1. Introduction
Idiopathic pulmonary arterial hypertension (IPAH) is a progressive and life-threatening disease with pulmonary vasculature remodeling leading to right-sided heart failure. According to mortality risk assessment, IPAH is treated with a combination therapy targeting three distinct signaling pathways (endothelin, nitric oxide, and prostacyclin) [
1,
2]. Epoprostenol is prostaglandin I2 with a high recommendation level for patients with severe pulmonary arterial hypertension (PAH) categorized in World Health Organization functional class III or IV [
3,
4]. In addition, the treatment of pulmonary hypertension is associated with various side effects and encounters situations where side effects are difficult to manage. In prostaglandin I2, such as epoprostenol, it has been reported that approximately 3.0-6.7% develop hyperthyroidism/Grave's disease/painless thyroiditis [
5,
6]. Thyroid disease requires management of thyroid function and thyroid gland enlargement, although benign thyroid disease with goiter resulting in severe airway stenosis has been reported, but is rare [7-9]. Thyroid hormones may directly affect the pulmonary vasculature and increase pulmonary arterial pressure. It is necessary to control thyroid function in PAH patients with thyroid dysfunction. This case report shows an IPAH patient with a giant goiter and airway stenosis after long-term intravenous epoprostenol therapy.
2. Case report
A 34-year-old woman was diagnosed with primary pulmonary hypertension (PPH). At that time, the current IPAH was called PPH. Then she underwent right heart catheterization, and her mean pulmonary artery pressure was 73 mmHg. She complained of dyspnea at rest corresponding to World Health Organization functional class IV. After diagnosing with PPH, she was treated with continuous intravenous epoprostenol. At 37 years of age, she started complaining of neck swelling. Thyroid studies showed Thyroid-stimulating hormone (TSH) = 0.01 μIU/mL (normal range: 0.35-4.94 μIU/mL), Free T3 = 19.88 pg/mL (normal range: 1.71-3.71 pg/mL), Free T4 = 4.86 pg/mL (normal range: 0.70-1.48 pg/mL), thyroid stimulating antibody = 465% (normal range: 0-180%), so she was diagnosed with Grave’s disease and prescribed thiamazole. Epoprostenol at this time was 15 ng/kg/min. Epoprostenol was titrated to decrease mean pulmonary artery pressure in combination with an endothelin receptor antagonist and a phosphodiesterase 5 inhibitor [
10]. Epoprostenol maintained at 60-70 ng/kg/min after 14 years of treatment, and mean pulmonary artery pressure decreased to 30-35 mmHg. Thyroid function was controlled by therapy for Grave’s disease using thiamazole and levothyroxine. Nevertheless, her thyroid gland enlargement worsened as epoprostenol was titrated (
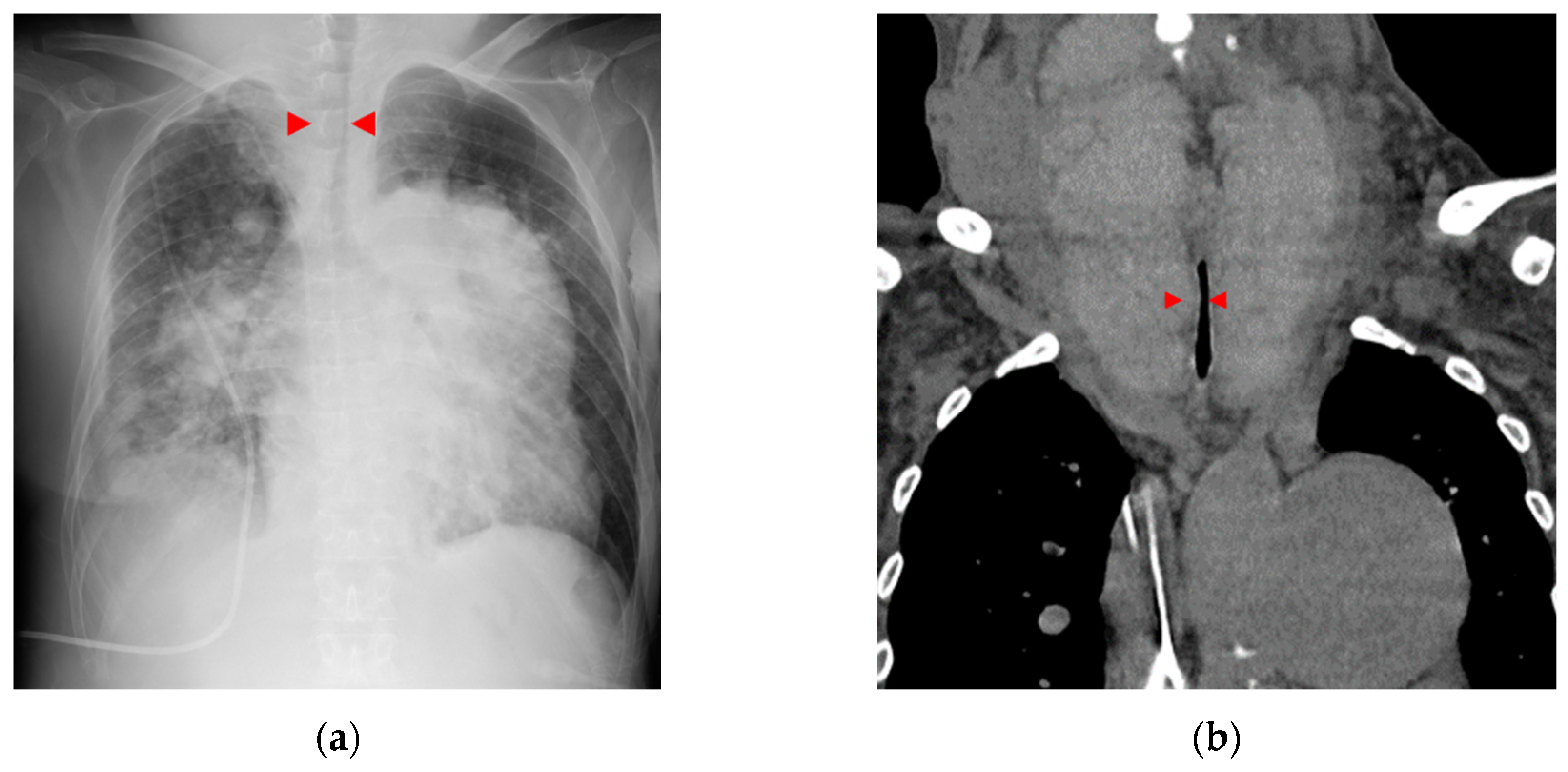
Figure 1a, b). At 56 years of age, she was admitted to the local hospital due to worsening dyspnea. However, cardiogenic shock and respiratory failure with hypercarbonemia were observed, and she was transferred to our hospital the next day. At admission to our hospital, the patient’s vital signs were blood pressure of 97/62 mmHg, pulse of 117 bpm, and oxygen saturation of 88% with oxygen at 10 L/min through the mask. On examination, the auscultation revealed coarse crackles in her lungs and strider in her neck. Radiography of the chest revealed bilateral pulmonary congestion and airway stenosis (
Figure 2a). Computed tomography of the chest also revealed airway stenosis by her giant goiter (
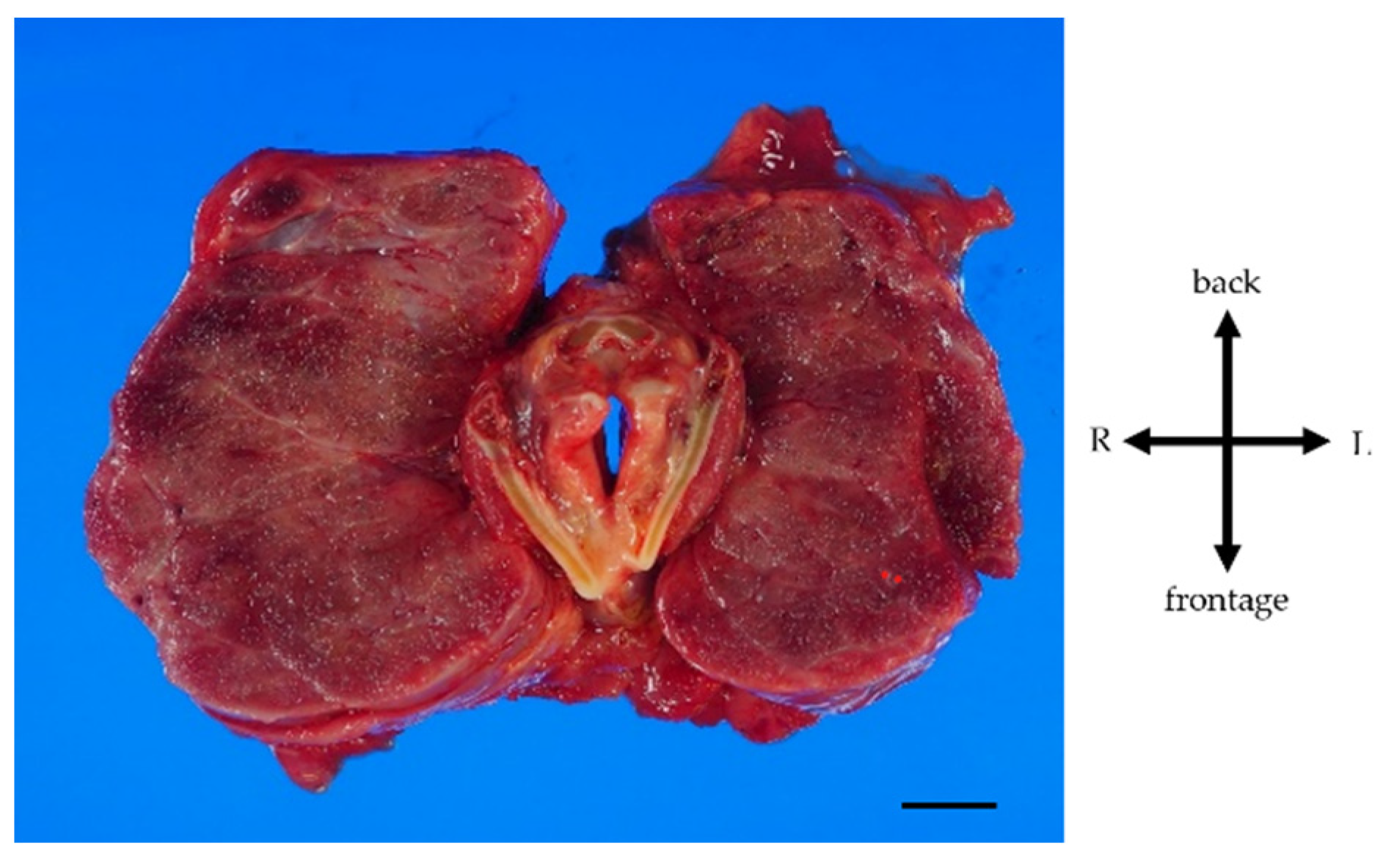
Figure 2b). Blood gas analysis on 10 L/min of oxygen showed pH: 7.2, PaO2: 63.7 mmHg, PaCO2: 73.9 mmHg and HCO3-: 28.4 mmol/L, indicating respiratory failure with hypercarbonemia. Thyroid studies showed TSH = 2.265 μIU/mL, Free T3 = 1.52 pg/mL, Free T4 = 0.90 pg/mL. Tracheal intubation was difficult due to severe airway stenosis. Treatment with noninvasive positive pressure ventilation, dobutamine, and noradrenaline was ineffective, and she passed away on the 12th day after admission. Pathological autopsy was performed. Gross thyroid findings included diffuse enlargement (weight 675 g, size 14 cm x 6 cm) and compressive constriction of the upper airway by the thyroid gland (
Figure 3). Histological findings of the thyroid gland showed increased thyroid follicle growth and increased colloidal resorption, suggesting a hyperthyroid state (
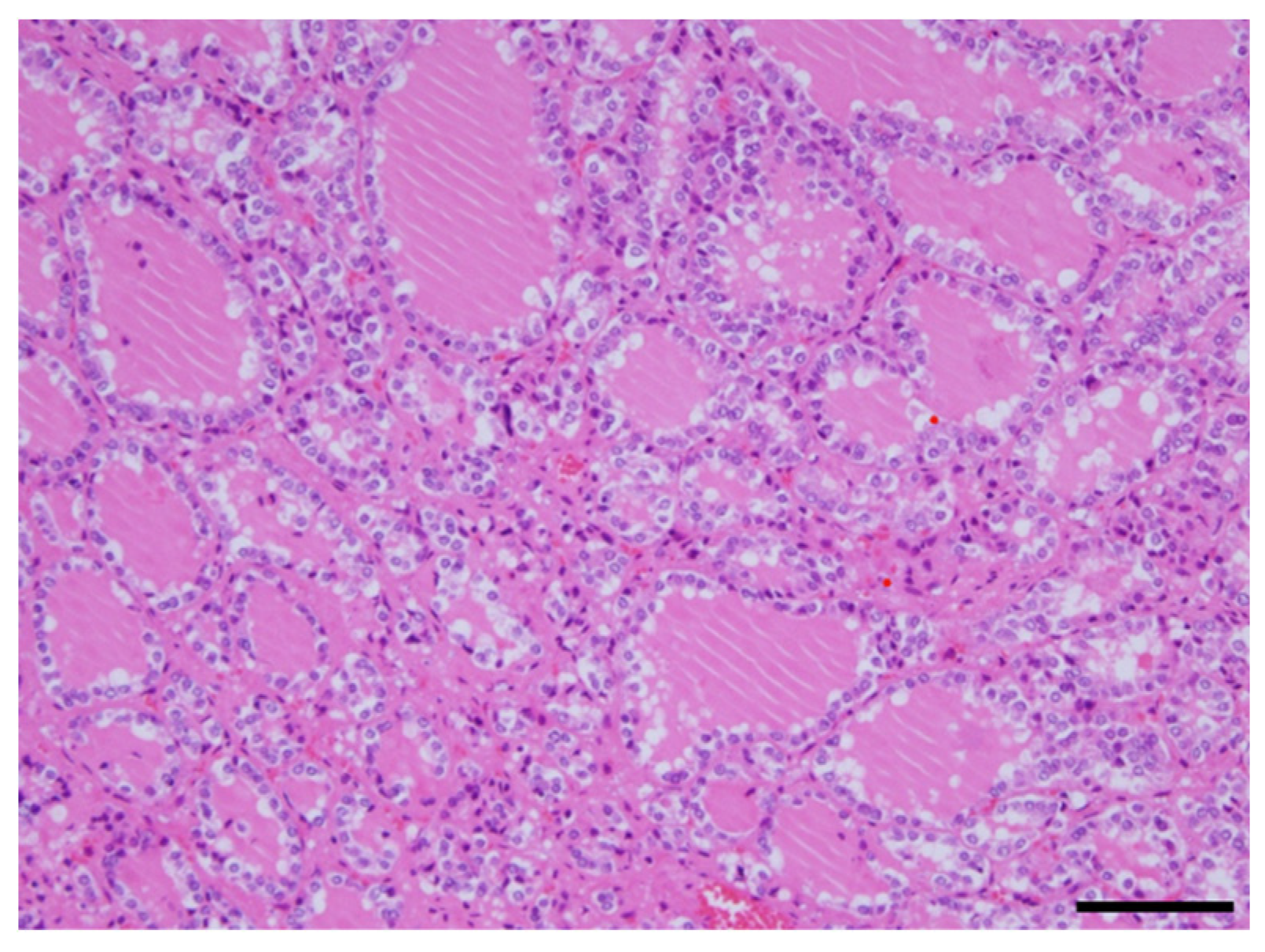
Figure 4). There were no obvious malignant findings. The cause of death was considered to be respiratory failure due to airway narrowing caused by thyroid gland enlargement. The enlarged goiter was thought to be due to continuous intravenous epoprostenol therapy for IPAH.
4. Discussion
We experienced a case of a massive goiter that led to airway stenosis during long-term continuous intravenous epoprostenol therapy. The enlarged goiter of this case doesn’t contradict hyperthyroidism according to Grave’s disease by the pathological findings. It is rare to report the enlarged goiter with airway stenosis by prostaglandin I
2 derivatives. There is only one previously reported case of airway stenosis as in this case [
11], but pathological findings are not discussed. This is the first case report of airway stenosis by giant goiter due to prostaglandin I
2 derivatives which was also pathologically autopsied. There are two mechanisms of goiter by prostaglandin I
2 derivatives. First, prostaglandin I
2 receptor is expressed in the thyroid follicular cells, and prostaglandin I
2 derivatives bind to this receptor and promote thyroid hormone synthesis [
12]. Second, prostaglandin I
2 derivatives active Th17 cells associated thyroid enlargement and Grave’s disease and promote the autoimmune response [
13,
14]. Although thyroid function was stable with thiamazole and levothyroxine in this case, her neck swelling was worsened. The cause was thought to be associated with the continuous intravenous epoprostenol therapy. Treatment for thyroid gland enlargement includes surgery or intra-iodine radiation therapy [
15]. But it was reported that it was difficult to remove the giant goiter weighing more than 200 g due to the increased risk of hemorrhage generally [
16], and the risk of perioperative mortality for patients with pulmonary hypertension is also high at approximately 8.3% [
17]. Thus, her predicted perioperative mortality was high. Furthermore, as the enlarged thyroid gland became huge, the need for radioactive iodine became excessive, making oral administration of radioactive iodine difficult. The patient also had severe pulmonary hypertension on high-flow continuous intravenous epoprostenol therapy, making it difficult to interrupt epoprostenol. Therefore, proactive intervention for goiter swelling might be considered in the earlier stages of epoprostenol therapy for PAH.
5. Conclusions
We experienced a rare case of IPAH with a giant goiter and airway stenosis after long-term intravenous epoprostenol therapy.
Author Contributions
Conceptualization, data curation and formal analysis, K.N. and T.Y.; Investigation and data collection, K.N., Y.S., Y.K., K.W., T.S., M.O., A.K. and K.S.; histopathological expertise, N.S., Y.H.; Writing-original draft, K.N.; Writing-review & editing, Y.T., K.N. and Y.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to their containing information that could compromise the privacy of research participants.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Runo, J.R.; Loyd, J.E. Primary pulmonary hypertension. Lancet 2003, 361, 1533–1544. [Google Scholar] [CrossRef] [PubMed]
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J.; Coats, A.J.S.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J 2022, 43, 3618–3731. [Google Scholar] [CrossRef] [PubMed]
- Degering, J.; Egenlauf, B.; Harutyunova, S.; Benjamin, N.; Salkić, A.; Xanthouli, P.; Eichstaedt, C.A.; Seeger, R.; Sitbon, O.; Grünig, E. Tolerability, safety and survival in patients with severe pulmonary arterial hypertension treated with intravenous epoprostenol (Veletri(®)): a prospective, 6-months, open label, observational, non-interventional study. Respir Res 2023, 24, 18. [Google Scholar] [CrossRef] [PubMed]
- Galiè, N.; Channick, R.N.; Frantz, R.P.; Grünig, E.; Jing, Z.C.; Moiseeva, O.; Preston, I.R.; Pulido, T.; Safdar, Z.; Tamura, Y.; et al. Risk stratification and medical therapy of pulmonary arterial hypertension. Eur Respir J 2019, 53. [Google Scholar] [CrossRef] [PubMed]
- Chadha, C.; Pritzker, M.; Mariash, C.N. Effect of epoprostenol on the thyroid gland: enlargement and secretion of thyroid hormone. Endocr Pract 2009, 15, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Satoh, M.; Aso, K.; Nakayama, T.; Saji, T. Effect of treatment with epoprostenol and endothelin receptor antagonists on the development of thyrotoxicosis in patients with pulmonary arterial hypertension. Endocr J 2017, 64, 1173–1180. [Google Scholar] [CrossRef]
- Kadhim, A.L.; Sheahan, P.; Timon, C. Management of life-threatening airway obstruction caused by benign thyroid disease. J Laryngol Otol 2006, 120, 1038–1041. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Zhou, H.; Ren, G.; Wang, Y.; Sui, Y. A new treatment strategy for airway obstruction induced by a giant benign goiter: A case report. Exp Ther Med 2023, 26, 376. [Google Scholar] [CrossRef]
- Raftos, J.R.; Ethell, A.T. Goitre causing acute respiratory arrest. Aust N Z J Surg 1996, 66, 331–332. [Google Scholar] [CrossRef]
- Akagi, S.; Nakamura, K.; Miyaji, K.; Ogawa, A.; Kusano, K.F.; Ito, H.; Matsubara, H. Marked hemodynamic improvements by high-dose epoprostenol therapy in patients with idiopathic pulmonary arterial hypertension. Circ J 2010, 74, 2200–2205. [Google Scholar] [CrossRef]
- Abughazaleh, S.; Safdar, Z. Goiter in a Patient with Pulmonary Arterial Hypertension Treated with Epoprostenol. Case Rep Pulmonol 2020, 1617253. [Google Scholar] [CrossRef]
- Kasai, K.; Hiraiwa, M.; Suzuki, Y.; Banba, N.; Emoto, T.; Nakamura, T.; Shimoda, S.I. Prostacyclin stimulation of adenylate cyclase activity in human thyroid membranes. Horm Metab Res 1986, 18, 625–629. [Google Scholar] [CrossRef]
- Torimoto, K.; Okada, Y.; Nakayamada, S.; Kubo, S.; Kurozumi, A.; Narisawa, M.; Tanaka, Y. Comprehensive immunophenotypic analysis reveals the pathological involvement of Th17 cells in Graves' disease. Sci Rep 2022, 12, 16880. [Google Scholar] [CrossRef]
- Boswell, M.G.; Zhou, W.; Newcomb, D.C.; Peebles, R.S., Jr. PGI2 as a regulator of CD4+ subset differentiation and function. Prostaglandins Other Lipid Mediat 2011, 96, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Ross, D.S.; Burch, H.B.; Cooper, D.S.; Greenlee, M.C.; Laurberg, P.; Maia, A.L.; Rivkees, S.A.; Samuels, M.; Sosa, J.A.; Stan, M.N.; et al. 2016 American Thyroid Association Guidelines for Diagnosis and Management of Hyperthyroidism and Other Causes of Thyrotoxicosis. Thyroid 2016, 26, 1343–1421. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Sako, H.; Nakane, Y.; Okugawa, K.; Nakano, K.; Yamano, T. A case of graves'disease with an enormous goiter. Nihon Rinsho Geka Gakkai Zasshi (Journal of Japan Surgical Association) 2004, 65, 2848–2852. [Google Scholar] [CrossRef]
- Price, L.C.; Martinez, G.; Brame, A.; Pickworth, T.; Samaranayake, C.; Alexander, D.; Garfield, B.; Aw, T.C.; McCabe, C.; Mukherjee, B.; et al. Perioperative management of patients with pulmonary hypertension undergoing non-cardiothoracic, non-obstetric surgery: a systematic review and expert consensus statement. Br J Anaesth 2021, 126, 774–790. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).