1. Introduction
Mosaicism is defined as the presence of two or several genomes in an individual or a tissue. Everyone acquires multitudes of mutations during development, growth and aging despite having efficacious DNA repair mechanisms. Earlier these somatic mutations were poorly explored; however, new generation sequencing technologies have allowed the more precise characterisation of somatic mosaicism. The number of mutations increases with aging and so increases the risk of developing malignant transformations [
1].
Mosaicism may appear in three different contexts: within a healthy organ, within a cancerous organ and within a cancer itself. Within a healthy organ the multiplication of a cell that has undergone somatic mutation may lead to the formation of mosaic tissue. Cancer cells usually hamper multiple mutations and their multiplication is more rapid compared to the surrounding tissue, so literally every cancerous organ is mosaic. When cancer cells become further differentiated they may form a mosaic cancer [
2].
Heterogeneity is a frequently used and broader term for expressing irregularity of cancers. Beyond genetic differences it refers to epigenetic modifications and also to microenvironmental influences including the pattern of tumor infiltrating immune cells, endothelial cells and stromal cells [
3]. The issue of the investigation of cancer heterogeneities would be the identification of therapeutically targetable alterations; hence heterogeneity of cancers has got limited clinical impact so far.
Recognition of the heterogeneity of prostate cancers is among others expressed by the Gleason grading system that classifies cellular differences at a microscopic level. The Gleason score correlates with the clinical outcome. Our attention was first caught by an exception, the neuroendocrine cancers of the prostate. Then we gradually realised that some of these tumours have a better prognosis as expected. In particular the cases that responded to both anticancer treatments, those usually directed for prostatic adenocarcinomas and those for high grade neuroendocrine cancers.
Neuroendocrine cells are normally present in the prostatic tissue [
4], but their identification by hematoxylin-eosin stain remains impossible due to their rarity [
5]. Neuroendocrine cancer of the prostate (NECP) may develop in single or multiple foci within an adenocarcinoma and also de novo as an individual tumour. The microscopic picture may present small cell and large cell carcinoma as well. Carcinoid tumours have also been described in the prostate [
6], but we have never identified any one. The diagnosis of NECP may be retained either by a positive immunohistochemistry (IHC) staining or by the observation of a favourable response to platinum based chemotherapy expressed also by the decrease of elevated neuroendocrine serum markers (
Table 1).
Table 1.
Diagnostic criteria of the neuroendocrine cancer of the prostate.
Table 1.
Diagnostic criteria of the neuroendocrine cancer of the prostate.
|
Major: positive IHC staining with either CD56, synaptophysin, chromogranine A or NSE |
|
Minor: elevation of serum NSE or chromogranine A |
|
Minor: favourable response to a platinum based chemotherapy |
Table 2.
Clinical situations when neuroendocrine cancer of the prostate should be suspected.
Table 2.
Clinical situations when neuroendocrine cancer of the prostate should be suspected.
| Clinical or radiological progression of prostate cancer without PSA rise |
| Diagnosis of an extended metastatic disease associated to a relatively low PSA |
| Presence of any non-pulmonary visceral metastases |
| Metastatic carcinoma of unknown primary |
Table 3.
Strategies to improve the detection rate of neuroendocrine prostate cancers.
Table 3.
Strategies to improve the detection rate of neuroendocrine prostate cancers.
| Avoiding to consider PSA as unique and to 100% sensitive marker of prostate cancer |
| Realisation of neuroendocrine IHC staining in the situations summarised in Table 2. |
| Determination of NSE and/or chromogranine A when biopsy or IHC are not feasible |
2. Results
Earlier results
The incidence of neuroendocrine prostate cancer has been considered to be extremely low [
7]. However by vigilant and rigorous search we found, that at least 2% of our prostate cancer patients were presenting neuroendocrine phenomena [
8]. The main reasons of missing the right diagnosis are the rarity of realising neuroendocrine IHC staining on prostate biopsy samples, and the erroneous elimination of the prostatic origin for a metastatic disease progression on the basis of a non-elevated PSA level. Furthermore, repeating the biopsy of the prostate for known prostate cancer patients and making neuroendocrine IHC staining for an adenocarcinoma of unknown primary are not part of the clinical routine. Bypassing the histological diagnosis by laboratory markers such as the non-specific neuron specific enolase (NSE) and the rather difficult to access chromogranine A is not convenient either.
Since the publication of our cohort [
8] we have been continuously observing the same incidence of NECP. We have published case reports presenting the pitfalls of the diagnosis and the possibility of several years of survival [
9]. The particularity of patients with favourable outcome was the parallel elevation of prostate specific antigen (PSA) and NSE. They received anticancer treatments in an alternating way directed according to the tumour marker that was actually in rise.
The aim of this paper is to show the pathological background of our clinical observations. A search in our histology archives was done. We were looking for representative samples that show mosaic neuroendocrine/adenocarcinoma of the prostate. A careful search of the literature was also made, to find any explanation that would fit with our clinical observations.
Recent results
Two pictures were selected for publication together with the clinical history of the patients.
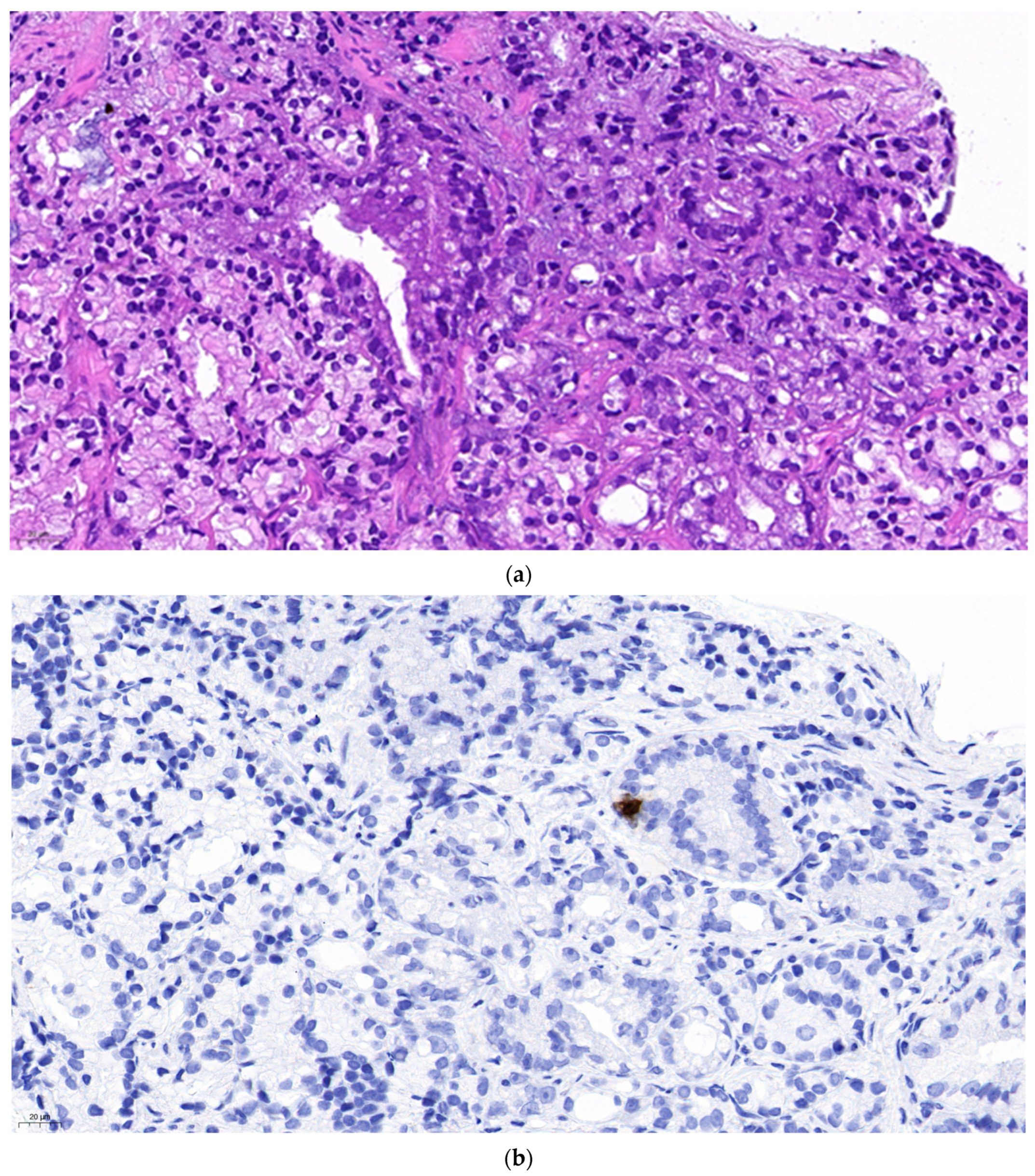
Figure 1 represents isolated neuroendocrine cells within an acinar adenocarcinoma (Gleason grade group 3) in a prostate biopsy specimen. The density of the neuroendocrine cells is not higher in the cancer than in the normal prostatic tissue. However, it can not be excluded that one of them might be the source of a malignant neuroendocrine line. The patient received 74 Gy radiotherapy to the prostate. A year later he was diagnosed in another hospital with intermediate grade neuroendocrine cancer affecting paraaortic lymph nodes and the liver, localisations of predilection for metastatic NECP. Since no primary tumour was found the suspicion that the earlier diagnosed prostate cancer be the origin may be raised.
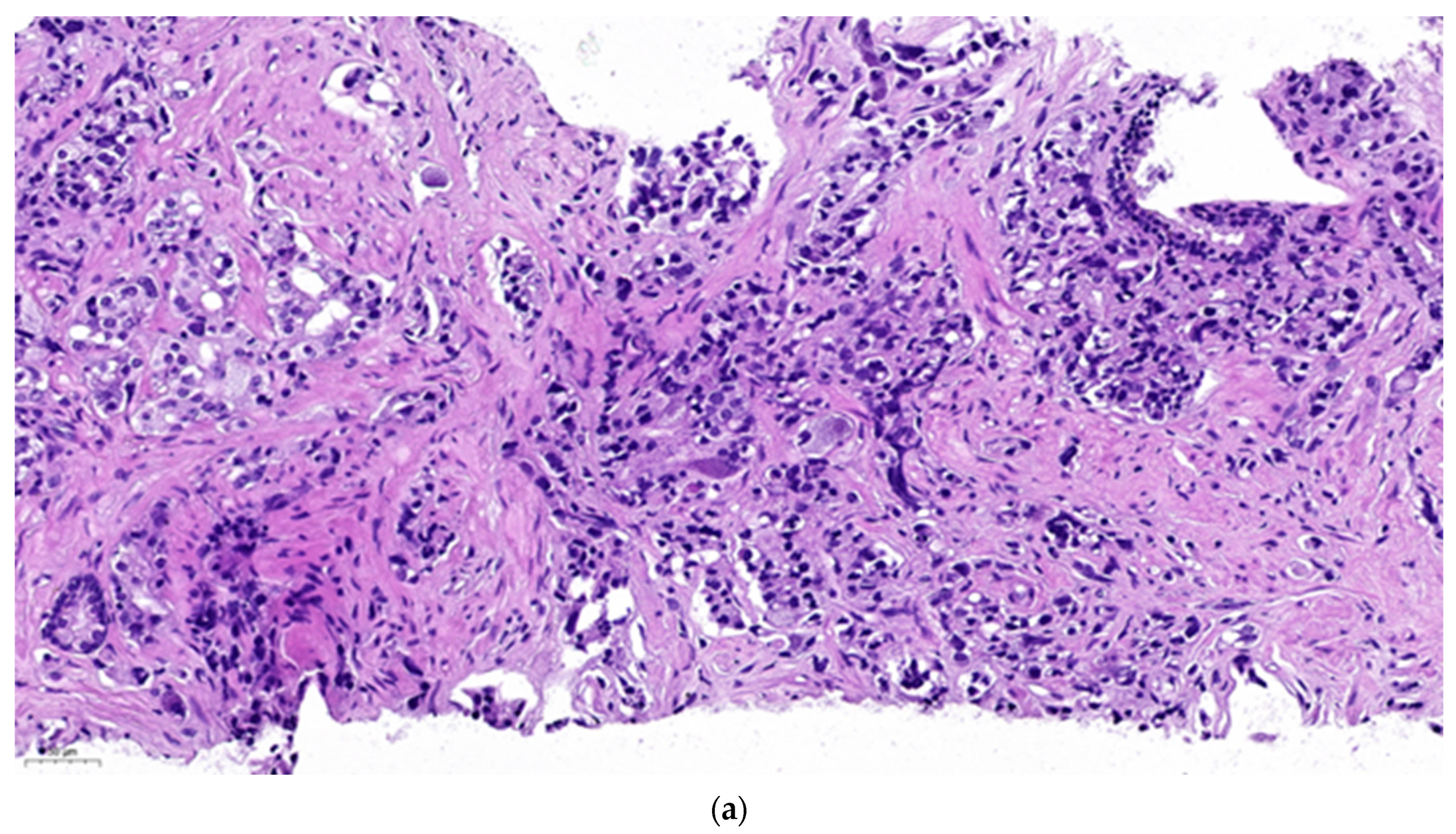
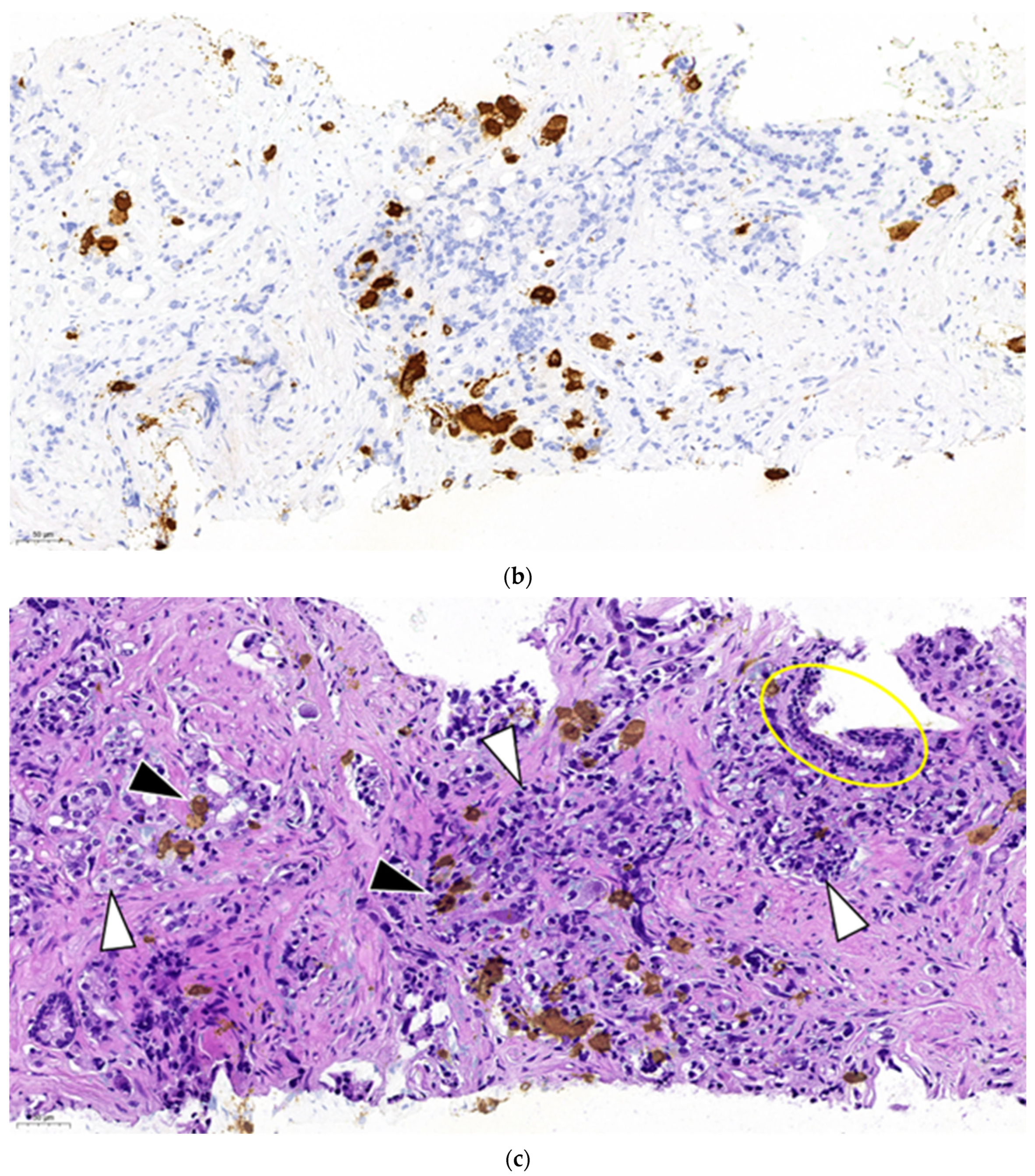
Figure 2a represents a primary prostate cancer (Gleason grade group 5).
Figure 2b shows positivity of chromogranine A IHC staining.
Figure 2c is the superposition of the two above figures showing clearly that only a part of the malignant cells are stained and thus have neuroendocrine feature. The remaining part is common adenocarcinoma. This figure demonstrates a primary mosaic prostate cancer. The patient was prostatectomised after receiving 3 cycles of primary cisplatin-etoposide treatment. The prostate did not show any positivity at neuroendocrine IHC, meaning that the neuroendocrine component was in complete pathological regression after chemotherapy. Currently we observe a slight increase of NSE while the PSA remains suppressed and staging examinations are negative.
3. Discussion
Our histological archives contained samples showing primary mosaic prostate cancer. While the parallel presence of neuroendocrine- and adenocarcinoma can only be speculated on the first sample it is clearly documented on the second one. It is not difficult to imagine that in the absence of preoperative chemotherapy and prostatectomy the disease would have become metastatic and it may also be suspected that the two parts might show different progression rate and also different spreading localisations.
Currently, the most efforts of prostate cancer research are concentrated to the understanding of mechanisms of resistance to castration. NECP is the sui generis castration resistant cancer. It is usually observed in late stage of the evolution of the cancer and is generally suspected to arise by dedifferenciation under long lasting androgen deprivation therapy [
10]. Our findings support the possibility of the parallel evolution of the two components from the beginning on.
So, the key question would sound how neuroendocrine foci may develop within a prostate cancer? For answering this question we rely on the works of Blackwood et al. [
11]. They demonstrated (through the observation of the transmission of changes in the mitochondrial DNA) that different cells of the normal prostatic epithelium originate from a common epithelial stem cell. With other words, an epithelial stem cell may supply the whole cellular population of the acini i.e., basal cells, luminal cells and neuroendocrine cells as well.
Although according to the current concept prostate cancer stem cells are among the luminal cells [
12,
13] with the above model it is enough to assume that prostate cancer may also arise from epithelial stem cells to explain the parallel development of adenocarcinoma and neuroendocrine cancer. Similarly to non-seminomatous germ cell tumours [
14] and chronic leukaemia the proliferation of a malignant-turned stem cell could be responsible of the mosaic pattern.
A further hypothesis could explain some of the unusual observations related to the hormone sensitivity of prostate cancers. Since the introduction of second line antihormonal therapies their optimal use has not been established. (We prefer using the term “second line” instead of the currently applied androgen receptor targeted agents abbreviated for “ARTA” as we find it less misleading. Please consider that there are medications that act on the androgen receptors still they are not considered among ARTAs and contrarily, not all ARTAs act on the androgen receptors.)
Subsequent studies showed that the more anticancer drugs were used in parallel from the beginning on for the treatment of a hormone sensitive metastatic prostate cancer the more additional clinical benefit could be obtained [
15,
16]. Administration of the second line antihormonal agents is recommended until clinical or radiological progression. Or, in a small set of patients of our single centre, patients whose treatment was stopped at PSA progression did not have a worse prognosis than those treated several months longer until clinical or radiological progression [
17]. The same antihormonal drug was reintroduced to some patients after progression under second line chemotherapy by cabazitaxel. A novel decrease in PSA level could be observed in patients treated earlier until PSA progression as well as until radiological progression (yet unpublished data). It means that in these patients the cancer lost its earlier gained resistance to the antihormonal drug. The most plausible explanation for this unusual phenomenon would be the simultaneous presence of different cell lines.
Let’s admit that adenocarcinoma cells of different characteristics may arise from a single malignant-turned stem cell. Even if these cells have the same phenotype they may differ in terms of their mechanism of resistance to androgen deprivation. In this case a treatment line may suppress one cell line more than the other. Parallel use of the treatments may suppress more cell lines. Sequential use of the treatments may block a cell line that was not suppressed by the preceding treatment. The deductions of the hypothesis correspond exactly to the clinical observations.
The limitations of our work are the small number of cases and the small number of literature citations. The number of cases could be raised if neuroendocrine histochemistry would be routinely practiced to all prostate biopsy and prostatectomy specimens. Such a survey would disclose the real incidence of NECP. There are 60 hits for mosaic neuroendocrine cancer in Pubmed, the majority of the articles dealing with digestive tumours and diffuse idiopathic pulmonary neuroendocrine cell hyperplasia. The only article about the prostate is that of Blackwood et al., that we discussed in detail.
Finally, for those who find the term “mosaic neuroendocrine-/adenocarcinoma of the prostate” to be too complicated and in addition like word games we propose the denomination of Petz NECP.
4. Methods
A prospective analysis of the patients treated in our hospital for prostate cancer was realised between 1st January 2015 and 31rd December 2018 [
8]. Neuroendocrine phenomena were tested by immunohistochemistry and laboratory chemistry when clinicians suspected the diagnosis. The neuroendocrine staining was realised in all but some rare cases on the explicit asking of the clinicians.
The diagnosis of neuroendocrine cancer was established in two cases: 1) either positive immunohistochemistry staining with neuroendocrine markers 2) or elevation of a neuroendocrine serum marker (NSE or chromogranine A) associated to a favourable radiological response to a platinum-etoposide combination chemotherapy delivered for metastatic disease.
10 prostate cancer patients out of a total of 521 showed neuroendocrine phenomena. The mean age at the diagnosis was 68 years (range: 59–79). 7 patients’ diagnosis was based on immunohistochemistry (on prostate, lymph node or liver biopsy specimens).
5 patients’ survival reached one year, the longest survivor lived over 5 years. 3 patients received 3 lines of palliative chemotherapy. A patient with localised disease underwent prostatectomy after neoadjuvant chemotherapy and was lost of an intercurrent disease without having signs of relapse.
The presented superposed images were realised with the Inkscape vektographic drawing software. The lower layer was the hematoxylin-eosin stained (Merck reagent) in normal mode, the upper layer was the chromogranine A stained (Cell Marque reagent, Dako autostainer) in darken mode.
5. Conclusions
Mosaic neuroendocrine-/adenocarcinoma of the prostate has been underestimated regarding its incidence as well as its prognosis.
References
- Machiela, M. Mosaicism, aging and cancer. Curr. Opin. Oncol. 2019, 31, 108–113. [Google Scholar] [PubMed]
- Thorpe, J.; Osei-Owusu, IA.; Avigdor, BE.; Tupler, R.; Pevsner, J. Mosaicism in Human Health and Disease. Annu. Rev. Genet. 2020, 54, 487–510. [Google Scholar] [PubMed]
- Prasetyanti, PR.; Medema, JP. Intra-tumor heterogeneity from a cancer stem cell perspective. Mol. Cancer. 2017, 16, 41. [Google Scholar] [PubMed]
- Honn, KV.; Aref, A.; Chen, YQ.; Cher, ML.; Crissman, JD.; Forman, JD.; Gao, X.; Grignon, D.; Hussain, M.; Porter, AT.; et al. Prostate Cancer—Old Problems and New Approaches. (Part II. Diagnostic and Prognostic Markers, Pathology and Biological Aspects). Pathol. Oncol. Res. 1996, 2, 191–211. [Google Scholar] [CrossRef] [PubMed]
- Parimi, V.; Goyal, R.; Poropatich, K.; Yang, XJ. Neuroendocrine differenciation of prostate cancer: a review. Am. J. Clin. Exp. Urol. 2014, 2, 273–285. [Google Scholar] [PubMed]
- Li, J.; Wang, Z. The Pathology of Unusual Subtypes of Prostate Cancer. Chin. J. Cancer. Res. 2016, 28, 130–143. [Google Scholar] [PubMed]
- Zaffuto, E.; Pompe, R.; Zanaty, M.; Bondarenko, HD.; Leyh-Bannurah, SR.; Moschini, M.; Dell’Oglio, P.; Gandaglia, G.; Fossati, N.; Stabile, A.; et al. Contemporary Incidence and Cancer Control Outcomes of Primary Neuroendocrine Prostate Cancer: A SEER Database Analysis. Clin. Genitourin. Cancer. 2017, 15, e793–e800. [Google Scholar] [CrossRef] [PubMed]
- Kránitz, N.; Szepesváry, Zs.; Kocsis, K.; Kullmann, T. Neuroendocrine cancer of the prostate. Pathol. Oncol. Res. 2020, 26, 1447–1450. [Google Scholar] [CrossRef]
- Kullmann, T.; Szigeti, A.; Kránitz, N.; Szepesváry, Zs. Neuroendocrine cancer of the prostate. Two case reports. J. Mens’ Health. 2022, 18, 65. [Google Scholar] [CrossRef]
- Kishore, K.; Rafeeq, A.; Chime, C.; Hassan, T.; Masooma, N.; Jasbir, M.; Aryo, I. Poorly Differentiated Small-Cell-Type Neuroendocrine Carcinoma of the Prostate: A Case Report and Literature Review. Case. Rep. Oncol. 2018, 11, 676–681. [Google Scholar]
- Blackwood, JK.; Williamson, SC.; Greaves, LC.; Wilson, L.; Rigas, AC.; Sandher, R.; Pickard, RS.; Robson, CN.; Turnbull, DM.; Taylor, RW.; Heer, R. In situ lineage tracking of human prostatic epithelial stem cell fate reveals a common clonal origin for basal and luminal cells. J. Pathol. 2011, 225, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Al Salhi, Y.; Sequi, MB.; Valenzi, FM.; Fuschi, A.; Martoccia, A.; Suraci, PP.; Carbone, A.; Tema, G.; Lombardo, R.; Cicione, A.; et al. Cancer Stem Cells and Prostate Cancer: A Narrative Review. Int. J. Mol. Sci. 2023, 24, 7746. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, T.; Jia, M.; Dai, R.; Wang, R. The Molecular Biology of Prostate Cancer Stem Cells: From the Past to the Future. Int. J. Mol. Sci. 2023, 24, 7482. [Google Scholar] [CrossRef] [PubMed]
- Lobo, J.; Gillis, AJM.; Jerónimo, C.; Henrique, R.; Looijenga, LHJ. Human Germ Cell Tumors are Developmental Cancers: Impact of Epigenetics on Pathobiology and Clinic. Int. J. Mol. Sci. 2019, 20, 258.
- Sweeney, CJ.; Chen, YH.; Carducci, M.; Liu, G.; Jarrard, DF.; Eisenberger, M.; Wong, YN.; Hahn, N.; Kohli, M.; Cooney, MM. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. N. Eng. J. Med. 2015, 373, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Smith, MR.; Hussain, M.; Saad, F.; Fizazi, K.; Sternberg, CN.; Crawford, ED.; Kopyltsov, E.; Park, CH.; Alekseev, B.; Montesa-Pino, Á.; et al. Darolutamide and Survival in Metastatic, Hormone-Sensitive Prostate Cancer. N. Eng. J. Med. 2022, 386, 1132–1142. [Google Scholar] [CrossRef] [PubMed]
- Kullmann, T.; Kocsis, K.; Ambrus, A.; Kránitz, N.; Herceg, Á.; Szepesváry, Zs. Optimisation of second line antihormonal treatment for castration resistant metastatic prostate cancer. J. Mens’ Health. 2022, 18, 93. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).