Submitted:
04 September 2023
Posted:
06 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
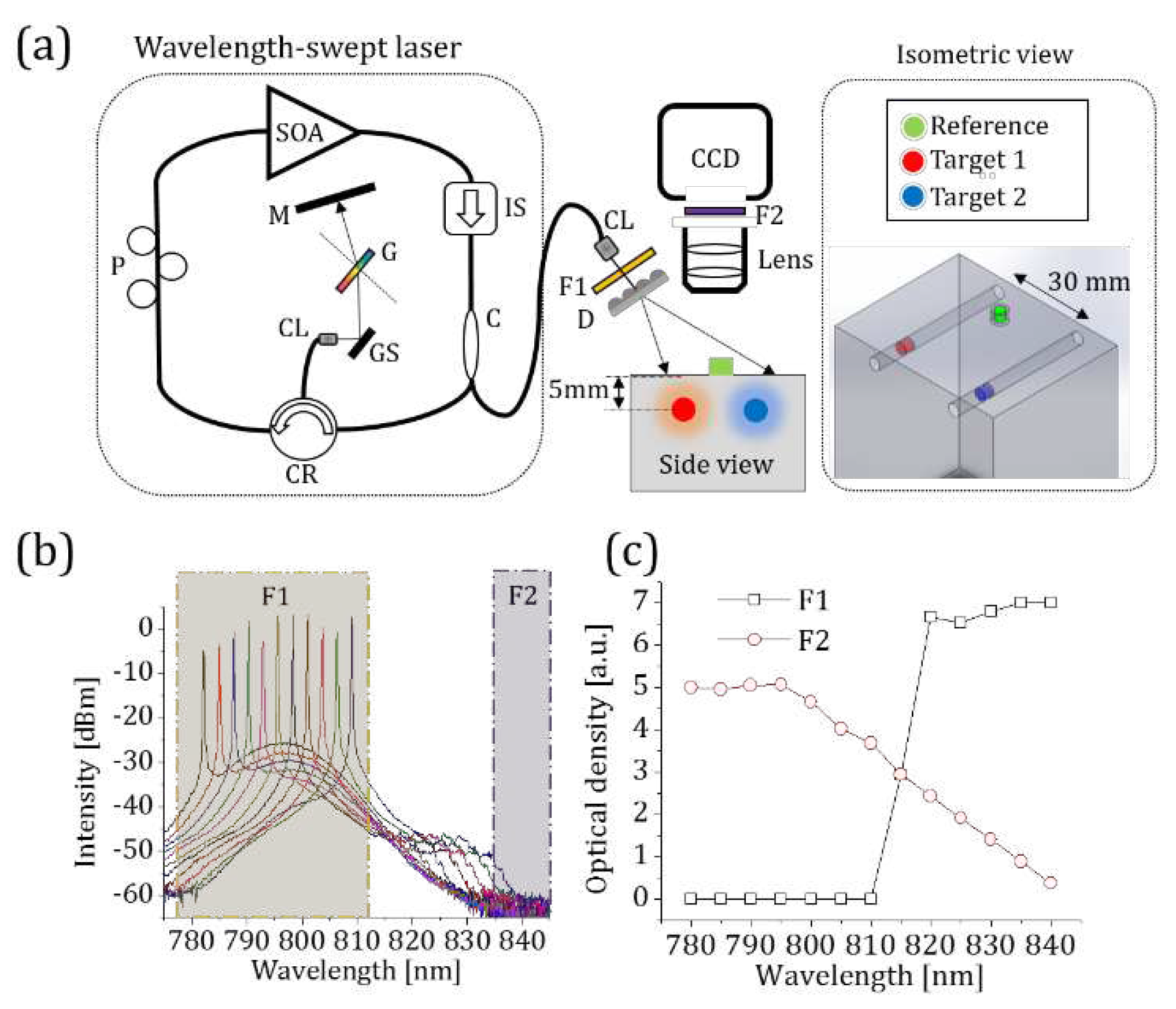
2.1. HER-NIRF System
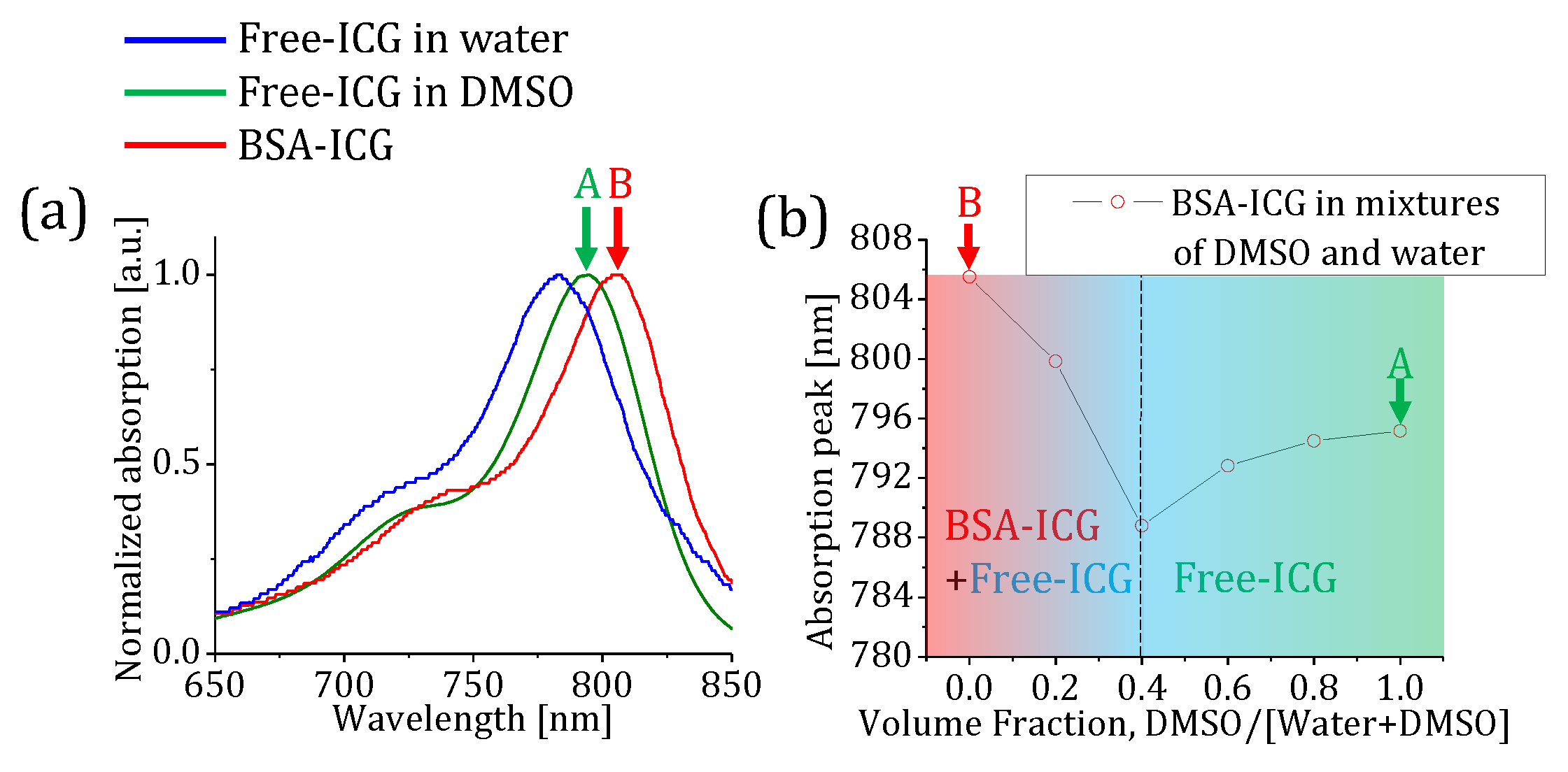
2.2. ICG characteristic in water, DMSO, and bounded to bovine serum albumin
3. Results
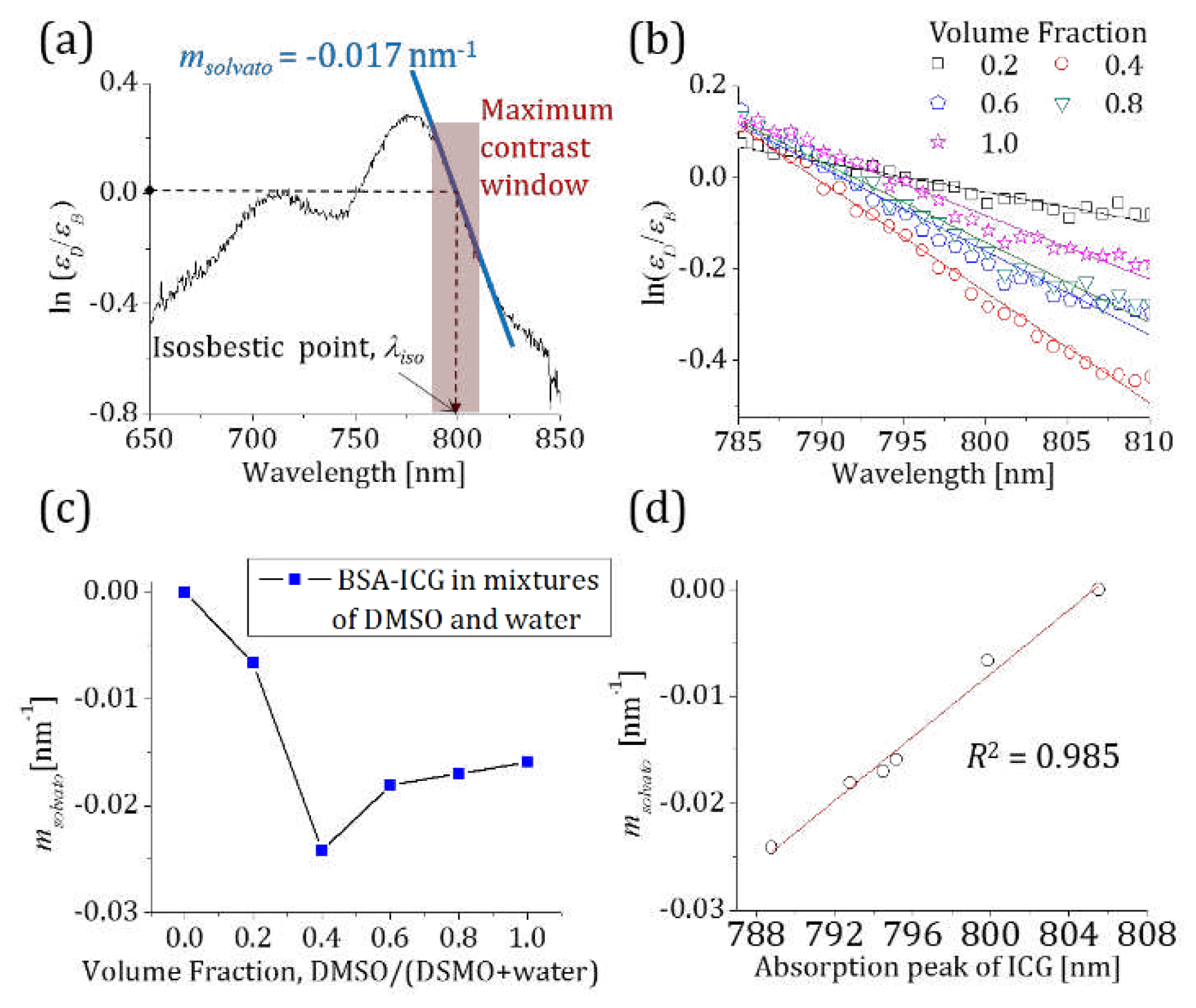
3.1. The ratio of extinction coefficients of DMSO- and BSA-ICG
3.2. Phantom Experiment Results
3.2.1. Free-space measurements using CCD
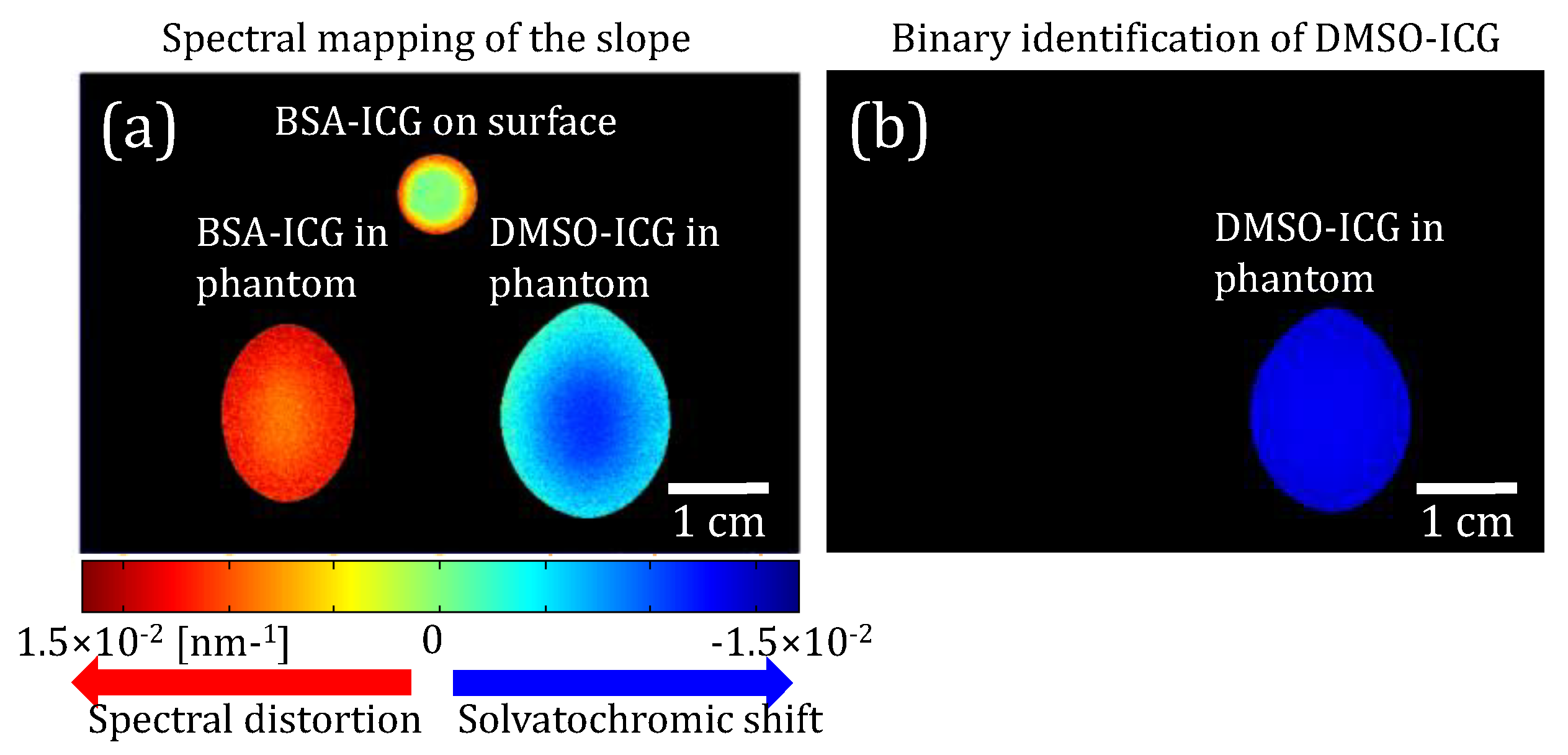
3.2.2. Phantom Measurements using HER-NIRF system
3.2.3. HER-NIRF spectral map for DSMO sensing
4. Discussion
5. Conclusion
Author Contributions
Funding
Acknowledgments
References
- Karim, M.; Boikess, R.S.; Schwartz, R.A.; Cohen, P.J. Dimethyl sulfoxide (DMSO): a solvent that may solve selected cutaneous clinical challenges. Arch Dermatol Res 2023, 315, 1465–1472. [Google Scholar] [CrossRef] [PubMed]
- Swanson, B.N. Medical use of dimethyl sulfoxide (DMSO). Rev Clin Basic Pharm 1985, 5, 1–33. [Google Scholar] [PubMed]
- Paul, M.M. Interval therapy with dimethyl sulfoxide. Ann N Y Acad Sci 1967, 141, 586–598. [Google Scholar] [CrossRef] [PubMed]
- Kulah, A.; Akar, M.; Baykut, L. Dimethyl sulfoxide in the management of patient with brain swelling and increased intracranial pressure after severe closed head injury. Neurochirurgia (Stuttg) 1990, 33, 177–180. [Google Scholar] [CrossRef]
- ROSENBAUM, E.E.; HERSCHLER, R.J.; JACOB, S.W. DIMETHYL SULFOXIDE IN MUSCULOSKELETAL DISORDERS. JAMA 1965, 192, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Peeker, R.; Haghsheno, M.A.; Holmäng, S.; Fall, M. Intravesical bacillus Calmette-Guerin and dimethyl sulfoxide for treatment of classic and nonulcer interstitial cystitis: a prospective, randomized double-blind study. J Urol 2000, 164, 1912–1915; discussion 1915. [Google Scholar] [CrossRef]
- Amemori, S.; Iwakiri, R.; Endo, H.; Ootani, A.; Ogata, S.; Noda, T.; Tsunada, S.; Sakata, H.; Matsunaga, H.; Mizuguchi, M.; et al. Oral dimethyl sulfoxide for systemic amyloid A amyloidosis complication in chronic inflammatory disease: a retrospective patient chart review. J Gastroenterol 2006, 41, 444–449. [Google Scholar] [CrossRef]
- Marren, K. Dimethyl sulfoxide: an effective penetration enhancer for topical administration of NSAIDs. Phys Sportsmed 2011, 39, 75–82. [Google Scholar] [CrossRef]
- Otterbach, A.; Lamprecht, A. Enhanced Skin Permeation of Estradiol by Dimethyl Sulfoxide Containing Transdermal Patches. Pharmaceutics 2021, 13. [Google Scholar] [CrossRef]
- Liu, G.; Li, J.; Deng, S. Applications of Supercritical Anti-Solvent Process in Preparation of Solid Multicomponent Systems. Pharmaceutics 2021, 13. [Google Scholar] [CrossRef]
- Pauli, G.; Tang, W.L.; Li, S.D. Development and Characterization of the Solvent-Assisted Active Loading Technology (SALT) for Liposomal Loading of Poorly Water-Soluble Compounds. Pharmaceutics 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Phiwayinkosi, V.D.a.B.B.N.a.S.E.M.-M.a.T.M.N.a.S.S.a.P.O.a.V.M.a.J.L.a. Chapter 25 - The impact of dimethyl sulfoxide on oxidative stress and cytotoxicity in various experimental models. In Toxicology, Vinood, B.P.a.V.R.P., Ed. Academic Press, 2021; pp. 243–261. [Google Scholar] [CrossRef]
- Morris, C.; de Wreede, L.; Scholten, M.; Brand, R.; van Biezen, A.; Sureda, A.; Dickmeiss, E.; Trneny, M.; Apperley, J.; Chiusolo, P.; et al. Should the standard dimethyl sulfoxide concentration be reduced? Results of a European Group for Blood and Marrow Transplantation prospective noninterventional study on usage and side effects of dimethyl sulfoxide. Transfusion 2014, 54, 2514–2522. [Google Scholar] [CrossRef] [PubMed]
- Kollerup Madsen, B.; Hilscher, M.; Zetner, D.; Rosenberg, J. Adverse reactions of dimethyl sulfoxide in humans: a systematic review. F1000Res 2018, 7, 1746. [Google Scholar] [CrossRef] [PubMed]
- Marini, A.; Muñoz-Losa, A.; Biancardi, A.; Mennucci, B. What is solvatochromism? J Phys Chem B 2010, 114, 17128–17135. [Google Scholar] [CrossRef]
- Truksa, J.; Kratochvíl, M.; Richtár, J.; Ivanová, L.; Weiter, M.; Krajčovič, J.; Lukeš, V. Spectroscopic behavior differences between lumazine and alloxazine in the DMSO-water mixture. Spectrochim Acta A Mol Biomol Spectrosc 2023, 302, 122998. [Google Scholar] [CrossRef] [PubMed]
- Chao, A.H.; Schulz, S.A.; Povoski, S.P. The application of indocyanine green (ICG) and near-infrared (NIR) fluorescence imaging for assessment of the lymphatic system in reconstructive lymphaticovenular anastomosis surgery. Expert Rev Med Devices 2021, 18, 367–374. [Google Scholar] [CrossRef]
- Schaafsma, B.E.; Verbeek, F.P.; Rietbergen, D.D.; van der Hiel, B.; van der Vorst, J.R.; Liefers, G.J.; Frangioni, J.V.; van de Velde, C.J.; van Leeuwen, F.W.; Vahrmeijer, A.L. Clinical trial of combined radio- and fluorescence-guided sentinel lymph node biopsy in breast cancer. Br J Surg 2013, 100, 1037–1044. [Google Scholar] [CrossRef]
- van der Vorst, J.R.; Hutteman, M.; Mieog, J.S.; de Rooij, K.E.; Kaijzel, E.L.; Lowik, C.W.; Putter, H.; Kuppen, P.J.; Frangioni, J.V.; van de Velde, C.J.; et al. Near-infrared fluorescence imaging of liver metastases in rats using indocyanine green. J Surg Res 2012, 174, 266–271. [Google Scholar] [CrossRef]
- Schaafsma, B.E.; van der Vorst, J.R.; Gaarenstroom, K.N.; Peters, A.A.; Verbeek, F.P.; de Kroon, C.D.; Trimbos, J.B.; van Poelgeest, M.I.; Frangioni, J.V.; van de Velde, C.J.; et al. Randomized comparison of near-infrared fluorescence lymphatic tracers for sentinel lymph node mapping of cervical cancer. Gynecol Oncol 2012. [Google Scholar] [CrossRef]
- Huang, P.; Intes, X.; Nioka, S.; Chance, a.B. Simulation of delivery of ICG injected intravenously into human subject for breast cancer detection. SPIE 2003, 4929, 322–329. [Google Scholar]
- Gurfinkel, M.; Thompson, A.B.; Ralston, W.; Troy, T.L.; Moore, A.L.; Moore, T.A.; Gust, J.D.; Tatman, D.; Reynolds, J.S.; Muggenburg, B.; et al. Pharmacokinetics of ICG and HPPH-car for the detection of normal and tumor tissue using fluorescence, near-infrared reflectance imaging: a case study. Photochem Photobiol 2000, 72, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Yoneya, S.; Saito, T.; Komatsu, Y.; Koyama, I.; Takahashi, K.; Duvoll-Young, J. Binding properties of indocyanine green in human blood. Invest Ophthalmol Vis Sci 1998, 39, 1286–1290. [Google Scholar] [PubMed]
- Berezin, M.Y.; Lee, H.; Akers, W.; Achilefu, S. Near infrared dyes as lifetime solvatochromic probes for micropolarity measurements of biological systems. Biophys J 2007, 93, 2892–2899. [Google Scholar] [CrossRef] [PubMed]
- Farouk Nouizi and Jaedu Cho and Maha Algarawi and Chang-Seok Kim and Gultekin, G. Application of a wavelength-swept laser for spectrally resolved wide-field near-infrared fluorescence imaging. Opt. Continuum 2022, 1, 1768. [Google Scholar] [CrossRef]
- Yun, H.L.a.J.F.d.B.a.B.H.P.a.E.C.W.L.a.R.Y.a.S.H. Optical frequency domain imaging with a rapidly swept laser in the 815--870 nm range. Opt. Express 2006, 14, 5937. [Google Scholar] [CrossRef]
- Joseph, D.M.a.M.T.E.-H.a.I.B.a.L.A.T.a.L.M.a.N.G.a.A.M.R.a.C.B.a. Simultaneous multimodal ophthalmic imaging using swept-source spectrally encoded scanning laser ophthalmoscopy and optical coherence tomography. Biomed. Opt. Express 2017, 8, 193. [Google Scholar] [CrossRef]
- Cho, J.; Gulsen, G.; Kim, C.-S. 800-nm-centered swept laser for spectroscopic optical coherence tomography. Laser Physics 2014, 24, 045605. [Google Scholar] [CrossRef]
- Mustari, A.; Nishidate, I.; Wares, M.A.; Maeda, T.; Kawauchi, S.; Sato, S.; Sato, M.; Aizu, Y. Agarose-based Tissue Mimicking Optical Phantoms for Diffuse Reflectance Spectroscopy. J Vis Exp 2018. [Google Scholar] [CrossRef]
- Lualdi, M.; Colombo, A.; Farina, B.; Tomatis, S.; Marchesini, R. A phantom with tissue-like optical properties in the visible and near infrared for use in photomedicine. Lasers Surg Med 2001, 28, 237–243. [Google Scholar] [CrossRef]
- Boas, D.A.; O'Leary, M.A.; Chance, B.; Yodh, A.G. Scattering and wavelength transduction of diffuse photon density waves. Physical Review. E. Statistical Physics, Plasmas, Fluids, and Related Interdisciplinary Topics 1993, 47, R2999–R3002. [Google Scholar] [CrossRef]
- Nouizi, F.; Brooks, J.; Zuro, D.M.; Hui, S.K.; Gulsen, G. Development of a theranostic preclinical fluorescence molecular tomography/cone beam CT-guided irradiator platform. Biomed Opt Express 2022, 13, 6100–6112. [Google Scholar] [CrossRef] [PubMed]
- Nouizi, F.; Kwong, T.C.; Ruiz, J.; Cho, J.; Chan, Y.W.; Ikemura, K.; Erkol, H.; Sampathkumaran, U.; Gulsen, G. A thermo-sensitive fluorescent agent based method for excitation light leakage rejection for fluorescence molecular tomography. Phys Med Biol 2019, 64, 035007. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.; Lim, G.; Hong, K.S.; Cho, J.; Gulsen, G.; Kim, C.S. Effect of Shot Noise on Simultaneous Sensing in Frequency Division Multiplexed Diffuse Optical Tomographic Imaging Process. Sensors (Basel) 2017, 17. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.B.; Sevick-Muraca, E.M. Near-infrared fluorescence contrast-enhanced imaging with intensified charge-coupled device homodyne detection: measurement precision and accuracy. J Biomed Opt 2003, 8, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Yao, T.; Yuan, B. An ICCD camera-based time-domain ultrasound-switchable fluorescence imaging system. Sci Rep 2019, 9, 10552. [Google Scholar] [CrossRef]
- Zhu, B.; Sevick-Muraca, E.M. A review of performance of near-infrared fluorescence imaging devices used in clinical studies. Br J Radiol 2015, 88, 20140547. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




