Submitted:
07 September 2023
Posted:
11 September 2023
You are already at the latest version
Abstract
Keywords:
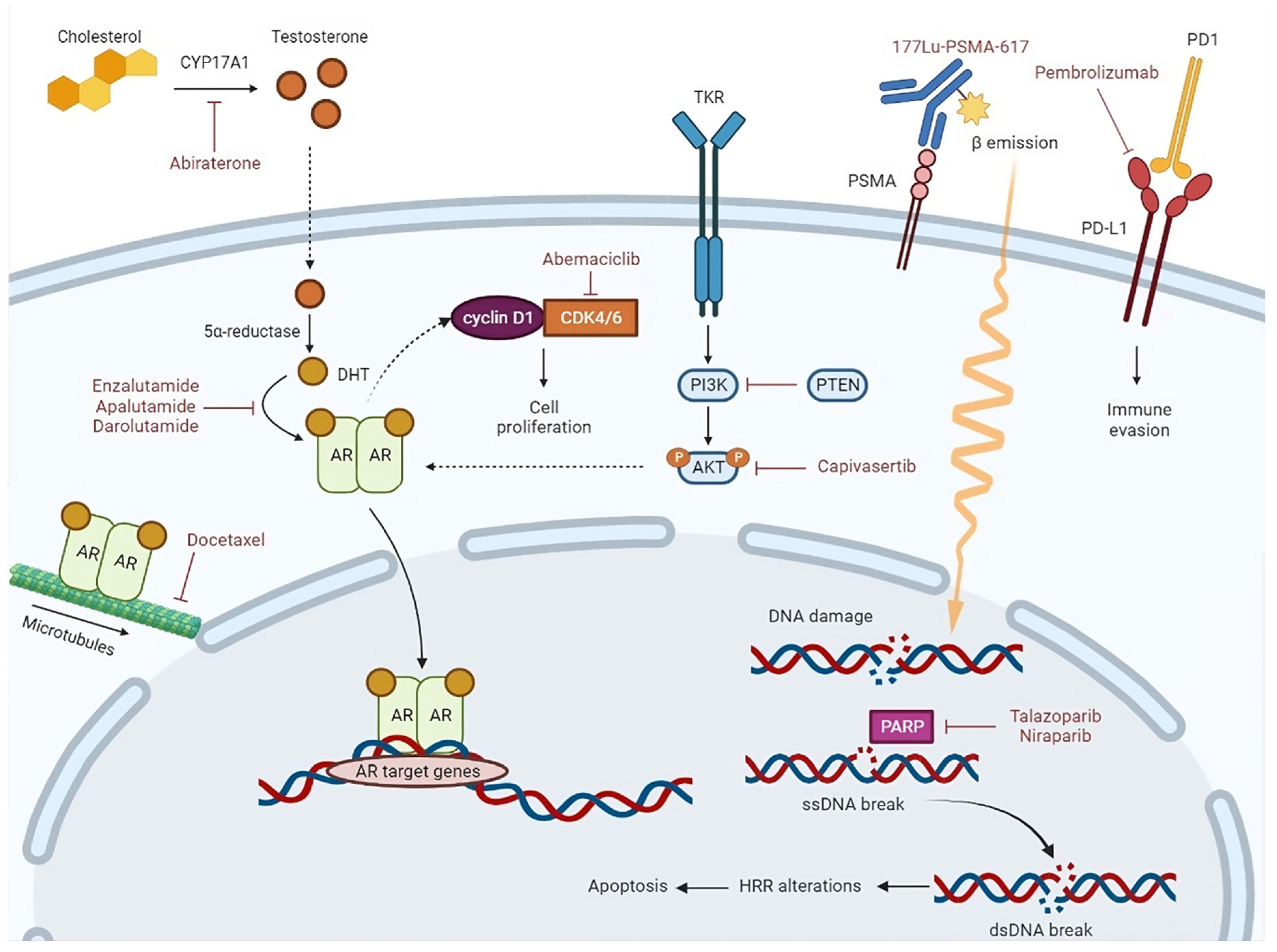
1. Introduction
2. Current therapeutic opportunities in de novo mHSPC
2.1. Doublet therapy
2.1.1. Docetaxel plus ADT
2.1.2. Abiraterone plus ADT
2.1.3. Enzalutamide plus ADT
2.1.3. Apalutamide plus ADT
2.2. Triplet therapy
2.3. Oligometastatic prostate cancer
3. Genomic features of mHSPC
3.1. The role of liquid biopsy
3.2. Prognostic information
4. Ongoing phase III clinical trials testing new therapeutic approaches in mHSPC
4.1. Immunotherapy
4.2. Radiopharmaceuticals
4.3. Molecular target agents
4.3.1. CDK4/6 inhibitors
4.3.2. PARP inhibitors
4.3.3. AKT inhibitors
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Prostate – Global Cancer Observatory. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/27-Prostate-fact-sheet.pdf (accessed on 7 August 2023).
- Helgstrand, J.T.; Røder, M.A.; Klemann, N.; Toft, B.G.; Lichtensztajn, D.Y.; Brooks, J.D.; Brasso, K.; Vainer, B.; Iversen, P. Trends in incidence and 5-year mortality in men with newly diagnosed, metastatic prostate cancer – A population-based analysis of 2 national cohorts. Cancer 2018, 124, 2931–2938. [Google Scholar] [CrossRef] [PubMed]
- Buzzoni, C.; Auvinen, A.; Roobol, M.J.; Carlsson, S.; Moss, S.M.; Puliti, D.; de Koning, H.J.; Bangma, C.H.; Denis, L.J.; Kwiatkowski, M.; et al. Metastatic prostate cancer incidence and prostate-specific antigen testing: new insights from the European Randomized Study of Screening for Prostate Cancer. Eur Urol 2015, 68, 885–890. [Google Scholar] [CrossRef] [PubMed]
- Weiner, A.B.; Matulewicz, R.S.; Eggener, S.E.; Schaeffer, E.M. Increasing incidence of metastatic prostate cancer in the United States (2004–2013). Prostate Cancer Prostat Dis 2016, 19, 395–397. [Google Scholar] [CrossRef]
- Hu, J.C.; Nguyen, P.; Mao, J.; Halpern, J.; Shoag, J.; Wright, J.D.; Sedrakyan, A. Increase in Prostate Cancer Distant Metastases at Diagnosis in the United States. JAMA Oncol 2017, 3, 705–707. [Google Scholar] [CrossRef] [PubMed]
- Perera, M.; Papa, N.; Roberts, M.; Williams, M.; Udovicich, C.; Vela, I.; Christidis, D.; Bolton, D.; Hofman, M.S.; Lawrentschuk, N.; et al. Gallium-68 Prostate-specific Membrane Antigen Positron Emission Tomography in Advanced Prostate Cancer-Updated Diagnostic Utility, Sensitivity, Specificity, and Distribution of Prostate-specific Membrane Antigen-avid Lesions: A Systematic Review and Meta-analysis. Eur Urol 2020, 77, 403–417. [Google Scholar] [CrossRef]
- Finianos, A.; Gupta, K.; Clark, B.; Simmens, S.J.; Aragon-Ching, J.B. Characterization of Differences Between Prostate Cancer Patients Presenting With De Novo Versus Primary Progressive Metastatic Disease. Clin Genitourin Cancer 2017, S1558-7673, 30247–1. [Google Scholar] [CrossRef]
- Sweeney, C.J.; Chen, Y.H.; Carducci, M.; Liu, G.; Jarrard, D.F.; Eisenberger, M.; Wong, Y.N.; Hahn, N.; Kohli, M.; Cooney, M.M.; et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med 2015, 373, 737–746. [Google Scholar] [CrossRef]
- James, N.D.; Sydes, M.R.; Clarke, N.W.; Mason, M.D.; Dearnaley, D.P.; Spears, M.R.; Ritchie, A.W.; Parker, C.C.; Russell, J.M.; Attard, G.; et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet 2016, 387, 1163–1177. [Google Scholar] [CrossRef]
- Fizazi, K.; Tran, N.; Fein, L.; Matsubara, N.; Rodriguez-Antolin, A.; Alekseev, B.Y.; Özgüroğlu, M.; Ye, D.; Feyerabend, S.; Protheroe, A.; et al. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med 2017, 377, 352–360. [Google Scholar] [CrossRef]
- James, N.D.; de Bono, J.S.; Spears, M.R.; Clarke, N.W.; Mason, M.D.; Dearnaley, D.P.; Ritchie, A.W.S.; Amos, C.L.; Gilson, C.; Jones, R.J.; et al. Abiraterone for prostate cancer not previously treated with hormone therapy. N Engl J Med 2017, 377, 338–351. [Google Scholar] [CrossRef]
- Davis, I.D.; Martin, A.J.; Stockler, M.R.; Begbie, S.; Chi, K.N.; Chowdhury, S.; Coskinas, X.; Frydenberg, M.; Hague, W.E.; Horvath, L.G.; et al. Enzalutamide with standard first-line therapy in metastatic prostate cancer. N Engl J Med 2019, 381, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.J.; Szmulewitz, R.Z.; Petrylak, D.P.; Holzbeierlein, J.; Villers, A.; Azad, A.; Alcaraz, A.; Alekseev, B.; Iguchi, T.; Shore, N.D.; et al. ARCHES: a randomized, phase III study of androgen deprivation therapy with enzalutamide or placebo in men with metastatic hormone-sensitive prostate cancer. J Clin Oncol 2019, 37, 2974–2986. [Google Scholar] [CrossRef] [PubMed]
- Chi, K.N.; Agarwal, N.; Bjartell, A.; Chung, B.H.; Pereira de Santana Gomes, A.J.; Given, R.; Juárez Soto, Á.; Merseburger, A.S.; Özgüroğlu, M.; Uemura, H.; et al. Apalutamide for metastatic, castration-sensitive prostate cancer. N Engl J Med 2019, 381, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Kyriakopoulos, C.E.; Chen, Y.H.; Carducci, M.A.; Liu, G.; Jarrard, D.F.; Hahn, N.M.; Shevrin, D.H.; Dreicer, R.; Hussain, M.; Eisenberger, M.; et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer: long-term survival analysis of the randomized phase III E3805 CHAARTED trial. J Clin Oncol 2018, 36, 1080–1087. [Google Scholar] [CrossRef] [PubMed]
- Hamid, A.A.; Huang, H.C.; Wang, V.; Chen, Y.H.; Feng, F.; Den, R.; Attard, G.; Van Allen, E.M.; Tran, P.T.; Spratt, D.E.; et al. Transcriptional profiling of primary prostate tumor in metastatic hormone-sensitive prostate cancer and association with clinical outcomes: correlative analysis of the E3805 CHAARTED trial. Ann Oncol 2021, 32, 1157–1166. [Google Scholar] [CrossRef]
- Spratt, D.E.; Yousefi, K.; Deheshi, S.; Ross, A.E.; Den, R.B.; Schaeffer, E.M.; Trock, B.J.; Zhang, J.; Glass, A.G.; Dicker, A.P.; et al. Individual Patient-Level Meta-Analysis of the Performance of the Decipher Genomic Classifier in High-Risk Men After Prostatectomy to Predict Development of Metastatic Disease. J Clin Oncol 2017, 35, 1991–1998. [Google Scholar] [CrossRef]
- Zhao, S.G.; Chang, S.L.; Erho, N.; Yu, M.; Lehrer, J.; Alshalalfa, M.; Speers, C.; Cooperberg, M.R.; Kim, W.; Ryan, C.J.; et al. Associations of Luminal and Basal Subtyping of Prostate Cancer With Prognosis and Response to Androgen Deprivation Therapy. JAMA Oncol 2017, 3, 1663–1672. [Google Scholar] [CrossRef]
- Spratt, D.E.; Alshalalfa, M.; Fishbane, N.; Weiner, A.B.; Mehra, R.; Mahal, B.A.; Lehrer, J.; Liu, Y.; Zhao, S.G.; Speers, C.; et al. Transcriptomic Heterogeneity of Androgen Receptor Activity Defines a de novo low AR-Active Subclass in Treatment Naïve Primary Prostate Cancer. Clin Cancer Res 2019, 25, 6721–6730. [Google Scholar] [CrossRef]
- Fizazi, K.; Tran, N.; Fein, L.; Matsubara, N.; Rodriguez-Antolin, A.; Alekseev, B.Y.; Özgüroğlu, M.; Ye, D.; Feyerabend, S.; Protheroe, A.; et al. Abiraterone acetate plus prednisone in patients with newly diagnosed high-risk metastatic castration-sensitive prostate cancer (LATITUDE): final overall survival analysis of a randomised, double-blind, phase 3 trial. Lancet Oncol 2019, 20, 686–700. [Google Scholar] [CrossRef]
- Roy, S.; Sun, Y.; Wallis, C.J.D.; Morgan, S.C.; Grimes, S.; Malone, J.; Kishan, A.U.; Mukherjee, D.; Spratt, D.E.; Saad, F.; Malone, S. Development and validation of a multivariable prognostic model in de novo metastatic castrate sensitive prostate cancer. Prostate Cancer Prostatic Dis 2023, 26, 119–125. [Google Scholar] [CrossRef]
- Azad, A.A.; Armstrong, A.J.; Alcaraz, A.; Szmulewitz, R.Z.; Petrylak, D.P.; Holzbeierlein, J.; Villers, A.; Alekseev, B.; Iguchi, T.; Shore, N.D.; et al. Efficacy of enzalutamide in subgroups of men with metastatic hormone-sensitive prostate cancer based on prior therapy, disease volume, and risk. Prostate Cancer Prostatic Dis 2022, 25, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Azad, A.A.; Villers, A.; Alekseev, B.; Szmulewitz, R.Z.; Alcaraz, A.; Shore, N.D.; Petrylak, D.P.; Holzbeierlein, J.; Gomez-Veiga, F.; Rosbrook, B.; et al. Efficacy of enzalutamide (ENZA) plus androgen deprivation therapy (ADT) in men with de novo (M1) metastatic hormone-sensitive prostate cancer (mHSPC) versus progression to mHSPC (M0): Post hoc analysis of the phase III ARCHES trial. J Clin Oncol 2021, 39, 6_suppl. [Google Scholar] [CrossRef]
- Armstrong, A.J.; Azad, A.A.; Iguchi, T.; Szmulewitz, R.Z.; Petrylak, D.P.; Holzbeierlein, J.; Villers, A.; Alcaraz, A.; Alekseev, B.; Shore, N.D.; et al. Improved Survival With Enzalutamide in Patients With Metastatic Hormone-Sensitive Prostate Cancer. J Clin Oncol 2022, 40, 1616–1622. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, C.J.; Martin, A.J.; Stockler, M.R.; Begbie, S.; Cheung, L.; Chi, K.N.; Chowdhury, S.; Frydenberg, M.; Horvath, L.G.; Joshua, A.M.; et al. Testosterone suppression plus enzalutamide versus testosterone suppression plus standard antiandrogen therapy for metastatic hormone-sensitive prostate cancer (ENZAMET): an international, open-label, randomised, phase 3 trial. Lancet Oncol 2023, 24, 323–334. [Google Scholar] [CrossRef]
- Chi, K.N.; Chowdhury, S.; Bjartell, A.; Chung, B.H.; Pereira de Santana Gomes, A.J.; Given, R.; Juárez, A.; Merseburger, A.S.; Özgüroğlu, M.; Uemura, H.; et al. Apalutamide in Patients With Metastatic Castration-Sensitive Prostate Cancer: Final Survival Analysis of the Randomized, Double-Blind, Phase III TITAN Study. J Clin Oncol 2021, 39, 2294–2303. [Google Scholar] [CrossRef]
- Feng, F.Y.; Thomas, S.; Aguilar-Bonavides, C.; Gormley, M.; Agarwal, N.; Attard, G.; Wyatt, A.W.; Davicioni, E.; Ricci, D.S.; Lopez-Gitlitz, A.; et al. Molecular determinants of outcome for metastatic castration-sensitive prostate cancer (mCSPC) with addition of apalutamide (APA) or placebo (PBO) to androgen deprivation therapy (ADT) in TITAN. J Clin Oncol 2020, 38 (Suppl. 15), 5535. [Google Scholar] [CrossRef]
- Feng, F.Y.; Thomas, S.; Saad, F.; Gormley, M.; Yu, M.K.; Ricci, D.S.; Rooney, B.; Brookman-May, S.; McCarthy, S.; Olmos, D.; et al. Association of Molecular Subtypes With Differential Outcome to Apalutamide Treatment in Nonmetastatic Castration-Resistant Prostate Cancer. JAMA Oncol 2021, 7, 1005–1014. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, N.; Lucas, J.; Aguilar-Bonavides, C.; Thomas, S.; Gormley, M.; Chowdhury, S.; Merseburger, A.S.; Bjartell, A.; Uemura, H.; Özgüroğlu, M.; et al. Genomic aberrations associated with overall survival (OS) in metastatic castration-sensitive prostate cancer (mCSPC) treated with apalutamide (APA) or placebo (PBO) plus androgen deprivation therapy (ADT) in TITAN. J Clin Oncol 2022, 40, suppl–16. [Google Scholar] [CrossRef]
- Smith, M.R.; Hussain, M.; Saad, F.; Fizazi, K.; Sternberg, C.N.; Crawford, E.D.; Kopyltsov, E.; Park, C.H.; Alekseev, B.; Montesa-Pino, Á.; et al. Darolutamide and Survival in Metastatic, Hormone-Sensitive Prostate Cancer. N Engl J Med 2022, 386, 1132–1142. [Google Scholar] [CrossRef]
- Hussain, M.; Tombal, B.; Saad, F.; Fizazi, K.; Sternberg, C.N.; Crawford, E.D.; Shore, N.; Kopyltsov, E.; Kalebasty, A.R.; Bögemann, M.; et al. Darolutamide Plus Androgen-Deprivation Therapy and Docetaxel in Metastatic Hormone-Sensitive Prostate Cancer by Disease Volume and Risk Subgroups in the Phase III ARASENS Trial. J Clin Oncol 2023, 41, 3595–3607. [Google Scholar] [CrossRef]
- Fizazi, K.; Foulon, S.; Carles, J.; Roubaud, G.; McDermott, R.; Fléchon, A.; Tombal, B.; Supiot, S.; Berthold, D.; Ronchin, P.; et al. Abiraterone plus prednisone added to androgen deprivation therapy and docetaxel in de novo metastatic castration-sensitive prostate cancer (PEACE-1): a multicentre, open-label, randomised, phase 3 study with 2 × 2 factorial design. Lancet 2022, 399, 1695–1707. [Google Scholar] [CrossRef]
- Katipally, R.R.; Pitroda, S.P.; Juloori, A.; Chmura, S.J.; Weichselbaum, R.R. The oligometastatic spectrum in the era of improved detection and modern systemic therapy. Nat Rev Clin Oncol 2022, 19, 585–599. [Google Scholar] [CrossRef] [PubMed]
- Mahjoub, S.; Heidenreich, A. Oligometastatic prostate cancer: definition and the role of local and systemic therapy: a narrative review. Transl Androl Urol 2021, 10, 3167–3175. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Yi, W.S.; Brasacchio, R.A.; Muhs, A.G.; Smudzin, T.; Williams, J.P.; Messing, E.; Okunieff, P. Is there a favorable subset of patients with prostate cancer who develop oligometastases? Int J Radiat Oncol Biol Phys 2004, 58, 3–10. [Google Scholar] [CrossRef]
- Gravis, G.; Fizazi, K.; Joly, F.; Oudard, S.; Priou, F.; Esterni, B.; Latorzeff, I.; Delva, R.; Krakowski, I.; Laguerre, B.; et al. Androgen-deprivation therapy alone or with docetaxel in non-castrate metastatic prostate cancer (GETUG-AFU 15): a randomised, open-label, phase 3 trial. Lancet Oncol 2013, 14, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Gravis, G.; Boher, J.M.; Chen, Y.H.; Liu, G.; Fizazi, K.; Carducci, M.A.; Oudard, S.; Joly, F.; Jarrard, D.M.; Soulie, M.; et al. Burden of Metastatic Castrate Naive Prostate Cancer Patients, to Identify Men More Likely to Benefit from Early Docetaxel: Further Analyses of CHAARTED and GETUG-AFU15 Studies. Eur Urol 2018, 73, 847–855. [Google Scholar] [CrossRef]
- Hoyle, A.P.; Ali, A.; James, N.D.; Cook, A.; Parker, C.C.; de Bono, J.S.; Attard, G.; Chowdhury, S.; Cross, W.R.; Dearnaley, D.P.; et al. Abiraterone in “High-” and “Low-risk” Metastatic Hormone-sensitive Prostate Cancer. Eur Urol 2019, 76, 719–728. [Google Scholar] [CrossRef]
- Boevé, L.M.S.; Hulshof, M.C.C.M.; Vis, A.N.; Zwinderman, A.H.; Twisk, J.W.R.; Witjes, W.P.J.; Delaere, K.P.J.; Moorselaar, R.J.A.V.; Verhagen, P.C.M.S.; van Andel, G. Effect on Survival of Androgen Deprivation Therapy Alone Compared to Androgen Deprivation Therapy Combined with Concurrent Radiation Therapy to the Prostate in Patients with Primary Bone Metastatic Prostate Cancer in a Prospective Randomised Clinical Trial: Data from the HORRAD Trial. Eur Urol 2019, 75, 410–418. [Google Scholar] [CrossRef]
- Parker, C.C.; James, N.D.; Brawley, C.D.; Clarke, N.W.; Hoyle, A.P.; Ali, A.; Ritchie, A.W.S.; Attard, G.; Chowdhury, S.; Cross, W.; et al. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled phase 3 trial. Lancet 2018, 392, 2353–2366. [Google Scholar] [CrossRef]
- Dai, B.; Zhang, S.; Wan, F.N.; Wang, H.K.; Zhang, J.Y.; Wang, Q.F.; Kong, Y.Y.; Ma, X.J.; Mo, M.; Zhu, Y.; et al. Combination of Androgen Deprivation Therapy with Radical Local Therapy Versus Androgen Deprivation Therapy Alone for Newly Diagnosed Oligometastatic Prostate Cancer: A Phase II Randomized Controlled Trial. Eur Urol Oncol 2022, 5, 519–525. [Google Scholar] [CrossRef]
- Bossi, A.; Foulon, S.; Maldonado, X.; Sargos, P.; McDermott, R.S.; Flechon, A.; Tombal, B.F.; Supiot, S.; Berthold, D.R.; Ronchin, P.; et al. Prostate irradiation in men with de novo, low-volume, metastatic, castration-sensitive prostate cancer (mCSPC): Results of PEACE-1, a phase 3 randomized trial with a 2 × 2 design. J Clin Oncol 2023, 41, LBA5000–LBA5000. [Google Scholar] [CrossRef]
- Parker, C.; Castro, E.; Fizazi, K.; Heidenreich, A.; Ost, P.; Procopio, G.; Tombal, B.; Gillessen, S.; ESMO Guidelines Committee. Prostate cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2020, 31, 1119–1134. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology. Prostate Cancer. Version 3.2023 – August 7, 2023.
- Ost, P.; Reynders, D.; Decaestecker, K.; Fonteyne, V.; Lumen, N.; De Bruycker, A.; Lambert, B.; Delrue, L.; Bultijnck, R.; Claeys, T.; et al. Surveillance or Metastasis-Directed Therapy for Oligometastatic Prostate Cancer Recurrence: A Prospective, Randomized, Multicenter Phase II Trial. J Clin Oncol 2018, 36, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Phillips, R.; Shi, W.Y.; Deek, M.; Radwan, N.; Lim, S.J.; Antonarakis, E.S.; Rowe, S.P.; Ross, A.E.; Gorin, M.A.; Deville, C.; et al. Outcomes of Observation vs. Stereotactic Ablative Radiation for Oligometastatic Prostate Cancer: The ORIOLE Phase 2 Randomized Clinical Trial. JAMA Oncol 2020, 6, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Gillessen, S.; Bossi, A.; Davis, I.D.; de Bono, J.; Fizazi, K.; James, N.D.; Mottet, N.; Shore, N.; Small, E.; Smith, M.; et al. Management of patients with advanced prostate cancer-metastatic and/or castration-resistant prostate cancer: Report of the Advanced Prostate Cancer Consensus Conference (APCCC) 2022. Eur J Cancer 2023, 185, 178–215. [Google Scholar] [CrossRef]
- O’Shaughnessy, M.J.; McBride, S.M.; Vargas, H.A.; Touijer, K.A.; Morris, M.J.; Danila, D.C.; Laudone, V.P.; Bochner, B.H.; Sheinfeld, J.; Dayan, E.S.; et al. A Pilot Study of a Multimodal Treatment Paradigm to Accelerate Drug Evaluations in Early-stage Metastatic Prostate Cancer. Urology 2017, 102, 164–172. [Google Scholar] [CrossRef]
- Reyes, D.K.; Rowe, S.P.; Schaeffer, E.M.; Allaf, M.E.; Ross, A.E.; Pavlovich, C.P.; Deville, C.; Tran, P.T.; Pienta, K.J. Multidisciplinary total eradication therapy (TET) in men with newly diagnosed oligometastatic prostate cancer. Med Oncol 2020, 37, 60. [Google Scholar] [CrossRef]
- Reyes, D.K.; Trock, B.J.; Tran, P.T.; Pavlovich, C.P.; Deville, C.; Allaf, M.E.; Greco, S.C.; Song, D.Y.; Bivalacqua, T.J.; Han, M.; et al. Interim analysis of companion, prospective, phase II, clinical trials assessing the efficacy and safety of multi-modal total eradication therapy in men with synchronous oligometastatic prostate cancer. Med Oncol 2022, 39, 63. [Google Scholar] [CrossRef]
- Deantoni, C.L.; Fodor, A.; Cozzarini, C.; Fiorino, C.; Brombin, C.; Di Serio, C.; Calandrino, R.; Di Muzio, N. Prostate cancer with low burden skeletal disease at diagnosis: outcome of concomitant radiotherapy on primary tumor and metastases. Br J Radiol 2020, 93, 20190353. [Google Scholar] [CrossRef]
- Nabrinsky, E.; Macklis, J.; Bitran, J. A Review of the Abscopal Effect in the Era of Immunotherapy. Cureus 2022, 14, e29620. [Google Scholar] [CrossRef]
- Gundem, G.; Van Loo, P.; Kremeyer, B.; Alexandrov, L.B.; Tubio, J.M.C.; Papaemmanuil, E.; Brewer, D.S.; Kallio, H.M.L.; Högnäs, G.; Annala, M.; et al. The evolutionary history of lethal metastatic prostate cancer. Nature 2015, 520, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Dorpe, J.; Fonteyne, V.; et al. Tissue- and Blood-derived Genomic Biomarkers for Metastatic Hormone-sensitive Prostate Cancer: A Systematic Review. Eur Urol Oncol 2021, 4, 914–923. [Google Scholar] [CrossRef]
- Deek, M.P.; Van der Eecken, K.; Phillips, R.; Parikh, N.R.; Isaacsson Velho, P.; Lotan, T.L.; Kishan, A.U.; Maurer, T.; GAP6 Consortium; Boutros, P. C. The Mutational Landscape of Metastatic Castration-sensitive Prostate Cancer: The Spectrum Theory Revisited. Eur Urol 2021, 80, 632–640. [Google Scholar] [CrossRef] [PubMed]
- Deek, M.P.; Van der Eecken, K.; Sutera, P.; Deek, R.A.; Fonteyne, V.; Mendes, A.A.; Decaestecker, K.; Kiess, A.P.; Lumen, N.; Phillips, R.; et al. Long-Term Outcomes and Genetic Predictors of Response to Metastasis-Directed Therapy Versus Observation in Oligometastatic Prostate Cancer: Analysis of STOMP and ORIOLE Trials. J Clin Oncol 2022, 40, 3377–3382, Van der Eecken, K.; Vanwelkenhuyzen, J.; Deek, M.P.; Tran, P.T.; Warner, E.; Wyatt, A.W.; Kwan, E.M.; Verbeke, S.; Van. [Google Scholar] [CrossRef] [PubMed]
- Armenia, J.; Wankowicz, S.A.M.; Liu, D.; Gao, J.; Kundra, R.; Reznik, E.; Chatila, W.K.; Chakravarty, D.; Han, G.C.; Coleman, I.; et al. The long tail of oncogenic drivers in prostate cancer. Nat Genet 2018, 50, 645–651. [Google Scholar] [CrossRef]
- Chung, J.H.; Dewal, N.; Sokol, E.; Mathew, P.; Whitehead, R.; Millis, S.Z.; Frampton, G.M.; Bratslavsky, G.; Pal, S.K.; Lee, R.J.; et al. Prospective Comprehensive Genomic Profiling of Primary and Metastatic Prostate Tumors. JCO Precis Oncol 2019, 3, PO–18. [Google Scholar] [CrossRef]
- Kumar, A.; White, T.A.; MacKenzie, A.P.; Clegg, N.; Lee, C.; Dumpit, R.F.; Coleman, I.; Ng, S.B.; Salipante, S.J.; Rieder, M.J.; et al. Exome sequencing identifies a spectrum of mutation frequencies in advanced and lethal prostate cancers. Proc Natl Acad Sci U S A 2011, 108, 17087–17092. [Google Scholar] [CrossRef]
- Abida, W.; Armenia, J.; Gopalan, A.; Brennan, R.; Walsh, M.; Barron, D.; Danila, D.; Rathkopf, D.; Morris, M.; Slovin, S.; et al. Prospective Genomic Profiling of Prostate Cancer Across Disease States Reveals Germline and Somatic Alterations That May Affect Clinical Decision Making. JCO Precis Oncol 2017, 2017, PO.17.00029. [Google Scholar] [CrossRef]
- Hamid, A.A.; Gray, K.P.; Shaw, G.; MacConaill, L.E.; Evan, C.; Bernard, B.; Loda, M.; Corcoran, N.M.; Van Allen, E.M.; Choudhury, A.D.; et al. Compound genomic alterations of TP53, PTEN, and RB1 tumor suppressors in localized and metastatic prostate cancer. Eur Urol 2019, 76, 89–97. [Google Scholar] [CrossRef]
- Gilson, C.; Ingleby, F.; Gilbert, D.C.; Parry, M.A.; Atako, N.B.; Ali, A.; Hoyle, A.; Clarke, N.W.; Gannon, M.; Wanstall, C.; et al. Genomic Profiles of De Novo High- and Low-Volume Metastatic Prostate Cancer: Results From a 2-Stage Feasibility and Prevalence Study in the STAMPEDE Trial. JCO Precis Oncol 2020, 4, 882–897. [Google Scholar] [CrossRef] [PubMed]
- Vandekerkhove, G.; Struss, W.J.; Annala, M.; Kallio, H.M.L.; Khalaf, D.; Warner, E.W.; Herberts, C.; Ritch, E.; Beja, K.; Loktionova, Y.; et al. Circulating Tumor DNA Abundance and Potential Utility in De Novo Metastatic Prostate Cancer. Eur Urol 2019, 75, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Fei, X.; Zhu, Y.; Pan, J.; Sha, J.; Chi, C.; Gong, Y.; Du, X.; Zhou, L.; Dong, B.; et al. Comparative Analysis of Genomic Alterations across Castration Sensitive and Castration Resistant Prostate Cancer via Circulating Tumor DNA Sequencing. J Urol 2021, 205, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Kohli, M.; Tan, W.; Zheng, T.; Wang, A.; Montesinos, C.; Wong, C.; Du, P.; Jia, S.; Yadav, S.; Horvath, L.G.; et al. Clinical and genomic insights into circulating tumor DNA-based alterations across the spectrum of metastatic hormone-sensitive and castrate-resistant prostate cancer. EbioMedicine 2020, 54, 102728. [Google Scholar] [CrossRef] [PubMed]
- Trujillo, B.; Wu, A.; Wetterskog, D.; Attard, G. Blood-based liquid biopsies for prostate cancer: clinical opportunities and challenges. Br J Cancer 2022, 127, 1394–1402. [Google Scholar] [CrossRef]
- Stopsack, K.H.; Nandakumar, S.; Wibmer, A.G.; Haywood, S.; Weg, E.S.; Barnett, E.S.; Kim, C.J.; Carbone, E.A.; Vasselman, S.E.; Nguyen, B.; et al. Oncogenic genomic alterations, clinical phenotypes, and outcomes in metastatic castration-sensitive prostate cancer. Clin Cancer Res 2020, 26, 3230–3238. [Google Scholar] [CrossRef]
- Velez, M.G.; Kosiorek, H.E.; Egan, J.B.; McNatty, A.L.; Riaz, I.B.; Hwang, S.R.; Stewart, G.A.; Ho, T.H.; Moore, C.N.; Singh, P.; et al. Differential impact of tumor suppressor gene (TP53, PTEN, RB1) alterations and treatment outcomes in metastatic, hormone-sensitive prostate cancer. Prostate Cancer Prostatic Dis 2022, 25, 479–483. [Google Scholar] [CrossRef]
- Mateo, J.; Seed, G.; Bertan, C.; Rescigno, P.; Dolling, D.; Figueiredo, I.; Miranda, S.; Nava Rodrigues, D.; Gurel, B.; Clarke, M.; et al. Genomics of lethal prostate cancer at diagnosis and castration resistance. J Clin Invest 2020, 130, 1743–1751. [Google Scholar] [CrossRef]
- Swami, U.; Isaacsson Velho, P.; Nussenzveig, R.; Chipman, J.; Sacristan Santos, V.; Erickson, S.; Dharmaraj, D.; Alva, A.S.; Vaishampayan, U.N.; Esther, J.; et al. Association of SPOP Mutations with Outcomes in Men with De Novo Metastatic Castration-sensitive Prostate Cancer. Eur Urol 2020, 78, 652–656. [Google Scholar] [CrossRef]
- Wang, Z.; Song, Y.; Ye, M.; Dai, X.; Zhu, X.; Wei, W. The diverse roles of SPOP in prostate cancer and kidney cancer. Nat Rev Urol 2020, 17, 339–350. [Google Scholar] [CrossRef]
- Swami, U.; Graf, R.P.; Nussenzveig, R.H.; Fisher, V.; Tukachinsky, H.; Schrock, A.B.; Li, G.; Ross, J.S.; Sayegh, N.; Tripathi, N.; et al. SPOP Mutations as a Predictive Biomarker for Androgen Receptor Axis-Targeted Therapy in De Novo Metastatic Castration-Sensitive Prostate Cancer. Clin Cancer Res 2022, 28, 4917–4925. [Google Scholar] [CrossRef] [PubMed]
- Bishop, J.L.; Sio, A.; Angeles, A.; Roberts, M.E.; Azad, A.A.; Chi, K.N.; Zoubeidi, A. PD-L1 is highly expressed in Enzalutamide resistant prostate cancer. Oncotarget 2015, 6, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Graff, J.N.; Beer, T.M.; Alumkal, J.J.; Slottke, R.E.; Redmond, W.L.; Thomas, G.V.; Thompson, R.F.; Wood, M.A.; Koguchi, Y.; Chen, Y.; et al. A phase II single-arm study of pembrolizumab with enzalutamide in men with metastatic castration-resistant prostate cancer progressing on enzalutamide alone. J Immunother Cancer 2020, 8, e000642. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Liu, Q.; Zeng, X.; Yu, W.; Xu, G. Pembrolizumab with or without enzalutamide in selected populations of men with previously untreated metastatic castration-resistant prostate cancer harbouring programmed cell death ligand-1 staining: a retrospective study. BMC Cancer 2021, 21, 399. [Google Scholar] [CrossRef] [PubMed]
- Gratzke, C.; Kwiatkowski, M.; De Giorgi, U.; Martins da Trindade, K.; De Santis, M.; Armstrong, A.J.; Niu, C.; Liu, Y.; Poehlein, C.H. KEYNOTE-991: pembrolizumab plus enzalutamide and androgen deprivation for metastatic hormone-sensitive prostate cancer. Future Oncol 2023. Epub ahead of print. [Google Scholar] [CrossRef]
- Arranz Arija, J. A.; Valderrama, B.P.; Alonso Gordoa, T.; Gallardo Diaz, E.; Sepulveda Sanchez, J. M.; Fernandez-Parra, E.; Piulats, J.M.; Mendez Vidal, M. J.; Sala González, N.; Vazquez Estevez, S. et. al. PROSTRATEGY: A Spanish Genitourinary Oncology Group (SOGUG) multi-arm multistage (MAMS) phase III trial of immunotherapy strategies in high-volume metastatic hormone-sensitive prostate cancer. Ann Oncol 2019, 30, suppl_5. V352–V353. [Google Scholar] [CrossRef]
- Fallah, J.; Agrawal, S.; Gittleman, H.; Fiero, M.H.; Subramaniam, S.; John, C.; Chen, W.; Ricks, T.K.; Niu, G.; Fotenos, A.; et al. FDA Approval Summary: Lutetium Lu 177 Vipivotide Tetraxetan for Patients with Metastatic Castration-Resistant Prostate Cancer. Clin Cancer Res 2023, 29, 1651–1657. [Google Scholar] [CrossRef]
- Núñez, M.I.; Villalobos, M.; Olea, N.; Valenzuela, M.T.; Pedraza, V.; McMillan, T.J.; Ruiz de Almodóvar, J.M. Radiation-induced DNA double-strand break rejoining in human tumour cells. Br J Cancer 1995, 71, 311–316. [Google Scholar] [CrossRef]
- Sartor, A.O.; Tagawa, S.T.; Saad, F.; De Bono, J.S.; Feng, F.Y.; Fizazi, K.; Sakharova, O.V.; Morris, M.J. PSMAddition: A phase 3 trial to compare treatment with 177Lu-PSMA-617 plus standard of care (SOC) versus SOC alone in patients with metastatic hormone-sensitive prostate cancer. J Clin Oncol 2022, 40, 6_suppl. TPS210. [Google Scholar] [CrossRef]
- Hamid, A.A.; Sayegh, N.; Tombal, B.; Hussain, M.; Sweeney, C.J.; Graff, J.N.; Agarwal, N. Metastatic Hormone-Sensitive Prostate Cancer: Toward an Era of Adaptive and Personalized Treatment. Am Soc Clin Oncol Educ Book. 2023, 5. [Google Scholar] [CrossRef]
- Kase, A.M.; Copland III, J.A.; Tan, W. Novel Therapeutic Strategies for CDK4/6 Inhibitors in Metastatic Castrate-Resistant Prostate Cancer. Onco Targets Ther 2020, 13, 10499–10513. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.R.; Matsubara, N.; McKay, R.R.; Piulats, J.M.; Todenhöfer, T.; Zhang, T.; Fasnacht, N.; Sherwood, S.; Johnston, E.L.; Schaverien, C.; et, a. CYCLONE 3: A phase III, randomized, double-blind, placebo-controlled study of abemaciclib in combination with abiraterone plus prednisone in men with high-risk metastatic hormone-sensitive prostate cancer (mHSPC), J Clin Oncol 2023, 41, 6_suppl:TPS289. [CrossRef]
- Rao, A.; Moka, N.; Hamstra, D.A.; Ryan, C.J. Co-Inhibition of Androgen Receptor and PARP as a Novel Treatment Paradigm in Prostate Cancer-Where Are We Now? Cancers (Basel) 2022, 14, 801. [Google Scholar] [CrossRef]
- Clarke, N.W.; Armstrong, A.J.; Thiery-Vuillemin, A.; Oya, M.; Shore, N.; Loredo, E.; Procopio, G.; de Menezes, J.; Girotto, G.; Arslan, C.; et al. Abiraterone and Olaparib for Metastatic Castration-Resistant Prostate Cancer. NEJM Evid 2022, 1, EVIDoa2200043. [Google Scholar] [CrossRef]
- Agarwal, N.; Azad, A.A.; Carles, J.; Fay, A.P.; Matsubara, N.; Heinrich, D.; Szczylik, C.; De Giorgi, U.; Young Joung, J.; Fong, P.C.C.; et al. Talazoparib plus enzalutamide in men with first-line metastatic castration-resistant prostate cancer (TALAPRO-2): a randomised, placebo-controlled, phase 3 trial. Lancet 2023, 402, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, N.; Saad, F.; Azad, A.; Mateo, J.; Matsubara, N.; Shore, N.D.; Chakrabarti, J.; Chen, H.; Lanzalone, S.; Niyazov, A.; et al. TALAPRO-3: A phase 3, double-blind, randomized study of enzalutamide (ENZA) plus talazoparib (TALA) vs. placebo plus ENZA in patients with DDR gene-mutated, metastatic castration-sensitive prostate cancer (mCSPC). J Clin Oncol, 2023, 41, 6_suppl. TPS279. [Google Scholar] [CrossRef]
- Rathkopf, D.E.; Chi, K.N.; Olmos, D.; Cheng, H.H.; Agarwal, N.; Graff, J.N.; Sandhu, S.K.; Hayreh, V.; Lopez-Gitlitz, A. St. John Francis, P.; et al. AMPLITUDE: A study of niraparib in combination with abiraterone acetate plus prednisone (AAP) versus AAP for the treatment of patients with deleterious germline or somatic homologous recombination repair (HRR) gene-altered metastatic castration-sensitive prostate cancer (mCSPC). J Clin Oncol 2021, 3. 39:6_suppl, TPS176. [Google Scholar] [CrossRef]
- Jamaspishvili, T.; Berman, D.M.; Ross, A.E.; Scher, H.I.; De Marzo, A.M.; Squire, J.A.; Lotan, T.L. Clinical implications of PTEN loss in prostate cancer. Nat Rev Urol 2018, 15, 222–234. [Google Scholar] [CrossRef]
- Sweeney, C.; Bracarda, S.; Sternberg, C.N.; Chi, K.N.; Olmos, D.; Sandhu, S.; Massard, C.; Matsubara, N.; Alekseev, B.; Parnis, F.; et al. Ipatasertib plus abiraterone and prednisolone in metastatic castration-resistant prostate cancer (IPATential150): a multicentre, randomised, double-blind, phase 3 trial. Lancet 2021, 398, 131–142. [Google Scholar] [CrossRef]
- Fizazi, K.; George, D.J.; De Santis, M.; Clarke, N.; Fay, A.P.; Uemura, H.; Grinsted, L.; Rooney, C.; Verheijen, R.B.; Anjum, R.; et al. A phase III trial of capivasertib and abiraterone versus placebo and abiraterone in patients with de novo metastatic hormone-sensitive prostate cancer characterized by PTEN deficiency (CAPItello-281). J Clin Oncol 2021, 3, 39. [Google Scholar] [CrossRef]
| Official Title NCT Number |
Control arm | Experimental arm(s) | Primary Endpoints | Status | Enrolment | Study start/completion date |
|---|---|---|---|---|---|---|
| KEYNOTE-991 NCT04191096 |
Placebo + Enzalutamide + ADT |
Pembrolizumab + Enzalutamide + ADT |
OS, rPFS | Active, not recruiting | 1251 (actual) |
2021-05-25/ 2026-02-02 |
|
PROSTRATEGY NCT03879122 |
ARM 1: ADT + Docetaxel for 6 cycles | ARM 2: ADT + Docetaxel for 6 cycle and then Nivolumab 3 mg/kg every 2 weeks for 12 months ARM 3: ADT + 2 cycles of Ipilimumab 3 mg/kg every 3 weeks, followed by 3 cycles of Docetaxel, 2 cycles of Ipilimumab, 3 cycles of Docetaxel, Nivolumab 3 mg/kg every 2 weeks for 12 months |
OS | Active, not recruiting | 135 (estimated) |
2019-02-11/ 2024-12-31 |
|
PSMAddition NCT04720157 |
NHA + ADT | 7.4 GBq (+/- 10%) 177Lu-PSMA-617, once every 6 weeks (+/- 1 week) for 6 cycles + NHA + ADT | rPFS | Recruiting | 1126 (estimated) |
2021-06-09/ 2026-02-11 |
|
CYCLONE-03 NCT05288166 |
Placebo + Abiraterone + Prednisone/Prednisolone | Abemaciclib + Abiraterone + Prednisone/Prednisolone | rPFS | Recruiting | 900 (estimated) |
2022-04-14/ 2027-10-01 |
|
TALAPRO-3 NCT04821622 |
Placebo + Enzalutamide | Talazoparib + Enzalutamide | rPFS | Active, not recruiting | 599 (actual) |
2021-05-12/ 2027-04-10 |
|
AMPLITUDE NCT04497844 |
Placebo + Abiraterone + Prednisone/Prednisolone |
Niraparib + Abiraterone + Prednisone/Prednisolone |
rPFS | Recruiting | 692 (estimated) |
2020-09-23/ 2027-05-27 |
|
CAPItello-281 NCT04493853 |
Placebo + Abiraterone + Prednisone/Prednisolone | Capivasertib + Abiraterone + Prednisone/Prednisolone | rPFS | Recruiting | 1000 (estimated) |
2020-07-13/ 2026-03-10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





