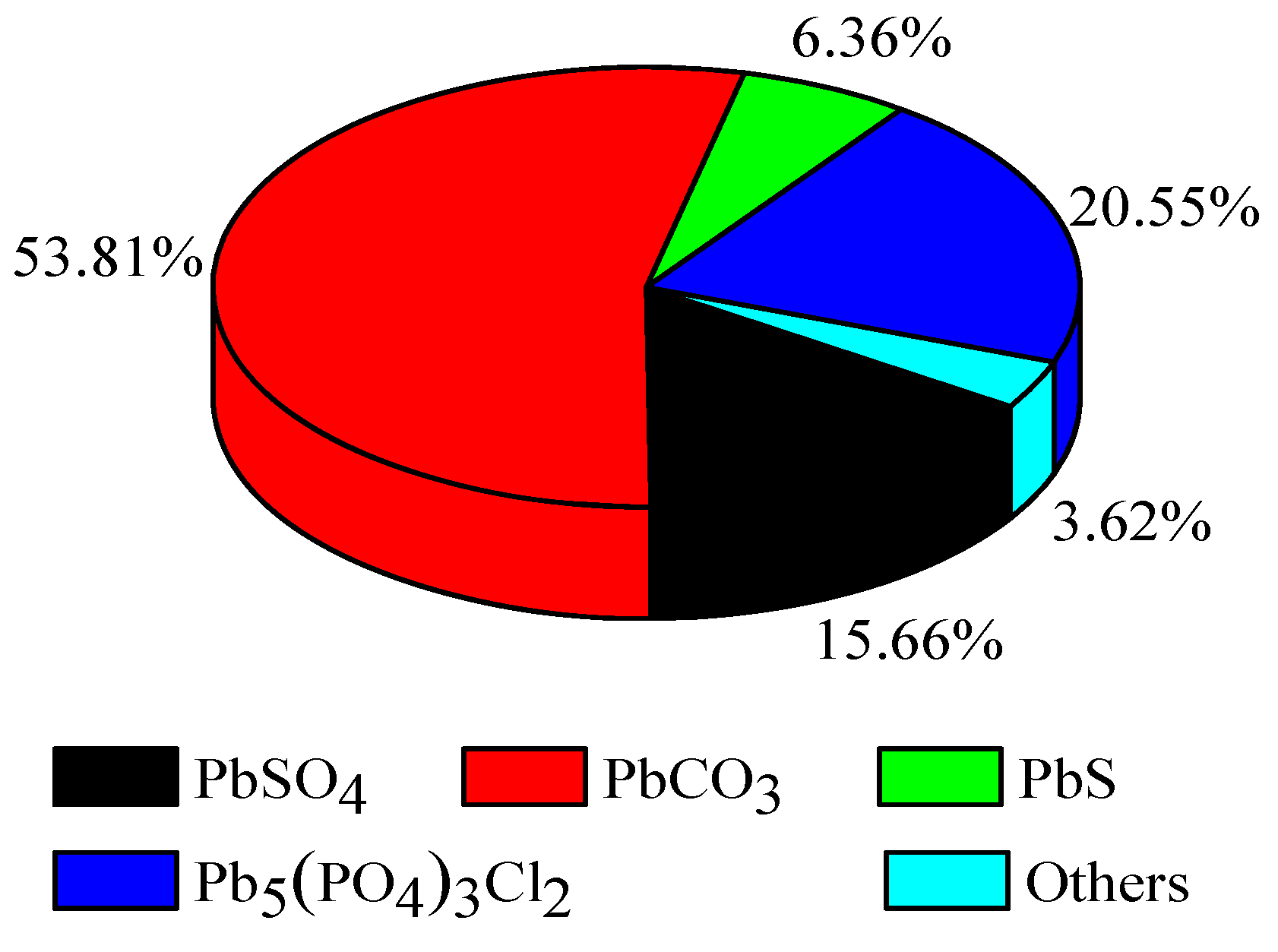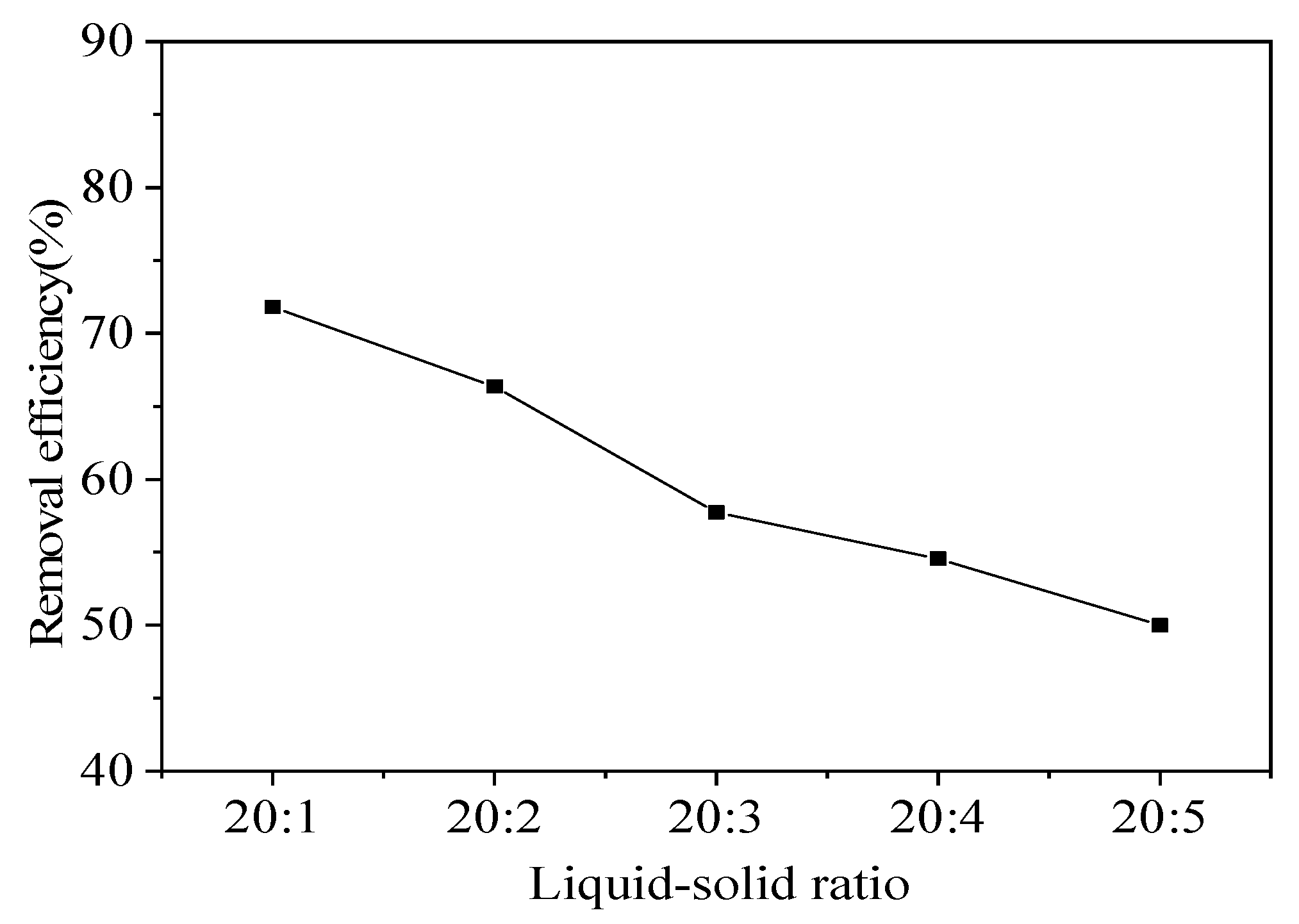1. Introduction
Heavy metal(loid)s are both biologically and industrially important, but the increased demand for these resources have often translated into the unscrupulous exploitation of heavy metal minerals[
1]. During the last few decades, human activities have altered the nature of the surface soil environment[
2,
3], increasing the contamination of soils with heavy metal(loid)s worldwide[
4]. Shahid et al. [5-8] have studied the influence of heavy metal(loid)s in the soil on soil physico-chemical properties, including the soil pH, electrical conductivity, cation exchange capacity, soil mineralogy, and microbial and biological conditions, some of which pose a significant threat to human health[9-13].
There are eight heavy metal(loid)s commonly present in soils: copper(Cu), lead(Pb), zinc(Zn), cadmium(Cd), arsenic(As), chromium(Cr), nickel(Ni), and mercury(Hg)[14-17]. Among these heavy metal(loid)s, As, Pb, Cd, and Hg are included in the top 20 hazardous substances listed by the Agency for Toxic Substances and Disease Registry(ATSDR,2012) and the United States Environmental Protection Agency(US EPA). It has been reported that Pb has a soil persistence period of 150–5000 years[
18]. Pb can cause human ailments such as stomach aches, headaches, and tremors. Therefore, remediating Pb contaminated soils is of vital importance to human and soil health.
In addition, increasing awareness of the impact of soil heavy metal contamination on human and environmental health will improve existing technologies and help to develop new technologies for the contamination remediation. Numerous Pb contaminated soil remediation techniques have been developed during the last two decades[19-21], such as soil replacement[
22],soil isolation[
23], vitrification[
24], electrokinetic remediation[
25,
26], immobilization techniques[
27], encapsulation[
28], soil washing[
4,
6,
29], and biological remediation[
30]. Soil washing refers to the removal of heavy metal(loid)s from the soil using various reagents and extractants that can leach the heavy metal(loid)s from the soil[29,31-34]. Soil washing is frequently used for remediating heavy-metal-contaminated sites because it has a number of potential advantages, such as cost-effectiveness, the complete removal of metals, faster, and it meets specific regulation criteria and reduces long-term liability[29,32-34]. Moreover, soil washing technology has certain limitations, such as the possibility of changing the physicochemical properties of the soil[
35], damaging the ecological structure of the soil[
36], and residues of detergents[
37]. Therefore, the selection of a detergent with high metal removal rate, low toxicity and low damage to soil properties is the key to enable the application of the washing technology. Commonly used drenching agents include[37-40] inorganic washing agents(acid, base, and salt)[41-43], chemical (synthetic) surfactants[
44,
45], biosurfactants (natural surfactants)[
39], Synthetic chelators[46-49], Low-molecular-weight organic acids, and citric acid has a high metal removal rate and is easily biodegradable[47,49-51], which has broad application prospects. This article finally chose citric acid as the leaching agent for detailed experiments.
Target farmland soils are affected by the atmospheric deposition of Pb smelting enterprises, and some heavy metal(loid)s in the soil exceed human health standards, which threatens food security. In this study, batch oscillating washing tests for remediating Pb contaminated soil are studied using soil washing. In addition, extensive continuous tests and plant growth tests were conducted to illustrate the larger-scale applicability of the soil washing technology.
The objectives of this study were to (1) select and compare the leaching effects of different leaching agent, select an environmentally friendly CA with a relatively high efficiency; (2) investigate the effects of different dosage, S/L, temperature and contact times ratios on CA for removing Pb; (3) conduct Extensive continuous tests to verify experimental indicators; and (4) assess changes in physicochemical properties and plant growth before and after soil washing.
2. Materials and Methods
2.1. Soil samples
All experiments were carried out on soil samples contaminated by the metallurgical industry. The sampling point is located in the woodland in the southeast of the smelter, 500~2000m away from the smelter chimney, with 50m as a gradient grid format sampling(E112°57′19″; N35°13′75″) located in Jiyuan (China), multi-point sampling mix, sampling method is soil sampler drilling, sampling depth of 20cm. The main chemical and physical characteristics of the Pb contaminated soil are presented in
Table 1.
The soil samples were heavily contaminated with Pb (Pb 2290 mg·kg-1), while the contents of the other heavy metal(loid)s were within the acceptable range: Cu 105 mg/kg, Zn 199 mg/g, Cd 8.30 mg/kg, Hg 0.902 mg/kg, As 10.50 mg/kg, Cr 67.90 mg/kg, and Ni 38.80 mg/kg.
The fine fraction (<0.0385 mm) largely contributed to the size composition of the soil samples, that is, 49.49% (W·W-1) of the total soil. The 0.045−0.0385 mm, 0.075−0.045 mm, 0.125−0.075 mm, and >0.125 mm fractions represented 12.66%, 13.10%, 16.26%, and 8.49% (W·W-1) of the total soil, respectively. The size distribution of the soil analysis above shows that the soil sample was a fine clay soil, and the influence of fine soil particles should be considered in the remediation tests.
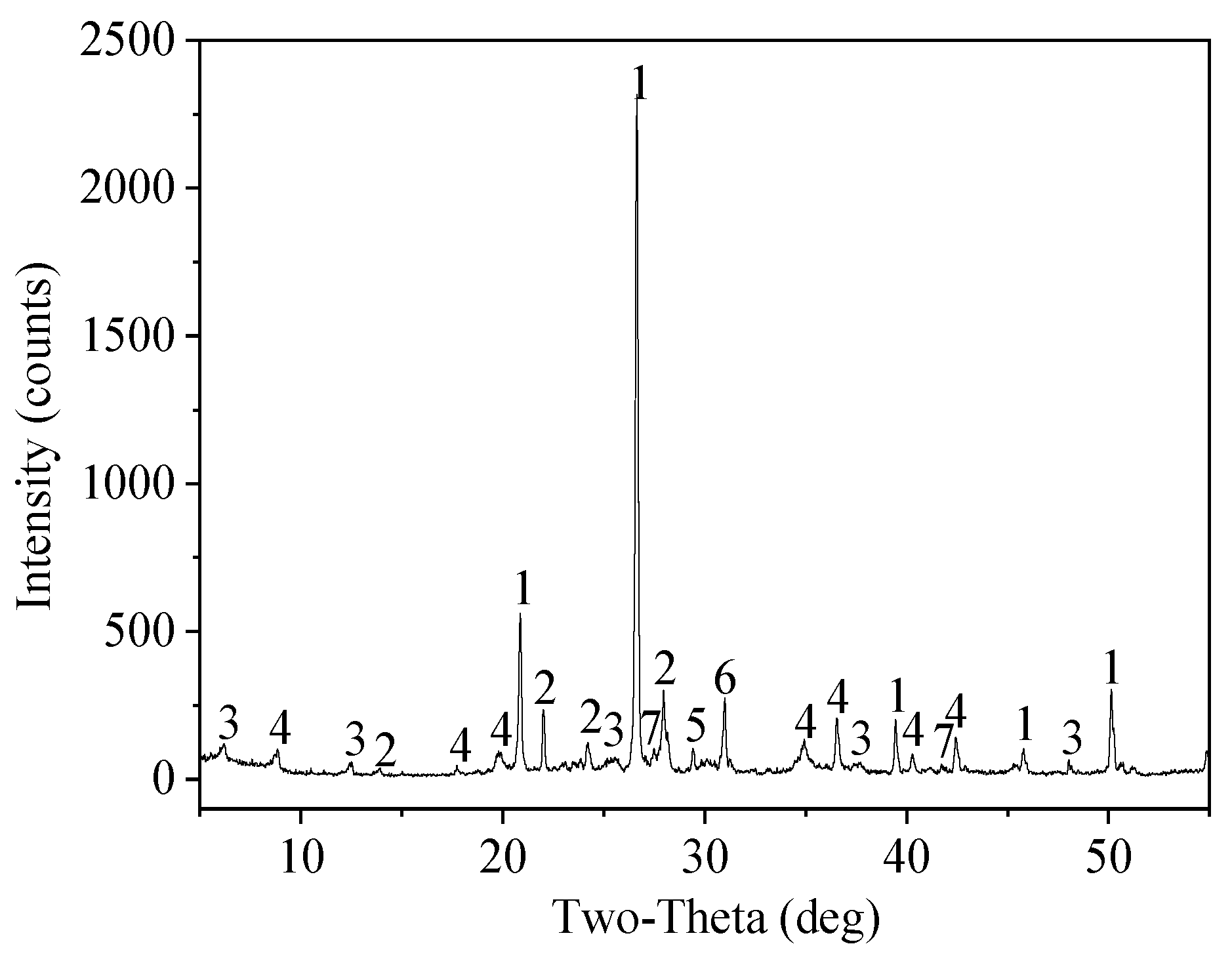
The mineral composition of the soil samples was analyzed by X-ray diffraction (XRD). According to the XRD analysis, the main mineral components in the soil samples were quartz, albite, clinochlore, muscovite, microcline, dolomite, and calcite (
Figure 1). However, Pb minerals were not detected, which was most likely because the Pb content of the remediated soil sample was lower than the lower limit of detection of the D/max-2500PC (D/max-2500PC, Japan).
The soil samples were fine grained. In addition, the flue gas and dust significantly contributed to the heavy metal(loid)s contamination of the soil sample, which came from the metallurgical industry and fine-grained particles[
52,
53]. Therefore, both spherical and square Pb were occasionally found under the condition of strong exposure of the scanning background (
Figure 2a−c). Pb was mainly present in spherical, square, and irregular forms (
Figure 2d−f) after elutriation, which indicates that elutriation removed the dust and fine Pb-containing pollutants and concentrated the Pb.
The phase analysis of the Pb showed that the Pb was mainly present as PbCO3 (53.82%), followed by Pb5(PO4)3Cl2 (20.55%) and PbSO4 (15.66%), and a small amount of PbS (6.36%). This correlated with the flue gas from the Pb smelters, which mainly contained spherical monomer Pb particles. Due to its small specific surface area and high activity, it easily forms PbSO4 in the presence of SO2 and forms square PbS in the presence of S. When PbSO4 is subjected to a carbonic acid solution, it forms PbCO3 as a secondary product.
2.2. Washing agents
The washing agent used in effects of different washing agents was a combination of analytical grade citric acid (CA), tartaric acid (TA), oxalic acid (OX), and acetic acid (HAc), all of which were purchased from the Tianjin Hengxing chemical reagent manufacturing Co., Ltd., and all of the chemicals used in the experiments were analytical grade. While, CA was selected as the washing agent for subsequent experiments through leaching tests.In addition, it should be noted that de-ionized water was used in the experiments.
2.3. Oscillating batch washing tests
The soil sample was air dried and sieved to remove debris, stones, and visible plant materials. It was then crushed (Φ200×125 mm, Wuhan Exploration Machinery Factory) and mixed (
Figure 4). We used a constant temperature shaking table (QYC-211, Shanghai Fuma Test Equipment Co., Ltd) in the soil washing test. First, a certain concentration of washing agent (0.1 mol/L, 0.2 mol/L, 0.3 mol/L, 0.4 mol/L, 0.5 mol/L)and 10 g soil samples were prepared. The detergent was mixed with the soil sample at a certain liquid-solid(L/S) ratio using a conical flask, and the mixture was placed on a constant temperature shaker to control the test parameters such as detergent concentration, L/S ratio, reaction temperature and reaction time. The washed mixture was filtered through 0.45 mm membrane filters (XTLZ-260, Sichuan Bureau of Geology and Mineral Resources), dried at 80°C overnight in a vacuum oven (GZX-9146 MBE, Shanghai Boxun Industrial Co., Ltd) before being weighed, ground (XPM – Φ120×3, Wuhan Exploration Machinery Factory), and content of Pb in washed solid was determined using inductively-coupled plasma optical emission spectroscopy (ICP-OES, Agilent 5100 SVDV ICP-OES, America Agilent Technologies) at the analysis center of Henan Province Rock and Minerals Testing Center. The Pb leaching efficiency was evaluated using Eq.1:
The mass after and mass before are the weight (in g) of the soil after washing and before washing, respectively; [Me] after and [Me] before are the heavy metal contents of the soil (in mg/kg) after washing and before washing, respectively.
2.4. Extensive continuous tests
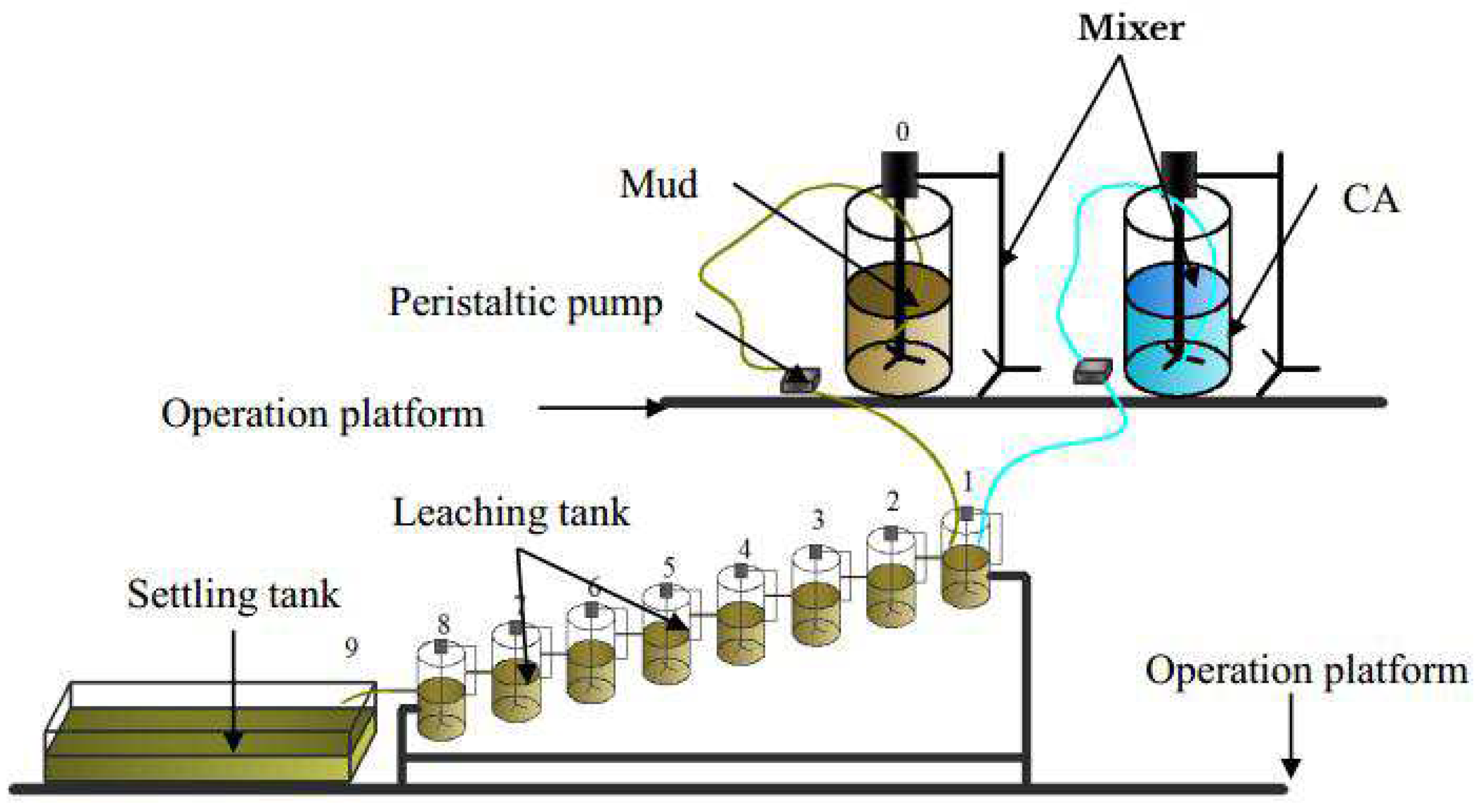
The air-agitation leaching system (
Figure 5), which was developed through independent research, was used in the soil washing extensive continuous tests. These tests involved eight stirring and leaching tanks (15 L), two buckets (200 L) for soil mixing and washing agent mixing, two peristaltic pumps (BT100S, Baoding leifu fluid technology Co., LTD) for accurately controlling the feeding speed, and one settling tank.
As can be seen from
Figure 5, a large volume mixture of washing agent and mud(mixture of soil and water) was prepared with a CA concentration of 0.8 M and a L/S ratio of 20:2. Secondly, the washing agent was mixed with an equal volume of mud, and the stirring and leaching tanks were filled completely. Third, the peristaltic pump parameters were adjusted to accurately control the feeding speed to 50 ml/min, and the valves between the tanks were adjusted to balance the speed of the mud (100 ml/min). It took 2.5 hours to fill a 15 L container and 20 hours to fill eight 15 L containers. Finally, after 24 hours of steady operation, the samples in each drum were separately processed by being filtered, dried, sufficiently ground, determined (as same as 2.3) and analyzed (Eq.1). The final sample, which was discarded into the settling tank, was used for the plant growth tests.
2.5. Plant growth test
After washing the soil with the extensive continuous test, the soil samples were air dried, crushed, and mixed. Then, 500 g of soil samples taken before and after washing were placed in separate pots (D 20cm and H 15cm). One hundred wheat seeds were planted in each pot to investigate the effects of soil Pb-remediation on wheat growth. After 30 days of growth, the stems and leaves of the wheat were collected, dried at 80°C overnight in a vacuum oven, sufficiently ground, and Pb contents been determined (as same as 2.3). The survival rate of the winter wheat was evaluated using Eq.2:
3. Results and Discussion
3.1. Oscillating batch washing tests
3.1.1. Effects of different washing agents on Pb removal efficiency
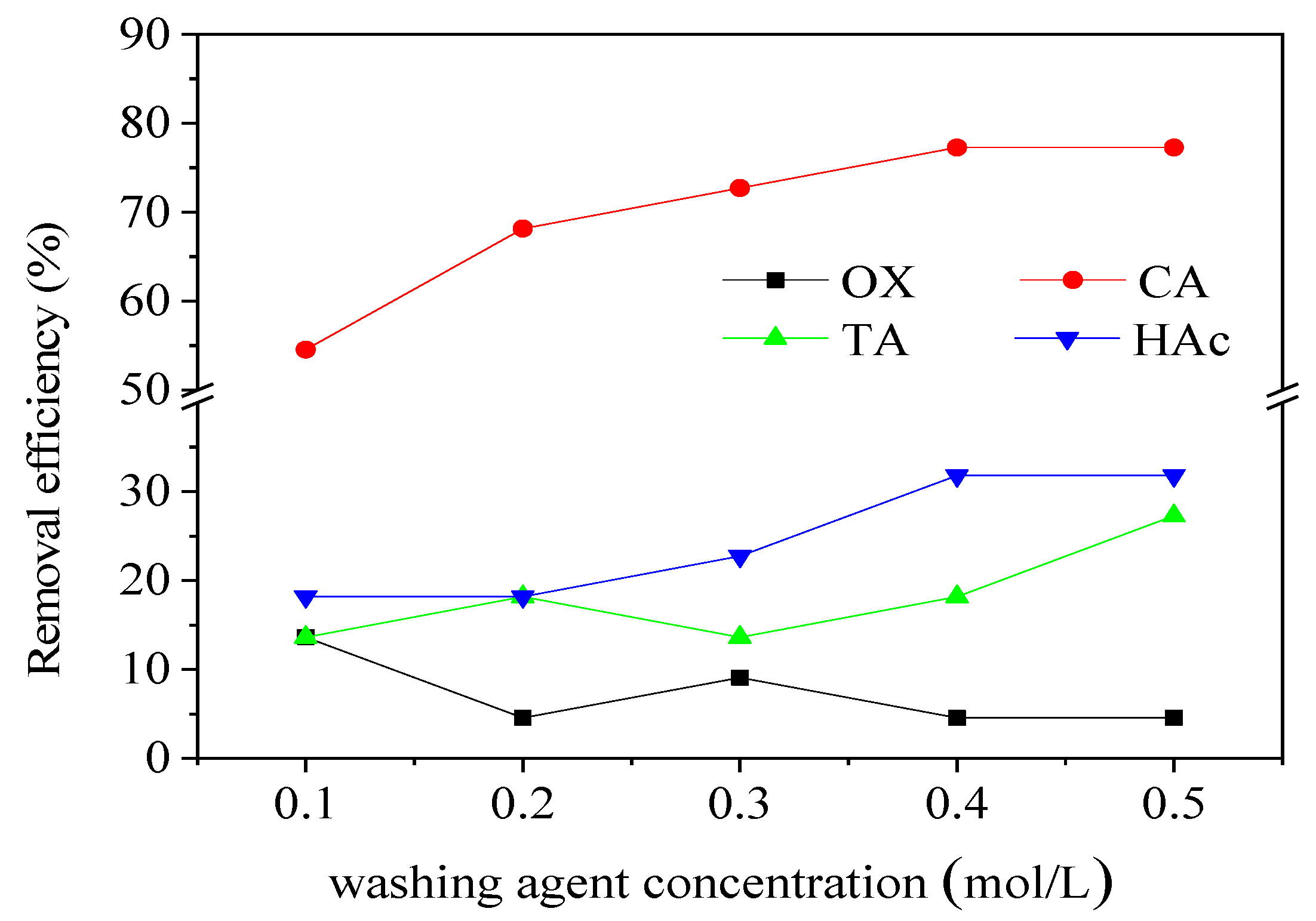
Batch experiments were performed to determine the removal efficiency of different washing agents. With increasing concentrations of oxalic acid (OX), tartaric acid (TA), and acetic acid (HAc), the removal efficiency of Pb was low, while the CA concentration had a significant impact on the Pb removal efficiency (
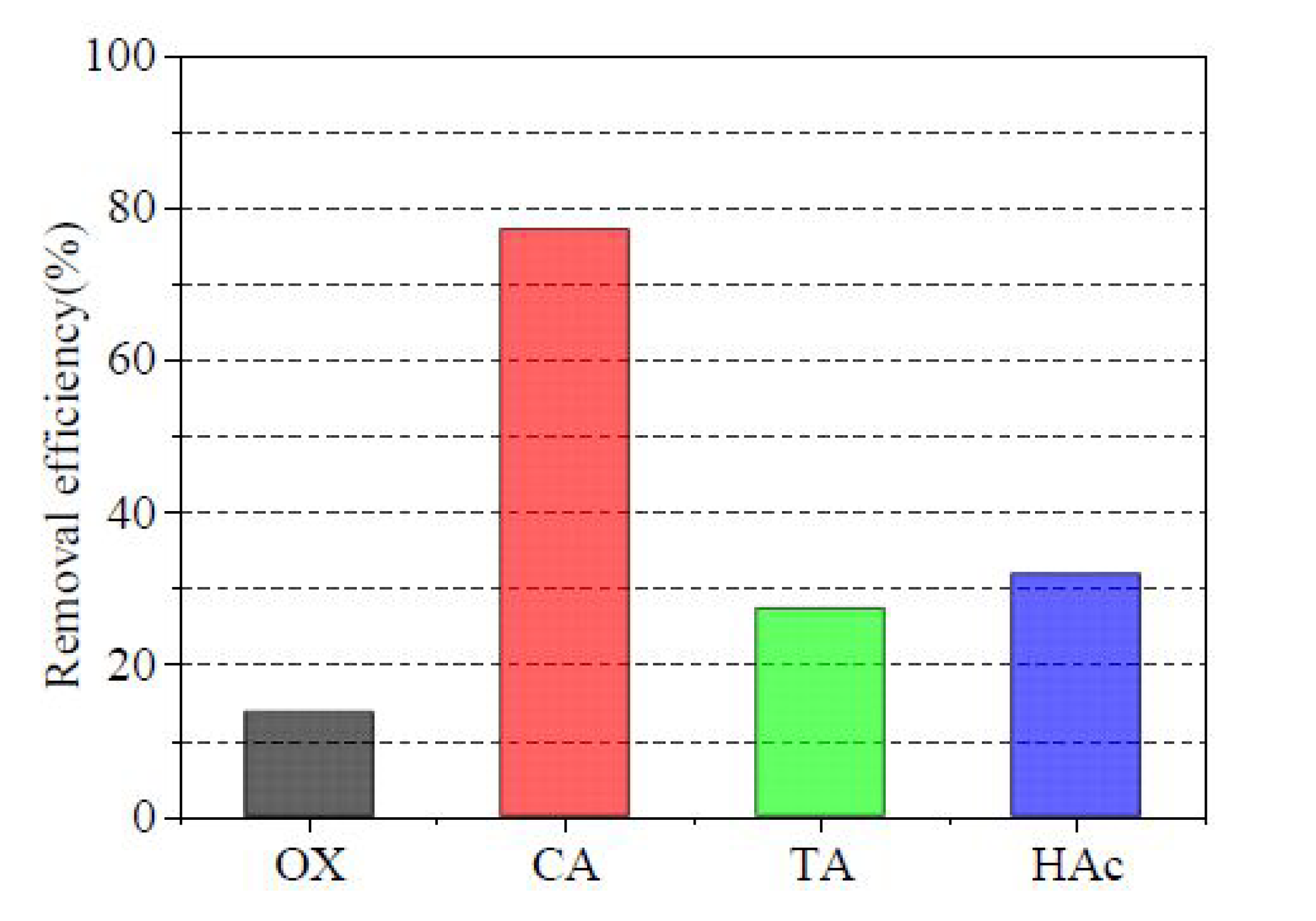
Figure 6). As the concentration of CA increased, the removal efficiency gradually increased. The removal efficiency was 54.55% and 77.27% for CA concentrations of 0.1M and 0.5M, respectively. However, this increase was not significant after a CA concentration of 0.4 M, making 0.4 M the optimal concentration. By a comprehensive comparison, CA had a significant effect on Pb removal efficiency compared with OX, TA, and HAc (
Figure 7). CA has betterPb removal efficiency mainly because, firstly, it can release hydrogen ions by ionization, thus promoting the dissolution of heavy metals through proton competition[
47]; secondly, CA is with carboxyl (-COOH) and hydroxyl (-OH) groups, which can complex heavy metals in soil to form water-soluble metal complexes[
48,
54,
55]. As the optimum washing agent, CA (0.4 M) was used in the other tests.
3.1.2. Effect of L/S ratio on Pb removal efficiency
The S/L ratio is another important factor affecting the removal of heavy metals[
56]. In this study, the effect of the L/S ratio on Pb removal efficiency was determined. As the L/S ratio decreased, the Pb removal efficiency gradually decreased, this is due to the fact that, as the liquid-to-solid ratio increases, the number of drencher functional groups increases during the reaction, which can provide more chelate sites to interact with heavy metals and thus improve the removal efficiency[
42,
57]. Which indicated that a low L/S ratio was not conducive to a high Pb removal efficiency (
Figure 8). The removal efficiency was 71.82% for a L/S ratio of 20:1, while the Pb removal efficiency decreased to 66.36% for a L/S ratio of 20:2. As the L/S ratio continued to decrease, the Pb removal efficiency also decreased significantly. When the L/S ratio was 20:5, the Pb removal efficiency was only 50.0%. However, a higher L/S ratio was not conducive to increasing the treatment capacity. Given the efficiency test results, detailed small-scale tests were conducted with a L/S ratio of 20:1.
3.1.3. Effect of L/S ratio on Pb removal efficiency
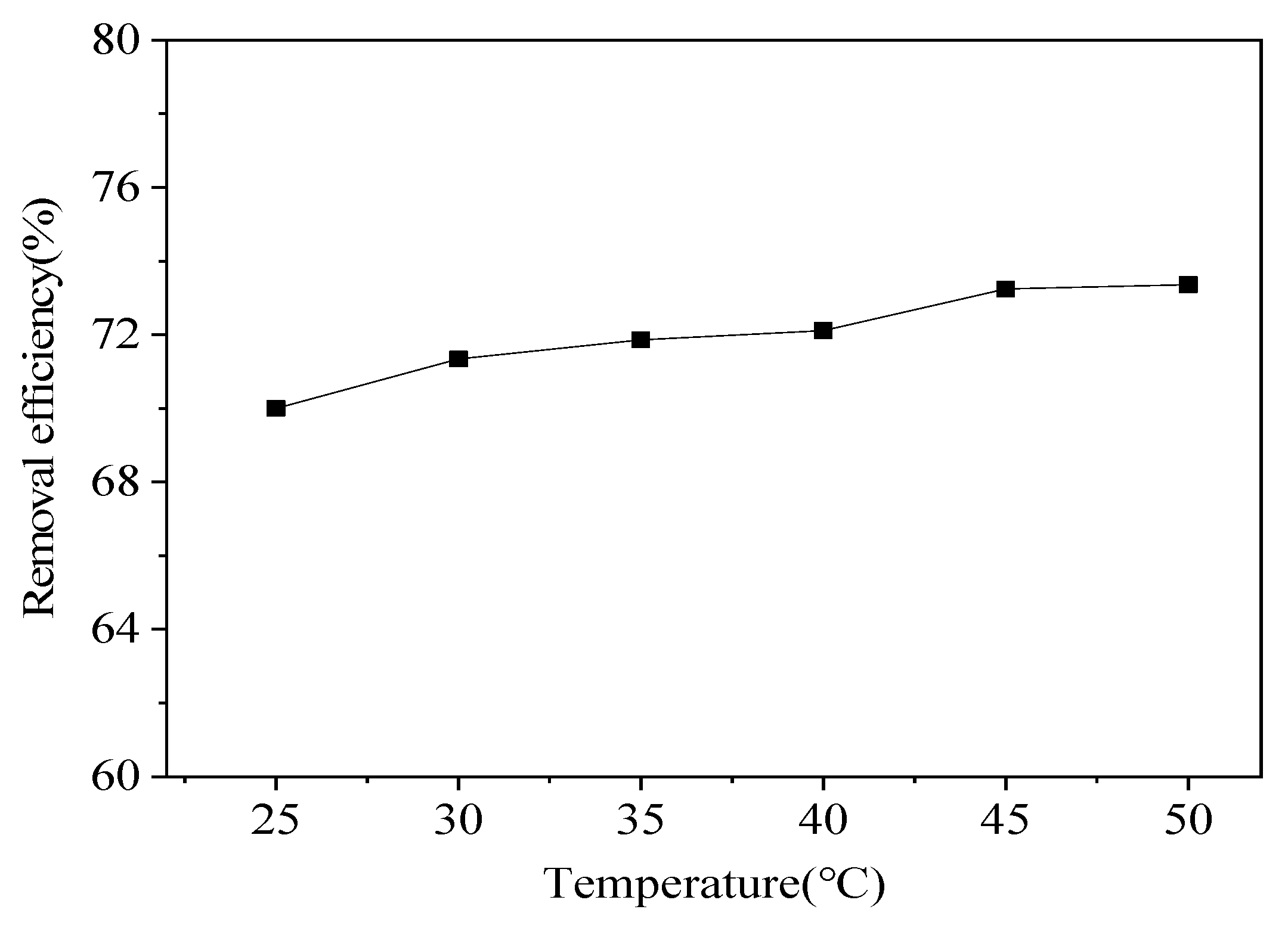
Temperature is known to have an effect on metal(loid) removal efficiency. Thus, tests were conducted to determine the effect of temperature on Pb removal efficiency (
Figure 9). As the leaching temperature increased, the Pb removal efficiency in the contaminated soil gradually increased, but the rate of increase was small. The removal efficiency was 70.0% at 25°C, and when the temperature was increased to 30°C, the efficiency only increased to 71.34%. As the temperature continued to increase, the Pb removal efficiency rate increased slightly, when the temperature was increased to 50°C, the Pb removal efficiency was 73.36%. The results of the temperature tests indicated that increasing the leaching temperature was marginally effective at increasing the Pb removal efficiency, but heating increased the treatment cost. Thus, the subsequent leaching tests were conducted at room temperature.
3.1.4. Effects of leaching time on Pb removal efficiency
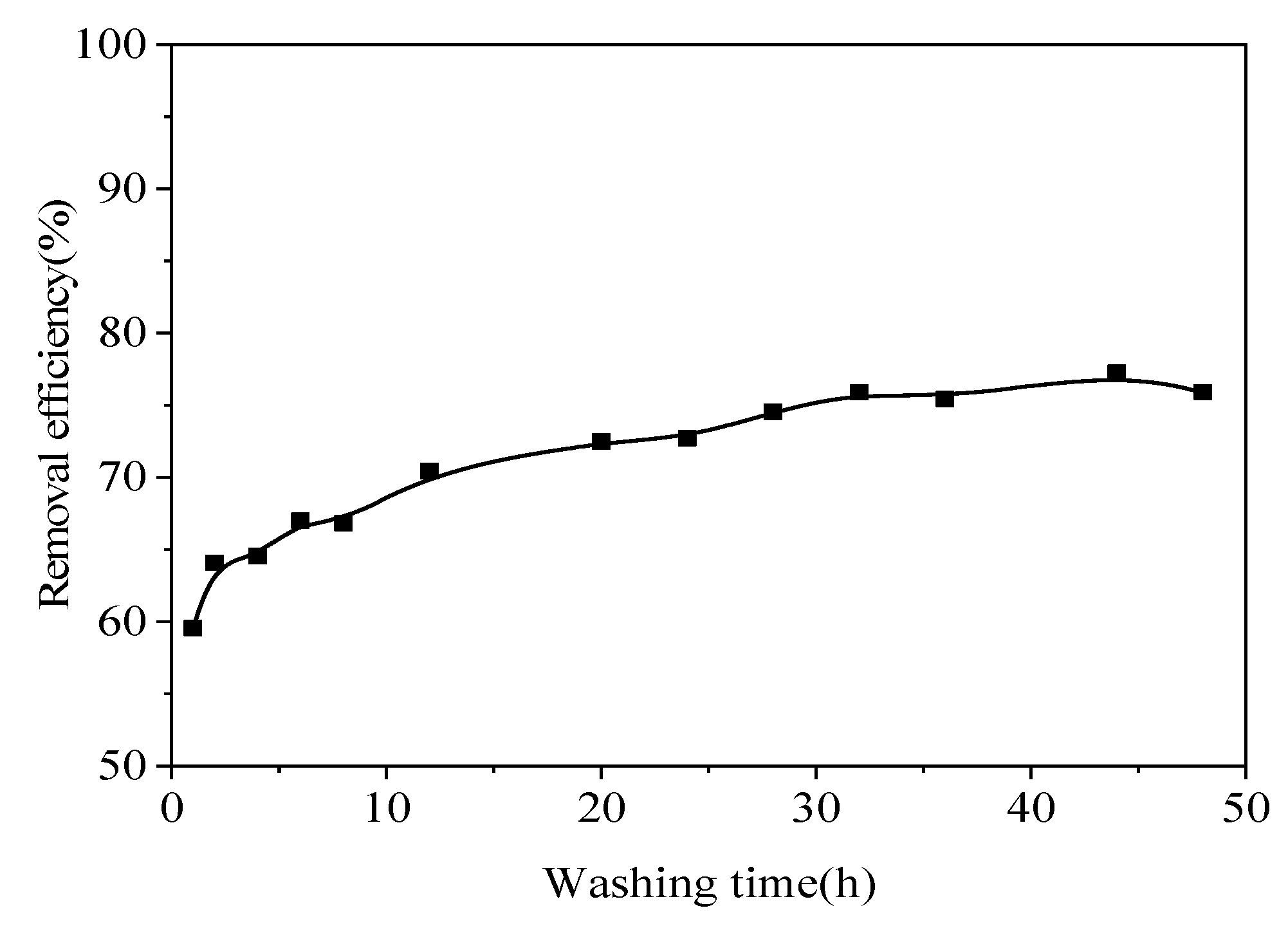
In the tests conducted on the contaminated soil, as the leaching time increased, the Pb removal efficiency gradually increased (
Figure 10). There was a significant difference in the removal efficiency of Pb up to a leaching time of 12 h. The removal efficiency was 59.55% for a leaching time of 1 h, but when the leaching time was increased to 12 h, the Pb removal efficiency increased significantly to 70.45%. With a 20 h leaching time, the Pb removal efficiency only reached 72.50%. After 20 h, the Pb removal efficiency stabilized, and continuing to increase the leaching time was not helpful. The equilibration time of washing may be related to the limited rate of heavy metal dissolution and desorption[
58], and heavy metal desorption/dissolution kinetics may be a more important factor in the heavy metal washing process[
59].Thus, the ideal leaching time was about 20 h, and a leaching time of 20 h was used in the extensive continuous tests.
3.2. Extensive continuous tests
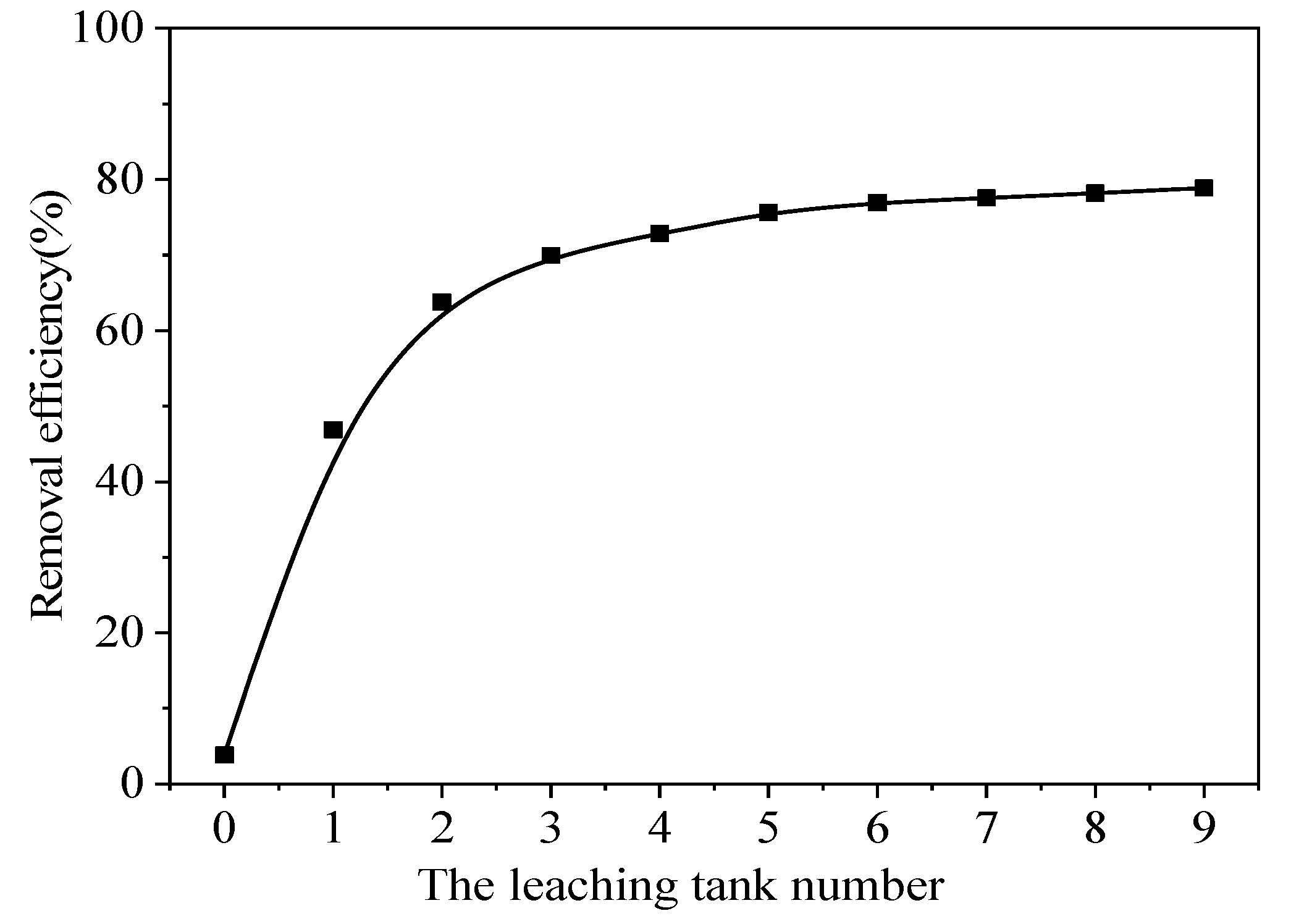
The Pb removal efficiency increased gradually with increased leaching time in the leaching tanks (
Figure 11). CA concentration in the stirring and leaching tanks was 0.4 M, L/S ratio in the stirring and leaching tanks was about 20:1, room temperature 25°C, stirring speed of 1000 r/min, 0 indicates taken from the bucket and a leaching time of 0 h. The numbers 1−8 indicate taken from stirring and leaching tanks 1−8 and leaching times of 0−2.5 h, 2.5−5.0 h, 5.0−7.5 h, 7.5−10.0 h, 10.0−12.5 h, 12.5−15.0 h, 15.0−17.5 h, and 17.5−20.0 h, respectively. Leaching tank 9 indicates the sample taken from the discharge port of the last stirring and leaching tank after a leaching time of 20.0 h. There was a significant difference in the Pb removal efficiency efficiencies of the first two leaching tanks, which meant that the rapid reaction stages occurred in the first 5 hours. The Pb removal efficiency was 63.76% in the second leaching tank, this is the so-called rapid response stage[
42,
57,
60], while the Pb removal efficiency in the third leaching tank (69.94%) experienced a slower increase compared to that of the second leaching tank. As the stirring and leaching tank number continued, the Pb removal efficiency increased slightly. After moving through three tanks with a total leaching time of approximately 7.5 h, the Pb removal efficiency stabilized, and increasing in leaching time the process was not effective. Thus, the optimum leaching time used in the extensive continuous test was 7.5 h.
3.3. Mechanism analysis
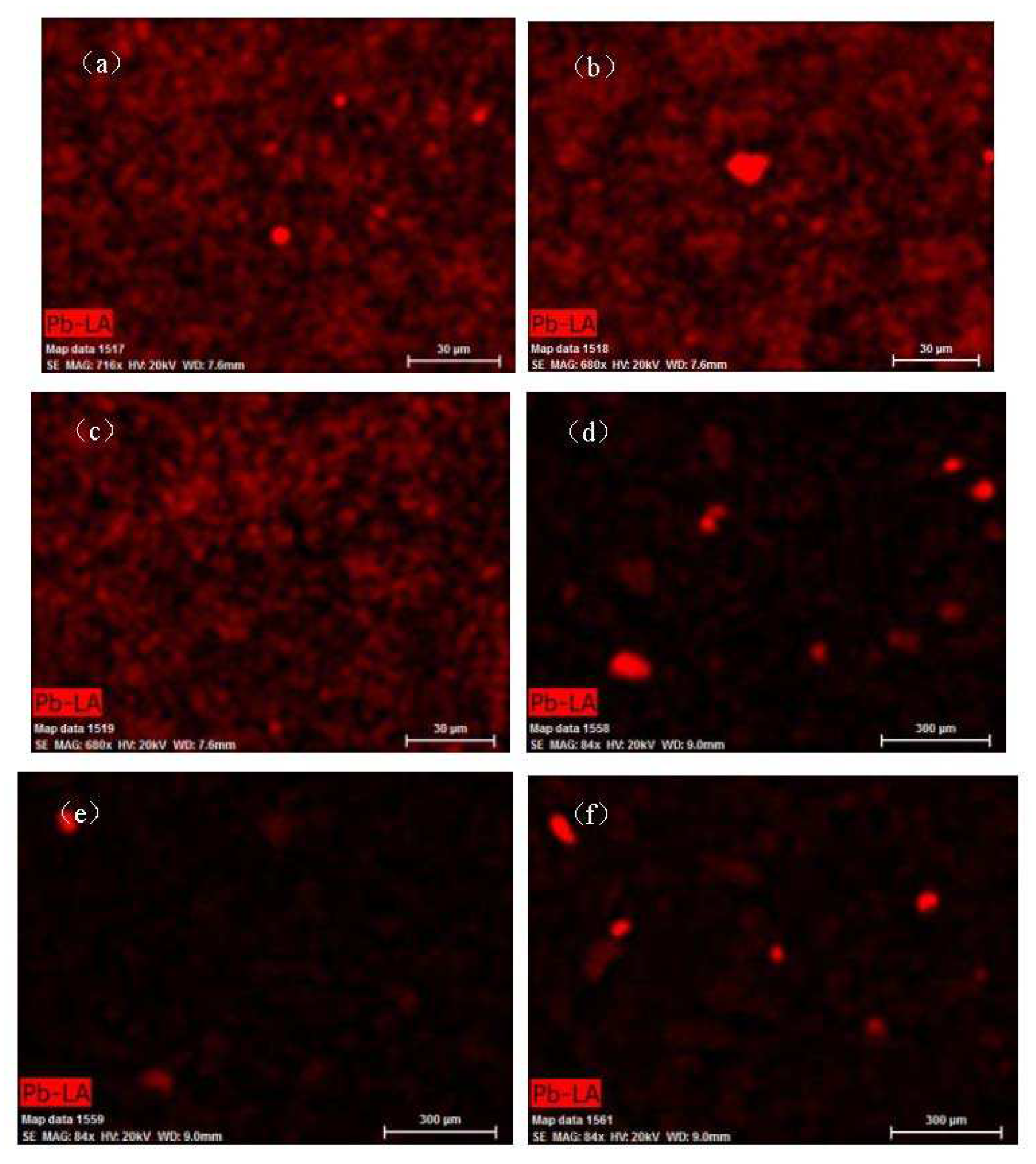
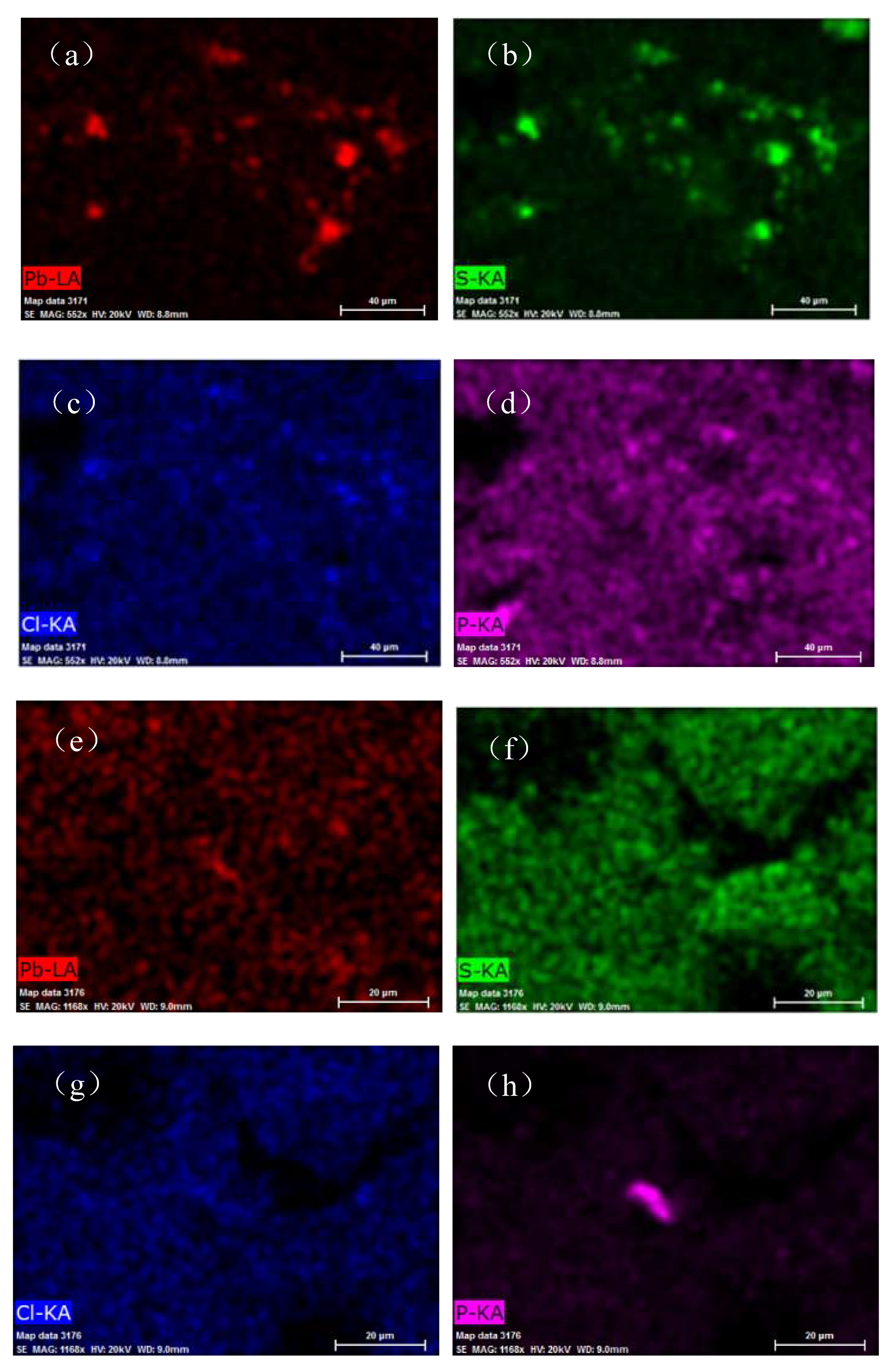
In
Figure 12, the bright areas in panels (a)−(d) corresponds to the distribution of Pb, S, Cl, and P in the contaminated soil, while the bright areas in panels (e)−(h) corresponds to the distribution of Pb, S, Cl, and P in the soil after leaching. As can be seen in
Figure 12a−d, the positions of the Pb and S basically overlap, which indicated that Pb and S were highly correlated. In addition, based on the fact that the bright areas were irregular, we can deduce that the Pb present mainly existed in the form of PbSO
4. Pb also partially overlapped with Cl and P, which indicated that Pb was also correlated with Cl and P. This was consistent with the phase analysis results (Pb
5(PO
4)
3Cl
2, 20.55%). The surface scanning of the soil remediation from the Pb leaching results exhibits no bright areas (
Figure 12e), which indicates that the Pb was removed from the contaminated soil. The surface scanning results for S also exhibited no bright areas, which indicated that PbS and PbSO
4 were removed from the contaminated soil through chemical reactions. The surface scanning results for P exhibited bright areas, but P was not correlated with Pb. Many factors can affect the reaction involved in the leaching of Pb contamination, including the oxygen content and the pH value. Based on the results of the EDS surface scan of the soil samples, PbSO
4, Pb
5(PO
4)
3Cl
2, and PbCO
3 reacted with CA to form Pb citrate, while PbS was produced by the oxidation of PbO, which also reacted with CA to form Pb citrate. The reaction can be described as follows:
3.4. Chemical and physical characteristics before and after the washing treatment
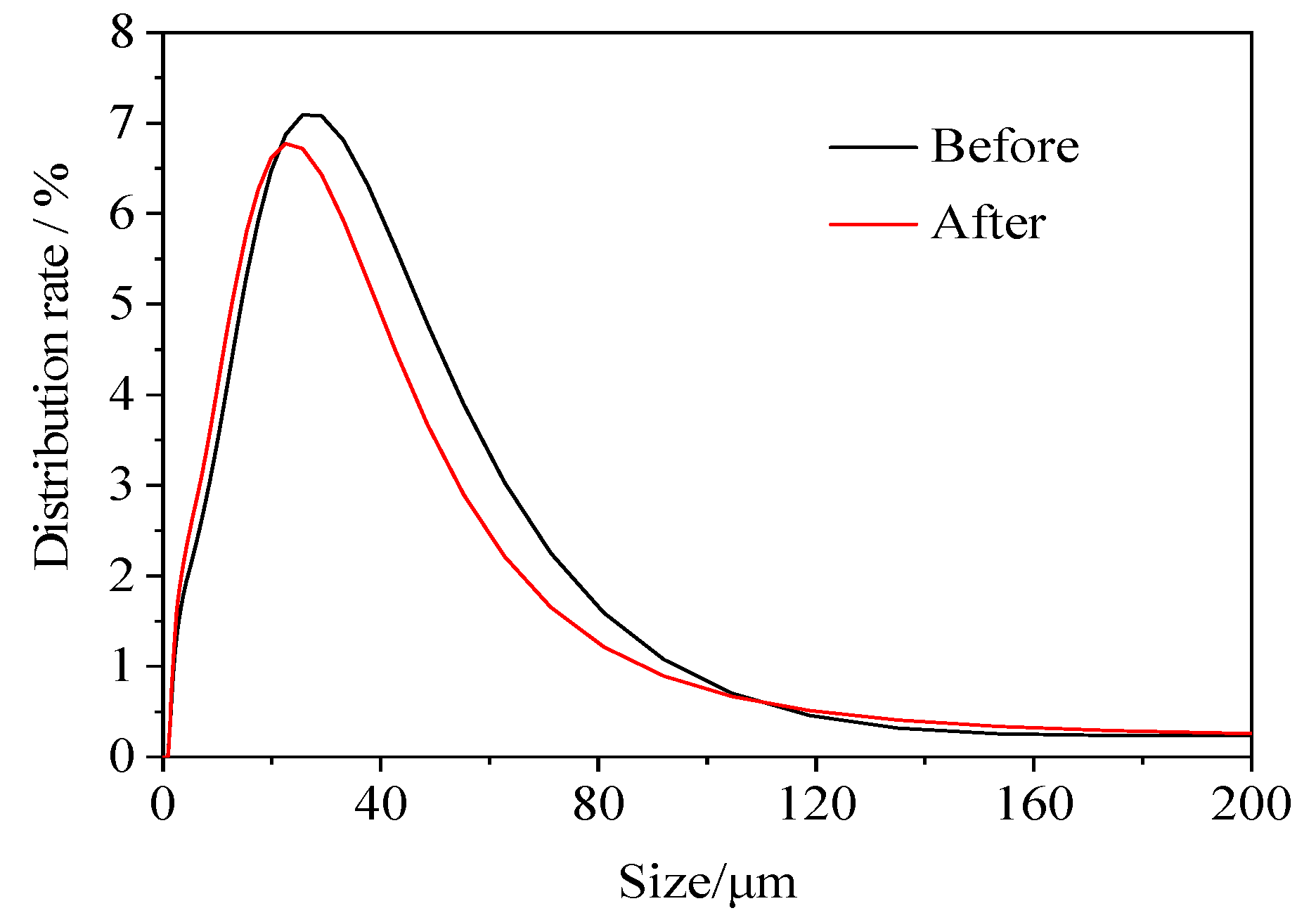
The particle size characteristics of the soil after the washing treatment were slightly different compared to those of the original soil (
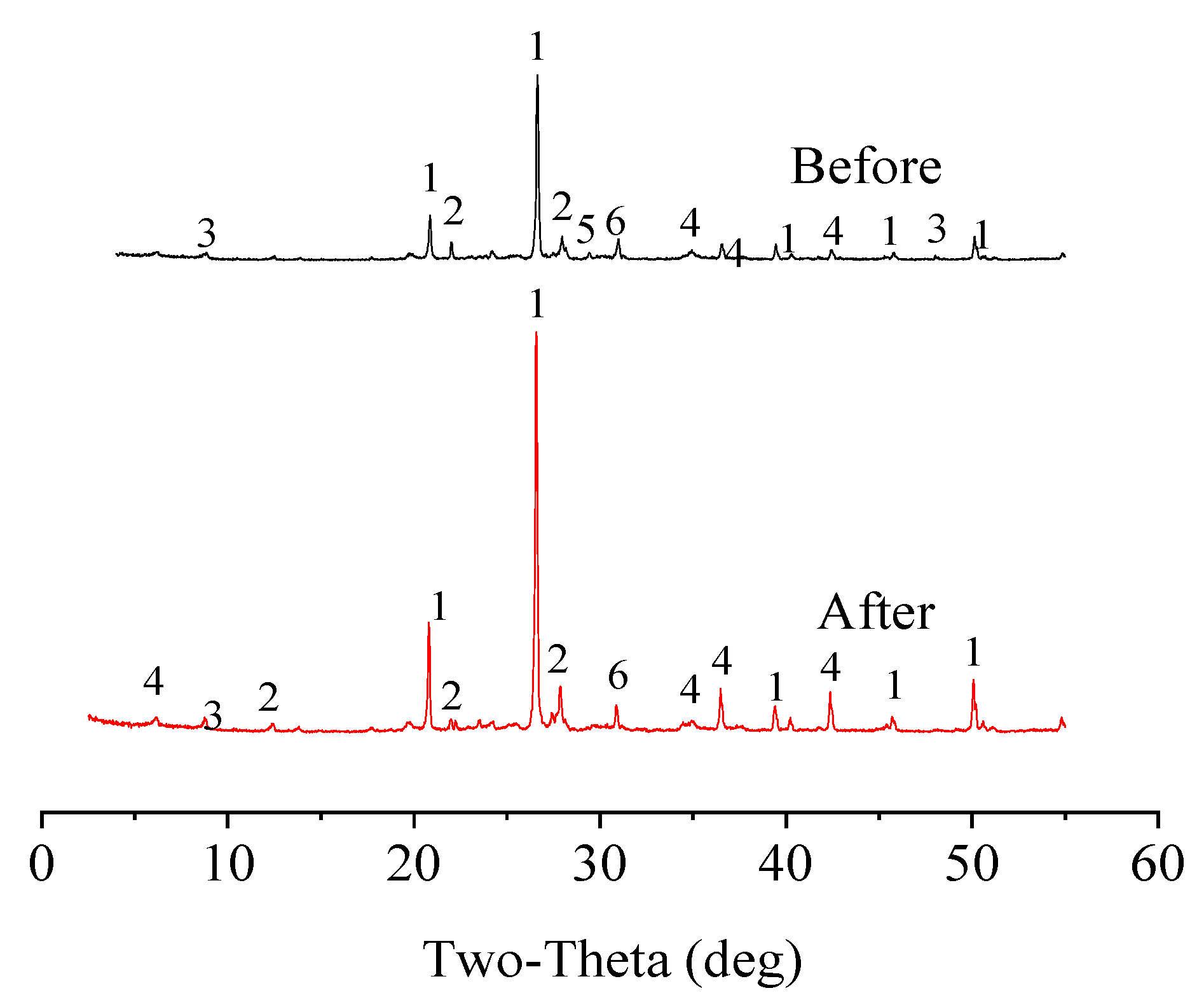
Figure 13). The d50 and d90 of the original soil were 21.90 μm and 62.00 μm, respectively, while those of the soil after the washing treatment were 18.90 μm and 58.10 μm, respectively. The results of the particle size analysis indicated that the leaching remediation had little effect on the particle size composition of the soil; therefore, leaching remediation had a minimal effect on the relative soil permeability. The XRD spectrum of the soil sample before and after the washing treatment is shown in
Figure 14. The peak types were basically the same, which indicated that the washing treatment did not have a significant impact on the mineral composition of the soil in this study. However, the washing treatment did have a negative effect on the soil, i.e., some of the nutrients were lost during the leaching process (
Table 2).
3.5. Plant growth
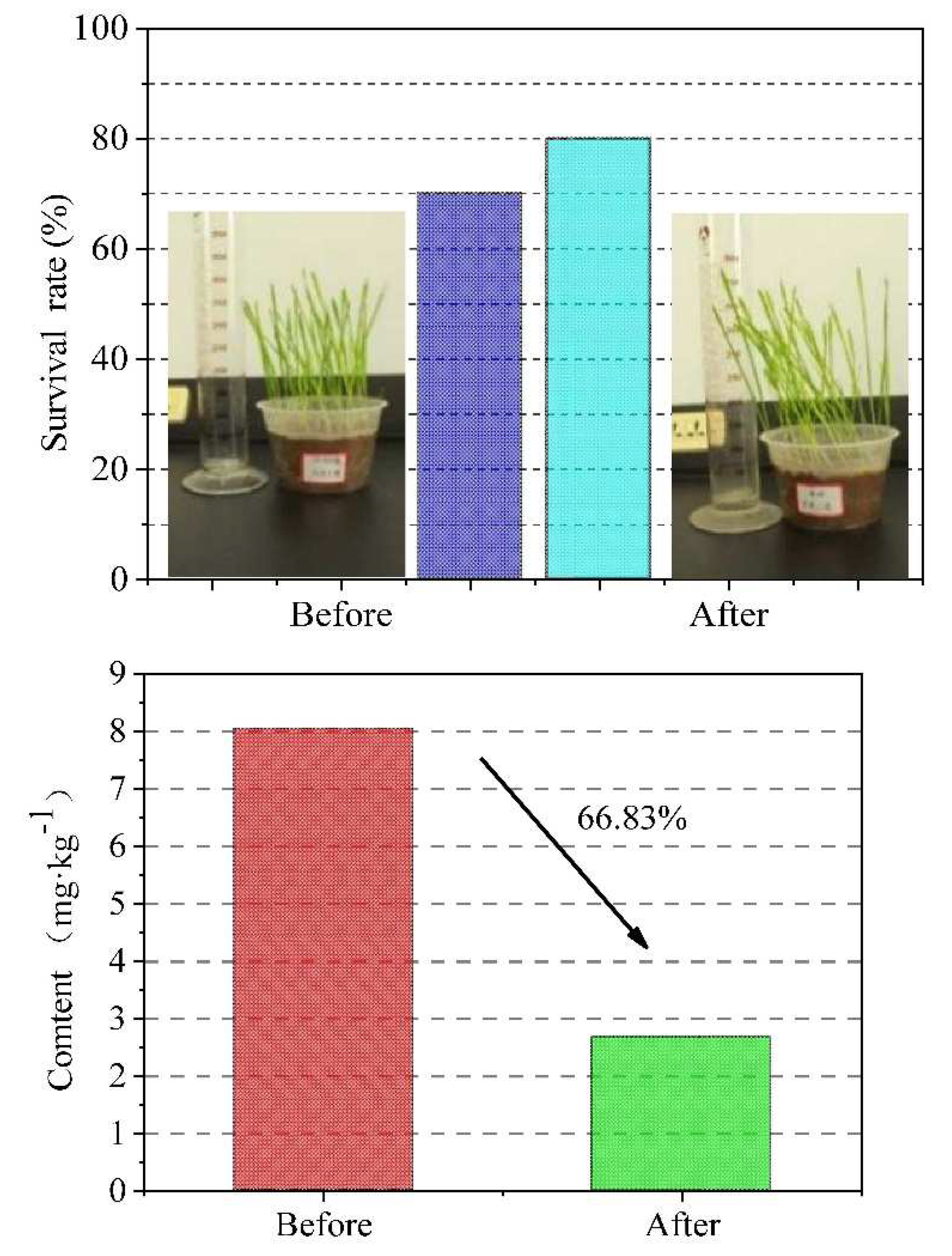
The remediation of the contaminated soil was beneficial to the survival and growth of winter wheat. Before washing, the survival rate was only 70% due to the influence of heavy metal(loid)s and Pb in the soil. After washing, the survival rate of the winter wheat increased significantly to 80%. As can be seen in
Figure 15, the plant height of the winter wheat grown in soils after the leaching restoration was slightly lower compared with the winter wheat potted in un-remediated soil, but the growth was still good.
After 30 days of maintenance and growth, the stems and leaves of the winter wheat were cut and dried, and the contents of the heavy metal(loid)s and Pb were determined. The results indicated that the Pb content of the stems and leaves of the wheat grown in the un-remediated soil (before the washing treatment) was 8.05 mg/kg, while the plants grown in the remediated soil (after the washing treatment) was 66.83% lower (2.67 mg/kg), in Figure 16.
In conclusion, the leaching technology used in this study does not have a negative impact on the survival rate of winter wheat, and theoretically, it will not have a negative impact on the survival rate of other crops. In addition, this leaching technology could reduce the accumulation of Pb in plants. We propose that the leaching technology used in this study is feasible for larger scale agriculture on contaminated soils.
4. Conclusions
Both the leaching regimes used in the batch oscillating washing tests and the extensive continuous test effectively removed Pb from the soil, thereby decreasing the environmental risk from heavy metal(loid) pollution. The PbSO4, Pb5(PO4)3Cl2, PbCO3, and PbS all reacted with the CA to form Pb citrate, which led to a Pb removal efficiency of 72.50% in the oscillating washing tests and 69.94% in the extensive continuous tests. The chemical and physical characteristics of the soil (soil mineralogy, the size characteristic curve, and the major element concentrations) were basically unchanged by the leaching regimes. Besides, the remediation of contaminated soil does not reduce the survival rate of winter wheat (before or after restoration, 70% and 80%, respectively). The Pb content of the stems and leaves of the winter wheat grown in soil before and after the washing treatment was 8.05 mg/kg and 2.67 mg/kg, respectively, demonstrating that the washing treatment resulted in a 66.83% decrease in the Pb content of the stems and leaves. This study demonstrated the feasibility of using citric acid (CA) as a leachate in the remediation of Pb contaminated soil and provides a reference for larger-scale remediation of contaminated soils in the future. However, the repeatability of achieved results and the possibility of field application should be verified in further study.
Author Contributions
Yehao Huang: Data curation, Methodology, Formal analysis, Writing – original draft. Runbo Gao: Writing – review & editing. Zhi Hua: Writing – review & editing. Jingmin Sun: Supervision, Formal analysis. Sensen Zhang: Supervision. Zipei Jia: Visualization, Software. Jia Yao: Visualization, Software. Xiangyu Song: Conceptualization, Resources, Writing – review & editing, Funding acquisition.
Funding
This research was funded by the National Natural Science Foundation of China, grant number 51874259 and 52074244;the Financial planning project of the Henan bureau of ge-exploration and mineral development,grant number 2018[37];the geological science and technology research project of Henan Provincial Institute of Geology in 2023,grant number 2023-904-XM009.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Restrictions apply to the availability of these data. Data were obtained from Zhengzhou University and Henan Academy of Geology, and are available from authors with the permission of Zhengzhou University and Henan Academy of Geology.
Acknowledgments
This research was partially supported by the Wuhan University of Science and Technology and the Jiyuan environmental monitoring station.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shahid, M.; Khalid, S.; Abbas, G.; Shahid, N.; Nadeem, M.; Sabir, M.; Aslam, M.; Dumat, C. Heavy Metal Stress and Crop Productivity. In Crop Production and Global Environmental Issues; 2015; pp. 1–25.
- Hsu, M.J.; Selvaraj, K.; Agoramoorthy, G. Taiwan's industrial heavy metal pollution threatens terrestrial biota. Environmental Pollution 2006, 143, 327–334. [Google Scholar] [CrossRef]
- Ikenaka, Y.; Nakayama, S.M.; Muzandu, K.; Choongo, K.; Teraoka, H.; Mizuno, N.; Ishizuka, M. Heavy metal contamination of soil and sediment in Zambia. African Journal of Environmental Science and Technology 2010, 4, 729–739. [Google Scholar]
- Khalid, S.; Shahid, M.; Niazi, N.K.; Murtaza, B.; Bibi, I.; Dumat, C. A comparison of technologies for remediation of heavy metal contaminated soils. Journal of Geochemical Exploration 2017, 182, 247–268. [Google Scholar] [CrossRef]
- Shahid, M.; Pinelli, E.; Dumat, C. Review of Pb availability and toxicity to plants in relation with metal speciation; role of synthetic and natural organic ligands. Journal of Hazardous Materials 2012, 219-220, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Arshad, M.; Kaemmerer, M.; Pinelli, E.; Probst, A.; Baque, D.; Pradere, P.; Dumat, C. Long-Term Field Metal Extraction by<i>Pelargonium:</i>Phytoextraction Efficiency in Relation to Plant Maturity. International Journal of Phytoremediation 2012, 14, 493–505. [Google Scholar] [CrossRef]
- Shahid, M.; Dumat, C.; Silvestre, J.; Pinelli, E. Effect of fulvic acids on lead-induced oxidative stress to metal sensitive Vicia faba L. plant. Biology and Fertility of Soils 2012, 48, 689–697. [Google Scholar] [CrossRef]
- Shahid, M.; Dumat, C.; Aslam, M.; Pinelli, E. Assessment of lead speciation by organic ligands using speciation models. Chemical Speciation & Bioavailability 2012, 24, 248–252. [Google Scholar] [CrossRef]
- Zotiadis, V.; Argyraki, A.; Theologou, E. Pilot-Scale Application of Attapulgitic Clay for Stabilization of Toxic Elements in Contaminated Soil. Journal of Geotechnical and Geoenvironmental Engineering 2012, 138, 633–637. [Google Scholar] [CrossRef]
- Wu, Y.J.; Zhou, H.; Zou, Z.J.; Zhu, W.; Yang, W.T.; Peng, P.Q.; Zeng, M.; Liao, B.H. A three-year in-situ study on the persistence of a combined amendment (limestone+sepiolite) for remedying paddy soil polluted with heavy metals. Ecotoxicol Environ Saf 2016, 130, 163–170. [Google Scholar] [CrossRef]
- Adelekan, B.; Abegunde, K. Heavy metals contamination of soil and groundwater at automobile mechanic villages in Ibadan, Nigeria. International Journal of the Physical Sciences 2011, 6, 1045–1058. [Google Scholar]
- Sun, Y.; Xu, Y.; Xu, Y.; Wang, L.; Liang, X.; Li, Y. Reliability and stability of immobilization remediation of Cd polluted soils using sepiolite under pot and field trials. Environmental Pollution 2016, 208, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Evangelou, M.W.H.; Ebel, M.; Schaeffer, A. Evaluation of the effect of small organic acids on phytoextraction of Cu and Pb from soil with tobacco Nicotiana tabacum. Chemosphere 2006, 63, 996–1004. [Google Scholar] [CrossRef]
- Gong, Y.; Zhao, D.; Wang, Q. An overview of field-scale studies on remediation of soil contaminated with heavy metals and metalloids: Technical progress over the last decade. Water Res 2018, 147, 440–460. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Mohamed, I.; Raleve, D.; Chen, W.; Huang, Q. RETRACTED ARTICLE: Field evaluation of intensive compost application on Cd fractionation and phytoavailability in a mining-contaminated soil. Environmental Geochemistry and Health 2016, 38, 1193–1201. [Google Scholar] [CrossRef] [PubMed]
- Babu, A.G.; Kim, J.-D.; Oh, B.-T. Enhancement of heavy metal phytoremediation by Alnus firma with endophytic Bacillus thuringiensis GDB-1. Journal of Hazardous Materials 2013, 250-251, 477–483. [Google Scholar] [CrossRef]
- Wang, C.; Wang, H. Carboxyl functionalized Cinnamomum camphora for removal of heavy metals from synthetic wastewater-contribution to sustainability in agroforestry. Journal of Cleaner Production 2018, 184, 921–928. [Google Scholar] [CrossRef]
- Kumar, P.B.A.N.; Dushenkov, V.; Motto, H.; Raskin, I. Phytoextraction: The Use of Plants To Remove Heavy Metals from Soils. Environmental Science & Technology 1995, 29, 1232–1238. [Google Scholar] [CrossRef]
- Verkleij, J.A.C.; Golan-Goldhirsh, A.; Antosiewisz, D.M.; Schwitzguébel, J.-P.; Schröder, P. Dualities in plant tolerance to pollutants and their uptake and translocation to the upper plant parts. Environmental and Experimental Botany 2009, 67, 10–22. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, X.; Sun, R.; Cao, Y. Neutralization of red mud using bio-acid generated by hydrothermal carbonization of waste biomass for potential soil application. Journal of Cleaner Production 2020, 271. [Google Scholar] [CrossRef]
- Wang, C.; Luo, D.; Zhang, X.; Huang, R.; Cao, Y.; Liu, G.; Zhang, Y.; Wang, H. Biochar-based slow-release of fertilizers for sustainable agriculture: A mini review. Environmental Science and Ecotechnology 2022, 10. [Google Scholar] [CrossRef]
- Yao, Z.; Li, J.; Xie, H.; Yu, C. Review on Remediation Technologies of Soil Contaminated by Heavy Metals. Procedia Environmental Sciences 2012, 16, 722–729. [Google Scholar] [CrossRef]
- Zheng, C.; Wang, P.P. A Field Demonstration of the Simulation Optimization Approach for Remediation System Design. Ground Water 2002, 40, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Mallampati, S.R.; Mitoma, Y.; Okuda, T.; Simion, C.; Lee, B.K. Dynamic immobilization of simulated radionuclide 133Cs in soil by thermal treatment/vitrification with nanometallic Ca/CaO composites. J Environ Radioact 2015, 139, 118–124. [Google Scholar] [CrossRef]
- Mao, X.; Han, F.X.; Shao, X.; Guo, K.; McComb, J.; Arslan, Z.; Zhang, Z. Electro-kinetic remediation coupled with phytoremediation to remove lead, arsenic and cesium from contaminated paddy soil. Ecotoxicology and Environmental Safety 2016, 125, 16–24. [Google Scholar] [CrossRef]
- Rozas, F.; Castellote, M. Electrokinetic remediation of dredged sediments polluted with heavy metals with different enhancing electrolytes. Electrochimica Acta 2012, 86, 102–109. [Google Scholar] [CrossRef]
- Khan, M.A.; Chattha, M.R.; Farooq, K.; Jawed, M.A.; Farooq, M.; Imran, M.; Iftkhar, M.; Kasana, M.I. Effect of farmyard manure levels and NPK applications on the pea plant growth, pod yield and quality. Life Sci. Int. J 2015, 9, 2. [Google Scholar]
- Ucaroglu, S.; Talinli, İ. Recovery and safer disposal of phosphate coating sludge by solidification/stabilization. Journal of Environmental Management 2012, 105, 131–137. [Google Scholar] [CrossRef]
- Gu, F.; Zhang, J.; Shen, Z.; Li, Y.; Ji, R.; Li, W.; Zhang, L.; Han, J.; Xue, J.; Cheng, H. A review for recent advances on soil washing remediation technologies. Bulletin of Environmental Contamination and Toxicology 2022, 109, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Marques, A.P.G.C.; Rangel, A.O.S.S.; Castro, P.M.L. Remediation of Heavy Metal Contaminated Soils: Phytoremediation as a Potentially Promising Clean-Up Technology. Critical Reviews in Environmental Science and Technology 2009, 39, 622–654. [Google Scholar] [CrossRef]
- Guo, X.; Wei, Z.; Wu, Q.; Li, C.; Qian, T.; Zheng, W. Effect of soil washing with only chelators or combining with ferric chloride on soil heavy metal removal and phytoavailability: Field experiments. Chemosphere 2016, 147, 412–419. [Google Scholar] [CrossRef]
- Park, B.; Son, Y. Ultrasonic and mechanical soil washing processes for the removal of heavy metals from soils. Ultrason Sonochem 2017, 35, 640–645. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xu, Y.; Kanyerere, T.; Wang, Y.-s.; Sun, M. Washing Reagents for Remediating Heavy-Metal-Contaminated Soil: A Review. Frontiers in Earth Science 2022, 10. [Google Scholar] [CrossRef]
- Zheng, X.-J.; Li, Q.; Peng, H.; Zhang, J.-X.; Chen, W.-J.; Zhou, B.-C.; Chen, M. Remediation of Heavy Metal-Contaminated Soils with Soil Washing: A Review. Sustainability 2022, 14. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, X.; Zhang, X.; Lu, Y.; Chen, M.; Sun, Y.; Ye, P. Experimental study on remediation of low permeability Cu-Zn contaminated clay by vacuum enhanced leaching combined with EDTA and hydrochloric acid. Chemosphere 2022, 298. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, C.; Deng, S.; Zhang, J.; Hou, J.; Wang, C.; Fu, Z. Effect of different washing solutions on soil enzyme activity and microbial community in agricultural soil severely contaminated with cadmium. Environmental Science and Pollution Research 2022, 29, 54641–54651. [Google Scholar] [CrossRef]
- Shukla, M.; Baksi, B.; Mohanty, S.P.; Mahanty, B.; Mansi, A.; Rene, E.R.; Behera, S.K. Remediation of chromium contaminated soil by soil washing using EDTA and N-acetyl-L-cysteine as the chelating agents. Progress in Organic Coatings 2022, 165. [Google Scholar] [CrossRef]
- Chen, Y.; Jiang, H.; Li, Y.; Liu, Y.; Chen, Y.; Chen, L.; Luo, X.; Tang, P.; Yan, H.; Zhao, M.; et al. A critical review on EDTA washing in soil remediation for potentially toxic elements (PTEs) pollutants. Reviews in Environmental Science and Bio-Technology 2022, 21, 399–423. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, L.; Liu, Q.; Li, J.; Qiao, Z.; Sun, P.; Yang, Y. A critical review on soil washing during soil remediation for heavy metals and organic pollutants. International Journal of Environmental Science and Technology 2022, 19, 601–624. [Google Scholar] [CrossRef]
- Qian, J.; Li, Y.-h.; Su, F.; Wu, J.-g.; Sun, J.-r.; Huang, T.-c. Citric acid-based deep eutectic solvent (CA-DES) as a new soil detergent for the removal of cadmium from coking sites. Environmental Science and Pollution Research 2022. [Google Scholar] [CrossRef]
- Navarro, A.; Martinez, F. The use of soil-flushing to remediate metal contamination in a smelting slag dumping area: Column and pilot-scale experiments. Engineering Geology 2010, 115, 16–27. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, H.; Wang, H.; Li, Q.; Li, Y. Effect of soil washing on heavy metal removal and soil quality: A two-sided coin. Ecotoxicology and Environmental Safety 2020, 203. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zeng, X.; Zhang, H.; Li, Y.; Zhao, S.; Su, S.; Bai, L.; Wang, Y.; Zhang, T. Effect of exogenous phosphate on the lability and phytoavailability of arsenic in soils. Chemosphere 2018, 196, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Paria, S. Surfactant-enhanced remediation of organic contaminated soil and water. Advances in Colloid and Interface Science 2008, 138, 24–58. [Google Scholar] [CrossRef]
- da Rocha Junior, R.B.; Meira, H.M.; Almeida, D.G.; Rufino, R.D.; Luna, J.M.; Santos, V.A.; Sarubbo, L.A. Application of a low-cost biosurfactant in heavy metal remediation processes. Biodegradation 2019, 30, 215–233. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.; Huang, F.; Liang, F.; Guo, Z. Continuous Soil Washing with EDTA/DTPA Combined with Citric Acid for Removing Cd and Pb and Its Impact on Soil Fertility. Mining and Metallurgical Engineering 2019, 39, 74–78. [Google Scholar]
- Hu, W.; Niu, Y.; Zhu, H.; Dong, K.; Wang, D.; Liu, F. Remediation of zinc-contaminated soils by using the two-step washing with citric acid and water-soluble chitosan. Chemosphere 2021, 282. [Google Scholar] [CrossRef] [PubMed]
- Piri, M.; Sepehr, E.; Rengel, Z. Citric acid decreased and humic acid increased Zn sorption in soils. Geoderma 2019, 341, 39–45. [Google Scholar] [CrossRef]
- Yu, Y.; Li, Y.; Chen, H.; Wang, Y.; Liu, M. Mild washing of uranium containing soil with citric acid combined with anion and cation exchange resin. Journal of Radioanalytical and Nuclear Chemistry 2022, 331, 145–163. [Google Scholar] [CrossRef]
- Chang, S.; Lee, H.-K.; Kang, H.-B.; Kim, T.-J.; Park, S.; Jeon, H. Decontamination of Uranium-Contaminated Soil by Acid Washing with Uranium Recovery. Water Air and Soil Pollution 2021, 232. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, S.; Ren, D.; Zhang, X.; Liu, S.; Pan, Z.; Wang, S. A Comparative Study of Ferric Nitrate and Citric Acid in Washing Heavy Metal-Contaminated Soil. Environmental Engineering Science 2021, 38, 764–776. [Google Scholar] [CrossRef]
- Cao, X.; Zhang, S.; Tan, C.; Hu, P.; Cui, X.; Zhou, T.; Wu, L.; Luo, Y. Heavy Metal Contamination Characteristics in Soils Around a Nonferrous Metal Smelter in Central Southern China. Soils 2015, 47, 94–99. [Google Scholar]
- Cheng, Y.; Zhao, Z.; Wang, Y.; Qiu, K.; Fu, Y.; Zhao, X.; Li, L. Soil Heavy Metals Pollution in the Farmland near a Lead Smelter in Henan Province. Chinese Journal of Soil Science 2014, 45, 1505–1510. [Google Scholar]
- Dang, J.; Wang, H.; Wang, C. Adsorption of Toxic Zinc by Functionalized Lignocellulose Derived from Waste Biomass: Kinetics, Isotherms and Thermodynamics. Sustainability 2021, 13. [Google Scholar] [CrossRef]
- Wang, C.; Wang, H.; Gu, G. Ultrasound-assisted xanthation of cellulose from lignocellulosic biomass optimized by response surface methodology for Pb(II) sorption. Carbohydrate Polymers 2018, 182, 21–28. [Google Scholar] [CrossRef]
- Cheng, S.; Lin, Q.; Wang, Y.; Luo, H.; Huang, Z.; Fu, H.; Chen, H.; Xiao, R. The removal of Cu, Ni, and Zn in industrial soil by washing with EDTA-organic acids. Arabian Journal of Chemistry 2020, 13, 5160–5170. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, Q.; Xiao, R.; Cheng, S.; Luo, H.; Wen, X.; Wu, L.; Zhong, Q. Removal of Cu and Pb from contaminated agricultural soil using mixed chelators of fulvic acid potassium and citric acid. Ecotoxicology and Environmental Safety 2020, 206. [Google Scholar] [CrossRef]
- Wei, M.; Chen, J.; Wang, X. Removal of arsenic and cadmium with sequential soil washing techniques using Na(2)EDTA, oxalic and phosphoric acid: Optimization conditions, removal effectiveness and ecological risks. Chemosphere 2016, 156, 252–261. [Google Scholar] [CrossRef]
- Zhang, W.; Huang, H.; Tan, F.; Wang, H.; Qiu, R. Influence of EDTA washing on the species and mobility of heavy metals residual in soils. Journal of Hazardous Materials 2010, 173, 369–376. [Google Scholar] [CrossRef]
- Yan, D.; Guo, Z.; Xiao, X.; Peng, C.; He, Y.; Yang, A.; Wang, X.; Hu, Y.; Li, Z. Cleanup of arsenic, cadmium, and lead in the soil from a smelting site using N,N-bis(carboxymethyl)-L-glutamic acid combined with ascorbic acid: A lab-scale experiment. Journal of Environmental Management 2021, 296. [Google Scholar] [CrossRef]
Figure 1.
XRD of the soil sample (1-Quartz, 2-Albite, 3-Clinochlore, 4-Muscovite, 5-Calcite, 6-Dolomite, and 7-Microcline).
Figure 1.
XRD of the soil sample (1-Quartz, 2-Albite, 3-Clinochlore, 4-Muscovite, 5-Calcite, 6-Dolomite, and 7-Microcline).
Figure 2.
EDS surface scans of the soil sample ((a)~(c) the original soil sample; (d)~(f) the heavy fractions after elutriation).
Figure 2.
EDS surface scans of the soil sample ((a)~(c) the original soil sample; (d)~(f) the heavy fractions after elutriation).
Figure 3.
Pb element chemical phase analysis.
Figure 3.
Pb element chemical phase analysis.
Figure 4.
Experimental flow diagram.
Figure 4.
Experimental flow diagram.
Figure 5.
The air-agitation leaching system.
Figure 5.
The air-agitation leaching system.
Figure 6.
Pb removal efficiency versus washing agent concentration (liquid solid ratio 20:1, 25°C, leaching time 20 h, speed 150 r/min).
Figure 6.
Pb removal efficiency versus washing agent concentration (liquid solid ratio 20:1, 25°C, leaching time 20 h, speed 150 r/min).
Figure 7.
Pb removal efficiency of different washing agents. (0.1 M OX, 0.4 M CA, 0.5 M TA, 0.4 M HAc; L/S ratio 20:1, 25°C, leaching time 20 h, speed 150 r/min).
Figure 7.
Pb removal efficiency of different washing agents. (0.1 M OX, 0.4 M CA, 0.5 M TA, 0.4 M HAc; L/S ratio 20:1, 25°C, leaching time 20 h, speed 150 r/min).
Figure 8.
Pb removal efficiency versus L/S ratio (0.4 M CA, room temperature 25°C, leaching time 10 h, speed 150 r/min).
Figure 8.
Pb removal efficiency versus L/S ratio (0.4 M CA, room temperature 25°C, leaching time 10 h, speed 150 r/min).
Figure 9.
Pb removal efficiency versus temperature. (0.4 M CA, concentration L/S ratio 20:1, leaching time 10 h, speed 150 r/min).
Figure 9.
Pb removal efficiency versus temperature. (0.4 M CA, concentration L/S ratio 20:1, leaching time 10 h, speed 150 r/min).
Figure 10.
Pb removal efficiency versus leaching time (CA concentration of 0.4 M, L/S ratio 20:1, room temperature 25°C, speed 150 r/min).
Figure 10.
Pb removal efficiency versus leaching time (CA concentration of 0.4 M, L/S ratio 20:1, room temperature 25°C, speed 150 r/min).
Figure 11.
Pb removal efficiency at different sampling points.
Figure 11.
Pb removal efficiency at different sampling points.
Figure 12.
EDS surface scan of the soil sample ((a)−(d) the original soil sample; (e)−(h) the soil after remediation).
Figure 12.
EDS surface scan of the soil sample ((a)−(d) the original soil sample; (e)−(h) the soil after remediation).
Figure 13.
Size characteristic curve of the soil sample before and after the washing treatment.
Figure 13.
Size characteristic curve of the soil sample before and after the washing treatment.
Figure 14.
XRD spectrum of the soil sample before and after the washing treatment (1-Quartz, 2-Albite, 3-Clinochlore, 4-Muscovite, 5-Calcite, and 6-Dolomite).
Figure 14.
XRD spectrum of the soil sample before and after the washing treatment (1-Quartz, 2-Albite, 3-Clinochlore, 4-Muscovite, 5-Calcite, and 6-Dolomite).
Figure 15.
(a) Survival rate of the wheat planted in soils before and after the washing treatment; (b) Pb contents of the stems and leaves of the wheat growing in the soil before and after the washing treatment.
Figure 15.
(a) Survival rate of the wheat planted in soils before and after the washing treatment; (b) Pb contents of the stems and leaves of the wheat growing in the soil before and after the washing treatment.
Table 1.
Chemical and physical characteristics of the heavy metal contaminated soil sample.
Table 1.
Chemical and physical characteristics of the heavy metal contaminated soil sample.
| Characteristics |
Value |
| pH-H2O |
7.55 |
| Density (g/cm3) |
2.17 |
| Granulometry |
|
| >0.125 mm |
8.49% |
| 0.125−0.075 mm |
16.26% |
| 0.075−0.045 mm |
13.10% |
| 0.045−0.0385 mm |
12.66% |
| <0.0385 mm |
49.49% |
| ∑ |
100.00% |
| Elemental composition |
|
| Total iron (Fe) |
3.38% |
| Magnesium (Mg) |
1.37% |
| Phosphorus (P) |
0.066% |
| Calcium (Ca) |
2.17% |
| Sodium (Na) |
0.99% |
| Potassium (K) |
1.93% |
| Copper (Cu) |
105 mg/kg |
| Lead (Pb) |
2290 mg/kg |
| Zinc (Zn) |
199 mg/kg |
| Cadmium (Cd) |
8.30 mg/kg |
| Mercury (Hg) |
0.902 mg/kg |
| Arsenic (As) |
10.50 mg/kg |
| Chromium (Cr) |
67.90 mg/kg |
| Nickel (Ni) |
38.80 mg/kg |
Table 2.
Chemical characteristics of the soil sample before and after the washing treatment.
Table 2.
Chemical characteristics of the soil sample before and after the washing treatment.
| Characteristics |
Before (%) |
After (%) |
Change (%) |
| Total iron (Fe) |
3.38 |
3.28 |
-2.96 |
| Magnesium (Mg) |
1.37 |
1.22 |
-10.95 |
| Phosphorus (P) |
0.066 |
0.052 |
-21.21 |
| Calcium (Ca) |
2.17 |
1.12 |
-48.39 |
| Sodium (Na) |
0.99 |
1.11 |
+12.12 |
| Potassium (K) |
1.93 |
1.96 |
+1.55 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).