Submitted:
08 September 2023
Posted:
11 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Experimental materials
2.2. Experimental methods
2.2.1. The fermentation process of blueberry wine
2.2.2. Extraction of genetic DNA from blueberry wine samples
2.2.3. PCR amplification and MiSeq sequencing of DNA extracted from blueberry wine fermentation broth samples
2.3. Data processing
3. Results
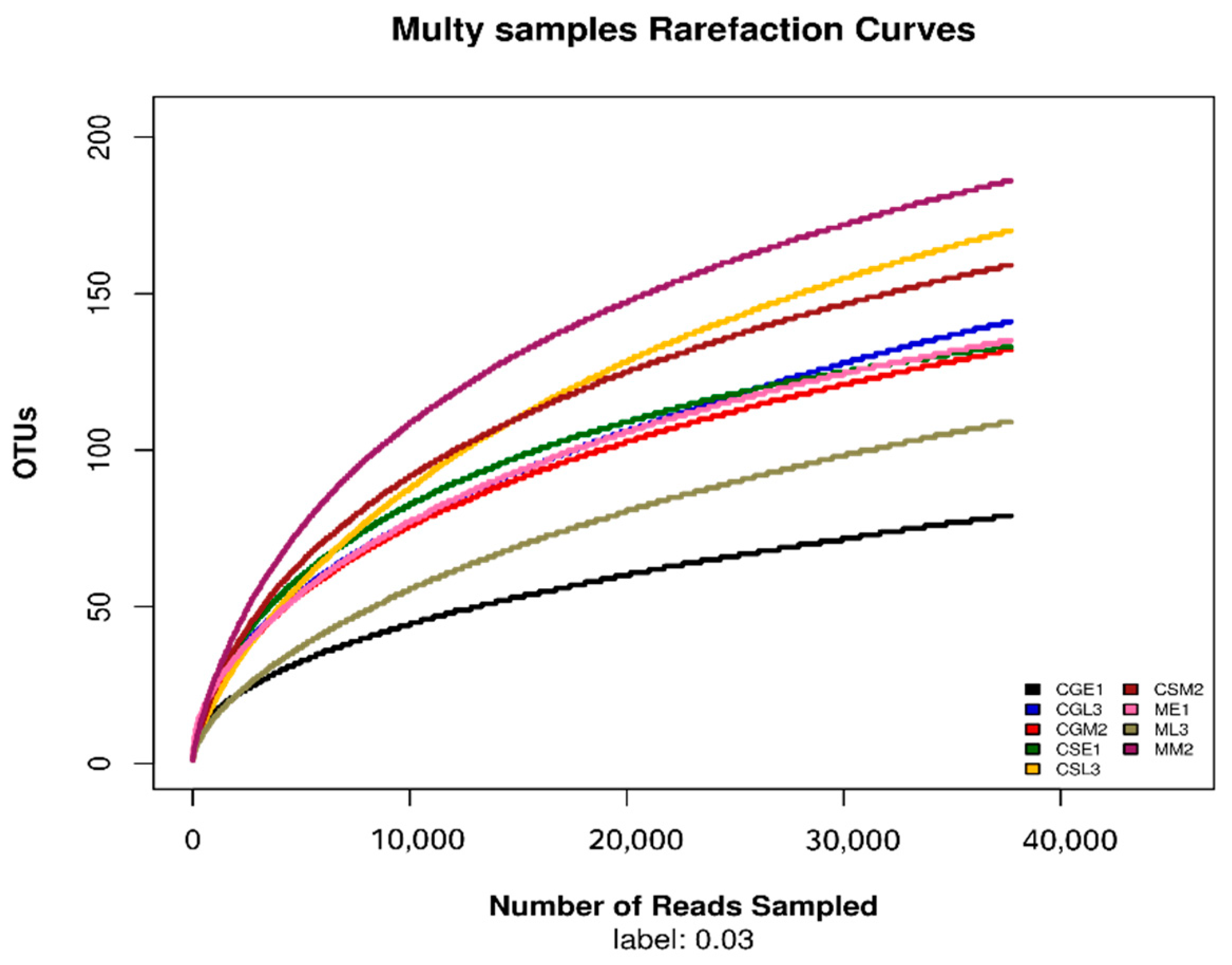
3.1. Sequence data and OTUs analysis
3.2. Analysis and discussion of alpha diversity data of fungal community zones during the fermentation of blueberry wine
3.3. Alpha diversity analysis of fungal flora during wine fermentation
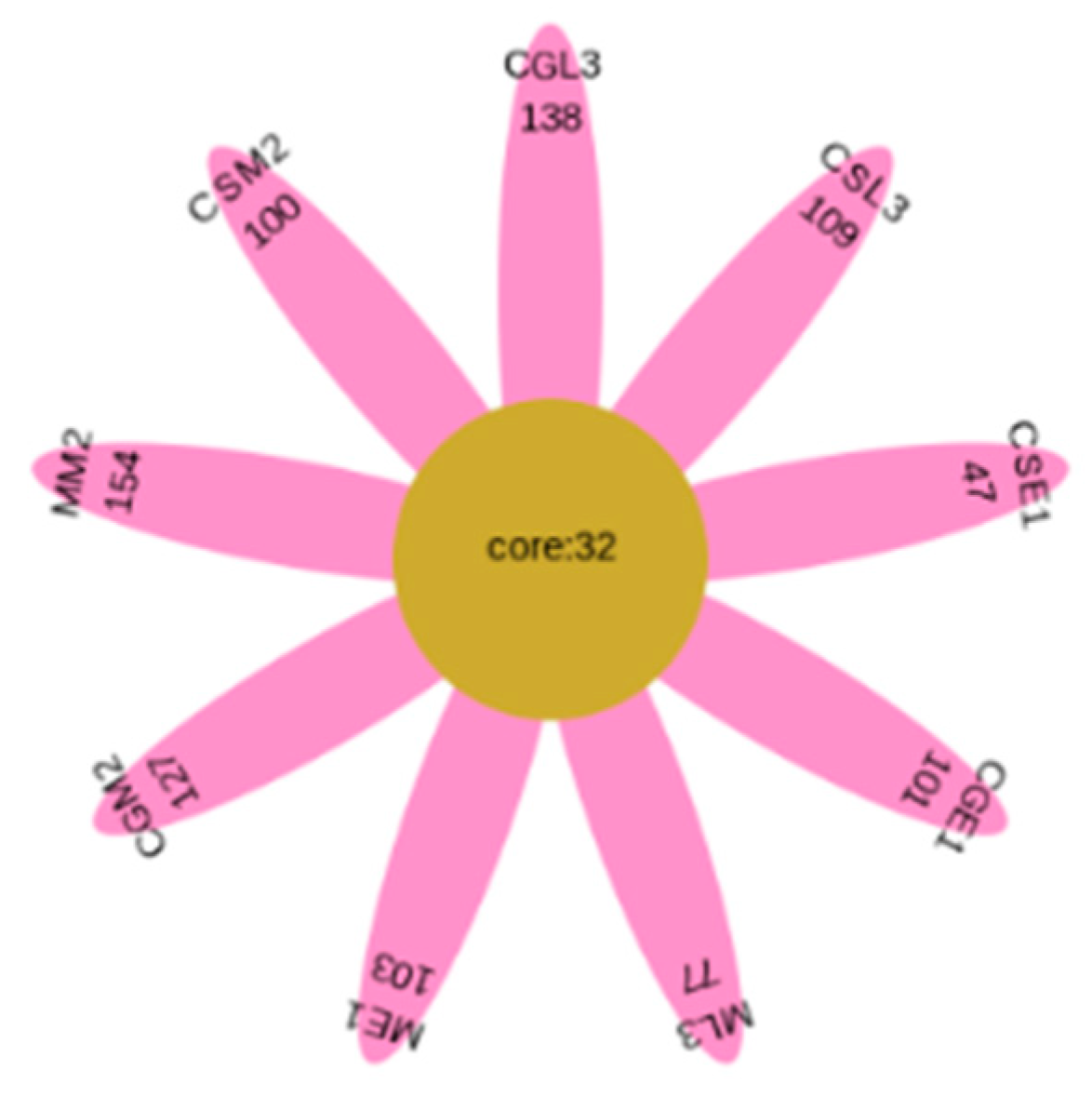
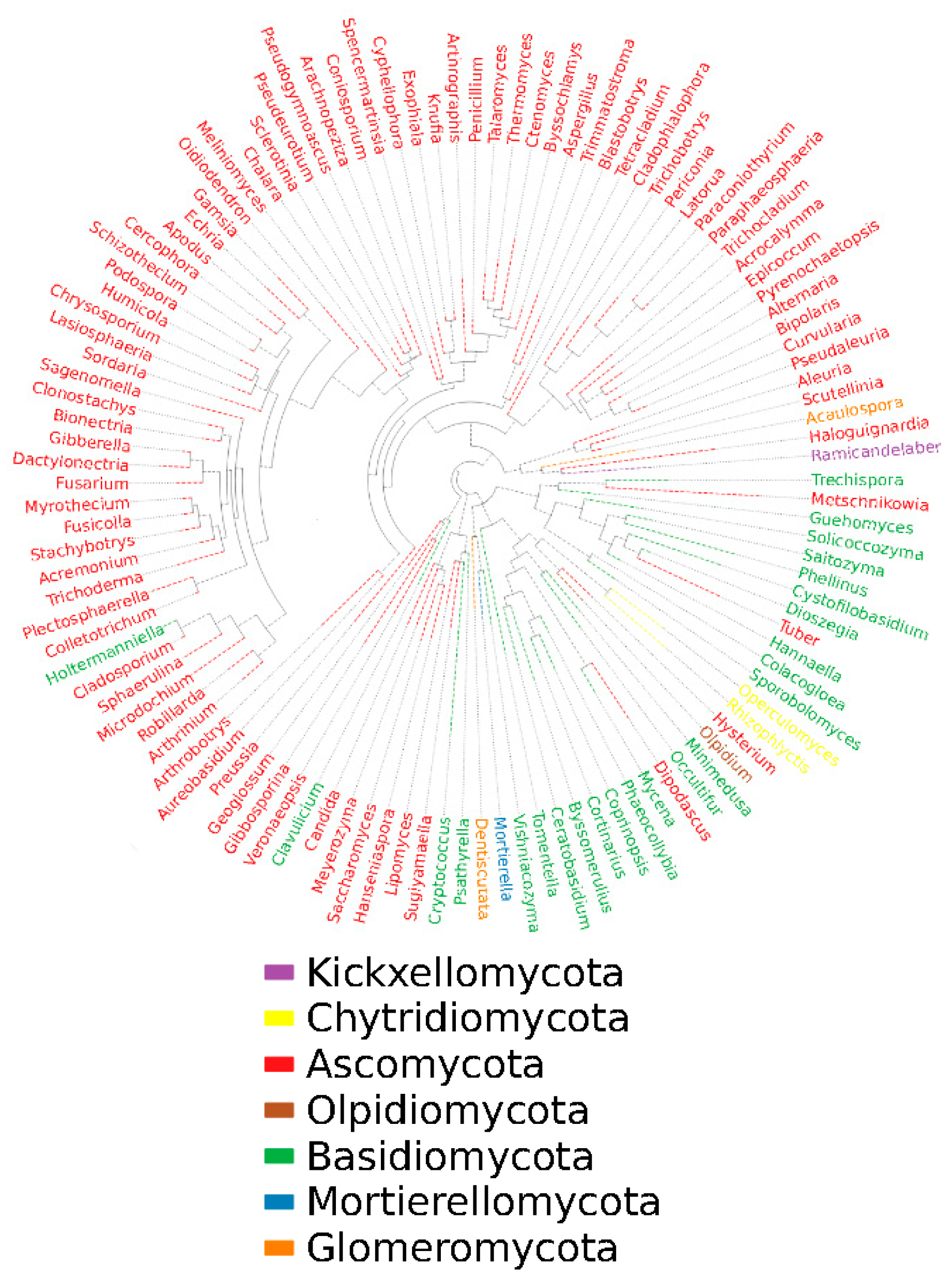
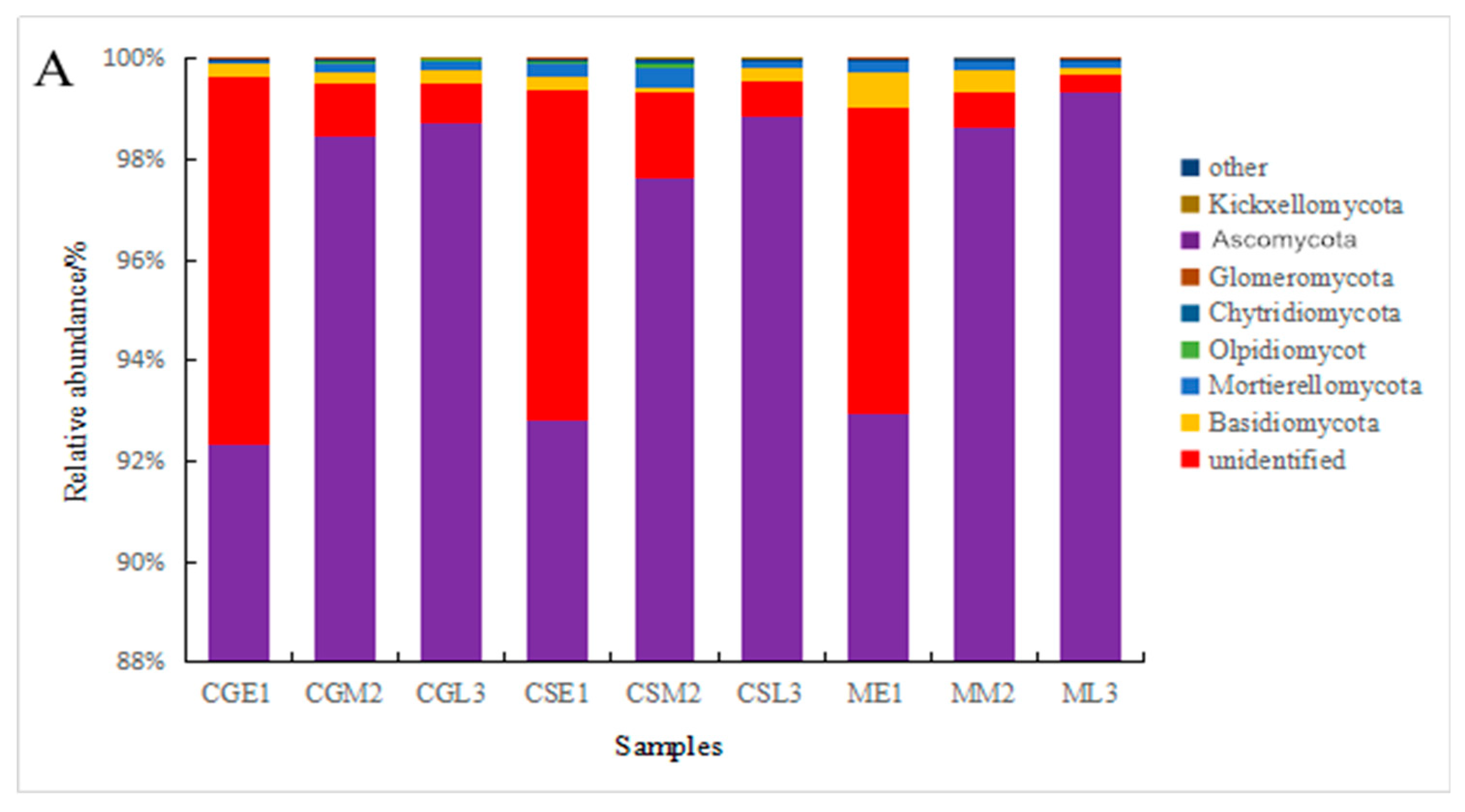
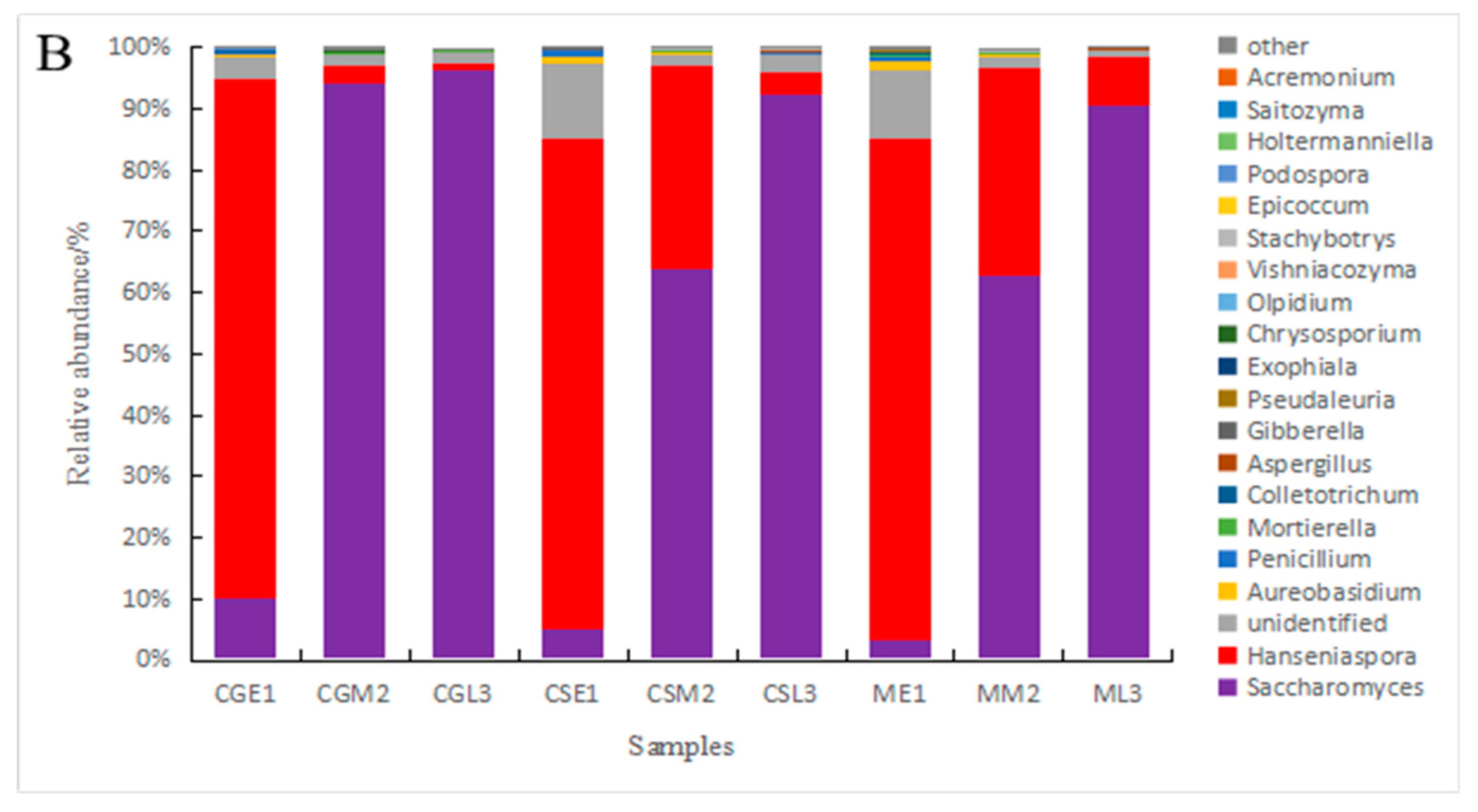
3.4. Analysis of fungal species and abundance
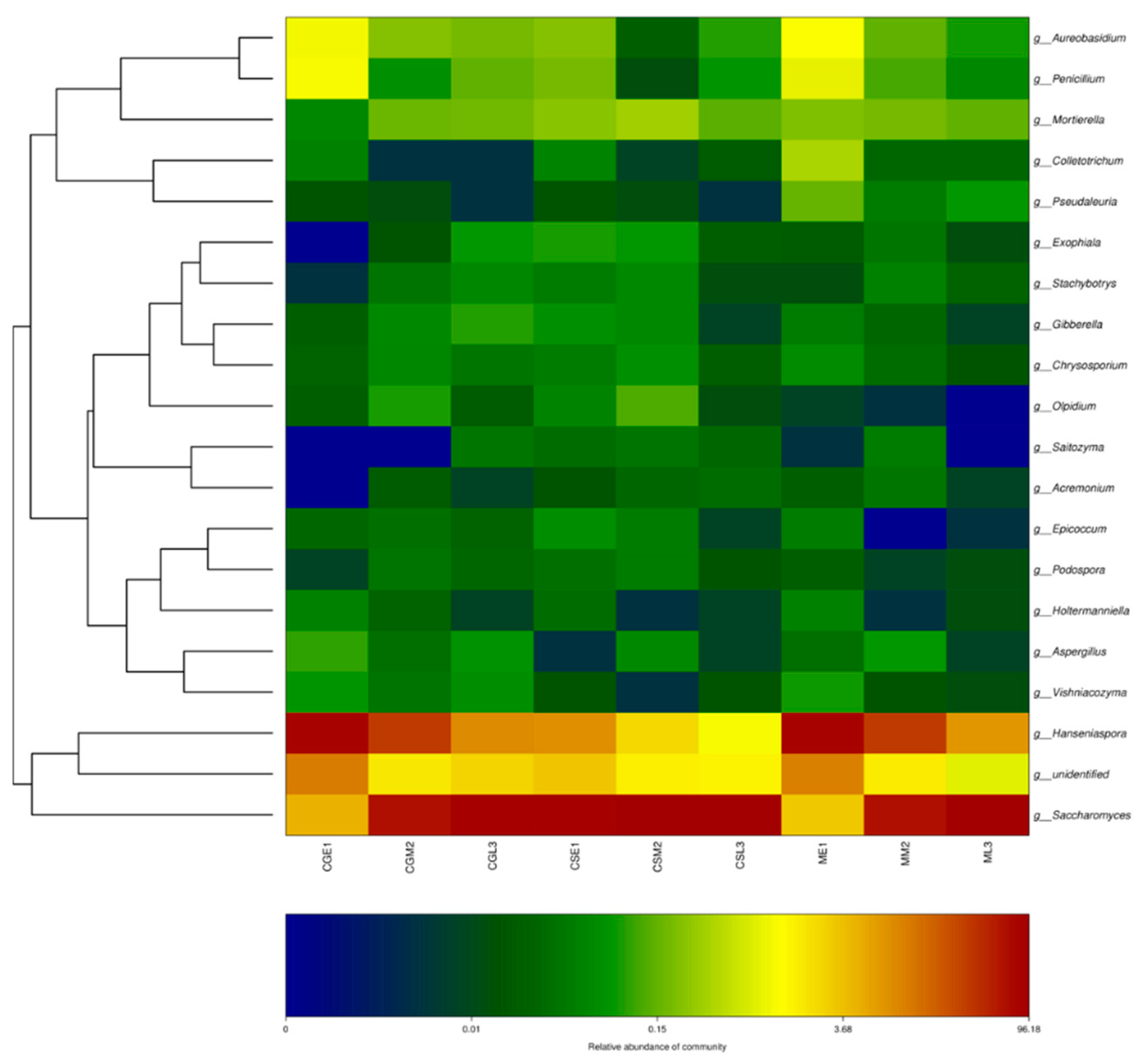
3.5. Dynamic changes of fungal flora
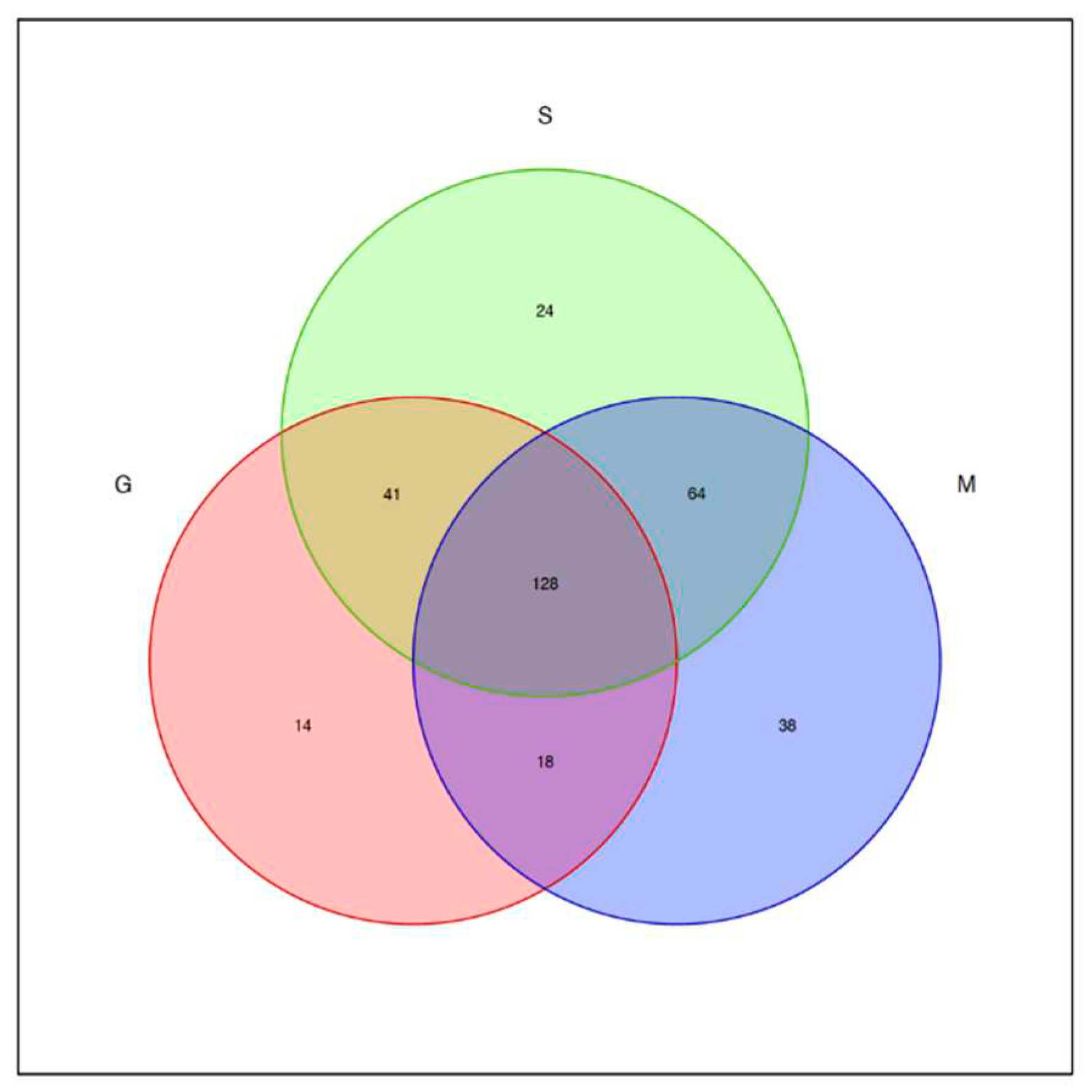
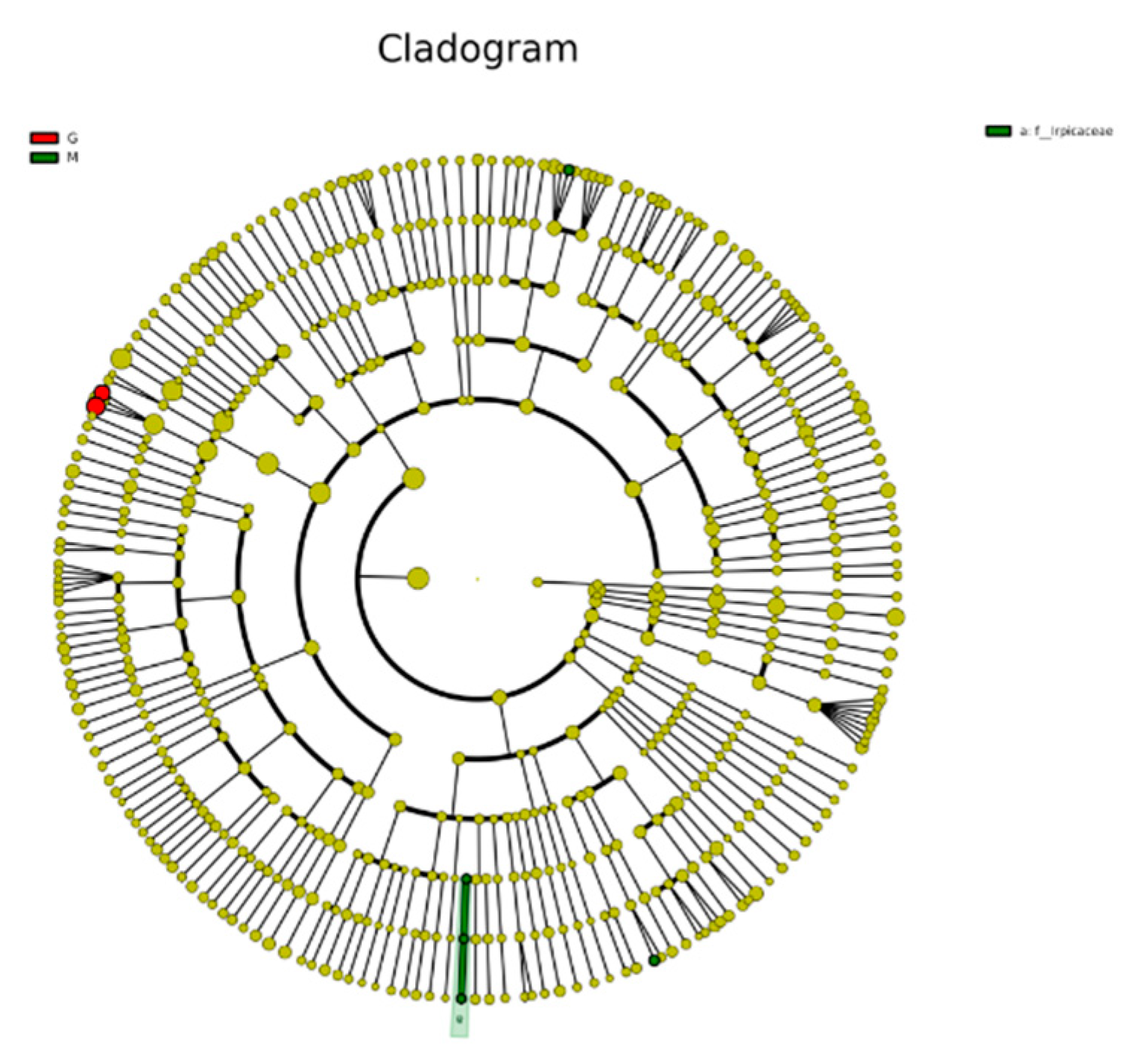
3.6. Analysis of fungal OTUs of different blueberry varieties before, during and after fermentation
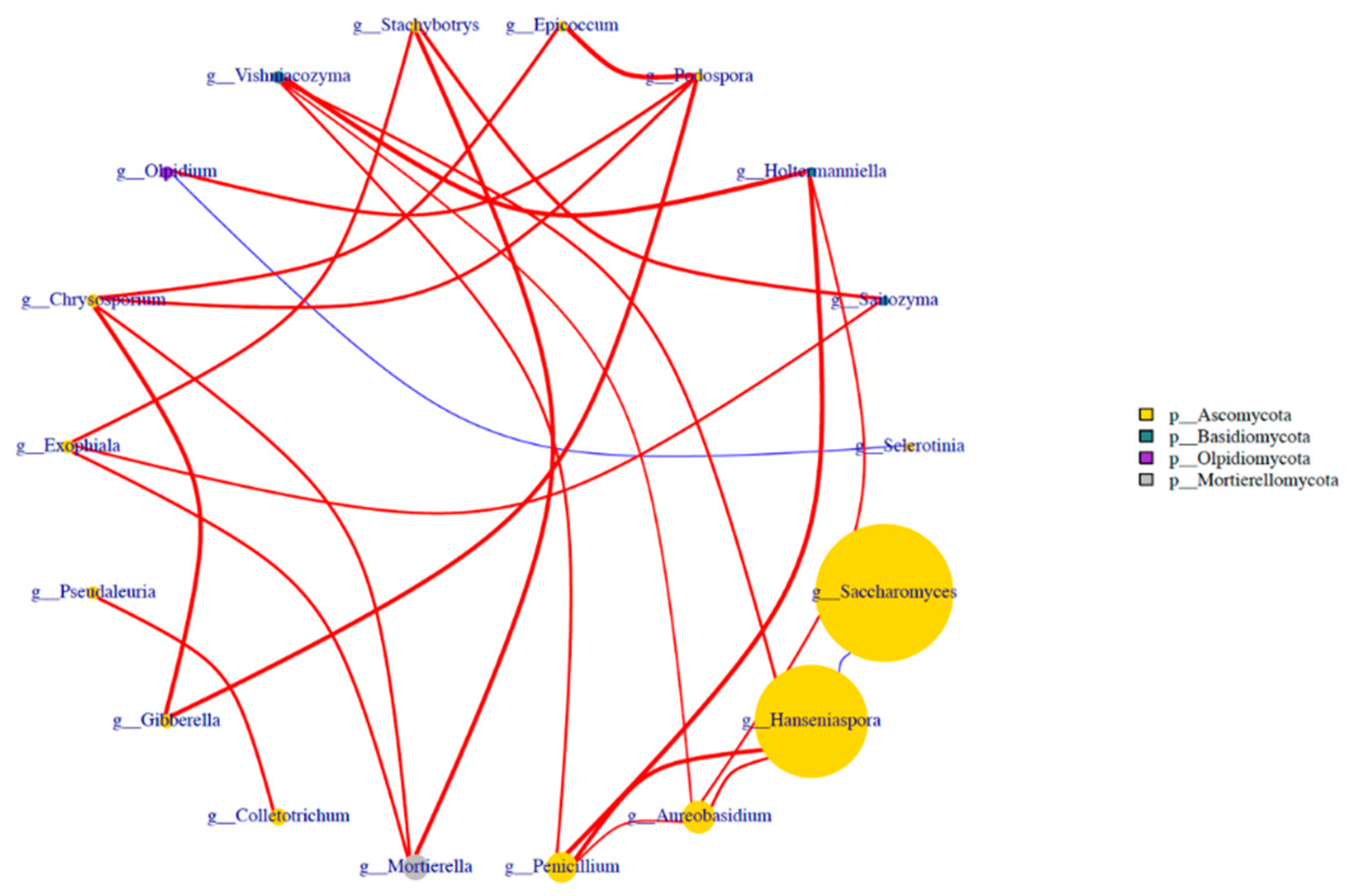
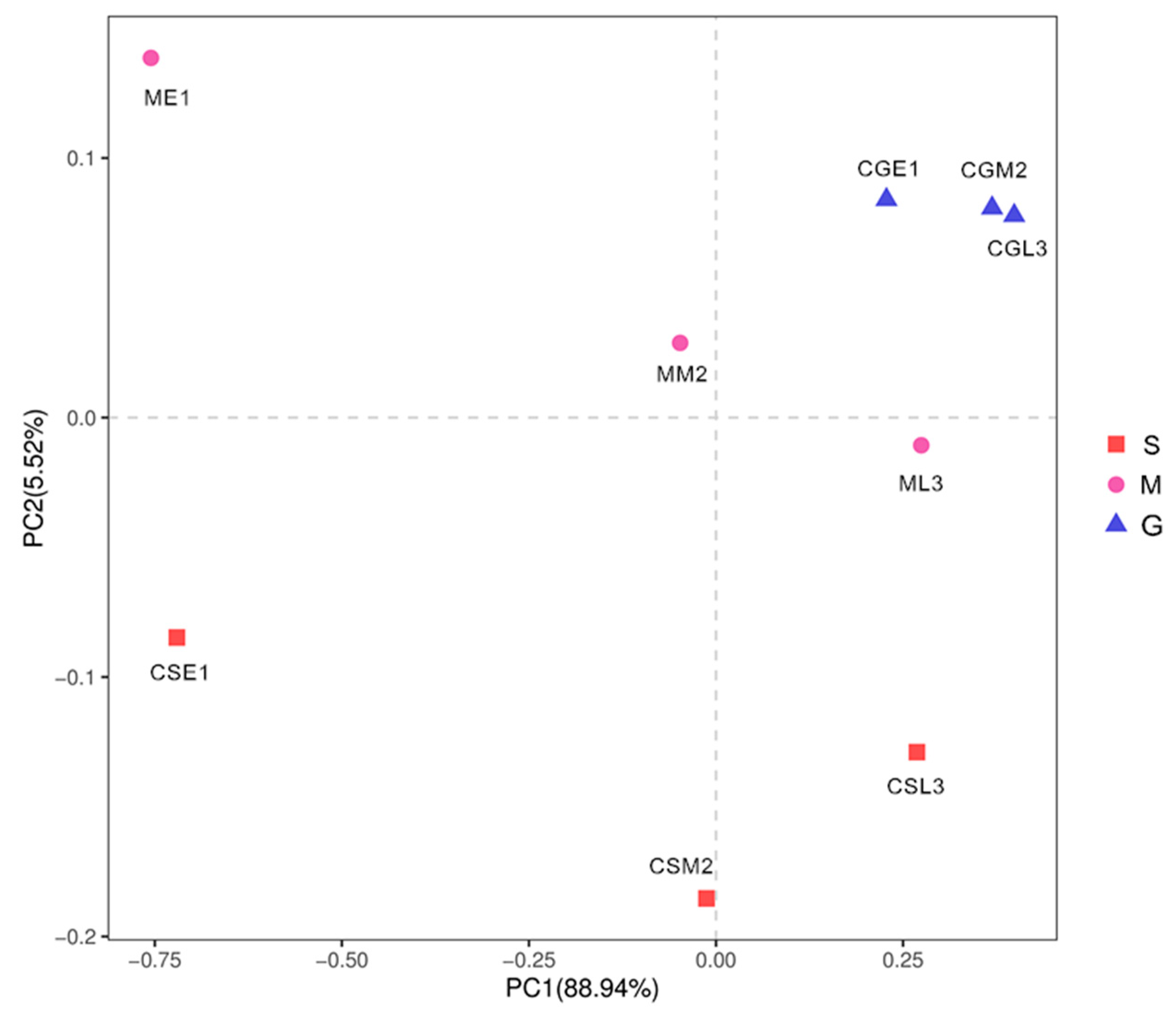
3.7. Beta diversity analysis of fungal flora during blueberry wine fermentation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eldarov, M.A.; Kishkovskaia, S.A.; Tanaschuk, T.N.; Mardanov, A.V. Genomics and biochemistry of Saccharomyces cerevisiae wine yeast strains. Biochemistry 2016, 81, 1650–1668. [Google Scholar] [CrossRef] [PubMed]
- Caridi, A.; Sidari, R.; Pulvirenti, A.; Blaiotta, G. Genetic Improvement of wine yeasts for opposite adsorption activity of phenolics and ochratoxin A during red winemaking. Food Biotechnol. 2020, 34, 352–370. [Google Scholar] [CrossRef]
- A, M. A.; B, A. P.; B, S. J.; C, G. B.; A, M. B.; A, J. C. , Influence of winemaking techniques on the resveratrol content, total phenolic content and antioxidant potential of red wines. Food Chemistry 2012, 131(2), 513–518. [Google Scholar]
- Bagheri, B.; Bauer, F.F.; Setati, M.E. The Impact of Saccharomyces cerevisiae on a Wine Yeast Consortium in Natural and Inoculated Fermentations. Front. Microbiol. 2017, 8, 1988. [Google Scholar] [CrossRef] [PubMed]
- Reuter, J.; Spacek, D. V.; Snyder, M. , High-Throughput Sequencing Technologies. Molecular Cell 2015, 58(4), 586–597. [Google Scholar] [CrossRef] [PubMed]
- Xu Hong, Planning and design study of special blueberry industry tourism park in Majiang County, Guizhou. China Agri-cultural Resources and Zoning 2016, 37, 204–207.
- Li, Q.; Li, C.; Wang, H.; Wei, X.; Liu, Y.; Yang, R.; Wen, X. Geochemical Characteristics of Heavy Metals in Soil and Blueberries of the Core Majiang Blueberry Production Area. Bull. Environ. Contam. Toxicol. 2021, 106, 57–64. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, F.; Wang, B.; Qiu, D. Identification, Characterization and Expression Analysis of Anthocyanin Biosynthesis-related bHLH Genes in Blueberry (Vaccinium corymbosum L.). 2021.
- Hu, B.; Gao, J.; Xu, S.; Zhu, J.; Fan, X.; Zhou, X. Quality evaluation of different varieties of dry red wine based on nuclear magnetic resonance metabolomics. Appl. Biol. Chem. 2020, 63, 1–8. [Google Scholar] [CrossRef]
- Hu, B.; Zhou, M.; Su, J.; Lin, L.; Xu, S. Study of Fungal Communities in Dry Red Wine Fermentation in Linfen Appellation, Shanxi. Fermentation 2022, 8, 475. [Google Scholar] [CrossRef]
- Walch, G.; Knapp, M.; Rainer, G.; Peintner, U. Colony-PCR Is a Rapid Method for DNA Amplification of Hyphomycetes. J. Fungi 2016, 2, 12. [Google Scholar] [CrossRef]
- Ghosh, S.; Namin, S.M.; Jung, C. Differential Bacterial Community of Bee Bread and Bee Pollen Revealed by 16s rRNA High-Throughput Sequencing. Insects 2022, 13, 863. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Li, Y.; Wang, Y.; Luo, X.; Du, F.; Liu, W.; Xie, L.; Chen, J.; Ren, Z.; Hou, S.; et al. The microbial diversity in industrial effluents makes high-throughput sequencing-based source tracking of the effluents possible. Environ. Res. 2022, 212, 113640. [Google Scholar] [CrossRef] [PubMed]
- Amato, K.R.; Yeoman, C.J.; Kent, A.; Righini, N.; Carbonero, F.; Estrada, A.; Gaskins, H.R.; Stumpf, R.M.; Yildirim, S.; Torralba, M.; et al. Habitat degradation impacts black howler monkey (Alouatta pigra) gastrointestinal microbiomes. ISME J. 2013, 7, 1344–1353. [Google Scholar] [CrossRef] [PubMed]
- Gail, M. H.; Yunhu, W.; Jianxin, S. , Power of Microbiome Beta-Diversity Analyses Based on Standard Reference Samples. American Journal of Epidemiology.
- Martínez-Salgado, S.; Romero-Arenas, O.; Morales-Mora, L. A.; Luna-Cruz, A.; Hoyos, P. A. , First Report of Macrophomina phaseolina Causing Charcoal Rot of Peanut (Arachis hypogaea L.) in Mexico. Plant Disease 2021.
- Zhao, Y.; Sun, Y.; Chen, W.; Zhao, Y.; Bai, Y. , remote sensing The Potential of Mapping Grassland Plant Diversity with the Links among Spectral Diversity, Functional Trait Diversity, and Species Diversity. Remote Sensing 2021, 13. [Google Scholar]
- Ken; Kelley; Francis; Bilson; Darku; Bhargab; Chattopadhyay, Sequential accuracy in parameter estimation for population correlation coefficients. Psychological Methods 2019.
- Oberauner, L.; Zachow, C.; Lackner, S.; Högenauer, C.; Smolle, K.-H.; Berg, G. The ignored diversity: complex bacterial communities in intensive care units revealed by 16S pyrosequencing. Sci. Rep. 2013, 3, srep01413. [Google Scholar] [CrossRef] [PubMed]
- Ameni, G.; Kebede, A.M.; Zewude, A.; Abdulla, M.G.; Asfaw, R.; Gobena, M.M.; Kyalo, M.; Stomeo, F.; Gumi, B.; Sori, T. Equine Histoplasmosis in Ethiopia: Phylogenetic Analysis by Sequencing of the Internal Transcribed Spacer Region of rRNA Genes. Front. Cell. Infect. Microbiol. 2022, 12, 789157. [Google Scholar] [CrossRef] [PubMed]
- Vidyasagar-E-mail, C.; Kumar–E-mail, P. S.; Vijayakumar–E-mail, P.; Alekya–E-mail, S.; Umamahesh, K.; Reddy, O. V. S. , Comparative structural and functional analysis of the PGU1 protein from Saccharomyces bayanus with other Saccharomyces species. Bioinformation 2022, 18(5), 464–469. [Google Scholar] [CrossRef] [PubMed]
- Manuel, S. L. J.; Sylwia, J.; Aslihan, E. K.; Jungho, L.; Roger, S.; Vanbogaert, I. N. A. , The oleaginous yeast Starmerella bombicola reveals limitations of Saccharomyces cerevisiae as a model for fatty acid transport studies. FEMS Yeast Research (1), 1.
- Santiago-Urbina, J.A.; Arias-García, J.A.; Ruiz-Terán, F. Yeast species associated with spontaneous fermentation of taberna, a traditional palm wine from the southeast of Mexico. Ann. Microbiol. 2015, 65, 287–296. [Google Scholar] [CrossRef]
- Hu, B.; Cao, Y.; Zhu, J.; Xu, W.; Wu, W. Analysis of metabolites in chardonnay dry white wine with various inactive yeasts by 1H NMR spectroscopy combined with pattern recognition analysis. AMB Express 2019, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Oh, E.J.; Jin, Y.-S. Engineering of Saccharomyces cerevisiae for efficient fermentation of cellulose. FEMS Yeast Res. 2020, 20. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Zhu, J.; Zhao, Q.; Gao, J.; Zhang, H.; Hu, B. Quality evaluation of Cabernet Sauvignon wines in different vintages by 1H nuclear magnetic resonance-based metabolomics. Open Chem. 2021, 19, 385–399. [Google Scholar] [CrossRef]
- Wendland, G. J. , The Whiff of Wine Yeast Innovation: Strategies for Enhancing Aroma Production by Yeast during Wine Fermentation. Journal of Agricultural and Food Chemistry 2019, 67. [Google Scholar]
- Quintero-Blanco, J.; Delodi, E.; Garzón, A.; Jimenez, J. Sexually-Driven Combinatorial Diversity in Native Saccharomyces Wine Yeasts. Fermentation 2022, 8, 569. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, K.; Liu, C.; Ma, L.; Li, J. Effects of glycosidase on glycoside-bound aroma compounds in grape and cherry juice. J. Food Sci. Technol. 2023, 60, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Viana, F.; Gil, J.V.; Vallés, S.; Manzanares, P. Increasing the levels of 2-phenylethyl acetate in wine through the use of a mixed culture of Hanseniaspora osmophila and Saccharomyces cerevisiae. Int. J. Food Microbiol. 2009, 135, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Bza, B.; Dxa, B.; Cda, B.; Gya, B. , Synergistic effect enhances 2-phenylethyl acetate production in the mixed fermentation of Hanseniaspora vineae and Saccharomyces cerevisiae. Process Biochemistry 2020, 90, 44–49. [Google Scholar]
- Li, T.; Yang, W.; Wu, S.; Selosse, M.-A.; Gao, J. Progress and Prospects of Mycorrhizal Fungal Diversity in Orchids. Front. Plant Sci. 2021, 12, 646325. [Google Scholar] [CrossRef]
- Merin, G.; Morata, V. I. Application of a grape surface majority pectinolytic species, Aureobasidium pullulans, to low-temperature red winemaking: development and stability of wine colour. Journal of Wine Research 2020, 31. [Google Scholar] [CrossRef]
- Yan, Q.; Pfleger, B.F. Revisiting metabolic engineering strategies for microbial synthesis of oleochemicals. Metab. Eng. 2019, 58, 35–46. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, C.; Feng, J.; Liu, Y.; Yang, Q.; Chen, H.; Gu, Z.; Zhang, H.; Chen, W.; Chen, Y.Q. Role of dihydrofolate reductase in tetrahydrobiopterin biosynthesis and lipid metabolism in the oleaginous fungus Mortierella alpina. Microbiology 2016, 162, 1544–1553. [Google Scholar] [CrossRef]
- Zhao, H.; Lv, M.; Liu, Z.; Zhang, M.; Wang, Y.; Ju, X.; Song, Z.; Ren, L.; Jia, B.; Qiao, M.; et al. High-yield oleaginous fungi and high-value microbial lipid resources from Mucoromycota. BioEnergy Res. 2020, 14, 1196–1206. [Google Scholar] [CrossRef]
- Shah, A.M.; Yang, W.; Mohamed, H.; Zhang, Y.; Song, Y. Microbes: A Hidden Treasure of Polyunsaturated Fatty Acids. Front. Nutr. 2022, 9, 827837. [Google Scholar] [CrossRef] [PubMed]
- Lane, S.; Dong, J.; Jin, Y.-S. Value-added biotransformation of cellulosic sugars by engineered Saccharomyces cerevisiae. Bioresource Technology: Biomass, Bioenergy, Biowastes, Conversion Technologies, Biotransformations. Bioresour. Technol. 2018, 260, 380–394. [Google Scholar] [CrossRef] [PubMed]
- Abdeljalil, S.; Ben Hmad, I.; Saibi, W.; Amouri, B.; Maalej, W.; Kaaniche, M.; Koubaa, A.; Gargouri, A. , Investigations on Hydrolytic Activities fromStachybotrys microsporaand Their Use as an Alternative in Yeast DNA Extraction. Applied Biochemistry & Biotechnology 2014, 172, 1599–1611. [Google Scholar]
- Cavello; Ivana; Cavalitto; Sebastian; Garmedia; Gabriela; Vero; Silvana; Albanesi; Agustin, Pectinolytic yeasts from cold environments: novel findings of Guehomyces pullulans, Cystofilobasidium infirmominiatum and Cryptococcus adeliensis producing pectinases. Extremophiles: Life under extreme conditions 2017, 21, 319–329.
- Liu, G. L. , The contribution of indigenous non-Saccharomyces wine yeast to improved aromatic quality of Cabernet Sauvignon wines by spontaneous fermentation. LWT-Food Science & Technology 2016, 71 (Null).
- Renault, P.; Coulon, J.; de Revel, G.; Barbe, J.-C.; Bely, M. Increase of fruity aroma during mixed T. delbrueckii/S. cerevisiae wine fermentation is linked to specific esters enhancement. Int. J. Food Microbiol. 2015, 207, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Miller, K. V.; Noguera, R.; Beaver, J.; Medina-Plaza, C.; Oberholster, A.; Block, D. E. , A Mechanistic Model for the Extraction of Phenolics from Grapes During Red Wine Fermentation. Molecules (Basel, Switzerland) 2019, 24. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, X.; Zhong, Q.; Zhuang, X.; Bai, Z. Microbial Community Analyses Associated with Nine Varieties of Wine Grape Carposphere Based on High-Throughput Sequencing. Microorganisms 2019, 7, 668. [Google Scholar] [CrossRef] [PubMed]
- Kioroglou, D.; Mas, A.; Portillo, M.C. High-Throughput Sequencing Approach to Analyze the Effect of Aging Time and Barrel Usage on the Microbial Community Composition of Red Wines. Front. Microbiol. 2020, 11. [Google Scholar] [CrossRef]
| Sequence length gradient | Number of Sequencs |
|---|---|
| 0-200 | 1109 |
| 200-260 | 28770 |
| 260-320 | 1521 |
| 320-360 | 17800 |
| 360-380 | 136776 |
| 380-400 | 11 |
| 400-420 | 11 |
| 420-440 | 296628 |
| 440-460 | 197 |
| 460-480 | 10 |
| 480-500 | 10 |
| 500-520 | 9 |
| 520-540 | 0 |
| 540-560 | 0 |
| 560-600 | 0 |
| Name of Samples | chao1 | coverage | observed_species | PD_whole_tree | shannon | simpson |
|---|---|---|---|---|---|---|
| CGE1 | 130.00 | 1.00 | 79.00 | 19.65 | 1.69 | 0.50 |
| CGM2 | 203.55 | 1.00 | 132.00 | 30.62 | 1.60 | 0.53 |
| CGL3 | 217.25 | 1.00 | 141.00 | 32.62 | 1.00 | 0.25 |
| CSE1 | 150.03 | 1.00 | 133.00 | 30.64 | 0.95 | 0.25 |
| CSM2 | 220.60 | 1.00 | 159.00 | 37.49 | 0.55 | 0.11 |
| CSL3 | 233.17 | 1.00 | 170.00 | 37.29 | 0.42 | 0.07 |
| ME1 | 176.93 | 1.00 | 135.00 | 29.58 | 1.43 | 0.36 |
| MM2 | 239.82 | 1.00 | 186.00 | 42.59 | 1.39 | 0.50 |
| ML3 | 156.00 | 1.00 | 109.00 | 26.59 | 0.65 | 0.17 |
| Name of strains | CGE1 | CGM2 | CGL3 | CSE1 | CSM2 | CSL3 | ME1 | MM2 | ML3 |
|---|---|---|---|---|---|---|---|---|---|
| S.cerevisiae | 4.9720% | 63.8434% | 86.3965% | 86.2348% | 94.4048% | 96.1841% | 3.4287% | 62.9180% | 90.6234% |
| H.uvarum | 68.9374% | 22.6035% | 3.4897% | 8.6447% | 2.5589% | 1.1376% | 79.5656% | 32.4918% | 6.4225% |
| A.pullulans | 1.1111% | 0.2413% | 0.1962% | 0.2493% | 0.0159% | 0.0955% | 1.2967% | 0.1511% | 0.0796% |
| M.elongata | 0.0345% | 0.0902% | 0.0796% | 0.1034% | 0.1405% | 0.0345% | 0.1671% | 0.0398% | 0.0902% |
| O.brassicae | 0.0159% | 0.0849% | 0.0133% | 0.0451% | 0.1246% | 0.0080% | 0.0053% | 0.0027% | - |
| S.microspora | 0.0027% | 0.0292% | 0.0477% | 0.0345% | 0.0530% | 0.0080% | 0.0080% | 0.0424% | 0.0186% |
| M.alpina | 0.0027% | 0.0265% | 0.0080% | 0.0265% | 0.0212% | 0.0504% | 0.0133% | 0.0796% | 0.0292% |
| H.takashimae | 0.0424% | 0.0186% | 0.0053% | 0.0239% | 0.0027% | 0.0053% | 0.0424% | 0.0027% | 0.0080% |
| P.sp | - | - | - | 0.0186% | 0.0159% | - | - | - | - |
| M.sp | 0.0106% | - | 0.0133% | 0.0133% | 0.0053% | - | 0.0053% | 0.0027% | - |
| D.erythropus | - | 0.0027% | - | 0.0106% | 0.0106% | 0.0027% | 0.0053% | - | 0.0053% |
| G.pullulans | 0.0053% | 0.0106% | 0.0186% | 0.0080% | 0.0106% | 0.0080% | 0.0106% | 0.0133% | 0.0133% |
| OTUs | level | taxonomy |
|---|---|---|
| OTU-1 | species | Saccharomyces cerevisiae |
| OTU-2 | species | Hanseniaspora uvarum |
| OTU-4 | species | Hanseniaspora osmophila |
| OTU-5 | species | Hanseniaspora vineae |
| OTU-48 | species | Pseudaleuria sp |
| OTU-102 | species | Aureobasidium pullulans |
| OTU-119 | species | Fungi sp |
| OTU-122 | species | Mortierella alpina |
| OTU-131 | species | Lasiosphaeriaceae sp |
| OTU-135 | species | Colletotrichum salsolae |
| OTU-136 | species | Chrysosporium synchronum |
| OTU-138 | species | Bipolaris drechsleri |
| OTU-148 | species | Fungi sp |
| OTU-192 | species | Stachybotrys microspora |
| OTU-196 | species | Podospora sp |
| OTU-217 | species | Holtermanniella takashimae |
| OTU-221 | species | Penicillium sp |
| OTU-222 | species | Guehomyces pullulans |
| OTU-241 | species | Gibberella baccata |
| OTU-262 | species | Mortierella elongata |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





