1. Introduction
Quantitative research on the balance of global greenhouse gases and their control mechanisms have become a focus among global environmental science research programs. Notable programs that are dedicated to addressing this issue include the International Geosphere-Biosphere Program, World Climate Research Program, International Human Dimensions Program on Global Environmental Change, and Global Change and Terrestrial Ecosystems. Wetland ecosystems play a crucial role in the global carbon cycle. Among the five major terrestrial ecosystems closely linked to the global carbon cycle—forests, grasslands, croplands, wetlands, and inland waters—wetlands constitute the largest component of the carbon reservoir of the terrestrial biosphere [
1]. Consequently, they play a vital role in the global carbon cycle [
2,
3]. The total global area of wetlands is approximately 5.7 × 10
6 km². This accounts for approximately 6% of the Earth's land area, which is significantly lower than the area covered by forests; however, due to their high productivity and redox potential, wetlands have become crucial sites for biogeochemical processes in the biosphere [
4]. Owing to prolonged or intermittent waterlogging, the anaerobic conditions in wetlands inhibit the decomposition of detritus, leading to the accumulation of organic matter in the soil. As a result, wetlands are carbon sinks that mitigate the rise in atmospheric CO
2 concentrations. According to estimates by the Intergovernmental Panel on Climate Change, global terrestrial ecosystems store approximately 2.48×10
6 TgC (1 Tg=1×10
12 g), of which peatlands store around 0.5×10
6 TgC. Peatlands alone store twice the carbon content of all forests combined, constituting 20% of the total carbon stock in global terrestrial ecosystems [
5]. This indicates that wetland ecosystems have significant potential to mitigate global warming. Although they have substantial soil carbon reservoirs, they are also significant sources of methane emissions, accounting for more than 20% of the global annual methane emissions each year [
6]. In the context of global warming, as soil moisture decreases and soil oxidation capacity intensifies, the anaerobic conditions in wetlands are mitigated. This leads to a significant acceleration in the decomposition rates of detritus and peat, resulting in an increase in CO
2 emissions, which can create a positive feedback mechanism that exacerbates global warming [
7].
Reeds are widely distributed in various types of salt marshes because of their adaptability and high reproductive capacity. They are extensively cultivated in various countries because of their significant economic and ecological value. Numerous studies [
8,
9,
10,
11]indicate that peatlands and reed wetlands serve as crucial carbon sinks. However, the carbon sink function of wetlands is influenced by various factors, including hydrology, temperature, atmospheric CO
2 concentrations, as well as nutrients like nitrogen (N) and phosphorus (P). Hence, studying variations of carbon fluxes in reed wetlands is important in understanding the sources and sinks of greenhouse gases.
The Momoge salt marshes are located in Jilin Province, China, and are characterized by arid and semi-arid lands with fragile ecosystems. Natural hydrological changes and human disturbances contribute to significant spatiotemporal variability in greenhouse gas emissions. Reed marshes, as one of the widely distributed wetland types in the region, play a crucial role in wetland carbon balances. Currently, due to the limited direct observations of CO2 exchange between reed marshes and the atmosphere, quantitatively analyzing the correlation between CO2 fluxes and environmental factors remains difficult. This limits our understanding of various physical and chemical processes that influence carbon accumulation and cycling in reed marshes. Advancements in eddy covariance technology has made accurate measurements of carbon exchange in wetland ecosystems possible. This study is based on four years of data from eddy correlation flux towers and automated meteorological stations within the study area. By investigating the CO2 fluxes in the Momoge salt marsh ecosystem, the study analyzes the daily, seasonal, and interannual variation of the net ecosystem exchange (NEE). Additionally, by exploring the correlations between net CO2 exchanges and major environmental factors at both daily and monthly scales, the results provide new insights into the local climate characteristics and land-atmosphere interactions in the Momoge wetlands. These findings can provide localized and accurate data for climate change modeling, global ecosystem modeling, and strategies for reducing greenhouse gas emissions.
2. Materials and Methods
2.1. Overview of the study area
The study area is on Juzhi Island within the Momoge National Nature Reserve. It is situated in the southeastern part of Zhenlai County, Baicheng City, in the western Songnen Plain of Jilin Province. This area represents a typical wetland-type protected area and is the largest wetland conservation area in Jilin Province. It serves as a significant stopover site for waterbirds in the northern part of China's eastern migratory route. The Momoge wetlands have a temperate continental monsoon climate. The average annual temperature in the area is 4.2°C, with an average annual precipitation of 391.8 mm. The predominant soil types are marsh soil, meadow soil, black soil (chernozem), and alluvial soil. The region boasts abundant water resources, primarily sourced from the Nen River system. The intricate network of ponds, marshes, and lakes within the basin formed due to the confluence of the Tao'er River and the Nen River. In this study, we selected widely distributed reed marshes to conduct long-term carbon flux monitoring.
2.2. Flux observations
An open-path eddy covariance system was installed in November 2014 in the Momoge wetlands. It was placed at a height of 2 m above the top of the vegetation. The vegetation within 1000 m around the instrument was dominated by reeds, with a sporadic distribution of balsam. The open-path eddy covariance system mainly consisted of an open-path infrared CO2/H2O analyzer (EC150), a three-dimensional ultrasonic anemometer (CS150, Campbell Scientific, USA) and a data collector (CR3000, Campbell Scientific, USA). The infrared CO2/H2O analyzer and the three-dimensional ultrasonic anemometer were mounted on a long arm of about 1 m. They were fixed to the monitoring tower and oriented towards the direction of the prevailing winds throughout the year in order to reduce the effect of disturbances formed by the air at the tower on the instruments. The data collector collects 10Hz of raw data in real time, calculates the flux and other covariance data online through the EasyFlux software, and records and stores the data in the PC card. As the sensor malfunctioned and was returned to the factory for repair in 2018, the range of data selected for this study is from January 2015 to December 2017, and the full year of 2021.
2.3. Micrometeorological observations
At the same time as the flux system installation, an automatic meteorological observation system (HOBO, USA) was installed, which continuously and automatically monitors and stores ten parameters, namely local air temperature, relative humidity, wind speed, wind direction, soil temperature, soil moisture, total radiation, photosynthetically active radiation, barometric pressure, and rainfall. This facilitates the study of changes in the meteorological environment on the impacts of the vegetation. The sensor was mounted at a height of 2.5 m.
2.4. Vegetation biomass survey and water level monitoring
Aboveground biomass was determined using the harvesting method. The survey was conducted once a month from June to September 2021 in four 0.5 m × 0.5 m sample plots surrounding the flux tower. The reeds in the sample plots were cut flush with the ground, returned to the laboratory, weighed, and dried at 65°C to a constant weight.
Onset's HOBO U20L series of hydrometers were used to monitor water level and water temperature changes, based on changes in pressure. U20L-04 water level range is 0-4 m (0-145 kPa), and the monitoring frequency was 1 hour.
2.5. Data processing
2.5.1. Flux data processing
To reduce the uncertainty caused by the observations, quality control and processing were performed on the data output from the EasyFlux software. Screening and quality control excluded approximately 25% of the data. The exclusion criteria included: 1) when the absolute value of CO
2 flux was greater than 30 g CO
2·m
-2·s
-1 (which is not biologically consistent); 2) when the endflow is weak, especially when the nighttime frictional wind speed U
star was less than 0.15 m/s (below which nighttime CO
2 flux data would show a significant discrete distribution); 3) when the PAR was <5 μmol·m
-2·s
-1, FCO
2<0[
12]. In this study, the following were used to interpolate the missing CO
2 data:
For missing data within 2 hours, linear interpolation was used; for missing data from 2 hours to 7 days, average daily variation was used, i.e., the average of the corresponding data points from the neighboring days was used for interpolation; for missing data for more than 7 days, nonlinear regression was used for interpolation by building a correlation model.
Daytime missing NEE data were obtained by interpolating using the Michaelis-Menten equation [
13]:
where
(mgCO
2μmol photons
-1) is the apparent light quantum efficiency of the ecosystem,
is the potential maximum CO
2 assimilation rate,
(mgCO
2·m
-2·s
-1) is the daytime ecosystem respiration rate, and
is given in μmol·m
-2·s
-1.
Due to insufficient nighttime turbulence, NEE data were presented as u* (friction wind speed) < 0.15 m/s. Nighttime missing data were obtained by respiration modeling [
14]:
where a and b are constants, Ts is the temperature at 5 cm soil depth, and
(mgCO
2m
-2s
-1) is the nighttime ecosystem respiration rate.
Directly observed CO
2 fluxes from flux towers represent NEE. Positive values represent net CO
2 emissions from the system and negative values represent net CO
2 uptake by the system. This produces the difference between ecosystem respiration (R
e) and gross primary productivity (GPP). Therefore, GPP can be defined as[
13]:
Daily ecosystem respiration is the sum of daytime ecosystem respiration (R
e,day) and nighttime ecosystem respiration (R
e,night)[
14]:
Nighttime ecosystem respiration was obtained from the observed nighttime net CO2 flux. Equation 2 was used to extrapolate daytime ecosystem respiration.
The interpolated data were divided into daytime (total radiation ≥l W·m) and nighttime (total radiation <l W·m). Data from clear and cloudless days each month were selected as representative data. The daily NEE dynamics and other variables throughout the growing season were analyzed. Additionally, the daytime and nighttime fluxes were integrated and summed at 30-minute intervals to represent the cumulative CO2 uptake and release of the ecosystem during the daytime and nighttime.
After undergoing data quality control and selection, the observed CO2 flux results from the Momoge wetlands can accurately represent the actual process of CO2 exchange between the wetland and the atmosphere.
2.5.2. Statistical analysis
Statistical analysis of all data was performed on Excel and SPSS22 software. Prior to conducting statistical analyses, a homogeneity of variance test was performed on all the data. If the variances were unequal, a logarithmic transformation was carried out. Impact of environmental and biological factors on ecosystem carbon flux was analyzed using correlation analysis. The resulting graphs were generated using SigmaPlot 12.5.
3. Results
3.1. Daily dynamics of net CO2 exchange in the salt marsh ecosystem
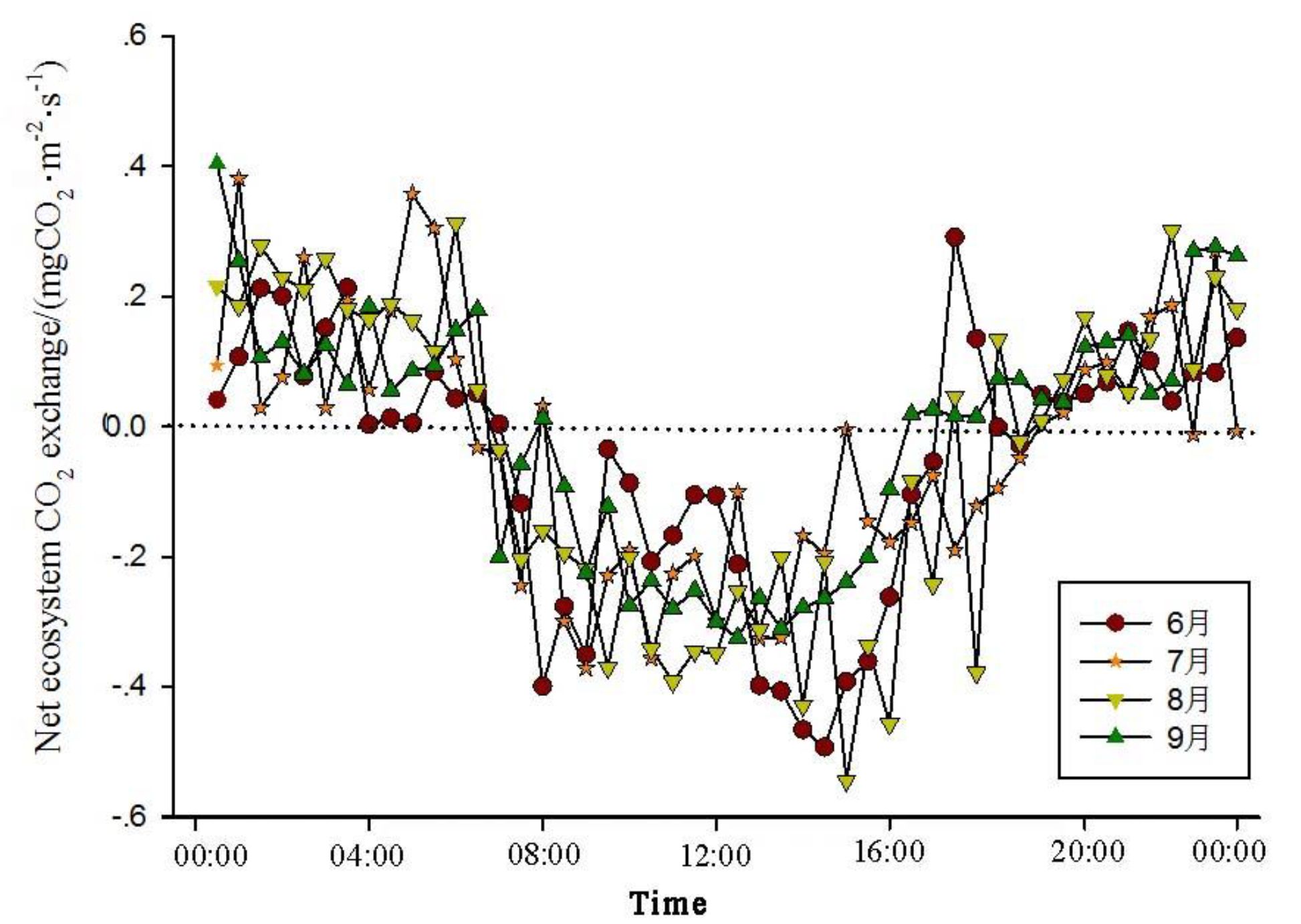
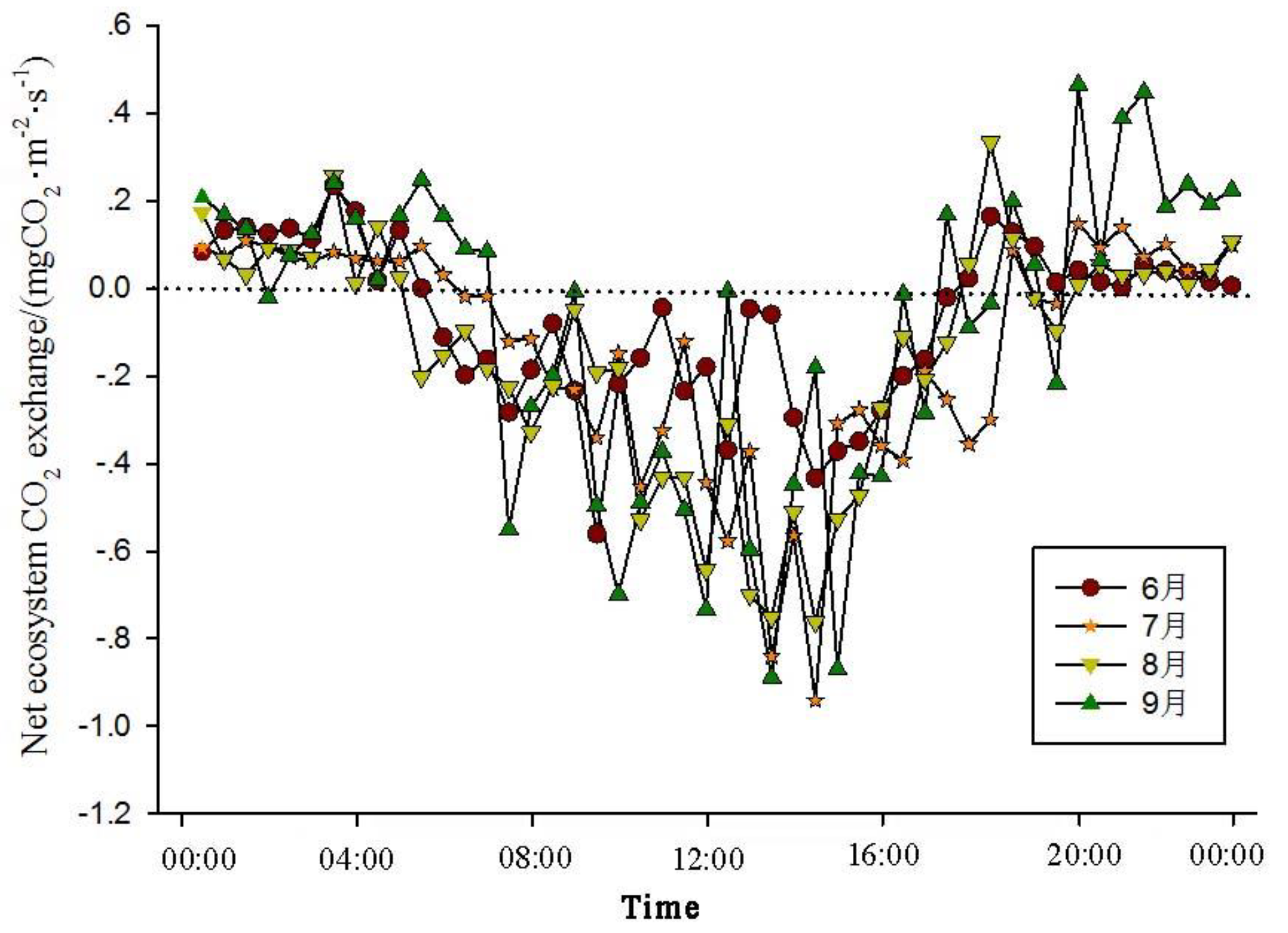
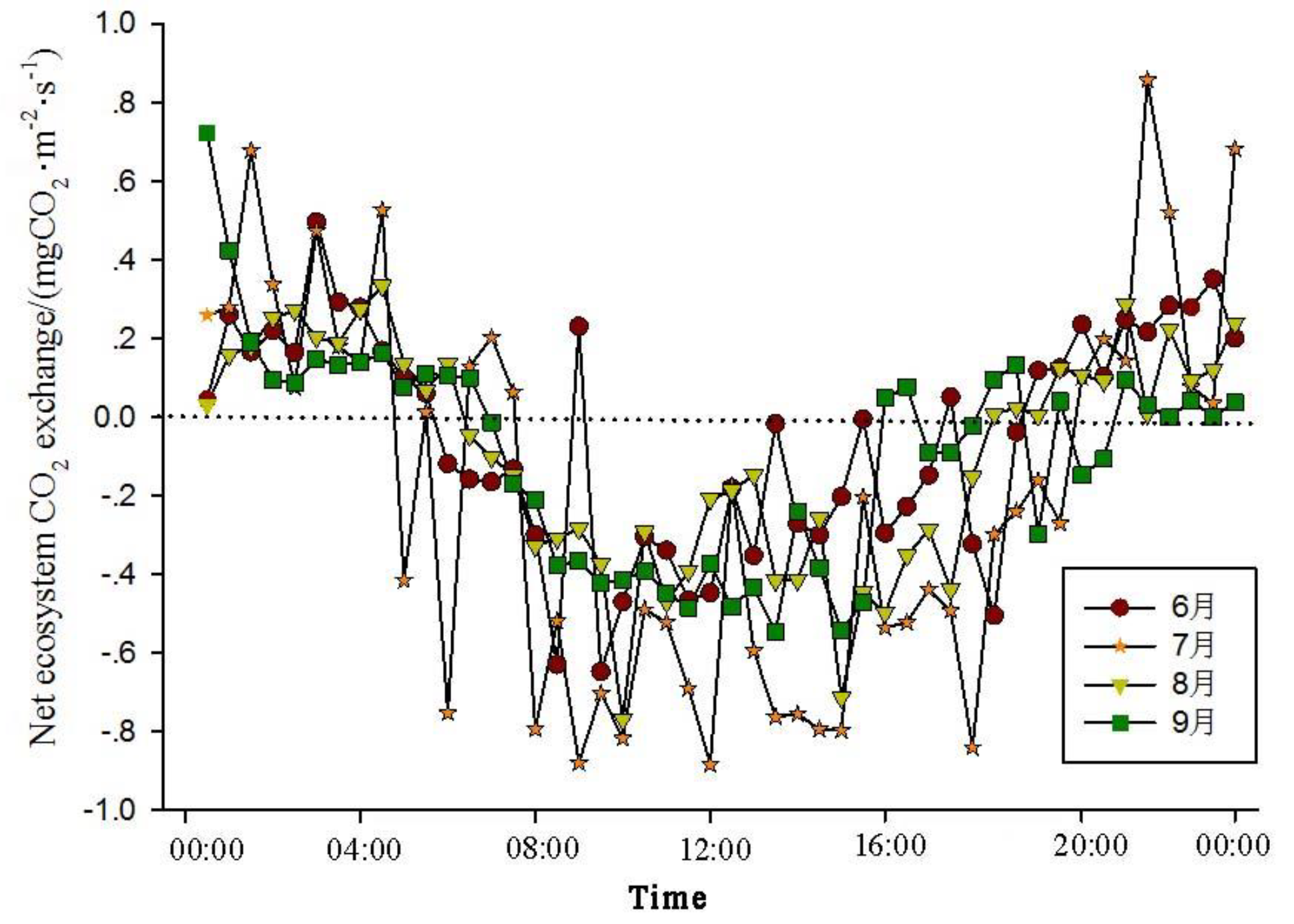
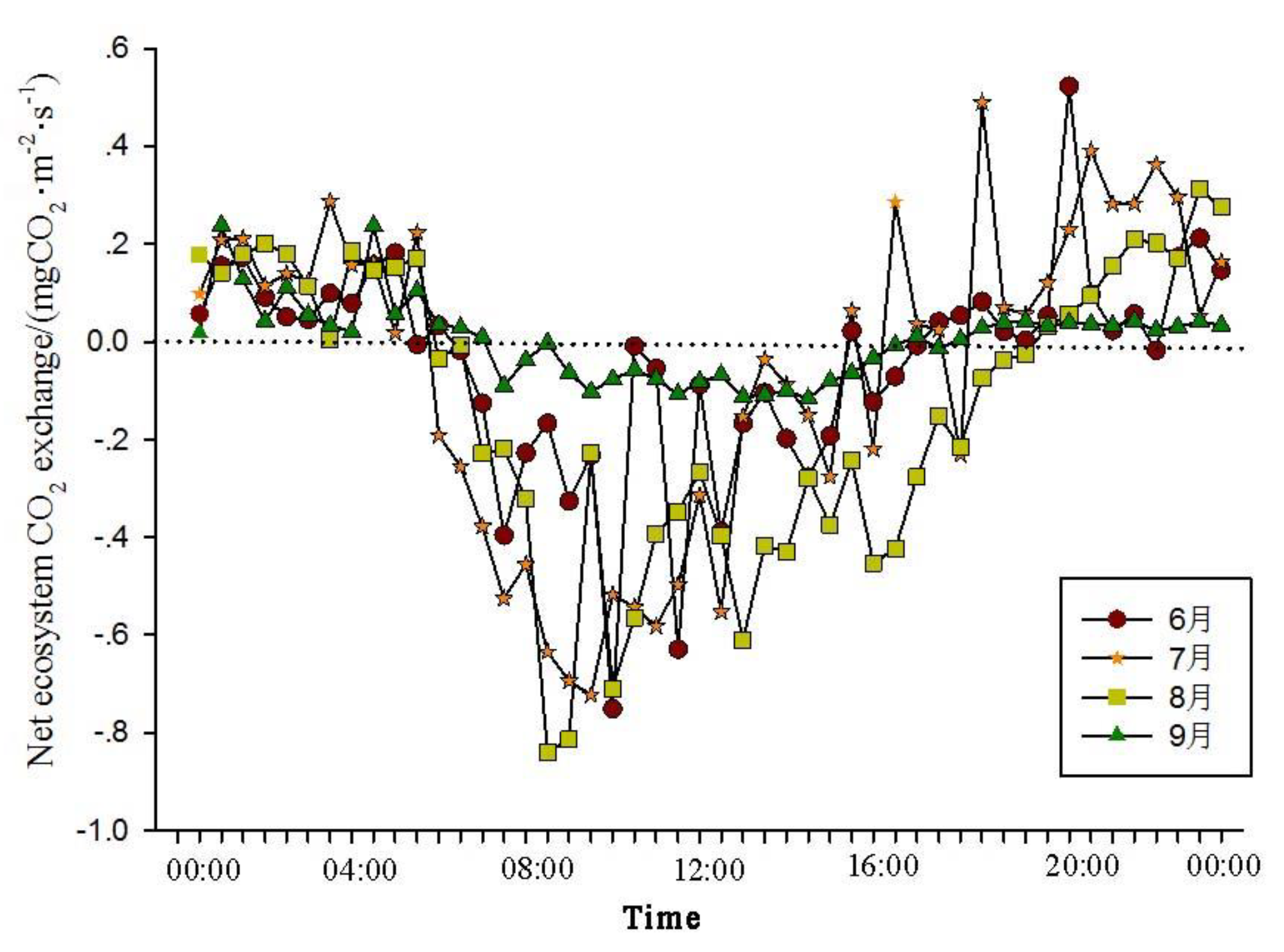
The analysis of the daily variations in CO
2 flux in the Momoge salt marsh ecosystem over four years during the growing season (June-September) shows two trends (
Figure 1,
Figure 2,
Figure 3 and
Figure 4). The CO
2 flux in the Momoge salt marsh demonstrates an overall "U" shaped distribution throughout the day. Prior to 6:00 AM, due to the respiration of wetland vegetation, the NEE is positive. After sunrise, around 7:00 AM, reeds initiate photosynthesis, which is stronger than the sum of autotrophic respiration and heterotrophic respiration of reeds and soils, resulting in carbon uptake. This uptake intensity increases with enhanced solar radiation. From 8:00 AM to 11:00 AM, the carbon uptake rate reaches its peak. In the first trend, during the midday period from 11:00 AM to 1:00 PM, carbon uptake diminishes due to light saturation, a phenomenon often referred to as midday depression. Around 4:00 PM in the afternoon, the ecosystem's carbon sequestration capacity starts to increase again, reaching the second peak of CO
2 uptake (
Figure 1 and
Figure 3). Subsequently, carbon uptake gradually reduces. Around 5:00 PM, the net carbon exchange shifts from negative to positive values, with NEE approaching zero. During this time, vegetation mainly engages in respiration, releasing CO
2. The ecosystem begins to emit CO
2 to the atmosphere, and photosynthesis essentially ceases. In the second trend, the ecosystem has only one uptake peak (
Figure 2) from 12:00 PM to 1:00 PM. After reaching the absorption peak around 12:00 PM, the absorption is suppressed in the afternoon due to light saturation. The amount of absorbed CO
2 begins to decrease, and the rate of carbon sequestration in the ecosystem declines gradually. From approximately 7:00 PM to 8:00 PM, the ecosystem becomes a CO
2 source.
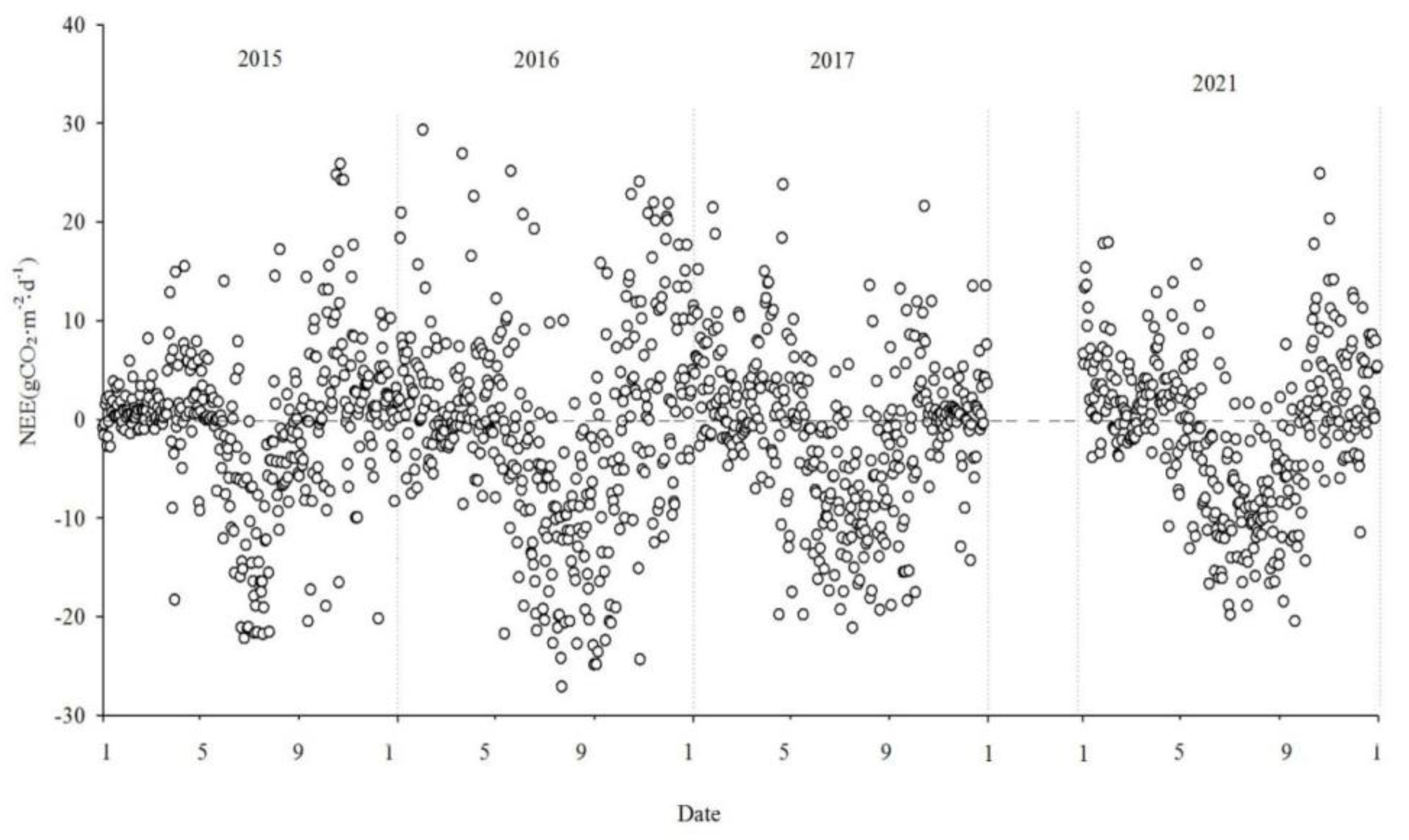
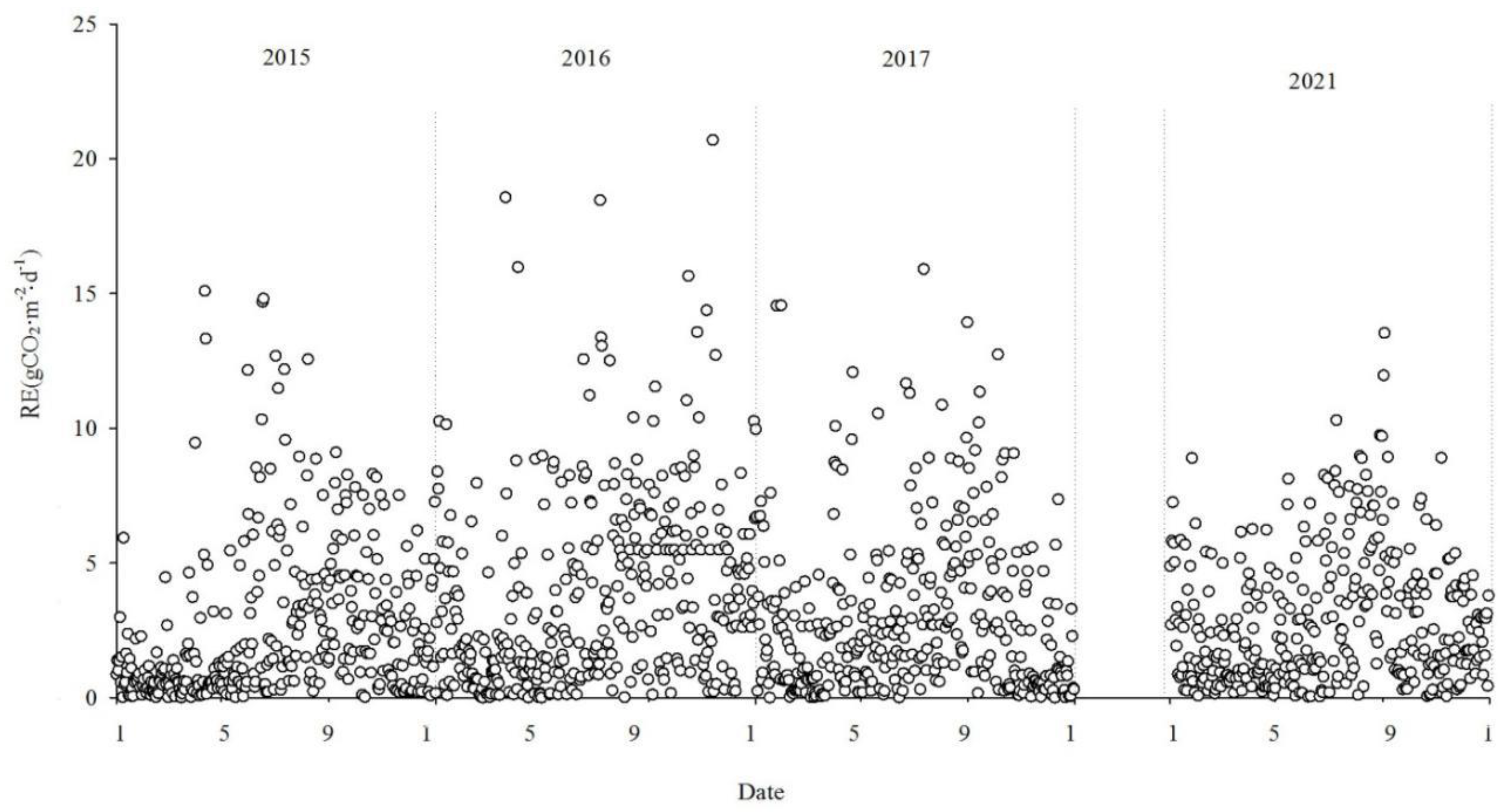
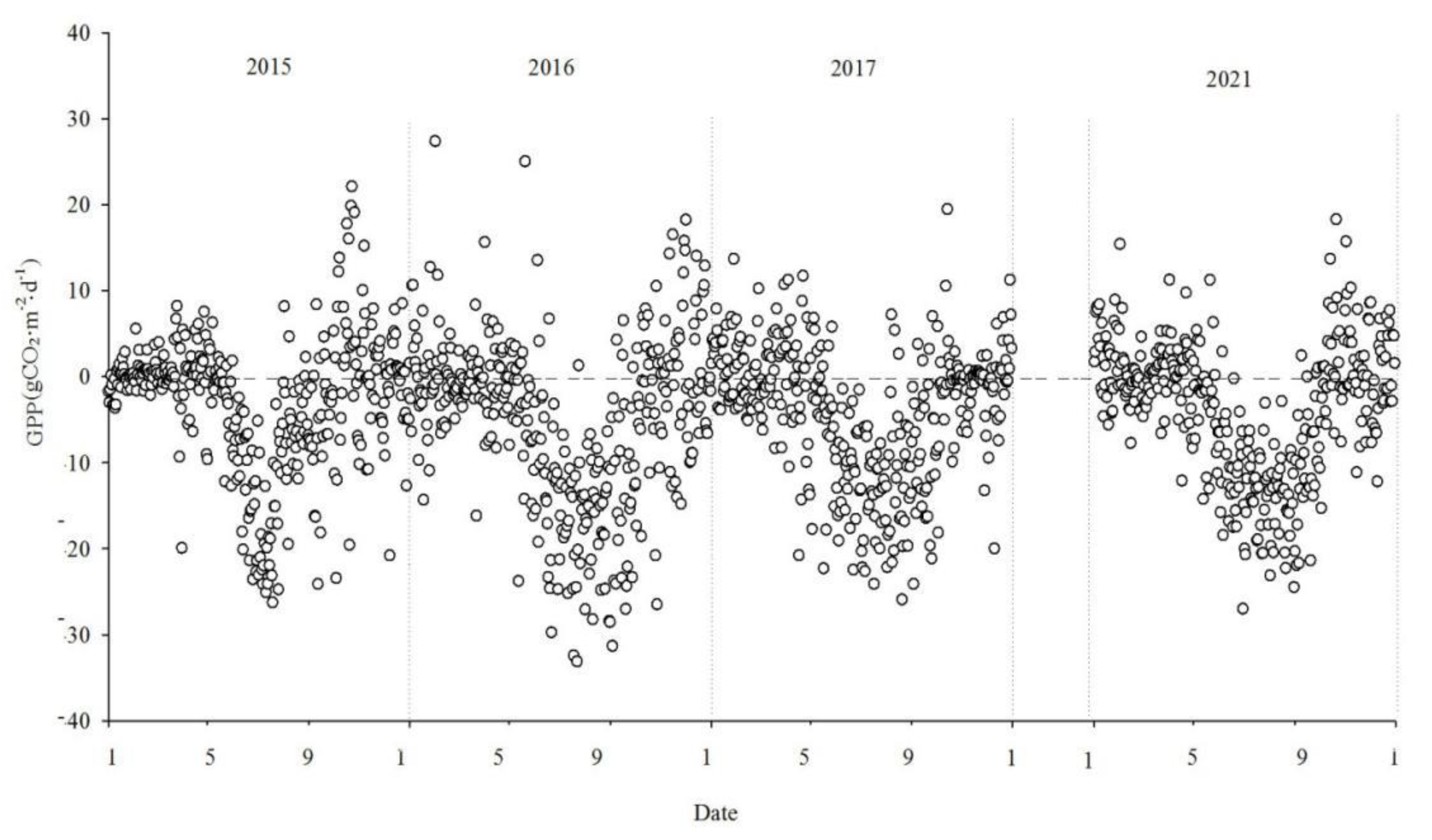
3.2. Seasonal and interannual dynamics in net CO2 exchange
Figure 5,
Figure 6 and
Figure 7 show the interannual dynamics of different components of CO
2 fluxes in the Momoge salt marsh from 2015 to 2017 and 2021, where NEE, GPP, and Reco are daily cumulative values (gCO
2·m
-2·d
-1). GPP and ecosystem respiration (Reco) are estimated by NEE.
During the study period, the NEE values illustrate the variations in carbon source/sink dynamics within the study area. Each year, the variation in carbon flux in the study area approximately follows a "V" shape. During the non-growing seasons, carbon flux tends to be positive, indicating CO2 emissions into the atmosphere. The Momoge salt marsh ecosystem acts as a CO2 source. During the growing season, carbon flux is predominantly negative, indicating the absorption of CO2 from the atmosphere. The Momoge salt marsh functions as a carbon sink during this period. Throughout the observation period, the average daily cumulative NEE value was approximately -1.03 gCO2·m-2·d-1. The number of days with negative carbon flux was 163, 202, 193, and 186 in 2015, 2016, 2017 and 2021, respectively. The proportion of negative carbon flux days was 51% for the entire observation period. During the high vegetation growth period in summer, carbon flux values can be relatively high, even reaching positive values. This situation is typically observed during rainy or cloudy days between June and September. Due to the low PAR under such conditions, NEE values generally tend to be small. With weaker vegetation photosynthesis and elevated temperatures, ecosystem respiration intensifies, resulting in the wetland ecosystem acting as a carbon source.
At the interannual scale, the monthly mean daily dynamics of NEE exhibit a characteristic "V" shaped curve, showing significant variations across seasons. During the non-growing season, the overall fluctuations were relatively small, whereas during the growing season, the overall fluctuations were more pronounced.
Reco encompasses both soil and plant respiration. During the non-growing season, Reco values were relatively low but still positive, indicating the emission of CO2 into the atmosphere. As temperatures rise, Reco gradually increased, reaching its peak during the summer months. During the study period, Reco values varied within the range of 0.002 gCO2·m-2·d-1-20.702 gCO2·m-2·d-1, with a mean value of 3.08 gCO2·m-2·d-1.
The total GPP of the ecosystem exhibited a yearly "V" shaped pattern. During the non-growing season, GPP values were relatively low. In the early growing season, with the emergence of reeds and the increase in leaf area, both GPP and Reco started to rise. As vegetation physiological activities intensify and photosynthesis dominates respiration, the wetland ecosystem acts as a carbon sink. During the main growing period (June to September), GPP and Reco grew gradually, and GPP growth was eventually higher than that of Reco.
3.3. Ecosystem CO2 balances
The dynamics of ecosystem CO2 exchange are closely linked to climate conditions and vegetation, displaying significant seasonal and interannual variations. The ecosystem acts as a carbon sink during the growing season (May-October) and a carbon source during the non-growing season (December-April).
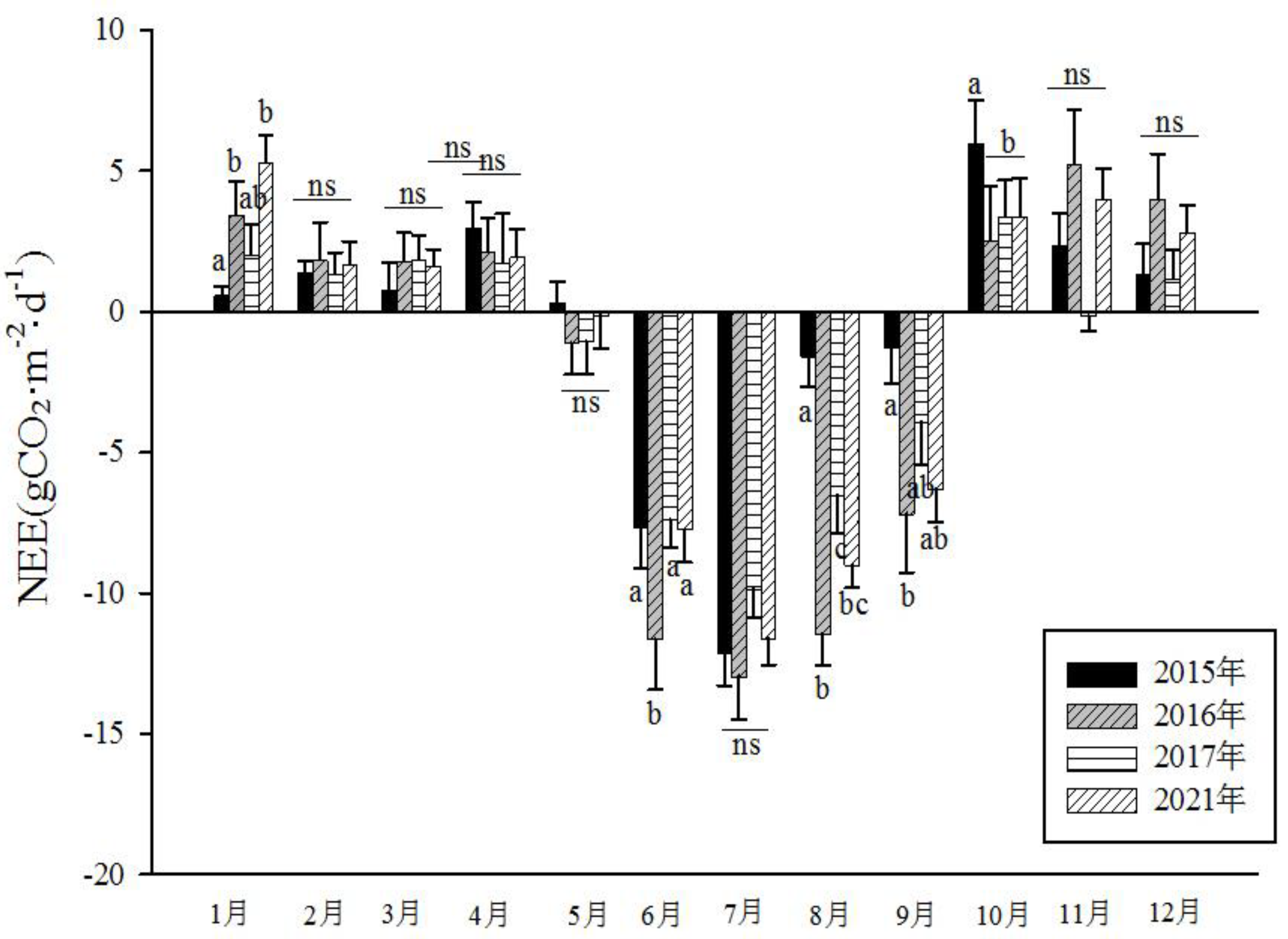
The four distinct seasons in climatic conditions and vegetation in the Momoge salt marsh lead to noticeable variations in the CO
2 flux of the wetland ecosystem. This can be observed from the average daily data for each month. By calculating the daily average CO
2 flux for each month from 2015 to 2017 and 2021, a monthly average CO
2 flux graph was generated. As shown in
Figure 8, the monthly average CO
2 flux was positive from January to April and from October to December. This indicates that the ecosystem emitted CO
2 to the atmosphere, acting as a carbon source. In May, as reeds started to sprout, photosynthesis intensified, and the amount of CO
2 absorbed by reeds gradually increased. From June to September, the wetland entered its growing season, and the monthly average carbon flux values were negative. July marks the peak, where the ecosystem absorbed CO
2 from the atmosphere. During this period, reed wetlands exhibited significant carbon sink activity during the day. Subsequently, as reeds entered the senescence phase, the amount of absorbed CO2 decreased, and the system shifted back to emitting carbon. Overall, within the study period, the wetland ecosystem exhibited its strongest carbon sequestration capacity in July, with a minimum monthly cumulative carbon flux of up to -359.98 gCO
2·m
-2 (July 2021). On the other hand, the month of January had the highest carbon emissions to the atmosphere, with a maximum monthly cumulative carbon flux of 185.89 gCO
2·m
-2 (October 2015). CO
2 flux differed significantly among different months in each year, with the CO
2 flux values during June to September notably lower than those in other months (F=29.79, P<0.001).
The NEE monitoring over the four years in the Momoge salt marsh revealed that it functions as a carbon sink overall. The carbon sequestration intensities for each year were as follows: 206.94g CO2·m-2·yr-1, 500.28g CO2·m-2·yr-1, 436.88 gCO2·m-2·yr-1 and 358.61 gCO2·m-2·yr-1, in 2015, 2016, 2017 and 2021, respectively. The cumulative GPP was -1152.62 gCO2·m-2·yr-1, -1928.62 gCO2·m-2·yr-1, -1516.79 gCO2·m-2·yr-1 and-1409.74 gCO2·m-2·yr-1. Cumulative Reco was 945.68 gCO2·m-2·yr-1, 1428.34gCO2·m-2·yr-1, 1079.91gCO2·m-2·yr-1 and 1051.13 gCO2·m-2·yr-1, respectively.
3.4. Effects of environmental factors on CO2 exchange in the wetland ecosystem
3.4.1. Daily scale
Pearson correlation was used to analyze the correlation between the factors and the dependent variable. The correlation coefficient matrix between independent variables is shown in
Table 1. NEE represents the daily average CO
2 exchange, Tsoil represents the temperature at 5cm soil depth at the daily scale, Tair represents the daily average air temperature, PAR represents the daily average photosynthetically active radiation, PPT represents the daily precipitation, and WL represents the water level.
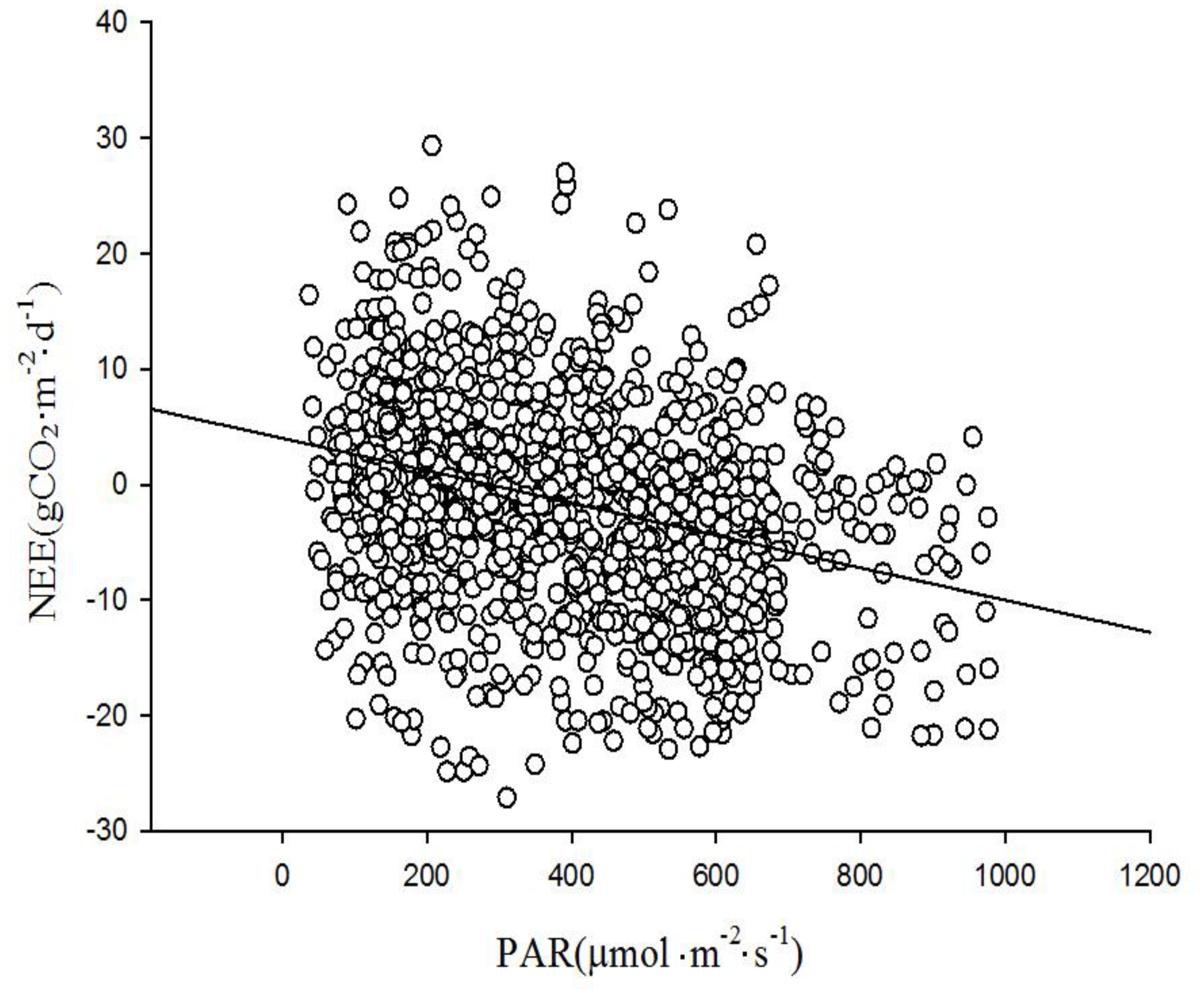
From the table, Tsoil, PAR, PPT, and WL all significantly influence the net CO2 exchange. Specifically, Tair, Tsoil, and PAR exhibited negative correlations with net CO2 exchange. As temperature and PAR increased, the net CO2 exchange of the ecosystem decreased, indicating a trend towards enhanced carbon absorption capacity. PPT was negatively correlated with net CO2 exchange. On rainy days, lower solar radiation results in reduced CO2 absorption. WL showed a positive correlation with CO2 net exchange. As WL rose, the proportion of plants exposed to the surface decreased, leading to a reduction in the wetland's carbon absorption capacity.
The role of PAR on the net exchange of CO
2 in wetlands was mainly reflected in the daytime of the growing season. In
Figure 9, net CO
2 exchange evidently increased rapidly with the rise in PAR. However, as PAR intensity surpassed a certain threshold, the rate of increase in net CO
2 exchange slowed, eventually reaching a steady state value. The influence of PAR was highly significant during different growth stages of the reeds. The phenomenon of net CO2 absorption is closely related to the growth activities of the reed. During the sprouting to flowering period from May 4th to August 18th, the wetland exhibited strong photosynthetic assimilation capacity, with maximum carbon absorption reaching up to 1.9 mg·m
-2s
-1. During the early and late stages of reed growth, carbon absorption capacity was relatively weak, with the maximum carbon absorption reaching only 0.94 mg·m
-2s
-1.
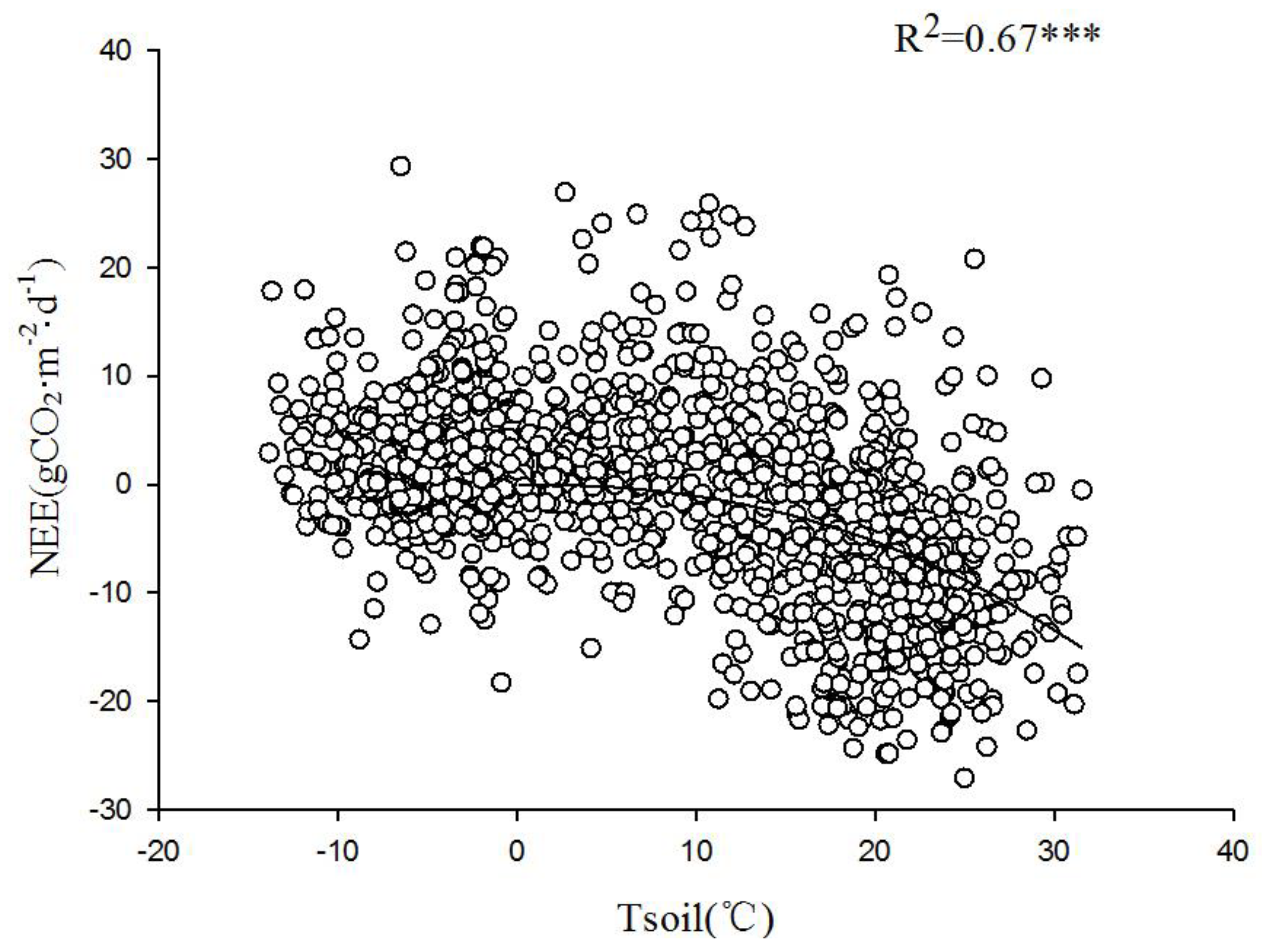
Temperature during the growing season has a significant impact on the photosynthetic physiology of vegetation and is the most important meteorological factor affecting the respiration intensity of various components within the ecosystem.
Figure 10 demonstrates a significant exponential correlation between nighttime CO
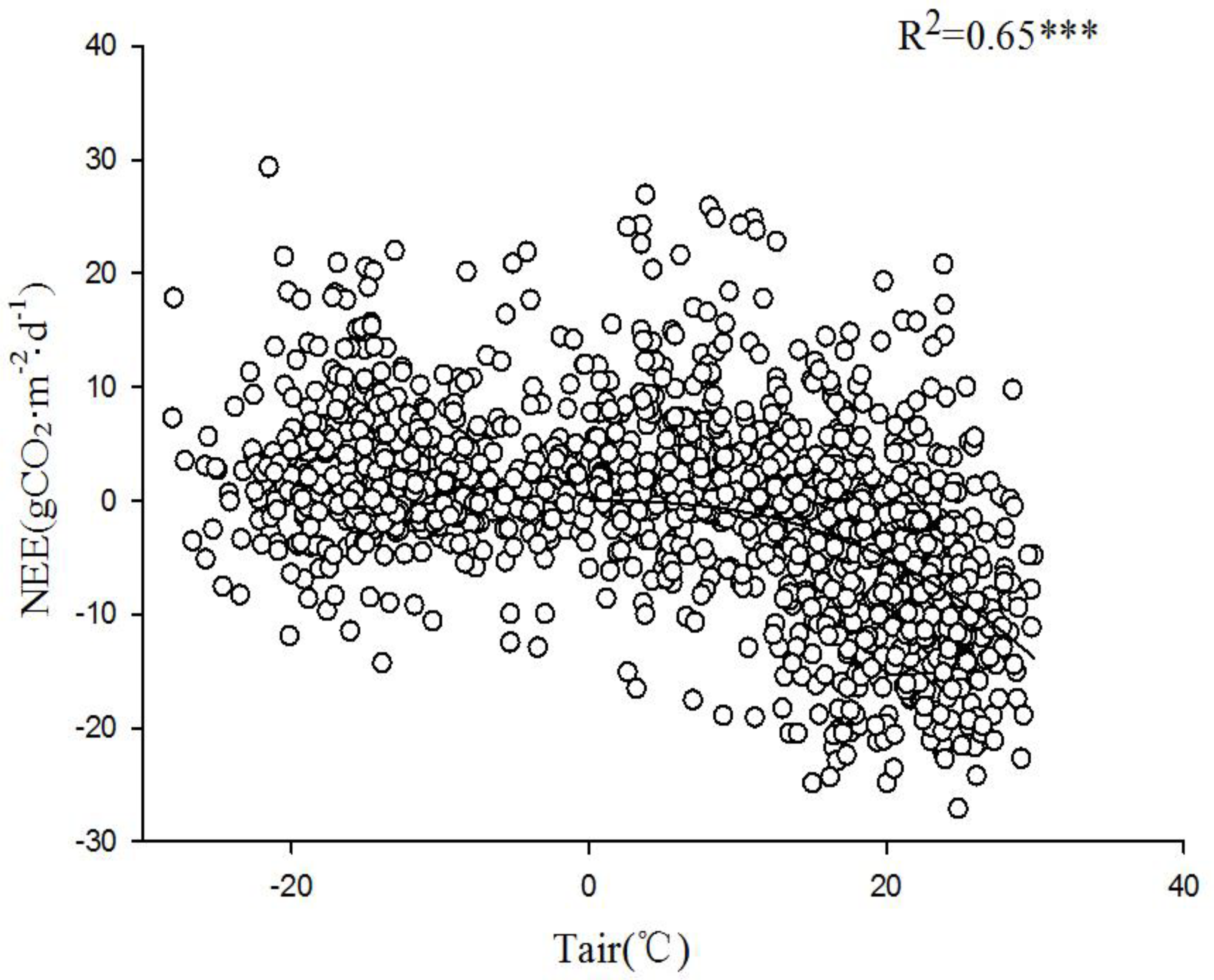
2 flux and Tsoil at 5 cm depth. Comparative analysis of the relationship between nighttime carbon flux and 2.5 m air temperature during the growing and non-growing seasons (
Figure 11) revealed an exponential growth trend in the response of nighttime carbon flux to Tair. This suggests that the intensity of nighttime carbon release is directly controlled by thermal conditions. Both Tair and upper Tsoil affect the respiration rate of the ecosystem. An increase in temperature accelerated the metabolism of plants and microorganisms, thus enhancing the intensity of carbon emissions.
3.4.2. Seasonal scale
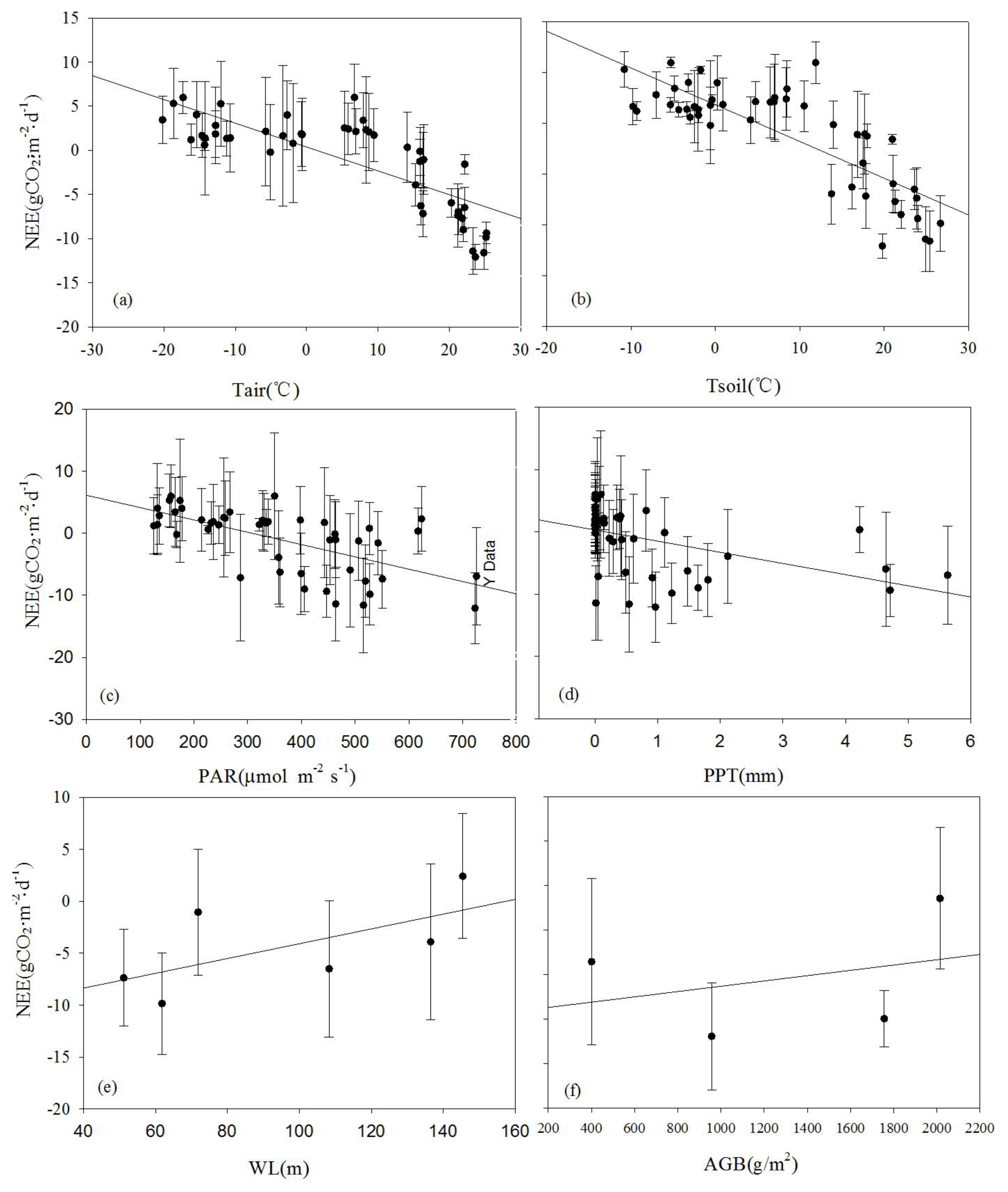
The effects of environmental control variables (Tair, Tsoil, PAR, PPT, WL, and AGB) on NEE are often complex and interrelated. To investigate the mechanisms influencing NEE, a correlation analysis was conducted between monthly average NEE and environmental factors at the seasonal scale (
Table 2). Apart from WL, a significant linear correlation between monthly average NEE and the main environmental and biological factors was found (
Figure 12), ranked in decreasing order of significance: Tsoil > Tair > PAR > PPT > biomass (AGB). Larger values of Tsoil, Tair, PAR, and AGB indicate a stronger carbon uptake capacity of the ecosystem. Tair significantly influenced Tsoil, indicating that Tair indirectly affects NEE flux by influencing Tsoil. Concurrently, the monthly carbon uptake capacity significantly increased with rising Tair. The dynamics of AGB are more dependent on the variations in PAR. This is primarily because the monitoring area experiences prolonged waterlogged conditions, where the anaerobic environment inhibits the decomposition of detritus, leading to the accumulation of organic matter in the soil. As a result, the wetland becomes a carbon sink that suppresses the rise of atmospheric CO
2 concentration. The impact of rainfall on biomass accumulation is relatively weak. Under waterlogged conditions, the reed biomass is relatively high, with the maximum biomass reaching up to 2.5kg·m
-2. The plant height may exceed 270 cm, and the ground cover density is relatively high.
4. Discussion
Different wetland ecosystems exhibit varying net ecosystem CO
2 exchange rates across different time scales. Similarly, CO
2 exchange rates differ among wetland types. Even within the same wetland type, interannual CO
2 exchange varies notably. Currently, wetlands generally act as carbon sinks, with an average carbon sequestration rate of 118 gC·m
-2·yr
-1 [
7]. Numerous studies have indicated that reed wetlands and peatlands are significant carbon sinks [
15]. Our study confirms this by indicating that the Momoge salt marsh acted as a carbon sink in the years 2015-2017 and 2021. A study monitoring the NEE in the reed wetland of the Liaohe Delta in 2006 showed that this area also acted as a carbon sink, with a carbon sequestration intensity of 230 gCO
2·m
-2·yr
-1[
16]. In the Nanji wetland of Poyang Lake, during the non-flooded period of 2015, the ecosystem acted as a carbon sink with a carbon sequestration rate of 667.62 gC·m
-2·yr
-1. However, in the subsequent years of 2016 and 2017, it shifted to a carbon source [
17]. A study of CO
2 fluxes for the entire year of 2005 in the Haibei wetland of the Qinghai-Tibet Plateau showed that it released around 86.18 gC·m
-2·yr
-1 into the atmosphere, making it a carbon source as well [
18]. The interannual variations of NEE in wetland ecosystems are closely linked to climate [
19,
20,
21], hydrology [
22], and vegetation conditions [
23]. These factors collectively play a significant role in shaping the carbon sink function of specific ecosystems. Furthermore, related studies have indicated that even within the same wetland ecosystem, the carbon source/sink status can exhibit uncertainty across interannual periods due to variations in vegetation composition, hydrological conditions, human disturbances, and climate change [
24]. Since wetland greenhouse gas balances are highly sensitive to changes in wetland area, the future impact of wetlands on the climate depend on the balance between degradation and restoration.
This study found that the NEE of wetlands exhibited a pattern of functioning as a carbon sink with greater fluctuations during the growing season and a carbon source with smaller fluctuations during the non-growing season. During the spring, as plants start sprouting and growing, the amount of carbon fixed through photosynthesis is initially less than the carbon released through respiration. As a result, the wetland continues to act as a carbon source. However, as plant growth progresses and photosynthesis becomes stronger than respiration, the wetland transitions to a carbon sink. Along with the peak of plant growth, the maximum values of NEE typically occur in July and August. Subsequently, as temperatures decrease and plants enter their senescence phase, their photosynthetic capacity gradually diminishes. This reduction in photosynthesis leads to a significant decrease in the ecosystem NEE. This pattern is consistent with findings from previous research [
25]. By November, the wetland vegetation is completely withered. Due to ecosystem respiration, the wetland transitions entirely to functioning as a carbon source.
A significant amount of research has indicated that when solar radiation intensity is low, net CO
2 exchange increases with the rise of PAR, following a hyperbolic trend. Many studies have shown a significant correlation between ecosystem NEE and PAR. Ecosystems such as forests, grasslands, and croplands exhibit a positive relationship between solar radiation intensity and net carbon exchange, following a hyperbolic curve [
26]. Similar conclusions were drawn by Beverland et al. [
27] in the context of wetland ecosystems. The current study also revealed that in the reed wetland, CO
2 net exchange initially increases rapidly with the rise of PAR. However, after PAR exceeds a certain threshold, the rate of increase in net CO
2 exchange slows, eventually converging to an asymptote value. The impact of PAR on CO
2 net uptake is significant across the various growth stages of reed. The phenomenon of net CO
2 absorption is closely tied to the growth activities of reed. Furthermore, related studies have suggested that the response of NEE to solar radiation is also influenced by other limiting factors [
28,
29]. The influence of radiation intensity on net CO
2 exchange is more pronounced within a suitable temperature range. Additionally, a larger leaf area index corresponds to a more distinct response of CO
2 net exchange to radiation intensity.
Most studies suggest that changes in WL and temperature increase can significantly affect the production of CO
2 in wetland ecosystems [
30,
31,
32]. Research on wetlands in southern Finland found that temperature is the primary factor leading to a decrease in net carbon uptake [
33]. This study found that nighttime carbon flux exhibits an exponential growth trend in response to Tair. Temperature affects ecosystem CO
2 flux through both direct and indirect mechanisms, ultimately influencing ecological processes [
34]. Firstly, the seasonal variation of photosynthesis is primarily controlled by temperature [
35]. Within a certain temperature range, higher temperatures are more favorable for photosynthesis [
36]. For example, studies on the seasonal cumulative GPP of corn and wheat ecosystems found that higher mean temperatures are associated with larger cumulative GPP values [
37]. Research on temperate bulrush marshes also showed that temperature is a key indicator for daily GPP [
38]. Furthermore, ecosystem respiration is primarily controlled by temperature, with respiration increasing exponentially as temperature rises [
39]. This is because higher temperatures can stimulate both soil and vegetation dark respiration. Research on northern marshes revealed that higher temperatures correspond to higher ecosystem respiration rates [
40]. At both interannual and seasonal scales, the Reco of salt marshes was found to be significantly correlated with temperature [
41]. Furthermore, CO
2 fluxes are controlled by temperature through its effect on vegetation cover, leaf area index, and length of the growing season. An increase in Tsoil is accompanied by an increase in ABG [
42]. The temperature in the environment controls the humidity by regulating vapor pressure. Research on wetlands in the southwestern plateau of China found that temperature can explain most of the seasonal variability in carbon fluxes. Warming can increase both GPP and Reco, thus influencing the carbon balance of the plateau wetlands [
43]. Although water level conditions significantly influence the uptake and release of CO
2 in the ecosystem, temperature remains the primary factor for carbon fluxes in the Momoge salt marsh.
5. Conclusions
This study analyzed the CO2 flux variation characteristics and the factors affecting them in the Momoge salt marsh ecosystem. In the daily variations of CO2 fluxes in the Momoge salt marsh during the growing season an overall "U" shaped distribution was observed, with uptake during the day (negative values) and release at night (positive values). Two patterns emerge (one peak and two peaks), with reduced carbon absorption during midday due to light saturation resulting in a midday depression phenomenon for the latter pattern. Within each annual variation, the carbon fluxes in the study area roughly exhibited a "V" shape. Carbon fluxes during the non-growing season predominantly showed positive values, indicating the release of CO2 into the atmosphere. The carbon sequestration intensities of the Momoge salt marsh ecosystem were 206.94 gCO2·m-2·yr-1, 500.28 gCO2·m-2·yr-1, 436.00g CO2·m-2·yr-1, and 358.61 gCO2·m-2·yr-1 for the years 2015-2017 and 2021, respectively. This indicates that the system behaves as a carbon sink.
At both hourly and daily scales, PAR was the primary influencing factor affecting daytime variations in NEE during the growing season, while temperature was the main factor influencing nighttime NEE dynamics. At the daily scale, Tair, Tsoil, PAR, PPT, and WL all showed significant effects on net CO2 exchange. At the monthly scale, larger values of Tsoil, Tair, PAR, and AGB corresponded to a stronger carbon absorption capacity of the ecosystem. Although WL conditions significantly influenced the uptake and release of CO2 in the ecosystem, temperature remained the primary factor for carbon fluxes in the Momoge salt marsh.