1. Introduction
Energy is one of the primary needs for everyday life and to ensure the progress of human civilization. Depleting fossil fuel resources, emissions of greenhouse gases into the environment and global warming encourage the search for renewable and environmentally friendly alternative energy sources worldwide [
1]. During the anaerobic digestion process, a wide variety of waste can be treated and bioenergy can be produced. Therefore, the industrial development of this technology would be an excellent alternative to fossil fuels [
2].
Various potential raw materials of plant origin are used for bioenergy production in order to reduce dependence on conventional fossil fuels [
3]. Algae are currently widely used as a third-generation renewable feedstock for sustainable biogas production and are a favorable and potentially sustainable source of biomass for the bioenergy industry in the future [
4,
5,
6].
Anaerobic processing of seaweed has been investigated as a positive energy conversion method. The potential and composition of biogas largely depends on its source (type of raw material, organic matter, etc.), fermentation mode (dry or wet) and the temperature maintained in the bioreactor (mesophilic or thermophilic mode). Biogas as an energy source is very useful because it has a high energy balance. 1 Nm
3 of methane can replace 2.1 Kg of wood, 1 L of oil, 1.15 L of gasoline or 1.3 Kg of coal [
7].
Methane gas is considered a clean fuel because it generates almost no soot or other pollutants. However, carbon dioxide (CO
2), which is the non-combustible part of biogas, reduces the calorific value of biogas. If the biogas contains 55% CH
4, its calorific value is 21.5 MJ/Nm
3, while the calorific value of pure CH
4 is about 35.8 MJ/Nm
3, so after removing CO
2 from the raw biogas, it can be called enriched biogas, i.e. biomethane (with high CH
4 content) [
8].
Macroalgae growing in the Sea of Azov play an important role in cleaning the water of the Sea of Azov from pollutants of anthropogenic origin. During storms, large amounts of macroalgae are washed ashore. As a result of coastal pollution by macroalgae, hydrogen sulfide and other pollutants of organic origin are released into the ambient air. Due to high waves, macroalgae that enter the sea form the secondary pollution of sea water. The average depth of the Sea of Azov is only 9 m, which creates favorable conditions for the growth of aquatic plants. It was established that due to excessive amounts of biomass, the ecosystem of the Sea of Azov cannot be cleaned under natural conditions. By rationally using macroalgae aggregates dumped on the coast, it is possible not only to clean the coast of the Sea of Azov, but also to extract energy [
9].
Due to the large amounts of biomass, it is very important to assess the energy potential of macroalgae growing in the Sea of Azov and Lithuanian water bodies. Macroalgae biomass could contribute to two important goals: reducing the effects of eutrophication and using renewable resources for bioenergy production [
4]. Macroalgae have been found to be different from land plants. They are high in nitrogen and sulfur, but low in carbon, hydrogen, and oxygen [
10]. They are high in carbohydrates but low in lignin and cellulose and can therefore be used for biogas production [
11]. Due to their high moisture content, which reaches between 80 and 90%, they are better suited for anaerobic digestion in bioreactors than for other thermal treatments [
9]. Currently, there is insufficient knowledge of how macroalgae improve biogas and methane yields when mixed with co-substrates. The use of macroalgae for energy production is promising due to its elemental composition.
The introduction of substrates with high energy potential can significantly increase the energy balance. Studies have shown that after adding 1 m
3 of co-substrate to sewage sludge, the amount of biogas increased from 18.3 to 32.2 Nm
3 / m
3. This allowed not only to increase the energy demand, but also the organic matter removal efficiency from 64% to 70% [
12]. In order to use macroalgae more efficiently, they can be used together with other organic wastes with high energy potential. The influence of marine macroalgae on the production of biogas is demonstrated by mixing them together with agricultural waste of animal origin - cattle manure [
13,
14]. Cattle manure has been extensively studied when mixed with other organic wastes [
15,
16]. To improve anaerobic digestion, it is recommended to treat macroalgae by crushing them, so that anaerobic microorganisms better absorb the volatile part of the macroalgae [
17]. Adding and acclimatizing inoculants to macroalgae results in a higher biogas yield [
18].
In addition to economic benefits from energy production, the use of macroalgae and manure in an anaerobic process provides additional environmental benefits (e.g., reduced water, soil, air pollution, etc.). Water pollution caused by slurry overflows or rainwater runoff in manure storage can severely affect aquatic life due to intense eutrophication [
19]. Air pollution caused by stored manure includes ammonia emissions and greenhouse gas (GHG) emissions such as carbon dioxide and methane [
15,
16].
The aim of this work is to evaluate the potential of macroalgae growing in the Sea of Azov, and in freshwater bodies, for biogas extraction. During the research, the yield and qualitative composition of biogas were determined during the anaerobic digestion of macroalgae mixed with cattle manure. Additionally, the aim of this work is to evaluate, on the basis of experimental and theoretical methods, the energy potential for biogas production of macroalgae growing in the Sea of Azov and in freshwater bodies.
2. Materials and Methods
2.1. The biomass used for research and its preparation
Macroalgae growing in the Sea of Azov (Ukraine) and in the Šventoji River (Lithuania) were used for this research. Green
Zostera marina (MGMA) and brown
Phaeophyceae sp. macroalgae (MBMA) from the Sea of Azov were collected on the seashore for research purposes. The collected macroalgae were stabilized and transsorted for testing.
Cladophora glomerata macroalgae (FGMA) growing in the Šventoji River were collected when they came to the surface of the water. The macroalgae species
Cladophora glomerata chosen for this research is one of the most widespread and abundant freshwater macroalgae in the world [
20]. Algal research does not require ethical approval. In this research, precise macroalgae are collected in compliance with all requirements. The composition of macroalgae was determined by scientists from Vilnius Gediminas Technical University and Pryazovskyi State Technical University. The macroalgae used for this research were mixed with cattle manure (CM). The cattle manure was collected in Lithuania on an organic cattle farm after the farm was grazed. Manure was transported to the research site in sealed containers. The biomass used for the research is presented in
Figure 1.
In order to evaluate the quality of biogas and the results of biogas yield, before preparing the substrates, the parameters of each raw material used for the anaerobic process (cattle manure, the Sea of Azov, and the Šventoji River) were determined: dry material fraction (TS), volatile solids (VS), carbon (C), nitrogen (N), hydrogen (H), sulfur (S) and oxygen (O
2). Also, in order to theoretically evaluate the energy potential of the macroalgae, the fat, protein, and carbohydrate content were determined. The amount of dry mass and organic load in the samples is presented in
Table 1:
The yield of biogas depends on the elemental composition of the substrate. Compared to other substrates, macroalgae contain more nitrogen and less carbon, so it can be used as a suitable co-substrate for maintaining an optimal C:N ratio. The concentrations of elements C, N, H, O
2 and S in biomass were determined using the elemental composition analyzer EA 3000. The elemental composition of the biomass is presented in
Table 2.
The yield and energy value of biogas depends on the amount of fat, protein, and carbohydrate content in the biomass. In order to theoretically evaluate the energy potential of the biomass, the amount of fat, protein, and carbohydrate content in biomass were determined. The research results are presented in
Table 3.
Cattle manure was used as a catalyst for experimental research. The substrate CM was considered as a control sample. The substrates MGMA, FGMA, and MBMA were prepared without mixing them together. All four substrates were diluted with equal amounts of water. In order to evaluate the influence of macroalgae on the quantity and quality of biogas, the macroalgae and CM were mixed in equal ratios based on dry mass (40 gTS). The cattle manure was mixed with the macroalgae at a ratio of 1:5 based on dry weight. The pH of the substrate was 8.5-8.7.
2.2. Description of the laboratory bench
Periodic two-module anaerobic bioreactors were used for the research. The volume of one bioreactor module is 4.8 L. Scheme of anaerobic bioreactors bench is presented in
Figure 2.
The module is made of organic glass, surrounded by a mat with integrated electric plates, which constantly maintained a temperature of 37 ±1 °C in the bioreactor. A temperature sensor was immersed inside the anaerobic bioreactor, which fed information to a digital display in order to maintain a constant temperature of the substrate. An on-board computer maintained a constant operating temperature of the substrate by supplying electricity to the electrical plates in the mats. The substrate was kept for 28 days in bioreactors, maintaining mesophilic conditions.
In the bioreactors, the substrate was mixed using stirrers. They could adjust the speed. The speed used during the tests was 90 rpm.
The biogas produced during the anaerobic process was stored in separate gas collection tanks. The volume of one calibrated container is 2,000 mL. Every day, at the same time (12h), the amount (mL/d) and composition of the produced biogas were determined.
2.3. Analytical methods used during research
Determination of total solids (TS) and volatile solids (VS) are important indicators on the basis of which it is possible to select a favorable medium for the metabolic processes of microorganisms. Samples of cattle manure and macroalgae from the Sea of Azov and the Sventoji River were placed in laboratory vessels and weighed with an analytical balance (scale error ±0.1 mg). The weighed samples were dried in a drying cabinet at a temperature of 100-105 °C for 4 hours until a constant mass of the sample with the jar was established. During drying, the water was evaporated from the sample and the dry mass of the sample remained. Three repetitions were performed for one sample. The dry mass of the sample was determined according to Equation 1 [
21]:
where
TS is the dry mass of the sample (g);
m0 is the mass of the sample before drying (g); and
mH2O is the mass of water removed from the sample (g).
Since the dry mass of the sample consists of organic and inorganic particles, the amount of organic particles present in the dry mass of the sample was further determined. The amount of nutrients in the substrate was characterized by the amount of organic matter, which depends on the processes of methanogenesis and metabolism taking place in the substrate. After drying the samples in the drying cabinet, the laboratory containers, together with the samples, were placed in the pyrolysis oven. In the furnace, at a temperature of 550 °C, the samples are burned for about 4 hours. The amount of inorganic particles present in the dry mass of the sample was determined by weighing the samples. The amount of organic particles in the material (m
org) was calculated according to Equation 2:
where
VS(g) is the amount of organic particles in the dry weight of the sample (g); m
s is the dry weight of the sample (g); and m
sn is the amount of inorganic particles in the dry weight of the sample (g).
The fraction of organic particles in the burned sample was determined by estimating the mass of the sample before burning and after burning according to equation 3:
where
VS(%) is the fraction of organic particles in the sample from the total mass of the sample before drying (%); m
org is the amount of organic particles in the dry mass of the sample (g); and m
o is the mass of the sample before drying (g).
Before determining the elemental composition of the sample (C, N, H, S, and O2), the sample was dried in a drying cabinet. The dry sample was crushed manually and placed in aluminum capsules (up to a 1 mg sample). An analytical balance was used to determine the exact weight of the filled capsule. The capsules filled with the sample were placed into the cavities in the capsule disc. In order to obtain more accurate test results, three capsules were administered to each sample. Next, the disc with the samples is placed in the combustion chamber of the elemental analyzer EURO EA. The sample and the aluminum capsule reacted with oxygen and burned at a temperature of 1,000 °C, as a result of which the sample was split into components (carbon, hydrogen, sulphur, nitrogen, and oxygen). Sulfur was not detected by the EURO EA analyzer, so the LST EN 15510:2017 method was used to determine the sulfuric content.
The content of fat, protein, and carbohydrates in the macroalgae and cattle manure was determined based on directives 71/393/EEC, 72/199/EEC, and 71/250/EEC.
The concentrations of compounds CH4, CO2, O2, and H2S present in the biogas were determined with a gas composition analyzer of the GasData series GFM 410. The error of the device is ±1%.
The pH of the substrate was determined using a Mettler Toledo MultiSeven meter, device error ±0.002.
The amount of biogas produced per day was calculated according to the following formula [
22]:
where
V0 dr is the volume of dry biogas under normal conditions (L);
V is the volume of biogas measured in the biogas storage tank (ml);
P is the biogas pressure measured in the biogas storage tank (hPa);
PW is the water vapor pressure dependent on the ambient temperature (hPa);
T0 is the normal temperature (
T0 = 273 K);
p0 is the normal pressure (
p0 = 1013hPa); and
T is the temperature of the biogas released during fermentation (K).
An honest significant difference test was used to examine the significance of differences between the analyzed variables. Statistical significance was determined at the p < 0.05 level. The Microsoft Office Excel 2019 program was used for the statistical calculations. The arithmetic averages of the measurements and experimental arithmetic variances, and the standard deviations of the arithmetic mean, were calculated using the following statistical functions of Microsoft Office Excel 2019: AVERAGE, VARP and STDEV. The measurements were repeated three times.
2.5. Energy value of biogas and energy potential of biomass
The energy potential of biomass for anaerobic processing is calculated based on the content of fat, protein, and carbohydrates in it. Considering the components of biomass organic matter, it is possible to determine the yield of biogas from treated biomass. The average theoretical amounts of biogas and methane produced can be calculated based on the results obtained during experimental studies [
23].
In order to assess the reliability of the results, the theoretical amount of biogas, the concentration of methane, and the energy value of the biogas were calculated, and the obtained values were compared with the values obtained during the experiment. Theoretically, these three parameters were calculated according to the formulas below [
23].
Amount of biogas derived from biologically degradable carbohydrates, proteins, and fats:
where
is the amount of biogas produced from the biodegradation of carbohydrates, proteins, and fats (m
3); 0.75 is average stoichiometric yield of biogas per kilogram of dry mass of carbohydrates, proteins, and fats;
is the amount of dry biomass (Kg); and
is the carbohydrate, protein, and fat content in the biomass, as a percentage of dry mass (%).
The amount of methane in biogas also depends on the amount of carbohydrates, proteins, and fats present in the biomass. The amount of methane in the released biogas can be calculated according to the equation 6:
where
is the concentration of methane in the biogas (%); 55, 77, and 85 are the average amounts of methane in the biogas from carbohydrates, proteins, and fats, respectively;
is the carbohydrate content in the biomass as a percentage of dry mass;
is the protein content in the biomass as a percentage of dry mass; and
is the fat content in the biomass as a percentage of dry mass.
By knowing the amount of methane formed in the biogas, its energy value can be calculated. The calorific value of methane is 21,500 KJ/Kg [
23]. The energy value of the emitted biogas was calculated according to the formula:
where
E is the energy value of the biogas (MJ/m
3);
is the calorific value of methane (35.8 MJ/m
3); and
is the concentration of methane in the biogas (%).
The energy potential of the biomass was calculated according to the formula:
where
Ep is the biomass energy potential (MJ/Kg);
is the biogas yield (m
3/Kg);
is the energy value of the biogas (MJ/m
3).
After performing calculations of biomass energy potential, the amount of energy obtained by the anaerobic treatment of CM with different microalgae was determined. The amount of energy was calculated according to formula 9 [
17]:
where
BS is the amount of energy obtained from 1 m
3 of biogas (kWh/m
3) and CH4 is the concentration of methane in the biogas (%).
Theoretical calculations of the energy value of the biogas and the energy potential of the biomass were performed based on the concentration of carbohydrates, proteins, and fats determined in the biomass. The energy value of the biogas and the energy potential of the biomass were determined based on the determinations of biogas and methane yield obtained during experimental studies.
4. Discussion
Depending on weather conditions and seasonality, up to 95% of drifting aquatic grasses and macroalgae reach the shores of marine regions. For example, brown and red macroalgae species are mostly found in the Baltic Sea region [
24]. Due to large amounts of macroalgae, the ecosystem is not able to clean itself, so the use of macroalgae for biogas production has a high energy potential. The maximum growth potential of macroalgae in marine regions depends on direct sunlight, water temperature, salinity and concentration of nutrients [
25]. Two species of macroalgae, such as Fucus vesiculosus and Fucus serratus, which have a high energy potential, dominate in the southwest of Sweden in the Baltic Sea [
26].
Theoretical and experimental studies have shown that mixing macroalgae with other organic substrates resulted in higher biogas and methane yields. In scientific works, in order to obtain a higher methane yield, macroalgae were mixed with other substrates, such as chicken manure or fruit waste. Studies have confirmed that macroalgae can be used successfully for methane production due to a better ratio of carbon to nitrogen. The anaerobic digestion of macroalgae also reduces nitrogen toxicity. Therefore, the microorganisms involved in the fermentation process see an improvement in the release of methane. Although macroalgae do not contain lignin, they are rich in carbohydrates and proteins, which are essential raw food materials for microorganisms [
27,
28].
Each plant or other raw material has its own composition of organic matter. For anaerobic digestion, biomass is evaluated based on fat, protein, and carbohydrate content. Different amounts of these elements lead to differing amounts of biogas and resulting methane concentration. If the substrate contains more carbohydrates, the biogas production process is faster because carbohydrates break down faster, in which case the methane concentration can reach 50%-60%. However, if the raw material contains more fat or protein, biogas production slows down while methane concentration is higher [
29]. The macroalgae used in this study increased the carbohydrate and protein content of the cattle manure, thereby contributing to higher methane yields.
Cattle manure is a very useful raw material at the beginning of anaerobic treatment because it already contains the necessary methanogenic bacteria. Later, the fermentation of manure as a substrate reduces methane production due to low anaerobic biodegradability, which is around 45%-50% [
29]. High water content in manure (70%-90%), however, has a positive effect on the stability of the anaerobic process [
30].
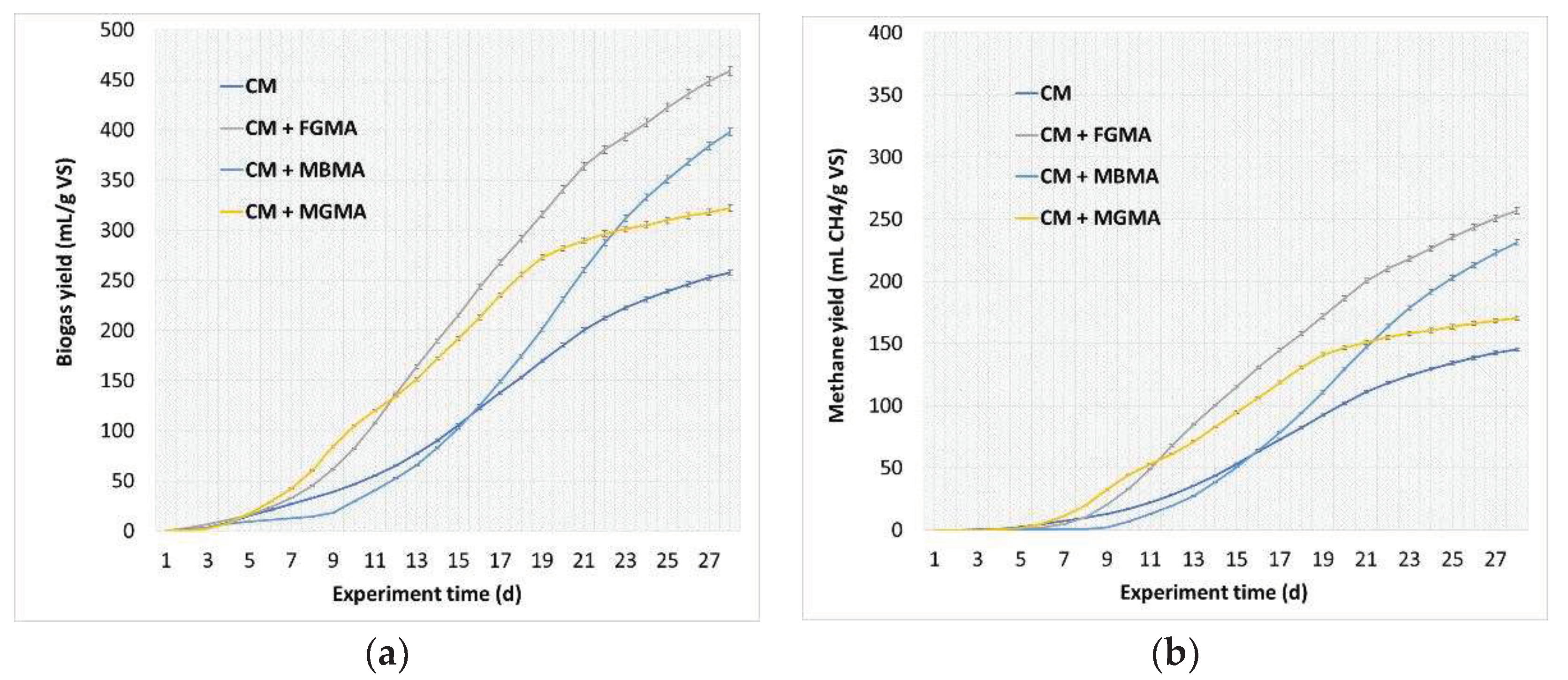
The research conducted in this work showed satisfactory biogas and methane yields when cattle manure was mixed with freshwater and Azov Sea macroalgae. Methane yield reached 170.7–256.9 mLCH4/g VS. Similar results were obtained by researchers Nielsen and Heiske. They conducted research with four species of macroalgae to evaluate their potential for biogas production. Before the tests, the algae were gently washed with water to remove sand and gravel, and then were treated in two ways. Algae were kept in flat-hatches where thermophilic conditions were maintained at 53 °C for 34 days. Algal homogenization resulted in a significant increase in methane yield in one macroalgal species (
U. lactuca), where methane yield increased from 152 mL gVS to 255 mL gVS. Lower methane yields were found when processing other species. The anaerobic digestion of
S. latissima macroalgae even reduced the methane yield from 340 mL gVS to 333 mL/gVS. The reason for this deviation is related to the biological composition of different algae and the biological conversion of the natural form. The cell walls of the
U. lactuca species are rich in dietary fiber (indigestible polysaccharides) that inhibit the uptake of nutrients by microbes. Therefore, homogenization facilitates algal degradation. A methane yield of 132 mL/gVS was determined by the anaerobic digestion of
Gracillaria vermiculophylla macroalgae [
31]. In comparison, Montingelli et al. [
32] conducted studies where they achieved methane yields of 169 mL/g VS and 240 mL/g VS methane by treating
Ascophyllum nodosum and
Laminaria sps.
Both theoretical and experimental studies have shown that the type of macroalgae has a significant effect on methane yield. The methane yield derived from different macroalgae cultures is presented in
Table 5.
During the anaerobic digestion of Cladophora glomerata macroalgae, the methane yield was 256.9 mLCH4/g VS. The anaerobic digestion of Zostera marina macroalgae achieved a lower methane yield of 170.7 mLCH4/g VS. Studies conducted by researchers have shown that methane yield is highly dependent on the type of macroalgae culture, the temperature maintained, and hydraulic residence time (HRT).
Depending on the type of macroalgae, the yield of methane varied. The methane yield from the anaerobic digestion of
Ulva sp.,
Ascophyllum sp., and
Laminaria sp. was between 110 L CH
4/Kg VS and 280 L CH
4/Kg VS [
39].
Akila et al. examined the effects of various concentrations of cattle manure mixed with the marine macroalgae
Ulva sp. The highest biogas yield occurred after 60 days and was found to be 574 ±26 mL/g VS when
Ulva sp. was mixed with cow manure at a ratio of 3:1. A biogas yield of 195 ±32 mL/g VS was also determined when the mixing ratio was 1:1. Experimental studies were conducted in mesophilic conditions at a temperature of 35 °C with pH levels of 7.5 ±0.2 [
13].
Stable relative values of methane and carbon monoxide were determined during the research, so we can assume that anaerobic digestion was stable. Any deviation may indicate process instability. The temperature, pH level, and pressure of the substrate all influence the ratio of methane to carbon dioxide in the biogas. Since CO
2 reduction is pH-dependent, pH instability affects changes in biogas composition [
40].
The calorific value of biogas has been extensively studied. Research has shown that the calorific value of biogas during the anaerobic processing of cattle manure was 19.50–22.62 MJ/m
3 [
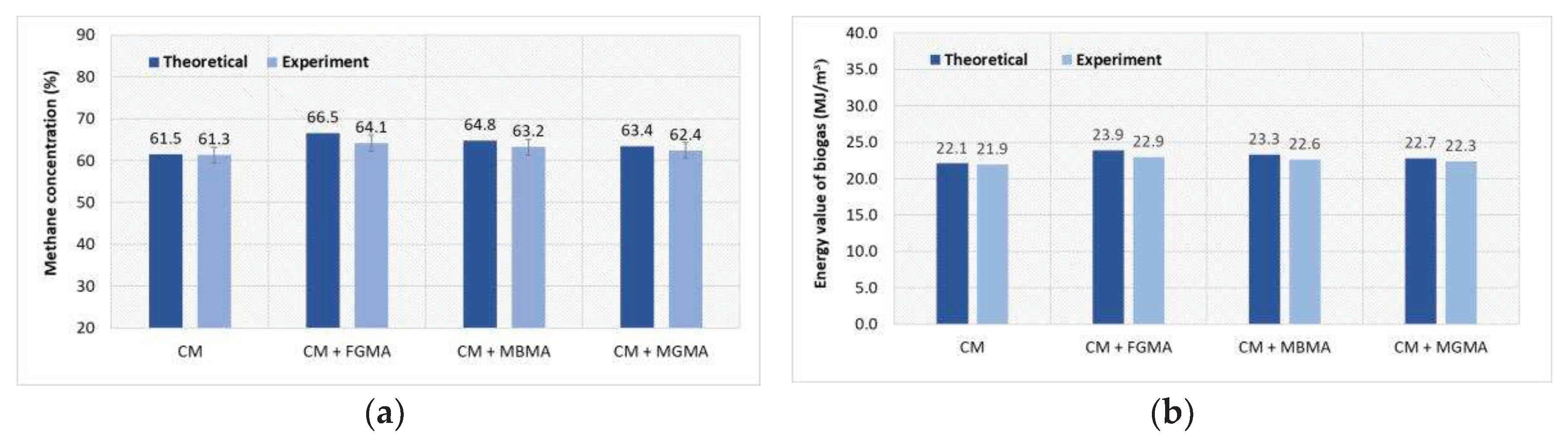
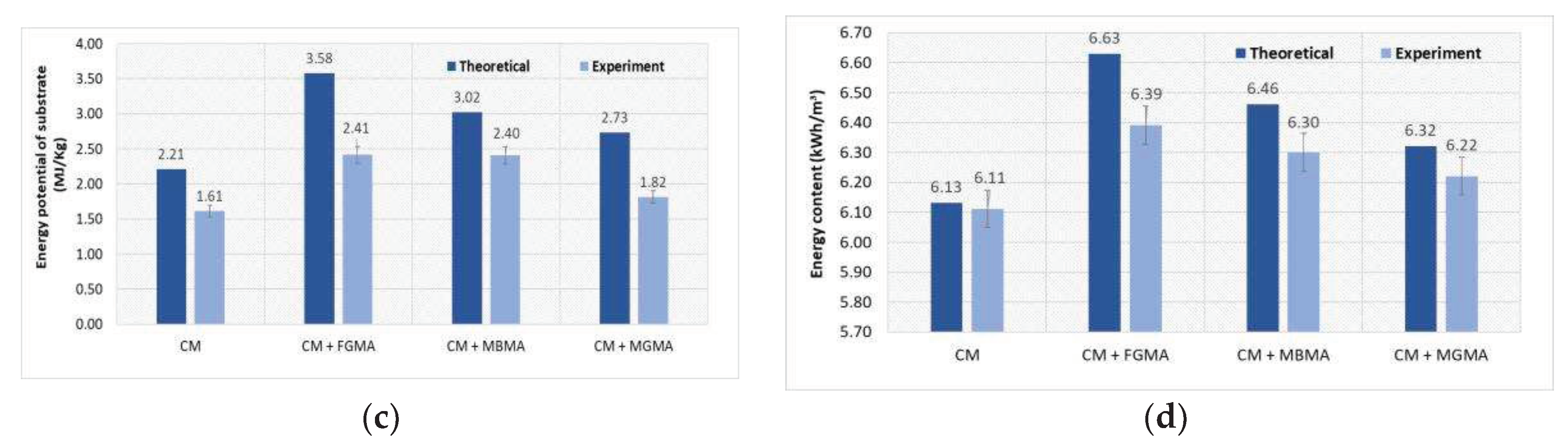
41]. Similar test results for the anaerobic treatment of CM were obtained in this study. During anaerobic treatment, the energy value of CM biogas was 21.90 MJ/m
3. By mixing CM with macroalgae, the energy value of the biogas increased to 22.90 MJ/m
3. Other authors also obtained similar results. By mixing cow manure with
Spirogyra varians macroalgae, the energy value of the biogas increased from 23.40 MJ/m
3 to 24.94 MJ/m
3. Conducted theoretical and experimental studies confirmed that the biogas obtained by mixing
Cladophora glomerata and
Zostera marina macroalgae with cattle manure was suitable for energy production and had an energy value necessary for combustion. The conducted studies of the energy potential of the biogas showed that from 1.82 MJ to 2.41 MJ of energy can be extracted from a mixture of 1 Kg of cattle manure and macroalgae. The obtained positive methane yield results showed that the use of macroalgae for biogas production is promising and can significantly contribute to energy production from sustainable, renewable energy sources. Biogas production using macroalgae is very relevant in regions where large amounts of macroalgae are produced. The energy value of biogas could be further increased by thermal treatment or by adding additional additives. After secondary treatment, the methane concentration in the biogas can be increased to 5% [
34].
The thermal properties of biogas are similar to those of other types of fuel. Therefore, they can be used as alternative fuels [
42]. Theoretical and experimental studies have shown that mixing CM with FGMA, MBMA, and MGMA increases the energy value of biogas. The values of the energy equivalent of the substrate CM are given in
Table 6.
Compared to fossil fuels, the energy potential of biogas is lower. However, they have a higher energy value than firewood or coal. As can be seen from the data presented in the table, fossil fuels can be replaced by alternative natural resources. Biogas produced from CM and FGMA, MBMA, and MGMA substrates is less polluting compared to fossil fuel. Therefore, it can significantly contribute to reducing climate change and improving the quality of life.
Author Contributions
Conceptualization, A.Z. and V.M.; methodology, A.Z.; validation, A.G., A.Z.; formal analysis, V.M.; investigation, A.G.; resources, A.Z., V.M.; data curation, A.Z.; writing—original draft preparation, A.Z.; writing—review and editing, V.M.; visualization, A.G.; supervision, A.Z.; project administration, A.Z.; funding acquisition, A.Z. All authors have read and agreed to the published version of the manuscript.
Figure 1.
The macroalgae used for the study: (a) Zostera marina macroalgae; (b) Cladophora glomerata macroalgae; (c) Phaeophyceae sp. macroalgae.
Figure 1.
The macroalgae used for the study: (a) Zostera marina macroalgae; (b) Cladophora glomerata macroalgae; (c) Phaeophyceae sp. macroalgae.
Figure 2.
Scheme of anaerobic bioreactors bench.
Figure 2.
Scheme of anaerobic bioreactors bench.
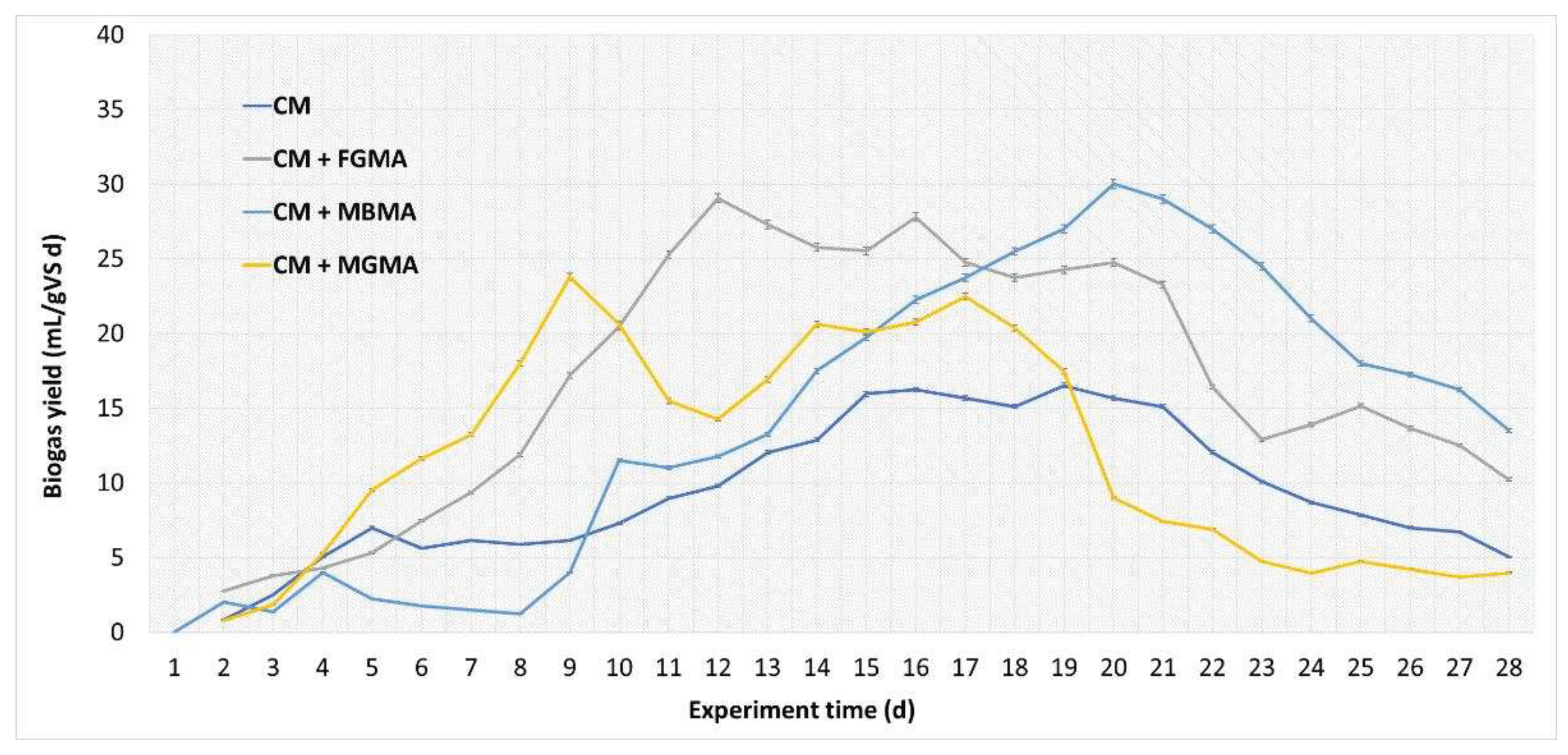
Figure 3.
Biogas yield per day during anaerobic digestion of cattle manure with different macroalgae cultures (FGMA, MBMA, MGMA). Values correspond to means of three replicates of independent values ± standard deviations. All values are significantly at p < 0.05 level.
Figure 3.
Biogas yield per day during anaerobic digestion of cattle manure with different macroalgae cultures (FGMA, MBMA, MGMA). Values correspond to means of three replicates of independent values ± standard deviations. All values are significantly at p < 0.05 level.
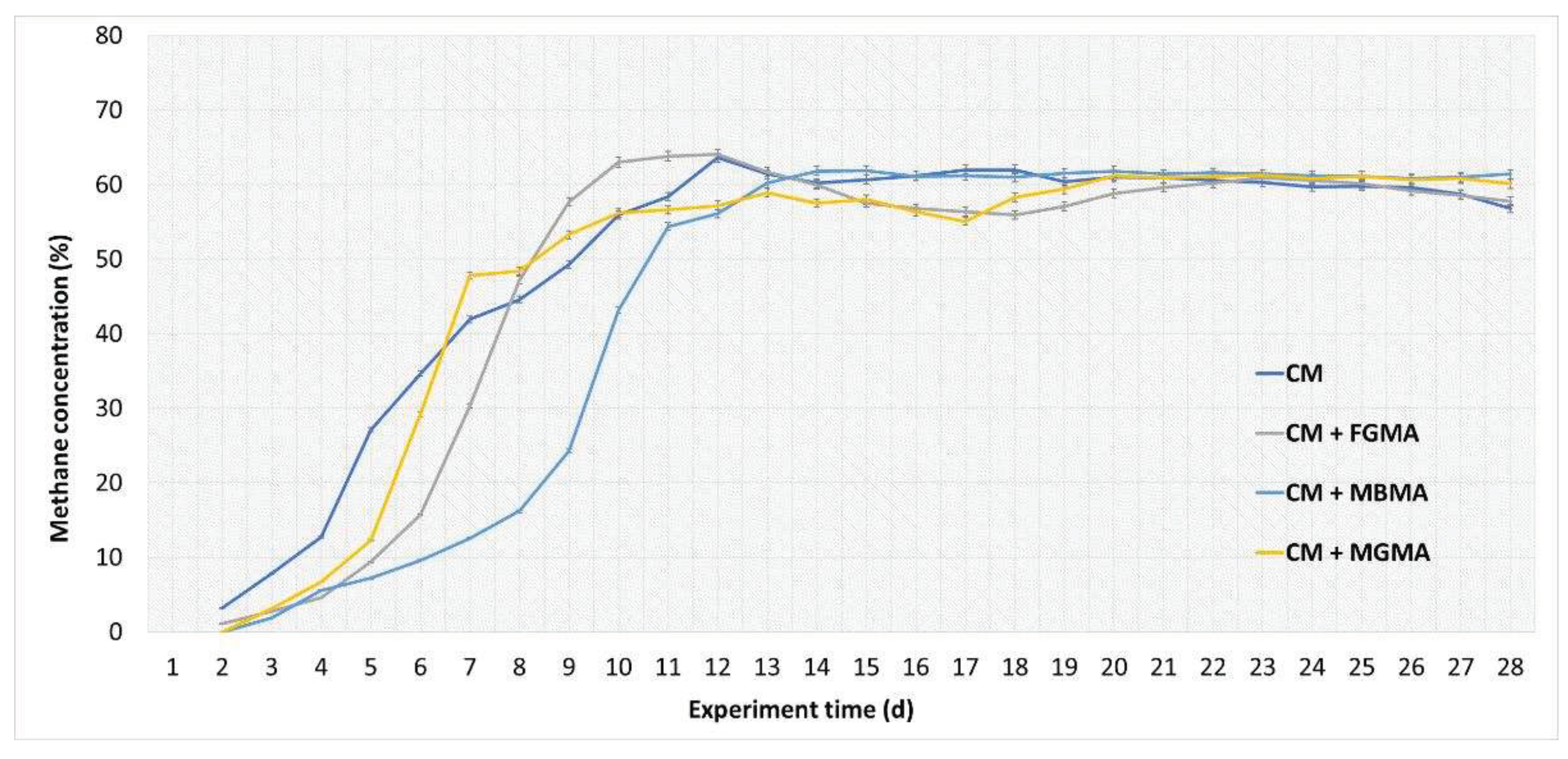
Figure 4.
Methane concentration in biogas during anaerobic digestion of cattle manure with different macroalgae cultures (FGMA, MBMA, MGMA). Values correspond to means of three replicates of independent values ± standard deviations. All values are significantly at p < 0.05 level.
Figure 4.
Methane concentration in biogas during anaerobic digestion of cattle manure with different macroalgae cultures (FGMA, MBMA, MGMA). Values correspond to means of three replicates of independent values ± standard deviations. All values are significantly at p < 0.05 level.
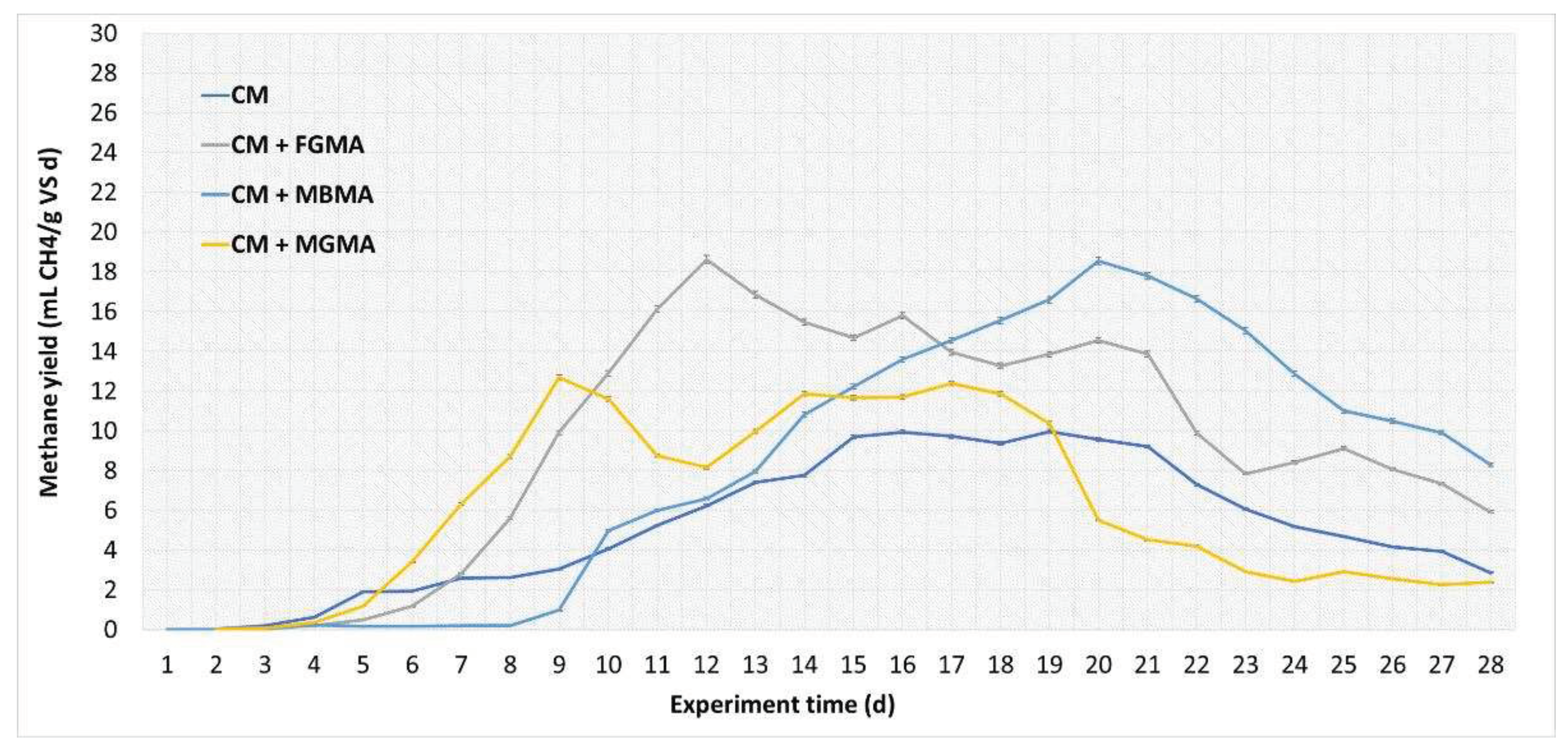
Figure 5.
Methane yield per day during the anaerobic digestion of cattle manure with different macroalgae cultures (FGMA, MBMA, MGMA). Values correspond to means of three replicates of independent values ± standard deviations. All values are significantly at p < 0.05 level.
Figure 5.
Methane yield per day during the anaerobic digestion of cattle manure with different macroalgae cultures (FGMA, MBMA, MGMA). Values correspond to means of three replicates of independent values ± standard deviations. All values are significantly at p < 0.05 level.
Figure 6.
Total biogas and methane yields during the anaerobic digestion of cattle manure with different macroalgae cultures (FGMA, MBMA, MGMA): (a) Total biogas yield during the 28 days of the experiment; (b) Total methane yield over the 28 days of the experiment. Values correspond to means of three replicates of independent values ± standard deviations. All values are significantly at p < 0.05 level.
Figure 6.
Total biogas and methane yields during the anaerobic digestion of cattle manure with different macroalgae cultures (FGMA, MBMA, MGMA): (a) Total biogas yield during the 28 days of the experiment; (b) Total methane yield over the 28 days of the experiment. Values correspond to means of three replicates of independent values ± standard deviations. All values are significantly at p < 0.05 level.
Figure 7.
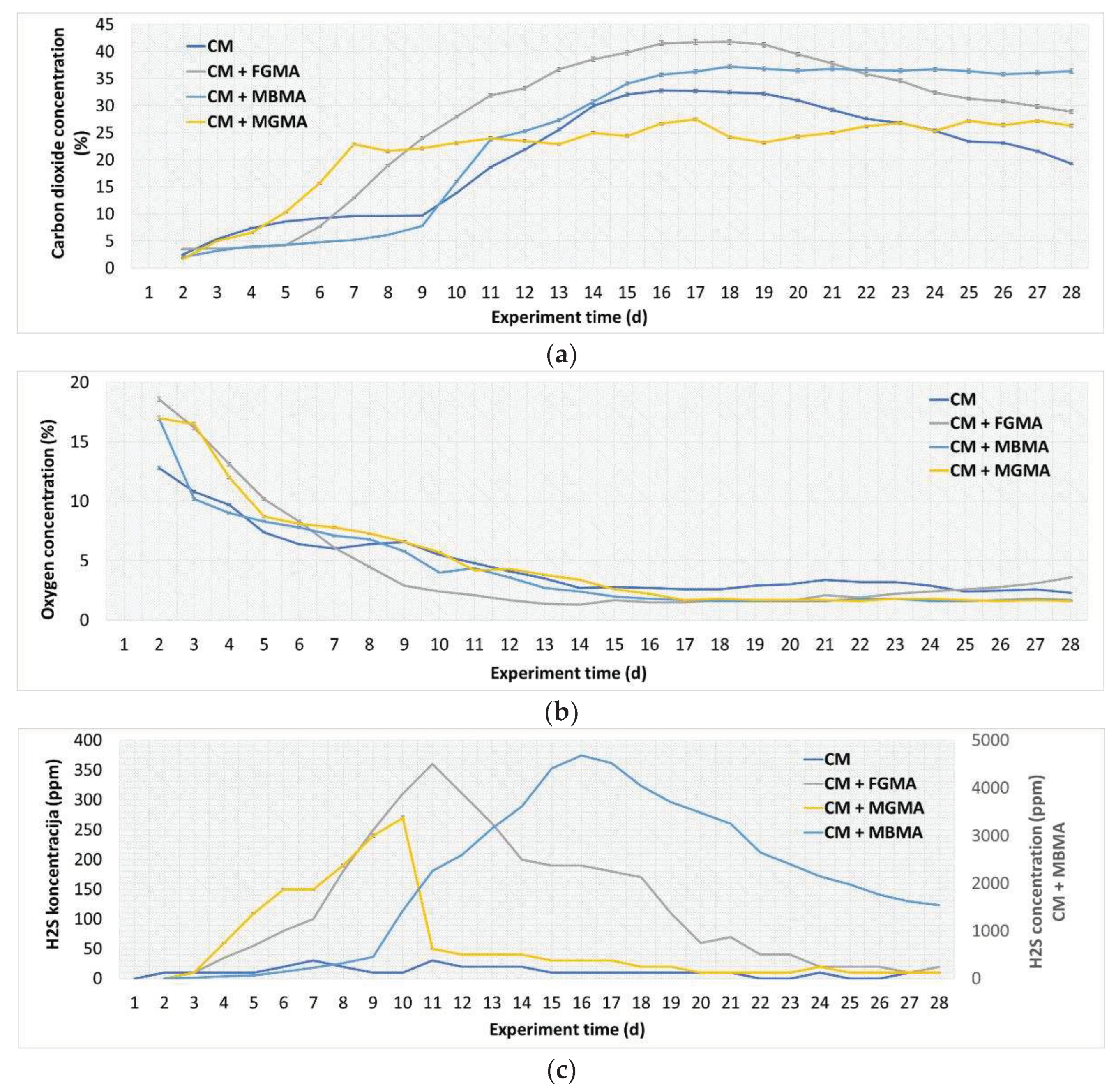
Biogas composition from the anaerobic digestion of cattle manure with different macroalgae cultures (FGMA, MBMA, MGMA): (a) Carbon dioxide (CO2) concentration; (b) Oxygen (O2) concentration; (c) Hydrogen sulfide (H2S) concentration. Values correspond to means of three replicates of independent values ± standard deviations. All values are significant at p < 0.05 level.
Figure 7.
Biogas composition from the anaerobic digestion of cattle manure with different macroalgae cultures (FGMA, MBMA, MGMA): (a) Carbon dioxide (CO2) concentration; (b) Oxygen (O2) concentration; (c) Hydrogen sulfide (H2S) concentration. Values correspond to means of three replicates of independent values ± standard deviations. All values are significant at p < 0.05 level.
Figure 8.
Comparison of the results obtained during the theoretical and experimental study to assess the energy potential when anaerobically digesting CM with different macroalgae cultures (FGMA, MBMA, MGMA): (a) Dependence of methane concentration on different substrates; (b) Dependence of energy value on different substrates; (c) Dependence of energy potential on different substrates; (d) Dependence of energy content on different substrates.
Figure 8.
Comparison of the results obtained during the theoretical and experimental study to assess the energy potential when anaerobically digesting CM with different macroalgae cultures (FGMA, MBMA, MGMA): (a) Dependence of methane concentration on different substrates; (b) Dependence of energy value on different substrates; (c) Dependence of energy potential on different substrates; (d) Dependence of energy content on different substrates.
Table 1.
Dry weight content and organic load in the samples.
Table 1.
Dry weight content and organic load in the samples.
| Biomass |
TS,
g TS/g |
VS,
g VS/g TS |
| CM |
0.28 ± 0.04 |
0.70 ± 0.05 |
| MBMA |
0.86 ± 0.05 |
0.79 ± 0.04 |
| FGMA |
0.53 ± 0.04 |
0.62 ± 0.06 |
| MGMA |
0.86 ± 0.04 |
0.58 ± 0.04 |
Table 2.
Elemental composition of the biomass. .
Table 2.
Elemental composition of the biomass. .
| Biomass |
C, % |
N, % |
H, % |
O2, % |
S, mg/Kg |
C:N |
| CM |
31.55 ± 0.21 |
2.41 ± 0.31 |
5.31 ± 0.08 |
51.5 ± 0.4 |
3.4 ± 0.4 |
13:1 |
| MBMA |
44.75 ± 0.32 |
1.44 ± 0.42 |
5.42 ± 0.09 |
13.4 ± 0.4 |
25.6 ± 0.4 |
31:1 |
| FGMA |
33.05 ± 0.20 |
1.05 ± 0.31 |
- |
32.1 ± 0.4 |
11.9 ± 0.4 |
31:1 |
| MGMA |
49.28 ± 0.40 |
1.48 ± 0.33 |
7.01 ± 0.10 |
41.5 ± 0.4 |
5.7 ± 0.4 |
33:1 |
Table 3.
The amount of fat, protein, and carbohydrate content in the dry mass of raw materials.
Table 3.
The amount of fat, protein, and carbohydrate content in the dry mass of raw materials.
| Method |
Directive
71/393/EEB |
Directive
72/199/EEB |
Directive
71/250/EEB |
| Biomass |
Fats,
% TS |
Proteins,
% TS |
Carbohydrates,
% TS |
| CM |
0.55 ± 0.04 |
15.25 ± 0.21 |
31.00 ± 0.08 |
| MGMA |
0.48 ± 0.05 |
13.38 ± 0.24 |
31.05 ± 0.07 |
| FGMA |
0.36 ± 0.03 |
17.06 ± 0.10 |
47.45 ± 0.06 |
| MBMA |
0.46 ± 0.02 |
13.35 ± 0.09 |
30.26 ± 0.05 |
Table 4.
General physicochemical properties of the obtained biogas.
Table 4.
General physicochemical properties of the obtained biogas.
| Biomass |
γ, Kg/m3
|
T, ºC |
H2O, % |
CH4, % |
CO2, % |
O2, % |
H2S, ppm |
Energy value, MJ/m3
|
| CM + FGMA |
1.20 |
20.0 ± 0.1 |
6.0 ± 0.2 |
59.6–66.5 |
28.9–34.6 |
2.2–3.6 |
20–40 |
22.9 |
| CM + MBMA |
61.0–63.2 |
35.8–36.7 |
1.6–1.8 |
1540–2400 |
22.6 |
| CM + MGMA |
58.0–62.4 |
24.4–27.5 |
1.6–2.6 |
10–30 |
22.3 |
Table 5.
Methane yield using different macroalgae cultures.
Table 5.
Methane yield using different macroalgae cultures.
| Macroalgae |
Temperature of digestion, ºC |
HRT, d |
Methane yield, mLCH4/g VS |
Reference |
| Cladophora glomerata |
37.0 |
28 |
256.9 |
This study |
| Zostera marina |
37.0 |
28 |
170.7 |
This study |
| Phaeophyceae sp. |
37.0 |
28 |
231.4 |
This study |
| Laminaria sp. |
35.0 |
22 |
139.0 |
[33] |
| Spirogyra varians |
20.0 |
70 |
340.0 |
[34] |
| P. canaliculata |
37.0 |
21 |
340.0 |
[35] |
| F. vesiculosus |
37.0 |
25 |
134.0 |
[36] |
| Laminaria sp. |
25.0 |
38 |
244.0 |
[17] |
| Fucus vesiculosus |
37.0 |
20 |
113.0 |
[37] |
| Ascophyllum nodosum |
38.0 |
14 |
169.0 |
[38] |
Table 6.
Substrate CM and FGMA, MBMA, MGMA energy equivalent.
Table 6.
Substrate CM and FGMA, MBMA, MGMA energy equivalent.
| Fuel |
Biogas
equivalent 1
|
CM + FGMA, Kg |
CM +
MBMA, Kg |
CM +
MGMA, Kg |
| 1 Kg of firewood |
0.29 m3
|
2.64 |
2.64 |
3.63 |
| 1 Kg charcoal |
0.50 m3
|
4.55 |
4.55 |
6.25 |
| 1 m3 of natural gas |
1.43 m3
|
13.00 |
13.00 |
17.88 |
| 1 Kg of petrol |
2.50 m3
|
22.73 |
22.73 |
31.25 |
| 1 Kg of fuel oil |
1.42 m3
|
12.91 |
12.91 |
17.75 |
| 1 Kg of carbon |
2.33 m3
|
21.18 |
21.18 |
29.13 |