Submitted:
29 August 2023
Posted:
31 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Experimental Section
2.1. Materials
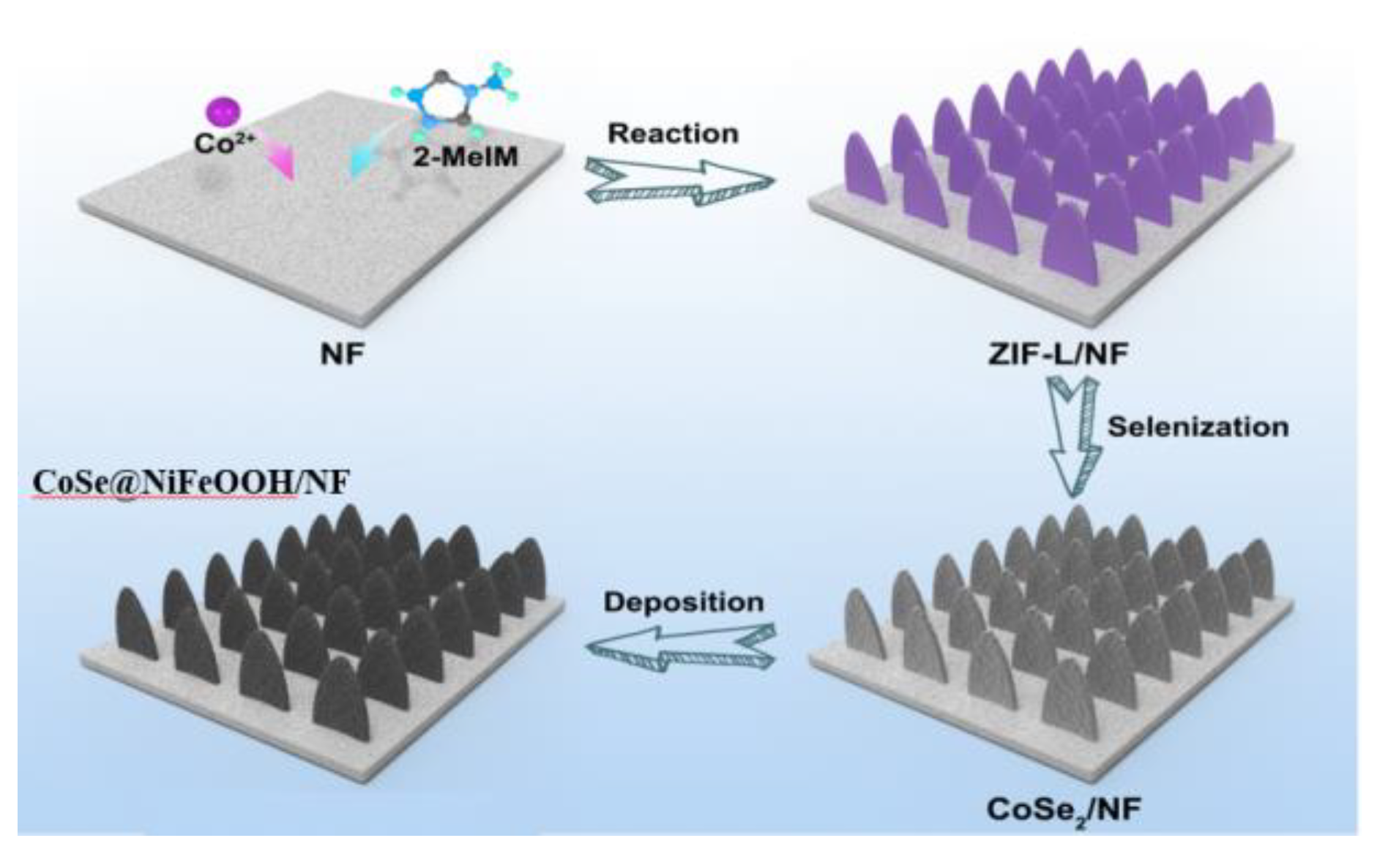
2.2. Preparation of cobalt-based ZIF-L/NF
2.3. Synthesis of CoSe2/NF
2.4. Synthesis of CoSe2@NiFeOOH/NF
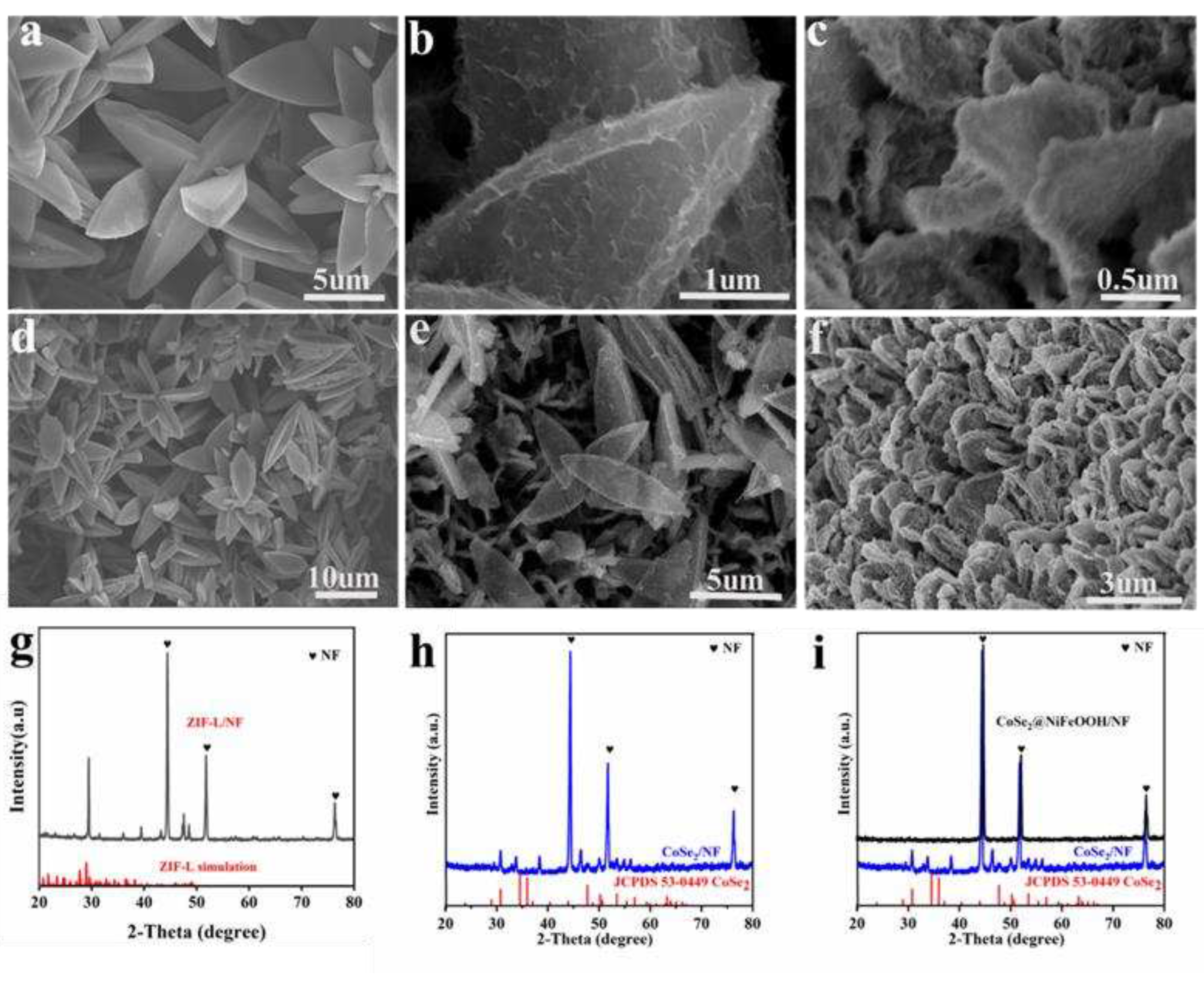
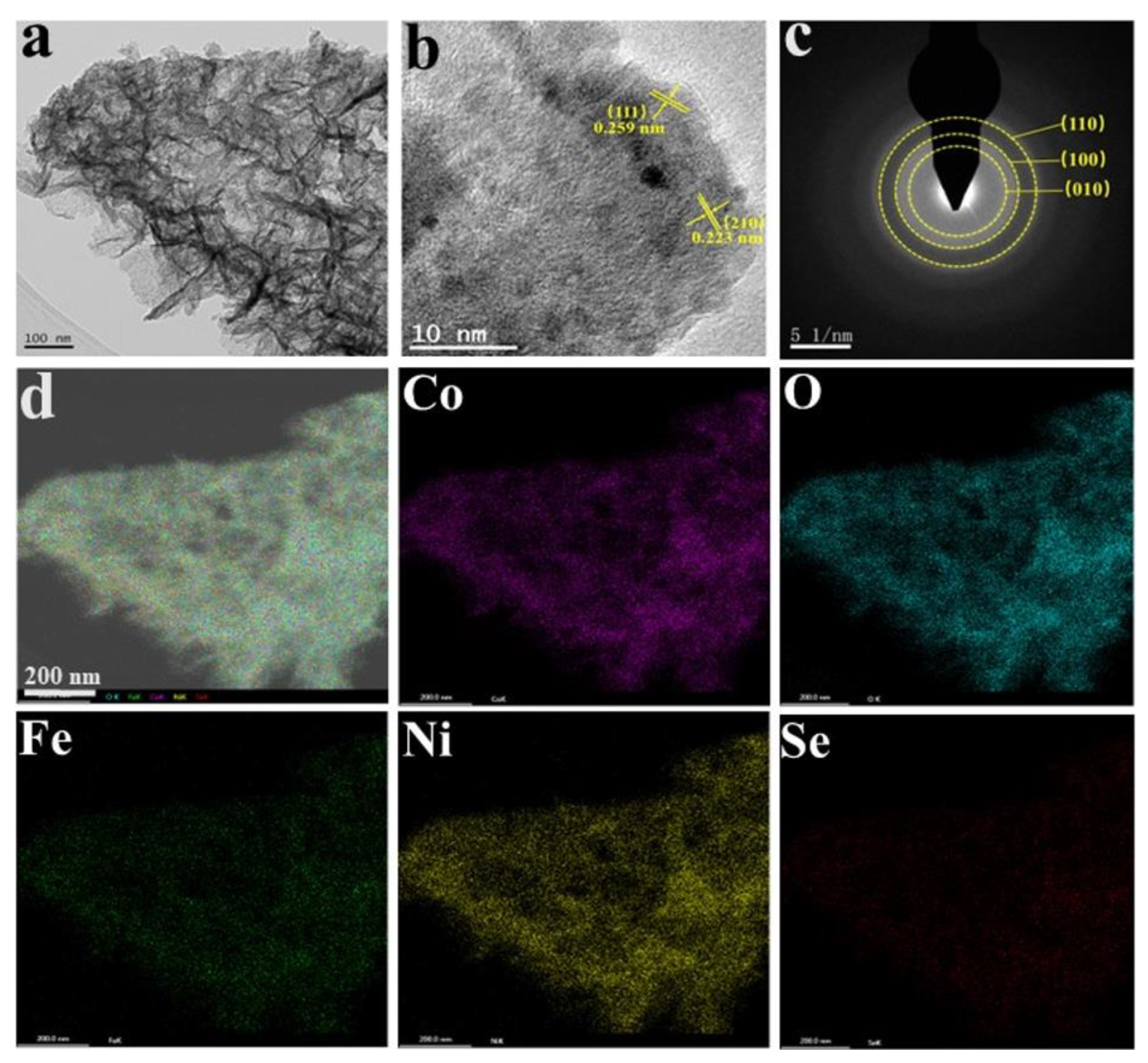
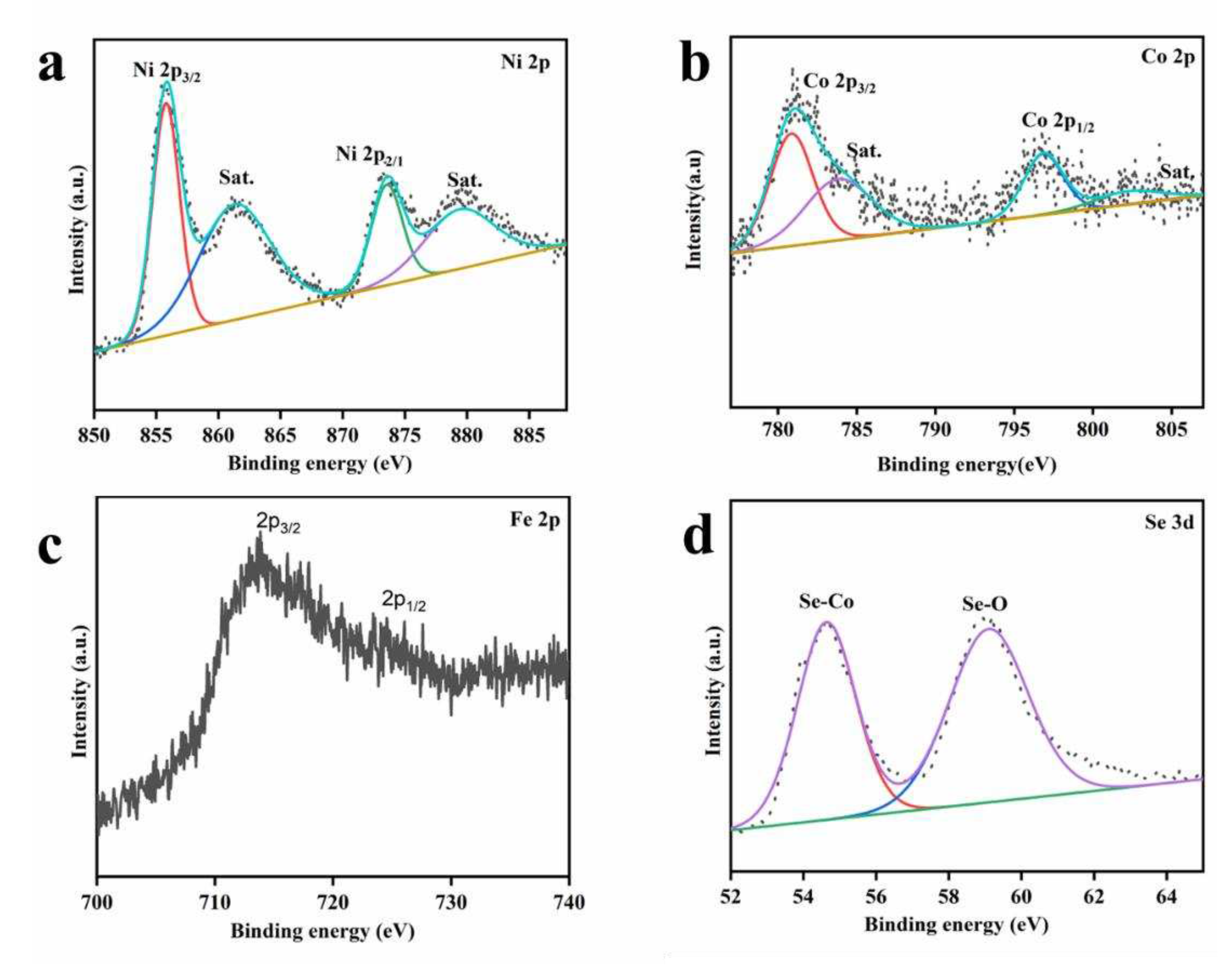
2.5. Characterization
2.6. Electrocatalytic measurement
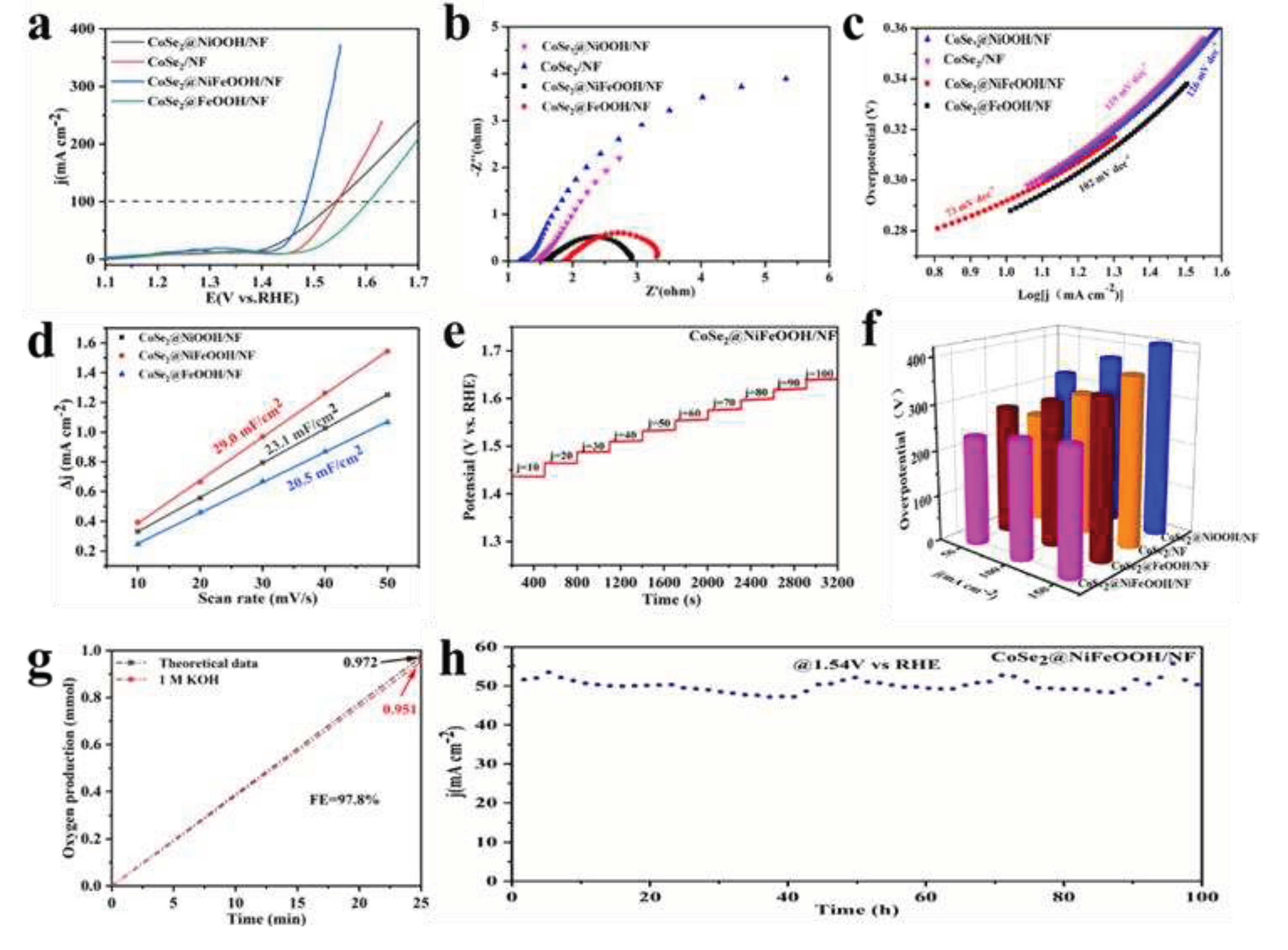
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dresp, S.; Ngo Thanh, T.; Klingenhof, M.; Brückner, S.; Hauke, P.; Strasser, P. Efficient direct seawater electrolysers using selective alkaline NiFe-LDH as OER catalyst in asymmetric electrolyte feeds. Energy Environ. Sci. 2020, 13, 1725–1729. [Google Scholar] [CrossRef]
- Wang, Y.; Tao, S.; Lin, H.; Wang, G.; Zhao, K.; Cai, R.; Tao, K.; Zhang, C.; Sun, M.; Hu, J.; Huang, B.; Yang, S. Atomically targeting NiFe LDH to create multivacancies for OER catalysis with a small organic anchor. Nano Energy. 2021, 81, 105606. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, W.; Li, J.-G.; Li, Z.; Ao, X.; Xue, K.-H.; Ostrikov, K. K.; Tang, J.; Wang, C. Rh-engineered ultrathin NiFe-LDH nanosheets enable highly-efficient overall water splitting and urea electrolysis. Appl. Catal. B Environ. 2021, 284, 119740. [Google Scholar] [CrossRef]
- Alobaid, A.; Wang, C.; Adomaitis, R. A. Mechanism and kinetics of HER and OER on NiFe LDH films in an alkaline electrolyte. J. Electrochem. Soc. 2018, 165, J3395–J3404. [Google Scholar] [CrossRef]
- Hunter, B. M.; Hieringer, W.; Winkler, J. R.; Gray, H. B.; Müller, A. M. Effect of interlayer anions on NiFe-LDH nanosheet water oxidation activity. Energy Environ. Sci. 2016, 9, 1734–1743. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, D.; El Hankari, S.; Zou, Y.; Wang, S. Recent progress on layered double hydroxides and their derivatives for electrocatalytic water splitting. Adv. Sci. 2018, 5, 1800064. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, Q.; Dong, L.; Wu, H. B.; Yu, X.-Y. Activating the hydrogen evolution and overall water splitting performance of NiFe LDH by cation doping and plasma reduction. Appl. Catal. B Environ. 2020, 266, 118627. [Google Scholar] [CrossRef]
- Hu, L.; Zeng, X.; Wei, X.; Wang, H.; Wu, Y.; Gu, W.; Shi, L.; Zhu, C. Interface engineering for enhancing electrocatalytic oxygen evolution of NiFe LDH/NiTe heterostructures. Appl. Catal. B Environ. 2020, 273, 119014. [Google Scholar] [CrossRef]
- Nayak, S.; Parida, K. Superactive NiFe-LDH/graphene nanocomposites as competent catalysts for water splitting reactions. Inorg. Chem. Front. 2020, 7, 3805–3836. [Google Scholar] [CrossRef]
- Yu, J.; Yu, F.; Yuen, M.-F.; Wang, C. Two-dimensional layered double hydroxides as a platform for electrocatalytic oxygen evolution. J. Mater. Chem. A 2021, 9, 9389–9430. [Google Scholar] [CrossRef]
- Chen, S.; Huang, H.; Jiang, P.; Yang, K.; Diao, J.; Gong, S.; Liu, S.; Huang, M.; Wang, H.; Chen, Q. Mn-doped RuO2 nanocrystals as highly active electrocatalysts for enhanced oxygen evolution in acidic media. ACS Catal. 2019, 10, 1152–1160. [Google Scholar] [CrossRef]
- Hubert, M. A.; Patel, A. M.; Gallo, A.; Liu, Y.; Valle, E.; Ben-Naim, M.; Sanchez, J.; Sokaras, D.; Sinclair, R.; Nørskov, J. K.; King, L. A.; Bajdich, M.; Jaramillo, T. F. Acidic oxygen evolution reaction activity–stability relationships in Ru-based pyrochlores. ACS Catal. 2020, 10, 12182–12196. [Google Scholar] [CrossRef]
- Su, J.; Ge, R.; Jiang, K.; Dong, Y.; Hao, F.; Tian, Z.; Chen, G.; Chen, L. Assembling ultrasmall Copper-doped ruthenium oxide nanocrystals into hollow porous polyhedra: highly robust electrocatalysts for oxygen evolution in acidic media. Adv. Mater. 2018, 30, 1801351. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Choi, S.; Bang, G. J.; Kwon, T.; Kim, H. S.; Lee, S. J.; Hong, Y.; Lee, D. W.; Park, H. S.; Baik, H.; Jung, Y.; Yoo, S. J.; Lee, K. Safeguarding the RuO2 phase against lattice oxygen oxidation during acidic water electrooxidation. Energy Environ. Sci. 2022, 15, 1119–1130. [Google Scholar] [CrossRef]
- Lin, Y.; Tian, Z.; Zhang, L.; Ma, J.; Jiang, Z.; Deibert, B. J.; Ge, R.; Chen, L. Chromium-ruthenium oxide solid solution electrocatalyst for highly efficient oxygen evolution reaction in acidic media. Nat. Commun. 2019, 10, 162–175. [Google Scholar] [CrossRef]
- Retuerto, M.; Pascual, L.; Calle-Vallejo, F.; Ferrer, P.; Gianolio, D.; Pereira, A. G.; Garcia, A.; Torrero, J.; Fernandez-Diaz, M. T.; Bencok, P.; Pena, M. A.; Fierro, J. L. G.; Rojas, S. Na-doped ruthenium perovskite electrocatalysts with improved oxygen evolution activity and durability in acidic media. Nat. Commun. 2019, 10, 2041–2050. [Google Scholar] [CrossRef]
- An, L.; Wei, C.; Lu, M.; Liu, H.; Chen, Y.; Scherer, G. G.; Fisher, A. C.; Xi, P.; Xu, Z. J.; Yan, C. H. Recent development of oxygen evolution electrocatalysts in acidic environment. Adv. Mater. 2021, 33, e2006328. [Google Scholar] [CrossRef]
- Li, L.; Wang, P.; Shao, Q.; Huang, X. Recent progress in advanced electrocatalyst design for acidic oxygen evolution reaction. Adv. Mater. 2021, 33, e2004243. [Google Scholar] [CrossRef]
- Spori, C.; Kwan, J. T. H.; Bonakdarpour, A.; Wilkinson, D. P.; Strasser, P. The stability challenges of oxygen evolving catalysts: towards a common fundamental understanding and mitigation of catalyst degradation. Angew. Chem. Int. Ed. 2017, 56, 5994–6021. [Google Scholar] [CrossRef]
- Zhang, T.; Hang, L.; Sun, Y.; Men, D.; Li, X.; Wen, L.; Lyu, X.; Li, Y. Hierarchical hetero-Ni3Se4@NiFe LDH micro/nanosheets as efficient bifunctional electrocatalysts with superior stability for overall water splitting. Nanoscale Horiz. 2019, 4, 1132–1138. [Google Scholar] [CrossRef]
- Suntivich, J.; May, K. J.; Gasteiger, H. A.; Goodenough, J. B.; Shao-Horn, Y. , A perovskite oxide optimized for oxygen evolution catalysis from molecular orbital principles. Science. 2011, 334, 1383–1385. [Google Scholar] [CrossRef]
- Liu, Y.; Cheng, H.; Lyu, M.; Fan, S.; Liu, Q.; Zhang, W.; Zhi, Y.; Wang, C.; Xiao, C.; Wei, S.; Ye, B.; Xie, Y. Low overpotential in vacancy-rich ultrathin CoSe2 nanosheets for water oxidation. J. Am. Chem. Soc. 2014, 136, 15670–15675. [Google Scholar] [CrossRef] [PubMed]
- Li, J. G.; Sun, H.; Lv, L.; Li, Z.; Ao, X.; Xu, C.; Li, Y.; Wang, C. Metal-organic framework-derived hierarchical (Co,Ni)Se2@NiFe LDH hollow nanocages for enhanced oxygen evolution. ACS Appl. Mater. Interfaces 2019, 11, 8106–8114. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Lee, S.; Hu, X. , Spectroscopic and electrokinetic evidence for a bifunctional mechanism of the oxygen evolution reaction. Angew. Chem. Int. Ed. Engl. 2021, 60, 3095–3103. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z. W.; Liu, J. Y.; Chen, X. M.; Zheng, X. L.; Mao, J.; Liu, H.; Ma, T.; Li, L.; Wang, W. C.; Du, X. W. , Engineering NiO/NiFe LDH intersection to bypass scaling relationship for oxygen evolution reaction via dynamic tridimensional adsorption of intermediates. Adv Mater 2019, 31, e1804769. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Wang, Q.; Dai, Z.; Qiu, H.; Dong, X. MOF-derived Zn-doped CoSe2 as an efficient and stable free-standing catalyst for oxygen evolution reaction. ACS Appl. Mater. Interfaces 2016, 8, 26902–26907. [Google Scholar] [CrossRef]
- He, K.; Tadesse Tsega, T.; Liu, X.; Zai, J.; Li, X. H.; Liu, X.; Li, W.; Ali, N.; Qian, X. Utilizing the space-charge region of the FeNi-LDH/CoP p-n junction to promote performance in oxygen evolution electrocatalysis. Angew. Chem., Int. Ed. 2019, 58, 11903–11909. [Google Scholar] [CrossRef]
- Yang, R.; Zhou, Y.; Xing, Y.; Li, D.; Jiang, D.; Chen, M.; Shi, W.; Yuan, S. Synergistic coupling of CoFe-LDH arrays with NiFe-LDH nanosheet for highly efficient overall water splitting in alkaline media. Appl. Catal. B Environ. 2019, 253, 131–139. [Google Scholar] [CrossRef]
- Liu, J.; Wang, J.; Zhang, B.; Ruan, Y.; Lv, L.; Ji, X.; Xu, K.; Miao, L.; Jiang, J. Hierarchical NiCo2S4@NiFe LDH heterostructures supported on nickel foam for enhanced overall-water-splitting activity. ACS Appl. Mater. Interfaces 2017, 9, 15364–15372. [Google Scholar] [CrossRef]
- Zhang, F. S.; Wang, J. W.; Luo, J.; Liu, R. R.; Zhang, Z. M.; He, C. T.; Lu, T. B. Extraction of nickel from NiFe-LDH into Ni2P@NiFe hydroxide as a bifunctional electrocatalyst for efficient overall water splitting. Chem. Sci. 2018, 9, 1375–1384. [Google Scholar] [CrossRef]
- Jia, B.; Xue, Z.; Liu, Q.; Liu, Q.; Liu, K.; Liu, M.; Chan, T.-S.; Li, Y.; Li, Z.; Su, C.-Y.; Li, G. Hierarchical nanotubes constructed from CoSe2 nanorods with an oxygen-rich surface for an efficient oxygen evolution reaction. J. Mater. Chem. A 2019, 7, 15073–15078. [Google Scholar] [CrossRef]
- Kwak, I. H.; Im, H. S.; Jang, D. M.; Kim, Y. W.; Park, K.; Lim, Y. R.; Cha, E. H.; Park, J. CoSe2 and NiSe2 nanocrystals as superior bifunctional catalysts for electrochemical and photoelectrochemical water splitting. ACS Appl. Mater. Interfaces 2016, 8, 5327–5357. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Jin, R.; Abroshan, H.; Zeng, C.; Zhang, H.; House, S. D.; Gottlieb, E.; Kim, H. J.; Yang, J. C.; Jin, R. , Gold nanoclusters promote electrocatalytic water oxidation at the nanocluster/CoSe2 interface. J. Am. Chem. Soc. 2017, 139, 1077–1080. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



