1. Introduction
According to the Task Force for Neck Pain, neck pain affects an estimated 12.1 to 71.5 percent of the population every year. For the working population, this figure is in the range of 27.1 to 47.8 percent. As neck pain follows a lifelong sinus curve with alternating epi- and remissions, it is estimated that between 50 to 85 percent of those affected will experience a new pain episode within the next one to five years [
1].
Previous studies have focused on general neck pain or the effect of pain on other regions, such as the lower trapezius. For example, it is already known that tension and overactivity of the upper trapezius, which tends to shorten and form trigger points, leads to weakness in the middle and lower parts [
2]. In one of his publications, Choudhari et al. [
3] found that in unilateral neck pain, the strength of the middle and lower trapezius is significantly reduced compared to the contralateral side. A second study assessed the strength capacity of the trapezius muscle in violinists with unilateral neck pain and showed a deficit in the parameter studied on the painful side of the neck [
4]. Looking at the thickness of the lower trapezius in patients with unilateral neck pain, it is reduced both at rest and during contraction compared to the control group [
5].
There have also been studies contrasting healthy individuals with those who suffer from neck pain. In terms of stiffness, for example, it has been shown that people with chronic neck pain have a significantly stiffer trapezius muscle than healthy people [
6,
7]. There also appears to be a linear, positive correlation between penetration force and elastomer stiffness for this parameter [
8]. Kocur et al. [
9] also describe a positive correlation between stiffness, age, body mass index (BMI) and trapezius elasticity.
However, there are no known trials that compare side-different measurements values of neck pain in the same person. This ensures better comparability and helps to determine how unilateral, severe neck pain affects the myofascial tissue properties of the upper trapezius in comparison to the opposite side. The importance of researching the myofascial tissue properties related to neck pain is evident as it can reduce work absenteeism, lower healthcare expenses, and alleviate pressure on national healthcare systems.
The purpose was to investigate whether biomechanical tissue properties, pressure painfulness, as well as active mobility on the affected side of the neck show detectable differences between the right and left upper trapezius.
2. Materials and Methods
2.1. Design and Ethics
The study was a cross-sectional study with one group according to the STROBE guidelines [
10]. The protocol was registered in the German Register for Clinical Studies (DRKS-ID: DRKS00031247). The measuring devices and the treatment instrument were granted by the Fascia Research Project of the Technical University of Munich and Ulm University in Germany. The study (No.: EB 1037/2022) obtained permission from the DIPLOMA Ethics Committee. All research procedures complied with the ethical principles of the Declaration of Helsinki. Each participant provided written informed consent at the outset of the study.
2.2. Participants
Patients between the ages of 18 and 75 with a confirmed clinical diagnosis of neck pain according to the ICD classification on one side of the body more than the other, as measured by a minimum difference of three on the Visual Analogue Scale (VAS), were included. Exclusion criteria were previous surgery or other scars in the neck region between C1 und Th1, use of medication that affects the muscle tone (e.g., analgesics and muscle relaxants), rheumatic diseases, skin changes (e.g., neurodermatitis, psoriasis) in the upper trapezius area and/or pregnancy. Based on these criteria, N=40 subjects from middle-size city in middle Germany were recruited for the measurements.
2.3. Measurements
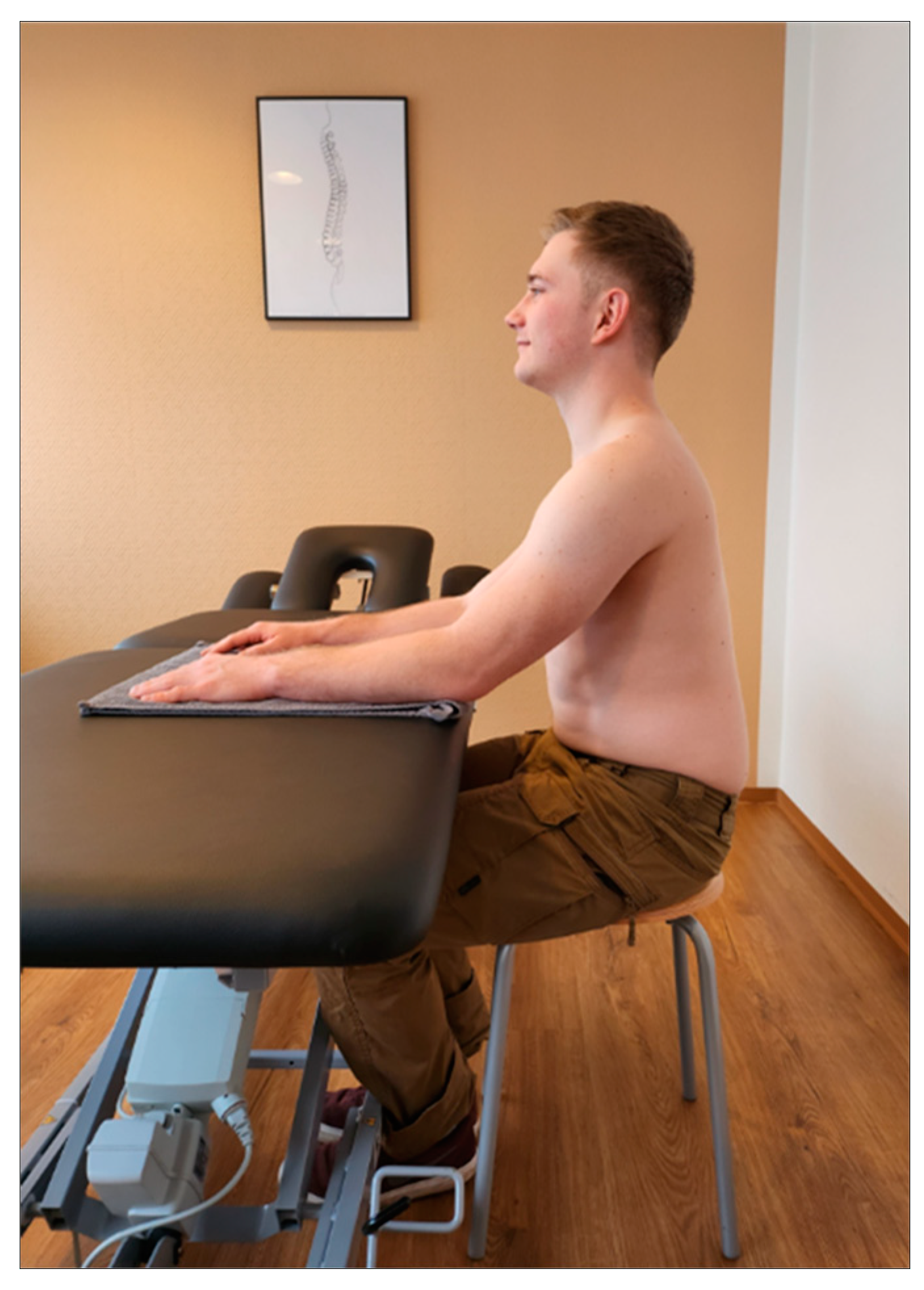
Setting: Before starting the upper trapezius measurements, patients were asked to complete a short data sheet (age, sex, height, weight, pain intensity on the affected side using VAS). In addition, the correlation between neck pain and headache was assessed using a subtest of the Neck Disability Index assessment tool [
11]. All examinations were performed by the same person and took approximately fifteen minutes. Sitting on a stool with forearms parallel and shoulder-width apart on a height-adjustable therapy couch (or table) was found to be an appropriate starting position. A towel was used to guide the patient. The upper body was aligned perpendicular to the floor, with the eyes forward and the shoulders not raised. The feet should be parallel and in firm contact with the floor (
Figure 1).
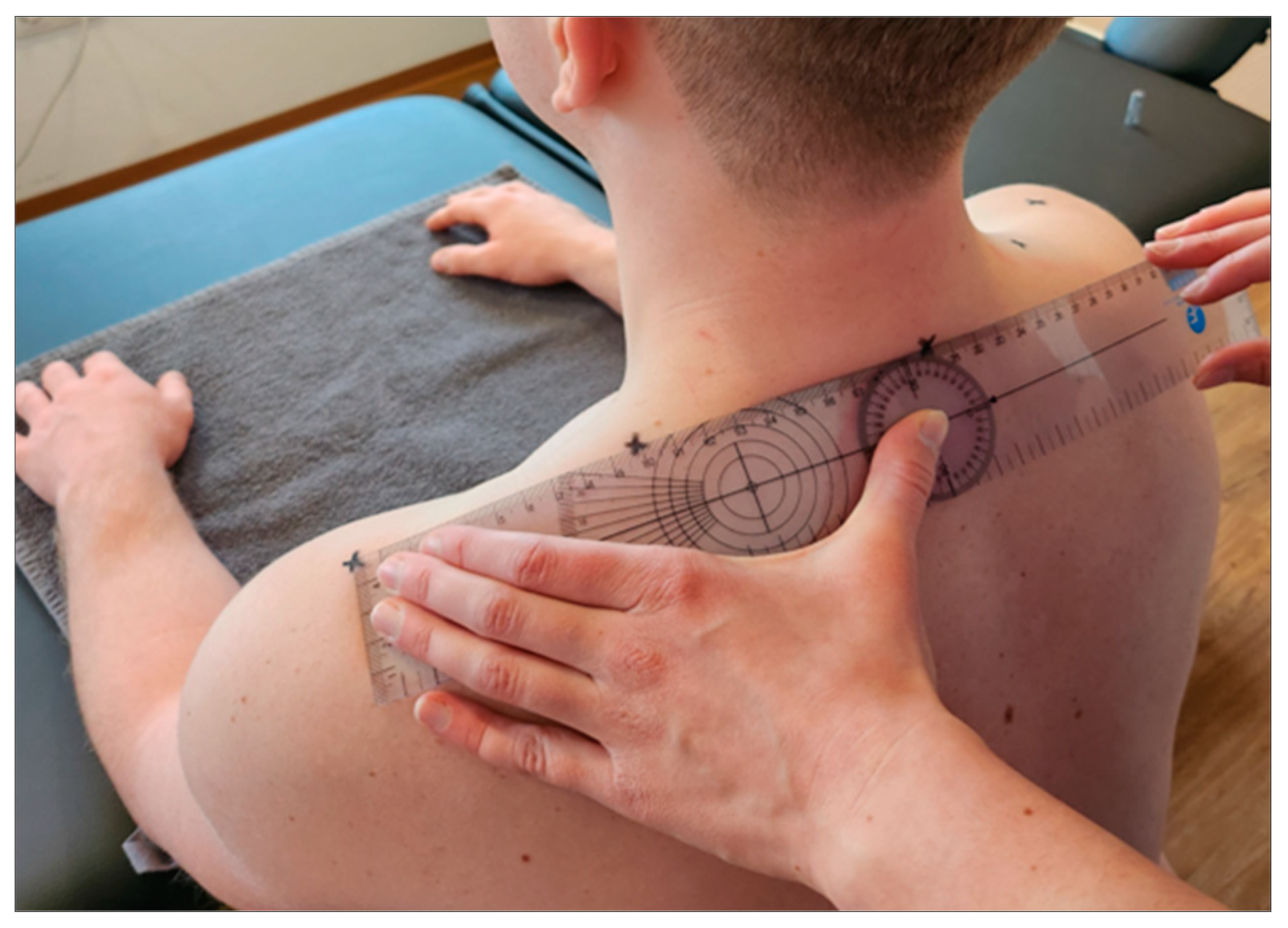
The measurement point was marked by the investigator and represented the midpoint between the processus spinosus of the 7th cervical vertebra and the tip of the acromion [
12,
13] (
Figure 2). All subsequent measurements were taken on alternating sides to allow the tissue to recover for one minute at a time.
Durometer: Various definitions of the parameters listed above can be found in the literature. In this context, the term 'tone' is often used. However, this term is not easy to define physiologically. Nonetheless, it comprises two vital properties: stiffness and elasticity. Stiffness describes how the muscle recovers its original shape after manual compression with the former property. Resistance remains independent of compression in rapidly repeated cycles [
14,
15]. The subsequent examination series measure these properties by applying vertical pressure above the skin and observing the resistance or deformation of the muscle [
12].
The first measurement was taken using a Shore durometer (Rex Gauge, Canada, ICC 6mm =0.77) [
16], which serves as a reference device and measures surface hardness, i.e., the depth of penetration into a material, based on the force generated on a standardised measuring attachment [
17]. Five consecutive measurements were taken on both sides at five second intervals. The plunger sank into the myofascial tissue due to the inherent gravity of the device (
Figure 3).
IndentoPro: Fascial tissue properties (stiffness, elastic storage capacity, pressure pain threshold) were then determined using the IndentoPro (Fascia Research Group, Germany, ICC 6mm=0.98) [
16] (
Figure 4). The myofascial elasticity, referring to the capability of maintaining the same tissue stiffness following mechanical deformation repetition. Thus, it is the opposite of high viscoelastic relaxation, i.e., stiffness decreases over time, or high damping capacity [
14,
18,
19]. The IndentoPro records the tissue's elastic storage capacity when subjected to repeated compression. Hence, taking multiple measurements within a span of thirty seconds can evaluate the tissue under examination's elastic storage capacity.
The pre-determined penetration depth was five mm [
20]. For stiffness, the measuring punch was pressed three times in thirty seconds and for elasticity five times in ten seconds (10 N/s) into the previously marked points until the predefined indentation depth was reached [
21]. The difference between the first and fifth measurement was used to determine the compressive elasticity of the tested tissue, the smaller the difference, the more elastic the tissue can be assumed to be.
Figure 4.
IndentoPro. (a) Attaching the IndentoPro measuring stamp; (b) Measurement of stiffness and elasticity by IndentoPro.
Figure 4.
IndentoPro. (a) Attaching the IndentoPro measuring stamp; (b) Measurement of stiffness and elasticity by IndentoPro.
To quantify algometry, the investigator used the pressure pain threshold (PPT). This measurement parameter is determined by applying the weakest pressure stimulus perceived as painful to the upper trapezius muscle [
22,
23]. This involved pressing the IndentoPro plunger into the myofascial tissue until the patient reported an additional pain sensation in addition to the mechanical pressure sensation. At this point the participant was asked to say the word "now". The QST guidelines were used as a guide for the related measurement protocol [
24].
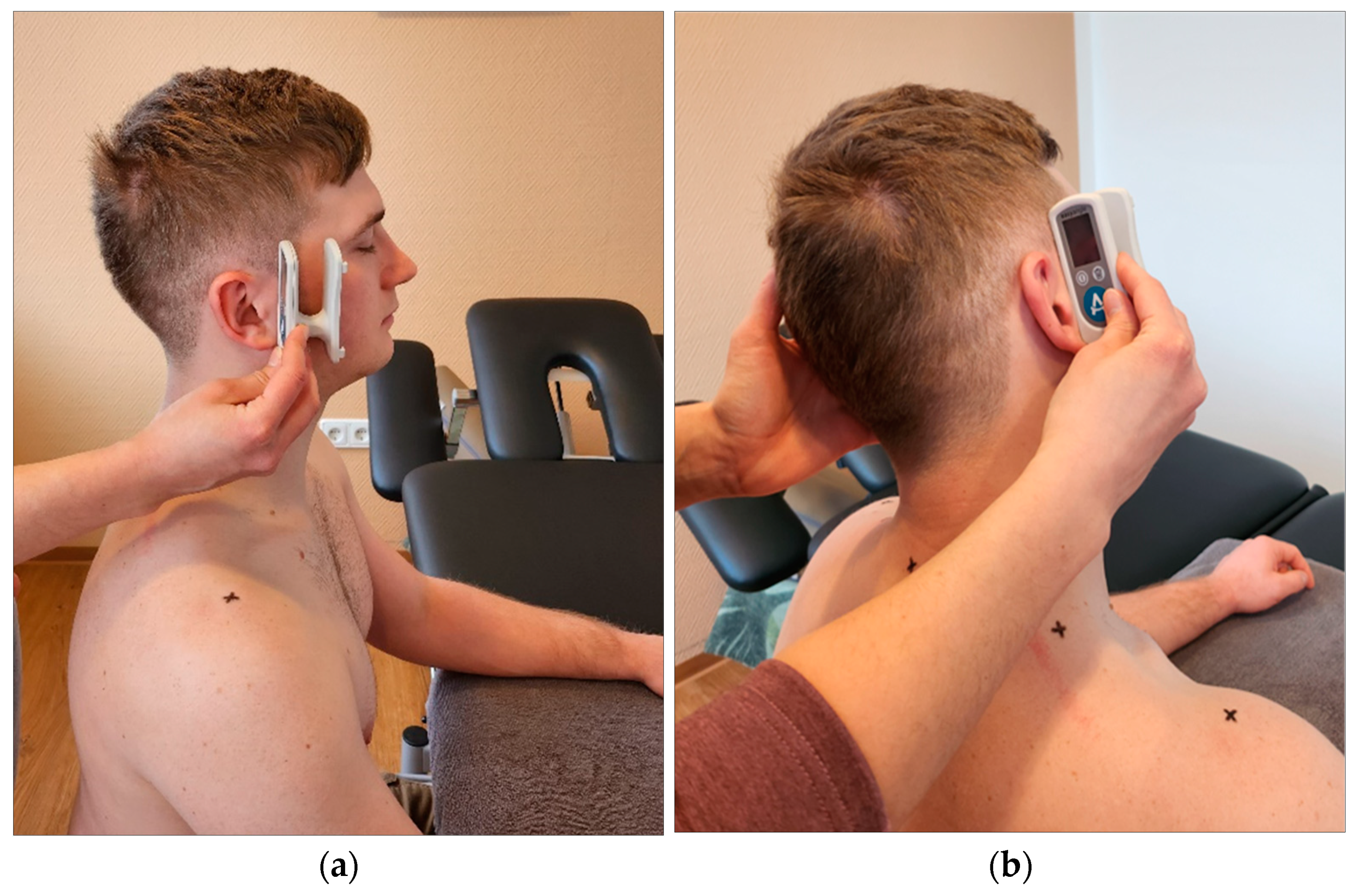
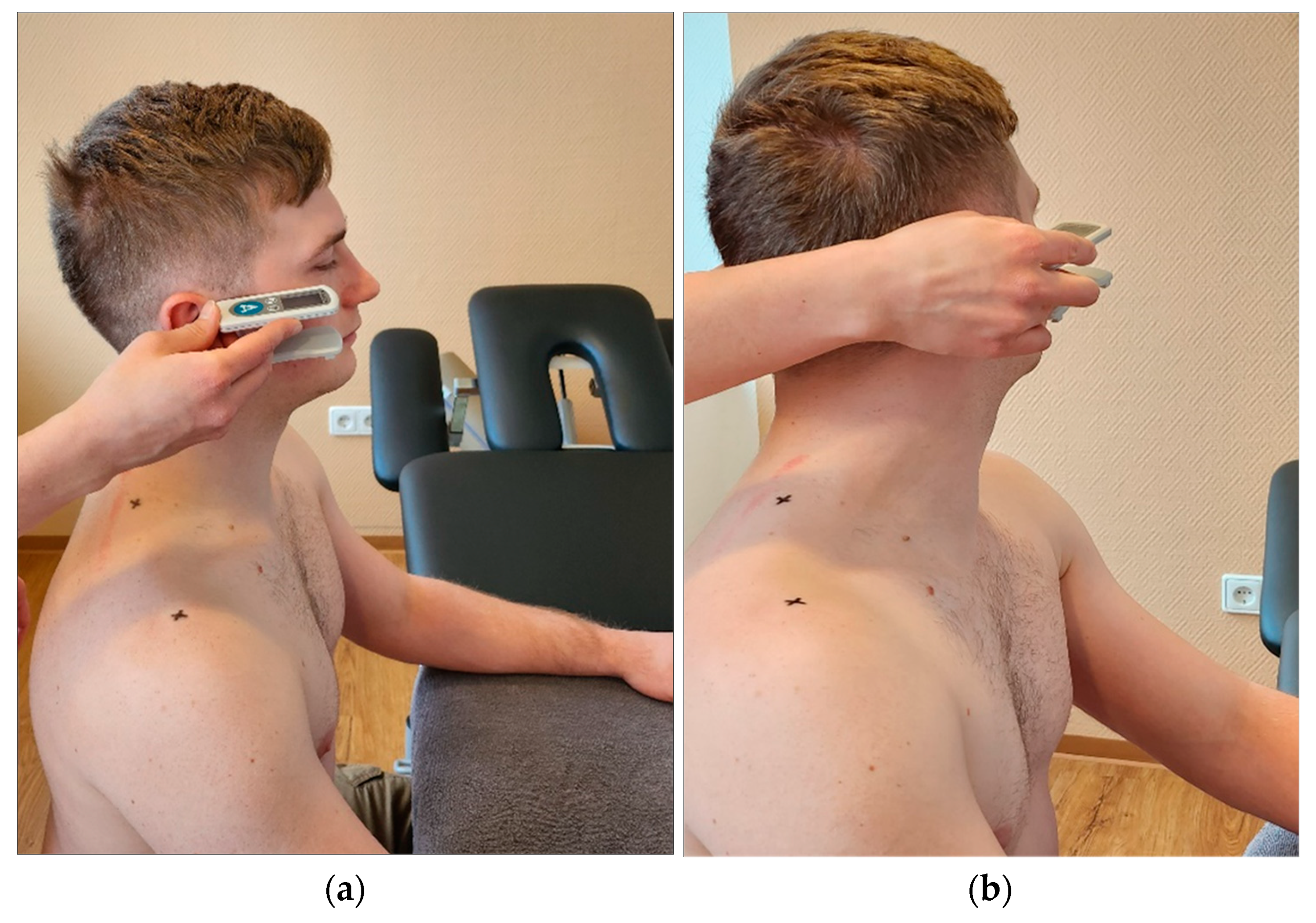
Digital goniometer: Finally, cervical spine mobility was quantified using a digital goniometer (Meloq AB, Sweden, ICC=0.66) [
25]. It was placed ventral to the subject's ears and the subject was asked to actively tilt the head sideways towards the shoulder (
Figure 5) or rotate the head (
Figure 6). Any evasive movement during the ventral lateral tilt or torso rotation was corrected and prevented by the experimenter [
25].
All measurements were painless! If the participant felt uncomfortable at any time during the examination series, he or she could indicate this by giving a hand signal, and the examination was then stopped.
2.4. Data Analysis and Statistics
The standard deviation, mean and 95% confidence interval were determined to evaluate the measurement results. In addition, the Pearson product-moment correlation was recorded for the following parameters Durometer and IndentoPro, as well as age groups and lateral flexion, age groups and rotation, and age groups and elasticity; each for the symptomatic and non-symptomatic side. Age groups were previously defined in three ranges (18-30; 31-60; 61-75 years). Somers' d was used for correlations between the following ordinal parameters Pain (VAS) and headache frequency, Pain (VAS) and headache intensity, gender and headache frequency, gender and headache intensity, and handedness and pain side. For multiple comparisons, the P value was adjusted using the Bonferroni correction. Correlations were interpreted according to Cohen as 'weak' (> 0.09, < 0.30), 'moderate' (> 0.29, < 0.50) and 'strong' (>= 0.50) [
26]. Comparisons between the symptomatic and non-symptomatic side were evaluated using a paired Student's t-test. There were no outliers, i.e., more than three times the interquartile range of the upper or lower quartile, in the data set. Normal distribution was assumed according to the central limit theorem [
27]. The significance level was set at p = 0.05. Descriptive statistics were performed using Libreoffice Calc version 6.4.7.2, Mozilla Public License v2.0. Inferential statistics were performed using R software, version 3.4.1., R Foundation for Statistical Computing, Vienna, Austria.
3. Results
3.1. Study Group Characteristics
Of the 48 participants in the study, 40 met the inclusion criteria. All epidemiological data are presented in
Table 1.
3.2. General correlations between age, gender, pain and mobility
Table 2 shows the extent to which there are general correlations between pain and the comparison variables.
3.3. Comparison of symptomatic and non-symptomatic side
Table 3 shows comparative measurements of both sides of the neck of the upper trapezius muscle.
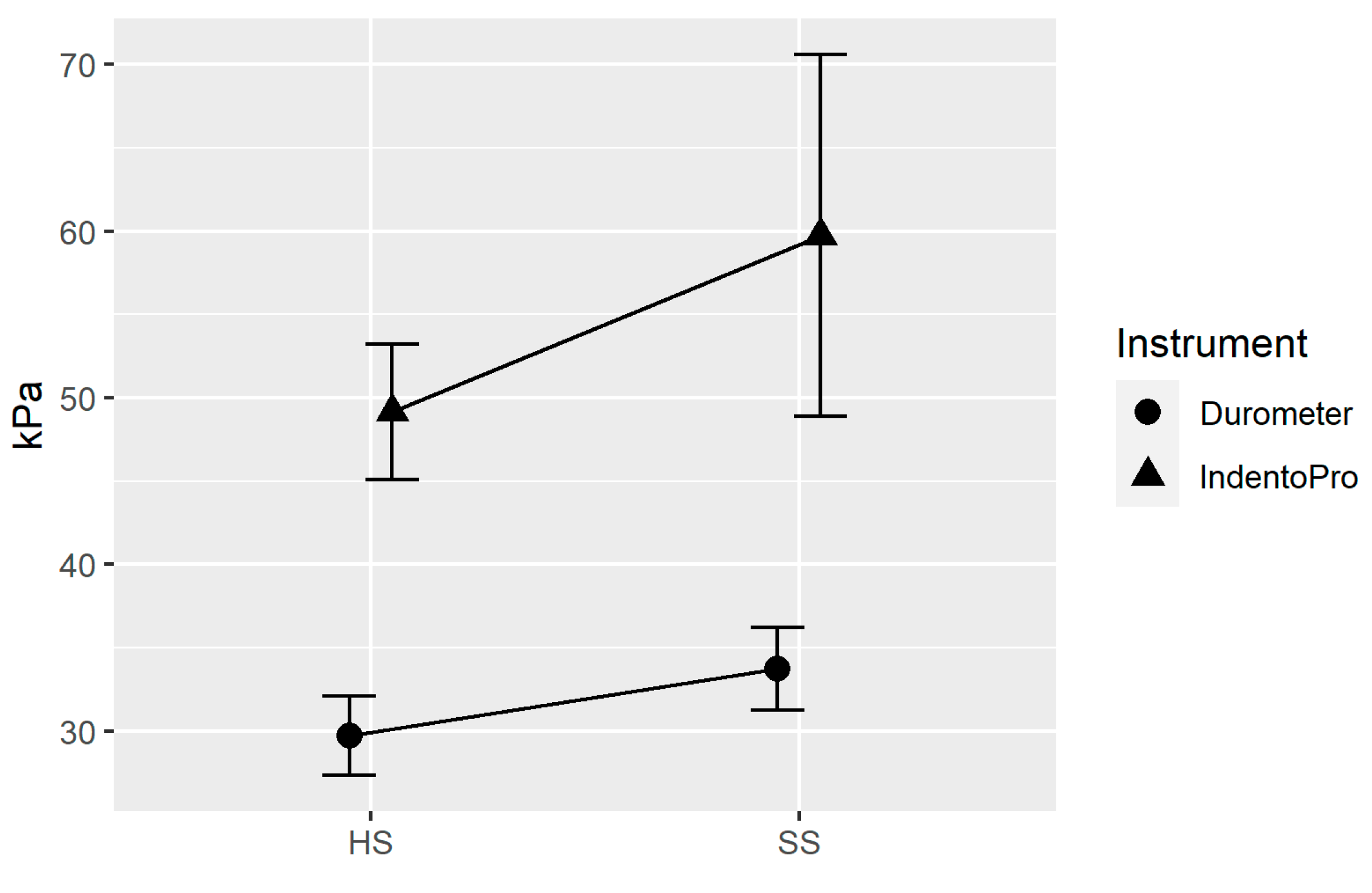
The following line plot (
Figure 7) shows that both devices used (IndentoPro, Durometer) show an increase in stiffness towards the painful side. In other words, there is a statistically significant correlation between stiffness and pain. Looking at both devices, there was a medium Pearson-Product-Moment-Correlation (r=0.29, p=0.010) between the two devices, with the Durometer presumably measuring mainly superficial tissue layers, while the IndentoPro also reaches deeper myofascial zones [
21,
28].
There was no significance between pain symptoms of unilateral neck pain and elasticity. Similarly, there was no correlation between side-dependent pain intensity and PPT. In addition, there was no statistical association between the more painful side of the neck and the opposite side for both lateral flexion and rotation, based on the unilateral function of the upper trapezius.
4. Discussion
The key finding of the study is the documentation of increased stiffness of the upper trapezius muscle on the symptomatic side, confirmed by duro- and indentometry. This finding is relevant for future treatment methods, as increased stiffness carries a higher risk of causing hydration dysfunction [
29], vasoconstriction, inflammation [
30], and further stress injuries [
31]. Furthermore, this knowledge can be used to better detect pathological tissue conditions, monitor the effects of therapy and training, and prevent sports injuries [
20].
Reasons for increased stiffness of the selected muscle may include altered tissue morphology of the myofascia and/or possible nerve irritation of the accessorius nerve [
32]. The former may be caused by ageing processes [
9] or pathogenic changes in the posterior-lateral fascial chain [
33], passively transmitted forces from the muscles to the fascial tissue [
34] or a fascia-triggered neuromuscular alteration [
35] as well as an altered hydration and viscosity state of the fascia [
36,
37]. An imbalance between the different muscle parts of the trapezius and the prevertebral and spinal extensors is also discussed in the literature as being relevant to increased stiffness. It should be noted that the upper trapezius has been reported of being already anatomically prone to shortening as a posture-dominant muscle [
3,
38,
39]. The significant change in elasticity with age may be explained by age-related processes in the body. From the age of sixty, the number, quality and strength of muscle fibres decrease, and the collagen content increases, leading to increased stiffness. This effect is more pronounced in women [
9,
40].
Despite more severe neck pain on one side with a clear localisation, no difference in pain intensity could be measured [
41]. This seems to contrast with the subjective perception of the patient. Reduced PPT and increased stiffness at trigger points, including the upper trapezius, have been documented in other studies [
42,
43]. However, as the present study did not focus on existing trigger points, it is unclear whether the painful side of the neck of the subjects was influenced by an increased expression of such trigger points. A second consideration relates to the assumption of deep nociceptive nerve endings. If these are dampened by the increased measured stiffness of the overlying tissue, this could affect the pressure sensitivity of the upper trapezius muscle, for example by attenuating it.
The present study has some limitations that warrant future research. Potential measurement errors and bias were largely avoided. Differences are possible in the positioning of the measuring device regarding the angle of pressure and the penetration force exerted when pressing the measuring punch into the myofascial tissue of the trapezius muscle. Due to the different locations, slight variations in the starting position are also realistic, which could also lead to different results. The investigator's influence is excluded by the blinded procedure, i.e., the investigator had no information about which of the two sides of the neck was reported by the patient to be more painful. The results only refer to one pressure point of the upper trapezius. It would be possible to extend this to other points on the same muscle or other muscle groups that are prone to pain. Another limitation is the age range used. The study included patients up to the age of 75, and it is known that fascia changes with age. There is a degree of inhomogeneity in the subject group, but this reflects clinical reality, as increasing age is associated with increasing pain. This should be considered in future studies and possibly supplemented by a control group. Furthermore, it would also be interesting to find out whether the results can be transferred to other starting positions such as standing or supine [
44].
5. Conclusion
The study shows unilateral increased stiffness of the upper trapezius muscle on the side of the neck with pain symptoms. However, other myofascial properties such as elasticity and algometry are not significantly altered. Similarly, despite the dominance of pain, there is no homolateral change in mobility of the cervical spine. If confirmed by future additional studies, our current data suggest that a palpatory (or tool-assisted) assessment of myofascial stiffness in the trapezius region may be a recommended feedback tool for evaluating treatments for myofascial neck pain; and most likely more reliable than an associated assessment of pressure tenderness, elastic recoil capacity, or neck mobility.
Author Contributions
Conceptualization, A.S. and R.S.; methodology, A.S.; software, A.B.; validation, A.S.; formal analysis, A.B.; investigation, A.S.; resources, A.S. and R.S.; data curation, A.S..; writing—original draft preparation, A.S.; writing—review and editing, A.S., A.B., C.E. and R.S..; visualization, A.S. and A.B.; supervision, C.E. and R.S.; project administration, A.S. and R.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Diploma University of Applied Sciences, Bad Sooden-Allendorf, Germany (approval number EB 1037/2022 and date of approval 10 October 2022).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets used and/or analysed in the current study or any query regarding the research process are available from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Haldeman, S.; Caroll, L.; Cassidy, D. The Bone and Joint Decade 2000 – 2010. Task Force on Neck Pain and Its Associated Disorders, Spine 2008, 33. [Google Scholar] [CrossRef]
- Petersen, S.M.; Wyatt, S.N. Lower trapezius muscle strength in individuals with unilateral neck pain. journal of orthopaedic & sports physical therapy 2011, 41, 260–265. [Google Scholar] [CrossRef]
- Choudhari, R.; Anap, D.; Rao, K.; Iyer, C. Comparison of upper, middle, and lower trapezius strength in individuals with unilateral neck pain. J Spine 2012, 1, 1–3. [Google Scholar] [CrossRef]
- Park, K.N.; Jung, D.Y.; Kim, S.H. Trapezius and serratus anterior muscle strength in violinists with unilateral neck pain. Journal of Back and Musculoskeletal Rehabilitation 2020, 33, 631–636. [Google Scholar] [CrossRef]
- Uthaikhup, S.; Pensri, C.; Kawsoiy, K. Decreased thickness of the lower trapezius muscle in patients with unilateral neck pain. Muscle & Nerve 2016, 54, 439–443. [Google Scholar] [CrossRef]
- Kuo, W.H.; Jian, D.W.; Wang, T.G.; Wang, Y.C. Neck muscle stiffness quantified by sonoelastography is correlated with body mass index and chronic neck pain symptoms. Ultrasound in medicine & biology 2013, 39, 1356–1361. [Google Scholar] [CrossRef]
- Kisilewicz, A.; Madeleine, P.; Ignasiak, Z.; Ciszek, B.; Kawczynski, A.; Larsen, R.G. Eccentric exercise reduces upper trapezius muscle stiffness assessed by shear wave elastography and myotonometry. Frontiers in Bioengineering and Biotechnology 2020, 8, 928. [Google Scholar] [CrossRef]
- Taş, S.; Korkusuz, F.; Erden, Z. Neck muscle stiffness in participants with and without chronic neck pain: a shear-wave elastography study. Journal of Manipulative and Physiological Therapeutics 2018, 41, 580–588. [Google Scholar] [CrossRef]
- Kocur, P.; Tomczak, M.; Wiernicka, M.; Goliwąs, M.; Lewandowski, J.; Łochyński, D. Relationship between age, BMI, head posture and superficial neck muscle stiffness and elasticity in adult women. Scientific reports 2019, 9, 8515. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. The Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef]
- MacDermid, J.C.; Walton, D.M.; Avery, S.; Blanchard, A.; Etruw, E.; Mcalpine, C.; Goldsmith, C.H. Measurement properties of the neck disability index: a systematic review. Journal of orthopaedic & sports physical therapy 2009, 39, 400–417. [Google Scholar]
- Sawada, T.; Okawara, H.; Nakashima, D.; Iwabuchi, S.; Matsumoto, M.; Nakamura, M.; Nagura, T. Reliability of trapezius muscle hardness measurement: a comparison between portable muscle hardness meter and ultrasound strain elastography. Sensors 2020, 20, 7200. [Google Scholar] [CrossRef] [PubMed]
- Hochschild, J. Strukturen und Funktionen begreifen. Grundlagen zur Wirbelsäule, HWS, Schädel, BWS und Brustkorb, Obere Extremität, Thieme-Verlag, 4. Auflage. 2015. [Google Scholar]
- Masi, A.T.; Andrade, C.K. Das Konzept Ruhemuskeltonus: Integrativ „übersetzt “. manuelletherapie 2014, 18, 69–73. [Google Scholar] [CrossRef]
- Dieterich, A. Gefühlt steif heißt nicht objektiv steif–Myth-Busters: die verspannte Nackenmuskulatur. Physiopraxis 2020, 18, 20–24. [Google Scholar] [CrossRef]
- Bartsch, K.; Brandl, A.; Weber, P.; Wilke, J.; Bensamoun, S.F.; Bauermeister, W.; Schleip, R. Assessing reliability and validity of different stiffness measurement tools on a multi-layered phantom tissue model. Scientific Reports 2023, 13, 815. [Google Scholar] [CrossRef] [PubMed]
- Dellalana, L.E.; Chen, F.; Vain, A.; Gandelman, J.S.; Põldemaa, M.; Chen, H.; Tkaczyk, E.R. Reproducibility of the durometer and myoton devices for skin stiffness measurement in healthy subjects. Skin Research and Technology 2019, 25, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Park, S.K.; Yang, D.J.; Kim, J.H.; Heo, J.W.; Uhm, Y.H.; Yoon, J.H. Analysis of mechanical properties of cervical muscles in patients with cervicogenic headache. Journal of physical therapy science 2017, 29, 332–335. [Google Scholar] [CrossRef] [PubMed]
- Akhbari, B.; Salavati, M.; Ebarahimi, I.; Ezzati, K.; Haghighat Khah, H. Association of ultrasonography findings with pain, range of motion, disability, and pressure pain threshold in subjects with upper trapezius myofascial pain syndrome. Physical Treatments-Specific Physical Therapy Journal 2015, 4, 221–227. [Google Scholar]
- Koch, V.; Wilke, J. Reliability of a new indentometer device for measuring myofascial tissue stiffness. Journal of Clinical Medicine 2022, 11, 5194. [Google Scholar] [CrossRef]
- Bartsch, K.; Brandl, A.; Weber, P.; Wilke, J.; Bensamoun, S. F.; Bauermeister, W.; Schleip, R. The princess and the pea-Comparison of different stiffness assessment tools on a multi-layered phantom tissue model. 2022. [Google Scholar] [CrossRef]
- Von Pickartz, H.; Andreotti, D.; Arendt-Nielsen, L. Kiefer, Gesichts- und Zervikalregion. Thieme-Verlag, Stuttgart, 2. Auflage. 2015. [Google Scholar]
- Grossi, D.B.; Chaves, T.C.; Gonçalves, M.C.; Moreira, V.C.; Canonica, A.C.; Florencio, L.L.; Bigal, M.E. Pressure pain threshold in the craniocervical muscles of women with episodic and chronic migraine: a controlled study. Arquivos de neuro-psiquiatria 2011, 69, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Rolke, R.; Magerl, W.; Campbell, K.A.; Schalber, C.; Caspari, S.; Birklein, F.; Treede, R.D. Quantitative sensory testing: a comprehensive protocol for clinical trials. European journal of pain 2006, 10, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Luedtke, K.; Schoettker-Königer, T.; Hall, T.; Reimer, C.; Grassold, M.; Hasselhoff-Styhler, P.; Schäfer, A. Concurrent validity and reliability of measuring range of motion during the cervical flexion rotation test with a novel digital goniometer. BMC musculoskeletal disorders 2020, 21, 1–10. [Google Scholar] [CrossRef]
- Stone, E. R.: t Test – Paired Samples. Encyclopedia of research design, N.J. Stalkind, Los Angeles: SAGE, 2010, 1560-1565. S: Stalkind, Los Angeles.
- Cohen, J. A power primer. Psychol Bull 1992, 112, 115–119. [Google Scholar] [CrossRef]
- Kim, C.Y.; Kang, J.H.; Park, T.S. Intra-Rater Reliability of the Shore Durometer in the Assessment of Upper Trapezius Muscle Hardness. Research Journal of Pharmacy and Technology 2019, 12, 2461–2464. [Google Scholar] [CrossRef]
- Brandl, A.; Egner, C.; Schwarze, M.; Reer, R.; Schmidt, T.; Schleip, R. Immediate Effects of Instrument-Assisted Soft Tissue Mobilization on Hydration Content in Lumbar Myofascial Tissues: A Quasi-Experiment. Journal of Clinical Medicine 2023, 12, 1009. [Google Scholar] [CrossRef]
- Brandl, A.; Egner, C.; Reer, R.; Schmidt, T.; Schleip, R. Immediate Effects of Myofascial Release Treatment on Lumbar Microcirculation: A Randomized, Placebo-Controlled Trial. Journal of Clinical Medicine 2023, 12, 1248. [Google Scholar] [CrossRef]
- Pruyn, E.C.; Watsford, M.L.; Murphy, A.J.; Pine, M.J.; Spurrs, R.W.; Cameron, M.L.; et al. Relationship between leg stiffness lower body injuries in professional Australian football, J. Sports Sci. 30 2012, 71–78. [Google Scholar] [CrossRef]
- Von Piekartz HJ, M. Untersuchung und Behandlung des kranialen Nervengewebes am Beispiel des N. accessorius. Manuelletherapie 2005, 9, 237–241. [Google Scholar] [CrossRef]
- Hockenholz, F.; Roos, K.; Emmert, A. Physiotherapie bei Schmerzen. Thieme-Verlag, Stuttgart. 2016. [Google Scholar]
- Schleip, R.; Jäger, H.; Klingler, W. What is 'fascia'? A review of different nomenclatures. J Bodyw Mov Ther 2012b, 16, 496–502. [Google Scholar] [CrossRef]
- Brandl, A.; Egner, C.; Reer, R.; Schmidt, T.; Schleip, R. Associations between Deformation of the Thoracolumbar Fascia and Activation of the Erector Spinae and Multifidus Muscle in Patients with Acute Low Back Pain and Healthy Controls: A Matched Pair Case-Control Study. Life 2022, 12, 1735. [Google Scholar] [CrossRef] [PubMed]
- Ng, C. P.; Hinz, B.; Swartz, M. A. Interstitial fluid flow induces myofibroblast differentiation and collagen alignment in vitro. J Cell Sci 2005, 118, 4731–4739. [Google Scholar] [CrossRef] [PubMed]
- Pavan, P. G.; Stecco, A.; Stern, R.; Stecco, C. Painful Connections: Densification Versus Fibrosis of Fascia. Cur Pain Head Rep 2014, 18, 44. [Google Scholar] [CrossRef]
- Böhni, U.; Lauper, M.; Lochner, H. Manuelle Medizin 2. Thieme-Verlag 2012, 298–302. [Google Scholar] [CrossRef]
- Petersen, S.M.; Wyatt, S.N. Lower trapezius muscle strength in individuals with unilateral neck pain. journal of orthopaedic & sports physical therapy 2011, 41, 260–265. [Google Scholar] [CrossRef]
- Heizelmann, A.; Tasdemir, S.; Schmidberger, J.; Gräter, T.; Kratzer, W.; Grüner, B. Measurements of the trapezius and erector spinae muscles using virtual touch imaging quantification ultrasound-Elastography: a cross section study. BMC Musculoskeletal Disorders 2017, 18, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Heredia-Rizo, A.M.; Petersen, K.K.; Arendt-Nielsen, L.; Madeleine, P. Eccentric training changes the pressure pain and stiffness maps of the upper trapezius in females with chronic neck-shoulder pain: a preliminary study. Pain Medicine 2020, 21, 1936–1946. [Google Scholar] [CrossRef]
- Geri, T.; Botticchio, A.; Rossettini, G.; Pournajaf, S.; Pellicciari, L.; Di Antonio, S.; Castaldo, M. Pressure Pain Threshold of the Upper Trapezius Trigger Point: A Systematic Review with Meta-Analysis of Baseline Values and Their Modification after Physical Therapy. Journal of Clinical Medicine 2022, 11, 7243. [Google Scholar] [CrossRef]
- Arokoski, J.P.; Surakka, J.; Ojala, T.; Kolari, P.; Jurvelin, J.S. Feasibility of the use of a novel soft tissue stiffness meter. Physiological measurement 2005, 26, 215. [Google Scholar] [CrossRef]
- Viir, R.; Virkus, A.; Laiho, K.; Rajaleid, K.; Selart, A.; Mikkelsson, M. Trapezius muscle tone and viscoelastic properties in sitting and supine positions. Scandinavian Journal of Work, Environment & Health 2007, 33, 76. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).