Submitted:
13 September 2023
Posted:
14 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Determination of flux composition
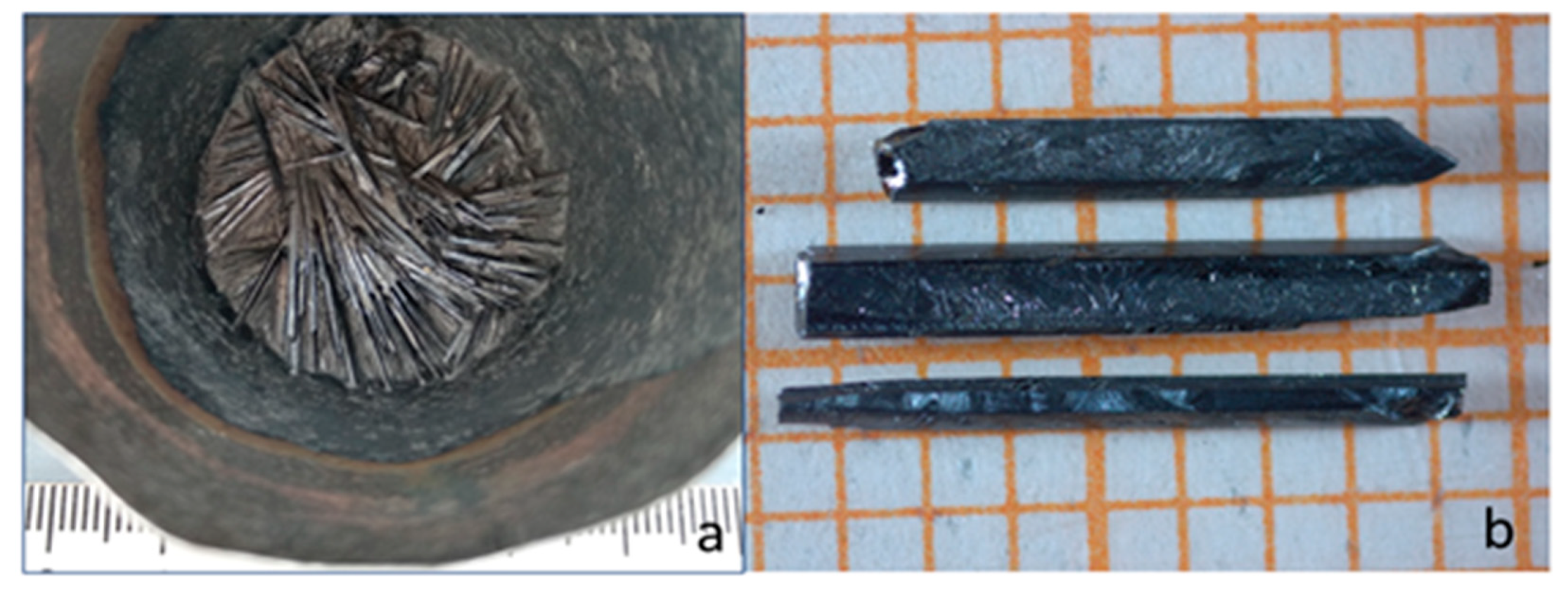
2.2. Single crystal growth
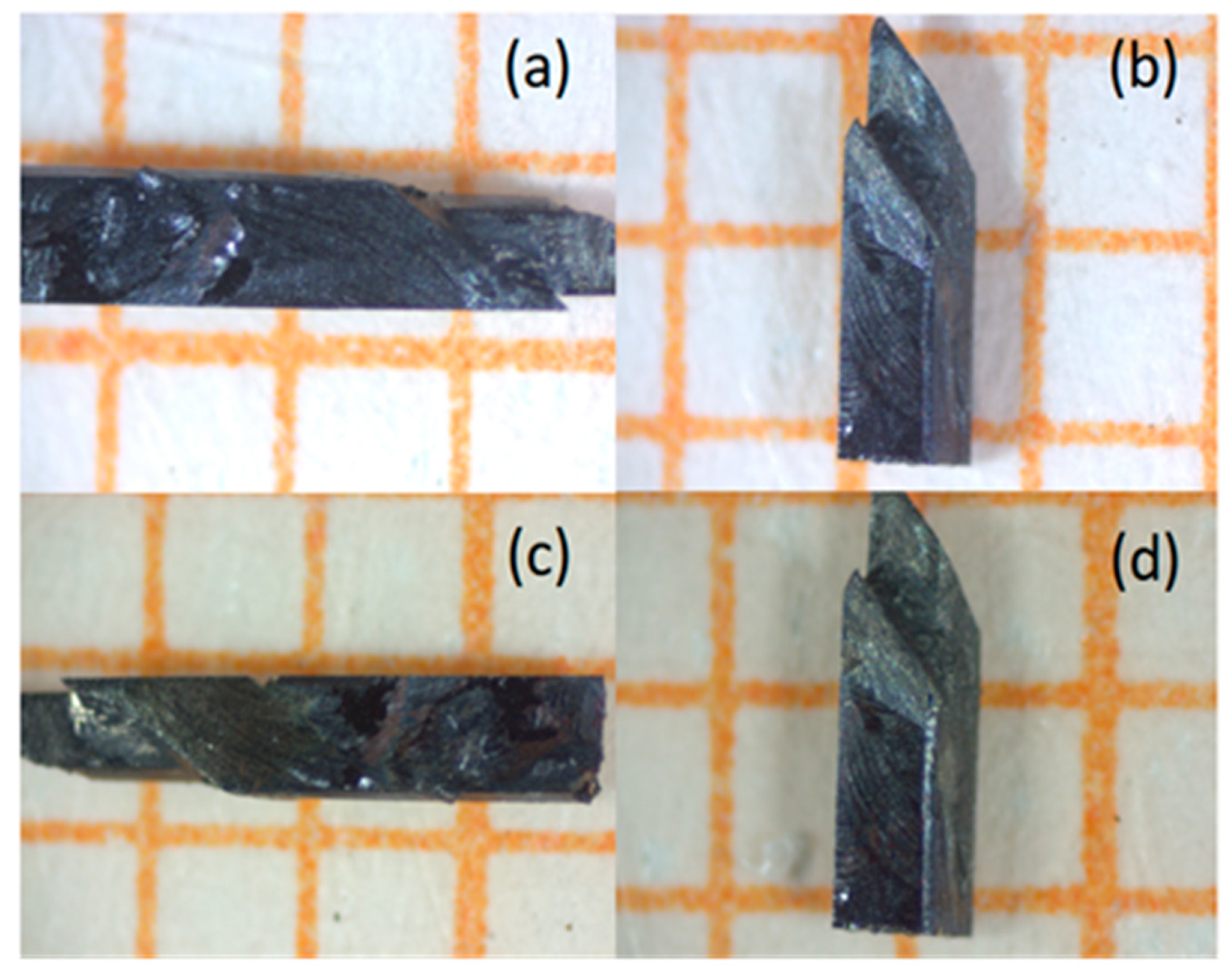
2.3. Sample characterization
3. Results and Discussion
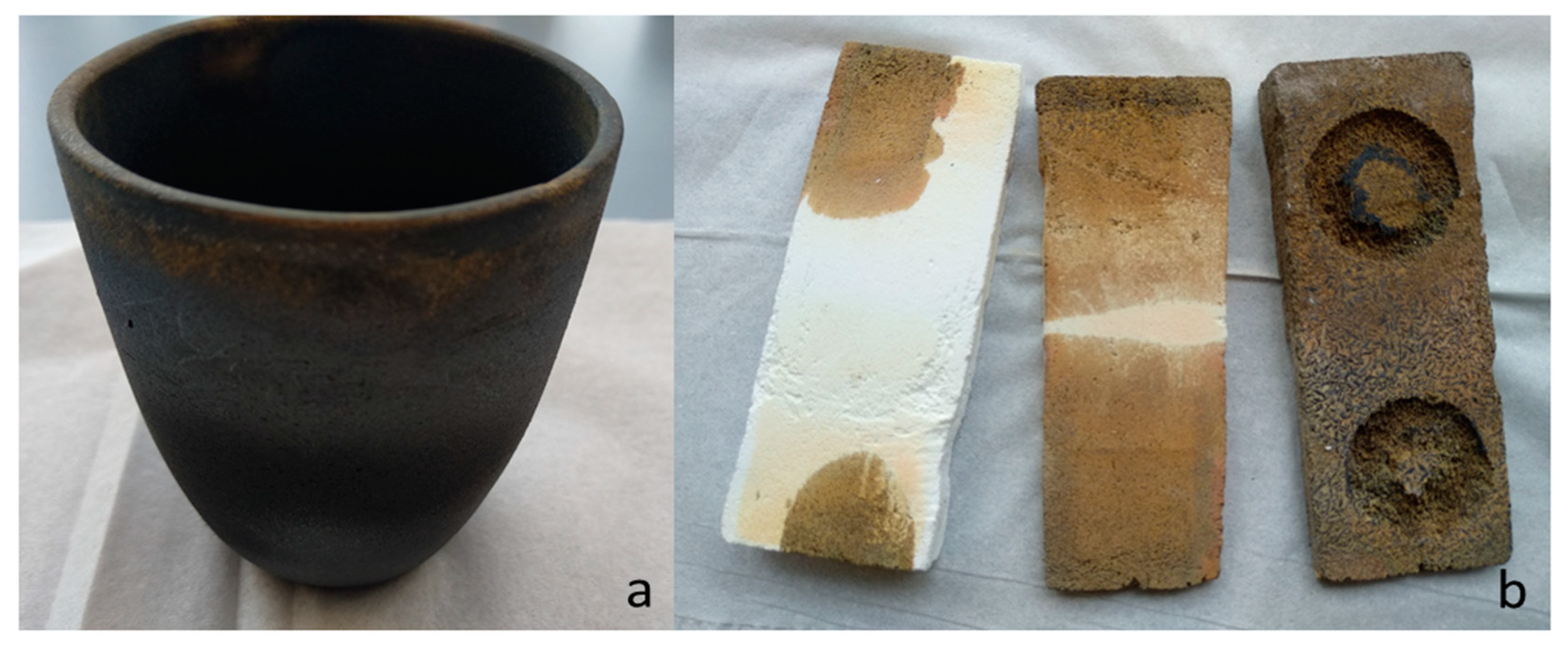
3.1. Characterization with XRD and EDX
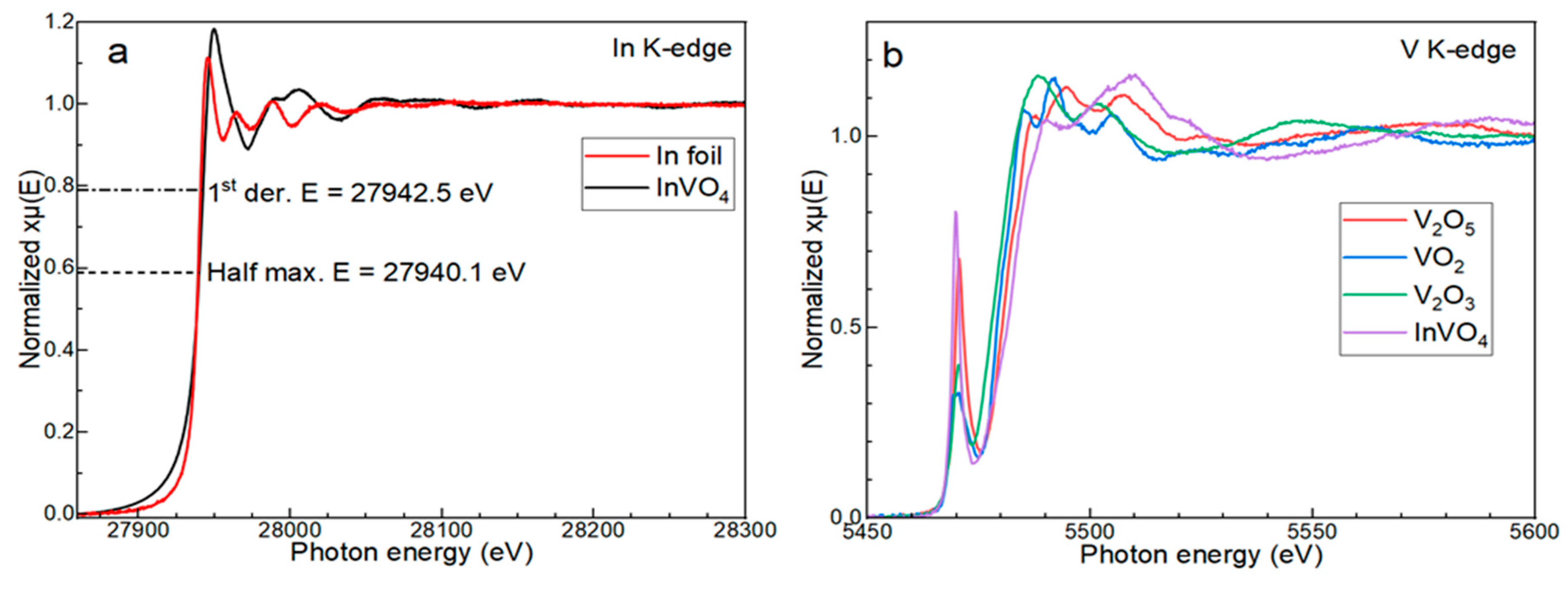
3.2. Characterization with XANES
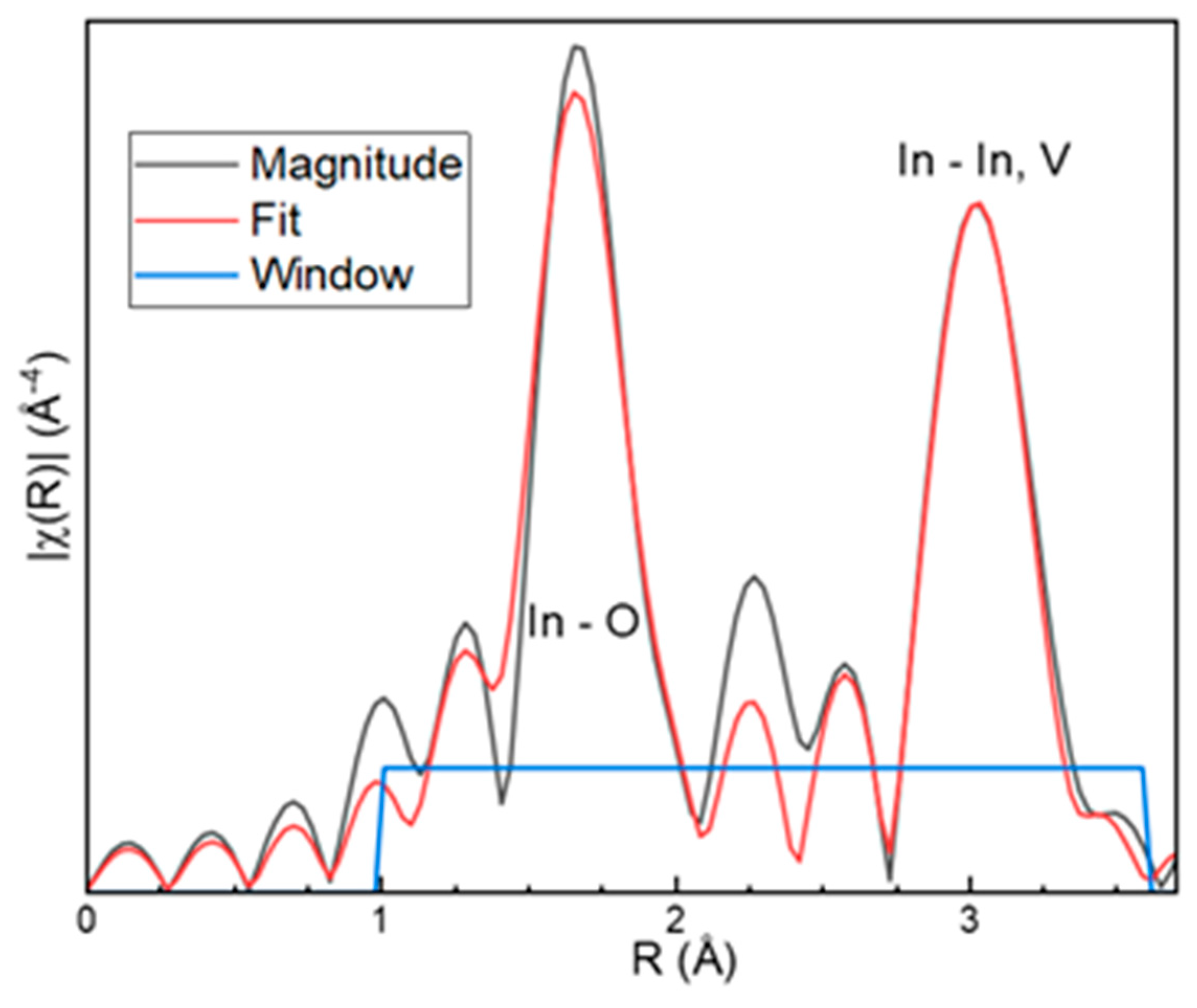
3.3. EXAFS (extended X-ray absorption fine structure)
3.4. UV-vis-NIR spectroscopy absorption
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Touboul, M.; Melghit, K.; Bénard, P.; Louër, D. Crystal Structure of a Metastable Form of Indium Orthovanadate, InVO4-I. J. Solid State Chem. 1995, 118, 93-98. [CrossRef]
- Touboul, M.; Tolédano, P. Structure du vanadate d’indium: InVO4. Acta Cryst. B 1980, 36, 240-245. [CrossRef]
- Touboul, M.; Ingrain, D. Synthèses et propriétés thermiques de InVO4 et TlVO4. Journal of the Less Common Metals 1980, 71, 55-62. [CrossRef]
- Errandonea, D.; Gomis, O.; Garcia-Domene, B.; Pellicer-Porres, J.; Katari, V.; Achary, S.N.; Tyagi, A.K.; Popescu, C. New polymorph of InVO4: a high-pressure structure with six-coordinated vanadium. Inorg. Chem. 2013, 52, 12790-12798. [CrossRef]
- Mondal, S.; Appalakondaiah, S.; Vaitheeswaran, G. High pressure structural, electronic, and optical properties of polymorphic InVO4 phases. J. Appl. Phys. 2016, 119, 085702 (8). [CrossRef]
- Errandonea, D. High pressure crystal structures of orthovanadates and their properties. J. Appl. Phys. 2020, 128, 040903 (17). [CrossRef]
- Botella, P.; Errandonea, D.; Garg, A.B.; Rodriguez-Hernandez, P.; Muñoz, A.; Achary, S.N.; Vomiero, A. High-pressure characterization of the optical and electronic properties of InVO4, InNbO4, and InTaO4. SN Appl. Sci. 2019, 1, 389. [CrossRef]
- Zou, Z.; Ye, J.; Sayama, K.; Arakawa, H. Direct splitting of water under visible light irradiation with an oxide semiconductor photocatalyst. Nature 2001, 414, 625. [CrossRef]
- Liu, Y.; Ren, W.; Shi, P.; Liu, D.; Liu, M.; Jing, W.; Tian, B.; Ye, Z.; Jiang, Z. Preparation and thermal volatility characteristics of In2O3/ITO thin film thermocouple by RF magnetron sputtering. AIP Advances 2017, 7, 115025-8. [CrossRef]
- Lamoreaux, R.H.; Hildenbrand, D.L.; Brewer, L. High-Temperature Vaporization Behavior of Oxides II. Oxides of Be, Mg, Ca, Sr, Ba, B, AI, Ga, In, TI, Si, Ge, Sn, Pb, Zn, Cd and Hg. Journal of Physical and Chemical Reference Data 1987, 16, 419-443. [CrossRef]
- Nakamura, S.; Maljuk, A.; Maruyama, Y.; Nagao, M.; Watauchi, S.; Hayashi, T.; Anzai, Y.; Furukawa, Y.; Ling, C.D.; Deng, G.; Avdeev, M.; Büchner, B.; Tanaka, I. Growth of LiCoO2 single crystals by the TSFZ method. Crystal Growth and Design 2019, 19, 415-420. [CrossRef]
- Bosacka, M.; Filipek, E.; Paczesna, A. Unknown phase equilibria in the ternary oxide V2O5-CuO-In2O3 system in subsolidus area. J. Therm. Anal. Calorim. 2016, 125, 1161-1170. [CrossRef]
- Nobe, Y.; Takashima, H.; Katsumata, T. Decoloration of yttrium orthovanadate laser host crystals by annealing. Optics Letters 1994, 19, 1216-1218. [CrossRef]
- Voloshyna, O.V.; Baumer, V.N.; Bondar, V.G.; Kurtsev, D.A.; Gorbacheva, T.E.; Zenya, I.M.; Zhukov, A.V.; Sidletskiy, O.Ts. Growth and scintillation properties of gadolinium and yttrium orthovanadate crystals. Nuclear Instruments and Methods in Physics Research A 2012, 664, 299–303. [CrossRef]
- Rietveld, H.M. A profile refinement method for nuclear and magnetic structures. J. Appl. Cryst. 1969, 2, 65-71. [CrossRef]
- Rodriguez-Carvajal, J.; Roisnel, T. FullProf.98 and WinPLOTR: New Windows95/NT Applications for Diffraction. Commission for Powder Diffraction, International Union of Crystallography, (May–August) Summer 1998, Newsletter N20.
- Calvo, C.; Faggiani, R. α-Cupric vanadate. Acta crystallogr. Section B 1975, 31, 603-605. [CrossRef]
- Caliebe, W.A.; Murzin, V.; Kalinko, A.; Görlitz, M. High-flux XAFS-beamline P64 at PETRA III. AIP Conf. Proc. 2019, 2054, 060031. [CrossRef]
- Ravel, B.; Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchr. Rad. 2005, 12, 537–541. [CrossRef]
- Denis, S.; Baudrin, E.; Touboul, M.; Tarascon, J.-M. Synthesis and electrochemical properties vs Li of amorphous vanadates of general formula RVO4 (R = In, Cr, Al, Fe, Y). J. Electrochem. Soc. 1997, 144, 4099-4109. https://dx.doi.org/10.1149/1.1838150.
- Vitova, T.; Mangold, S.; Paulmann, C.; Gospodinov, M.; Marinova, V.; Mihailova, B. X-ray absorption spectroscopy of Ru-doped relaxor ferroelectrics with a perovskite-type structure. Phys. Rev. B: Condens. Matter Mater. Phys. 2014, 89, 144112. [CrossRef]
- Bearden, J.A.; Burr, A.F. Reevaluation of X-Ray Atomic Energy Levels. Rev. Mod. Phys. 1967, 39, 125. [CrossRef]
- Tsoukalou, A.; Abdala, P.M.; Armutlulu, A.; Willinger, E.; Fedorov, A.; Müller, C.R. Operando X-ray Absorption Spectroscopy Identifies a Monoclinic ZrO2:In Solid Solution as the Active Phase for the Hydrogenation of CO2 to Methanol. ACS Catal. 2020, 10, 10060–10067. [CrossRef]
- Chaurand, P.; Rose, J.; Briois, V.; Salome, M.; Proux, O.; Nassif, V.; Olivi, L.; Susini, J.; Hazemann, J.-L.; Bottero, J.-Y. New Methodological Approach for the Vanadium K-Edge X-ray Absorption Near-Edge Structure Interpretation: Application to the Speciation of Vanadium in Oxide Phases from Steel Slag. J. Phys. Chem. B 2007, 111, 5101-5110. [CrossRef]
- Nga, T.T.T.; Huang, Y.-C.; Chen, J.-L.; Chen, C.-L.; Lin, B.-H.; Yeh, P.-H.; Du, C.-H.; Chiou, J.-W.; Pong, W.-F.; Arul, K.T.; Dong, C.-L.; Chou, W.-C. Effect of Ag-Decorated BiVO4 on Photoelectrochemical Water Splitting: An X-ray Absorption Spectroscopic Investigation. Nanomaterials 2022, 12, 3659. [CrossRef]
- Newville, M. Fundamentals of XAFS, Revision 1.7; University of Chicago: Chicago, IL, USA, 2004.
- Rehr, J.J.; de Leon, J.M.; Zabinsky, S.I.; Albers, R.C. Theoretical X-ray Absorption Fine Structure Standards. J. Am. Chem. Soc. 1991, 113, 5135–5140. [CrossRef]
- Ye, J.; Zou, Z.; Arakawa, H.; Oshikiri, M.; Shimoda, M.; Matsushita, A.; Shishido, T. Correlation of crystal and electronic structures with photophysical properties of water splitting photocatalysts InMO4 (M=V5+, Nb5+, Ta5+). J. Photochem. Photobiol. A 2002, 148, 79-83. [CrossRef]
- Ye, J.; Zou, Z.; Oshikiri, M.; Matsushita, A.; Shimoda, M.; Imai, M.; Shishido, T. A novel hydrogen-evolving photocatalyst InVO4 active under visible light irradiation. Chem. Phys. Lett. 2002, 356, 221-226. [CrossRef]
- Oshikiri, M.; Boero, M.; Ye, J.; Zou, Z.; Kido, G. Electronic structures of promising photocatalysts InMO4 (M=V, Nb, Ta) and BiVO4 for water decomposition in the visible wavelength region. J. Chem. Phys. 2002, 117, 7313. [CrossRef]
- Enache, C.S.; Lloyd, D.; Damen, M.R.; Schoonman, J.; van de Krol, R. Photo-electrochemical Properties of Thin-Film InVO4 Photoanodes: the Role of Deep Donor States. J. Phys. Chem. C 2009, 113, 19351–19360. [CrossRef]
- Oshikiri, M.; Boero, M.; Ye, J.; Aryasetiwan, F.; Kido, G. The electronic structure of the thin films of InVO4 and TiO2 by first principles calculations. Thin Solid Films 2003, 445, 168-174. [CrossRef]




| Parameter | InVO4 | Cu2V2O7 |
|---|---|---|
| Space group, Prototype |
Cmcm (#64) CrVO4-type |
Fdd2 (#43) Cu2V2O7-type |
| a, Å | 5.7329(8) | 8.3631(0) |
| b, Å | 8.5010(8) | 20.6495(6) |
| c, Å | 6.5590(3) | 6.4497(6) |
| V, Å3 | 319.664(0.014) | 1113.837(0.000) |
| Reflections measured | 73 | 114 |
| Weighted profile R-factor (Rwp) |
4.64 | 16.1 |
| Expected R factor (Rexp) | 1.94 | 6.76 |
| Goodness of fit (χ2) | 5.69 | 5.69 |
| Content in the sample, wt.% | 97.13 | 2.87 |
| Distance (in Å) | Str. model | EXAFS calc. | Rf = 0.036 |
| In–O1(×4) | 2.1184 | 2.11(4) | |
| In–O2 (×2) | 2.2219 | 2.22(6) | |
| In–In | 3.2795 | 3.29(2) | |
| In–V | 3.4985 | 3.53(9) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





