Introduction
Hepatitis C virus (HCV) infection–related end-stage liver disease is the leading indication for a liver transplant (LT) in the Western world [
1]. Because of the availability of highly effective anti-HCV agents with direct-acting antivirals (DAAs), the composition of the LT waiting list has changed considerably; DAA use has also resulted in the improvement of post-LT outcomes, including lower likelihood of graft failure, death, and retransplant procedures [
2,
3].
In formal pre-LT evaluation protocols, a series of laboratory tests, particularly viral serological tests such as those for hepatitis A, B, and C, are performed to confirm the irreversible nature of the patient’s liver disease [
4,
5]. Anti-HCV antibody titers are utilized as a serological screening marker in clinical diagnosis of HCV infection. A positive serum anti-HCV antibody test and negative polymerase chain reaction (PCR) test for HCV RNA indicate no evidence of current (active) HCV infection [
6]; however, current serological tests for anti-HCV antibodies only detect antibodies to HCV antigens, which are unrelated to protection from HCV [
7]. Moreover, recent clinical research show that viral clearance by DAA treatment does not eliminate HCV-related extrahepatic diseases, such as B-cell lymphoma and mixed cryoglobulinemia (MC), which are resulted from dysregulation of immune functions particularly in B cells, abnormal lymphoproliferation and the production of autoantibodies [
8]. The causes and molecular mechanisms of these antibody response to HCV infection remained poorly understood.
The role of anti-HCV antibodies and their association with the dysregulation of immune functions have been investigated. Toyoda et al. (2005) reported that anti-HCV antibody titers decreased during a 10-year follow-up after the eradication of HCV through interferon therapy [
9]. The mechanisms underlying the alteration of serum anti-HCV antibody levels have yet to be clarified. A previous study conducted in Taiwanese population concluded that anti-HCV titer strongly predicted HCV viremia; this excellent performance could be generalized to either HCV mono-infected or HBV dually infected patients [
10]. The association between changes in anti-HCV antibody titers and immune status in patients with sustained virologic response require further investigation, especially in the era of DAAs. Although recent breakthroughs in research on antibody responses to chronic HCV infection have provided valuable insights into neutralizing antibodies, several crucial aspects of such responses must still be uncovered [
11,
12]. HCV-specific T-cell response and humoral immunity have been reported to be associated with control of HCV infection [
13,
14]. Moreover, T-cell dysfunction and exhaustion induced by immune tolerance have been demonstrated to be strongly associated with successful LT outcomes [
15,
16]. However, most studies have demonstrated that DAA treatment for HCV infection may result in at least partial restoration of T-cell immune function [
17]. Few studies, nevertheless, have examined whether pre-LT DAA therapy contributes to restoration of immune function, and therefore affects post-LT graft damage.
In liver transplantation settings, biliary complications (BCs) and acute cellular rejection (ACR) often lead to post-transplant acute jaundice or graft dysfunction. In our previous study, we have demonstrated that liver graft microRNAs expression associated with different etiologies of post-LT acute jaundice, including acute rejection, acute cholangitis, recurrent hepatitis, non-specific pathological change and fatty change [
18]. However, we have not investigated the immune microenvironment of damaged liver graft, especially in HCV-infected recipients.
Whether the fluctuation of anti-HCV antibody titers before and after an LT reflects the altered immune response of HCV-infected patients is unclear. Accordingly, in this study, we included patients with chronic HCV who received pre-LT DAA therapy to examine the association between changes in anti-HCV antibody titers in such patients and allograft injury, including biliary complications (BCs) and acute cellular rejection (ACR).
Materials and Methods
Study population and study design
In this observational cohort study, we selected 153 liver allograft recipients with positive serum anti-HCV antibody titers detected during pre-LT evaluation from January 2015 to February 2021 from our liver transplantation program. We excluded those with negative serum anti-HCV antibodies, positive serum HBsAg levels, underlying psychiatric conditions, primary biliary cirrhosis, or alcohol-related liver disease, in addition to those with a pediatric LT history.
Among 153 recipients, 31 (20.3%) were administered pre-transplant DAAs (defined as DAA group) for 3 months based on their HCV genotype; the other 122 (79.7%) recipients did not have pre-transplant DAAs (defined as DAA naïve group) [
19]. All transplant recipients were followed up till September 2021.
Serial blood samples collected from all HCV-infected recipients were tested for anti-HCV antibodies (by using electrochemiluminescence immunoassays) and for HCV RNA viral load (by using quantitative real-time reverse transcription PCR) on the day before the LT and on the 30th day after the LT, respectively. Changes in anti-HCV antibody titers before and after the LT were recorded.
All recipients received the same immunosuppression protocol and post-LT anti-HCV therapy for recurrent HCV viremia in accordance with practice guidelines [
20]. Serum tests regarding hepatobiliary function were routinely monitored after the LT. When abnormal laboratory tests were repeatedly measured, further investigations and imaging studies such as doppler ultrasound of the liver and magnetic resonance cholangiopancreatography were arranged depending on the persistence and severity of the liver test abnormalities. In some cases, a post-LT percutaneous liver biopsy for histological confirmation of immune-mediated damage was also performed on the basis of the clinical decision of LT surgeons [
21].
Criteria for histopathological diagnosis of allograft rejection were defined on the basis of the 1995 Banff classification, and severity grades were characterized according to the rejection activity index [
22]. BCs in our study were determined clinically as a requirement of post-LT endoscopic retrograde cholangiopancreatography for biliary stenting or surgical revision of biliary tract anastomosis and episodes of post-LT biliary tract infection requiring systemic parenteral antibiotics and hospitalization.
Ethics
All procedures involving human participants were performed in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study protocol was approved and authorized by the Ethics Committee of our hospital (approval number: 202300159B0). Informed consent was obtained from all patients included in the study. We followed the Strengthening the Reporting of Observational Studies in Epidemiology statement guidelines for reporting observational studies. No allograft donors or recipients were from a vulnerable population.
Statistics
Statistical analyses were performed using SPSS (version 22.0; SPSS Inc., Chicago, IL, USA) and SAS software (version 9.4; SAS Institute, Inc., Cary, NC, USA). Descriptive values are expressed as mean ± standard deviation and percentages. Categorical variables were compared using the chi-square or Fisher’s exact test, and continuous variables were compared using Student’s t test. All tests were two-tailed, and a P value of <0.05 was considered statistically significant.
Results
Patient Characteristics
The clinical characteristics of the 153 allograft recipients who were seropositive for anti-HCV antibodies. Among these recipients, 31 (20.3%) constituted the DAA group and 122 (79.7%) constituted the DAA-naïve group. The mean age at the LT was 54.5 years, and men accounted for 47.7% of the population. The mean post-LT follow-up period was 43.6 months. Before the LT, only 1 (3.2%) patient in the DAA group and 69 patients (56.6%) in the DAA-naïve group still had detectable serum HCV RNA. Those with detectable viral load in the DAA-naïve group were patients without anti-viral therapy (n=49, 71%) or without effective response to interferon therapy (n=20, 29%). After the LT, none of the patients in the DAA group had HCV viremia; 61 (50%) patients with detectable serum HCV viral loads in the DAA-naïve group required further post-LT anti-viral therapy. The majority of liver donors were living donors (136/153, 88.9%). Moreover, 73 (47.7%) allograft recipients were diagnosed as having hepatocellular carcinoma (HCC) at the LT time, and 6 (4.9%) patients in the DAA-naïve group had de novo HCC after the LT (
Table 1).
Incidence of allograft injury: BCs and ACR
Among the 31 patients in the DAA group, 32.3% (10/31) had BCs and 25.8% (8/31) had ACR. Of the patients in the DAA-naïve group (n = 122), 14.8% (18/122) had BCs and 27.1% (33/122) had ACR (
Table 2). The incidence of BCs was higher in the DAA group than in the DAA-naïve group (32.3% vs. 14.8%,
P = 0.028). The incidence of ACR was comparable between the two groups (25.8% vs. 27.1%,
P = 0.56;
Figure 1).
Table 3 demonstrated that there are no significant differences in terms of age, sex, graft ischemic time for recipients with or without BCs and ACR.
Fluctuation of anti-HCV antibody titers and allograft injury
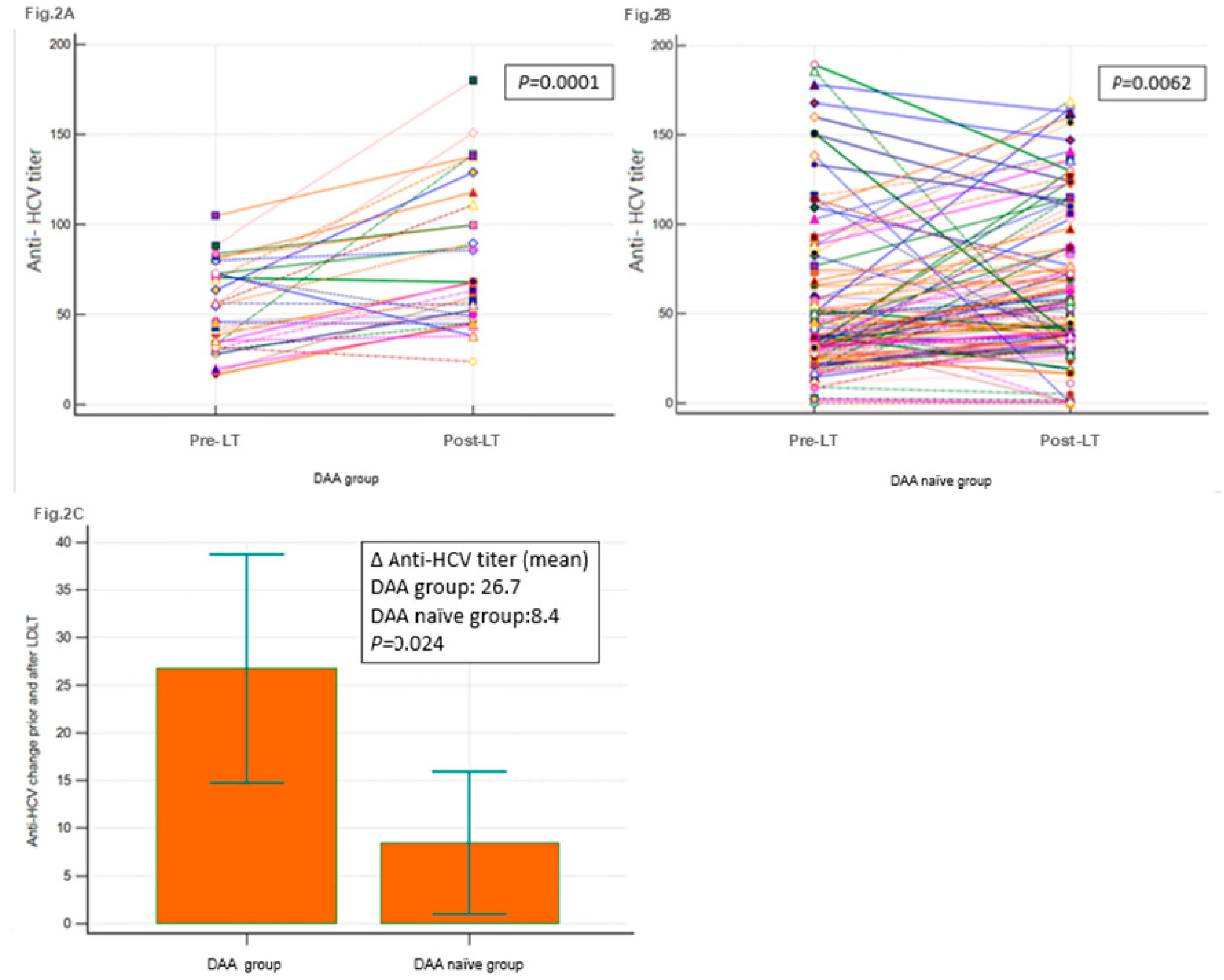
Most of the 153 patients with HCV infection who received liver allografts, the post-LT anti-HCV antibody titers were upregulated (
Table 4 and
Figure 2A,2B). Compared with the DAA-naïve group, the mean level of anti-HCV antibody titer upregulation was significantly higher in the DAA group (
p = 0.0024;
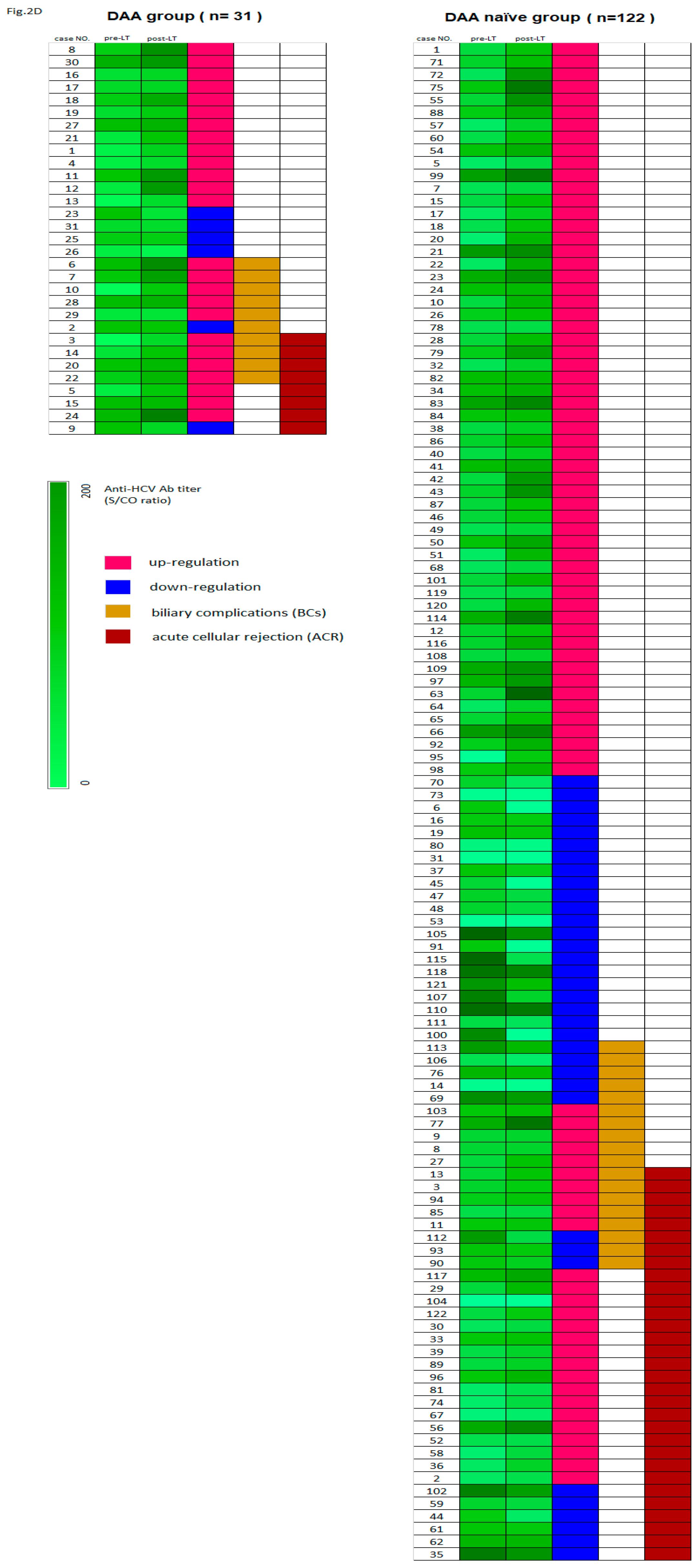
Figure 2C). Heapmap demonstrated pre-LT and post-LT anti-HCV titer of all patients (n=153) and patients with biliary complications (BCs) and acute cellular rejection (ACR) (
Figure 2D). Among the allograft recipients with BCs (n = 28) and ACR (n = 41), those in the DAA group had higher mean levels of anti-HCV antibody titer upregulation (
P = 0.05 and
P < 0.005, respectively) (
Table 5).
Discussion
This study determined the role of anti-HCV antibodies, which are considered a useful diagnostic and screening tool for HCV infection in the LT setting. Studies in the literature have rarely investigated antibody responses in HCV-infected recipients of liver allografts. According to our review of the literature, this is the first study to assess the association between changes in anti-HCV antibody titers before and after an LT and allograft injury, including BCs and ACR, in patients with chronic HCV who received pre-LT DAA therapy. The significant finding of this study is as following: first, the mean level of anti-HCV antibody titers upregulation was significantly higher in the DAA group, and there is no strong correlation to the post-LT HCV viremia. Second, the incidence of BCs was higher in the DAA group than in the DAA-naïve group. Furthermore, we observed that among allograft recipients with BCs and ACR, those in the DAA group had had higher mean levels of anti-HCV antibody titer upregulation after the LT. The comparable incidence of ACR between the DAA and DAA-naïve groups might be explained by the difficulty in differentiating between ACR and recurrent HCV after an LT [
23]. We postulated that the incidence of ACR in DAA naïve group would be overestimated, according to the result of our recent study, which revealed that 85% of allograft recipients had positive hepatic HCV RNA [
19].
The crucial role of antibody responses in HCV infection has been noted. HCV-specific T-cell dysfunction and viral escape mutations are the main factors leading to chronic HCV infection [
16,
24]. HCV clearance is associated with both innate immunity and adaptive immunity, including that associated with CD8
+ T cells, CD4
+ T cells, and B cells [
25]; notably, antibody responses and the development of neutralizing antibodies to antiviral immunity are closely related to CD4
+ T-cell and humoral immune responses [
11,
26]. Recently, numerous studies have demonstrated the reversal of HCV-specific immune response after successful viral elimination by DAA treatment [
17,
27]. However, changes in antibody responses, especially the fluctuation of anti-HCV antibody titers before and after DAA-mediated immune responses and HCV elimination, have never been investigated. The results of the current study reveal that the mean level of post-LT anti-HCV antibody titer upregulation was significantly higher in the DAA group, suggesting the restoration of immunity to HCV infection by pre-LT DAA therapy. This clinical finding still requires further validation to establish the exact immunological mechanisms underlying anti-HCV antibody titer fluctuation and DAA therapy.
We considered chronic HCV infection in liver transplantation to be a unique model of human immunology. On one hand, the phenomenon of significant upregulation of anti-HCV antibody titers in the DAA group after the LT might indicate immune reactivation in the liver allograft recipients; on the other hand, our study revealed that among the recipients with BCs and ACR, those in the DAA group had significantly higher mean levels of post-LT anti-HCV antibody titer upregulation. We can reasonably speculate that the reactivated immune response induced by DAA treatment and the possible presence of high levels of IgG and immune complexes resulted in the immune-mediated BCs and ACR. BCs are among the most complex post-LT problems and often result from biliary stricture. According to previous studies, in addition to ischemia-related factors, immune-mediated injury targeting the biliary epithelium plays a major role in the development of post-LT BCs [
28,
29,
30,
31]. ACR, defined as a histological feature of portal infiltrative inflammatory cells, nonsuppurative cholangitis and endotheliitis (Snover’s triad), is a prominent post-LT immunopathologic injury mediated by CD4
+ T cells [
22]. To explain our clinical findings, we postulated that immunological response alteration and immune function restoration after DAA therapy might not only prompt allograft rejection, but also interfere healing and fibrosis process of biliary anastomosis, consequently increasing the rate of post-LT BCs.
This study has several strengths. First, although the mechanisms underlying post-DAA anti-HCV antibody titer upregulation and BCs and ACR have yet to be fully elucidated, our study adds to the body of knowledge about the possible DAA-mediated immunopathologic response in the field of post-LT alloimmunity and also demonstrates the significance of antibody responses to HCV infection before and after an LT. It may be involved the genetic manipulation in chronic C hepatitis infection. By the genetic background, the immune response for the environmental factor such as viral infections regarding to MHC, HLA class II and DR3/4 as we know. Proliferation of T-cell associated with immune checkpoint expressed the blockade or agonism is closely related to the positive and negative costimulation of T cell activation. At the meanwhile, the positive costimulation provides the proliferation and cytokine production, prevention of anergy and making T helper and cytotoxic T lymphocyte differentiation. In contrast, the negative costimulation causing inhibition of proliferation and cytokine production, enhancing anergy and induction of T reg. This mechanism needs to through the Fas ligand (FASL) and LIGHT pathway of IFN gamma and IL-2 from cytotoxic T lymphocytes and majority adhesion with decoy receptor 3 (DcR3) to attack the target organs. Therefore, it is easily to explain the FASL is liked with HCV infection by DcR3 touching the infected hepatocytes and leading failure to spontaneous HCV clearance. Finally, B cell expressed the immunoglobin to finish the immune reaction regards to a so-called detective antibody (anti-HCV). In which, this antibody cannot protect the host hepatocytes not only, but also the DcR3 may cause the fluctuation of the anti-HCV title to regulate the immune response in the infected hepatocytes [
32]. In particular, we discovered the relationship between HCV clearance mediated by DAA therapy, fluctuations in anti-HCV antibody titers induced by cellular and humoral immune responses, and allograft injury mediated by immune responses in the field of liver transplantation, all of which can provide innovative ideas for further human research for developing HCV antibody–based vaccines to protect new liver grafts from HCV reinfection.
Our study has some limitations. First, this was not a prospective study. Nevertheless, we consider the risk of bias to be very small due to the standardization of pre-LT clinical and laboratory assessments, sophisticated LT surgical techniques, and post-LT protocols for immunosuppression regimens and graft outcome surveillance. Second, we could determine the association between changes in anti-HCV antibody titers before and after LT procedures and allograft injury only through a retrospective review of medical records. Future prospective studies may be warranted to identify the role of molecular expression levels in allograft rejection and to clarify the mechanisms underlying the association between DAA-induced anti-HCV antibody titer fluctuation and immune reaction to post-LT allograft injury; the findings of such studies may shed light on measures for optimizing pre-LT plans and donor selection and may serve as a reference for modifying anti-HCV treatment protocols for potential liver allograft recipients in order to reduce the incidence and severity of both BCs and ACR.
In conclusion, our study figured out the phenomenon of significant up-regulation of anti-HCV antibody titers after liver transplantation in recipients with pre-LT DAA therapy. The fluctuations of anti-HCV antibody titer might have been engendered by restoration of immunity induced by pre-LT DAA use for treating chronic HCV infection, and this immune response might have impact on allograft injury, including BCs and ACR.
Funding
This work was supported by the grant number CMRPG8N0761 from the Chang Gung Memorial Hospital of Taiwan.
Ethical Approval
The study protocol was approved and authorized by the Ethics Committee of CGMH (approval number: 202300159B0).
Statement of Informed Consent
Informed consent was obtained from all patients included in the study.
Data Availability
The datasets used and analyzed in this study are available from the corresponding author upon reasonable request.
Acknowledgments
We gratefully acknowledge all the participants who participated in the study and the study team for their support.
Disclosure
The authors declare no conflict of interest.
Statement of Human Rights
All procedures involving human participants were performed in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. We followed the Strengthening the Reporting of Observational Studies in Epidemiology statement guidelines for reporting observational studies. No allograft donors or recipients were from a vulnerable population.
References
- Crespo, G.; Mariño, Z.; Navasa, M.; Forns, X. Viral hepatitis in liver transplantation. Gastroenterology 2012, 142, 1373–1383.e1371. [Google Scholar] [CrossRef] [PubMed]
- Crespo, G.; et al. The efficacy of direct anti-HCV drugs improves early post-liver transplant survival and induces significant changes in waiting list composition. J Hepatol 2018, 69, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Young, K.; Liu, B.; Bhuket, T.; Wong, R.J. Lower Likelihood of Post-transplant Graft Failure, Death, and Retransplantation in the Era of Direct-Acting Antivirals. J Clin Exp Hepatol 2020, 10, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Millson, C.; et al. Adult liver transplantation: A UK clinical guideline - part 1: pre-operation. Frontline Gastroenterol 2020, 11, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.; DiMartini, A.; Feng, S.; Brown, R., Jr.; Fallon, M. Evaluation for liver transplantation in adults: 2013 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Hepatology 2014, 59, 1144–1165. [Google Scholar] [CrossRef]
- Ghany, M.G.; Morgan, T.R.; Panel, A.-I.H.C.G. Hepatitis C Guidance 2019 Update: American Association for the Study of Liver Diseases-Infectious Diseases Society of America Recommendations for Testing, Managing, and Treating Hepatitis C Virus Infection. Hepatology 2020, 71, 686–721. [Google Scholar] [CrossRef]
- Tang, W.; et al. Diagnostic accuracy of tests to detect Hepatitis C antibody: a meta-analysis and review of the literature. BMC Infect Dis 2017, 17, 695. [Google Scholar] [CrossRef]
- Bonacci, M.; et al. Long-Term Outcomes of Patients With HCV-Associated Cryoglobulinemic Vasculitis After Virologic Cure. Gastroenterology 2018, 155, 311–315.e316. [Google Scholar] [CrossRef]
- Toyoda, H.; et al. Changes in hepatitis C virus (HCV) antibody status in patients with chronic hepatitis C after eradication of HCV infection by interferon therapy. Clin Infect Dis 2005, 40, e49–54. [Google Scholar] [CrossRef]
- Liu, H.-Y.; Lin, Y.-H.; Lin, P.-J.; Tsai, P.-C.; Liu, S.F.; Huang, Y.-C.; et al. Anti-HCV antibody titer highly predicts HCV viremia in patients with hepatitis B virus dual-infection. PLoS ONE 2021, 16, e0254028. [Google Scholar] [CrossRef]
- Law, M. Antibody Responses in Hepatitis C Infection. Cold Spring Harb Perspect Med 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Luxenburger, H.; Neumann-Haefelin, C.; Thimme, R.; Boettler, T. HCV-Specific T Cell Responses During and After Chronic HCV Infection. Viruses 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Osburn, W.O.; et al. Clearance of hepatitis C infection is associated with the early appearance of broad neutralizing antibody responses. Hepatology 2014, 59, 2140–2151. [Google Scholar] [CrossRef] [PubMed]
- Pestka, J.M.; et al. Rapid induction of virus-neutralizing antibodies and viral clearance in a single-source outbreak of hepatitis C. Proc Natl Acad Sci U S A 2007, 104, 6025–6030. [Google Scholar] [CrossRef]
- Ding, M.; He, Y.; Zhang, S.; Guo, W. Recent Advances in Costimulatory Blockade to Induce Immune Tolerance in Liver Transplantation. Front Immunol 2021, 12, 537079. [Google Scholar] [CrossRef]
- Angeletti, A.; Cantarelli, C.; Riella, L.V.; Fribourg, M.; Cravedi, P. T Cell Exhaustion in Organ Transplantation. Transplantation 2021. [Google Scholar] [CrossRef]
- Osuch, S.; Metzner, K.J.; Caraballo Cortés, K. Reversal of T Cell Exhaustion in Chronic HCV Infection. Viruses 2020, 12. [Google Scholar] [CrossRef]
- Lin, S.-H.; Wu, K.-T.; Wang, C.-C.; Huang, K.-T.; Chen, K.-D.; Hsu, L.-W.; Eng, H.-L.; Chiu, K.-W. Liver Graft MicroRNAs Expression in Different Etiology of Acute Jaundice after Living Donor Liver Transplantation. Biology 2022, 11, 1228. [Google Scholar] [CrossRef]
- Lin, S.H.; et al. HCV RNA in serum and liver samples of patients undergoing living donor liver transplantation. J Int Med Res 2021, 49, 3000605211034945. [Google Scholar] [CrossRef]
- Lucey, M.R.; et al. Long-term management of the successful adult liver transplant: 2012 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Liver Transpl 2013, 19, 3–26. [Google Scholar] [CrossRef]
- Chiu, K.W.; et al. Characterization of liver enzymes on living related liver transplantation patients with acute rejection. Hepatogastroenterology 2005, 52, 1825–1827. [Google Scholar]
- Panqueva, R.P.L. e. a. Liver Biopsies in Transplant Pathology Histopathological Diagnosis and Clinicopathological Correlation in the Early Post-Transplant Period. Rev Col Gastroenterol 2016, 31, 2016. [Google Scholar]
- Grassi, A.; Ballardini, G. Post-liver transplant hepatitis C virus recurrence: an unresolved thorny problem. World J Gastroenterol 2014, 20, 11095–11115. [Google Scholar] [CrossRef]
- Rehermann, B.; Thimme, R. Insights From Antiviral Therapy Into Immune Responses to Hepatitis B and C Virus Infection. Gastroenterology 2019, 156, 369–383. [Google Scholar] [CrossRef] [PubMed]
- Heim, M.H.; Thimme, R. Innate and adaptive immune responses in HCV infections. J Hepatol 2014, 61, S14–25. [Google Scholar] [CrossRef] [PubMed]
- Sepulveda-Crespo, D.; Resino, S.; Martinez, I. Hepatitis C virus vaccine design: focus on the humoral immune response. J Biomed Sci 2020, 27, 78. [Google Scholar] [CrossRef] [PubMed]
- Casey, J.L.; Feld, J.J.; MacParland, S.A. Restoration of HCV-Specific Immune Responses with Antiviral Therapy: A Case for DAA Treatment in Acute HCV Infection. Cells 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; et al. Nonanastomotic Biliary Strictures After Liver Transplantation. Am Surg 2020, 86, 1363–1367. [Google Scholar] [CrossRef] [PubMed]
- Karimian, N.; Op den Dries, S.; Porte, R.J. The origin of biliary strictures after liver transplantation: is it the amount of epithelial injury or insufficient regeneration that counts? J Hepatol 2013, 58, 1065–1067. [Google Scholar] [CrossRef]
- de Vries, Y.; von Meijenfeldt, F.A.; Porte, R.J. Post-transplant cholangiopathy: Classification, pathogenesis, and preventive strategies. Biochim Biophys Acta Mol Basis Dis 2018, 1864, 1507–1515. [Google Scholar] [CrossRef]
- Op den Dries, S.; Sutton, M.E.; Lisman, T.; Porte, R.J. Protection of bile ducts in liver transplantation: looking beyond ischemia. Transplantation 2011, 92, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Demirci, G.; Li, X.C. Negative T cell costimulation and islet tolerance. Diabetes Metab Res Rev 2003, 19, 179–85. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).