Submitted:
25 August 2023
Posted:
31 August 2023
You are already at the latest version
Abstract
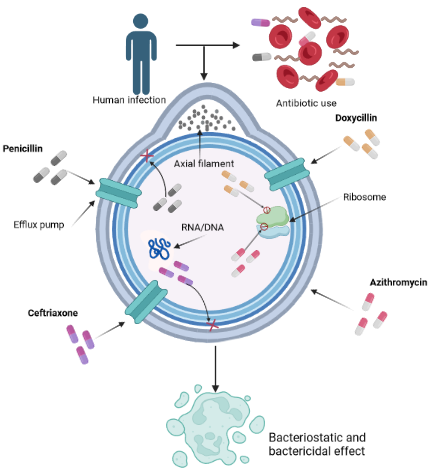
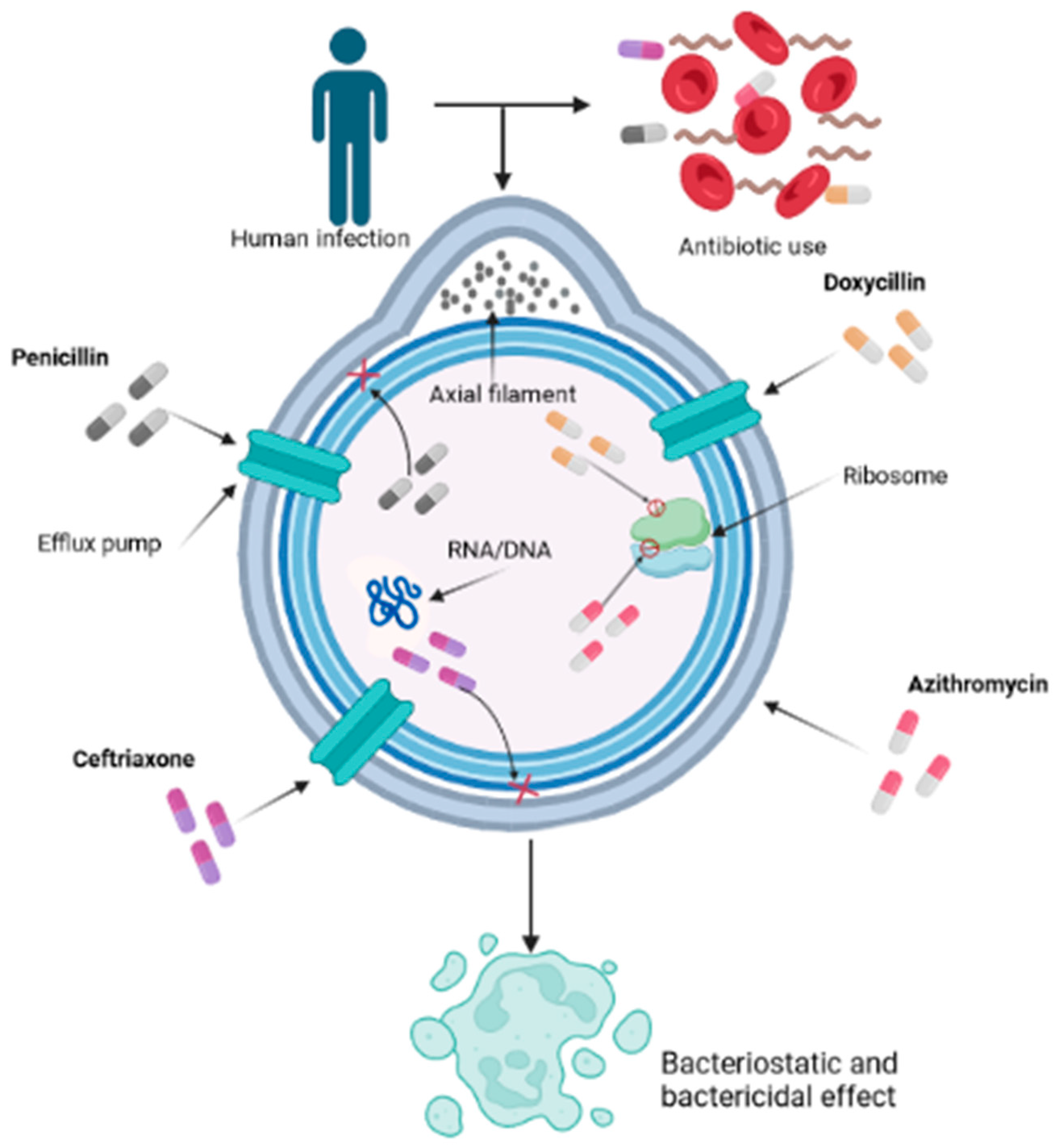
Keywords:
1. Introduction
2. Epidemiology of Syphilis: Current Trends and Future Challenges
3. Diagnosis of Syphilis: Advances and Challenges
4. Pharmacological Interventions
5. Future Approaches in Syphilis Prevention and Control
5.1. Development of Effective Vaccines
5.2. Innovative Point-of-Care Diagnostics
5.3. Targeted Prevention Strategies
5.4. Integration of Syphilis Services into Primary Healthcare
6. Future Directions
References
- Kojima, N.; Klausner, J.D. An Update on the Global Epidemiology of Syphilis. Curr. Epidemiol. Reports 2018, 5, 24–38. [Google Scholar] [CrossRef]
- Tiecco, G.; Degli Antoni, M.; Storti, S.; Marchese, V.; Focà, E.; Torti, C.; Castelli, F.; Quiros-Roldan, E. A 2021 Update on Syphilis: Taking Stock from Pathogenesis to Vaccines. Pathogens 2021, 10, 1364. [Google Scholar] [CrossRef]
- Li, S.; Li, W.; Jin, Y.; Wu, B.; Wu, Y. Advancements in the Development of Nucleic Acid Vaccines for Syphilis Prevention and Control. Hum. Vaccin. Immunother. 2023, 19. [Google Scholar] [CrossRef] [PubMed]
- Osias, E.; Hung, P.; Giacani, L.; Stafylis, C.; Konda, K.A.; Vargas, S.K.; Reyes-Díaz, E.M.; Comulada, W.S.; Haake, D.A.; Haynes, A.M.; et al. Investigation of Syphilis Immunology and Treponema Pallidum Subsp. Pallidum Biology to Improve Clinical Management and Design a Broadly Protective Vaccine: Study Protocol. BMC Infect. Dis. 2020, 20, 444. [Google Scholar] [CrossRef] [PubMed]
- Mues, N.; Chu, H.W. Out-Smarting the Host: Bacteria Maneuvering the Immune Response to Favor Their Survival. Front. Immunol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Scott, B.N. V; Sarkar, T.; Kratofil, R.M.; Kubes, P.; Thanabalasuriar, A. Unraveling the Host’s Immune Response to Infection: Seeing Is Believing. J. Leukoc. Biol. 2019, 106, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Miethke, M.; Pieroni, M.; Weber, T.; Brönstrup, M.; Hammann, P.; Halby, L.; Arimondo, P.B.; Glaser, P.; Aigle, B.; Bode, H.B.; et al. Towards the Sustainable Discovery and Development of New Antibiotics. Nat. Rev. Chem. 2021, 5, 726–749. [Google Scholar] [CrossRef]
- Murugaiyan, J.; Kumar, P.A.; Rao, G.S.; Iskandar, K.; Hawser, S.; Hays, J.P.; Mohsen, Y.; Adukkadukkam, S.; Awuah, W.A.; Jose, R.A.M.; et al. Progress in Alternative Strategies to Combat Antimicrobial Resistance: Focus on Antibiotics. Antibiotics 2022, 11, 200. [Google Scholar] [CrossRef]
- Huemer, M.; Mairpady Shambat, S.; Brugger, S.D.; Zinkernagel, A.S. Antibiotic Resistance and Persistence—Implications for Human Health and Treatment Perspectives. EMBO Rep. 2020, 21. [Google Scholar] [CrossRef]
- Tuite, A.R.; Testa, C.; Rönn, M.; Bellerose, M.; Gift, T.; Fridge, J.; Molotnikov, L.; Desmarais, C.; Berruti, A.; Menzies, N.; et al. Exploring How Epidemic Context Influences Syphilis Screening Impact: A Mathematical Modeling Study. Sex. Transm. Dis. 2020, 47, 798–810. [Google Scholar] [CrossRef]
- Schmidt, R.; Carson, P.J.; Jansen, R.J. Resurgence of Syphilis in the United States: An Assessment of Contributing Factors. Infect. Dis. Res. Treat. 2019, 12, 117863371988328. [Google Scholar] [CrossRef] [PubMed]
- Peeling, R.W.; Mabey, D.; Kamb, M.L.; Chen, X.-S.; Radolf, J.D.; Benzaken, A.S. Syphilis. Nat. Rev. Dis. Prim. 2017, 3, 17073. [Google Scholar] [CrossRef] [PubMed]
- de Souza, T.A.; Teixeira, K.K.; Santana, R.L.; Penha, C.B.; Medeiros, A. de A.; de Lima, K.C.; Barbosa, I.R. Intra-Urban Differentials of Congenital and Acquired Syphilis and Syphilis in Pregnant Women in an Urban Area in Northeastern Brazil. Trans. R. Soc. Trop. Med. Hyg. 2021, 115, 1010–1018. [Google Scholar] [CrossRef] [PubMed]
- Pereira Nogueira, W.; Figueiredo Nogueira, M.; de Almeida Nogueira, J.; Freire, M.E.M.; Gir, E.; Silva, A.C. de O. e Syphilis in Riverine Communities: Prevalence and Associated Factors. Rev. da Esc. Enferm. da USP 2022, 56. [Google Scholar] [CrossRef]
- Macêdo, V.C. de; Lira, P.I.C. de; Frias, P.G. de; Romaguera, L.M.D.; Caires, S. de F.F.; Ximenes, R.A. de A. Risk Factors for Syphilis in Women: Case-Control Study. Rev. Saude Publica 2017, 51, 78. [Google Scholar] [CrossRef]
- Amerson, E.H.; Castillo Valladares, H.B.; Leslie, K.S. Resurgence of Syphilis in the US—USPSTF Reaffirms Screening Guidelines. JAMA Dermatology 2022, 158, 1241. [Google Scholar] [CrossRef]
- Copen, C.E.; Brookmeyer, K.A.; Haderxhanaj, L.T.; Hogben, M.; Torrone, E.A. Sexual Risk Behaviors Among Persons Diagnosed With Primary and Secondary Syphilis Who Reported High-Risk Substance Use: Data From the National Notifiable Diseases Surveillance System, 2018. Sex. Transm. Dis. 2022, 49, 99–104. [Google Scholar] [CrossRef]
- Šmit, R.; Wojtalewicz, N.; Vierbaum, L.; Nourbakhsh, F.; Schellenberg, I.; Hunfeld, K.-P.; Lohr, B. Epidemiology, Management, Quality of Testing and Cost of Syphilis in Germany: A Retrospective Model Analysis. Front. Public Heal. 2022, 10. [Google Scholar] [CrossRef]
- Ramos Jr., A. N. Persistence of Syphilis as a Challenge for the Brazilian Public Health: The Solution Is to Strengthen SUS in Defense of Democracy and Life. Cad. Saude Publica 2022, 38. [Google Scholar] [CrossRef]
- Valentine, J.A.; Bolan, G.A. Syphilis Elimination: Lessons Learned Again. Sex. Transm. Dis. 2018, 45, S80–S85. [Google Scholar] [CrossRef]
- Kitayama, K.; Segura, E.R.; Lake, J.E.; Perez-Brumer, A.G.; Oldenburg, C.E.; Myers, B.A.; Pourjavaheri, P.; Okorie, C.N.; Cabello, R.L.; Clark, J.L. Syphilis in the Americas: A Protocol for a Systematic Review of Syphilis Prevalence and Incidence in Four High-Risk Groups, 1980–2016. Syst. Rev. 2017, 6, 195. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Xie, Y.; Xiao, Y. Laboratory Diagnostic Tools for Syphilis: Current Status and Future Prospects. Front. Cell. Infect. Microbiol. 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Wolgemuth, C.W. Flagellar Motility of the Pathogenic Spirochetes. Semin. Cell Dev. Biol. 2015, 46, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Forrestel, A.K.; Kovarik, C.L.; Katz, K.A. Sexually Acquired Syphilis. J. Am. Acad. Dermatol. 2020, 82, 17–28. [Google Scholar] [CrossRef]
- Hook, E.W.; Roddy, R.E.; Lukehart, S.A.; Hom, J.; Holmes, K.K.; Tam, M.R. Detection of Treponema Pallidum in Lesion Exudate with a Pathogen-Specific Monoclonal Antibody. J. Clin. Microbiol. 1985, 22, 241–244. [Google Scholar] [CrossRef]
- Ito, F.; Hunter, E.F.; George, R.W.; Pope, V.; Larsen, S.A. Specific Immunofluorescent Staining of Pathogenic Treponemes with a Monoclonal Antibody. J. Clin. Microbiol. 1992, 30, 831–838. [Google Scholar] [CrossRef]
- Morshed, M.G. Current Trend on Syphilis Diagnosis: Issues and Challenges. In; 2014; pp. 51–64.
- Graham, R.P.; Naini, B. V.; Shah, S.S.; Arnold, C.A.; Kannangai, R.; Torbenson, M.S.; Lam-Himlin, D.M. Treponema Pallidum Immunohistochemistry Is Positive in Human Intestinal Spirochetosis. Diagn. Pathol. 2018, 13, 7. [Google Scholar] [CrossRef]
- Radolf, J.D.; Deka, R.K.; Anand, A.; Šmajs, D.; Norgard, M. V.; Yang, X.F. Treponema Pallidum, the Syphilis Spirochete: Making a Living as a Stealth Pathogen. Nat. Rev. Microbiol. 2016, 14, 744–759. [Google Scholar] [CrossRef]
- Morshed, M.G.; Singh, A.E. Recent Trends in the Serologic Diagnosis of Syphilis. Clin. Vaccine Immunol. 2015, 22, 137–147. [Google Scholar] [CrossRef]
- Gao, K.; Shen, X.; Lin, Y.; Zhu, X.-Z.; Lin, L.-R.; Tong, M.-L.; Xiao, Y.; Zhang, H.-L.; Liang, X.-M.; Niu, J.-J.; et al. Origin of Nontreponemal Antibodies During Treponema Pallidum Infection: Evidence From a Rabbit Model. J. Infect. Dis. 2018, 218, 835–843. [Google Scholar] [CrossRef]
- Tuddenham, S.; Katz, S.S.; Ghanem, K.G. Syphilis Laboratory Guidelines: Performance Characteristics of Nontreponemal Antibody Tests. Clin. Infect. Dis. 2020, 71, S21–S42. [Google Scholar] [CrossRef] [PubMed]
- Marra, C.M.; Tantalo, L.C.; Maxwell, C.L.; Ho, E.L.; Sahi, S.K.; Jones, T. The Rapid Plasma Reagin Test Cannot Replace the Venereal Disease Research Laboratory Test for Neurosyphilis Diagnosis. Sex. Transm. Dis. 2012, 39, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Park, I.U.; Fakile, Y.F.; Chow, J.M.; Gustafson, K.J.; Jost, H.; Schapiro, J.M.; Novak-Weekley, S.; Tran, A.; Nomura, J.H.; Chen, V.; et al. Performance of Treponemal Tests for the Diagnosis of Syphilis. Clin. Infect. Dis. 2019, 68, 913–918. [Google Scholar] [CrossRef] [PubMed]
- Larsen, S.A.; Steiner, B.M.; Rudolph, A.H. Laboratory Diagnosis and Interpretation of Tests for Syphilis. Clin. Microbiol. Rev. 1995, 8, 1–21. [Google Scholar] [CrossRef]
- Centurion-Lara, A.; Castro, C.; Shaffer, J.M.; Van Voorhis, W.C.; Marra, C.M.; Lukehart, S.A. Detection of Treponema Pallidum by a Sensitive Reverse Transcriptase PCR. J. Clin. Microbiol. 1997, 35, 1348–1352. [Google Scholar] [CrossRef]
- Gayet-Ageron, A.; Sednaoui, P.; Lautenschlager, S.; Ferry, T.; Toutous-Trellu, L.; Cavassini, M.; Yassir, F.; Martinez de Tejada, B.; Emonet, S.; Combescure, C.; et al. Use of Treponema Pallidum PCR in Testing of Ulcers for Diagnosis of Primary Syphilis1. Emerg. Infect. Dis. 2015, 21, 127–129. [Google Scholar] [CrossRef]
- Xiao, Y.; Xie, Y.; Xu, M.; Liu, S.; Jiang, C.; Zhao, F.; Zeng, T.; Liu, Z.; Yu, J.; Wu, Y. Development and Evaluation of a Loop-Mediated Isothermal Amplification Assay for the Detection of Treponema Pallidum DNA in the Peripheral Blood of Secondary Syphilis Patients. Am. J. Trop. Med. Hyg. 2017, 97, 1673–1678. [Google Scholar] [CrossRef]
- Morshed, M.G.; Lee, M.-K.; Jorgensen, D.; Isaac-Renton, J.L. Molecular Methods Used in Clinical Laboratory: Prospects and Pitfalls. FEMS Immunol. Med. Microbiol. 2007, 49, 184–191. [Google Scholar] [CrossRef]
- Singhal, N.; Kumar, M.; Kanaujia, P.K.; Virdi, J.S. MALDI-TOF Mass Spectrometry: An Emerging Technology for Microbial Identification and Diagnosis. Front. Microbiol. 2015, 6. [Google Scholar] [CrossRef]
- Stamm, L. V. Syphilis: Re-Emergence of an Old Foe. Microb. Cell 2016, 3, 363–370. [Google Scholar] [CrossRef]
- Douglas, J.M. Penicillin Treatment of Syphilis. JAMA 2009, 301, 769. [Google Scholar] [CrossRef] [PubMed]
- Katz, K.A.; Klausner, J.D. Azithromycin Resistance in Treponema Pallidum. Curr. Opin. Infect. Dis. 2008, 21, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-S.; Yin, Y.-P.; Wei, W.-H.; Wang, H.-C.; Peng, R.-R.; Zheng, H.-P.; Zhang, J.-P.; Zhu, B.-Y.; Liu, Q.-Z.; Huang, S.-J. High Prevalence of Azithromycin Resistance to Treponema Pallidum in Geographically Different Areas in China. Clin. Microbiol. Infect. 2013, 19, 975–979. [Google Scholar] [CrossRef] [PubMed]
- Orbe-Orihuela, Y.C.; Sánchez-Alemán, M.Á.; Hernández-Pliego, A.; Medina-García, C.V.; Vergara-Ortega, D.N. Syphilis as Re-Emerging Disease, Antibiotic Resistance, and Vulnerable Population: Global Systematic Review and Meta-Analysis. Pathogens 2022, 11, 1546. [Google Scholar] [CrossRef]
- Cameron, C.E.; Lukehart, S.A. Current Status of Syphilis Vaccine Development: Need, Challenges, Prospects. Vaccine 2014, 32, 1602–1609. [Google Scholar] [CrossRef]
- Miller, J.N. Immunity in Experimental Syphilis. J. Immunol. 1973, 110, 1206–1215. [Google Scholar] [CrossRef]
- Haynes, A.M.; Giacani, L.; Mayans, M.V.; Ubals, M.; Nieto, C.; Pérez-Mañá, C.; Quintó, L.; Romeis, E.; Mitjà, O. Efficacy of Linezolid on Treponema Pallidum, the Syphilis Agent: A Preclinical Study. EBioMedicine 2021, 65, 103281. [Google Scholar] [CrossRef]
- Stamm, L. V. Hope for New Antibiotics for Syphilis Treatment. EBioMedicine 2021, 66, 103320. [Google Scholar] [CrossRef]
- Vickram, A.S.; Dhama, K.; Thanigaivel, S.; Chakraborty, S.; Anbarasu, K.; Dey, N.; Karunakaran, R. Strategies for Successful Designing of Immunocontraceptive Vaccines and Recent Updates in Vaccine Development against Sexually Transmitted Infections - A Review. Saudi J. Biol. Sci. 2022, 29, 2033–2046. [Google Scholar] [CrossRef]
- Cameron, C.E. Syphilis Vaccine Development: Requirements, Challenges, and Opportunities. Sex. Transm. Dis. 2018, 45, S17–S19. [Google Scholar] [CrossRef]
- Kojima, N.; Konda, K.A.; Klausner, J.D. Notes on Syphilis Vaccine Development. Front. Immunol. 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Giacani, L. Strategies for Syphilis Vaccine Development. J. Bras. Doenças Sex. Transm. 2022, 34. [Google Scholar] [CrossRef]
- Pham, M.D.; Ong, J.J.; Anderson, D.A.; Drummer, H.E.; Stoové, M. Point-of-Care Diagnostics for Diagnosis of Active Syphilis Infection: Needs, Challenges and the Way Forward. Int. J. Environ. Res. Public Health 2022, 19, 8172. [Google Scholar] [CrossRef] [PubMed]
- Mabey, D.C.; Sollis, K.A.; Kelly, H.A.; Benzaken, A.S.; Bitarakwate, E.; Changalucha, J.; Chen, X.-S.; Yin, Y.-P.; Garcia, P.J.; Strasser, S.; et al. Point-of-Care Tests to Strengthen Health Systems and Save Newborn Lives: The Case of Syphilis. PLoS Med. 2012, 9, e1001233. [Google Scholar] [CrossRef] [PubMed]
- Causer, L.M.; Kaldor, J.M.; Conway, D.P.; Leslie, D.E.; Denham, I.; Karapanagiotidis, T.; Ryan, C.; Wand, H.; Anderson, D.A.; Robertson, P.W.; et al. An Evaluation of a Novel Dual Treponemal/Nontreponemal Point-of-Care Test for Syphilis as a Tool to Distinguish Active From Past Treated Infection. Clin. Infect. Dis. 2015, 61, 184–191. [Google Scholar] [CrossRef]
- Caya, C.; Maheu-Giroux, M.; Xia, Y.; Serhir, B.; Morin, V.; Libman, M.; Corsini, R.; Goldfarb, D.M.; Wong, T.; Singh, A.E.; et al. Stopping Syphilis Transmission in Arctic Communities through Rapid Diagnostic Testing: The STAR Study Protocol. PLoS One 2022, 17, e0273713. [Google Scholar] [CrossRef]
- Toskin, I.; Blondeel, K.; Peeling, R.W.; Deal, C.; Kiarie, J. Advancing Point of Care Diagnostics for the Control and Prevention of STIs: The Way Forward. Sex. Transm. Infect. 2017, 93, S81–S88. [Google Scholar] [CrossRef]
- Basing, L.A.W.; Simpson, S.V.; Adu-Sarkodie, Y.; Linnes, J.C. A Loop-Mediated Isothermal Amplification Assay for the Detection of Treponema Pallidum Subsp. Pertenue. Am. J. Trop. Med. Hyg. 2020, 103, 253–259. [Google Scholar] [CrossRef]
- Tharakan, S.; Faqah, O.; Asghar, W.; Ilyas, A. Microfluidic Devices for HIV Diagnosis and Monitoring at Point-of-Care (POC) Settings. Biosensors 2022, 12, 949. [Google Scholar] [CrossRef]
- Christodouleas, D.C.; Kaur, B.; Chorti, P. From Point-of-Care Testing to EHealth Diagnostic Devices (EDiagnostics). ACS Cent. Sci. 2018, 4, 1600–1616. [Google Scholar] [CrossRef]
- Naeem, F.; Karellis, A.; Nair, S.; Routy, J.-P.; Yansouni, C.P.; Kim, J.; Pai, N. Multiplexed Technologies for Sexually Transmitted Infections: Global Evidence on Patient-Centered and Clinical Health Outcomes. BMJ Glob. Heal. 2021, 6, e005670. [Google Scholar] [CrossRef] [PubMed]
- Karellis, A.; Naeem, F.; Nair, S.; Mallya, S.D.; Routy, J.-P.; Gahagan, J.; Yansouni, C.P.; Kim, J.; Pai, N.P. Multiplexed Rapid Technologies for Sexually Transmitted Infections: A Systematic Review. The Lancet Microbe 2022, 3, e303–e315. [Google Scholar] [CrossRef] [PubMed]
- Osbak, K.K.; Van Raemdonck, G.A.; Dom, M.; Cameron, C.E.; Meehan, C.J.; Deforce, D.; Ostade, X. Van; Kenyon, C.R.; Dhaenens, M. Candidate Treponema Pallidum Biomarkers Uncovered in Urine from Individuals with Syphilis Using Mass Spectrometry. Future Microbiol. 2018, 13, 1497–1510. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, M.; Wang, Z.; Li, S.; Deng, Y.; He, N. Point-of-Care Diagnostics for Infectious Diseases: From Methods to Devices. Nano Today 2021, 37, 101092. [Google Scholar] [CrossRef] [PubMed]
- Peterman, T.A.; Cha, S. Context-Appropriate Interventions to Prevent Syphilis: A Narrative Review. Sex. Transm. Dis. 2018, 45, S65–S71. [Google Scholar] [CrossRef]
- Copen, C.E.; Rushmore, J.; De Voux, A.; Kirkcaldy, R.D.; Fakile, Y.F.; Tilchin, C.; Duchen, J.; Jennings, J.M.; Spahnie, M.; Norris Turner, A.; et al. Factors Associated With Syphilis Transmission and Acquisition Among Men Who Have Sex With Men: Protocol for a Multisite Egocentric Network Study. JMIR Res. Protoc. 2022, 11, e40095. [Google Scholar] [CrossRef]
- Welch, J. Antenatal Screening for Syphilis. BMJ 1998, 317, 1605–1606. [Google Scholar] [CrossRef]
- Paiva, J.C. de L.; Dias-Trindade, S.; Gonzalez, M.O.A.; Barros, D.M. da S.; Cardoso, P.H.; Bezerra, P.H.C.; Lima, T.G.F. de M.S.; Lacerda, J. de S.; Muneiro, L.C.; Cunha-Oliveira, A.; et al. Analysis of the Impact of Communication Campaigns under the Project “Syphilis No”: A National Tool for Inducing and Promoting Health. Int. J. Environ. Res. Public Health 2022, 19, e15884. [Google Scholar] [CrossRef]
- Varshney, K.; Ikanovic, A.; Ghosh, P.; Shet, P.; Di Sipio, M.; Khatri, C.; Mahmood, M.Q. A Global Scoping Review of the Factors Associated with HIV and Syphilis Co-Infection: Findings from 40 Countries. Venereology 2022, 1, 98–113. [Google Scholar] [CrossRef]
- Nguyen, S.H.; Dang, A.K.; Vu, G.T.; Nguyen, C.T.; Le, T.H.T.; Truong, N.T.; Hoang, C.L.; Tran, T.T.; Tran, T.H.; Pham, H.Q.; et al. Lack of Knowledge about Sexually Transmitted Diseases (STDs): Implications for STDs Prevention and Care among Dermatology Patients in an Urban City in Vietnam. Int. J. Environ. Res. Public Health 2019, 16, 1080. [Google Scholar] [CrossRef]
- Valentine, J.A.; Delgado, L.F.; Haderxhanaj, L.T.; Hogben, M. Improving Sexual Health in U.S. Rural Communities: Reducing the Impact of Stigma. AIDS Behav. 2022, 26, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Grieb, S.M.; Jackman, K.-M.; Jennings, J.M. Recommendations From Black Sexual Minority Men: Building Trust to Improve Engagement and Impact of HIV/STI Research. Health Promot. Pract. 2021, 22, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Peterman, T.A.; Furness, B.W. Public Health Interventions to Control Syphilis. Sex. Health 2015, 12, 126. [Google Scholar] [CrossRef] [PubMed]
- Saes, M. de O.; Duro, S.M.S.; Gonçalves, C. de S.; Tomasi, E.; Facchini, L.A. Assessment of the Appropriate Management of Syphilis Patients in Primary Health Care in Different Regions of Brazil from 2012 to 2018. Cad. Saude Publica 2022, 38. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.M. dos; Rosendo, T.M.S. de S.; Lopes, A.K.B.; Roncalli, A.G.; Lima, K.C. de Weaknesses in Primary Health Care Favor the Growth of Acquired Syphilis. PLoS Negl. Trop. Dis. 2021, 15, e0009085. [Google Scholar] [CrossRef]
- van Weel, C.; Kidd, M.R. Why Strengthening Primary Health Care Is Essential to Achieving Universal Health Coverage. Can. Med. Assoc. J. 2018, 190, E463–E466. [Google Scholar] [CrossRef]
- McCormack, H.; Guy, R.; Bourne, C.; Newman, C.E. Integrating Testing for Sexually Transmissible Infections into Routine Primary Care for Aboriginal Young People: A Strengths-based Qualitative Analysis. Aust. N. Z. J. Public Health 2022, 46, 370–376. [Google Scholar] [CrossRef]
- Guedes, A.L. de L.; Guimarães, D.C. da S.; Sarkis, D.J.; Gabriel, T.T.; Delgado, C.S.; Campos, A.A.L.; Nogueira, M.C.; Ribeiro, L.C. Factors Associated with Women Diagnosed with Syphilis Who Received Prenatal Care in a Primary Healthcare Unit. einstein (São Paulo) 2023, 31. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




