Submitted:
07 September 2023
Posted:
11 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
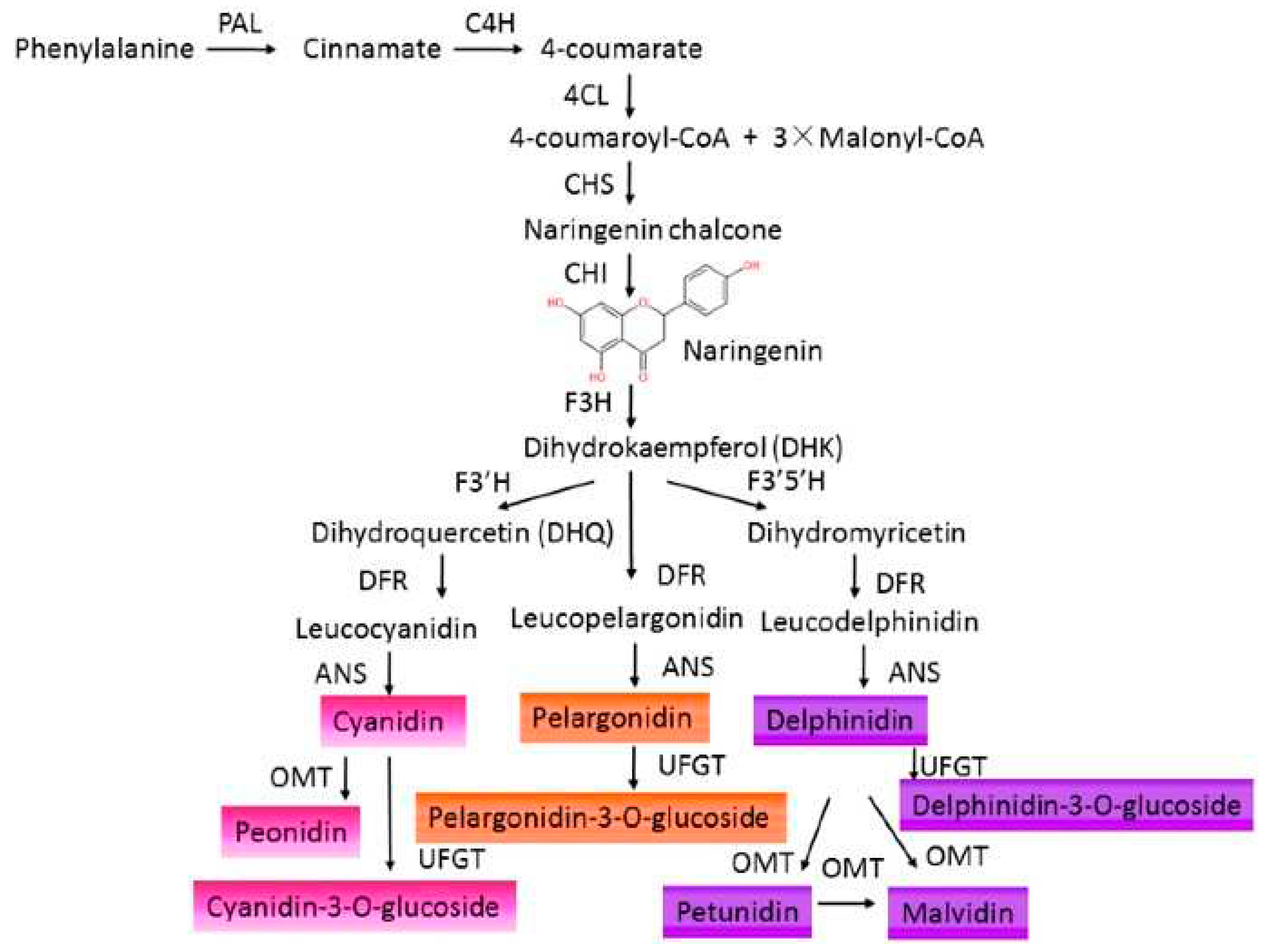
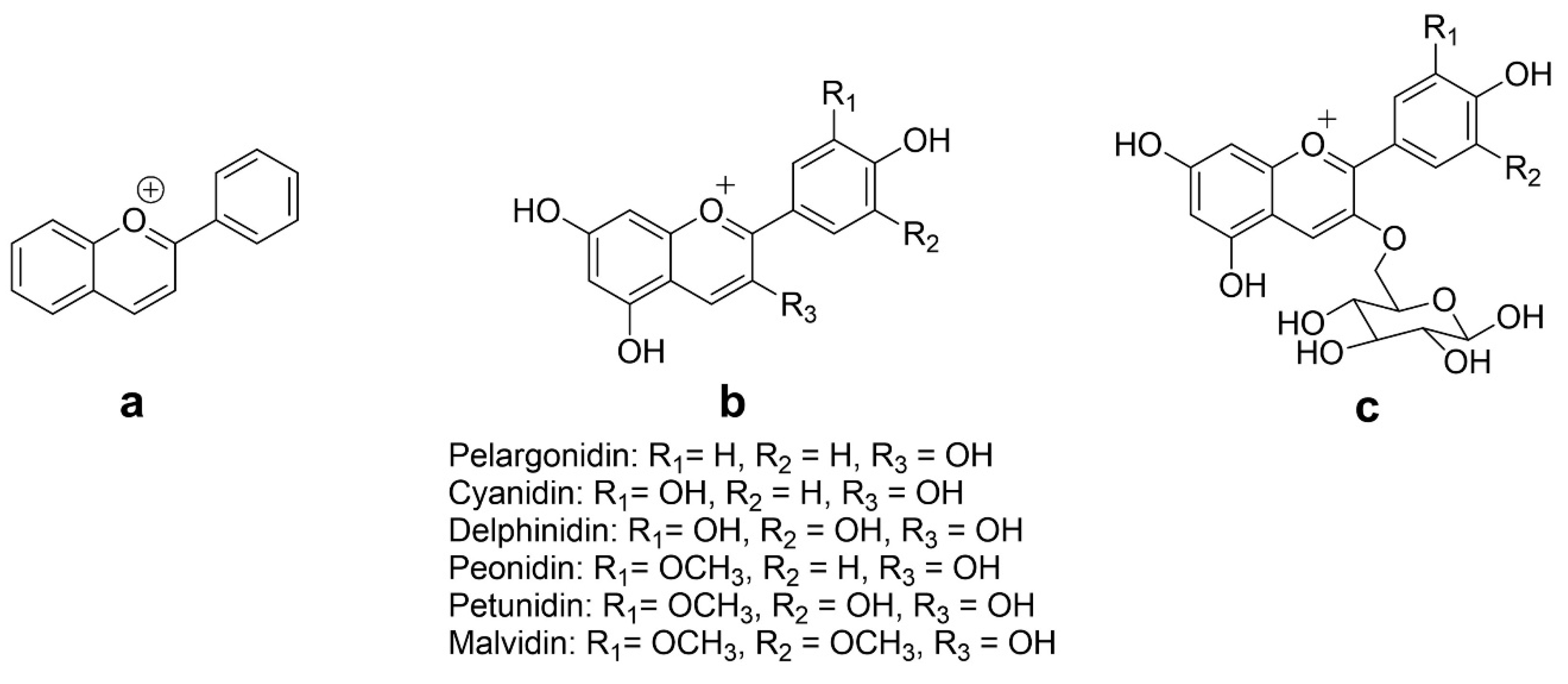
2. Chemistry and Natural Sources of ACNs
3. Immunomodulatory Potential of ACNs
- Inflammation disrupts immune homeostasis, leading to diverse diseases or disease conditions. However, ACNs have been proven to play a crucial role in regulating inflammatory pathways by inhibiting the production of proinflammatory cytokines (IL-1β, IL-6, and TNF-α) and proinflammatory mediators (COX, LOX, MPO, and PGE2), thereby offering protection against the development of various inflammatory conditions [29,32,50].
- ACNs influence immune cell activation and proliferation by modulating various pathways. Moreover, they have a significant impact on gene expression within immune cells, leading to heightened expression of genes responsible for antioxidant defense, anti-inflammatory pathways, and immune cell activation [29,51,52].
- Recent research indicates that ACNs can influence gut microbiota composition and functionality. Given the pivotal role of the gut microbiota in regulating the immune system, this interaction has the potential to contribute significantly to the immunomodulatory effects exhibited by ACNs [53,54,55,56,57].
4. Gut Microbiota in Immunity and Metabolism of ACNs
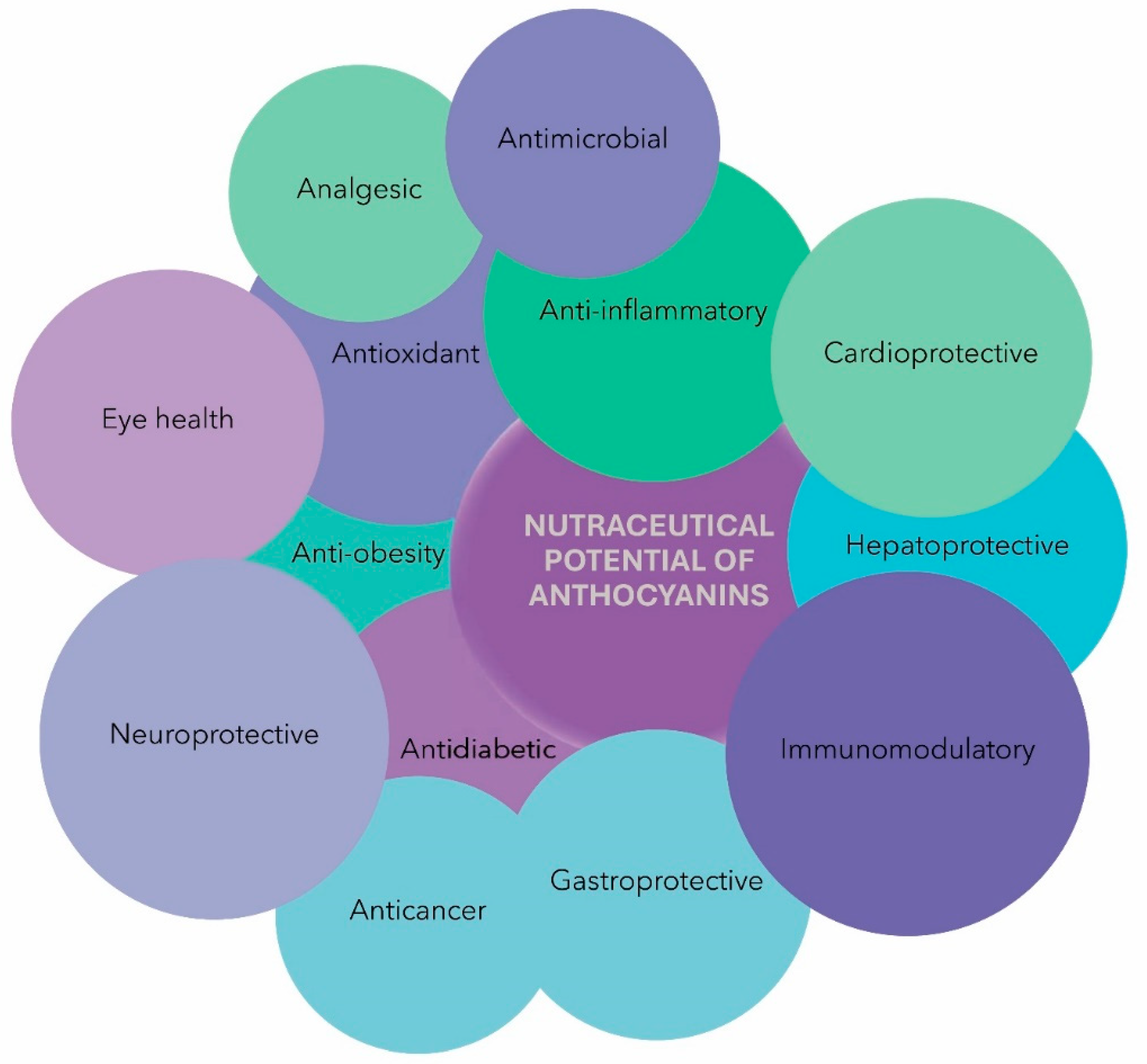
5. Nutraceutical Potential of ACNs
6. Factors Influencing Pharmacokinetics of ACNs
7. Strategies for Enhanced ACN Bioavailability
8. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mei, H.; Dong, X.; Wang, Y.; Tang, L.; Hu, Y. Managing Patients with Cancer during the COVID-19 Pandemic: Frontline Experience from Wuhan. Lancet Oncol. 2020, 21, 634–636. [Google Scholar] [CrossRef]
- Mastrandrea, L.D. An Overview of Organ-Specific Autoimmune Diseases Including Immunotherapy. Immunol. Invest. 2015, 44, 803–816. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Jia, S.; Xu, H. Potential Therapeutic Applications of Exosomes in Different Autoimmune Diseases. Clin. Immunol. 2019, 205, 116–124. [Google Scholar] [CrossRef]
- Rusek, P.; Wala, M.; Druszczyńska, M.; Fol, M. Infectious Agents as Stimuli of Trained Innate Immunity. Int. J. Mol. Sci. 2018, 19, 456. [Google Scholar] [CrossRef]
- Mrak, D.; Tobudic, S.; Koblischke, M.; Graninger, M.; Radner, H.; Sieghart, D.; Hofer, P.; Perkmann, T.; Haslacher, H.; Thalhammer, R.; et al. SARS-CoV-2 Vaccination in Rituximab-Treated Patients: B Cells Promote Humoral Immune Responses in the Presence of T-Cell-Mediated Immunity. Ann. Rheum. Dis. 2021, 80, 1345–1350. [Google Scholar] [CrossRef]
- Wang, Y.; Jia, A.; Bi, Y.; Wang, Y.; Yang, Q.; Cao, Y.; Li, Y.; Liu, G. Targeting Myeloid-Derived Suppressor Cells in Cancer Immunotherapy. Cancers (Basel) 2020, 12, 2626. [Google Scholar] [CrossRef]
- Ivashkiv, L.B. IFNγ: Signalling, Epigenetics and Roles in Immunity, Metabolism, Disease and Cancer Immunotherapy. Nat. Rev. Immunol. 2018, 18, 545–558. [Google Scholar] [CrossRef]
- Ouyang, W.; O’Garra, A. IL-10 Family Cytokines IL-10 and IL-22: From Basic Science to Clinical Translation. Immunity 2019, 50, 871–891. [Google Scholar] [CrossRef]
- Galon, J.; Bruni, D. Tumor Immunology and Tumor Evolution: Intertwined Histories. Immunity 2020, 52, 55–81. [Google Scholar] [CrossRef]
- Bilanges, B.; Posor, Y.; Vanhaesebroeck, B. PI3K Isoforms in Cell Signalling and Vesicle Trafficking. Nat. Rev. Mol. Cell Biol. 2019, 20, 515–534. [Google Scholar] [CrossRef]
- Peluso, I.; Yarla, N.S.; Ambra, R.; Pastore, G.; Perry, G. MAPK Signalling Pathway in Cancers: Olive Products as Cancer Preventive and Therapeutic Agents. Semin. Cancer Biol. 2019, 56, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Clara, J.A.; Monge, C.; Yang, Y.; Takebe, N. Targeting Signalling Pathways and the Immune Microenvironment of Cancer Stem Cells - a Clinical Update. Nat. Rev. Clin. Oncol. 2020, 17, 204–232. [Google Scholar] [PubMed]
- Macedo, A.C; de Faria, A.O.V.; Ghezzi, P. Boosting the Immune System, from Science to Myth: Analysis the Infosphere with Google. Front. Med. (Lausanne) 2019, 6, 165. [Google Scholar] [CrossRef] [PubMed]
- Wagner, D.N.; Marcon, A.R.; Caulfield, T. “Immune Boosting” in the Time of COVID: Selling Immunity on Instagram. Allergy Asthma Clin. Immunol. 2020, 16. [Google Scholar] [CrossRef] [PubMed]
- Clarke, T.C.; Black, L.I.; Stussman, B.J.; Barnes, P.M.; Nahin, R.L. Trends in the Use of Complementary Health Approaches among Adults: United States, 2002-2012. Natl. Health Stat. Report. 2015, 1–16. [Google Scholar]
- Grand View Research, Dietary Supplements Market Size, Share & Trend Analysis Report by Ingredient (Botanicals, Vitamins, Minerals, Amino Acids, Enzymes), By Product, By Application, By End-use, And Segment Forecasts, 2018 - 2024. (2018). Archived at: https://web.archive.org/web/20190115112358/https://www.grandviewresearch.com/industry-analysis/dietary-supplements-market (accessed June 2, 2023).
- Wainwright, C.L.; Teixeira, M.M.; Adelson, D.L.; Buenz, E.J.; David, B.; Glaser, K.B.; Harata-Lee, Y.; Howes, M.-J.R.; Izzo, A.A.; Maffia, P.; et al. Future Directions for the Discovery of Natural Product-Derived Immunomodulating Drugs: An IUPHAR Positional Review. Pharmacol. Res. 2022, 177, 106076. [Google Scholar] [CrossRef]
- Alhazmi, H.A.; Najmi, A.; Javed, S.A.; Sultana, S.; Al Bratty, M.; Makeen, H.A.; Meraya, A.M.; Ahsan, W.; Mohan, S.; Taha, M.M.E.; et al. Medicinal Plants and Isolated Molecules Demonstrating Immunomodulation Activity as Potential Alternative Therapies for Viral Diseases Including COVID-19. Front. Immunol. 2021, 12, 637553. [Google Scholar] [CrossRef]
- Demaria, O.; Cornen, S.; Daëron, M.; Morel, Y.; Medzhitov, R.; Vivier, E. Publisher Correction: Harnessing Innate Immunity in Cancer Therapy. Nature 2019, 576, E3. [Google Scholar] [CrossRef]
- Di Sotto, A.; Vitalone, A.; Di Giacomo, S. Plant-Derived Nutraceuticals and Immune System Modulation: An Evidence-Based Overview. Vaccines (Basel) 2020, 8, 468. [Google Scholar] [CrossRef]
- Maheshwari, S.; Kumar, V.; Bhadauria, G.; Mishra, A. Immunomodulatory Potential of Phytochemicals and Other Bioactive Compounds of Fruits: A Review. Food Front. 2022, 3, 221–238. [Google Scholar] [CrossRef]
- Jantan, I.; Ahmad, W.; Bukhari, S.N.A. Corrigendum: Plant-Derived Immunomodulators: An Insight on Their Preclinical Evaluation and Clinical Trials. Front. Plant Sci. 2018, 9, 1178. [Google Scholar] [CrossRef] [PubMed]
- Gupta, B.; Singh, V.R.; Verma, S.; Meshram, N.; Dhruw, L.; Sharma, R.; Ghosh, K.K.; Gupta, R.C. Plant and Food Derived Immunomodulators as Nutraceuticals for Performance Enhancing Activities. In Nutraceuticals in Veterinary Medicine; Springer International Publishing: Cham, 2019; pp. 593–601. [Google Scholar]
- Kim, J.H.; Kim, D.H.; Jo, S.; Cho, M.J.; Cho, Y.R.; Lee, Y.J.; Byun, S. Immunomodulatory Functional Foods and Their Molecular Mechanisms. Exp. Mol. Med. 2022, 54, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Smeriglio, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Chemistry, Pharmacology and Health Benefits of Anthocyanins: Anthocyanins and Human Health. Phytother. Res. 2016, 30, 1265–1286. [Google Scholar] [CrossRef]
- do Rosario, V.A.; Chang, C.; Spencer, J.; Alahakone, T.; Roodenrys, S.; Francois, M.; Weston-Green, K.; Hölzel, N.; Nichols, D.S.; Kent, K.; et al. Anthocyanins Attenuate Vascular and Inflammatory Responses to a High Fat High Energy Meal Challenge in Overweight Older Adults: A Cross-over, Randomized, Double-Blind Clinical Trial. Clin. Nutr. 2021, 40, 879–889. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Amaya, D.B. Natural Food Pigments and Colorants. In Reference Series in Phytochemistry; Springer International Publishing: Cham, 2018; pp. 1–35. ISBN 9783319264783. [Google Scholar]
- Qi, Q.; Chu, M.; Yu, X.; Xie, Y.; Li, Y.; Du, Y.; Liu, X.; Zhang, Z.; Shi, J.; Yan, N. Anthocyanins and Proanthocyanidins: Chemical Structures, Food Sources, Bioactivities, and Product Development. Food Rev. Int. 2022, 1–29. [Google Scholar] [CrossRef]
- Kozłowska, A.; Dzierżanowski, T. Targeting Inflammation by Anthocyanins as the Novel Therapeutic Potential for Chronic Diseases: An Update. Molecules 2021, 26, 4380. [Google Scholar] [CrossRef]
- Wallace, T.C.; Giusti, M.M. Anthocyanins. Adv. Nutr. 2015, 6, 620–622. [Google Scholar] [CrossRef]
- Riaz, M.; Zia-Ul-Haq, M.; Saad, B. Biosynthesis and Stability of Anthocyanins. In Anthocyanins and Human Health: Biomolecular and Therapeutic Aspects; Springer International Publishing: Cham, 2016; pp. 71–86. [Google Scholar]
- Lee, Y.-M.; Yoon, Y.; Yoon, H.; Park, H.-M.; Song, S.; Yeum, K.-J. Dietary Anthocyanins against Obesity and Inflammation. Nutrients 2017, 9, 1089. [Google Scholar] [CrossRef]
- Tena, N.; Martín, J.; Asuero, A.G. State of the Art of Anthocyanins: Antioxidant Activity, Sources, Bioavailability, and Therapeutic Effect in Human Health. Antioxidants (Basel) 2020, 9, 451. [Google Scholar] [CrossRef]
- Calderaro, A.; Barreca, D.; Bellocco, E.; Smeriglio, A.; Trombetta, D.; Laganà, G. Colored Phytonutrients: Role and Applications in the Functional Foods of Anthocyanins. In Phytonutrients in Food: From Traditional to Rational Usage; Nabavi, S.M., Suntar, I., Barreca, D., Khan, H., Eds.; Elsevier: Woodhead Publishing, 2020; pp. 177–195. [Google Scholar]
- Onyilagha, J. C.; Grotewold, E. The Biology and Structural Distribution of Surface Flavonoids. Recent Res. Dev. Plant Sci 2004, 2, 53–71. [Google Scholar]
- Harbone, J.B. The Flavonoids: Recent Advances. In Plant Pigments; Goodwin, T.W., Ed.; Academic Press: San Diego, CA, USA, 1988; pp. 299–343. [Google Scholar]
- Tanaka, Y.; Sasaki, N.; Ohmiya, A. Biosynthesis of Plant Pigments: Anthocyanins, Betalains and Carotenoids. Plant J. 2008, 54 (4), 733–749. [Google Scholar] [CrossRef]
- Sasaki, N.; Nishizaki, Y.; Ozeki, Y.; Miyahara, T. The Role of Acyl-Glucose in Anthocyanin Modifications. Molecules 2014, 19, 18747–18766. [Google Scholar] [CrossRef]
- Jokioja, J.; Yang, B.; Linderborg, K.M. Acylated Anthocyanins: A Review on Their Bioavailability and Effects on Postprandial Carbohydrate Metabolism and Inflammation. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5570–5615. [Google Scholar] [CrossRef]
- Zhao, C.-L.; Yu, Y.-Q.; Chen, Z.-J.; Wen, G.-S.; Wei, F.-G.; Zheng, Q.; Wang, C.-D.; Xiao, X.-L. Stability-Increasing Effects of Anthocyanin Glycosyl Acylation. Food Chem. 2017, 214, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Alappat, B.; Alappat, J. Anthocyanin Pigments: Beyond Aesthetics. Molecules 2020, 25, 5500. [Google Scholar] [CrossRef]
- Nile, S.H.; Park, S.W. Edible Berries: Bioactive Components and Their Effect on Human Health. Nutrition 2014, 30, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Virgous, C.; Si, H. Synergistic Anti-Inflammatory Effects and Mechanisms of Combined Phytochemicals. J. Nutr. Biochem. 2019, 69, 19–30. [Google Scholar] [CrossRef]
- Zamora-Ros, R.; Knaze, V.; Luján-Barroso, L.; Slimani, N.; Romieu, I.; Touillaud, M.; Kaaks, R.; Teucher, B.; Mattiello, A.; Grioni, S.; et al. Estimation of the Intake of Anthocyanidins and Their Food Sources in the European Prospective Investigation into Cancer and Nutrition (EPIC) Study. Br. J. Nutr. 2011, 106, 1090–1099. [Google Scholar] [CrossRef] [PubMed]
- Bellocco, E.; Barreca, D.; Laganà, G.; Calderaro, A.; El Lekhlifi, Z.; Chebaibi, S.; Smeriglio, A.; Trombetta, D. Cyanidin-3- O -Galactoside in Ripe Pistachio (Pistachia vera L. Variety Bronte) Hulls: Identification and Evaluation of Its Antioxidant and Cytoprotective Activities. J. Funct. Foods 2016, 27, 376–385. [Google Scholar] [CrossRef]
- Giusti, M.M.; Wrolstad, R.E. Acylated Anthocyanins from Edible Sources and Their Applications in Food Systems. Biochem. Eng. J. 2003, 14, 217–225. [Google Scholar] [CrossRef]
- Liu, Y.; Tikunov, Y.; Schouten, R.E.; Marcelis, L.F.M.; Visser, R.G.F.; Bovy, A. Anthocyanin Biosynthesis and Degradation Mechanisms in Solanaceous Vegetables: A Review. Front. Chem. 2018, 6. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and Anthocyanins: Colored Pigments as Food, Pharmaceutical Ingredients, and the Potential Health Benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef]
- Sobhani, M.; Farzaei, M.H.; Kiani, S.; Khodarahmi, R. Immunomodulatory; Anti-Inflammatory/Antioxidant Effects of Polyphenols: A Comparative Review on the Parental Compounds and Their Metabolites. Food Rev. Int. 2021, 37, 759–811. [Google Scholar] [CrossRef]
- Ma, Z.; Du, B.; Li, J.; Yang, Y.; Zhu, F. An Insight into Anti-Inflammatory Activities and Inflammation Related Diseases of Anthocyanins: A Review of Both in Vivo and in Vitro Investigations. Int. J. Mol. Sci. 2021, 22, 11076. [Google Scholar] [CrossRef] [PubMed]
- Fallah, A.A.; Sarmast, E.; Fatehi, P.; Jafari, T. Impact of Dietary Anthocyanins on Systemic and Vascular Inflammation: Systematic Review and Meta-Analysis on Randomised Clinical Trials. Food Chem. Toxicol. 2020, 135, 110922. [Google Scholar] [CrossRef]
- Samarpita, S.; Ganesan, R.; Rasool, M. Cyanidin Prevents the Hyperproliferative Potential of Fibroblast-like Synoviocytes and Disease Progression via Targeting IL-17A Cytokine Signalling in Rheumatoid Arthritis. Toxicol. Appl. Pharmacol. 2020, 391, 114917. [Google Scholar] [CrossRef] [PubMed]
- Hair, R.; Sakaki, J.R.; Chun, O.K. Anthocyanins, Microbiome and Health Benefits in Aging. Molecules 2021, 26, 537. [Google Scholar] [CrossRef] [PubMed]
- Verediano, T.A.; Stampini Duarte Martino, H.; Dias Paes, M.C.; Tako, E. Effects of Anthocyanin on Intestinal Health: A Systematic Review. Nutrients 2021, 13, 1331. [Google Scholar] [CrossRef]
- Chen, K.; Kortesniemi, M.K.; Linderborg, K.M.; Yang, B. Anthocyanins as Promising Molecules Affecting Energy Homeostasis, Inflammation, and Gut Microbiota in Type 2 Diabetes with Special Reference to Impact of Acylation. J. Agric. Food Chem. 2023, 71, 1002–1017. [Google Scholar] [CrossRef]
- Kapoor, P.; Tiwari, A.; Sharma, S.; Tiwari, V.; Sheoran, B.; Ali, U.; Garg, M. Effect of Anthocyanins on Gut Health Markers, Firmicutes-Bacteroidetes Ratio and Short-Chain Fatty Acids: A Systematic Review via Meta-Analysis. Sci. Rep. 2023, 13, 1729. [Google Scholar] [CrossRef]
- Liang, A.; Leonard, W.; Beasley, J.T.; Fang, Z.; Zhang, P.; Ranadheera, C.S. Anthocyanins-Gut Microbiota-Health Axis: A Review. Crit. Rev. Food Sci. Nutr. 2023, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Kumazawa, Y.; Takimoto, H.; Matsumoto, T.; Kawaguchi, K. Potential Use of Dietary Natural Products, Especially Polyphenols, for Improving Type-1 Allergic Symptoms. Curr. Pharm. Des. 2014, 20, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Percival, S.S. Grape Consumption Supports Immunity in Animals and Humans. J. Nutr. 2009, 139, 1801S–5S. [Google Scholar] [CrossRef]
- Park, K.H.; Gu, D.R.; So, H.S.; Kim, K.J.; Lee, S.H. Dual Role of Cyanidin-3-Glucoside on the Differentiation of Bone Cells. J. Dent. Res. 2015, 94, 1676–1683. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-H.; Kim, J.-Y.; Cheon, Y.-H.; Baek, J.M.; Ahn, S.-J.; Yoon, K.-H.; Lee, M.S.; Oh, J. Protocatechuic Acid Attenuates Osteoclastogenesis by Downregulating JNK/c-Fos/NFATc1 Signaling and Prevents Inflammatory Bone Loss in Mice: PCA Attenuates Osteoclastogenesis. Phytother. Res. 2016, 30, 604–612. [Google Scholar] [CrossRef]
- Jara, E.; Hidalgo, M.A.; Hancke, J.L.; Hidalgo, A.I.; Brauchi, S.; Nuñez, L.; Villalobos, C.; Burgos, R.A. Delphinidin Activates NFAT and Induces IL-2 Production through SOCE in T Cells. Cell Biochem. Biophys. 2014, 68, 497–509. [Google Scholar] [CrossRef]
- Hou, D.-X.; Kai, K.; Li, J.-J.; Lin, S.; Terahara, N.; Wakamatsu, M.; Fujii, M.; Young, M.R.; Colburn, N. Anthocyanidins Inhibit Activator Protein 1 Activity and Cell Transformation: Structure-Activity Relationship and Molecular Mechanisms. Carcinogenesis 2003, 25, 29–36. [Google Scholar] [CrossRef]
- Hou, D.-X.; Yanagita, T.; Uto, T.; Masuzaki, S.; Fujii, M. Anthocyanidins Inhibit Cyclooxygenase-2 Expression in LPS-Evoked Macrophages: Structure–Activity Relationship and Molecular Mechanisms Involved. Biochem. Pharmacol. 2005, 70, 417–425. [Google Scholar] [CrossRef]
- Kiselova-Kaneva, Y.; Nashar, M.; Roussev, B.; Salim, A.; Hristova, M.; Olczyk, P.; Komosinska-Vassev, K.; Dincheva, I.; Badjakov, I.; Galunska, B.; et al. Sambucus Ebulus (Elderberry) Fruits Modulate Inflammation and Complement System Activity in Humans. Int. J. Mol. Sci. 2023, 24. [Google Scholar] [CrossRef]
- Semmarath, W.; Mapoung, S.; Umsumarng, S.; Arjsri, P.; Srisawad, K.; Thippraphan, P.; Yodkeeree, S.; Dejkriengkraikul, P. Cyanidin-3-O-Glucoside and Peonidin-3-O-Glucoside-Rich Fraction of Black Rice Germ and Bran Suppresses Inflammatory Responses from SARS-CoV-2 Spike Glycoprotein S1-Induction in Vitro in A549 Lung Cells and THP-1 Macrophages via Inhibition of the NLRP3 Inflammasome Pathway. Nutrients 2022, 14, 2738. [Google Scholar]
- Li, J.; Zou, C.; Liu, Y. Amelioration of Ovalbumin-Induced Food Allergy in Mice by Targeted Rectal and Colonic Delivery of Cyanidin-3-O-Glucoside. Foods 2022, 11. [Google Scholar] [CrossRef] [PubMed]
- Amer, S.A.; Al-Khalaifah, H.S.; Gouda, A.; Osman, A.; Goda, N.I.A.; Mohammed, H.A.; Darwish, M.I.M.; Hassan, A.M.; Mohamed, S.K.A. Potential Effects of Anthocyanin-Rich Roselle (Hibiscus Sabdariffa L.) Extract on the Growth, Intestinal Histomorphology, Blood Biochemical Parameters, and the Immune Status of Broiler Chickens. Antioxidants (Basel) 2022, 11, 544. [Google Scholar] [CrossRef] [PubMed]
- Trinei, M.; Carpi, A.; Menabo’, R.; Storto, M.; Fornari, M.; Marinelli, A.; Minardi, S.; Riboni, M.; Casciaro, F.; DiLisa, F.; et al. Dietary Intake of Cyanidin-3-Glucoside Induces a Long-Lasting Cardioprotection from Ischemia/Reperfusion Injury by Altering the Microbiota. J. Nutr. Biochem. 2022, 101, 108921. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, V.; Park, M.; Lee, H.-J. Comparative Transcriptome Analysis of the Expression of Antioxidant and Immunity Genes in the Spleen of a Cyanidin 3-O-Glucoside-Treated Alzheimer’s Mouse Model. Antioxidants (Basel) 2021, 10, 1435. [Google Scholar] [CrossRef]
- Shaw, O.M.; Hurst, R.D.; Cooney, J.; Sawyer, G.M.; Dinnan, H.; Martell, S. Boysenberry and Apple Juice Concentrate Reduced Acute Lung Inflammation and Increased M2 Macrophage-Associated Cytokines in an Acute Mouse Model of Allergic Airways Disease. Food Sci. Nutr. 2021, 9, 1491–1503. [Google Scholar] [CrossRef]
- Pitsillou, E.; Liang, J.; Ververis, K.; Lim, K.W.; Hung, A.; Karagiannis, T.C. Identification of Small Molecule Inhibitors of the Deubiquitinating Activity of the SARS-CoV-2 Papain-like Protease: In Silico Molecular Docking Studies and in Vitro Enzymatic Activity Assay. Front. Chem. 2020, 8, 623971. [Google Scholar] [CrossRef]
- Pitsillou, E.; Liang, J.; Ververis, K.; Hung, A.; Karagiannis, T.C. Interaction of Small Molecules with the SARS-CoV-2 Papain-like Protease: In Silico Studies and in Vitro Validation of Protease Activity Inhibition Using an Enzymatic Inhibition Assay. J. Mol. Graph. Model. 2021, 104, 107851. [Google Scholar] [CrossRef]
- Aloud, B.M.; Petkau, J.C.; Yu, L.; McCallum, J.; Kirby, C.; Netticadan, T.; Blewett, H. Effects of Cyanidin 3-O-Glucoside and Hydrochlorothiazide on T-Cell Phenotypes and Function in Spontaneously Hypertensive Rats. Food Funct. 2020, 11, 8560–8572. [Google Scholar] [CrossRef]
- Wang, H.; Li, S.; Zhang, G.; Wu, H.; Chang, X. Potential Therapeutic Effects of Cyanidin-3-O-Glucoside on Rheumatoid Arthritis by Relieving Inhibition of CD38+ NK Cells on Treg Cell Differentiation. Arthritis Res. Ther. 2019, 21, 220. [Google Scholar] [CrossRef]
- Mazewski, C.; Kim, M.S.; Gonzalez de Mejia, E. Anthocyanins, Delphinidin-3-O-Glucoside and Cyanidin-3-O-Glucoside, Inhibit Immune Checkpoints in Human Colorectal Cancer Cells in Vitro and in Silico. Sci. Rep. 2019, 9, 11560. [Google Scholar] [CrossRef]
- Cremonini, E.; Daveri, E.; Mastaloudis, A.; Adamo, A.M.; Mills, D.; Kalanetra, K.; Hester, S.N.; Wood, S.M.; Fraga, C.G.; Oteiza, P.I. Anthocyanins Protect the Gastrointestinal Tract from High Fat Diet-Induced Alterations in Redox Signaling, Barrier Integrity and Dysbiosis. Redox Biol. 2019, 26, 101269. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Wang, H.; Yi, J.; Yang, B.; Li, M.; He, D.; Yang, W.; Zhang, Y.; Ni, H. Anti-Tumor Properties of Anthocyanins from Lonicera caerulea ‘Beilei’ Fruit on Human Hepatocellular Carcinoma: In Vitro and in Vivo Study. Biomed. Pharmacother. 2018, 104, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, D.; Cimino, F.; Fratantonio, D.; Molonia, M.S.; Bashllari, R.; Busà, R.; Saija, A.; Speciale, A. Cyanidin-3-O-Glucoside Modulates the in Vitro Inflammatory Crosstalk between Intestinal Epithelial and Endothelial Cells. Mediators Inflamm. 2017, 3454023. [Google Scholar] [CrossRef] [PubMed]
- Simonyi, A.; Chen, Z.; Jiang, J.; Zong, Y.; Chuang, D.Y.; Gu, Z.; Lu, C.-H.; Fritsche, K.L.; Greenlief, C.M.; Rottinghaus, G.E.; et al. Inhibition of Microglial Activation by Elderberry Extracts and Its Phenolic Components. Life Sci. 2015, 128, 30–38. [Google Scholar] [CrossRef]
- Pyo, M.Y.; Yoon, S.J.; Yu, Y.; Park, S.; Jin, M. Cyanidin-3-Glucoside Suppresses Th2 Cytokines and GATA-3 Transcription Factor in EL-4 T Cells. Biosci. Biotechnol. Biochem. 2014, 78, 1037–1043. [Google Scholar] [CrossRef] [PubMed]
- Mace, T.A.; King, S.A.; Ameen, Z.; Elnaggar, O.; Young, G.; Riedl, K.M.; Schwartz, S.J.; Clinton, S.K.; Knobloch, T.J.; Weghorst, C.M.; et al. Bioactive Compounds or Metabolites from Black Raspberries Modulate T Lymphocyte Proliferation, Myeloid Cell Differentiation and Jak/STAT Signaling. Cancer Immunol. Immunother. 2014, 63, 889–900. [Google Scholar] [CrossRef]
- Köchling, J.; Schmidt, M.; Rott, Y.; Sagner, M.; Ungefroren, H.; Wittig, B.; Henze, G. Can Anthocyanins Improve Maintenance Therapy of Ph(+) Acute Lymphoblastic Leukaemia? Eur. J. Haematol. 2013, 90, 291–300. [Google Scholar] [CrossRef]
- Bub, A.; Watzl, B.; Blockhaus, M.; Briviba, K.; Liegibel, U.; Müller, H.; Pool-Zobel, B.L.; Rechkemmer, G. Fruit Juice Consumption Modulates Antioxidative Status, Immune Status and DNA Damage. J. Nutr. Biochem. 2003, 14, 90–98. [Google Scholar] [CrossRef]
- Ryyti, R.; Hämäläinen, M.; Leppänen, T.; Peltola, R.; Moilanen, E. Phenolic Compounds Known to Be Present in Lingonberry (Vaccinium Vitis-Idaea L.) Enhance Macrophage Polarization towards the Anti-Inflammatory M2 Phenotype. Biomedicines 2022, 10, 3045. [Google Scholar] [CrossRef]
- Neves, B.R.O.; de Freitas, S.; Borelli, P.; Rogero, M.M.; Fock, R.A. Delphinidin-3-O-Glucoside in Vitro Suppresses NF-ΚB and Changes the Secretome of Mesenchymal Stem Cells Affecting Macrophage Activation. Nutrition 2023, 105, 111853. [Google Scholar] [CrossRef]
- Mazewski, C.; Luna-Vital, D.; Berhow, M.; Gonzalez de Mejia, E. Reduction of Colitis-Associated Colon Carcinogenesis by a Black Lentil Water Extract through Inhibition of Inflammatory and Immunomodulatory Cytokines. Carcinogenesis 2020, 41, 790–803. [Google Scholar] [CrossRef]
- Hyun, K.H.; Gil, K.C.; Kim, S.G.; Park, S.-Y.; Hwang, K.W. Delphinidin Chloride and Its Hydrolytic Metabolite Gallic Acid Promote Differentiation of Regulatory T Cells and Have an Anti-Inflammatory Effect on the Allograft Model. J. Food Sci. 2019, 84, 920–930. [Google Scholar] [CrossRef] [PubMed]
- Dayoub, O.; Le Lay, S.; Soleti, R.; Clere, N.; Hilairet, G.; Dubois, S.; Gagnadoux, F.; Boursier, J.; Martínez, M.C.; Andriantsitohaina, R. Estrogen Receptor α/HDAC/NFAT Axis for Delphinidin Effects on Proliferation and Differentiation of T Lymphocytes from Patients with Cardiovascular Risks. Sci. Rep. 2017, 7, 9378. [Google Scholar] [CrossRef] [PubMed]
- Chamcheu, J.C.; Adhami, V.M.; Esnault, S.; Sechi, M.; Siddiqui, I.A.; Satyshur, K.A.; Syed, D.N.; Dodwad, S.-J.M.; Chaves-Rodriquez, M.-I.; Longley, B.J.; et al. Dual Inhibition of PI3K/Akt and MTOR by the Dietary Antioxidant, Delphinidin, Ameliorates Psoriatic Features in Vitro and in an Imiquimod-Induced Psoriasis-like Disease in Mice. Antioxid. Redox Signal. 2017, 26, 49–69. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.; Jeong, M.-H.; Jeon, H.; Sung, G.-J.; So, Y.; Kim, I.; Son, J.; Lee, S.-W.; Yoon, H.-G.; Choi, K.-C. Delphinidin Sensitizes Prostate Cancer Cells to TRAIL-Induced Apoptosis, by Inducing DR5 and Causing Caspase-Mediated HDAC3 Cleavage. Oncotarget 2015, 6, 9970–9984. [Google Scholar] [CrossRef] [PubMed]
- Seong, A.-R.; Yoo, J.-Y.; Choi, K.; Lee, M.-H.; Lee, Y.-H.; Lee, J.; Jun, W.; Kim, S.; Yoon, H.-G. Delphinidin, a Specific Inhibitor of Histone Acetyltransferase, Suppresses Inflammatory Signaling via Prevention of NF-ΚB Acetylation in Fibroblast-like Synoviocyte MH7A Cells. Biochem. Biophys. Res. Commun. 2011, 410, 581–586. [Google Scholar] [CrossRef]
- Chung, I.-C.; Yuan, S.-N.; OuYang, C.-N.; Hu, S.-I.; Lin, H.-C.; Huang, K.-Y.; Lin, W.-N.; Chuang, Y.-T.; Chen, Y.-J.; Ojcius, D.M.; et al. EFLA 945 Restricts AIM2 Inflammasome Activation by Preventing DNA Entry for Psoriasis Treatment. Cytokine 2020, 127, 154951. [Google Scholar] [CrossRef]
- Iban-Arias, R.; Sebastian-Valverde, M.; Wu, H.; Lyu, W.; Wu, Q.; Simon, J.; Pasinetti, G.M. Role of Polyphenol-Derived Phenolic Acid in Mitigation of Inflammasome-Mediated Anxiety and Depression. Biomedicines 2022, 10, 1264. [Google Scholar] [CrossRef]
- Wang, J.; Hodes, G.E.; Zhang, H.; Zhang, S.; Zhao, W.; Golden, S.A.; Bi, W.; Menard, C.; Kana, V.; Leboeuf, M.; et al. Epigenetic Modulation of Inflammation and Synaptic Plasticity Promotes Resilience against Stress in Mice. Nat. Commun. 2018, 9. [Google Scholar] [CrossRef]
- Eisenstein, M. Microbiome: Bacterial Broadband. Nature 2016, 533, S104–6. [Google Scholar] [CrossRef]
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef]
- Williamson, G.; Clifford, M.N. Colonic Metabolites of Berry Polyphenols: The Missing Link to Biological Activity? Br. J. Nutr. 2010, 104 Suppl 3, S48–66. [Google Scholar] [CrossRef]
- Etxeberria, U.; Fernández-Quintela, A.; Milagro, F.I.; Aguirre, L.; Martínez, J.A.; Portillo, M.P. Impact of Polyphenols and Polyphenol-Rich Dietary Sources on F. Han et al. Trends in Food. J. Agric. Food Chem. 2013, 83, 9517–9533. [Google Scholar] [CrossRef] [PubMed]
- Belkaid, Y.; Hand, T.W. Role of the Microbiota in Immunity and Inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef] [PubMed]
- Hanske, L.; Engst, W.; Loh, G.; Sczesny, S.; Blaut, M.; Braune, A. Contribution of Gut Bacteria to the Metabolism of Cyanidin 3-Glucoside in Human Microbiota-Associated Rats. Br. J. Nutr. 2013, 109, 1433–1441. [Google Scholar] [CrossRef]
- Mayta-Apaza, A.C.; Pottgen, E.; De Bodt, J.; Papp, N.; Marasini, D.; Howard, L.; Abranko, L.; Van de Wiele, T.; Lee, S.-O.; Carbonero, F. Impact of Tart Cherries Polyphenols on the Human Gut Microbiota and Phenolic Metabolites in Vitro and in Vivo. J. Nutr. Biochem. 2018, 59, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Bresciani, L.; Angelino, D.; Vivas, E.I.; Kerby, R.L.; García-Viguera, C.; Del Rio, D.; Rey, F.E.; Mena, P. Differential Catabolism of an Anthocyanin-Rich Elderberry Extract by Three Gut Microbiota Bacterial Species. J. Agric. Food Chem. 2020, 68, 1837–1843. [Google Scholar] [CrossRef]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut Microbiota Functions: Metabolism of Nutrients and Other Food Components. Eur. J. Nutr. 2018, 57, 1–24. [Google Scholar] [CrossRef]
- Eker, M.E.; Aaby, K.; Budic-Leto, I.; Rimac Brnčić, S.; El, S.N.; Karakaya, S.; Simsek, S.; Manach, C.; Wiczkowski, W.; de Pascual-Teresa, S. A Review of Factors Affecting Anthocyanin Bioavailability: Possible Implications for the Inter-Individual Variability. Foods 2019, 9, 2. [Google Scholar] [CrossRef]
- Han, F.; Yang, P.; Wang, H.; Fernandes, I.; Mateus, N.; Liu, Y. Digestion and Absorption of Red Grape and Wine Anthocyanins through the Gastrointestinal Tract. Trends Food Sci. Technol. 2019, 83, 211–224. [Google Scholar] [CrossRef]
- Tian, L.; Tan, Y.; Chen, G.; Wang, G.; Sun, J.; Ou, S.; Chen, W.; Bai, W. Metabolism of Anthocyanins and Consequent Effects on the Gut Microbiota. Crit. Rev. Food Sci. Nutr. 2019, 59, 982–991. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, Q.; Zhao, T.; Zhang, Z.; Mao, G.; Feng, W.; Wu, X.; Yang, L. Biotransformation and Metabolism of Three Mulberry Anthocyanin Monomers by Rat Gut Microflora. Food Chem. 2017, 237, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Makarewicz, M.; Drożdż, I.; Tarko, T.; Duda-Chodak, A. The Interactions between Polyphenols and Microorganisms, Especially Gut Microbiota. Antioxidants (Basel) 2021, 10, 188. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, I.; Faria, A.; Calhau, C.; de Freitas, V.; Mateus, N. Bioavailability of Anthocyanins and Derivatives. J. Funct. Foods 2014, 7, 54–66. [Google Scholar] [CrossRef]
- Fleschhut, J.; Kratzer, F.; Rechkemmer, G.; Kulling, S.E. Stability and Biotransformation of Various Dietary Anthocyanins in Vitro. Eur. J. Nutr. 2006, 45, 7–18. [Google Scholar] [CrossRef]
- Zhao, R.; Shen, G.X. Impact of Anthocyanin Component and Metabolite of Saskatoon Berry on Gut Microbiome and Relationship with Fecal Short Chain Fatty Acids in Diet-Induced Insulin Resistant Mice. J. Nutr. Biochem. 2023, 111, 109201. [Google Scholar] [CrossRef]
- Dong, A.; Lin, C.-W.; Echeveste, C.E.; Huang, Y.-W.; Oshima, K.; Yearsley, M.; Chen, X.; Yu, J.; Wang, L.-S. Protocatechuic Acid, a Gut Bacterial Metabolite of Black Raspberries, Inhibits Adenoma Development and Alters Gut Microbiome Profiles in Apcmin/+ Mice. J. Cancer Prev. 2022, 27, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhu, R.; Ye, X.; Sun, Y.; Tang, Q.; Liu, Y.; Yan, F.; Yu, T.; Zheng, X.; Tu, P. Food-Derived Cyanidin-3-O-Glucoside Reverses Microplastic Toxicity via Promoting Discharge and Modulating the Gut Microbiota in Mice. Food Funct. 2022, 13, 1447–1458. [Google Scholar] [CrossRef] [PubMed]
- Mu, J.; Xu, J.; Wang, L.; Chen, C.; Chen, P. Anti-Inflammatory Effects of Purple Sweet Potato Anthocyanin Extract in DSS-Induced Colitis: Modulation of Commensal Bacteria and Attenuated Bacterial Intestinal Infection. Food Funct. 2021, 12, 11503–11514. [Google Scholar] [CrossRef]
- Liu, X.; Wang, L.; Jing, N.; Jiang, G.; Liu, Z. Biostimulating Gut Microbiome with Bilberry Anthocyanin Combo to Enhance Anti-PD-L1 Efficiency against Murine Colon Cancer. Microorganisms 2020, 8, 175. [Google Scholar] [CrossRef]
- Nakano, H.; Wu, S.; Sakao, K.; Hara, T.; He, J.; Garcia, S.; Shetty, K.; Hou, D.-X. Bilberry Anthocyanins Ameliorate NAFLD by Improving Dyslipidemia and Gut Microbiome Dysbiosis. Nutrients 2020, 12, 3252. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Wang, T.T.Y.; Tang, Q.; Xue, C.; Li, R.W.; Wu, V.C.H. Malvidin 3-glucoside Modulated Gut Microbial Dysbiosis and Global Metabolome Disrupted in a Murine Colitis Model Induced by Dextran Sulfate Sodium. Mol. Nutr. Food Res. 2019, 63, 1900455. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Li, Y.; Hou, D.-X.; Wu, S. The Effects and Mechanisms of Cyanidin-3-Glucoside and Its Phenolic Metabolites in Maintaining Intestinal Integrity. Antioxidants (Basel) 2019, 8, 479. [Google Scholar] [CrossRef] [PubMed]
- Overall, J.; Bonney, S.; Wilson, M.; Beermann, A., III; Grace, M.; Esposito, D.; Lila, M.; Komarnytsky, S. Metabolic Effects of Berries with Structurally Diverse Anthocyanins. Int. J. Mol. Sci. 2017, 18, 422. [Google Scholar] [CrossRef]
- Esposito, D.; Damsud, T.; Wilson, M.; Grace, M.H.; Strauch, R.; Li, X.; Lila, M.A.; Komarnytsky, S. Black Currant Anthocyanins Attenuate Weight Gain and Improve Glucose Metabolism in Diet-Induced Obese Mice with Intact, but Not Disrupted, Gut Microbiome. J. Agric. Food Chem. 2015, 63, 6172–6180. [Google Scholar] [CrossRef]
- Hidalgo, M.; Oruna-Concha, M.J.; Kolida, S.; Walton, G.E.; Kallithraka, S.; Spencer, J.P.E.; Gibson, G.R.; de Pascual-Teresa, S. Metabolism of Anthocyanins by Human Gut Microflora and Their Influence on Gut Bacterial Growth. J. Agric. Food Chem. 2012, 60, 3882–3890. [Google Scholar] [CrossRef]
- Wang, H.; Cao, G.; Prior, R.L. Oxygen Radical Absorbing Capacity of Anthocyanins. J. Agric. Food Chem. 1997, 45, 304–309. [Google Scholar] [CrossRef]
- Stintzing, F.C.; Stintzing, A.S.; Carle, R.; Frei, B.; Wrolstad, R.E. Colour and Antioxidant Properties of Cyanidin-Based Anthocyanin Pigments. J. Agric. Food Chem. 2002, 50, 6172–6181. [Google Scholar] [CrossRef]
- Tamura, H.; Yamagami, A. Antioxidative Activity of Monoacylated Anthocyanins Isolated from Muscat Bailey A Grape. J. Agric. Food Chem. 1994, 42, 1612–1615. [Google Scholar] [CrossRef]
- Tsuda, T.; Shiga, K.; Ohshima, K.; Kawakishi, S.; Osawa, T. Inhibition of Lipid Peroxidation and the Active Oxygen Radical Scavenging Effect of Anthocyanin Pigments Isolated from Phaseolus Vulgaris L. Biochem. Pharmacol. 1996, 52, 1033–1039. [Google Scholar] [CrossRef]
- Brown, J.E.; Kelly, M.F. Inhibition of Lipid Peroxidation by Anthocyanins, Anthocyanidins and Their Phenolic Degradation Products. Eur. J. Lipid Sci. Technol. 2007, 109, 66–71. [Google Scholar] [CrossRef]
- Speciale, A.; Chirafisi, J.; Saija, A.; Cimino, F. Nutritional Antioxidants and Adaptive Cell Responses: An Update. Curr. Mol. Med. 2011, 11, 770–789. [Google Scholar] [CrossRef] [PubMed]
- Speciale, A.; Anwar, S.; Canali, R.; Chirafisi, J.; Saija, A.; Virgili, F.; Cimino, F. Cyanidin-3-O -Glucoside Counters the Response to TNF-Alpha of Endothelial Cells by Activating Nrf2 Pathway. Mol. Nutr. Food Res. 2013, 57, 1979–1987. [Google Scholar] [CrossRef]
- Vendrame, S.; Klimis-Zacas, D. Anti-Inflammatory Effect of Anthocyanins via Modulation of Nuclear Factor- B and Mitogen-Activated Protein Kinase Signaling Cascades. Nutr. Rev. 2015, 73, 348–358. [Google Scholar] [CrossRef]
- Poulose, S.M.; Fisher, D.R.; Larson, J.; Bielinski, D.F.; Rimando, A.M.; Carey, A.N.; Schauss, A.G.; Shukitt-Hale, B. Anthocyanin-Rich Açai (Euterpe Oleracea Mart.) Fruit Pulp Fractions Attenuate Inflammatory Stress Signaling in Mouse Brain BV-2 Microglial Cells. J. Agric. Food Chem. 2012, 60, 1084–1093. [Google Scholar] [CrossRef]
- Hassimotto, N.M.A.; Moreira, V.; Nascimento, N.G. do; Souto, P.C.M. de C.; Teixeira, C.; Lajolo, F.M. Inhibition of Carrageenan-Induced Acute Inflammation in Mice by Oral Administration of Anthocyanin Mixture from Wild Mulberry and Cyanidin-3-Glucoside. Biomed Res. Int. 2013, 1–10. [Google Scholar]
- Cui, H.-X.; Chen, J.-H.; Li, J.-W.; Cheng, F.-R.; Yuan, K. Protection of Anthocyanin from Myrica Rubra against Cerebral Ischemia-Reperfusion Injury via Modulation of the TLR4/NF-ΚB and NLRP3 Pathways. Molecules 2018, 23, 1788. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Wang, Y.; He, Q.; Li, L.; Xie, H.; Zhao, Y.; Zhao, J. Nrf2 Inhibits NLRP3 Inflammasome Activation through Regulating Trx1/TXNIP Complex in Cerebral Ischemia Reperfusion Injury. Behav. Brain Res. 2018, 336, 32–39. [Google Scholar] [CrossRef]
- Putta, S.; Yarla, N.S.; Peluso, I.; Tiwari, D.K.; Reddy, G.V.; Giri, P.V.; Kumar, N.; Malla, R.; Rachel, V.; Bramhachari, P.V.; et al. Anthocyanins: Multi-Target Agents for Prevention and Therapy of Chronic Diseases. Curr. Pharm. Des. 2018, 23, 6321–6346. [Google Scholar] [CrossRef]
- Zhou, P.; Pan, Y.; Yang, W.; Yang, B.; Ou, S.; Liu, P.; Zheng, J. Hepatoprotective Effect of Cyanidin-3-O-Glucoside–Lauric Acid Ester against H2O2-Induced Oxidative Damage in LO2 Cells. J. Funct. Foods 2023, 107, 105642. [Google Scholar] [CrossRef]
- Romualdo, G.R.; de Souza, I.P.; de Souza, L.V.; Prata, G.B.; Fraga-Silva, T.F. de C.; Sartori, A.; Borguini, R.G.; Santiago, M.C.P. de A.; Fernandes, A.A.H.; Cogliati, B.; et al. Beneficial Effects of Anthocyanin-Rich Peels of Myrtaceae Fruits on Chemically-Induced Liver Fibrosis and Carcinogenesis in Mice. Food Res. Int. 2021, 139, 109964. [Google Scholar] [CrossRef]
- Zuo, A.; Wang, S.; Liu, L.; Yao, Y.; Guo, J. Understanding the Effect of Anthocyanin Extracted from Lonicera Caerulea L. on Alcoholic Hepatosteatosis. Biomed. Pharmacother. 2019, 117, 109087. [Google Scholar] [CrossRef] [PubMed]
- Popović, D.; Kocić, G.; Katić, V.; Zarubica, A.; Veličković, L.J.; Ničković, V.P.; Jović, A.; Veljković, A.; Petrović, V.; Rakić, V.; et al. Anthocyanins Protect Hepatocytes against CCl4-Induced Acute Liver Injury in Rats by Inhibiting pro-Inflammatory Mediators, Polyamine Catabolism, Lipocalin-2, and Excessive Proliferation of Kupffer Cells. Antioxidants (Basel) 2019, 8, 451. [Google Scholar] [CrossRef] [PubMed]
- Nussbaum, L.; Hogea, L.M.; Călina, D.; Andreescu, N.; Grădinaru, R.; Ştefănescu, R. Modern Treatment Approaches in Psychoses. Pharmacogenetic, Neuroimagistic and Clinical Implications. Farmacia 2017, 65, 75–81. [Google Scholar]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef]
- Russo, M.V.; McGavern, D.B. Immune Surveillance of the CNS Following Infection and Injury. Trends Immunol. 2015, 36, 637–650. [Google Scholar] [CrossRef]
- Min, J.; Yu, S.-W.; Baek, S.-H.; Nair, K.M.; Bae, O.-N.; Bhatt, A.; Kassab, M.; Nair, M.G.; Majid, A. Neuroprotective Effect of Cyanidin-3-O-Glucoside Anthocyanin in Mice with Focal Cerebral Ischemia. Neurosci. Lett. 2011, 500, 157–161. [Google Scholar] [CrossRef]
- Cásedas, G.; González-Burgos, E.; Smith, C.; López, V.; Gómez-Serranillos, M.P. Regulation of Redox Status in Neuronal SH-SY5Y Cells by Blueberry (Vaccinium Myrtillus L.) Juice, Cranberry (Vaccinium Macrocarpon A.) Juice and Cyanidin. Food Chem. Toxicol. 2018, 118, 572–580. [Google Scholar] [CrossRef]
- Pacheco, S.M.; Soares, M.S.P.; Gutierres, J.M.; Gerzson, M.F.B.; Carvalho, F.B.; Azambuja, J.H.; Schetinger, M.R.C.; Stefanello, F.M.; Spanevello, R.M. Anthocyanins as a Potential Pharmacological Agent to Manage Memory Deficit, Oxidative Stress and Alterations in Ion Pump Activity Induced by Experimental Sporadic Dementia of Alzheimer’s Type. J. Nutr. Biochem. 2018, 56, 193–204. [Google Scholar] [CrossRef]
- Amin, F.U.; Shah, S.A.; Badshah, H.; Khan, M.; Kim, M.O. Anthocyanins Encapsulated by PLGA@PEG Nanoparticles Potentially Improved Its Free Radical Scavenging Capabilities via P38/JNK Pathway against Aβ1–42-Induced Oxidative Stress. J. Nanobiotechnology 2017, 15. [Google Scholar] [CrossRef]
- Kim, M.J.; Rehman, S.U.; Amin, F.U.; Kim, M.O. Enhanced Neuroprotection of Anthocyanin-Loaded PEG-Gold Nanoparticles against Aβ1-42-Induced Neuroinflammation and Neurodegeneration via the NF-KB /JNK/GSK3β Signaling Pathway. Nanomedicine 2017, 13, 2533–2544. [Google Scholar] [CrossRef]
- Mulabagal, V.; Lang, G.A.; DeWitt, D.L.; Dalavoy, S.S.; Nair, M.G. Anthocyanin Content, Lipid Peroxidation and Cyclooxygenase Enzyme Inhibitory Activities of Sweet and Sour Cherries. J. Agric. Food Chem. 2009, 57, 1239–1246. [Google Scholar] [CrossRef]
- Joseph, J.A.; Arendash, G.; Gordon, M.; Diamond, D.; Shukitt-Hale, B.; Morgan, D.; Denisova, N.A. Blueberry Supplementation Enhances Signaling and Prevents Behavioral Deficits in an Alzheimer Disease Model. Nutr. Neurosci. 2003, 6, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Cassidy, A.; Schwarzschild, M.A.; Rimm, E.B.; Ascherio, A. Habitual Intake of Dietary Flavonoids and Risk of Parkinson Disease. Neurology 2012, 78, 1138–1145. [Google Scholar] [CrossRef] [PubMed]
- Krikorian, R.; Nash, T.A.; Shidler, M.D.; Shukitt-Hale, B.; Joseph, J.A. Concord Grape Juice Supplementation Improves Memory Function in Older Adults with Mild Cognitive Impairment. Br. J. Nutr. 2010, 103, 730–734. [Google Scholar] [CrossRef]
- Krikorian, R.; Shidler, M.D.; Nash, T.A.; Kalt, W.; Vinqvist-Tymchuk, M.R.; Shukitt-Hale, B.; Joseph, J.A. Blueberry Supplementation Improves Memory in Older Adults. J. Agric. Food Chem. 2010, 58, 3996–4000. [Google Scholar] [CrossRef] [PubMed]
- Ockermann, P.; Headley, L.; Lizio, R.; Hansmann, J. A Review of the Properties of Anthocyanins and Their Influence on Factors Affecting Cardiometabolic and Cognitive Health. Nutrients 2021, 13, 2831. [Google Scholar] [CrossRef]
- Yang, Y.; Andrews, M.C.; Hu, Y.; Wang, D.; Qin, Y.; Zhu, Y.; Ni, H.; Ling, W. Anthocyanin Extract from Black Rice Significantly Ameliorates Platelet Hyperactivity and Hypertriglyceridemia in Dyslipidemic Rats Induced by High Fat Diets. J. Agric. Food Chem. 2011, 59, 6759–6764. [Google Scholar] [CrossRef] [PubMed]
- García-Conesa, M.-T.; Chambers, K.; Combet, E.; Pinto, P.; Garcia-Aloy, M.; Andrés-Lacueva, C.; de Pascual-Teresa, S.; Mena, P.; Konic Ristic, A.; Hollands, W.; et al. Meta-Analysis of the Effects of Foods and Derived Products Containing Ellagitannins and Anthocyanins on Cardiometabolic Biomarkers: Analysis of Factors Influencing Variability of the Individual Responses. Int. J. Mol. Sci. 2018, 19, 694. [Google Scholar] [CrossRef]
- Luís, Â.; Domingues, F.; Pereira, L. Association between Berries Intake and Cardiovascular Diseases Risk Factors: A Systematic Review with Meta-Analysis and Trial Sequential Analysis of Randomized Controlled Trials. Food Funct. 2018, 9, 740–757. [Google Scholar] [CrossRef]
- Cassidy, A.; Mukamal, K.J.; Liu, L.; Franz, M.; Eliassen, A.H.; Rimm, E.B. High Anthocyanin Intake Is Associated with a Reduced Risk of Myocardial Infarction in Young and Middle-Aged Women. Circulation 2013, 127, 188–196. [Google Scholar] [CrossRef]
- Cassidy, A.; Bertoia, M.; Chiuve, S.; Flint, A.; Forman, J.; Rimm, E.B. Habitual Intake of Anthocyanins and Flavanones and Risk of Cardiovascular Disease in Men. Am. J. Clin. Nutr. 2016, 104, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Joo, H.; Choi, S.; Lee, Y.; Lee, E.; Park, M.; Park, K.; Kim, C.-S.; Lim, Y.; Park, J.-T.; Jeon, B. Anthocyanin-Rich Extract from Red Chinese Cabbage Alleviates Vascular Inflammation in Endothelial Cells and APO E−/− Mice. Int. J. Mol. Sci. 2018, 19, 816. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wei, X.; Yan, X.; Jin, T.; Ling, W. Protocatechuic Acid, a Metabolite of Anthocyanins, Inhibits Monocyte Adhesion and Reduces Atherosclerosis in Apolipoprotein E-Deficient Mice. J. Agric. Food Chem. 2010, 58, 12722–12728. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Kang, J.; Xie, C.; Burris, R.; Ferguson, M.E.; Badger, T.M.; Nagarajan, S. Dietary Blueberries Attenuate Atherosclerosis in Apolipoprotein E-Deficient Mice by Upregulating Antioxidant Enzyme Expression. J. Nutr. 2010, 140, 1628–1632. [Google Scholar] [CrossRef] [PubMed]
- Petroni, K.; Trinei, M.; Fornari, M.; Calvenzani, V.; Marinelli, A.; Micheli, L.A.; Pilu, R.; Matros, A.; Mock, H.-P.; Tonelli, C.; et al. Dietary Cyanidin 3-Glucoside from Purple Corn Ameliorates Doxorubicin-Induced Cardiotoxicity in Mice. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 462–469. [Google Scholar] [CrossRef]
- Skates, E.; Overall, J.; DeZego, K.; Wilson, M.; Esposito, D.; Lila, M.A.; Komarnytsky, S. Berries Containing Anthocyanins with Enhanced Methylation Profiles Are More Effective at Ameliorating High Fat Diet-Induced Metabolic Damage. Food Chem. Toxicol. 2018, 111, 445–453. [Google Scholar] [CrossRef]
- Bognar, E.; Sarszegi, Z.; Szabo, A.; Debreceni, B.; Kalman, N.; Tucsek, Z.; Sumegi, B.; Gallyas, F. Antioxidant and Anti-Inflammatory Effects in RAW264.7 Macrophages of Malvidin, a Major Red Wine Polyphenol. PLoS One 2013, 8, e65355. [Google Scholar] [CrossRef]
- Hardie, D.G.; Ashford, M.L.J. AMPK: Regulating Energy Balance at the Cellular and Whole Body Levels. Physiology (Bethesda) 2014, 29, 99–107. [Google Scholar] [CrossRef]
- López, M. Hypothalamic AMPK and Energy Balance. Eur. J. Clin. Invest. 2018, 48, e12996. [Google Scholar] [CrossRef]
- Park, S.; Kang, S.; Jeong, D.-Y.; Jeong, S.-Y.; Park, J.J.; Yun, H.S. Cyanidin and Malvidin in Aqueous Extracts of Black Carrots Fermented with Aspergillus Oryzae Prevent the Impairment of Energy, Lipid and Glucose Metabolism in Estrogen-Deficient Rats by AMPK Activation. Genes Nutr. 2015, 10. [Google Scholar] [CrossRef]
- Wu, T.; Jiang, Z.; Yin, J.; Long, H.; Zheng, X. Anti-Obesity Effects of Artificial Planting Blueberry (Vaccinium Ashei) Anthocyanin in High-Fat Diet-Treated Mice. Int. J. Food Sci. Nutr. 2016, 67, 257–264. [Google Scholar] [CrossRef]
- Jamar, G.; Estadella, D.; Pisani, L.P. Contribution of Anthocyanin-Rich Foods in Obesity Control through Gut Microbiota Interactions: Anthocyanin-Rich Foods in Obesity Control. Biofactors 2017, 43, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Kang, M.; Xie, Q.; Xu, B.; Sun, C.; Chen, K.; Wu, Y. Anthocyanins from Chinese Bayberry Extract Protect β Cells from Oxidative Stress-Mediated Injury via HO-1 Upregulation. J. Agric. Food Chem. 2011, 59, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Fu, D.X.; Wilkinson, M.; Simmons, B.; Wu, M.; Betts, N.M.; Du, M.; Lyons, T.J. Strawberries Decrease Atherosclerotic Markers in Subjects with Metabolic Syndrome. Nutr. Res. 2010, 30, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Wu, X.; Gu, L.; Hager, T.J.; Hager, A.; Howard, L.R. Whole Berries versus Berry Anthocyanins: Interactions with Dietary Fat Levels in the C57BL/6J Mouse Model of Obesity. J. Agric. Food Chem 2008, 56, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Vendrame, S.; Daugherty, A.; Kristo, A.S.; Riso, P.; Klimis-Zacas, D. Wild Blueberry (Vaccinium Angustifolium) Consumption Improves Inflammatory Status in the Obese Zucker Rat Model of the Metabolic Syndrome. J. Nutr. Biochem. 2013, 24, 1508–1512. [Google Scholar] [CrossRef]
- Tomay, F.; Marinelli, A.; Leoni, V.; Caccia, C.; Matros, A.; Mock, H.-P.; Tonelli, C.; Petroni, K. Purple Corn Extract Induces Long-Lasting Reprogramming and M2 Phenotypic Switch of Adipose Tissue Macrophages in Obese Mice. J. Transl. Med. 2019, 17. [Google Scholar] [CrossRef]
- Pojer, E.; Mattivi, F.; Johnson, D.; Stockley, C.S. The Case for Anthocyanin Consumption to Promote Human Health: A Review: Anthocyanins and Human Health. Compr. Rev. Food Sci. Food Saf. 2013, 12, 483–508. [Google Scholar] [CrossRef]
- Lin, B.-W.; Gong, C.-C.; Song, H.-F.; Cui, Y.-Y. Effects of Anthocyanins on the Prevention and Treatment of Cancer: Anthocyanins and Prevention & Treatment of Cancer. Br. J. Pharmacol. 2017, 174, 1226–1243. [Google Scholar]
- Dreesen, O.; Brivanlou, A.H. Signaling Pathways in Cancer and Embryonic Stem Cells. Stem Cell Rev. 2007, 3, 7–17. [Google Scholar] [CrossRef]
- Kausar, H.; Jeyabalan, J.; Aqil, F.; Chabba, D.; Sidana, J.; Singh, I.P.; Gupta, R.C. Berry Anthocyanidins Synergistically Suppress Growth and Invasive Potential of Human Non-Small-Cell Lung Cancer Cells. Cancer Lett. 2012, 325, 54–62. [Google Scholar] [CrossRef]
- Pratheeshkumar, P.; Son, Y.-O.; Wang, X.; Divya, S.P.; Joseph, B.; Hitron, J.A.; Wang, L.; Kim, D.; Yin, Y.; Roy, R.V.; et al. Cyanidin-3-Glucoside Inhibits UVB-Induced Oxidative Damage and Inflammation by Regulating MAP Kinase and NF-ΚB Signaling Pathways in SKH-1 Hairless Mice Skin. Toxicol. Appl. Pharmacol. 2014, 280, 127–137. [Google Scholar] [CrossRef]
- Choi, Y.J.; Choi, Y.J.; Kim, N.; Nam, R.H.; Lee, S.; Lee, H.S.; Lee, H.-N.; Surh, Y.-J.; Lee, D.H. Açaí Berries Inhibit Colon Tumorigenesis in Azoxymethane/Dextran Sulfate Sodium-Treated Mice. Gut Liver 2017, 11, 243–252. [Google Scholar] [CrossRef]
- Fragoso, M.F.; Romualdo, G.R.; Vanderveer, L.A.; Franco-Barraza, J.; Cukierman, E.; Clapper, M.L.; Carvalho, R.F.; Barbisan, L.F. Lyophilized Açaí Pulp (Euterpe Oleracea Mart) Attenuates Colitis-Associated Colon Carcinogenesis While Its Main Anthocyanin Has the Potential to Affect the Motility of Colon Cancer Cells. Food Chem. Toxicol. 2018, 121, 237–245. [Google Scholar] [CrossRef]
- Long, H.-L.; Zhang, F.-F.; Wang, H.-L.; Yang, W.-S.; Hou, H.-T.; Yu, J.-K.; Liu, B. Mulberry Anthocyanins Improves Thyroid Cancer Progression Mainly by Inducing Apoptosis and Autophagy Cell Death. Kaohsiung J. Med. Sci. 2018, 34, 255–262. [Google Scholar] [CrossRef]
- Salehi, B.; Sharifi-Rad, J.; Cappellini, F.; Reiner, Ž.; Zorzan, D.; Imran, M.; Sener, B.; Kilic, M.; El-Shazly, M.; Fahmy, N.M.; et al. The Therapeutic Potential of Anthocyanins: Current Approaches Based on Their Molecular Mechanism of Action. Front. Pharmacol. 2020, 11, 1300. [Google Scholar] [CrossRef]
- Lippert, E.; Ruemmele, P.; Obermeier, F.; Goelder, S.; Kunst, C.; Rogler, G.; Dunger, N.; Messmann, H.; Hartmann, A.; Endlicher, E. Anthocyanins Prevent Colorectal Cancer Development in a Mouse Model. Digestion 2017, 95, 275–280. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, Y.; Zhang, W.; Peng, X.; Zhou, J.; Li, F.; Han, B.; Liu, X.; Ou, Y.; Yu, X. Delphinidin Induced Protective Autophagy via MTOR Pathway Suppression and AMPK Pathway Activation in HER-2 Positive Breast Cancer Cells. BMC Cancer 2018, 18. [Google Scholar] [CrossRef]
- Nomi, Y; Iwasaki-Kurashige, K; Matsumoto, H. Therapeutic Effects of Anthocyanins for Vision and Eye Health. Molecules 2019, 24, 3311. [Google Scholar] [CrossRef]
- Miyake, S.; Takahashi, N.; Sasaki, M.; Kobayashi, S.; Tsubota, K.; Ozawa, Y. Vision Preservation during Retinal Inflammation by Anthocyanin-Rich Bilberry Extract: Cellular and Molecular Mechanism. Lab. Invest. 2012, 92, 102–109. [Google Scholar] [CrossRef]
- Shim, S.H.; Kim, J.M.; Choi, C.Y.; Kim, C.Y.; Park, K.H. Ginkgo Biloba Extract and Bilberry Anthocyanins Improve Visual Function in Patients with Normal Tension Glaucoma. J. Med. Food 2012, 15, 818–823. [Google Scholar] [CrossRef] [PubMed]
- Ohguro, H.; Ohguro, I.; Katai, M.; Tanaka, S. Two-Year Randomized, Placebo-Controlled Study of Black Currant Anthocyanins on Visual Field in Glaucoma. Ophthalmologica 2012, 228, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Thiraphatthanavong, P.; Wattanathorn, J.; Muchimapura, S.; Wipawee, T.-M.; Wannanon, P.; Terdthai, T.-U.; Suriharn, B.; Lertrat, K. Preventive Effect of Zea Mays L. (Purple Waxy Corn) on Experimental Diabetic Cataract. Biomed Res. Int. 2014, 507435. [Google Scholar]
- Paik, S.-S.; Jeong, E.; Jung, S.W.; Ha, T.J.; Kang, S.; Sim, S.; Jeon, J.H.; Chun, M.-H.; Kim, I.-B. Anthocyanins from the Seed Coat of Black Soybean Reduce Retinal Degeneration Induced by N-Methyl-N-Nitrosourea. Exp. Eye Res. 2012, 97, 55–62. [Google Scholar] [CrossRef]
- Genskowsky, E.; Puente, L.A.; Pérez-Álvarez, J.A.; Fernández-López, J.; Muñoz, L.A.; Viuda-Martos, M. Determination of Polyphenolic Profile, Antioxidant Activity and Antibacterial Properties of Maqui [Aristotelia Chilensis (Molina) Stuntz] a Chilean Blackberry: Antioxidant and Antibacterial Properties of Maqui. J. Sci. Food Agric. 2016, 96, 4235–4242. [Google Scholar] [CrossRef] [PubMed]
- Iturriaga, L.; Olabarrieta, I.; de Marañón, I.M. Antimicrobial Assays of Natural Extracts and Their Inhibitory Effect against Listeria Innocua and Fish Spoilage Bacteria, after Incorporation into Biopolymer Edible Films. Int. J. Food Microbiol. 2012, 158, 58–64. [Google Scholar] [CrossRef]
- Côté, J.; Caillet, S.; Doyon, G.; Dussault, D.; Sylvain, J.-F.; Lacroix, M. Antimicrobial Effect of Cranberry Juice and Extracts. Food Control 2011, 22, 1413–1418. [Google Scholar] [CrossRef]
- Puupponen-Pimiä, R.; Nohynek, L.; Meier, C.; Kähkönen, M.; Heinonen, M.; Hopia, A.; Oksman-Caldentey, K.M. Antimicrobial Properties of Phenolic Compounds from Berries. J. Appl. Microbiol. 2001, 90, 494–507. [Google Scholar] [CrossRef]
- Cisowska, A.; Wojnicz, D.; Hendrich, A.B. Anthocyanins as Antimicrobial Agents of Natural Plant Origin. Nat. Prod. Commun. 2011, 6, 149–156. [Google Scholar] [CrossRef]
- Manolescu, B.N.; Oprea, E.; Mititelu, M.; Ruta, L.L.; Farcasanu, I.C. Dietary Anthocyanins and Stroke: A Review of Pharmacokinetic and Pharmacodynamic Studies. Nutrients 2019, 11, 1479. [Google Scholar] [CrossRef]
- Ferrer-Gallego, R.; Soares, S.; Mateus, N.; Rivas-Gonzalo, J.; Escribano-Bailón, M.T.; Freitas, V. de New Anthocyanin–Human Salivary Protein Complexes. Langmuir 2015, 31, 8392–8401. [Google Scholar] [CrossRef] [PubMed]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A Standardised Staticin VitroDigestion Method Suitable for Food – an International Consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Sandhu, A.; Huang, Y.; Park, E.; Edirisinghe, I.; Burton-Freeman, B.M. The Effect of Dietary Factors on Strawberry Anthocyanins Oral Bioavailability. Food Funct. 2017, 8, 3970–3979. [Google Scholar] [CrossRef]
- Kamonpatana, K.; Giusti, M.M.; Chitchumroonchokchai, C.; MorenoCruz, M.; Riedl, K.M.; Kumar, P.; Failla, M.L. Susceptibility of Anthocyanins to Ex Vivo Degradation in Human Saliva. Food Chem. 2012, 135, 738–747. [Google Scholar] [CrossRef] [PubMed]
- Kamonpatana, K.; Failla, M.L.; Kumar, P.S.; Giusti, M.M. Anthocyanin Structure Determines Susceptibility to Microbial Degradation and Bioavailability to the Buccal Mucosa. J. Agric. Food Chem. 2014, 62, 6903–6910. [Google Scholar] [CrossRef] [PubMed]
- Lila, M.A.; Burton-Freeman, B.; Grace, M.; Kalt, W. Unraveling Anthocyanin Bioavailability for Human Health. Annu. Rev. Food Sci. Technol. 2016, 7, 375–393. [Google Scholar] [CrossRef]
- Pedersen, A.M.; Bardow, A.; Jensen, S.B.; Nauntofte, B. Saliva and Gastrointestinal Functions of Taste, Mastication, Swallowing and Digestion: Saliva and Gastrointestinal Functions. Oral Dis. 2002, 8, 117–129. [Google Scholar] [CrossRef]
- Henriques, J.F.; Serra, D.; Dinis, T.C.P.; Almeida, L.M. The Anti-Neuroinflammatory Role of Anthocyanins and Their Metabolites for the Prevention and Treatment of Brain Disorders. Int. J. Mol. Sci. 2020, 21, 8653. [Google Scholar] [CrossRef]
- He, J.; Magnuson, B.A.; Lala, G.; Tian, Q.; Schwartz, S.J.; Giusti, M.M. Intact Anthocyanins and Metabolites in Rat Urine and Plasma after 3 Months of Anthocyanin Supplementation. Nutr. Cancer 2006, 54, 3–12. [Google Scholar] [CrossRef]
- Borges, G.; Roowi, S.; Rouanet, J.-M.; Duthie, G.G.; Lean, M.E.J.; Crozier, A. The Bioavailability of Raspberry Anthocyanins and Ellagitannins in Rats. Mol. Nutr. Food Res. 2007, 51, 714–725. [Google Scholar] [CrossRef]
- Stalmach, A.; Edwards, C.A.; Wightman, J.D.; Crozier, A. Gastrointestinal Stability and Bioavailability of (Poly)Phenolic Compounds Following Ingestion of Concord Grape Juice by Humans. Mol. Nutr. Food Res. 2012, 56, 497–509. [Google Scholar] [CrossRef]
- Oliveira, H.; Roma-Rodrigues, C.; Santos, A.; Veigas, B.; Brás, N.; Faria, A.; Calhau, C.; de Freitas, V.; Baptista, P.V.; Mateus, N.; et al. GLUT1 and GLUT3 Involvement in Anthocyanin Gastric Transport- Nanobased Targeted Approach. Sci. Rep. 2019, 9, 789. [Google Scholar] [CrossRef]
- Passamonti, S.; Vrhovsek, U.; Mattivi, F. The Interaction of Anthocyanins with Bilitranslocase. Biochem. Biophys. Res. Commun. 2002, 296, 631–636. [Google Scholar] [CrossRef]
- Krga, I.; Milenkovic, D. Anthocyanins: From Sources and Bioavailability to Cardiovascular-Health Benefits and Molecular Mechanisms of Action. J. Agric. Food Chem. 2019, 67, 1771–1783. [Google Scholar] [CrossRef] [PubMed]
- Talavéra, S.; Felgines, C.; Texier, O.; Besson, C.; Lamaison, J.-L.; Rémésy, C. Anthocyanins Are Efficiently Absorbed from the Stomach in Anesthetized Rats. J. Nutr. 2003, 133, 4178–4182. [Google Scholar] [CrossRef]
- Shahidi, F.; Peng, H. Bioaccessibility and Bioavailability of Phenolic Compounds. J. Food Bioact. 2018, 4. [Google Scholar] [CrossRef]
- Hribar, U.; Ulrih, N.P. The Metabolism of Anthocyanins. Curr. Drug Metab. 2014, 15, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, A.; Minihane, A.-M. The Role of Metabolism (and the Microbiome) in Defining the Clinical Efficacy of Dietary Flavonoids. Am. J. Clin. Nutr. 2017, 105, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Passamonti, S.; Terdoslavich, M.; Franca, R.; Vanzo, A.; Tramer, F.; Braidot, E.; Petrussa, E.; Vianello, A. Bioavailability of Flavonoids: A Review of Their Membrane Transport and the Function of Bilitranslocase in Animal and Plant Organisms. Curr. Drug Metab. 2009, 10, 369–394. [Google Scholar] [CrossRef]
- Zou, T.-B.; Feng, D.; Song, G.; Li, H.-W.; Tang, H.-W.; Ling, W.-H. The Role of Sodium-Dependent Glucose Transporter 1 and Glucose Transporter 2 in the Absorption of Cyanidin-3-o-β-Glucoside in Caco-2 Cells. Nutrients 2014, 6, 4165–4177. [Google Scholar] [CrossRef]
- Kamiloglu, S.; Capanoglu, E.; Grootaert, C.; Van Camp, J. Anthocyanin Absorption and Metabolism by Human Intestinal Caco-2 Cells—A Review. Int. J. Mol. Sci. 2015, 16, 21555–21574. [Google Scholar] [CrossRef]
- Figueira, I.; Menezes, R.; Macedo, D.; Costa, I.; Dos Santos, C.N. Polyphenols beyond Barriers: A Glimpse into the Brain. Curr. Neuropharmacol. 2017, 15, 562–594. [Google Scholar] [CrossRef]
- Faria, A.; Pestana, D.; Azevedo, J.; Martel, F.; de Freitas, V.; Azevedo, I.; Mateus, N.; Calhau, C. Absorption of Anthocyanins through Intestinal Epithelial Cells - Putative Involvement of GLUT2. Mol. Nutr. Food Res. 2009, 53, 1430–1437. [Google Scholar] [CrossRef]
- Alzaid, F.; Cheung, H.-M.; Preedy, V.R.; Sharp, P.A. Regulation of Glucose Transporter Expression in Human Intestinal Caco-2 Cells Following Exposure to an Anthocyanin-Rich Berry Extract. PLoS One 2013, 8, e78932. [Google Scholar] [CrossRef]
- Lewandowska, U.; Szewczyk, K.; Hrabec, E.; Janecka, A.; Gorlach, S. Overview of Metabolism and Bioavailability Enhancement of Polyphenols. J. Agric. Food Chem. 2013, 61, 12183–12199. [Google Scholar] [CrossRef]
- Czank, C.; Cassidy, A.; Zhang, Q.; Morrison, D.J.; Preston, T.; Kroon, P.A.; Botting, N.P.; Kay, C.D. Human Metabolism and Elimination of the Anthocyanin, Cyanidin-3-Glucoside: A 13C-Tracer Study. Am. J. Clin. Nutr. 2013, 97, 995–1003. [Google Scholar] [CrossRef]
- Felgines, C.; Krisa, S.; Mauray, A.; Besson, C.; Lamaison, J.-L.; Scalbert, A.; Mérillon, J.-M.; Texier, O. Radiolabelled Cyanidin 3-O-Glucoside Is Poorly Absorbed in the Mouse. Br. J. Nutr. 2010, 103, 1738–1745. [Google Scholar] [CrossRef]
- Kalt, W.; McDonald, J.E.; Vinqvist-Tymchuk, M.R.; Liu, Y.; Fillmore, S.A.E. Human Anthocyanin Bioavailability: Effect of Intake Duration and Dosing. Food Funct. 2017, 8, 4563–4569. [Google Scholar] [CrossRef]
- Cooney, J.M.; Jensen, D.J.; McGhie, T.K. LC-MS Identification of Anthocyanins in Boysenberry Extract and Anthocyanin Metabolites in Human Urine Following Dosing. J. Sci. Food Agric. 2004, 84, 237–245. [Google Scholar] [CrossRef]
- Felgines, C.; Talavera, S.; Texier, O.; Gil-Izquierdo, A.; Lamaison, J.-L.; Remesy, C. Blackberry Anthocyanins Are Mainly Recovered from Urine as Methylated and Glucuronidated Conjugates in Humans. J. Agric. Food Chem. 2005, 53, 7721–7727. [Google Scholar] [CrossRef]
- Kalt, W.; McDonald, J.E.; Liu, Y.; Fillmore, S.A.E. Flavonoid Metabolites in Human Urine during Blueberry Anthocyanin Intake. J. Agric. Food Chem. 2017, 65, 1582–1591. [Google Scholar] [CrossRef] [PubMed]
- Fornasaro, S.; Ziberna, L.; Gasperotti, M.; Tramer, F.; Vrhovšek, U.; Mattivi, F.; Passamonti, S. Determination of Cyanidin 3-Glucoside in Rat Brain, Liver and Kidneys by UPLC/MS-MS and Its Application to a Short-Term Pharmacokinetic Study. Sci. Rep. 2016, 6, 22815. [Google Scholar] [CrossRef] [PubMed]
- Kalt, W.; Blumberg, J.B.; McDonald, J.E.; Vinqvist-Tymchuk, M.R.; Fillmore, S.A.E.; Graf, B.A.; O’Leary, J.M.; Milbury, P.E. Identification of Anthocyanins in the Liver, Eye, and Brain of Blueberry-Fed Pigs. J. Agric. Food Chem. 2008, 56, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Milbury, P.E.; Kalt, W. Xenobiotic Metabolism and Berry Flavonoid Transport across the Blood-Brain Barrier. J. Agric. Food Chem. 2010, 58, 3950–3956. [Google Scholar] [CrossRef]
- Chen, T.-Y.; Kritchevsky, J.; Hargett, K.; Feller, K.; Klobusnik, R.; Song, B.J.; Cooper, B.; Jouni, Z.; Ferruzzi, M.G.; Janle, E.M. Plasma Bioavailability and Regional Brain Distribution of Polyphenols from Apple/Grape Seed and Bilberry Extracts in a Young Swine Model. Mol. Nutr. Food Res. 2015, 59, 2432–2447. [Google Scholar] [CrossRef] [PubMed]
- Sakakibara, H.; Ogawa, T.; Koyanagi, A.; Kobayashi, S.; Goda, T.; Kumazawa, S.; Kobayashi, H.; Shimoi, K. Correction to Distribution and Excretion of Bilberry Anthocyanins in Mice. J. Agric. Food Chem. 2009, 57, 9856–9856. [Google Scholar] [CrossRef]
- Zimman, A.; Waterhouse, A.L. Enzymatic Synthesis of [3‘-O-Methyl-3H]Malvidin-3-Glucoside from Petunidin-3-Glucoside. J. Agric. Food Chem. 2002, 50, 2429–2431. [Google Scholar] [CrossRef]
- Wen, X.; Walle, T. Methylated Flavonoids Have Greatly Improved Intestinal Absorption and Metabolic Stability. Drug Metab. Dispos. 2006, 34, 1786–1792. [Google Scholar] [CrossRef]
- El Mohsen, M.A.; Marks, J.; Kuhnle, G.; Moore, K.; Debnam, E.; Kaila Srai, S.; Rice-Evans, C.; Spencer, J.P.E. Absorption, Tissue Distribution and Excretion of Pelargonidin and Its Metabolites Following Oral Administration to Rats. Br. J. Nutr. 2006, 95, 51–58. [Google Scholar] [CrossRef]
- Wu, X.; Pittman, H.E., 3rd; Prior, R.L. Fate of Anthocyanins and Antioxidant Capacity in Contents of the Gastrointestinal Tract of Weanling Pigs Following Black Raspberry Consumption. J. Agric. Food Chem. 2006, 54, 583–589. [Google Scholar] [CrossRef]
- Carkeet, C.; Clevidence, B.A.; Novotny, J.A. Anthocyanin Excretion by Humans Increases Linearly with Increasing Strawberry Dose. J. Nutr. 2008, 138, 897–902. [Google Scholar] [CrossRef] [PubMed]
- Müller, L.; Bednár, P.; Barták, P.; Lemr, K.; Sevcík, J. Estimation of Partition Coefficients by MEKC Part 2: Anthocyanins. J. Sep. Sci. 2005, 28, 1285–1290. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Muccitelli, H.U.; Sánchez-Moreno, C.; Prior, R.L. Anthocyanins Are Absorbed in Glycated Forms in Elderly Women: A Pharmacokinetic Study. Am. J. Clin. Nutr. 2001, 73, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, A.K.; Huang, Y.; Xiao, D.; Park, E.; Edirisinghe, I.; Burton-Freeman, B. Pharmacokinetic Characterization and Bioavailability of Strawberry Anthocyanins Relative to Meal Intake. J. Agric. Food Chem. 2016, 64, 4891–4899. [Google Scholar] [CrossRef] [PubMed]
- Baron, G.; Altomare, A.; Regazzoni, L.; Redaelli, V.; Grandi, S.; Riva, A.; Morazzoni, P.; Mazzolari, A.; Carini, M.; Vistoli, G.; et al. Pharmacokinetic Profile of Bilberry Anthocyanins in Rats and the Role of Glucose Transporters: LC–MS/MS and Computational Studies. J. Pharm. Biomed. Anal. 2017, 144, 112–121. [Google Scholar] [CrossRef]
- Kay, C.D.; Mazza, G.; Holub, B.J.; Wang, J. Anthocyanin Metabolites in Human Urine and Serum. Br. J. Nutr. 2004, 91, 933–942. [Google Scholar] [CrossRef]
- Kay, C.D. Aspects of Anthocyanin Absorption, Metabolism and Pharmacokinetics in Humans. Nutr. Res. Rev. 2006, 19, 137–146. [Google Scholar] [CrossRef]
- Felgines, C.; Talavéra, S.; Texier, O.; Lamaison, J.-L.; Gonthier, M.-P.; Scalbert, A.; Rémésy, C. Strawberry Anthocyanins Are Recovered in Urine as Glucuro- and Sulfoconjugates in Humans. J. Nutr. 2003, 133, 1296–1301. [Google Scholar] [CrossRef]
- Kay, C.D.; Mazza, G. (joe); Holub, B.J. Anthocyanins Exist in the Circulation Primarily as Metabolites in Adult Men. J. Nutr. 2005, 135, 2582–2588. [Google Scholar] [CrossRef]
- Xie, L.; Lee, S.G.; Vance, T.M.; Wang, Y.; Kim, B.; Lee, J.-Y.; Chun, O.K.; Bolling, B.W. Bioavailability of Anthocyanins and Colonic Polyphenol Metabolites Following Consumption of Aronia Berry Extract. Food Chem. 2016, 211, 860–868. [Google Scholar] [CrossRef]
- Li, A.; Xiao, R.; He, S.; An, X.; He, Y.; Wang, C.; Yin, S.; Wang, B.; Shi, X.; He, J. Research Advances of Purple Sweet Potato Anthocyanins: Extraction, Identification, Stability, Bioactivity, Application, and Biotransformation. Molecules 2019, 24, 3816. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Zhang, Y. Biopolymer-Based Encapsulation of Anthocyanins as Reinforced Natural Colorants for Food Applications. J. Agric. Food Res. 2023, 11, 100488. [Google Scholar] [CrossRef]
- Yuan, Y.; Fan, Q.; Xu, X.; Wang, O.; Zhao, L.; Zhao, L. Nanocarriers Based on Polysaccharides for Improving the Stability and Bioavailability of Anthocyanins: A Review. Carbohydr. Polym. Technol. Appl. 2023, 100346. [Google Scholar] [CrossRef]
- Mehran, M.; Masoum, S.; Memarzadeh, M. Improvement of Thermal Stability and Antioxidant Activity of Anthocyanins of Echium Amoenum Petal Using Maltodextrin/Modified Starch Combination as Wall Material. Int. J. Biol. Macromol. 2020, 148, 768–776. [Google Scholar] [CrossRef]
- Wang, M.; Li, L.; Wan, M.; Lin, Y.; Tong, Y.; Cui, Y.; Deng, H.; Tan, C.; Kong, Y.; Meng, X. Preparing, Optimising, and Evaluating Chitosan Nanocapsules to Improve the Stability of Anthocyanins from Aronia Melanocarpa. RSC Adv. 2021, 11, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Li, Z.; Wang, C.; Liu, S.; Wang, Y.; Zhang, M.; Tian, Y.; Lv, J.; Xu, H.; Xia, G. Stability and Antioxidant Activity of Chitosan/β-Lactoglobulin on Anthocyanins from Aronia Melanocarpa. Lebenson. Wiss. Technol. 2023, 173, 114335. [Google Scholar] [CrossRef]
- Fernandes, A.; Rocha, M.A.A.; Santos, L.M.N.B.F.; Brás, J.; Oliveira, J.; Mateus, N.; de Freitas, V. Blackberry Anthocyanins: β-Cyclodextrin Fortification for Thermal and Gastrointestinal Stabilization. Food Chem. 2018, 245, 426–431. [Google Scholar] [CrossRef]
- Vukoja, J.; Buljeta, I.; Pichler, A.; Šimunović, J.; Kopjar, M. Formulation and Stability of Cellulose-Based Delivery Systems of Raspberry Phenolics. Processes (Basel) 2021, 9, 90. [Google Scholar] [CrossRef]
- Kanokpanont, S.; Yamdech, R.; Aramwit, P. Stability Enhancement of Mulberry-Extracted Anthocyanin Using Alginate/Chitosan Microencapsulation for Food Supplement Application. Artif. Cells Nanomed. Biotechnol. 2018, 46, 773–782. [Google Scholar] [CrossRef]
- Ahmad, M.; Ashraf, B.; Gani, A.; Gani, A. Microencapsulation of Saffron Anthocyanins Using β Glucan and β Cyclodextrin: Microcapsule Characterization, Release Behaviour & Antioxidant Potential during in-Vitro Digestion. Int. J. Biol. Macromol. 2018, 109, 435–442. [Google Scholar]
- Flores, F.P.; Singh, R.K.; Kerr, W.L.; Phillips, D.R.; Kong, F. In Vitro Release Properties of Encapsulated Blueberry (Vaccinium Ashei) Extracts. Food Chem. 2015, 168, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Lanna, E.G.; Bittencourt, V.C.E.; Moreira, A.M.S.; Da Silva, J.G.; Sousa, O.V.; Denadai, Â.M.L. Physicochemical Characterization and Biological Activities of the Ethanol Extract of Bryophyllum Pinnatum (Lam.) Oken Incorporated in β-Cyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 2016, 85, 247–259. [Google Scholar] [CrossRef]
- Norkaew, O.; Thitisut, P.; Mahatheeranont, S.; Pawin, B.; Sookwong, P.; Yodpitak, S.; Lungkaphin, A. Effect of Wall Materials on Some Physicochemical Properties and Release Characteristics of Encapsulated Black Rice Anthocyanin Microcapsules. Food Chem. 2019, 294, 493–502. [Google Scholar] [CrossRef]
- Oancea, A.-M.; Hasan, M.; Vasile, A.M.; Barbu, V.; Enachi, E.; Bahrim, G.; Râpeanu, G.; Silvi, S.; Stănciuc, N. Functional Evaluation of Microencapsulated Anthocyanins from Sour Cherries Skins Extract in Whey Proteins Isolate. Lebenson. Wiss. Technol. 2018, 95, 129–134. [Google Scholar] [CrossRef]
- Mueller, D.; Jung, K.; Winter, M.; Rogoll, D.; Melcher, R.; Kulozik, U.; Schwarz, K.; Richling, E. Encapsulation of Anthocyanins from Bilberries – Effects on Bioavailability and Intestinal Accessibility in Humans. Food Chem. 2018, 248, 217–224. [Google Scholar] [CrossRef]
- Santos, S.S. dos; Paraíso, C.M.; Romanini, E.B.; Correa, V.G.; Peralta, R.M.; Costa, S.C. da; Santos Junior, O. de O.; Visentainer, J.V.; Reis, M.H.M.; Madrona, G.S. Bioavailability of Blackberry Pomace Microcapsules by Using Different Techniques: An Approach for Yogurt Application. Innov. Food Sci. Emerg. Technol. 2022, 81, 103111. [Google Scholar] [CrossRef]
- Flores, G.; Ruiz del Castillo, M.L.; Costabile, A.; Klee, A.; Bigetti Guergoletto, K.; Gibson, G.R. In Vitro Fermentation of Anthocyanins Encapsulated with Cyclodextrins: Release, Metabolism and Influence on Gut Microbiota Growth. J. Funct. Foods 2015, 16, 50–57. [Google Scholar] [CrossRef]
- Ge, J.; Yue, X.; Wang, S.; Chi, J.; Liang, J.; Sun, Y.; Gao, X.; Yue, P. Nanocomplexes Composed of Chitosan Derivatives and β-Lactoglobulin as a Carrier for Anthocyanins: Preparation, Stability and Bioavailability in Vitro. Food Res. Int. 2019, 116, 336–345. [Google Scholar] [CrossRef]
- Oidtmann, J.; Schantz, M.; Mäder, K.; Baum, M.; Berg, S.; Betz, M.; Kulozik, U.; Leick, S.; Rehage, H.; Schwarz, K.; et al. Preparation and Comparative Release Characteristics of Three Anthocyanin Encapsulation Systems. J. Agric. Food Chem. 2012, 60, 844–851. [Google Scholar] [CrossRef]
- Betz, M.; Kulozik, U. Whey Protein Gels for the Entrapment of Bioactive Anthocyanins from Bilberry Extract. Int. Dairy J. 2011, 21, 703–710. [Google Scholar] [CrossRef]
- Ji, Y. Synthesis of Porous Starch Microgels for the Encapsulation, Delivery and Stabilization of Anthocyanins. J. Food Eng. 2021, 302, 110552. [Google Scholar] [CrossRef]
- Chatterjee, N.S.; Dara, P.K.; Perumcherry Raman, S.; Vijayan, D.K.; Sadasivam, J.; Mathew, S.; Ravishankar, C.N.; Anandan, R. Nanoencapsulation in Low-molecular-weight Chitosan Improves in Vivo Antioxidant Potential of Black Carrot Anthocyanin. J. Sci. Food Agric. 2021, 101, 5264–5271. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhang, X.; Tie, S.; Hou, S.; Wang, H.; Song, Y.; Rai, R.; Tan, M. Facile Synthesis of Nano-Nanocarriers from Chitosan and Pectin with Improved Stability and Biocompatibility for Anthocyanins Delivery: An in Vitro and in Vivo Study. Food Hydrocoll. 2020, 109, 106114. [Google Scholar] [CrossRef]
- Wang, W.; Jung, J.; Zhao, Y. Chitosan-Cellulose Nanocrystal Microencapsulation to Improve Encapsulation Efficiency and Stability of Entrapped Fruit Anthocyanins. Carbohydr. Polym. 2017, 157, 1246–1253. [Google Scholar] [CrossRef] [PubMed]
- Jafari, S.M.; Ghalegi Ghalenoei, M.; Dehnad, D. Influence of Spray Drying on Water Solubility Index, Apparent Density, and Anthocyanin Content of Pomegranate Juice Powder. Powder Technol. 2017, 311, 59–65. [Google Scholar] [CrossRef]
- Akhavan, S.; Assadpour, E.; Katouzian, I.; Jafari, S.M. Lipid Nano Scale Cargos for the Protection and Delivery of Food Bioactive Ingredients and Nutraceuticals. Trends Food Sci. Technol. 2018, 74, 132–146. [Google Scholar] [CrossRef]
- Assadpour, E.; Jafari, S.M. A Systematic Review on Nanoencapsulation of Food Bioactive Ingredients and Nutraceuticals by Various Nanocarriers. Crit. Rev. Food Sci. Nutr. 2019, 59, 3129–3151. [Google Scholar] [CrossRef]
- Demirci, M.; Caglar, M.Y.; Cakir, B.; Gülseren, İ. Encapsulation by Nanoliposomes. In Nanoencapsulation Technologies for the Food and Nutraceutical Industries; Jafari, S.M., Ed.; Elsevier: Academic Press, 2017; pp. 74–113. [Google Scholar]
- Ravanfar, R.; Tamaddon, A.M.; Niakousari, M.; Moein, M.R. Preservation of Anthocyanins in Solid Lipid Nanoparticles: Optimization of a Microemulsion Dilution Method Using the Placket–Burman and Box–Behnken Designs. Food Chem. 2016, 199, 573–580. [Google Scholar] [CrossRef]
- Mulia, K.; Putri, G.A.; Krisanti, E. Encapsulation of Mangosteen Extract in Virgin Coconut Oil Based Nanoemulsions: Preparation and Characterization for Topical Formulation. Mater. Sci. For. 2018, 929, 234–242. [Google Scholar] [CrossRef]
- Rafiee, Z.; Barzegar, M.; Sahari, M.A.; Maherani, B. Nanoliposomal Carriers for Improvement the Bioavailability of High – Valued Phenolic Compounds of Pistachio Green Hull Extract. Food Chem. 2017, 220, 115–122. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




