Submitted:
14 September 2023
Posted:
14 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Centesimal composition of whole ryegrass and maize flour
2.2.2. Ryegrass germination
2.2.3. Obtaining the whole flours of unsprouted, sprouted and maize
2.2.4. Experimental design, test preparation, and thermoplastic extrusion processing to produce breakfast cereals
2.2.5. Physico-chemical properties of breakfast cereals
2.2.4.1. Expansion index, bulk density
2.2.4.2. Instrumental color
2.2.4.3. Instrumental texture of dry breakfast cereals and after immersion in refrigerated whole milk (bowl-life)
2.2.4.4. Water absorption index and water solubility index
2.2.4.5. γ -aminobutiric acid
2.2.4.6. Total soluble phenolic compounds
2.2.6. Statistical analysis
3. Results e Discussion
3.1. Nutritional, technological, and physicochemical properties of raw material
3.2. Technological properties of breakfast cereals
3.2.1. Radial expansion index and bulk density of breakfast cereal
3.2.2. Water absorption index and water solubility index of breakfast cereals
3.2.3. Instrumental color of breakfast cereals
3.2.4. Total soluble phenolic compounds and γ-aminobutyric acid for breakfast cereals
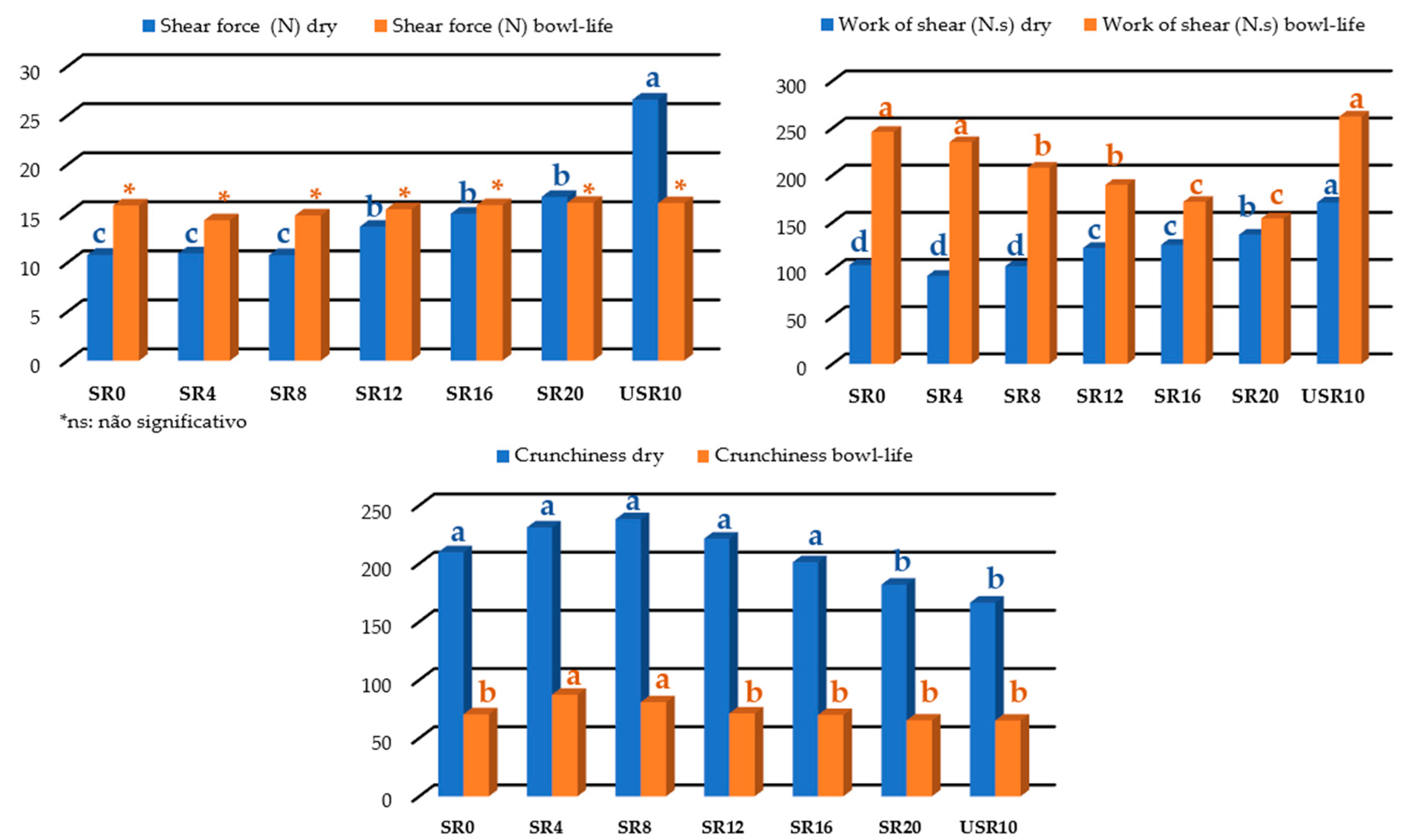
3.2.5. Instrumental texture for breakfast cereals
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Caporizzi, R.; Schönlechner, R.; D’amico, S.; Severini, C.; Derossi, A. Novel gluten-free breakfast cereals produced by extrusion cooking from rice and teff: Effects on microstructural, physical and nutritional properties. Foods 2023, 12, 609. [CrossRef]
- Lima, C.T.; Lima, N.G.; Rodrigues, S.M.; Schmiele, M. Enteropatias e perspectivas de um mundo sem glúten como alternativa: uma revisão. In I SICITAL - Simpósio de Ciência e Tecnologia de Alimentos, Diamantina, Brasil, 05-07 July 2022.
- Paucar-Menacho, L.M.; Castillo-Martínez, W.E.; Simpalo-Lopez, W.D.; Verona-Ruiz, A.L.; Lavado-Cruz, A.A.; Martínez-Villuenga, C.; Peñas, E; Frias, J.; Schmiele, M. Performance of thermoplastic extrusion, germination, fermentation, and hydrolysis techniques on phenolic compounds in cereals and pseudocereals. Foods, 2022, 11, 1957. [CrossRef]
- Mittelmann, A. Azevém BRS Integração. Embrapa Gado de Leite, 39-41, 2017. Available online: https://ainfo.cnptia.embrapa.br/digital/bitstream/item/165502/1/Cnpgl-2017-Cap-Mittelmann-Doc-Cpact.pdf (accessed on 11 September 2023).
- Lemmens, E.; Montori, A.V.; Pangand, J.; Heirbaut, P.; Ritala, A.; Karlen, Y.; Delcour, J.A. Impact of cereal seed sprouting on its nutritional and technological properties: A critical review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 305-328. [CrossRef]
- Carvalho, H.J.M; Lima, C.T; Leoro, M.G.V; Schmiele, M. Sprouted sorghum impacts the grain radicle development and carbohydrates and protein levels in water-soluble extracts. In V Congreso Internacional en Investigación e Innovación en Ingeniería, Ciencia y Tecnología de los Alimentos – IICTA, Manizales, Colombia, 07-09 Octubre 2022.
- Bakhshy, E.; Zarinkamar, F.; Nazari, M. Structural and quantitative changes of starch in seed of Trigonella persica during germination. Int. J. Biol. Macromol. 2020, 164, 1284-1293. [CrossRef]
- Lima, C.T.; Santos, T.M.; Leite, I.A.; Gomes, L.R.; Meza, S.L.R.; Schmiele, M. Ryegrass as a novel source of food protein. In IX International Conference on Food Proteins and Colloids (CIPCA 2023), Rio de Janeiro, Brazil, 09-11 May 2023.
- Srichuwong, S.; Curti, D.; Austin, S.; Kimg, R.; Lamothe, L.; Gloria-Thabet, S.G.; Moursi, Y.S., Karam, M.A.; Ggraner, A.; Alqudar, A.M. Genetic basis of drought tolerance during seed germination in barley. Plos one 2018, 13, e0206682.
- Xia, Q. Investigating the influence of selected texture-improved pretreatment techniques on storage stability of wholegrain brown rice: Involvement of processing-induced mineral changes with lipid degradation. Food Res. Int. 2017, 99, 510-521. [CrossRef]
- Paucar-Menacho, L.M.; Schmiele M.; Lavado-Cruz, A.A.; Verona-Ruiz A.L.; Mollá C.; Peñas E.; Frias J.; Simpalo-Lopez, W.D.; Castillo-Martínez, W.E.; Martínez-Villuenga, C. Andean sprouted pseudocereals to produce healthier extrudates: Impact in nutritional and physicochemical properties. Foods 2022, 11, 3259. [CrossRef]
- Xiang, Z., Deng, J., Yang, K., Zhu, Y., Xia, C., Chen, J., Liu, T. Effect of processing on the release of phenolic compounds and antioxidant activity during in vitro digestion of hulless barley. Arab. J. Chem. 2021, 14, 103447. [CrossRef]
- Meza, S.L.R.; Sinnecker, P.; Schmiele, M.; Massaretto, I.L.; Chang, Y.K.; Marquez, U.M.L. Production of innovative gluten-free breakfast cereals based on red black rice by extrusion processing technology. J. Food Sci. Technol. 2019, 56, 4855-4866. [CrossRef]
- AACCI. American Association of Cereal Chemists International. Approved Methods of AACCI, 11th ed.; St. Paul, MN, USA, 2010.
- AOAC. Association of Official Analysis Chemists International. Official Methods of Analysis of AOAC International, 21st ed., Gaithersburg, MG, USA, 2019.
- Oliveira, L.C.; Schmiele, M.; Steel C.J. Development of whole grain wheat flour extruded cereal and process impacts on color, expansion, and dry and bowl-life texture. LWT – Food Sci. Tech. 2017, 75, 261-270. [CrossRef]
- Schmiele, M.; Jaekel, L. Z.; Ishida, P.M.G.; Chang, Y.K.; Steel, C.J. Gluten-free pasta with high protein content obtained by conventional processing. Ciência Rural, 2013, 43, 908-914.
- Genčić, M.S.; Stojanović, N.M.; Mladenović, M.Z.; Radulović, N.S. An HPLC-based assay for improved measurement of glutamate decarboxylase inhibition/activation. Neurochem. Int. 2022, 161, 105433. [CrossRef]
- Lima, C.T.; Santos, T.M.; Silva, L.E.P.; Leite, I.A.; Vendruscolo, R.G.; Neves, N.A.; Meza, S.L.R.; Schmiele, M. Optimization of extracting solution of total soluble phenolic compounds from ryegrass using white technology assisted by low-frequency ultrasound. In XV Latin American Symposium on Food Science and Nutrition - 15 SLACAN - The Food and Nutrition Science Revolution: Feeding the World Sustainably, Campinas, Brazil, 12-14 November 2023.
- Shewry, P.R.; Koksel, H.; Taylor, J.J. ICC Handbook of 21st Century Cereal Science and Technology, 1st ed.; Elsevier Science, Cambridge, USA, 2023; 400p.
- Pimenta, T.S. Prospecção do nanoamido nativo de azevém. Dissertation, Master in Food Science and Technology, Federal University of Jequitinhonha and Mucuri Valleys, Diamantina, 2022.
- Torbica, A.; Radosavljević, M., Belović, M. Tamilselvan, T.; Prabhasankar, P. Biotechnological tools for cereal and pseudocereal dietary fibre modification in the bakery products creation–Advantages, disadvantages and challenges. Trends Food Sci. Technol. 2022, 129, 194-209.
- Spotti, M.J.; Campanella, O.H. Functional modifications by physical treatments of dietary fibers used in food formulations. Curr. Opin. Food Sci. 2017, 15, 70-78. [CrossRef]
- El Sohaimy, S.A.; Mohamed, S.E.; Shehata, M.G.; Mehany, T.; Zaitoun, M. A. Compositional analysis and functional characteristics of quinoa flour. Annu. Res. Rev.Biol. 2018, 22, 1-11. [CrossRef]
- Dalbhagat, C.G.; Mahato, D.K.; Mishra, H.N. Effect of extrusion processing on physicochemical, functional and nutritional characteristics of rice and rice-based products: A review. Trends Food Sci. Technol. 2019, 85, 226-240. [CrossRef]
- Barros, S.K.A.; Souza, A.R.M.; Silva, F.S.; Pires, C.R.F.; Damiani, C.; Silveira, M.F.A.; Silva, C.R.E. Preparation of fresh gluten free pasta enriched with açaí waste flour (Euterpe oleracea Mart.) and bacaba (Oenocarpus bacaba Mart.) Res. Soc. Dev. 2021, 10, e1810613722.
- Osório, C.; Machado, S.; Peixoto, J.; Bessada, S.; Pimentel, F.B.; Alves, R.C.; Oliveira, M.B.P.P. Pigments content (chlorophylls, fucoxanthin and phycobiliproteins) of different commercial dried algae. Separations, 2020, 7, 33. [CrossRef]
- Silva, L.E.P.; Rodrigues, S.M.; Leoro, M.G.V.; Neves, N.A.; Schmiele, M. Prospecção da radiação por micro-ondas e da liofilização na elaboração e características tecnológicas e nutricionais da farinha de palma. In I SICITAL - Simpósio de Ciência e Tecnologia de Alimentos, Diamantina, Brasil, 05-07 July 2022.
- Hitayezu, R.; Baakdah, M. M.; Kinnin, J.; Henderson, K.; Tsopmo, A. Antioxidant activity, avenanthramide and phenolic acid contents of oat milling fractions. J. Cereal Sci., 2015, 63, 35-40. [CrossRef]
- Kasote, D.; Tiozon, R.N.J.; Sartagoda, K.J.D.; Itagi, H.; Roy, P.; Kohili, A.; Regina, A. Sreenivasulu N. Food processing technologies to develop functional foods with enriched bioactive phenolic compounds in cereals. Front. Plant Sci., 2021, 12, 771276. [CrossRef]
- Kagan, I.A.; Goodman, J.P.; Seman, D.H.; Lawrence, L.M.; Smith, S. R. Effects of harvest date, sampling tie, and cultivar on total phenolic concentrations, water-soluble carbohydrate concentrations, and phenolic profiles of selected cool-season grasses in central Kentucky. J. Equine Vet. Sci. 2019, 79, 86-93. [CrossRef]
- Hashimoto, J.M.; Schmiele, M.; Nabeshima, E.H. Modelling to obtain expanded cowpea products in a twin screw extruder. Braz. J. Food Technol. 2021, 24, 11120. [CrossRef]
- Menis-Henrique, M.E.C.; Scarton, M.; Piran, M.V.F.; Clerici, M.T.P.S. Cereal fiber: Extrusion modifications for food industry. Curr. Opin. Food Sci. 2020, 33, 141-148. [CrossRef]
- Pereira, A.M.; Schmiele, M.; Souza, E.J.D.; Ávila, B.P.; Ramos, A.H.; Zavareze, E.R.; Gularte, M.A. Extrudate gluten-free breakfast cereals frow rice and corn flours with different amylose content: technological and sensory properties. Food Sci. Technol. 2021, 56, 4182-4190.
- Andressa, I.; Nascimento, G.K.S.; Santos, T.M.; Oliveira, J.R.; Benassi, V.M.; Schmiele, M. Physicochemical changes induced by the sprouting process of purple pericap corn kernels. Braz. J. Dev. 2023, 9, 8051-8070.
- Liang, M.H.; He, Y.J.; Liu, D.M.; Jiang, J.G. Regulation of carotenoid degradation and production of apocarotenoids in natural and engineered organisms. Crit. Rev. Biotechnol. 2021, 41, 513-534. [CrossRef]
- Los, F.G.B.; Zielinski, A.A.F.; Wojeicchowski, J.P.; Nogueira, A.; Demiate, I.M. Beans (Phaseolus vulgaris L.): whole seeds with complex chemical composition. Curr. Opin. Food Sci., 2018, 19, 63-71. [CrossRef]
- Bešlo, D.; Došlić, G.; Agić, D.; Rastija, V.; Šperanda, M.; Gantner, V.; Lučić, B. Polyphenols in ruminant nutrition and their effects on reproduction. Antioxidants 2022; 11, 970. [CrossRef]
- Kagan, I.A. Soluble phenolic compounds of perennial ryegrass (Lolium perenne L.): Potential effects on animal performance, and challenges in determining profiles and concentrations. Anim. Feed Sci. Technol. 2021, 277, 114960. [CrossRef]
- Cao, M.; Fraser, K.; Jones, C.; Stewart, A.; Lyons, T.; Faville, M.; Barrett, B. Untargeted metabotyping Lolium perenne reveals population-level variation in plant flavonoids and alkaloids. Front. Plant Sci. 2017, 8, 133. [CrossRef]
- Fariaszewska, A.; Aper, J.; Van Huylenbroeck, J.; De Swaef, T.; Baert, J.; Pecio, Ł. Physiological and biochemical responses of forage grass varieties to mild drought stress under field conditions. Int. J. Plant Prod. 2020, 14, 335-353.
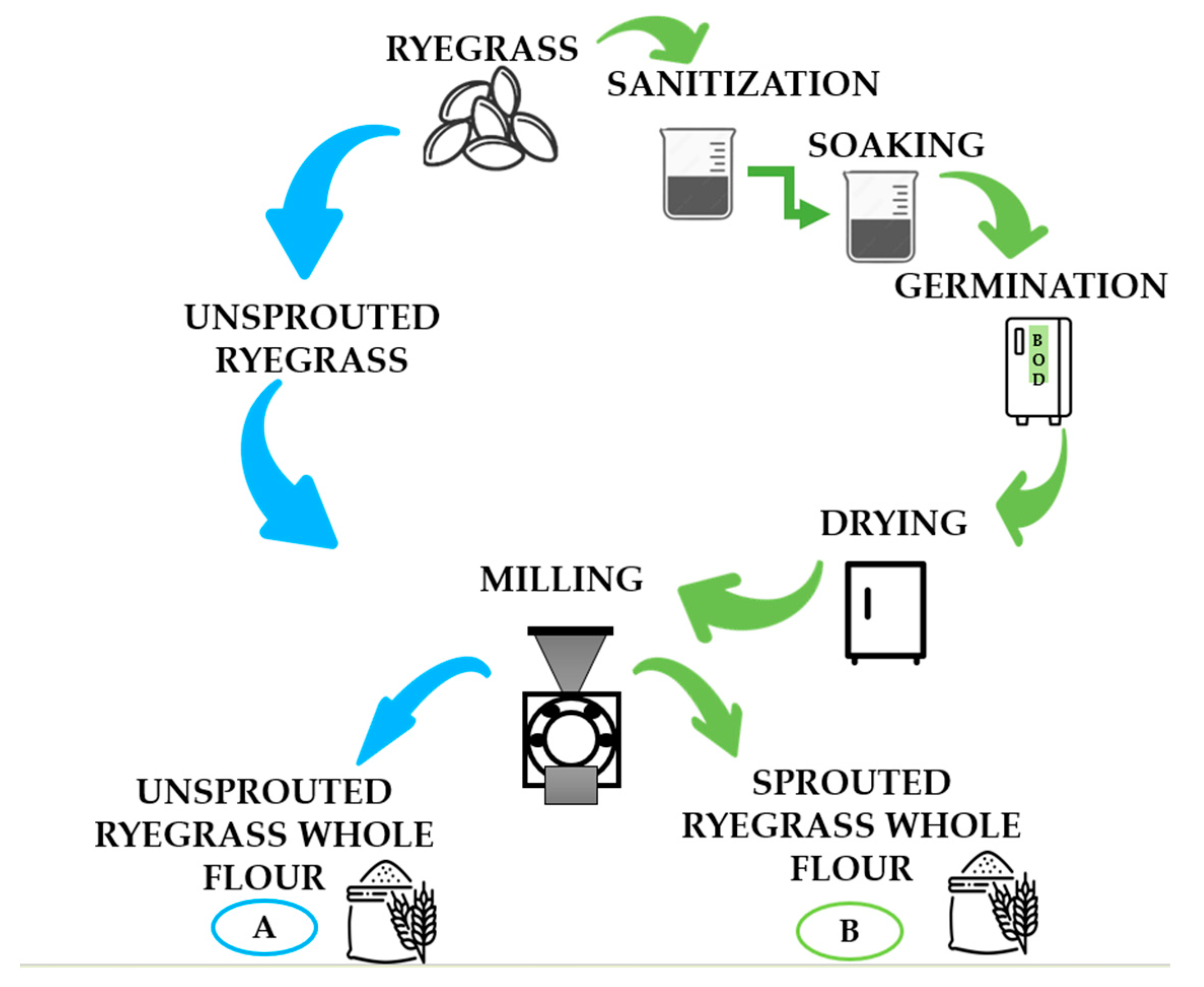


| Component | Maize flour | Sprouted ryegrass | Unsprouted ryegrass |
|---|---|---|---|
| Proteins | 7.03 ± 0.50 | 13.12 ± 0.16 | 13.11 ± 0.37 |
| Lipids | 6.27 ± 0.05 | 2.70 ± 0.10 | 2.15 ± 0.18 |
| Ashes | 1.51 ± 0.68 | 5.09 ± 0.02 | 5.78 ± 0.01 |
| Digestible carbohydrates (starch and sugars) | 80.19 ± 0.64 | 65.17 ± 0.56 | 63.69 ± 0.43 |
| Total dietary fibers* | 5.00 ± 0.87# | 13.92 ± 0.77# | 15.27 ± 0.35# |
| Parameters | Maize flour | Sprouted ryegrass | Unsprouted ryegrass | |
|---|---|---|---|---|
| Water absorption index (g of gel.g-1 of sample, d.b.) | 2.75 ± 0.01c | 3.82 ± 0.02a | 3.48 ± 0.17 b | |
| Water solubility index (%, d.b.) | 5.31 ± 0.07c | 11.72 ± 0.19a | 6.80 ± 0.21b | |
| Instrumental color | L* | 77.37 ± 0.10a | 67.60 ± 0.04c | 68.75 ± 0.04b |
| a* | 7.28 ± 0.01a | 2.72 ± 0.01b | 2.57 ± 0.01c | |
| b* | 39.74 ± 0.05a | 14.75 ± 0.01b | 14.43 ± 0.02c | |
| γ-aminobutic acid (mg.100 g-1, d.b.) | 4.37 ± 0.02b | 40.83 ± 3.78a | 6.07 ± 0.04b | |
| TSPC (mg GAE.100 g-1, d.b.) | 537.19 ± 31.48c | 1002.58 ± 37.67a | 841.46 ± 14.56b | |
| Trials | Expansion index | Bulk density (g.cm-3) | Water absorption index (g of gel/g of sample, d.b.) |
Water solubility index (%, d.b.) |
|---|---|---|---|---|
| SR0 | 1.93 ± 0.15d | 0.18 ± 0.03a | 7.71 ± 0.02a | 25.68 ± 1.05b |
| SR4 | 2.36 ± 0.13a | 0.13 ± 0.02c | 7.31 ± 0.14b | 25.28 ± 1.95b |
| SR8 | 2.30 ± 0.10b | 0.13 ± 0.01c | 7.59 ± 0.30a | 24.48 ± 0.75b |
| SR12 | 2.05 ± 0.08b | 0.15 ± 0.02b | 7.42 ± 0.03b | 30.47 ± 1.50a |
| SR16 | 2.03 ± 0.09c | 0.15 ± 0.01b | 7.36 ± 0.05b | 22.96 ± 0.77b |
| SR20 | 2.18 ± 0.08c | 0.14 ± 0.01c | 7.26 ± 0.07b | 27.63 ± 1.15a |
| USR10 | 2.50 ± 0.09a | 0.15 ± 0.01b | 7.60 ± 0.09a | 22.18 ± 1.22b |
| p-value | <0.001 | <0.001 | 0.038 | <0.001 |
| Trials | L* | a* | b* | ΔE |
|---|---|---|---|---|
| SR0 | 60.88±0.28a | 7.11±0.11a | 39.73±0.22a | - |
| SR4 | 54.26±1.42b | 7.06±0.14a | 34.96±0.39b | 8.12±1.34b |
| SR8 | 51.79±1.25c | 6.60±0.14b | 30.66±0.61c | 12.80±1.32b |
| SR12 | 50.44±0.53c | 5.80±0.05d | 27.40±0.39d | 16.15±0.61a |
| SR16 | 50.67±0.37c | 5.86±0.06d | 25.77±0.21e | 17.29±0.22b |
| SR20 | 50.29±0.48c | 5.46±0.10e | 24.13±0.11f | 18.88±0.30a |
| USR10 | 50.69±0.70c | 6.19±0.20c | 27.26±0.30d | 16.09±0.38d |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 |
| Trials | Total soluble phenolic compounds (mg GAE.100 g-1) |
γ-aminobutyric acid (mg. 100 g ) |
|---|---|---|
| SR0 | 372.19±28.04f | 3.50 ± 0.10e |
| SR4 | 485.96±17.31e | 7.96 ± 0.55c |
| SR8 | 535.52±44.56d | 8.48 ± 0.71c |
| SR12 | 586.37±45.82c | 8.64 ± 0.41c |
| SR16 | 644.52±34.75b | 11.70 ± 0.60b |
| SR20 | 699.36±39.92a | 14.52 ± 0.97a |
| USR10 | 548.38±30.18d | 6.55 ± 0.20d |
| p-value | <0.001 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





