Submitted:
29 August 2023
Posted:
30 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials for orthopedic implants
3. Features of AM techniques as related to printing porous structures
3.1. AM techniques to print porous biocompatibe products of Ti alloys
-
Powders for SLM printing of titanium medical devices must meet strict requirements (purity, density, homogeneity, particle shape and size) [51,52] to ensure high print quality, biocompatibility, and product reliability [53,54]:
- Homogeneity: Powders must be homogeneous in terms of particle shape and size. This is necessary to ensure uniform sintering and to obtain smooth printed surface.
- Purity: Powders must not contain any impurities inherited from atomizing processes which can affect the biomedical and mechanical response of the products. This avoids the risk of rejection, increases service life, and accelerates osseointegration.
- Density: The density of powders should be enough to ensure proper sintering and to avoid the formation of voids and other printing defects.
- Particle size: The powder particle size should be small enough to ensure good adhesion to the platform and prevent the formation of internal defects in the products. Particle size also defines the minimum possible inaccuracy of the printed structures as compared with the designed ones.
- Particle shape: The particle shape should be spherical or close to spherical to ensure uniform sintering and form smooth printed surfaces.
- AM equipment settings. Laser processing parameters, such as power and speed, should be properly adjusted to ensure optimal powder melting and solidification. Before the final production, the printer settings should be calibrated for each particular material and design. Testing different parameters and settings can help determine the optimal settings for a specific task and material [55].
- Environmental conditions, such as humidity, dustiness, and temperature in the facility, can have an impact on the print quality. It is important to ensure that proper environmental conditions are monitored during SLM printing [56].
3.2. Features of as-printed materials
4. Computer-assisted design of pore/cell geometry
4.1. Design of cell geometry to build up porous products
- 1)
- Skeleton structure (Solid-Networks, φ < k or φ > k). In this case, one of the volumes bounded by the minimal surface is considered as a solid region, while the other is considered as an empty region. This is achieved by considering the volume bounded by the minimal surface such that φ(x, y, z) > k or φ(x, y, z) < k, in order to create the lattice TPMS structure.
- 2)
- Sheet structure (Sheet-Networks, k ≤ φ ≥ k). In this case, the creation of the lattice structure of TPMS is achieved by creating a double surface and transforming it into a solid structure based on the blending of isosurfaces along its normal and anti-normal direction by solving −k ≤ φ(x, y, z) ≤ k. The resulting structure is a lattice created based on the isosurface by thickening it within a certain limit −k ≤ φ(x, y, z) ≤ k.
4.2. Consistency of the designed and printed structures
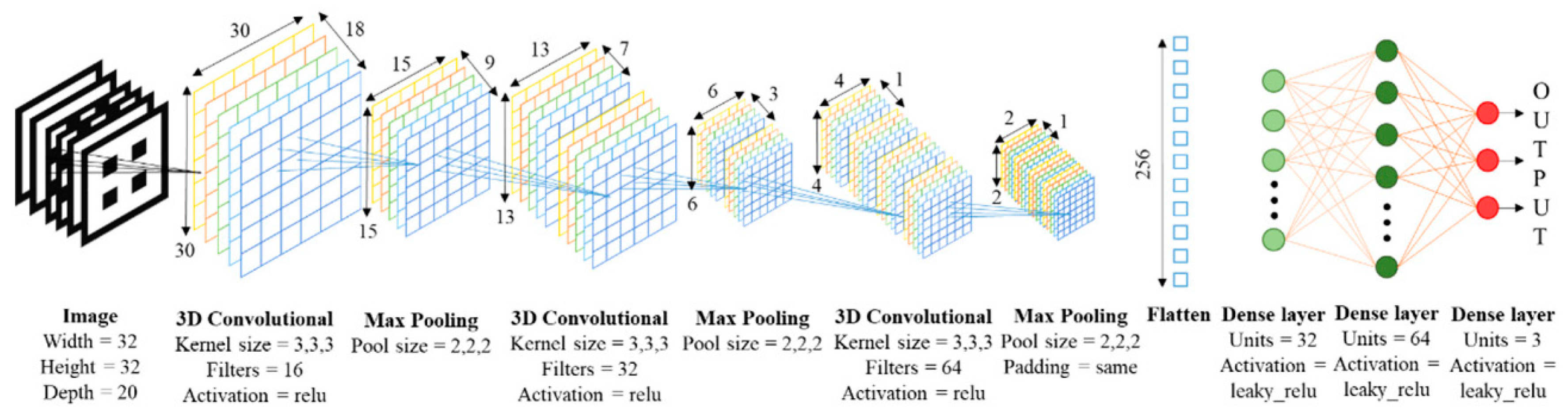
4.3. Neural networks to optimize porous structures for biomedical applications
- Improved diagnostic function. AI analyzes medical data, images and symptoms with high accuracy and is useful for early detection of diseases and more accurate diagnosis.
- Optimized treatment. AI can create a personalized treatment plan for each patient based on their unique characteristics and response to medications.
- Fewer mistakes. AI can help minimize diagnostic and treatment errors, as well as improve the quality of care and patient safety.
- Speed up research. AI can process and analyze vast amounts of data, accelerating the research and development of new treatments.
- Automation of tasks. AI allows the automation of routine tasks such as processing medical records, freeing healthcare workers to focus on more complex tasks.
- The main disadvantages of using artificial intelligence in medicine:
- Lack of transparency. Some AI algorithms are difficult to understand and explain, which creates trust issues between medical professionals and patients.
- Ethical issues. The use of AI in medicine raises important ethical issues such as data privacy, decision making, and liability for errors.
- Data dependencies. The performance of AI in healthcare depends on the quality and quantity of data available. Inaccurate or limited data may affect the accuracy of your results.
- High cost. Developing, deploying, and maintaining AI systems in healthcare can be costly, especially in smaller clinics or countries with limited resources.
- Responsibility for errors. The use of AI in medicine raises questions about who is responsible for errors made by automated systems, which can be legally difficult.
- Drug inclusion mode [103].
- Shape of the implant. Shape, size, and configuration of porous implants. This includes parameters such as pore diameter and depth, pore location, total pore volume, and total implant surface [104].
- Material microstructure. Porous implants may have different microstructures and phase compositions, which may affect their ability to interact with or release drugs.
- Physical and mechanical properties of materials. It includes parameters such as material density, strength, elasticity, and hardness. These properties can affect the material's ability to retain and release drugs over time [105].
- Chemical properties of materials. A material's chemical composition can affect drug interactions and stability.
- Physicochemical properties of medicinal products. Parameters such as water solubility, particle size and diffusion rate. These properties determine how the drug penetrates the pores of the implant and how quickly it is released.
- Pore size and shape. Large, open pores can provide faster drug release rates than small, closed pores.
- Porosity of the material. Greater porosity increases the availability of interactions with the drug on the material surface, thus increasing the release rate.
- Drug concentration. A high drug concentration within the pores of the implant can accelerate the release process.
- Physicochemical interactions: Interactions between drugs and implant materials can affect drug release.
- Drug diffusion. The rate of drug diffusion through the implant material determines how quickly the drug is released.
- Environmental conditions. Temperature, humidity, and other environmental conditions can affect drug diffusion and release from implants.
5. Biomimetic scaffolds for bone tissue engineering: biomedical issues
5.1. Porous matrices
5.2. Cell geometry
5.3. Biocoatings of porous structures
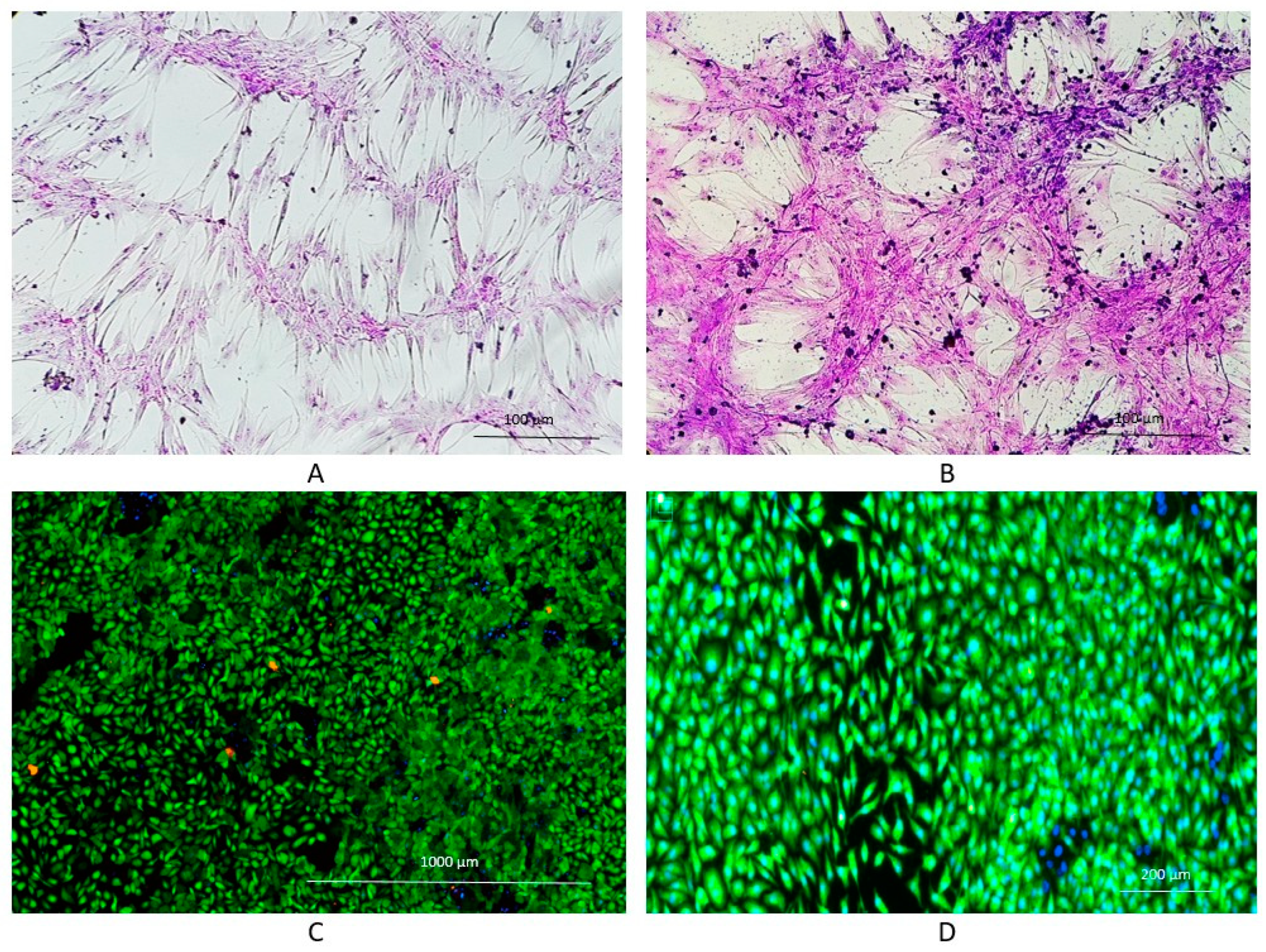
5.4. Cell colonization
5.5. Clinical studies of porous T-based materials
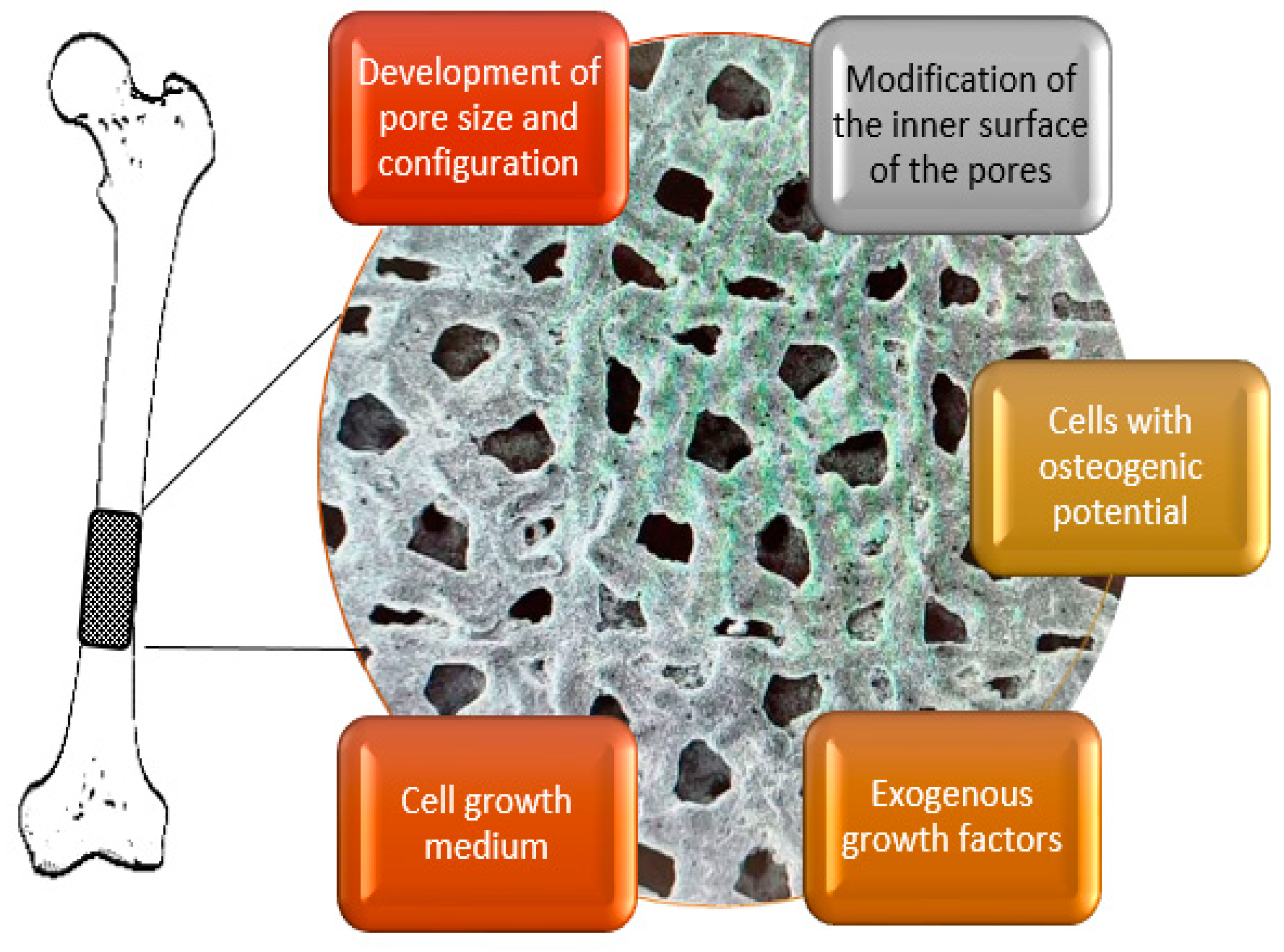
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Davoodi, E.; Montazerian, H.; Mirhakimi, A.S.; Zhianmanesh, M.; Ibhadode, O.; Shahabad, S.I.; Esmaeilizadeh, R.; Sarikhanig, E.; Toorandaz, S.; Sarabi, S.A.; Nasiri, R.; Zhu, Y.; Kadkhodapour, J.; Li, B.; Khademhosseini, A.; Toyserkani, E. Additively manufactured metallic biomaterials. Bioactive Mater. 2022, 15, 214–249. [Google Scholar] [CrossRef]
- Vesvoranan, O.; Anup, A.; Hixon, K.R. Current Concepts and Methods in Tissue Interface Scaffold Fabrication. Biomimetics 2022, 7, 151. [Google Scholar] [CrossRef]
- Lv, Y; Wang, B; Liu, G; Tang, Y; Lu, E; Xie, K; Lan, C; Liu, J; Qin, Z; Wang, L. Metal Material, Properties and Design Methods of Porous Biomedical Scaffolds for Additive Manufacturing: A Review. Front. Bioeng. Biotechnol 2021, 9, 641130. [Google Scholar] [CrossRef]
- Koju, N.; Niraula, S.; Fotovvati, B. Additively Manufactured Porous Ti6Al4V for Bone Implants: A Review. Metals 2022, 12, 687. [Google Scholar] [CrossRef]
- du Plessis, A.; Razavi, S.M.J.; Benedetti, M.; Murchio, S.; Leary, M.; Watson, M.; Bhate, D.; Berto, F. Properties and applications of additively manufactured metallic cellular materials: A review. Prog. Mater. Sci. 2022, 125, 100918. [Google Scholar] [CrossRef]
- Marsell, R.; Einhorn, T. A. The Biology of Fracture Healing. Injury 2011, 42, 551–555. [Google Scholar] [CrossRef]
- Ho-Shui-Ling, A.; Bolander, J.; Rustom, L. E.; Johnson, A. W.; Luyten, F. P.; Picart, C. Bone regeneration strategies: Engineered scaffolds, bioactive molecules and stem cells current stage and future perspectives. Biomaterials 2018, 180, 143–162. [Google Scholar] [CrossRef]
- Newman, H.; Shih, Y. V.; Varghese, S. Resolution of inflammation in bone regeneration: From understandings to therapeutic applications. Biomaterials 2021, 277, 121114. [Google Scholar] [CrossRef]
- The orthopaedic industry annual report 2023. Available online: https://www.orthoworld.com/the-orthopaedic-industry-annual-report/ (accessed on 23 August 2023).
- de Villiers, T.J.; Goldstein, S.R. Bone Health 2022: An Update. Climacteric 2022, 25, 1–3. [Google Scholar] [CrossRef]
- Wu, Ai-Min et al. Global, regional, and national burden of bone fractures in 204 countries and territories, 1990–2019: a systematic analysis from the Global Burden of Disease Study 2019, Lancet Healthy Longev. 2021, 2, e580–e592. [CrossRef]
- Brown, H.K.; Schiavone, K.; Gouin, F.; Heymann, M.F.; Heymann, D. Biology of Bone Sarcomas and New Therapeutic Developments. Calcif Tissue Int. 2018, 102, 174–195. [Google Scholar] [CrossRef] [PubMed]
- Thanindratarn, P; Dean, D.C.; Nelson, S.D.; Hornicek, F.J.; Duan, Z. Advances in immune checkpoint inhibitors for bone sarcoma therapy. J. Bone Oncol. 2019, 15, 100221. [CrossRef] [PubMed]
- Ma, X.; Gao, Y.; Zhao, D.; Zhang, W.; Zhao, W.; Wu, M.; Cui, Y.; Li, Q.; Zhang, Z.; Ma, C. Titanium Implants and Local Drug Delivery Systems Become Mutual Promoters in Orthopedic Clinics. Nanomaterials 2022, 12, 47. [Google Scholar] [CrossRef]
- Massaro, C.; Rotolo, P.; De Riccardis, F.; Milella, E.; Napoli, A.; Wieland, M.; Textor, M.; Spencer, N. D.; Brunette, D. M. Comparative investigation of the surface properties of commercial titanium dental implants. Part I: chemical composition. J. Mater. Sci. Mater. Med. 2002, 13, 535–548. [Google Scholar] [CrossRef]
- Dutta, B.; Froes, F. H. (Sam) The Additive Manufacturing (AM) of Titanium Alloys. Met. Powder Rep. 2017, 72, 96–106. [Google Scholar] [CrossRef]
- Antunes, L. H. M.; de Lima, C. R. P. Cobalt-chromium alloys – properties and applications. Reference Module in Materials Science and Materials Engineering 2018. [Google Scholar] [CrossRef]
- Goharian, A. Further development of trauma plating fixation. Trauma Plating Systems ed. by A. Goharian, Elsevier, 2017, 361–381. [CrossRef]
- Zysset, P. K.; Edward Guo, X.; Edward Hoffler, C.; Moore, K. E.; Goldstein, S. A. Elastic modulus and hardness of cortical and trabecular bone lamellae measured by nanoindentation in the human femur. J. Biomech. 1999, 32, 1005–1012. [Google Scholar] [CrossRef] [PubMed]
- Savio, D.; Bagno, A. When the total hip replacement fails: A review on the stress-shielding effect. Processes 2022, 10, 612. [Google Scholar] [CrossRef]
- Kim, T.; See, C.W.; Li, X.; Zhu, D. Orthopedic implants and devices for bone fractures and defects: Past, present and perspective. Eng. Regen. 2020, 1, 6–18. [Google Scholar] [CrossRef]
- Derome, P.; Sternheim, A.; Backstein, D.; Malo, M. Treatment of large bone defects with trabecular metal cones in revision total Knee Arthroplasty. J. Arthroplasty 2014, 29, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, C.; Zhao, H.; Qu, S.; Li, X.; Li, Y. New developments of Ti-based alloys for biomedical applications. Materials 2014, 7, 1709–1800. [Google Scholar] [CrossRef]
- Coffigniez, M.; Gremillard, L.; Balvay, S.; Lachambre, J.; Adrien, J.; Boulnat, X. Direct-ink writing of strong and biocompatible titanium scaffolds with bimodal interconnected porosity. Addit. Manuf. 2021, 39, 101859. [Google Scholar] [CrossRef]
- Kumar, P.; Ramamurty, U. Microstructural optimization through heat treatment for enhancing the fracture toughness and fatigue crack growth resistance of selective laser melted Ti-6Al-4V alloy. Acta Mater. 2019, 169, 45–59. [Google Scholar] [CrossRef]
- Renner, P.; Jha, S.; Chen, Y.; Raut, A.; Mehta, S. G.; Liang, H. A review on corrosion and wear of additively manufactured alloys. J. Tribol. 2021, 143. [Google Scholar] [CrossRef]
- Pattanayak, D. K.; Fukuda, A.; Matsushita, T.; Takemoto, M.; Fujibayashi, S.; Sasaki, K.; Nishida, N.; Nakamura, T.; Kokubo, T. Bioactive Ti metal analogous to human cancellous bone: Fabrication by selective laser melting and chemical treatments. Acta Biomater. 2011, 7, 1398–1406. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, N.; Fujibayashi, S.; Takemoto, M.; Sasaki, K.; Otsuki, B.; Nakamura, T.; Matsushita, T.; Kokubo, T.; Matsuda, S. Effect of pore size on bone ingrowth into porous titanium implants fabricated by additive manufacturing: an in vivo experiment. Mater. Sci. Eng. C 2016, 59, 690–701. [Google Scholar] [CrossRef] [PubMed]
- Davoodi, E.; Zhianmanesh, M.; Montazerian, H.; Milani, A. S.; Hoorfar, M. Nano-porous anodic alumina: Fundamentals and applications in tissue engineering. J. Mater. Sci. Mater. Med. 2020, 31, 60. [Google Scholar] [CrossRef]
- Al-Tamimi, A. A.; Peach, C.; Fernandes, P. R.; Cseke, A.; Bartolo, P. J. D. S. Topology optimization to reduce the stress shielding effect for orthopedic applications. Procedia CIRP 2017, 65, 202–206. [Google Scholar] [CrossRef]
- Rahbari, A.; Montazerian, H.; Davoodi, E.; Homayoonfar, S. Predicting permeability of regular tissue engineering scaffolds: Scaling analysis of pore architecture, scaffold length, and fluid flow rate effects. Comput. Methods Biomech. Biomed. Eng. 2016, 20, 231–241. [Google Scholar] [CrossRef]
- Zhianmanesh, M.; Varmazyar, M.; Montazerian, H. Fluid permeability of graded porosity scaffolds architectured with minimal surfaces. ACS Biomater. Sci. Eng. 2019, 5, 1228–1237. [Google Scholar] [CrossRef]
- Kim, F. H.; Moylan, S. P. Literature review of Metal Additive Manufacturing Defects 2018. Advanced Manufacturing Series (NIST AMS), National Institute of Standards and Technology, Gaithersburg, MD, [online], https://doi.org/10.6028/NIST.AMS.100-16 (Accessed 23 August 2023). [CrossRef]
- Li, X.; Jia, X.; Yang, Q.; Lee, J. Quality Analysis in metal additive manufacturing with Deep Learning. J. Intell. Manuf. 2020, 31, 2003–2017. [Google Scholar] [CrossRef]
- Cui, W.; Zhang, Y.; Zhang, X.; Li, L.; Liou, F. Metal additive manufacturing parts inspection using convolutional neural network. Appl. Sci. 2020, 10, 545. [Google Scholar] [CrossRef]
- Mellor, S.; Hao, L.; Zhang, D. Additive manufacturing: A framework for implementation. Int. J. Prod. Econ. 2014, 149, 194–201. [Google Scholar] [CrossRef]
- Mondal, P.; Wazeer, A.; Das, A.; Karmakar, A. Low cost porous Ti-6Al-4 V structures by additive manufacturing for orthopaedic applications. Mater. Today Proc. 2022, 67, 398–403. [Google Scholar] [CrossRef]
- Xiong, Y.; Han, Z.; Qin, J.; Dong, L.; Zhang, H.; Wang, Y.; Chen, H.; Li, X. Effects of porosity gradient pattern on mechanical performance of additive manufactured Ti-6Al-4V functionally graded porous structure. Mater. Des. 2021, 208, 109911. [Google Scholar] [CrossRef]
- Awad, A.; Fina, F.; Goyanes, A.; Gaisford, S.; Basit, A. W. 3D printing: Principles and pharmaceutical applications of Selective Laser Sintering. Int. J. Pharm. 2020, 586, 119594. [Google Scholar] [CrossRef] [PubMed]
- Herzog, D.; Seyda, V.; Wycisk, E.; Emmelmann, C. Additive Manufacturing of Metals. Acta Mater. 2016, 117, 371–392. [Google Scholar] [CrossRef]
- Ataee, A.; Li, Y.; Wen, C. A comparative study on the nanoindentation behavior, wear resistance and in vitro biocompatibility of SLM manufactured CP–TI and EBM manufactured TI64 gyroid scaffolds. Acta Biomater. 2019, 97, 587–596. [Google Scholar] [CrossRef]
- Murr, L. E. Metallurgy principles applied to powder bed fusion 3D printing/additive manufacturing of personalized and optimized metal and alloy biomedical implants: An overview. J Mater. Res. Technol. 2020, 9, 1087–1103. [Google Scholar] [CrossRef]
- Gu, D.; Shi, X.; Poprawe, R.; Bourell, D.L.; Setchi, R.; Zhu, J. Material-structure-performance integrated laser-metal additive manufacturing. Science 2021, 372, eabg1487, 2023, 1–33. [Google Scholar] [CrossRef]
- Dumpa, N.; Butreddy, A.; Wang, H.; Komanduri, N.; Bandari, S.; Repka, M. A. 3D printing in Personalized Drug Delivery: An overview of hot-melt extrusion-based fused deposition modeling. Int. J. Pharm. 2021, 600, 120501. [Google Scholar] [CrossRef]
- Kumar, V.; Kaur, H.; Kumari, A.; Hooda, G.; Garg, V.; Dureja, H. Drug delivery and testing via 3D printing. Bioprinting 2023, e00298. [Google Scholar] [CrossRef]
- Jiao, Y.; Li, X.; Zhang, X.; Li, G.; Fang, J.; Xuan, S.; Liu, L.; Wang, S.; Xie, H. Silver antibacterial surface adjusted by hierarchical structure on 3D printed porous titanium alloy. Appl. Surf. Sci. 2023, 610, 155519. [Google Scholar] [CrossRef]
- Tshephe, T. S.; Akinwamide, S. O.; Olevsky, E.; Olubambi, P. A. Additive manufacturing of titanium-based alloys- a review of methods, properties, challenges, and prospects. Heliyon 2022, 8, e09041. [Google Scholar] [CrossRef] [PubMed]
- Farazin, A.; Zhang, C.; Gheisizadeh, A.; Shahbazi, A. 3D bio-printing for use as bone replacement tissues: A review of Biomedical Application. Biomed. Eng. Adv. 2023, 5, 100075. [Google Scholar] [CrossRef]
- Weaver, J. S.; Heigel, J. C.; Lane, B. M. Laser spot size and scaling laws for laser beam additive manufacturing. J. Manuf. Processes 2022, 73, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Dutta, B.; Froes, F. H. (Sam) The Additive Manufacturing (AM) of Titanium Alloys. Met. Powder Rep. 2017, 72, 96–106. [Google Scholar] [CrossRef]
- Keaveney, S.; Shmeliov, A.; Nicolosi, V.; Dowling, D. P. Investigation of process by-products during the selective laser melting of Ti6Al4V powder. Additive Manufacturing 2020, 36, 101514. [Google Scholar] [CrossRef]
- Dowling, L.; Kennedy, J.; O’Shaughnessy, S.; Trimble, D. A review of critical repeatability and reproducibility issues in powder bed fusion. Materials and Design 2020, 186, 108346. [Google Scholar] [CrossRef]
- Guo, A. X. Y.; Cheng, L.; Zhan, S.; Zhang, S.; Xiong, W.; Wang, Z.; Wang, G.; Cao, S. C. Biomedical applications of the powder-based 3D Printed Titanium Alloys: A Review. Journal of Materials Science and Technology 2022, 125, 252–264. [Google Scholar] [CrossRef]
- Ma, K.; Chen, H.; Shen, Y.; Guo, Y.; Li, W.; Wang, Y.; Zhang, Y.; Sun, Y. Feasibility Study and material selection for powder-bed fusion process in printing of Denture clasps. Computers in Biology and Medicine 2023, 157, 106772. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.; An, S.; Robles, U.; Rumpf, R. C. Process parameter optimization for removable partial denture frameworks manufactured by Selective Laser Melting. The Journal of Prosthetic Dentistry 2023, 129, 191–198. [Google Scholar] [CrossRef]
- Roberson, G. A.; Sinha, P. K. 3D printing in Orthodontics: A practical guide to the Printer Technology and selection. Seminars in Orthodontics 2022, 28, 100–106. [Google Scholar] [CrossRef]
- Gu, D.; Hagedorn, Y.C.; Meiners, W.; Meng, G.; Batista, R.J.S.; Wissenbach, K.; Poprawe, R. Densification behavior, microstructure evolution, and wear performance of selective laser melting processed commercially pure titanium. Acta Mater. 2012, 60, 3849–3860. [Google Scholar] [CrossRef]
- Barbas, A.; Bonnet, A.-S.; Lipinski, P.; Pesci, R.; Dubois, G. Development and mechanical characterization of porous titanium bone substitutes. Journal of the Mechanical Behavior of Biomedical Materials 2012, 9, 34–44. [Google Scholar] [CrossRef]
- Guo, S.; Li, Y.; Gu, J.; Liu, J.; Peng, Y.; Wang, P.; Zhou, Q.; Wang, K. Microstructure and mechanical properties of Ti6Al4V/ B4C titanium matrix composite fabricated by selective laser melting (SLM). Journal of Materials Research and Technology 2023, 23, 1934–1946. [Google Scholar] [CrossRef]
- d18 Xiao, Y. K.; Bian, Z. Y.; Wu, Y.; Ji, G.; Lian, Q.; Wang, H. Z.; Chen, Z.; Wang, H. W. Simultaneously minimizing residual stress and enhancing strength of selective laser melted nano-TiB2 decorated Al alloy via post-uphill quenching and ageing. Mater. Charact. 2021, 178, 111242. [Google Scholar] [CrossRef]
- Thijs, L.; Verhaeghe, F.; Craeghs, T.; Humbeeck, J. V.; Kruth, J.-P. A study of the microstructural evolution during selective laser melting of ti–6al–4v. Acta Materialia 2010, 58, 3303–3312. [Google Scholar] [CrossRef]
- He, K.; Zhao, X. 3D thermal finite element analysis of the SLM 316L parts with microstructural correlations. Complexity 2018, 2018, 1–13. [Google Scholar] [CrossRef]
- Kruth, J. P.; Froyen, L.; Van Vaerenbergh, J.; Mercelis, P.; Rombouts, M.; Lauwers, B. Selective laser melting of iron-based powder. Journal of Materials Processing Technology 2004, 149, 616–622. [Google Scholar] [CrossRef]
- Xu, W.; Brandt, M.; Sun, S.; Elambasseril, J.; Liu, Q.; Latham, K.; Xia, K.; Qian, M. Additive manufacturing of strong and ductile ti–6al–4v by selective laser melting via in situ martensite decomposition. Acta Materialia 2015, 85, 74–84. [Google Scholar] [CrossRef]
- Murr, L. E.; Quinones, S. A.; Gaytan, S. M.; Lopez, M. I.; Rodela, A.; Martinez, E. Y.; Hernandez, D. H.; Martinez, E.; Medina, F.; Wicker, R. B. Microstructure and mechanical behavior of ti–6al–4v produced by rapid-layer manufacturing, for biomedical applications. Journal of the Mechanical Behavior of Biomedical Materials 2009, 2, 20–32. [Google Scholar] [CrossRef]
- Wysocki, B.; Maj, P.; Krawczyńska, A.; Rożniatowski, K.; Zdunek, J.; Kurzydłowski, K. J.; Święszkowski, W. Microstructure and mechanical properties investigation of CP titanium processed by selective laser melting (SLM). Journal of Materials Processing Technology 2017, 241, 13–23. [Google Scholar] [CrossRef]
- Kruth, J.; Mercelis, P.; Van Vaerenbergh, J.; Froyen, L.; Rombouts, M. Binding mechanisms in selective laser sintering and Selective Laser melting. Rapid Prototyping Journal 2005, 11, 26–36. [Google Scholar] [CrossRef]
- Jin, B.; Wang, Q.; Zhao, L.; Pan, A.; Ding, X.; Gao, W.; Song, Y.; Zhang, X. A Review of Additive Manufacturing Techniques and Post-Processing for High-Temperature Titanium Alloys. Metals 2023, 13, 1327. [Google Scholar] [CrossRef]
- Wei, J.; Sun, H.; Zhang, D.; Gong, L.; Lin, J.; Wen, C. Influence of heat treatments on microstructure and mechanical properties of TI–26NB alloy elaborated in situ by laser additive manufacturing with ti and NB mixed powder. Materials 2018, 12, 61. [Google Scholar] [CrossRef]
- Vrancken, B.; Thijs, L.; Kruth, J.-P.; Van Humbeeck, J. Heat treatment of ti6al4v produced by selective laser melting: Microstructure and mechanical properties. Journal of Alloys and Compounds 2012, 541, 177–185. [Google Scholar] [CrossRef]
- Barriobero-Vila, P.; Gussone, J.; Stark, A.; Requena, G.; Schell, N.; Haubrich, J. Peritectic Titanium Alloys for 3D Printing. Nat. Commun. 2018, 9, 3426. [Google Scholar] [CrossRef]
- Phani Babu, V. V.; GB, V. K. A review on 3D printing process on metals and their surface roughness and dimensional accuracy. Materials Today: Proceedings 2022, 64, 523–530. [Google Scholar] [CrossRef]
- Khrapov, D.; Paveleva, A.; Kozadayeva, M.; Evsevleev, S.; Mishurova, T.; Bruno, G.; Surmenev, R.; Koptyug, A.; Surmeneva, M. Trapped powder removal from sheet-based porous structures based on triply periodic minimal surfaces fabricated by electron beam powder bed fusion. Materials Science and Engineering: A 2023, 862, 144479. [Google Scholar] [CrossRef]
- Wang, S.; Ning, J.; Zhu, L.; Yang, Z.; Yan, W.; Dun, Y.; Xue, P.; Xu, P.; Bose, S.; Bandyopadhyay, A. Role of porosity defects in metal 3d printing: Formation Mechanisms, impacts on properties and mitigation strategies. Materials Today 2022, 59, 133–160. [Google Scholar] [CrossRef]
- Gibson, L. J.; Ashby, M. F.; Harley, B. A. Cellular materials in nature and medicine; Cambridge University Press: Cambridge, 2010. [Google Scholar]
- Olivares, A. L.; Lacroix, D. Simulation of cell seeding within a three-dimensional porous scaffold: A fluid-particle analysis. Tissue Engineering Part C: Methods 2012, 18, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Rahbari, A.; Montazerian, H.; Davoodi, E.; Homayoonfar, S. Predicting permeability of regular tissue engineering scaffolds: Scaling analysis of pore architecture, scaffold length, and fluid flow rate effects. Computer Methods in Biomechanics and Biomedical Engineering 2016, 20, 231–241. [Google Scholar] [CrossRef]
- Castro, A. P.; Ruben, R. B.; Gonçalves, S. B.; Pinheiro, J.; Guedes, J. M.; Fernandes, P. R. Numerical and experimental evaluation of TPMS gyroid scaffolds for bone tissue engineering. Comp. Meth. Biomech. Biomed. Eng. 2019, 22, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Castilho, M.; Pires, I.; Gouveia, B.; Rodrigues, J. Structural evaluation of scaffolds prototypes produced by three-dimensional printing. The International Journal of Advanced Manufacturing Technology 2011, 56, 561–569. [Google Scholar] [CrossRef]
- Papantoniou, I.; Guyot, Y.; Sonnaert, M.; Kerckhofs, G.; Luyten, F. P.; Geris, L.; Schrooten, J. Spatial optimization in perfusion bioreactors improves bone tissue-engineered construct quality attributes. Biotechnology and Bioengineering 2014, 111, 2560–2570. [Google Scholar] [CrossRef]
- Bertassoni, L.E.; Coelho, P.G. Engineering mineralized and load bearing tissues; Springer: Cham, 2015. [Google Scholar] [CrossRef]
- Bobbert, F. S. L.; Lietaert, K.; Eftekhari, A. A.; Pouran, B.; Ahmadi, S. M.; Weinans, H.; Zadpoor, A. A. Additively manufactured metallic porous biomaterials based on minimal surfaces: A unique combination of topological, mechanical, and mass transport properties. Acta Biomaterialia 2017, 53, 572–584. [Google Scholar] [CrossRef]
- Lu, T.; Li, Y.; Chen, T. Techniques for fabrication and construction of three-dimensional scaffolds for tissue engineering. International Journal of Nanomedicine 2013, 337. [Google Scholar] [CrossRef]
- Yoo, D.-J. Advanced porous scaffold design using multi-void triply periodic minimal surface models with high surface area to volume ratios. International Journal of Precision Engineering and Manufacturing 2014, 15, 1657–1666. [Google Scholar] [CrossRef]
- Shi, J.; Wei, F.; Chouraki, B.; Sun, X.; Wei, J.; Zhu, L. Study on Performance Simulation of Vascular-like Flow Channel Model Based on TPMS Structure. Biomimetics 2023, 8, 69. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Fu, J.; Shang, C.; Lin, Z.; Li, B. Porous scaffold design by solid T-splines and triply periodic minimal surfaces. Computer Methods in Applied Mechanics and Engineering 2018, 336, 333–352. [Google Scholar] [CrossRef]
- Al-Ketan, O.; Abu Al-Rub, R. K. Multifunctional mechanical metamaterials based on triply periodic minimal surface lattices. Adv. Eng. Mater. 2019, 21, 1900524. [Google Scholar] [CrossRef]
- Liu, F.; Mao, Z.; Zhang, P.; Zhang, D. Z.; Jiang, J.; Ma, Z. Functionally graded porous scaffolds in multiple patterns: New design method, physical and mechanical properties. Materials and Design 2018, 160, 849–860. [Google Scholar] [CrossRef]
- Al-Ketan, O.; Abu Al-Rub, R. K. MSLattice: A free software for generating uniform and graded lattices based on triply periodic minimal surfaces. Material Design and Processing Communications 2020, 3, 6, 205. [Google Scholar] [CrossRef]
- Gabbrielli, R.; Turner, I. G.; Bowen, C. R. Development of modelling methods for materials to be used as bone substitutes. Bioceramics 20 2007, 903–906. [Google Scholar] [CrossRef]
- Baravalle, R.; Scandolo, L.; Delrieux, C.; García Bauza, C.; Eisemann, E. Realistic modeling of porous materials. Comput Animat Virtual Worlds. 2017, 28, e1719. [Google Scholar] [CrossRef]
- Bermejillo Barrera, M.D.; Franco-Martínez, F.; Díaz Lantada, A. Artificial Intelligence Aided Design of Tissue Engineering Scaffolds Employing Virtual Tomography and 3D Convolutional Neural Networks. Materials 2021, 14, 5278. [Google Scholar] [CrossRef] [PubMed]
- Merayo, D.; Rodriguez-Prieto, A.; Camacho, A. M. Prediction of physical and mechanical properties for metallic materials selection using big data and Artificial Neural Networks. IEEE Access 2020, 8, 13444–13456. [Google Scholar] [CrossRef]
- Javaid, S.; Gorji, H. T.; Soulami, K. B.; Kaabouch, N. Identification and ranking biomaterials for bone scaffolds using machine learning and PROMETHEE. Res. Biomed. Eng. 2023, 39, 129–138. [Google Scholar] [CrossRef]
- Jafari Chashmi, M.; Fathi, A.; Shirzad, M.; Jafari-Talookolaei, R.-A.; Bodaghi, M.; Rabiee, S. M. Design and analysis of porous functionally graded femoral prostheses with improved stress shielding. Designs 2020, 4, 12. [Google Scholar] [CrossRef]
- Vora, L. K.; Gholap, A. D.; Jetha, K.; Thakur, R. R.; Solanki, H. K.; Chavda, V. P. Artificial Intelligence in pharmaceutical technology and Drug Delivery Design. Pharmaceutics 2023, 15, 1916. [Google Scholar] [CrossRef] [PubMed]
- Conev, A.; Litsa, E. E.; Perez, M. R.; Diba, M.; Mikos, A. G.; Kavraki, L. E. Machine learning-guided three-dimensional printing of tissue engineering scaffolds. Tissue Eng. Part A 2020, 26, 1359–1368. [Google Scholar] [CrossRef] [PubMed]
- Sujeeun, L. Y.; Goonoo, N.; Ramphul, H.; Chummun, I.; Gimié, F.; Baichoo, S.; Bhaw-Luximon, A. Correlating in vitro performance with physico-chemical characteristics of nanofibrous scaffolds for skin tissue engineering using supervised machine learning algorithms. Royal Society Open Science 2020, 7, 201293. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Xiong, S.; Liu, M.; Yang, H.; Wei, P.; Yi, F.; Ouyang, M.; Xi, H.; Long, Z.; Liu, Y.; Li, J.; Ding, L.; Xiong, L. Study on the influence of scaffold morphology and structure on osteogenic performance. Front. Bioeng. Biotechnol. 2023, 11. [Google Scholar] [CrossRef] [PubMed]
- Quan, H.; Zhang, T.; Xu, H.; Luo, S.; Nie, J.; Zhu, X. Photo-curing 3D printing technique and its challenges. Bioact. Mater. 2020, 5, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Lo Re, G.; Lopresti, F.; Petrucci, G.; Scaffaro, R. A facile method to determine pore size distribution in porous scaffold by using image processing. Micron 2015, 76, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zou, S.; Mu, Y.; Wang, J.; Jin, Y. Additively manufactured scaffolds with optimized thickness based on triply periodic minimal surface. Materials 2022, 15, 7084. [Google Scholar] [CrossRef] [PubMed]
- Ferracini, R.; Martínez Herreros, I.; Russo, A.; Casalini, T.; Rossi, F.; Perale, G. Scaffolds as structural tools for bone-targeted drug delivery. Pharmaceutics 2018, 10, 122. [Google Scholar] [CrossRef] [PubMed]
- Domsta, V.; Hänsch, C.; Lenz, S.; Gao, Z.; Matin-Mann, F.; Scheper, V.; Lenarz, T.; Seidlitz, A. The influence of shape parameters on unidirectional drug release from 3D printed implants and prediction of release from implants with individualized shapes. Pharmaceutics 2023, 15, 1276. [Google Scholar] [CrossRef]
- Zamoume, O.; Thibault, S.; Regnié, G.; Mecherri, M. O.; Fiallo, M.; Sharrock, P. Macroporous calcium phosphate ceramic implants for sustained drug delivery. Mater. Sci. Eng. C 2011, 31, 1352–1356. [Google Scholar] [CrossRef]
- Roseti, L.; Parisi, V.; Petretta, M.; Cavallo, C.; Desando, G.; Bartolotti, I.; Grigolo, B. Scaffolds for bone tissue engineering: State of the art and new perspectives. Materials Science and Engineering: C 2017, 78, 1246–1262. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Tare, R. S.; Yang, L.-Y.; Williams, D. F.; Ou, K.-L.; Oreffo, R. O. C. Biofabrication of bone tissue: Approaches, challenges and translation for Bone Regeneration. Biomaterials 2016, 83, 363–382. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, M.; Fan, X.; Zhou, H. Recent advances in bioprinting techniques: Approaches, applications and future prospects. Journal of Translational Medicine 2016, 14. [Google Scholar] [CrossRef]
- Aljohani, W.; Ullah, M. W.; Zhang, X.; Yang, G. Bioprinting and its applications in tissue engineering and Regenerative Medicine. International Journal of Biological Macromolecules 2018, 107, 261–275. [Google Scholar] [CrossRef]
- Adepu, S.; Dhiman, N.; Laha, A.; Sharma, C. S.; Ramakrishna, S.; Khandelwal, M. Three-dimensional bioprinting for bone tissue regeneration. Current Opinion in Biomedical Engineering 2017, 2, 22–28. [Google Scholar] [CrossRef]
- Lin, K.-F.; He, S.; Song, Y.; Wang, C.-M.; Gao, Y.; Li, J.-Q.; Tang, P.; Wang, Z.; Bi, L.; Pei, G.-X. Low-temperature additive manufacturing of Biomimic three-dimensional hydroxyapatite/collagen scaffolds for bone regeneration. ACS Applied Materials and Interfaces 2016, 8, 6905–6916. [Google Scholar] [CrossRef] [PubMed]
- Park, J. Y.; Shim, J.-H.; Choi, S.-A.; Jang, J.; Kim, M.; Lee, S. H.; Cho, D.-W. 3D printing technology to control BMP-2 and VEGF delivery spatially and temporally to promote large-volume bone regeneration. Journal of Materials Chemistry B 2015, 3, 5415–5425. [Google Scholar] [CrossRef] [PubMed]
- Ratheesh, G.; Venugopal, J. R.; Chinappan, A.; Ezhilarasu, H.; Sadiq, A.; Ramakrishna, S. 3D fabrication of polymeric scaffolds for regenerative therapy. ACS Biomaterials Science and Engineering 2017, 3, 1175–1194. [Google Scholar] [CrossRef] [PubMed]
- Leong, K. F.; Cheah, C. M.; Chua, C. K. Solid freeform fabrication of three-dimensional scaffolds for engineering replacement tissues and organs. Biomater. 2003, 24, 2363–2378. [Google Scholar] [CrossRef] [PubMed]
- Kruth, J.-P.; Levy, G.; Klocke, F.; Childs, T. H. C. Consolidation phenomena in laser and powder-bed based layered manufacturing. CIRP Annals 2007, 56, 730–759. [Google Scholar] [CrossRef]
- Thijs, L.; Verhaeghe, F.; Craeghs, T.; Humbeeck, J. V.; Kruth, J.-P. A study of the microstructural evolution during selective laser melting of ti–6al–4v. Acta Materialia 2010, 58, 3303–3312. [Google Scholar] [CrossRef]
- Warnke, P. H.; Douglas, T.; Wollny, P.; Sherry, E.; Steiner, M.; Galonska, S.; Becker, S. T.; Springer, I. N.; Wiltfang, J.; Sivananthan, S. Rapid prototyping: Porous titanium alloy scaffolds produced by selective laser melting for bone tissue engineering. Tissue Engineering Part C: Methods 2009, 15, 115–124. [Google Scholar] [CrossRef]
- Liang, H.; Yang, Y.; Xie, D.; Li, L.; Mao, N.; Wang, C.; Tian, Z.; Jiang, Q.; Shen, L. Trabecular-like Ti-6Al-4V scaffolds for orthopedic: Fabrication by selective laser melting and in vitro biocompatibility. Journal of Materials Science and Technology 2019, 35, 1284–1297. [Google Scholar] [CrossRef]
- Yang, Y.; Yuan, F.; Gao, C.; Feng, P.; Xue, L.; He, S.; Shuai, C. A combined strategy to enhance the properties of Zn by Laser Rapid Solidification and laser alloying. Journal of the Mechanical Behavior of Biomedical Materials 2018, 82, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Giannitelli, S. M.; Accoto, D.; Trombetta, M.; Rainer, A. Current trends in the design of scaffolds for computer-aided tissue engineering. Acta Biomaterialia 2014, 10, 580–594. [Google Scholar] [CrossRef]
- Wang, X.; Xu, S.; Zhou, S.; Xu, W.; Leary, M.; Choong, P.; Qian, M.; Brandt, M.; Xie, Y. M. Topological design and additive manufacturing of porous metals for bone scaffolds and orthopaedic implants: A Review. Biomaterials 2016, 83, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Van Bael, S.; Chai, Y. C.; Truscello, S.; Moesen, M.; Kerckhofs, G.; Van Oosterwyck, H.; Kruth, J.-P.; Schrooten, J. The effect of pore geometry on the in vitro biological behavior of human periosteum-derived cells seeded on selective laser-melted ti6al4v bone scaffolds. Acta Biomater. 2012, 8, 2824–2834. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, C.; Li, C.; Qin, Y.; Zhong, L.; Chen, B.; Li, Z.; Liu, H.; Chang, F.; Wang, J. Analysis of factors influencing bone ingrowth into three-dimensional printed porous metal scaffolds: A Review. Journal of Alloys and Compounds 2017, 717, 271–285. [Google Scholar] [CrossRef]
- Lv, X.; Wang, S.; Xu, Z.; Liu, X.; Liu, G.; Cao, F.; Ma, Y. Structural Mechanical Properties of 3D Printing Biomimetic Bone Replacement Materials. Biomimetics 2023, 8, 166. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.; Humayun, A.; Cohen, D. J.; Boyan, B. D.; Schwartz, Z. Additively manufactured 3D porous ti-6al-4v constructs mimic trabecular bone structure and regulate osteoblast proliferation, differentiation and local factor production in a porosity and surface roughness dependent manner. Biofabrication 2014, 6, 045007. [Google Scholar] [CrossRef] [PubMed]
- Kou, X. Y.; Tan, S. T. A simple and effective geometric representation for irregular porous structure modeling. Computer-Aided Design 2010, 42, 930–941. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, L.; Liu, Z.; Jiang, Z.; Liu, Y.; Wu, Y. Yield properties of closed-cell aluminum foam under triaxial loadings by a 3D Voronoi model. Mechanics of Materials 2017, 104, 73–84. [Google Scholar] [CrossRef]
- Honda, H.; Nagai, T. Cell models lead to understanding of multi-cellular morphogenesis consisting of successive self-construction of cells. Journal of Biochemistry 2014, 157, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Shen, L.; Zhao, J.; Liang, H.; Xie, D.; Tian, Z.; Wang, C. Design and compressive behavior of controllable irregular porous scaffolds: Based on Voronoi-tessellation and for additive manufacturing. ACS Biomater. Sci. Eng. 2018, 4, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Fantini, M.; Curto, M.; De Crescenzio, F. A method to design biomimetic scaffolds for bone tissue engineering based on Voronoi lattices. Virtual and Physical Prototyping 2016, 11, 77–90. [Google Scholar] [CrossRef]
- Gómez, S.; Vlad, M. D.; López, J.; Fernández, E. Design and properties of 3D scaffolds for bone tissue engineering. Acta Biomaterialia 2016, 42, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Costa, A. Permeability-porosity relationship: A reexamination of the kozeny-carman equation based on a fractal pore-space geometry assumption. Geophysical Research Letters 2006, 33. [Google Scholar] [CrossRef]
- Hulbert, S. F.; Young, F. A.; Mathews, R. S.; Klawitter, J. J.; Talbert, C. D.; Stelling, F. H. Potential of ceramic materials as permanently implantable skeletal prostheses. J Biomed Mater Res 1970, 4, 433–456. [Google Scholar] [CrossRef]
- Bobyn, J. D.; Pilliar, R. M.; Cameron, H. U.; Weatherly, G. C. The optimum pore size for the fixation of porous-surfaced metal implants by the ingrowth of bone. Clinical Orthopaedics and Related Research 1980, 150. [Google Scholar] [CrossRef]
- Pilliar, R.M. Porous-surfaces metallic implants for orthopedic applications. J. Biomed. Mater. Res. 1987, 21(A1 Suppl), 1– 33.
- Lu, J. X.; Flautre, B.; Anselme, K.; Gallur, A.; Descamps, M.; Thierry, B.; Hardouin, P. Study of porous interconnections of bioceramic on cellular rehabilitation in vitro and in vivo. Bioceramics 1997, 583–586. [Google Scholar]
- Itälä, A.I.; Ylänen, H.O.; Ekholm, C.; Karlsson, K.H.; Aro, H.T. Pore diameter of more than 100 μm is not requisite for bone ingrowth in Rabbits. J. Biomed. Mater. Res. 2001, 58, 679–683. [Google Scholar] [CrossRef]
- Jones, A. C.; Arns, C. H.; Hutmacher, D. W.; Milthorpe, B. K.; Sheppard, A. P.; Knackstedt, M. A. The correlation of pore morphology, interconnectivity and physical properties of 3D ceramic scaffolds with bone ingrowth. Biomaterials 2009, 30, 1440–1451. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.; Song, W.; Han, T.; Yan, J.; Li, F.; Zhao, L.; Kou, H.; Zhang, Y. Influence of pore size of porous titanium fabricated by vacuum diffusion bonding of titanium meshes on cell penetration and Bone Ingrowth. Acta Biomater. 2016, 33, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Krishna, B. V.; Bandyopadhyay, A.; Bose, S. Processing and biocompatibility evaluation of laser processed porous titanium. Acta Biomaterialia 2007, 3, 1007–1018. [Google Scholar] [CrossRef]
- Knychala, J.; Bouropoulos, N.; Catt, C. J.; Katsamenis, O. L.; Please, C. P.; Sengers, B. G. Pore geometry regulates early stage human bone marrow cell tissue formation and organisation. Ann Biomed Eng 2013, 41, 917–930. [Google Scholar] [CrossRef]
- Lu, X.; Wang, Y.; Jin, F. Influence of a non-biodegradable porous structure on Bone Repair. RSC Advances 2016, 6, 80522–80528. [Google Scholar] [CrossRef]
- Shor, L.; Güçeri, S.; Wen, X.; Gandhi, M.; Sun, W. Fabrication of three-dimensional polycaprolactone/hydroxyapatite tissue scaffolds and osteoblast-scaffold interactions in vitro. Biomaterials 2007, 28, 5291–5297. [Google Scholar] [CrossRef] [PubMed]
- Dias, M. R.; Fernandes, P. R.; Guedes, J. M.; Hollister, S. J. Permeability analysis of scaffolds for bone tissue engineering. Journal of Biomechanics 2012, 45, 938–944. [Google Scholar] [CrossRef]
- Porter, A. E.; Buckland, T.; Hing, K.; Best, S. M.; Bonfield, W. The structure of the bond between bone and porous silicon-substituted hydroxyapatite bioceramic implants. Journal of Biomedical Materials Research Part A 2006, 78A, 25–33. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef]
- Fukuda, A.; Takemoto, M.; Saito, T.; Fujibayashi, S.; Neo, M.; Pattanayak, D. K.; Matsushita, T.; Sasaki, K.; Nishida, N.; Kokubo, T.; Nakamura, T. Osteoinduction of porous Ti implants with a channel structure fabricated by selective laser melting. Acta Biomaterialia 2011, 7, 2327–2336. [Google Scholar] [CrossRef]
- Wauthle, R.; van der Stok, J.; Amin Yavari, S.; Van Humbeeck, J.; Kruth, J.-P.; Zadpoor, A. A.; Weinans, H.; Mulier, M.; Schrooten, J. Additively manufactured porous tantalum implants. Acta Biomater. 2015, 14, 217–225. [Google Scholar] [CrossRef]
- Wally, Z. J.; Haque, A. M.; Feteira, A.; Claeyssens, F.; Goodall, R.; Reilly, G. C. Selective laser melting processed ti6al4v lattices with graded porosities for Dental Applications. J. Mech. Behav. Biomed. Mater. 2019, 90, 20–29. [Google Scholar] [CrossRef]
- Taniguchi, N.; Fujibayashi, S.; Takemoto, M.; Sasaki, K.; Otsuki, B.; Nakamura, T.; Matsushita, T.; Kokubo, T.; Matsuda, S. Effect of pore size on bone ingrowth into porous titanium implants fabricated by additive manufacturing: An in vivo experiment. Mater. Sci. Eng. C 2016, 59, 690–701. [Google Scholar] [CrossRef]
- Wieding, J.; Lindner, T.; Bergschmidt, P.; Bader, R. Biomechanical stability of novel mechanically adapted open-porous titanium scaffolds in metatarsal bone defects of sheep. Biomaterials 2015, 46, 35–47. [Google Scholar] [CrossRef]
- Li, F.; Li, J.; Xu, G.; Liu, G.; Kou, H.; Zhou, L. Fabrication, pore structure and compressive behavior of anisotropic porous titanium for human trabecular bone implant applications. J. Mech. Behav. Biomed. Mater. 2015, 46, 104–114. [Google Scholar] [CrossRef]
- Chen, Z.; Yan, X.; Yin, S.; Liu, L.; Liu, X.; Zhao, G.; Ma, W.; Qi, W.; Ren, Z.; Liao, H.; Liu, M.; Cai, D.; Fang, H. Influence of the pore size and porosity of selective laser melted ti6al4v Eli porous scaffold on cell proliferation, osteogenesis and bone ingrowth. Mater. Sci. Eng. C 2020, 106, 110289. [Google Scholar] [CrossRef]
- Huang, G.; Pan, S.-T.; Qiu, J.-X. The osteogenic effects of porous tantalum and titanium alloy scaffolds with different unit cell structure. Colloids and Surfaces B: Biointerfaces 2022, 210, 112229. [Google Scholar] [CrossRef]
- Lee, Y.; Jung, A.; Heo, S.-J.; Gweon, B.; Lim, D. Influences of surface topography of porous titanium scaffolds manufactured by powder bed fusion on osteogenesis. J. Mater. Res. Technol. 2023, 23, 2784–2797. [Google Scholar] [CrossRef]
- Otsuki, B.; Takemoto, M.; Fujibayashi, S.; Neo, M.; Kokubo, T.; Nakamura, T. Pore throat size and connectivity determine bone and tissue ingrowth into porous implants: three-dimensional micro-CT based structural analyses of porous bioactive titanium implants. Biomaterials. 2006, 27, 5892–900. [Google Scholar] [CrossRef] [PubMed]
- Takemoto, M.; Fujibayashi, S.; Neo, M.; Suzuki, J.; Kokubo, T.; Nakamura, T. Mechanical properties and osteoconductivity of porous bioactive titanium. Biomaterials. 2005, 30, 6014–6023. [Google Scholar] [CrossRef]
- Habibovic, P.; Li, J.; van der Valk, C.M.; Meijer, G.; Layrolle, P.; van Blitterswijk, C.A.; de Groot, K. Biological performance of uncoated and octacalcium phosphate-coated Ti6Al4V. Biomaterials. 2005, 26, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Baino, F.; Yamaguchi, S. The Use of Simulated Body Fluid (SBF) for Assessing Materials Bioactivity in the Context of Tissue Engineering: Review and Challenges. Biomimetics. 2020, 5, 57. [Google Scholar] [CrossRef] [PubMed]
- Groeger, S.; Meyle, J. Reactivity of Titanium Dental Implant Surfaces in Simulated Body Fluid. ACS Appl. Bio. Mater. 2021, 4, 5575–5584. [Google Scholar] [CrossRef] [PubMed]
- Kon, M.; Hirakata, L.M.; Asaoka, K. Porous Ti-6Al-4 V alloy fabricated by spark plasma sintering for biomimetic surface modification. J. Biomed Mater. Res. B Appl. Biomater. 2004, 68, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Phillips, A.M. Overview of the fracture healing cascade. Injury. 2005, 36, S5–7. [Google Scholar] [CrossRef]
- Ai-Aql, Z.S.; Alagl, A.S.; Graves, D.T.; Gerstenfeld, L.C.; Einhorn, T.A. Molecular mechanisms controlling bone formation during fracture healing and distraction osteogenesis. J. Dent. Res. 2008, 87, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Schmid GJ, Kobayashi C, Sandell LJ, Ornitz DM. Fibroblast growth factor expression during skeletal fracture healing in mice. Dev. Dyn. 2009, 238, 766–774. [Google Scholar] [CrossRef]
- Yu, Y.Y.; Lieu, S.; Lu, C.; Miclau, T.; Marcucio, R.S.; Colnot, C. Immunolocalization of BMPs, BMP antagonists, receptors, and effectors during fracture repair. Bone. 2010, 46, 841–851. [Google Scholar] [CrossRef]
- Burkus, J.K.; Gornet, M.F.; Dickman, C.A.; Zdeblick, T.A. Anterior lumbar interbody fusion using rhBMP-2 with tapered interbody cages. J. Spinal Disord. Tech. 2002, 15, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Burkus, J.K.; Transfeldt, E.E.; Kitchel, S.H.; Watkins, R.G.; Balderston, R.A. Clinical and radiographic outcomes of anterior lumbar interbody fusion using recombinant human bone morphogenetic protein-2. Spine. 2002, 27, 2396–2408. [Google Scholar] [CrossRef] [PubMed]
- Turgeman, G.; Zilberman, Y.; Zhou, S.; Kelly, P.; Moutsatsos, I.K.; Kharode, Y.P.; Borella, L.E.; Bex, F.J.; Komm, B.S.; Bodine, P.V. et al. Systemically administered rhBMP-2 promotes MSC activity and reverses bone and cartilage loss in osteopenic mice. J. Cell Biochem 2002, 86, 461–474. [Google Scholar] [CrossRef] [PubMed]
- Dumic-Cule, I.; Brkljacic, J.; Rogic, D.; Bordukalo, N.T.; Tikvica, L.A.; Draca, N.; Kufner, V.; Trkulja, V.; Grgurevic, L.; Vukicevic, S. Systemically available bone morphogenetic protein two and seven affect bone metabolism. Int. Orthop. 2014, 38, 1979–1985. [Google Scholar] [CrossRef] [PubMed]
- Akkiraju, H.; Bonor, J.; Olli, K.; Bowen, C.; Bragdon, B.; Coombs, H.; Donahue, L. R.; Duncan, R.; Nohe, A. Systemic injection of CK2.3, a novel peptide acting downstream of bone morphogenetic protein receptor bmpria, leads to increased trabecular bone mass. J. Orthop. Res. 2014, 33, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Zhao, M.; Mundy, G.R. Bone morphogenetic proteins. Growth Factors. 2004. 22, 233– 241. 22. [CrossRef]
- Lee, J.; Decker, J.F.; Polimeni, G.; Cortella, C.A.; Rohrer, M.D.; Wozney, J.M.; Hall, J.; Susin, C.; Wikesjo, U.M. Evaluation of implants coated with rhbmp-2 using two different coating strategies: a critical-size supraalveolar peri-implant defect study in dogs. J. Clin. Periodontol. 2010, 37, 582–590. [Google Scholar] [CrossRef]
- Hunziker, E.B.; Jovanovic, J.; Horner, A.; Keel, M.J.; Lippuner, K.; Shintani, N. Optimisation of BMP-2 dosage for the osseointegration of porous titanium implants in an ovine model. Eur. Cell Mater. 2016, 32, 241–256. [Google Scholar] [CrossRef] [PubMed]
- Faundez, A.; Tournier, C.; Garcia, M.; Aunoble, S.; Le Huec, J.C. Bone morphogenetic protein use in spine surgery-complications and outcomes: a systematic review. Int. Orthop. 2016, 40, 1309–1319. [Google Scholar] [CrossRef] [PubMed]
- James, A.W.; LaChaud, G.; Shen, J.; Asatrian, G.; Nguyen, V.; Zhang, X.; Ting, K.; Soo, C. A review of the clinical side effects of bone morphogenetic protein-2. Tissue Eng. Part. B. Rev. 2016, 22, 284–297. [Google Scholar] [CrossRef] [PubMed]
- Hofstetter, C.P.; Hofer, A.S.; Levi, A.D. Exploratory meta-analysis on dose-related efficacy and morbidity of bone morphogenetic protein in spinal arthrodesis surgery. J. Neurosurg Spine. 2016, 24, 457–475. [Google Scholar] [CrossRef]
- Vavken, J.; Mameghani, A.; Vavken, P.; Schaeren, S. Complications and cancer rates in spine fusion with recombinant human bone morphogenetic protein-2 (rhBMP-2). Eur. Spine J. 2015, 25, 3979–3989. [Google Scholar] [CrossRef] [PubMed]
- Herberg, S.; Kondrikova, G.; Periyasamy-Thandavan, S.; Howie, R.N.; Elsalanty, M.E.; Weiss, L.; Campbell, P.; Hill, W.D.; Cray, J.J. Inkjet-based biopatterning of SDF-1β augments BMP-2-induced repair of critical size calvarial bone defects in mice. Bone. 2014, 7, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Pobloth, A.M.; Duda, G.N.; Giesecke, M.T.; Dienelt, A.; Schwabe, P. High-dose recombinant human bone morphogenetic protein-2 impacts histological and biomechanical properties of a cervical spine fusion segment: results from a sheep model. J. Tissue Eng. Regen. Med. 2017, 11, 1514–1523. [Google Scholar] [CrossRef]
- Lin, H.S.; Pang, W.P.; Yuan, H.; Kong, Y.Z.; Long, F.L.; Zhang, R.Z.; Yang, L.; Fang, Q,L,; Pan, A.P.; Fan, X.H.; Li, M.F. Molecular subtypes based on DNA sensors predict prognosis and tumor immunophenotype in hepatocellular carcinoma. Aging (Albany NY). 2023. 14, 15. [CrossRef]
- Aro, H.T.; Govender, S.; Patel, A.D.; Hernigou, P.; Perera de Gregorio, A.; Popescu, G.I.; Golden, J.D.; Christensen, J.; Valentin, A. Recombinant human bone morphogenetic protein-2: a randomized trial in open tibial fractures treated with reamed nail fixation. J. Bone Joint. Surg. Am. 2011, 93, 801–808. [Google Scholar] [CrossRef] [PubMed]
- Mroz, T.E.; Wang, J.C.; Hashimoto, R.; Norvell, D.C. Complications related to osteobiologics use in spine surgery: a systematic review. Spine. 2010, 35, S86–S104. [Google Scholar] [CrossRef]
- Guillot, R.; Gilde, F.; Becquart, P.; Sailhan, F.; Lapeyrere, A.; Logeart-Avramoglou, D.; Picart, C. The stability of BMP loaded polyelectrolyte multilayer coatings on titanium. Biomaterials. 2013, 34, 5737–5746. [Google Scholar] [CrossRef]
- Baltzer, A.W.; Lattermann, C.; Whalen, J.D.; Ghivizzani, S.; Wooley, P.; Krauspe, R.; Robbins, P.D.; Evans, C.H. Potential role of direct adenoviral gene transfer in enhancing fracture repair. Clin. Orthop. Relat. Res. 2000, 379, S120–S125. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Zhao, Z.; Koh, J.T.; Jin, T.; Franceschi, R.T. Combinatorial gene therapy for bone regeneration: cooperative interactions between adenovirus vectors expressing bone morphogenetic proteins 2, 4, and 7. J. Cell Biochem. 2005, 95, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Lutz, R.; Park, J.; Felszeghy, E.; Wiltfang, J.; Nkenke, E.; Schlegel, K.A. Bone regeneration after topical bmp-2-gene delivery in circumferential peri-implant bone defects. J. Clin. Oral Implants Res. 2008, 19, 590–559. [Google Scholar] [CrossRef]
- Chen, C.F.; Chen ,Y.C.; Fu, Y.S.; Tsai, S.W.; Wu, P.K.; Chen, C.M.; Chang, M.C.; Chen, W.M. Characterization of Osteogenesis and Chondrogenesis of Human Decellularized Allogeneic Bone with Mesenchymal Stem Cells Derived from Bone Marrow, Adipose Tissue, and Wharton’s Jelly. Int. J. Mol. Sci. 2021, 22, 8987. [CrossRef]
- Cohen, D.J.; Cheng, A.; Sahingur, K.; Clohessy, R.M.; Hopkins, L.B.; Boyan, B.D.; Schwartz, Z. Performance of laser sintered Ti-6Al-4V implants with bone-inspired porosity and micro/nanoscale surface roughness in the rabbit femur. Biomed. Mater. 2017, 12, 025021. [Google Scholar] [CrossRef] [PubMed]
- Sakisaka, Y.; Ishihata, H.; Maruyama, K.; Nemoto, E.; Chiba, S.; Nagamine, M.; Hasegawa, H.; Hatsuzawa, T.; Yamada, S. Serial Cultivation of an MSC-Like Cell Line with Enzyme-Free Passaging Using a Microporous Titanium Scaffold. Materials 2023, 16, 1165. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Liu, P.; Zhao, R.; Yang, X.; Xiao, Z.; Zhang, K; Zhu, X.; Zhang, X. Functionalized 3D-printed porous titanium scaffold induces in situ vascularized bone regeneration by orchestrating bone microenvironment. J. Mater. Sci. Techol. 2023, 153, 92–105. [Google Scholar] [CrossRef]
- Steiner, D.; Reinhardt, L.; Fischer, L.; Popp, V.; Körner, C.; Geppert, C.I.; Bäuerle, T.; Horch, R.E.; Arkudas, A. Impact of Endothelial Progenitor Cells in the Vascularization of Osteogenic Scaffolds. Cells 2022, 11, 926. [Google Scholar] [CrossRef] [PubMed]
- Seebach, C.; Henrich, D.; Kahling, C.; Wilhelm, K.; Tami, A.E.; Alini, M.; Marzi, I. Endothelial progenitor cells and mesenchymal stem cells seeded onto β-TCP granules enhance early vascularization and bone healing in a critical-sized bone defect in rats. Tissue Eng. Part. A. 2010, 16, 1961–1970. [Google Scholar] [CrossRef] [PubMed]
- Duffy, G.P.; Ahsan, T.; O’Brien, T.; Barry, F.; Nerem, R.M. Bone marrow-derived mesenchymal stem cells promote angiogenic processes in a time- and dose-dependent manner in vitro. Tissue Eng. Part. A. 2009, 15, 2459–2470. [Google Scholar] [CrossRef]
- Arts, M.; Torensma, B.; Wolfs, J. Porous titanium cervical interbody fusion device in the treatment of degenerative cervical radiculopathy; 1-year results of a prospective controlled trial. Spine J. 2020, 20, 1065–1072. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, Y.; Chai, X.; Zhuo, H. Application of three-dimensional printed porous titanium alloy cage and poly-ether-ether-ketone cage in posterior lumbar interbody fusion. 2022, 36, 1126–1131. [CrossRef]
- Arts, M.; Torensma, B.; Wolfs, J. Porous titanium cervical interbody fusion device in the treatment of degenerative cervical radiculopathy; 1-year results of a prospective controlled trial. Spine J. 2020, 20, 1065–1072. [Google Scholar] [CrossRef]
- Salemyr, M.; Muren, O.; Eisler, T.; Bodén, H.; Chammout, G.; Stark, A.; Sköldenberg, O. Porous titanium construct cup compared to porous coated titanium cup in total hip arthroplasty. A randomised controlled trial. Int. Orthop. 2015, 39, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, S.; Nakano, S.; Kinoshita, Y.; Nakamura, M.; Goto, T.; Hamada, D.; Sairyo, K. Comparison of a highly porous titanium cup (Tritanium) and a conventional hydroxyapatite-coated porous titanium cup: A retrospective analysis of clinical and radiological outcomes in hip arthroplasty among Japanese patients. J. Orthop. Sci. 2018, 23, 967–972. [Google Scholar] [CrossRef] [PubMed]
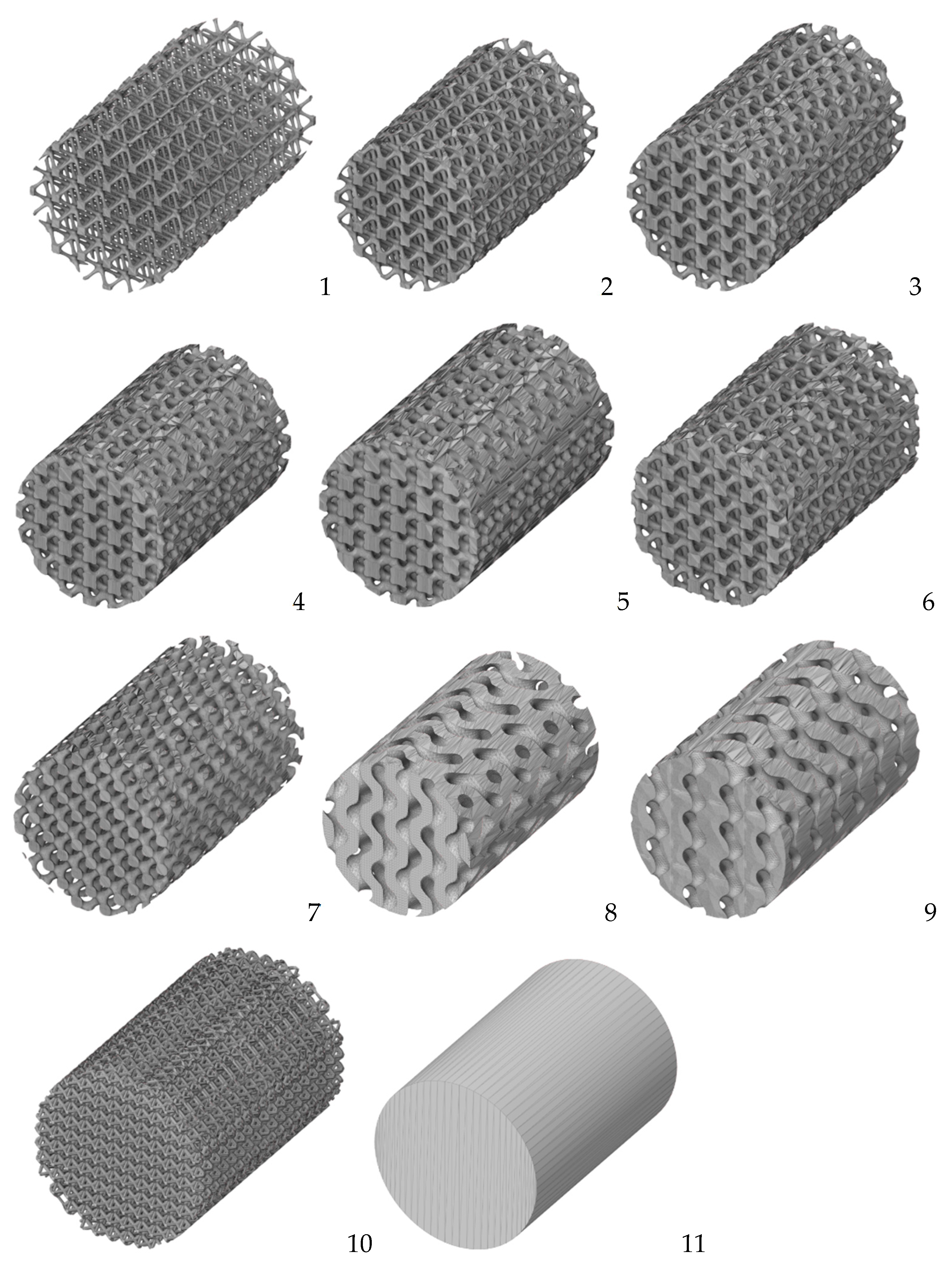
| Model | Expression |
|---|---|
| Diamond | cos X cos Y cos Z – sin X sin Y sin Z =c |
| IWP | 2 (cos X cos Y +cos Y cos Z + cos Z cos X) − (cos 2X +cos 2Y +cos 2Z) = 0 |
| Gyroid | sin Y cos X + sin Z cos Y + sin X cos Z = 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





