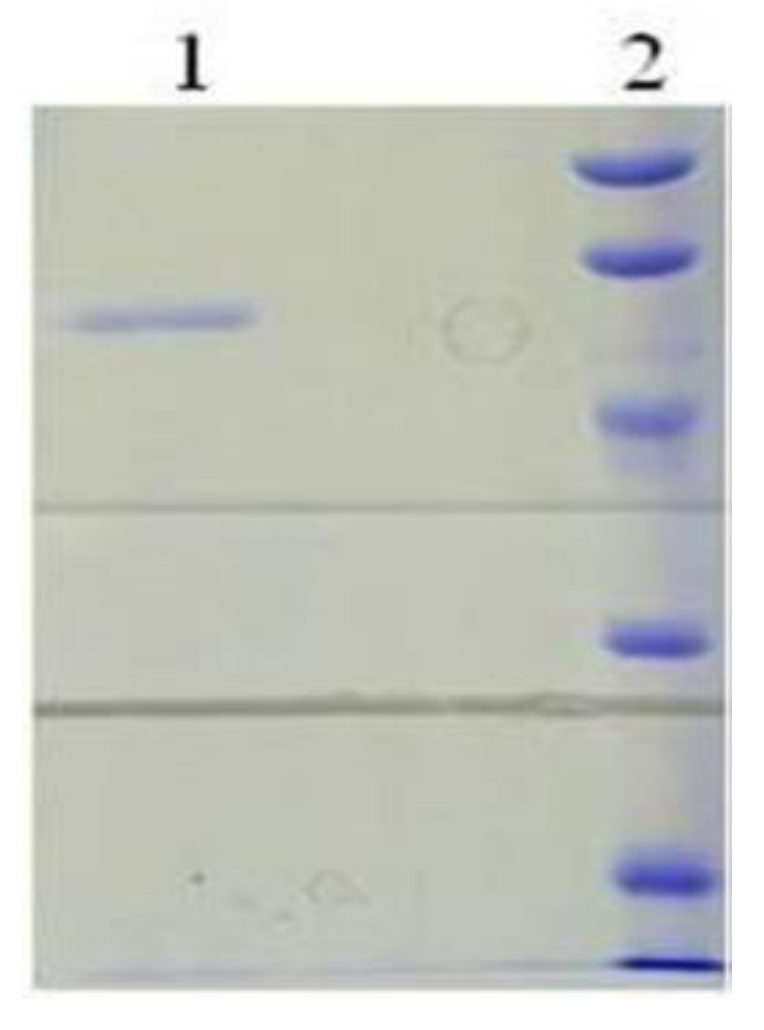
2.3. Purification of the Recombinant bcLAP
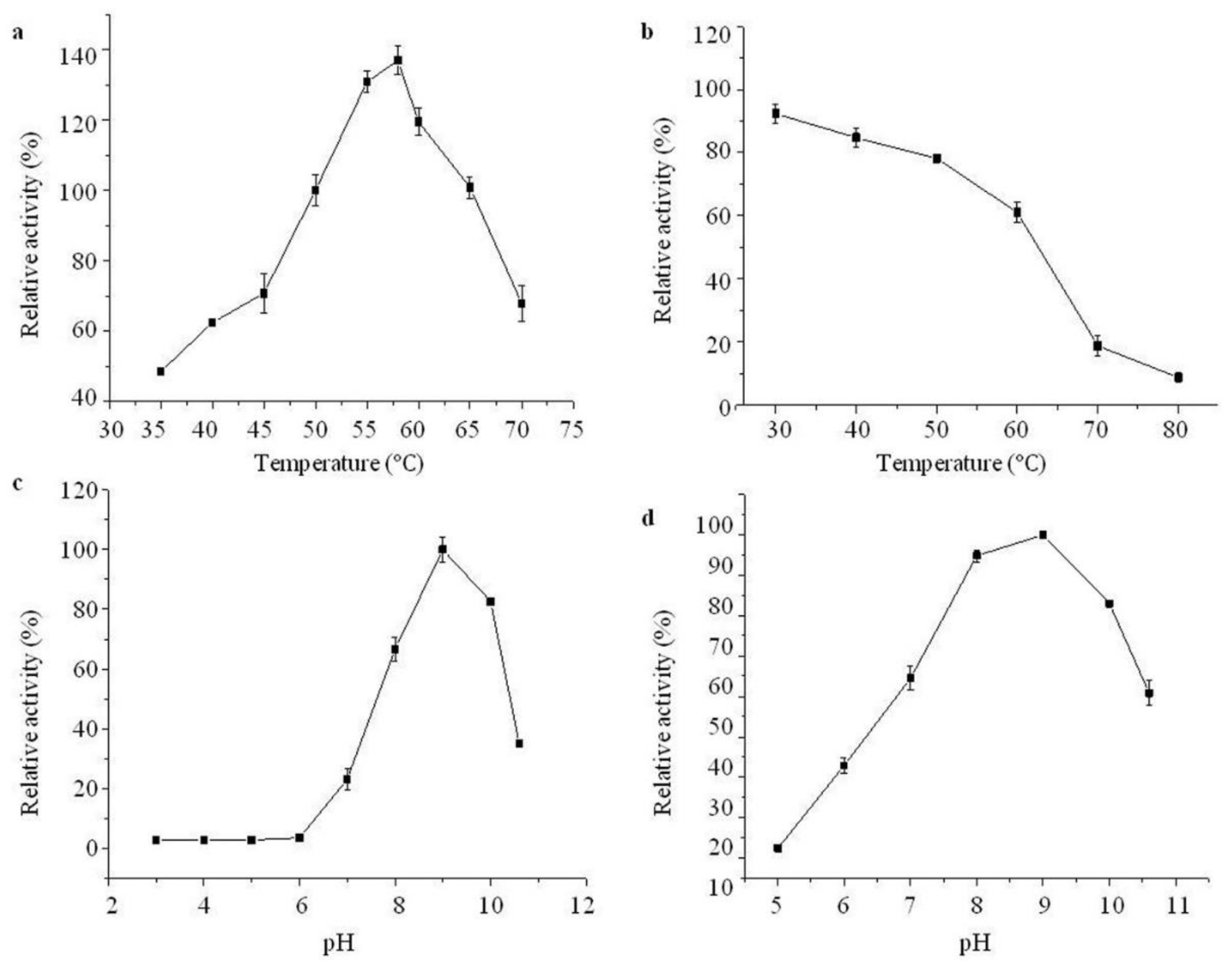
2.4. Effects of Temperature and pH on Purified Aminopeptidase
2.5. Effect of Metal Ions and Chemical Reagents on Purified Aminopeptidase
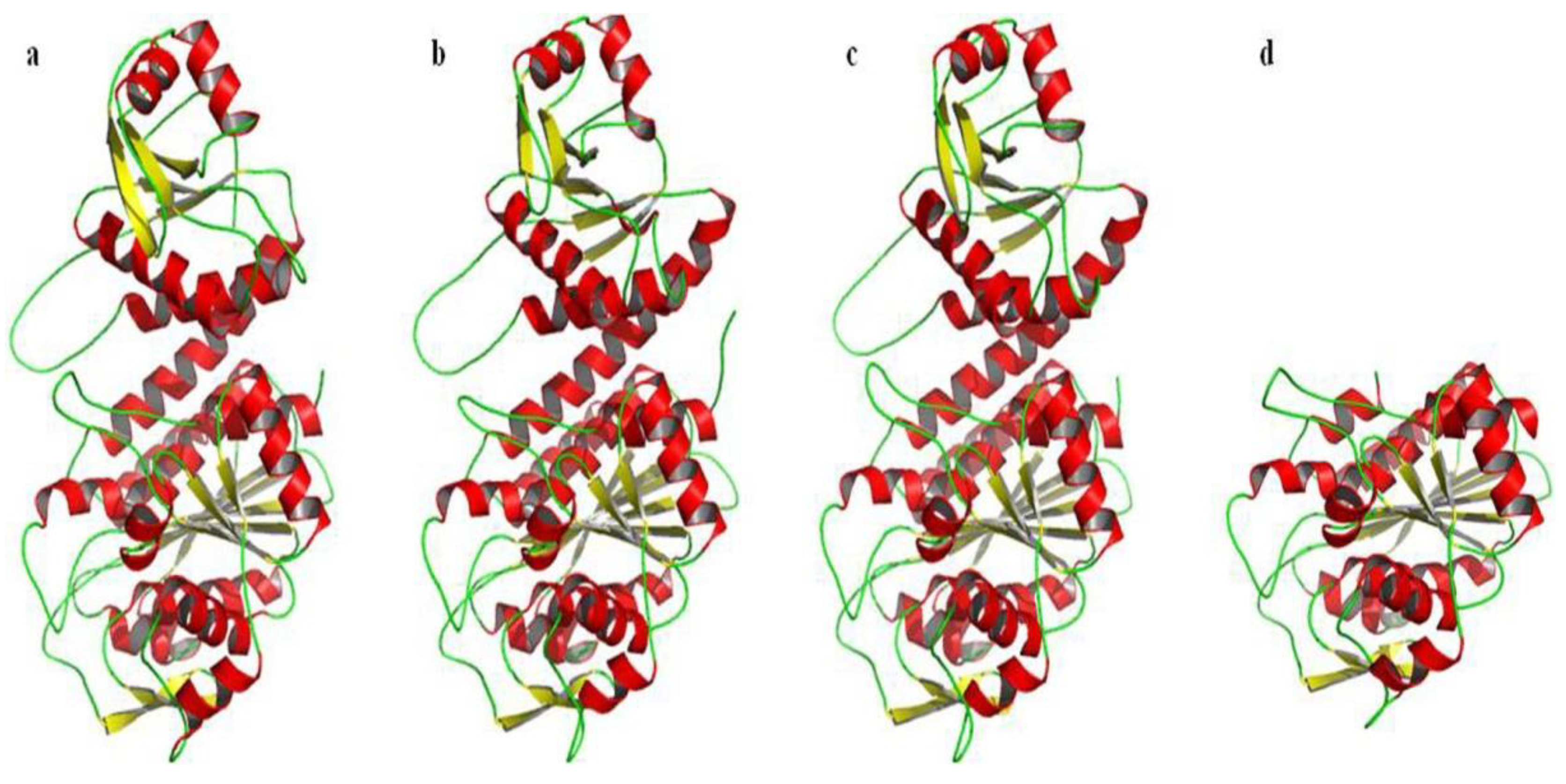
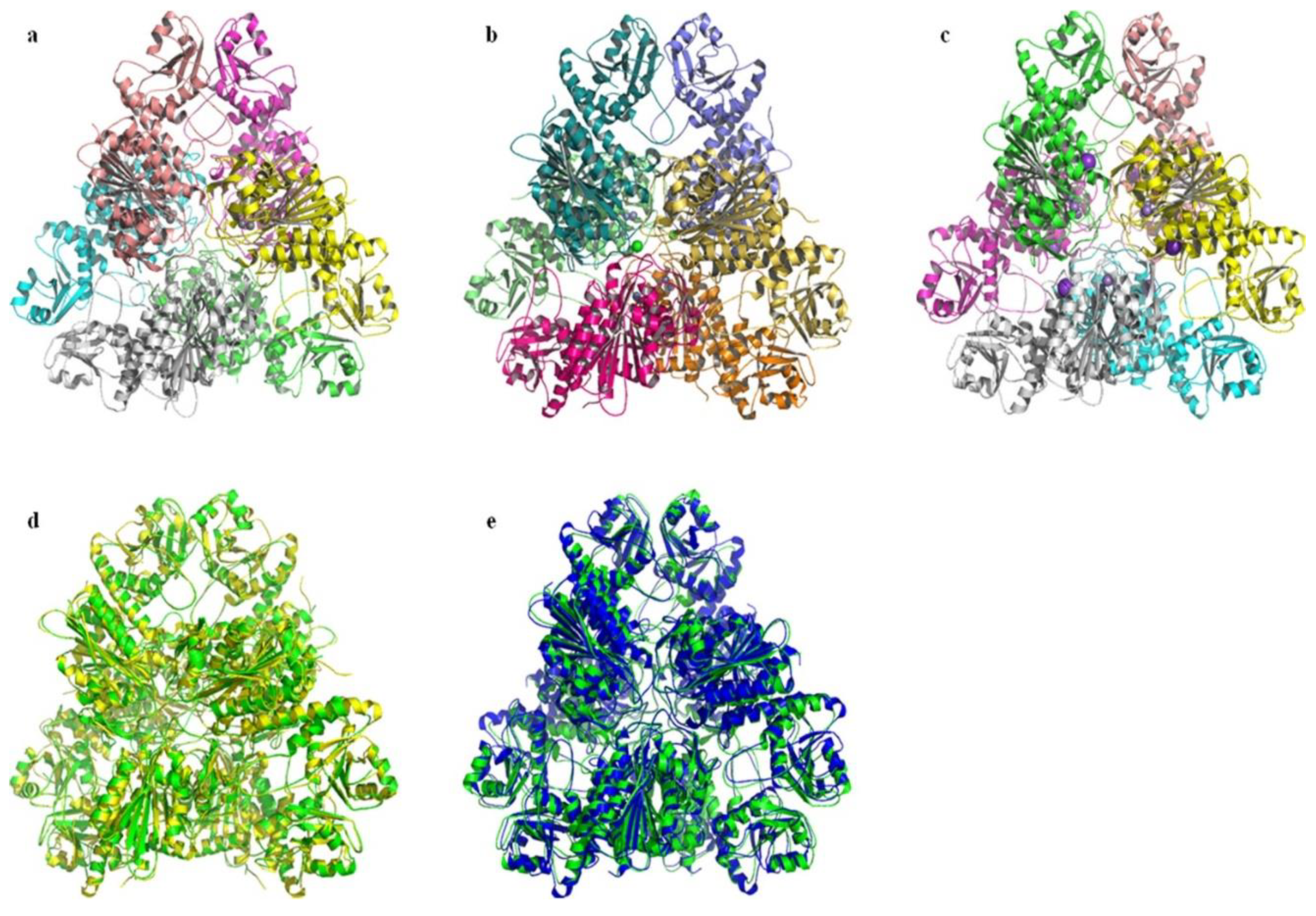
2.6. Prediction and Analysis of the Secondary and Tertiary Structure of bcLAP
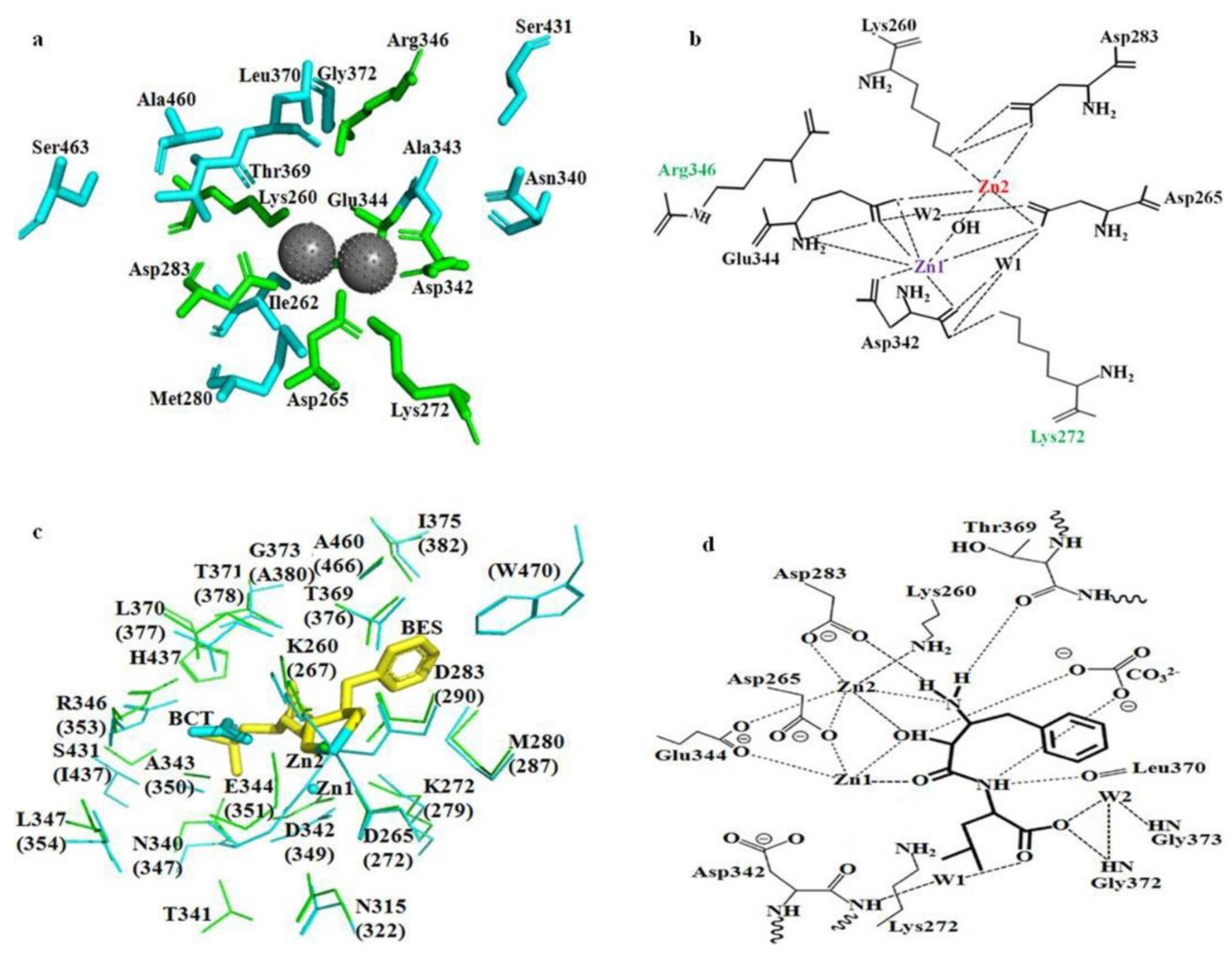
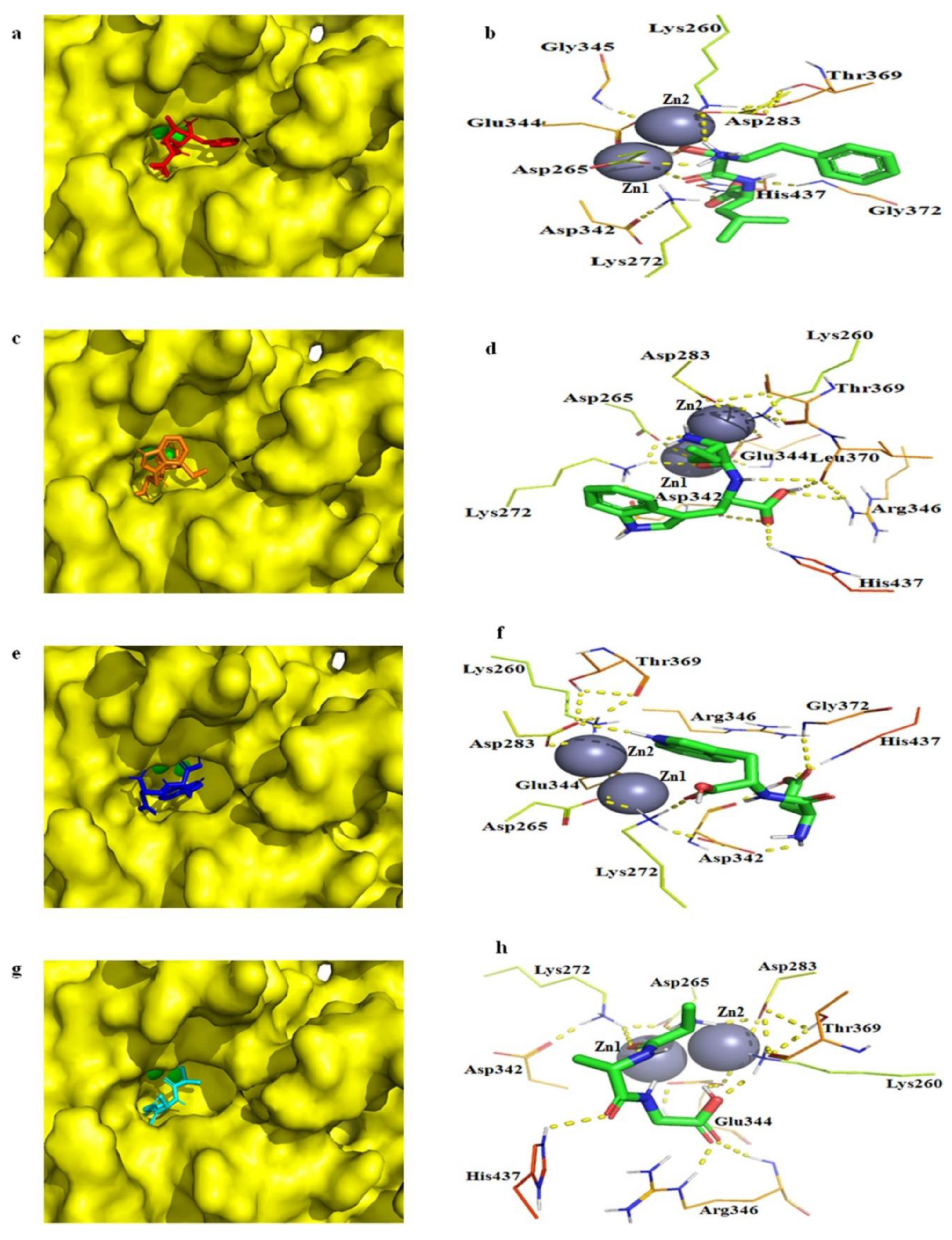
2.7. Molecular docking of bcLAP
3. Materials and Methods
3.1. Bacterial Strains, Vectors, and Culture Conditions
3.2. Reagents
3.3. PCR Amplification of the LAP Gene (Bclap)
3.4. Transformation of Recombinant Plasmids and Expression of Bclap
3.5. Optimisation of Recombinant bcLAP Induction Conditions in Shake Flasks
3.6. Preparation of Crude Enzyme Solution
3.7. SDS-PAGE and Analysis of Enzyme Activity
3.8. Purification of Recombinant Aminopeptidase
3.9. Temperature and pH Characterisation of bcLAP
3.10. Effect of Metal Ions and Chemical Agents
3.11. Structure Prediction and Molecular Docking Analysis
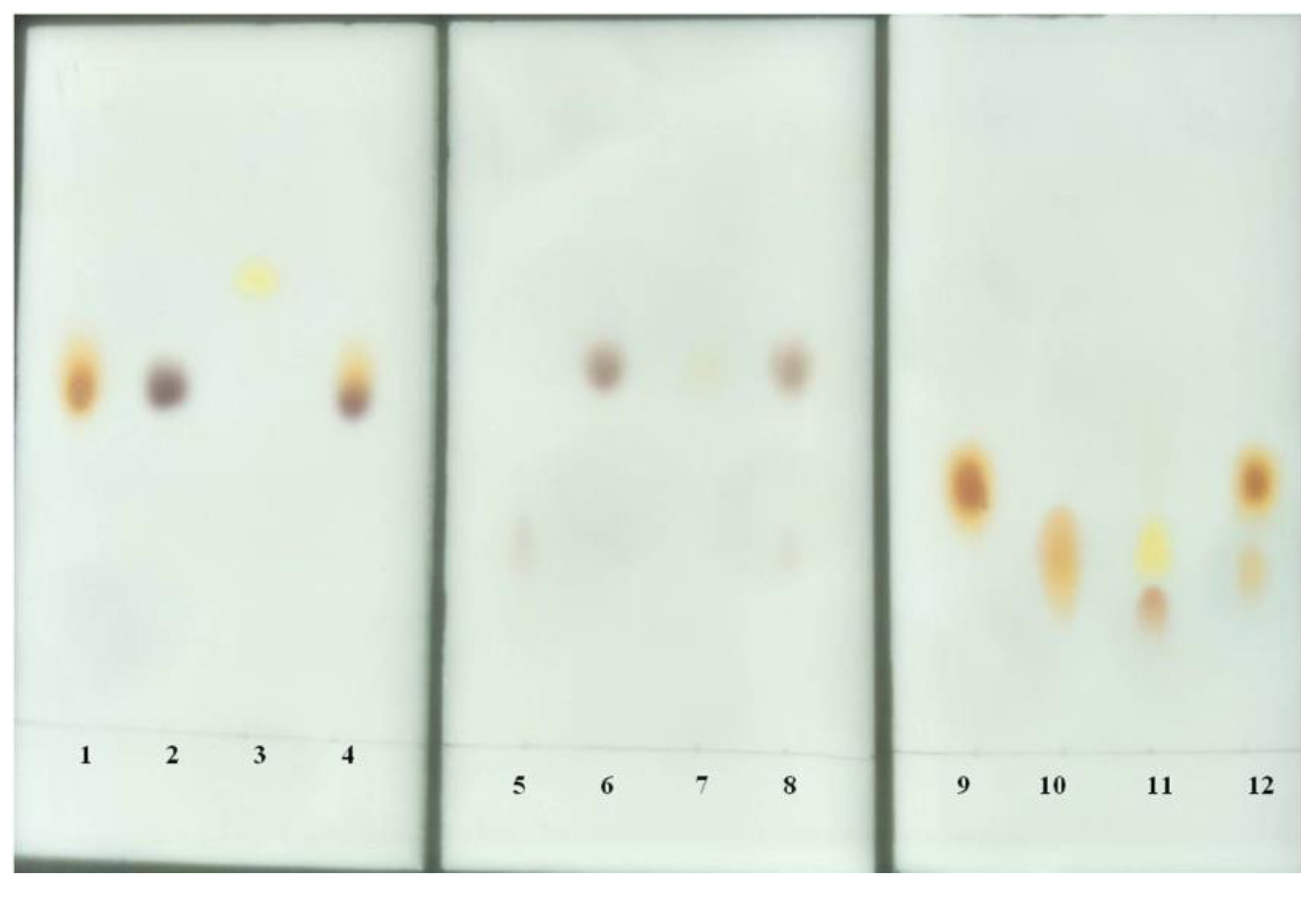
3.12. Thin Layer Chromatography
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sanderink, G.J.; Artur, Y.; Siest, G. Human aminopeptidases: a review of the literature. Clin. Chem. Lab. Med. 1998, 26, 795–808. [Google Scholar] [CrossRef]
- Taylor, A. Aminopeptidases: structure and function. FASEB. J. 1993, 7, 290–298. [Google Scholar] [CrossRef]
- Rawlings, N.D.; Barrett, A.J. Introduction: Metallopeptidases and their clans, In: Handbook of Proteolytic Enzymes, 2nd Edition (San Diego, USA: Elsevier/Academic Press). 2004; pp. 231–263.
- Kim, H.; Lipscomb, W.N. Differentiation and identification of the two catalytic metal and binding sites in bovine lens leucine aminopeptidase by X-ray crystallography. Proc. Natl. Acad. Sci. USA. 1993, 90, 5006–5010. [Google Scholar] [CrossRef]
- Smith, E.L.; Hill, R.L. The enzymes, vol 4, part A, 2nd edn, Boyer PD, Lardy H, Myrback K (eds), Academic, NewYork. 1960; pp. 37.
- Matsui, M.; Fowler, J.H.; Walling, L.L. Leucine aminopeptidases: diversity in structure and function. Biol. Chem. 2006, 387, 1535–1544. [Google Scholar] [CrossRef]
- Burley, S.K.; David, P.R.; Taylor, A.; Lipscomb, W. Molecular structure of leucine aminopeptidase at 2.7 Å resolution. Proc. Natl. Acad. Sci. USA. 1990, 87, 6878–6882. [Google Scholar] [CrossRef]
- Herrera-Camacho, I.; Rosas-Murrieta, N.H.; Rojo-Domínguez, A.; Millán, L.; Reyes-Leyva, J.; Santos-López, G.; Suárez-Rendueles, P. Biochemical characterization and structural prediction of a novel cytosolic leucyl aminopeptidase of the M17 family from Schizosaccharomyces pombe. FEBS J. 2007, 274, 6228–6240. [Google Scholar] [CrossRef]
- Sträter, N. D.; Sherrat, J.; Collons, S.D. X-ray structure of aminopeptidase A from Escherichia coli and a model for the nucleoprotein complex in Xer-site specific recombination. EMBO J. 1999, 18, 4513–4522. [Google Scholar] [CrossRef]
- Gotou, T.; Shinoda, T.; Mizuno, S.; Yamamoto, N. Purification and identification of proteolytic enzymes from Aspergillus oryzae capable of producing the antihypertensive peptide Ile-Pro-Pro. J. Biosci. Bioeng. 2009, 107, 615–619. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.Q.; Zhong, L.F.; Meng, Z.Z.; You, Z.J.; Li, J.Z.; Luo, X.C. The structure and enzyme characteristics of a recombinant Leucine aminopeptidase rLap1 from Aspergillus sojae and its application in debittering. Appl. Biotechnol. 2015, 177, 190–206. [Google Scholar] [CrossRef]
- Wang, F.H.; Ning, Z.X.; Lan, D.M.; Liu, Y.Y.; Yang, B.; Wang, Y.H. Biochemical properties of recombinant Leucine aminopeptidase II from Bacillus stearothermophilus and potential applications in the hydrolysis of Chinese anchovy (Engraulis japonicus) proteins. J. Agri. Food Chem. 2012, 60, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.F.; Wang, F.H.; Lan, D.M.; Liu, Y.Y.; Yang, B.; Wang, Y.H. Biochemical Properties and potential applications of recombinant Leucine aminopeptidase from Bacillus kaustophilus CCRC 11223. Int. J. Mol. Sci. 2011, 12, 7609–7625. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Natarajan, S.; Park, H.; Huynh, K.H.; Lee, S.H.; Kim, J.G.; Ahn, Y.J.; Kang, L.W. Crystal structure of XoLAP, a leucine aminopeptidase, from xanthomonas oryzae pv. Oryzae. J. Microbiol. 2013, 51, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Moreno, A.V.; Perdomo-Abúndez, F.C.; Pérez-Medina Martínez, V.; Luna-Bárcenas, G.; Villaseñor-Ortega, F.; Pérez, N.O.; López-Morales, C.A.; Flores-Ortiz, L.F.; Medina-Rivero, E. Structural and functional characterization of a recombinant leucine aminopeptidase. J. Mol. Catal. B Enzym. 2015, 113, 39–46. [Google Scholar] [CrossRef]
- Katrolia, P.; Liu, X.L.; Zhao, Y.Y.; Kopparapu, N. K.; Zheng, X.Q. Gene cloning, expression and homology modeling of first fibrinolytic enzyme from mushroom (cordyceps militaris). Int. J. Biol. Macromol. 2020, 146, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Pitman, M. R.; Menz, R.I. Methods for Protein Homology Modelling. in: Applied Mycology and Biotechnology, 2006, 6; pp. 37-59.
- Tang, Z.Z.; Jin, W.Q.; Tang, Y.J.; Wang, Y.S.; Wang, C.; Zheng, X.; Sun, W.J.; Liu, M.Y.; Zheng, T.R.; Chen, H.; Wu, Q.; Shan, Z.; Bu, T.L.; Li, C.L. Research on homology modeling, molecular docking of the cellulase and highly expression of the key enzyme (Bgl) in Pichia pastoris. Int. J. Biol. Macromol. 2018, 115, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Guinebretière, M.H.; Auger, S.; Galleron, N.; Contzen, M.; De Sarrau, B.; De Buyser, M.L.; Lamberet, G.; Fagerlund, A.; Granum, P.E.; Ler-eclus, D.; De Vos, P.; Nguyen-The, C.; Sorokin, A. Bacillus cytotoxicus sp. nov. is a novel thermotolerant species of the Bacillus cereus Group occasionally associated with food poisoning. Int. J. Syst. Evol. Microbiol. 2013, 63, 31–40. [Google Scholar] [CrossRef]
- Vilas-Bôas, G.T.; Peruca, A.P.; Arantes, O.M. Biology and taxonomy of Bacillus cereus, Bacillus anthracis, and Bacillus thuringiensi. Can. J. Microbiol. 2007, 53, 673–687. [Google Scholar] [CrossRef]
- Carroll, R.K.; Robison, T.M.; Rivera, F.E.; Davenport, J.E.; Jonsson, I.M.; Florczyk, D.; Tarkowski, A.; Potempa, J.; Koziel, J.; Shaw, L.N. Identification of an intracellular M17 family leucine aminopeptidase that is required for virulence in Staphylococcus aureus. Microb. Infect. 2012, 14, 989–999. [Google Scholar] [CrossRef]
- Bhosale, M.; Pande, S.; Kumar, A.; Kairamkonda, S.; Nandi, D. Characterization of two M17 family members in Escherichia coli, Peptidase A and Peptidase B. Biochem. Biophys. Res. Commun. 2010, 395, 76–81. [Google Scholar] [CrossRef]
- Taylor, A. Aminopeptidases: structure and function. FASEB J. 1993, 7, 290–298. [Google Scholar] [CrossRef]
- Vujčić, Z.; Lončar, N.; Dojnov, B.; Milovanović, A.; Vujčić, M.; Božić, N. Characterisation of leucyl aminopeptidase from Solanum tuberosum tuber. Food Chem. 2010, 121, 418–423. [Google Scholar] [CrossRef]
- Cueva, R.; García-Alvarez, N.; Suárez-Rendueles, P. Yeast vacuolar aminopeptidase yscI: Isolation and regulation of the APE1 (LAP4) structural gene. FEBS Lett. 1989, 259, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Kale, A.; Pijning, T.; Sonke, T.; Dijkstra, B.W.; Thunnissen, A.M.W.H. Crystal structure of the Leucine aminopeptidase from Pseudomonas putida reveals the molecular basis for its enantioselectivity and broad substrate specificity. J. Mol. Biol. 2010, 398, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Pokharel, D.R.; Rathaur, S. Purification and characterization of a leucine aminopeptidase from the bovine filarial parasite Setariacervi. Acta Trop. 2008, 106, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.L.; Hsu, W.H.; Wu, C.P.; Chi, M.C.; Chou, W.M.; Hu, H.Y. A thermostable leucine aminopeptidase from Bacillus kaustophilus CCRC 11223. Extremophiles 2004, 8, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Xi, H.X.; Tian, Y.P.; Zhou, N.D.; Zhou, Z.M.; Shen, W. Characterization of an N-glycosylated Bacillus Subtilis Leucine aminopeptidase expressed in Pichia pastoris. J. Basic Microbiol. 2015, 55, 236–246. [Google Scholar] [CrossRef]
- Wang, F.H.; Guo, S.H.; Liu, Y.Y.; Lan, D.M.; Yang, B.; Wang, Y.H. Biochemical and conformational characterization of a leucine aminopeptidase from Geobacillus thermodenitrificans NG80-2. World J. Microbiol. Biotechnol. 2012, 28, 3227–3237. [Google Scholar] [CrossRef]
- Gobbetti, M.; CorsettiP, A.; Fox, F. Purification and characterization of intracellular aminopeptidase from Pseudomonas fluorescens ATCC 948. J. Dairy Sci. 1995, 78, 44–54. [Google Scholar] [CrossRef]
- Aphale, J.S.; Strohl, W.R. Purification and Properties of an Extracellular Aminopeptidase from Streptomyces Lividans 1326. J. Gen. Microbiol. 1993, 139, 417–424. [Google Scholar] [CrossRef]
- Tang, W.; Li, Z.Z.; Li, C.H.; Yu, X.H.; Wang, F.; Wan, X.; Wang, Y.P.; Ma, L.X. High-level expression and characterization of the Bacillus subtilis subsp. subtilis str. BSP1 YwaD aminopeptidase in Pichia pastoris. Protein Expres. Purif. 2016, 122, 23–30. [Google Scholar] [CrossRef]
- Tian, Y.P.; Xu, Y.M. Purification and characteristic of a kind of aminopeptidase from Bacillus subtilis. Food Fermentation Ind. (Chinese). 2006, 32, 7–10. [Google Scholar]
- Izawa, N.; Ishikawa, S.; Tanokura, T.; Ohta, K.; Hayashi, K. Purification and characterization of Aeromonas caviae aminopeptidase possessing debittering activity. J.Agr. Food Chem. 1997, 45, 4897–4902. [Google Scholar] [CrossRef]
- Raksakulthai, R.; Haard, N.F. Exopeptidases and their application to reduce bitterness in food: A review. Crit. Rev. Food Sci. 2003, 43, 401–445. [Google Scholar] [CrossRef] [PubMed]
- Rawlings, N.D.; Barrett, A.J. Evolutionary families of metallopeptidases. Methods Enzymol. 1995, 248, 183–228. [Google Scholar] [PubMed]
- Sträter, N.; Lipscomb, W.N. Two metal ion mechanism of bovine lens leucine aminopeptidase: active site, solvent structure and binding mode of L-leucinal, a gem-biolato transition state analogue by X-ray crystallography. Biochemistry 1995, 34, 14792–14800. [Google Scholar] [CrossRef]
- Singh, A.K.; Singh, R.; Tomar, D.; Pandya, C.D.; Singh, R. The leucine aminopeptidase of Staphylococcus aureus is secreted and contributes to biofilm formation. Int. J. Infect. Dis. 2012, 16, e375–e381. [Google Scholar] [CrossRef]
- Arima, J.; Iwabuchi, M.; Hatanaka, T. Gene cloning and overproduction of an aminopeptidase from Streptomyces septatus TH-2, and comparison with a calcium-activated enzyme from Streptomyces griseus. Biochem. Bioph. Res. Co. 2004, 317, 531–538. [Google Scholar] [CrossRef]
- Dong, L.; Cheng, N.; Wang, M.W.; Zhang, J.F.; Shu, C.; Zhu, D.X. The leucyl aminopeptidase from Helicobacter pylori is an allosteric enzyme. Microbiology. 2005, 151, 2017–2023. [Google Scholar] [CrossRef]
- Hatta, T.; Kazama, K.; Miyoshi, T.; Umemiya, R.; Liao, M.; Inoue, N.; Xuan, X.N.; Tsuji, N.; Fujisaki, K. Identification and characterisation of a leucine aminopeptidase from the hard tick Haemaphysalis longicornis. Int. J. Parasitol. 2006, 36, 1123–1132. [Google Scholar] [CrossRef]
- Cadavid-Restrepo, G.; Gastardelo, T.S.; Faudry, E.; de Almeida, H.; Bastos, I.M.D.; Negreiros, R.S.; Lima, M.M.; Assumpção, T.C.; Almeida, K.C.; Ragno, M.; Ebel, C.; Ribeiro, B.M.; Felix, C.R.; Santana, J.M. The major leucyl aminopeptidase of Trypanosoma cruzi (LAPTc) assembles into a homohexamer and belongs to the M17 family of metallopeptidases. BMC Biochem. 2011, 12, 46. [Google Scholar] [CrossRef]
- Acosta, D.; Cancela, M.; Piacenza, L.; Roche, L.; Carmona, C.; Tort, J.F. Fasciola hepatica leucine aminopeptidase, a promising candidate for vaccination against ruminant fasciolosis. Mol. Biochem. Parasit. 2008, 158, 52–64. [Google Scholar] [CrossRef]
- Maggioli, G.; Rinaldi, G.; Giaudrone, I.; Berasain, P.; Tort, J.F.; Brindley, P.J.; Carmona, C. Expression, purification and characterization of two leucine aminopeptidases of the blood fluke, Schistosoma mansoni. Mol. Biochem. Parasit. 2018, 219, 17–23. [Google Scholar] [CrossRef]
- He, B.; Zhang, Y.; Zhang, T.; Wang, H.R.; Zhou, H.M. Inactivation and unfolding of aminoacylase during denaturation in sodium dodecyl sulfate solutions. J. Protein Chem. 1995, 14, 349–357. [Google Scholar] [CrossRef]
- Wang, Z.F.; Huang, M.Q.; Zou, X.M.; Zou, H.M. Unfolding, conformational change of active sites and inactivation of creatine kinase in SDS solutions. Biochim. Biophys. Acta Protein Struct. Mol. Enzymol. 1995, 1251, 109–114. [Google Scholar] [CrossRef]
- Levitt, M.; Chottia, C. Structural patterns in globular proteins. Nature 1976, 261, 552–558. [Google Scholar] [CrossRef]
- Baker, D.; Sali, A. Protein structure prediction and structural genomics. Science 2001, 294, 93–96. [Google Scholar] [CrossRef]
- Allen, M. P.; Yamada, A. H.; Carpenter, F. H. Kinetic parameters of metal-substituted leucine aminopeptidase from bovine lens. Biochemistry 1983, 22, 3778–3783. [Google Scholar] [CrossRef]
- Burley, S.K.; David, P.R.; Lipscomb, W.N. Leucine aminopeptidase: bestatin inhibition and a model for enzyme-catalyzed peptide hydrolysis. Proc. Natl Acad. Sci. USA 1991, 88, 6916–6920. [Google Scholar] [CrossRef]
- Lowther, W.T.; Matthews, B.W. Metalloaminopeptidases: common functional themes in disparate structural surroundings. Chem. Rev. 2002, 102, 4581–4607. [Google Scholar] [CrossRef]
- Barage, S.H.; Jalkute, C.B.; Dhanavade, M.J.; Sonawane, K.D. Simulated interactions between endothelin converting enzyme and Aβ peptide: insights into subsite recognition and cleavage mechanism. Int J Pept Res Ther. 2014, 20, 409–420. [Google Scholar] [CrossRef]
- Dhanavade, M.J.; Sonawane, K.D. Insights into the molecular interactions between aminopeptidase and amyloid beta peptide using molecular modeling techniques. Amino Acids 2014, 46, 1853–1866. [Google Scholar] [CrossRef] [PubMed]
- Duprez, K.; Scranton, M.A.; Walling, L.L.; Fan, L. Structure of tomato wound-induced leucine aminopeptidase sheds light on substrate specificity. Acta Cryst. 2014, 70, 1649–1658. [Google Scholar] [CrossRef] [PubMed]
- Modak, J.K.; Rut, W.; Wijeyewickrema, L.C.; Pike, R.N.; Drag, M.; Roujeinikova, A. Structural basis for substrate specificity of Helicobacter pylori M17 aminopeptidase. Biochimie 2016, 121, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Goodsell, D.S.; Halliday, R.S.; Huey, R.; Hart, W.E.; Belew, R.K.; Olson, A.J. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J. Comput. Chem. 1998, 19, 1639–1662. [Google Scholar] [CrossRef]
- Kumar, D.T.; Doss, C.G.P. Chapter nine-investigating the inhibitory effect of Wortmannin in the hotspot mutation at codon1047 of PIK3CA kinase domain: a molecular docking and molecular dynamics approach. Adv. Protein. Chem. Struct. Biol. 2016, 102, 267. [Google Scholar]
| Purification step | Activity (U) | Protein (mg) | SpecificActivity (U/mg) | Purification (fold) | Yield (%) |
|---|---|---|---|---|---|
| Crude extract | 86806 | 5695.54 | 15 | 1.00 | 100 |
| Ammonium sulfate precipitation | 51950 | 150.40 | 345 | 22.66 | 59.85 |
| Sephadex G-50 | 45467 | 115.84 | 393 | 25.75 | 52.38 |
| Capto DEAE | 38165 | 41.09 | 929 | 60.94 | 43.97 |
| phenyl HP | 14382 | 10.09 | 1426 | 93.56 | 16.57 |
| Concentration | Enzyme activity fold* | ||||||
|---|---|---|---|---|---|---|---|
| Ni2+ | Mn2+ | Co2+ | Mg2+ | Zn2+ | Ba2+ | Cu2+ | |
| 0.1 mM | 8.75 ± 0.029 | 3.63 ± 0.019 | 2.81 ± 0.012 | 1.40 ± 0.004 | 1.12 ± 0.014 | 0.96 ± 0.006 | 0.93 ± 0.005 |
| 0.5 mM | 14.92 ± 0.056 | 3.32 ± 0.005 | 1.88 ± 0.011 | 1.45 ± 0.006 | 0.99 ± 0.017 | 0.95 ± 0.002 | 0.69 ±0 .001 |
| 1 mM | 13.60 ± 0.037 | 2.74 ± 0.009 | 1.81 ± 0.008 | 1.58 ± 0.015 | 1.14 ± 0.004 | 0.83 ± 0.012 | 0.37 ± 0.010 |
| Chemical reagents | Residual activity (%)a |
|---|---|
| None | 100 |
| EDTA | 15.18 ± 1.21 |
| PMSF | 94.60 ± 2.02 |
| Pepstain | 97.92 ± 1.85 |
| Leupeptin | 90.45 ± 3.15 |
| Bestatin | 35.25 ± 0.89 |
| SDS | 31.68 ± 2.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





