Submitted:
15 September 2023
Posted:
18 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Material and Methods
2.1. Exposure Data
2.2. Outcome Data
2.3. Ethics Statement
2.4. Statistical Analysis
3. Results
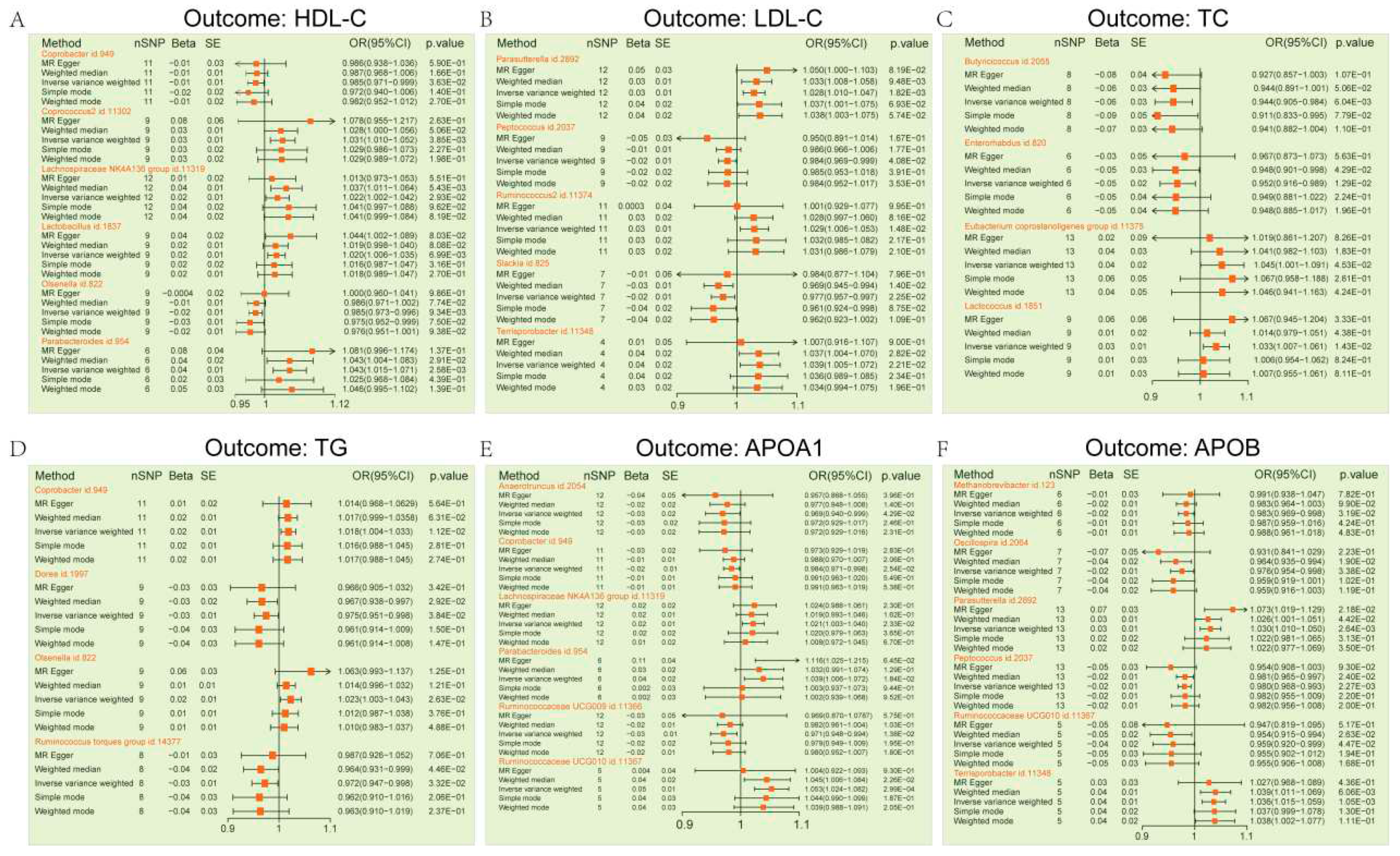
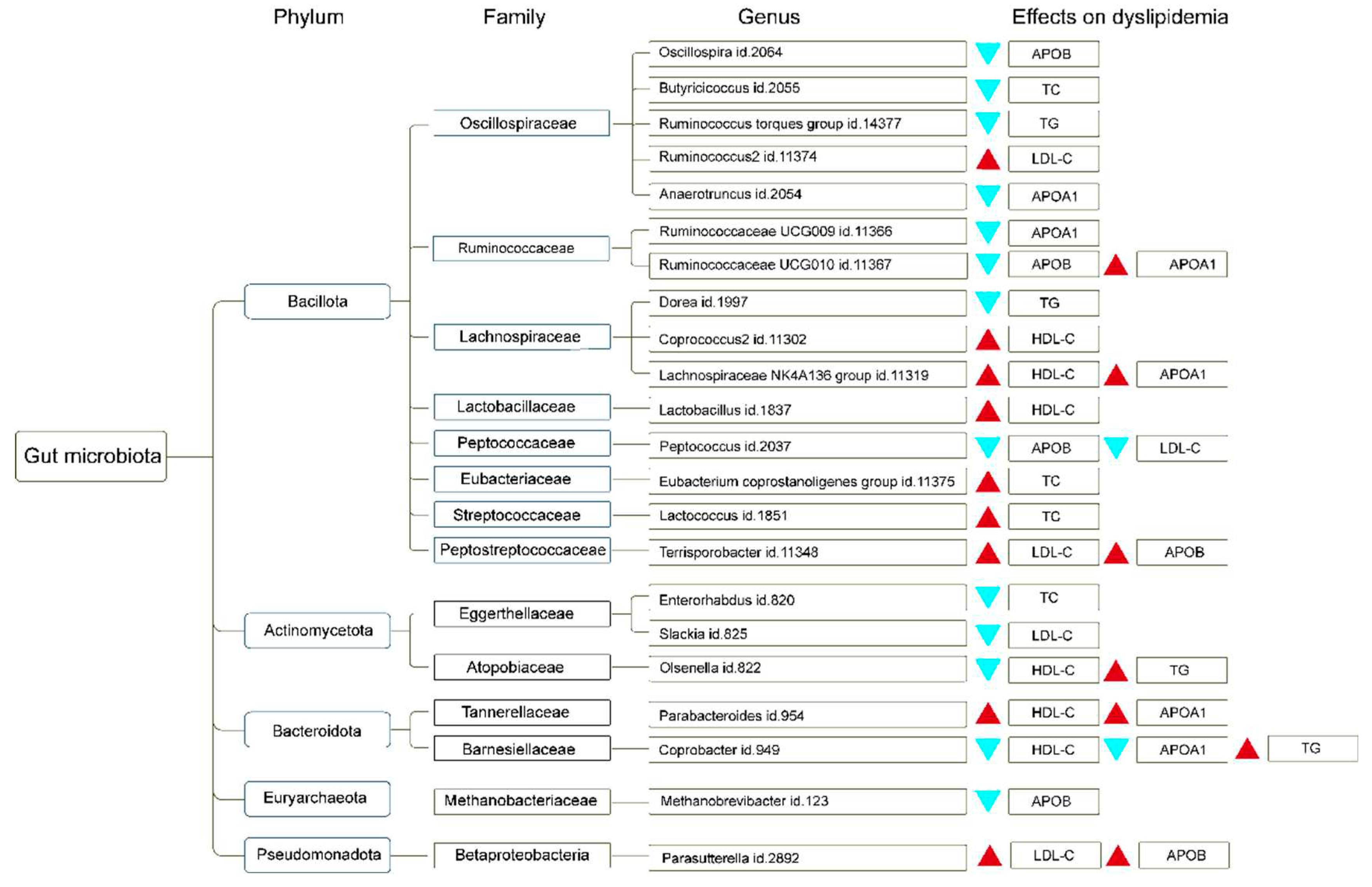
3.1. Dyslipidemia MR Estimates
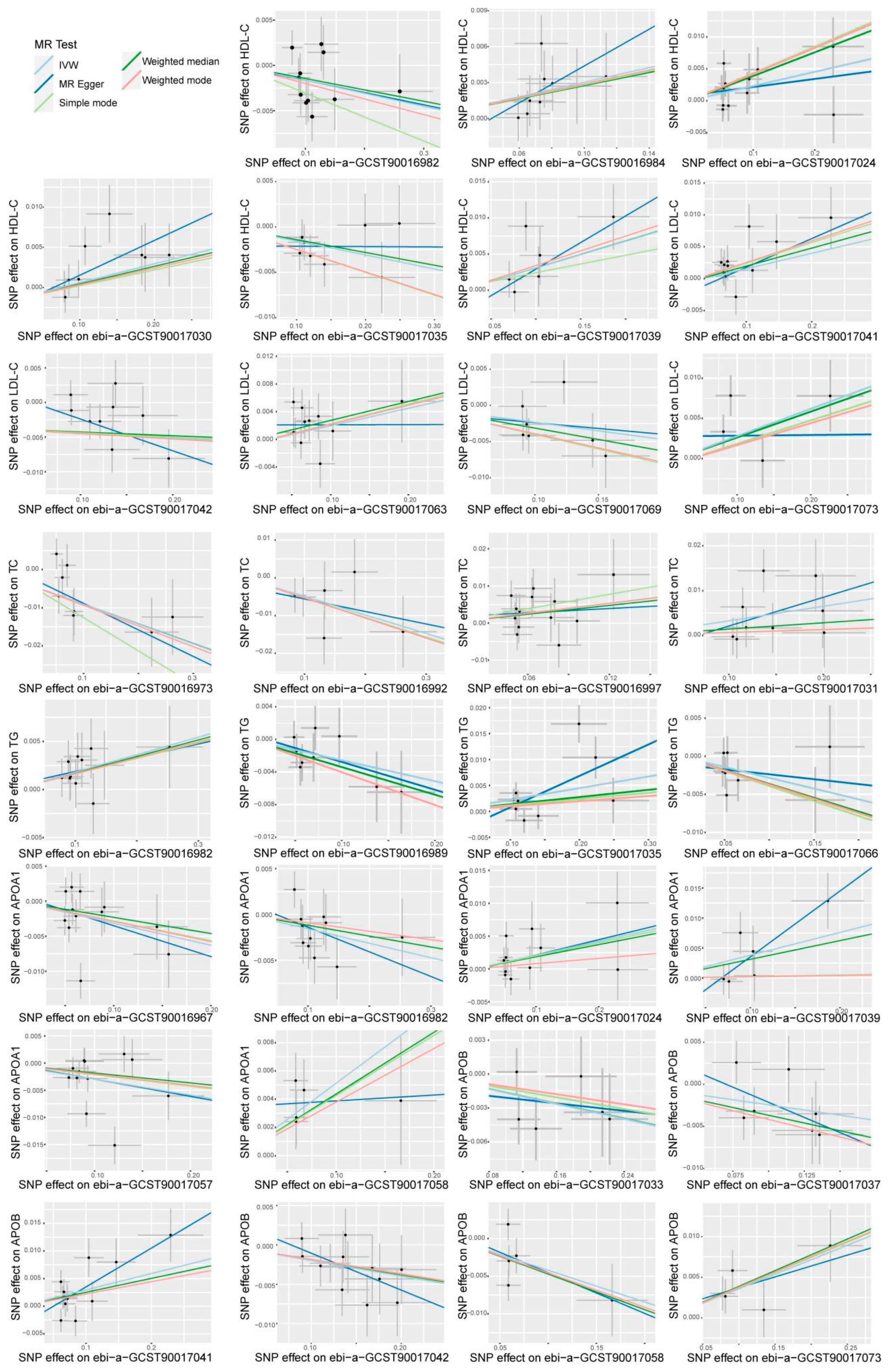
3.2. Sensitivity Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kamada, N.; Seo, S.-U.; Chen, G.Y.; Núñez, G. Role of the gut microbiota in immunity and inflammatory disease. Nature Reviews Immunology 2013, 13, 321–335. [Google Scholar] [CrossRef]
- Ley, R.E.; Peterson, D.A.; Gordon, J.I. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell 2006, 124, 837–848. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lian, J.; Zheng, Q.; Wang, L.; Wang, Y.; Yang, D. Composition analysis and prebiotics properties of polysaccharides extracted from Lepista sordida submerged cultivation mycelium. Frontiers in microbiology 2022, 13, 1077322. [Google Scholar] [CrossRef]
- Bi, C.R.; Sun, J.T.; Du, J.; Chu, L.Y.; Li, Y.J.; Jia, X.Y.; Liu, Y.; Zhang, W.P.; Li, Y.C.; Liu, Y.J. Effects of Zhishi Daozhi Decoction on the intestinal flora of nonalcoholic fatty liver disease mice induced by a high-fat diet. Frontiers in cellular and infection microbiology 2022, 12, 1005318. [Google Scholar] [CrossRef]
- Luo, Q.; Lei, X.; Xu, J.; Jahangir, A.; He, J.; Huang, C.; Liu, W.; Cheng, A.; Tang, L.; Geng, Y. , et al. An altered gut microbiota in duck-origin parvovirus infection on cherry valley ducklings is associated with mucosal barrier dysfunction. Poultry science 2021, 100, 101021. [Google Scholar] [CrossRef]
- Makrgeorgou, A.; Leonardi-Bee, J.; Bath-Hextall, F.J.; Murrell, D.F.; Tang, M.L.; Roberts, A.; Boyle, R.J. Probiotics for treating eczema. Cochrane Database Syst Rev 2018, 11, Cd006135. [Google Scholar] [CrossRef]
- Mishra, A.K.; Dubey, V.; Ghosh, A.R. Obesity: An overview of possible role(s) of gut hormones, lipid sensing and gut microbiota. Metabolism: clinical and experimental 2016, 65, 48–65. [Google Scholar] [CrossRef]
- Wei, M.Y.; Shi, S.; Liang, C.; Meng, Q.C.; Hua, J.; Zhang, Y.Y.; Liu, J.; Zhang, B.; Xu, J.; Yu, X.J. The microbiota and microbiome in pancreatic cancer: more influential than expected. Molecular cancer 2019, 18, 97. [Google Scholar] [CrossRef]
- Gilbert, J.A.; Blaser, M.J.; Caporaso, J.G.; Jansson, J.K.; Lynch, S.V.; Knight, R. Current understanding of the human microbiome. Nat Med 2018, 24, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Vuotto, C.; Longo, F.; Donelli, G. Probiotics to counteract biofilm-associated infections: promising and conflicting data. International journal of oral science 2014, 6, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Wu, K.; Xu, L.; Cen, Y.; Ni, J.; Chen, J.; Zheng, W.; Liu, W. Methanol extract of Inonotus obliquus improves type 2 diabetes mellitus through modifying intestinal flora. Frontiers in endocrinology 2022, 13, 1103972. [Google Scholar] [CrossRef] [PubMed]
- Pasini, E.; Corsetti, G.; Assanelli, D.; Testa, C.; Romano, C.; Dioguardi, F.S.; Aquilani, R. Effects of chronic exercise on gut microbiota and intestinal barrier in human with type 2 diabetes. Minerva medica 2019, 110, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.B.; Jun, D.W.; Kang, B.K.; Lim, J.H.; Lim, S.; Chung, M.J. Randomized, Double-blind, Placebo-controlled Study of a Multispecies Probiotic Mixture in Nonalcoholic Fatty Liver Disease. Scientific reports 2019, 9, 5688. [Google Scholar] [CrossRef] [PubMed]
- Al-Assal, K.; Martinez, A.C.; Torrinhas, R.S.; Cardinelli, C.; Waitzberg, D. Gut microbiota and obesity. Clinical Nutrition Experimental 2018, 20, 60–64. [Google Scholar] [CrossRef]
- Baker, R.G.; Hayden, M.S.; Ghosh, S. NF-κB, inflammation, and metabolic disease. Cell metabolism 2011, 13, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhao, Q.; Liu, J.; Huang, A.; Xia, X. Buyang Huanwu decoction affects gut microbiota and lipid metabolism in a ZDF rat model of co-morbid type 2 diabetes mellitus and obesity: An integrated metabolomics analysis. Frontiers in chemistry 2022, 10, 1036380. [Google Scholar] [CrossRef]
- Aron-Wisnewsky, J.; Clément, K. The gut microbiome, diet, and links to cardiometabolic and chronic disorders. Nature reviews. Nephrology 2016, 12, 169–181. [Google Scholar] [CrossRef]
- Chung, S.; Barnes, J.L.; Astroth, K.S. Gastrointestinal Microbiota in Patients with Chronic Kidney Disease: A Systematic Review. Advances in nutrition (Bethesda, Md.) 2019, 10, 888–901. [Google Scholar] [CrossRef]
- Feng, Y.L.; Cao, G.; Chen, D.Q.; Vaziri, N.D.; Chen, L.; Zhang, J.; Wang, M.; Guo, Y.; Zhao, Y.Y. Microbiome-metabolomics reveals gut microbiota associated with glycine-conjugated metabolites and polyamine metabolism in chronic kidney disease. Cellular and molecular life sciences : CMLS 2019, 76, 4961–4978. [Google Scholar] [CrossRef]
- Li, J.; Zhao, F.; Wang, Y.; Chen, J.; Tao, J.; Tian, G.; Wu, S.; Liu, W.; Cui, Q.; Geng, B. , et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome 2017, 5, 14. [Google Scholar] [CrossRef]
- Jie, Z.; Xia, H.; Zhong, S.L.; Feng, Q.; Li, S.; Liang, S.; Zhong, H.; Liu, Z.; Gao, Y.; Zhao, H. , et al. The gut microbiome in atherosclerotic cardiovascular disease. Nat Commun 2017, 8, 845. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, C. Analysis of changes in intestinal flora and intravascular inflammation and coronary heart disease in obese patients. Experimental and therapeutic medicine 2018, 15, 4538–4542. [Google Scholar] [CrossRef] [PubMed]
- Lau, E.; Carvalho, D.; Freitas, P. Gut Microbiota: Association with NAFLD and Metabolic Disturbances. BioMed research international 2015, 2015, 979515. [Google Scholar] [CrossRef] [PubMed]
- Rezasoltani, S.; Asadzadeh-Aghdaei, H.; Nazemalhosseini-Mojarad, E.; Dabiri, H.; Ghanbari, R.; Zali, M.R. Gut microbiota, epigenetic modification and colorectal cancer. Iranian journal of microbiology 2017, 9, 55–63. [Google Scholar] [PubMed]
- Wang, X.; Yang, Y.; Huycke, M.M. Commensal-infected macrophages induce dedifferentiation and reprogramming of epithelial cells during colorectal carcinogenesis. Oncotarget 2017, 8, 102176–102190. [Google Scholar] [CrossRef]
- Li, S.; Wang, Z.; Yang, Y.; Yang, S.; Yao, C.; Liu, K.; Cui, S.; Zou, Q.; Sun, H.; Guo, G. Lachnospiraceae shift in the microbial community of mice faecal sample effects on water immersion restraint stress. AMB Express 2017, 7, 82. [Google Scholar] [CrossRef]
- Armstrong, H.; Alipour, M.; Valcheva, R.; Bording-Jorgensen, M.; Jovel, J.; Zaidi, D.; Shah, P.; Lou, Y.; Ebeling, C.; Mason, A.L. , et al. Host immunoglobulin G selectively identifies pathobionts in pediatric inflammatory bowel diseases. Microbiome 2019, 7, 1. [Google Scholar] [CrossRef]
- Furness, J.B. The enteric nervous system and neurogastroenterology. Nature Reviews Gastroenterology & Hepatology 2012, 9, 286–294. [Google Scholar] [CrossRef]
- Mayer, E.A.; Aziz, Q.; Coen, S.; Kern, M.; Labus, J.S.; Lane, R.; Kuo, B.; Naliboff, B.; Tracey, I. Brain imaging approaches to the study of functional GI disorders: a Rome working team report. Neurogastroenterology and motility 2009, 21, 579–596. [Google Scholar] [CrossRef]
- Subramanya, S.H.; Sharan, N.K.; Baral, B.P.; Hamal, D.; Nayak, N.; Prakash, P.Y.; Sathian, B.; Bairy, I.; Gokhale, S. Diversity, in-vitro virulence traits and antifungal susceptibility pattern of gastrointestinal yeast flora of healthy poultry, Gallus gallus domesticus. BMC microbiology 2017, 17, 113. [Google Scholar] [CrossRef]
- Round, J.L.; Mazmanian, S.K. The gut microbiota shapes intestinal immune responses during health and disease. Nature reviews. Immunology 2009, 9, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Liu, Y.; Chen, J.; Bai, Y.; He, J.; Cao, H.; Che, Q.; Guo, J.; Su, Z. Marine Chitooligosaccharide Alters Intestinal Flora Structure and Regulates Hepatic Inflammatory Response to Influence Nonalcoholic Fatty Liver Disease. Marine drugs 2022, 20. [Google Scholar] [CrossRef] [PubMed]
- Bo, L.; Li, J.; Tao, T.; Bai, Y.; Ye, X.; Hotchkiss, R.S.; Kollef, M.H.; Crooks, N.H.; Deng, X. Probiotics for preventing ventilator-associated pneumonia. Cochrane Database Syst Rev 2014, 10, Cd009066. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.M.; Surette, M.; Bercik, P. The interplay between the intestinal microbiota and the brain. Nature Reviews Microbiology 2012, 10, 735–742. [Google Scholar] [CrossRef]
- Bercik, P. The microbiota–gut–brain axis: learning from intestinal bacteria? 2011, 60, 288–289. [CrossRef]
- Collins, S.M.; Bercik, P. The relationship between intestinal microbiota and the central nervous system in normal gastrointestinal function and disease. Gastroenterology 2009, 136, 2003–2014. [Google Scholar] [CrossRef]
- Yi, X.; Zhou, K.; Deng, N.; Cai, Y.; Peng, X.; Tan, Z. Simo decoction curing spleen deficiency constipation was associated with brain-bacteria-gut axis by intestinal mucosal microbiota. Frontiers in microbiology 2023, 14, 1090302. [Google Scholar] [CrossRef]
- El-Salhy, M.; Winkel, R.; Casen, C.; Hausken, T.; Gilja, O.H.; Hatlebakk, J.G. Efficacy of Fecal Microbiota Transplantation for Patients With Irritable Bowel Syndrome at 3 Years After Transplantation. Gastroenterology 2022, 163, 982–994. [Google Scholar] [CrossRef]
- Kindt, A.; Liebisch, G.; Clavel, T.; Haller, D.; Hörmannsperger, G.; Yoon, H.; Kolmeder, D.; Sigruener, A.; Krautbauer, S.; Seeliger, C. , et al. The gut microbiota promotes hepatic fatty acid desaturation and elongation in mice. Nat Commun 2018, 9, 3760. [Google Scholar] [CrossRef]
- You, H.; Deng, X.; Bai, Y.; He, J.; Cao, H.; Che, Q.; Guo, J.; Su, Z. The Ameliorative Effect of COST on Diet-Induced Lipid Metabolism Disorders by Regulating Intestinal Microbiota. Marine drugs 2022, 20. [Google Scholar] [CrossRef]
- Qin, S.; He, Z.; Wu, Y.; Zeng, C.; Zheng, Z.; Zhang, H.; Lv, C.; Yuan, Y.; Wu, H.; Ye, J. , et al. Instant Dark Tea Alleviates Hyperlipidaemia in High-Fat Diet-Fed Rat: From Molecular Evidence to Redox Balance and Beyond. Frontiers in nutrition 2022, 9, 819980. [Google Scholar] [CrossRef]
- Liu, H.; Meng, W.; Zhao, D.; Ma, Z.; Zhang, W.; Chen, Z.; Li, Z.; Zhao, P. Study on mechanism of action of total flavonoids from Cortex Juglandis Mandshuricae against alcoholic liver disease based on “gut-liver axis”. Frontiers in pharmacology 2022, 13, 1074286. [Google Scholar] [CrossRef] [PubMed]
- Chapman, M.J.; Ginsberg, H.N.; Amarenco, P.; Andreotti, F.; Borén, J.; Catapano, A.L.; Descamps, O.S.; Fisher, E.; Kovanen, P.T.; Kuivenhoven, J.A. , et al. Triglyceride-rich lipoproteins and high-density lipoprotein cholesterol in patients at high risk of cardiovascular disease: evidence and guidance for management. European heart journal 2011, 32, 1345–1361. [Google Scholar] [CrossRef] [PubMed]
- Voight, B.F.; Peloso, G.M.; Orho-Melander, M.; Frikke-Schmidt, R.; Barbalic, M.; Jensen, M.K.; Hindy, G.; Hólm, H.; Ding, E.L.; Johnson, T. , et al. Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study. Lancet (London, England) 2012, 380, 572–580. [Google Scholar] [CrossRef]
- Holmes, M.V.; Asselbergs, F.W.; Palmer, T.M.; Drenos, F.; Lanktree, M.B.; Nelson, C.P.; Dale, C.E.; Padmanabhan, S.; Finan, C.; Swerdlow, D.I. , et al. Mendelian randomization of blood lipids for coronary heart disease. European heart journal 2015, 36, 539–550. [Google Scholar] [CrossRef] [PubMed]
- groups, E. Emergency expert consensus on diagnosis and treatment of hypertriglyceridemic acute pancreatitis. Chinese General Practice 2021, 24, 3781–3793. [Google Scholar]
- Klop, B.; Elte, J.W.; Cabezas, M.C. Dyslipidemia in obesity: mechanisms and potential targets. Nutrients 2013, 5, 1218–1240. [Google Scholar] [CrossRef]
- Soremekun, O.; Karhunen, V.; He, Y.; Rajasundaram, S.; Liu, B.; Gkatzionis, A.; Soremekun, C.; Udosen, B.; Musa, H.; Silva, S. , et al. Lipid traits and type 2 diabetes risk in African ancestry individuals: A Mendelian Randomization study. EBioMedicine 2022, 78, 103953. [Google Scholar] [CrossRef]
- Bulbul, M.C.; Dagel, T.; Afsar, B.; Ulusu, N.N.; Kuwabara, M.; Covic, A.; Kanbay, M. Disorders of Lipid Metabolism in Chronic Kidney Disease. Blood purification 2018, 46, 144–152. [Google Scholar] [CrossRef]
- Moradi, H.; Vaziri, N.D. Molecular mechanisms of disorders of lipid metabolism in chronic kidney disease. Frontiers in bioscience (Landmark edition) 2018, 23, 146–161. [Google Scholar] [CrossRef]
- Ferro, C.J.; Mark, P.B.; Kanbay, M.; Sarafidis, P.; Heine, G.H.; Rossignol, P.; Massy, Z.A.; Mallamaci, F.; Valdivielso, J.M.; Malyszko, J. , et al. Lipid management in patients with chronic kidney disease. Nature reviews. Nephrology 2018, 14, 727–749. [Google Scholar] [CrossRef]
- Ou, M.; Li, X.; Zhao, S.; Cui, S.; Tu, J. Long non-coding RNA CDKN2B-AS1 contributes to atherosclerotic plaque formation by forming RNA-DNA triplex in the CDKN2B promoter. EBioMedicine 2020, 55, 102694. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgözoğlu, L.; Lewis, E.F. Atherosclerosis. Nature reviews. Disease primers 2019, 5, 56. [Google Scholar] [CrossRef] [PubMed]
- Qin, T.; Sheng, W.; Hu, G. To Analyze the Influencing Factors of Senile Coronary Heart Disease Patients Complicated with Frailty Syndrome. Journal of healthcare engineering 2022, 2022, 7619438. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zheng, L.; Xie, X.; Luo, J.; Yu, J.; Zhang, L.; Meng, W.; Zhou, Y.; Chen, L.; Ouyang, D. , et al. Targeting PLA2G16, a lipid metabolism gene, by Ginsenoside Compound K to suppress the malignant progression of colorectal cancer. Journal of advanced research 2022, 36, 265–276. [Google Scholar] [CrossRef]
- Bian, X.; Liu, R.; Meng, Y.; Xing, D.; Xu, D.; Lu, Z. Lipid metabolism and cancer. The Journal of experimental medicine 2021, 218. [Google Scholar] [CrossRef]
- Cheng, C.; Geng, F.; Cheng, X.; Guo, D. Lipid metabolism reprogramming and its potential targets in cancer. Cancer communications (London, England) 2018, 38, 27. [Google Scholar] [CrossRef]
- Cao, Y. Adipocyte and lipid metabolism in cancer drug resistance. The Journal of clinical investigation 2019, 129, 3006–3017. [Google Scholar] [CrossRef]
- Virani, S.S.; Morris, P.B.; Agarwala, A.; Ballantyne, C.M.; Birtcher, K.K.; Kris-Etherton, P.M.; Ladden-Stirling, A.B.; Miller, M.; Orringer, C.E.; Stone, N.J. 2021 ACC Expert Consensus Decision Pathway on the Management of ASCVD Risk Reduction in Patients With Persistent Hypertriglyceridemia: A Report of the American College of Cardiology Solution Set Oversight Committee. Journal of the American College of Cardiology 2021, 78, 960–993. [Google Scholar] [CrossRef]
- Averna, M.; Banach, M.; Bruckert, E.; Drexel, H.; Farnier, M.; Gaita, D.; Magni, P.; März, W.; Masana, L.; Mello, E.S.A. , et al. Practical guidance for combination lipid-modifying therapy in high- and very-high-risk patients: A statement from a European Atherosclerosis Society Task Force. Atherosclerosis 2021, 325, 99–109. [Google Scholar] [CrossRef]
- Huang, Y.; Zheng, S.; Guo, Z.; de Mollerat du Jeu, X.; Liang, X.J.; Yang, Z.; Zhang, H.Y.; Gao, S.; Liang, Z. Ionizable liposomal siRNA therapeutics enables potent and persistent treatment of Hepatitis B. Signal transduction and targeted therapy 2022, 7, 38. [Google Scholar] [CrossRef]
- Richardson, T.G.; Sanderson, E.; Palmer, T.M.; Ala-Korpela, M.; Ference, B.A.; Davey Smith, G.; Holmes, M.V. Evaluating the relationship between circulating lipoprotein lipids and apolipoproteins with risk of coronary heart disease: A multivariable Mendelian randomisation analysis. PLoS medicine 2020, 17, e1003062. [Google Scholar] [CrossRef] [PubMed]
- Boekholdt, S.M.; Arsenault, B.J.; Hovingh, G.K.; Mora, S.; Pedersen, T.R.; Larosa, J.C.; Welch, K.M.; Amarenco, P.; Demicco, D.A.; Tonkin, A.M. , et al. Levels and changes of HDL cholesterol and apolipoprotein A-I in relation to risk of cardiovascular events among statin-treated patients: a meta-analysis. Circulation 2013, 128, 1504–1512. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.C.; Watts, G.F.; Coll, B.; Wasserman, S.M.; Marcovina, S.M.; Barrett, P.H.R. Lipoprotein(a) Particle Production as a Determinant of Plasma Lipoprotein(a) Concentration Across Varying Apolipoprotein(a) Isoform Sizes and Background Cholesterol-Lowering Therapy. Journal of the American Heart Association 2019, 8, e011781. [Google Scholar] [CrossRef] [PubMed]
- Di Angelantonio, E.; Sarwar, N.; Perry, P.; Kaptoge, S.; Ray, K.K.; Thompson, A.; Wood, A.M.; Lewington, S.; Sattar, N.; Packard, C.J. , et al. Major lipids, apolipoproteins, and risk of vascular disease. Jama 2009, 302, 1993–2000. [Google Scholar] [CrossRef]
- Sahm, A.; Bens, M.; Platzer, M.; Cellerino, A. Parallel evolution of genes controlling mitonuclear balance in short-lived annual fishes. Aging cell 2017, 16, 488–496. [Google Scholar] [CrossRef]
- Wilson, P.W.; Garrison, R.J.; Castelli, W.P.; Feinleib, M.; McNamara, P.M.; Kannel, W.B. Prevalence of coronary heart disease in the Framingham Offspring Study: role of lipoprotein cholesterols. The American journal of cardiology 1980, 46, 649–654. [Google Scholar] [CrossRef]
- Zhou, J.; Zhao, L.; Meng, L.; Liang, H.; Zhou, T.; Ye, S.; Qi, Z.; Huang, X.; Zhou, P.; Fu, W. Acupuncture treatment for carotid atherosclerotic plaques: study protocol for a pilot randomized, single blinded, controlled clinical trial. Trials 2020, 21, 768. [Google Scholar] [CrossRef]
- Emdin, C.A.; Khera, A.V.; Kathiresan, S. Mendelian Randomization. Jama 2017, 318, 1925–1926. [Google Scholar] [CrossRef]
- Birney, E. Mendelian Randomization. Cold Spring Harbor perspectives in medicine 2022, 12. [Google Scholar] [CrossRef]
- Lawlor, D.A.; Harbord, R.M.; Sterne, J.A.; Timpson, N.; Davey Smith, G. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Statistics in medicine 2008, 27, 1133–1163. [Google Scholar] [CrossRef]
- Borges, M.C.; Oliveira, I.O.; Freitas, D.F.; Horta, B.L.; Ong, K.K.; Gigante, D.P.; Barros, A.J.D. Obesity-induced hypoadiponectinaemia: the opposite influences of central and peripheral fat compartments. International journal of epidemiology 2017, 46, 2044–2055. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.D.; Ebrahim, S. Mendelian randomization: prospects, potentials, and limitations. International journal of epidemiology 2004, 33, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.D.; Ebrahim, S. ‘Mendelian randomization’: can genetic epidemiology contribute to understanding environmental determinants of disease? International journal of epidemiology 2003, 32, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Riaz, H.; Khan, M.S.; Siddiqi, T.J.; Usman, M.S.; Shah, N.; Goyal, A.; Khan, S.S.; Mookadam, F.; Krasuski, R.A.; Ahmed, H. Association Between Obesity and Cardiovascular Outcomes: A Systematic Review and Meta-analysis of Mendelian Randomization Studies. JAMA network open 2018, 1, e183788. [Google Scholar] [CrossRef] [PubMed]
- Lyon, M.S.; Andrews, S.J.; Elsworth, B.; Gaunt, T.R.; Hemani, G.; Marcora, E. The variant call format provides efficient and robust storage of GWAS summary statistics. Genome Biol 2021, 22, 32. [Google Scholar] [CrossRef] [PubMed]
- Ben, E.; Matthew, L.; Tessa, A.; Yi, L.; Peter, M.; Jon, H.; Phil, B.; Tom, P.; Valeriia, H.; George Davey, S. , et al. The MRC IEU OpenGWAS data infrastructure. bioRxiv, 1101. [Google Scholar] [CrossRef]
- Bowden, J.; Davey Smith, G.; Burgess, S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. International journal of epidemiology 2015, 44, 512–525. [Google Scholar] [CrossRef]
- Rasooly, D.; Peloso, G.M. Two-Sample Multivariable Mendelian Randomization Analysis Using R. Current protocols 2021, 1, e335. [Google Scholar] [CrossRef]
- Li, Y.; Ma, L. Relationship between telomere length and the prognosis of breast cancer based on estrogen receptor status: A Mendelian randomization study. Frontiers in oncology 2022, 12, 1024772. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, M.; Chen, X.; Ruan, W.; Yao, J.; Lian, X. Telomere length and multiple sclerosis: a Mendelian randomization study. The International journal of neuroscience, 1080. [Google Scholar] [CrossRef]
- Kamat, M.A.; Blackshaw, J.A.; Young, R.; Surendran, P.; Burgess, S.; Danesh, J.; Butterworth, A.S.; Staley, J.R. PhenoScanner V2: an expanded tool for searching human genotype-phenotype associations. Bioinformatics (Oxford, England) 2019, 35, 4851–4853. [Google Scholar] [CrossRef]
- Staley, J.R.; Blackshaw, J.; Kamat, M.A.; Ellis, S.; Surendran, P.; Sun, B.B.; Paul, D.S.; Freitag, D.; Burgess, S.; Danesh, J. , et al. PhenoScanner: a database of human genotype-phenotype associations. Bioinformatics (Oxford, England) 2016, 32, 3207–3209. [Google Scholar] [CrossRef]
- Davey Smith, G.; Hemani, G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Human molecular genetics 2014, 23, R89–R98. [Google Scholar] [CrossRef] [PubMed]
- Liao, Q.; He, J.; Tian, F.F.; Bi, F.F.; Huang, K. A causal relationship between leukocyte telomere length and multiple sclerosis: A Mendelian randomization study. Frontiers in immunology 2022, 13, 922922. [Google Scholar] [CrossRef] [PubMed]
- Bowden, J.; Del Greco, M.F.; Minelli, C.; Davey Smith, G.; Sheehan, N.; Thompson, J. A framework for the investigation of pleiotropy in two-sample summary data Mendelian randomization. Statistics in medicine 2017, 36, 1783–1802. [Google Scholar] [CrossRef] [PubMed]
- Kamiza, A.B.; Fatumo, S.; Singini, M.G.; Yeh, C.C.; Chikowore, T. Hepatitis B infection is causally associated with extrahepatic cancers: A Mendelian randomization study. EBioMedicine 2022, 79, 104003. [Google Scholar] [CrossRef]
- Yuan, S.; Carter, P.; Bruzelius, M.; Vithayathil, M.; Kar, S.; Mason, A.M.; Lin, A.; Burgess, S.; Larsson, S.C. Effects of tumour necrosis factor on cardiovascular disease and cancer: A two-sample Mendelian randomization study. EBioMedicine 2020, 59, 102956. [Google Scholar] [CrossRef]
- Yang, J.; He, X.; Qian, L.; Zhao, B.; Fan, Y.; Gao, F.; Yan, B.; Zhu, F.; Ma, X. Association between plasma proteome and childhood neurodevelopmental disorders: A two-sample Mendelian randomization analysis. EBioMedicine 2022, 78, 103948. [Google Scholar] [CrossRef]
- Ye, M.; Wang, Y.; Zhan, Y. Genetic association of leukocyte telomere length with Graves’ disease in Biobank Japan: A two-sample Mendelian randomization study. Frontiers in immunology 2022, 13, 998102. [Google Scholar] [CrossRef]
- Verbanck, M.; Chen, C.Y.; Neale, B.; Do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nature genetics 2018, 50, 693–698. [Google Scholar] [CrossRef]
- Zhan, Y.; Song, C.; Karlsson, R.; Tillander, A.; Reynolds, C.A.; Pedersen, N.L.; Hägg, S. Telomere Length Shortening and Alzheimer Disease--A Mendelian Randomization Study. JAMA neurology 2015, 72, 1202–1203. [Google Scholar] [CrossRef]
- Bourebaba, L.; Łyczko, J.; Alicka, M.; Bourebaba, N.; Szumny, A.; Fal, A.M.; Marycz, K. Inhibition of Protein-tyrosine Phosphatase PTP1B and LMPTP Promotes Palmitate/Oleate-challenged HepG2 Cell Survival by Reducing Lipoapoptosis, Improving Mitochondrial Dynamics and Mitigating Oxidative and Endoplasmic Reticulum Stress. Journal of clinical medicine 2020, 9. [Google Scholar] [CrossRef]
- Huang, P.L. A comprehensive definition for metabolic syndrome. Disease models & mechanisms 2009, 2, 231–237. [Google Scholar] [CrossRef]
- Qu, J.; Fourman, S.; Fitzgerald, M.; Liu, M.; Nair, S.; Oses-Prieto, J.; Burlingame, A.; Morris, J.H.; Davidson, W.S.; Tso, P. , et al. Low-density lipoprotein receptor-related protein 1 (LRP1) is a novel receptor for apolipoprotein A4 (APOA4) in adipose tissue. Scientific reports 2021, 11, 13289. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Yin, B.; Li, W.; Chai, T.; Liang, W.; Huang, Y.; Tan, X.; Zheng, P.; Wu, J.; Li, Y. , et al. Age-related changes in microbial composition and function in cynomolgus macaques. Aging 2019, 11, 12080–12096. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Zhao, N.; Zhang, L.; Chen, J.; Liu, X.; Piao, S. Gut microbiota is a potential goalkeeper of dyslipidemia. Frontiers in endocrinology 2022, 13, 950826. [Google Scholar] [CrossRef] [PubMed]
- Pi, X.; Hua, H.; Wu, Q.; Wang, X.; Wang, X.; Li, J. Effects of Different Feeding Methods on the Structure, Metabolism, and Gas Production of Infant and Toddler Intestinal Flora and Their Mechanisms. Nutrients 2022, 14. [Google Scholar] [CrossRef]
- Zang, Y.; Lai, X.; Li, C.; Ding, D.; Wang, Y.; Zhu, Y. The Role of Gut Microbiota in Various Neurological and Psychiatric Disorders-An Evidence Mapping Based on Quantified Evidence. Mediators of inflammation 2023, 2023, 5127157. [Google Scholar] [CrossRef]
- Xing, D.; Ren, N.; Li, Q.; Lin, M.; Wang, A.; Zhao, L. Ethanoligenens harbinense gen. nov., sp. nov., isolated from molasses wastewater. International journal of systematic and evolutionary microbiology 2006, 56, 755–760. [Google Scholar] [CrossRef]
- Konikoff, T.; Gophna, U. Oscillospira: a Central, Enigmatic Component of the Human Gut Microbiota. Trends in microbiology 2016, 24, 523–524. [Google Scholar] [CrossRef]
- Chen, Y.R.; Zheng, H.M.; Zhang, G.X.; Chen, F.L.; Chen, L.D.; Yang, Z.C. High Oscillospira abundance indicates constipation and low BMI in the Guangdong Gut Microbiome Project. Scientific reports 2020, 10, 9364. [Google Scholar] [CrossRef]
- Yang, J.; Li, Y.; Wen, Z.; Liu, W.; Meng, L.; Huang, H. Oscillospira - a candidate for the next-generation probiotics. Gut microbes 2021, 13, 1987783. [Google Scholar] [CrossRef]
- Eeckhaut, V.; Machiels, K.; Perrier, C.; Romero, C.; Maes, S.; Flahou, B.; Steppe, M.; Haesebrouck, F.; Sas, B.; Ducatelle, R. , et al. Butyricicoccus pullicaecorum in inflammatory bowel disease. Gut 2013, 62, 1745–1752. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.T. Pathophysiology of Inflammatory Bowel Diseases. The New England journal of medicine 2020, 383, 2652–2664. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Kabir, I.; Tietelman, G.; Huan, C.; Fan, J.; Worgall, T.; Jiang, X.C. Sphingolipid de novo biosynthesis is essential for intestine cell survival and barrier function. Cell death & disease 2018, 9, 173. [Google Scholar] [CrossRef]
- Rajilić-Stojanović, M.; de Vos, W.M. The first 1000 cultured species of the human gastrointestinal microbiota. FEMS Microbiology Reviews 2014, 38, 996–1047. [Google Scholar] [CrossRef]
- Nagao-Kitamoto, H.; Kamada, N. Host-microbial Cross-talk in Inflammatory Bowel Disease. Immune network 2017, 17, 1–12. [Google Scholar] [CrossRef]
- Hill-Burns, E.M.; Debelius, J.W.; Morton, J.T.; Wissemann, W.T.; Lewis, M.R.; Wallen, Z.D.; Peddada, S.D.; Factor, S.A.; Molho, E.; Zabetian, C.P. , et al. Parkinson’s disease and Parkinson’s disease medications have distinct signatures of the gut microbiome. Movement disorders : official journal of the Movement Disorder Society 2017, 32, 739–749. [Google Scholar] [CrossRef]
- Rowin, J.; Xia, Y.; Jung, B.; Sun, J. Gut inflammation and dysbiosis in human motor neuron disease. Physiological reports 2017, 5. [Google Scholar] [CrossRef]
- Brenner, D.; Hiergeist, A.; Adis, C.; Mayer, B.; Gessner, A.; Ludolph, A.C.; Weishaupt, J.H. The fecal microbiome of ALS patients. Neurobiology of aging 2018, 61, 132–137. [Google Scholar] [CrossRef]
- Henke, M.T.; Kenny, D.J.; Cassilly, C.D.; Vlamakis, H.; Xavier, R.J.; Clardy, J. Ruminococcus gnavus, a member of the human gut microbiome associated with Crohn’s disease, produces an inflammatory polysaccharide. Proc Natl Acad Sci U S A 2019, 116, 12672–12677. [Google Scholar] [CrossRef]
- Ponzo, V.; Fedele, D.; Goitre, I.; Leone, F.; Lezo, A.; Monzeglio, C.; Finocchiaro, C.; Ghigo, E.; Bo, S. Diet-Gut Microbiota Interactions and Gestational Diabetes Mellitus (GDM). Nutrients 2019, 11. [Google Scholar] [CrossRef]
- Lee, G.; You, H.J.; Bajaj, J.S.; Joo, S.K.; Yu, J.; Park, S.; Kang, H.; Park, J.H.; Kim, J.H.; Lee, D.H. , et al. Distinct signatures of gut microbiome and metabolites associated with significant fibrosis in non-obese NAFLD. Nat Commun 2020, 11, 4982. [Google Scholar] [CrossRef] [PubMed]
- Munukka, E.; Rintala, A.; Toivonen, R.; Nylund, M.; Yang, B.; Takanen, A.; Hänninen, A.; Vuopio, J.; Huovinen, P.; Jalkanen, S. , et al. Faecalibacterium prausnitzii treatment improves hepatic health and reduces adipose tissue inflammation in high-fat fed mice. The ISME journal 2017, 11, 1667–1679. [Google Scholar] [CrossRef] [PubMed]
- Ai, D.; Pan, H.; Li, X.; Gao, Y.; Liu, G.; Xia, L.C. Identifying Gut Microbiota Associated With Colorectal Cancer Using a Zero-Inflated Lognormal Model. Frontiers in microbiology 2019, 10, 826. [Google Scholar] [CrossRef] [PubMed]
- Meehan, C.J.; Beiko, R.G. A phylogenomic view of ecological specialization in the Lachnospiraceae, a family of digestive tract-associated bacteria. Genome biology and evolution 2014, 6, 703–713. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yu, D.; Wu, D.; Gao, X.; Shao, F.; Zhao, M.; Wang, J.; Ma, J.; Wang, W.; Qin, X. , et al. Tissue-resident Lachnospiraceae family bacteria protect against colorectal carcinogenesis by promoting tumor immune surveillance. Cell host & microbe 2023, 31, 418–432. [Google Scholar] [CrossRef]
- Ćesić, D.; Lugović Mihić, L.; Ozretić, P.; Lojkić, I.; Buljan, M.; Šitum, M.; Zovak, M.; Vidović, D.; Mijić, A.; Galić, N. , et al. Association of Gut Lachnospiraceae and Chronic Spontaneous Urticaria. Life (Basel, Switzerland) 2023, 13. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Z.; Cao, J.; Dong, Y.; Chen, Y. Gut microbiota-derived metabolites mediate the neuroprotective effect of melatonin in cognitive impairment induced by sleep deprivation. Microbiome 2023, 11, 17. [Google Scholar] [CrossRef]
- Takeuchi, T.; Kameyama, K.; Miyauchi, E.; Nakanishi, Y.; Kanaya, T.; Fujii, T.; Kato, T.; Sasaki, T.; Tachibana, N.; Negishi, H. , et al. Fatty acid overproduction by gut commensal microbiota exacerbates obesity. Cell metabolism 2023, 35, 361–375. [Google Scholar] [CrossRef]
- Duar, R.M.; Lin, X.B.; Zheng, J.; Martino, M.E.; Grenier, T.; Pérez-Muñoz, M.E.; Leulier, F.; Gänzle, M.; Walter, J. Lifestyles in transition: evolution and natural history of the genus Lactobacillus. FEMS Microbiol Rev 2017, 41, S27–s48. [Google Scholar] [CrossRef]
- Martín, R.; Miquel, S.; Ulmer, J.; Kechaou, N.; Langella, P.; Bermúdez-Humarán, L.G. Role of commensal and probiotic bacteria in human health: a focus on inflammatory bowel disease. Microbial cell factories 2013, 12, 71. [Google Scholar] [CrossRef]
- Yun, S.W.; Kim, J.K.; Lee, K.E.; Oh, Y.J.; Choi, H.J.; Han, M.J.; Kim, D.H. A Probiotic Lactobacillus gasseri Alleviates Escherichia coli-Induced Cognitive Impairment and Depression in Mice by Regulating IL-1β Expression and Gut Microbiota. Nutrients 2020, 12. [Google Scholar] [CrossRef] [PubMed]
- Asgharian, H.; Homayouni-Rad, A.; Mirghafourvand, M.; Mohammad-Alizadeh-Charandabi, S. Effect of probiotic yoghurt on plasma glucose in overweight and obese pregnant women: a randomized controlled clinical trial. European journal of nutrition 2020, 59, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.L.; Yu, H.Q.; Wu, R.J.; He, C.; Li, M.; Yan, H.; Li, J.J.; Wang, S.; Liu, Z.G.; Liu, Z.J. , et al. Thrombospondin 1 Modulates Monocyte Properties to Suppress Intestinal Mucosal Inflammation. Journal of innate immunity 2015, 7, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Kong, Q.; He, G.Q.; Jia, J.L.; Zhu, Q.L.; Ruan, H. Oral administration of Clostridium butyricum for modulating gastrointestinal microflora in mice. Current microbiology 2011, 62, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Turroni, F.; Peano, C.; Pass, D.A.; Foroni, E.; Severgnini, M.; Claesson, M.J.; Kerr, C.; Hourihane, J.; Murray, D.; Fuligni, F. , et al. Diversity of bifidobacteria within the infant gut microbiota. PLoS One 2012, 7, e36957. [Google Scholar] [CrossRef]
- Pinzone, M.R.; Celesia, B.M.; Di Rosa, M.; Cacopardo, B.; Nunnari, G. Microbial translocation in chronic liver diseases. International journal of microbiology 2012, 2012, 694629. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




