Submitted:
26 August 2023
Posted:
29 August 2023
You are already at the latest version
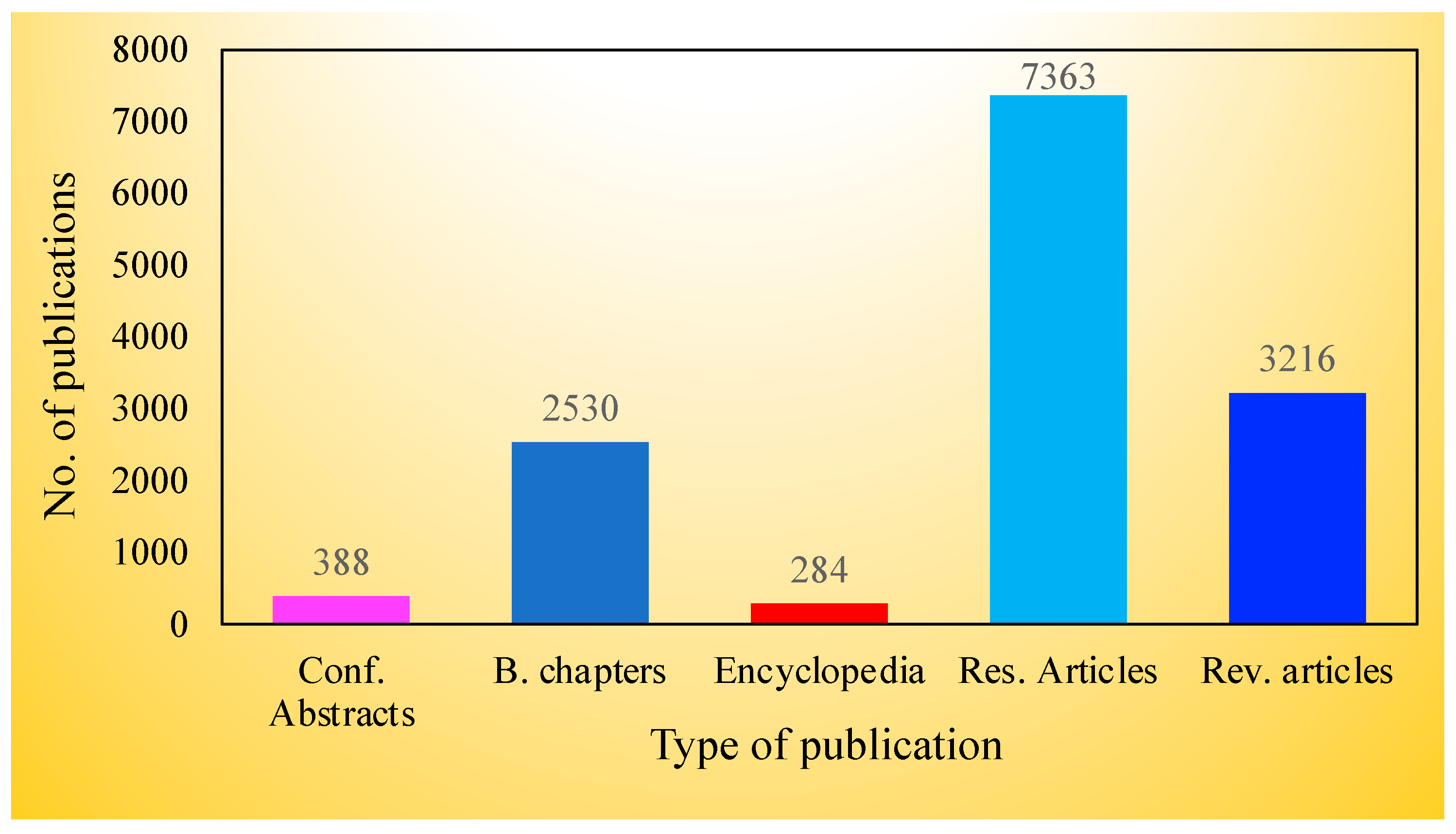
Abstract
Keywords:
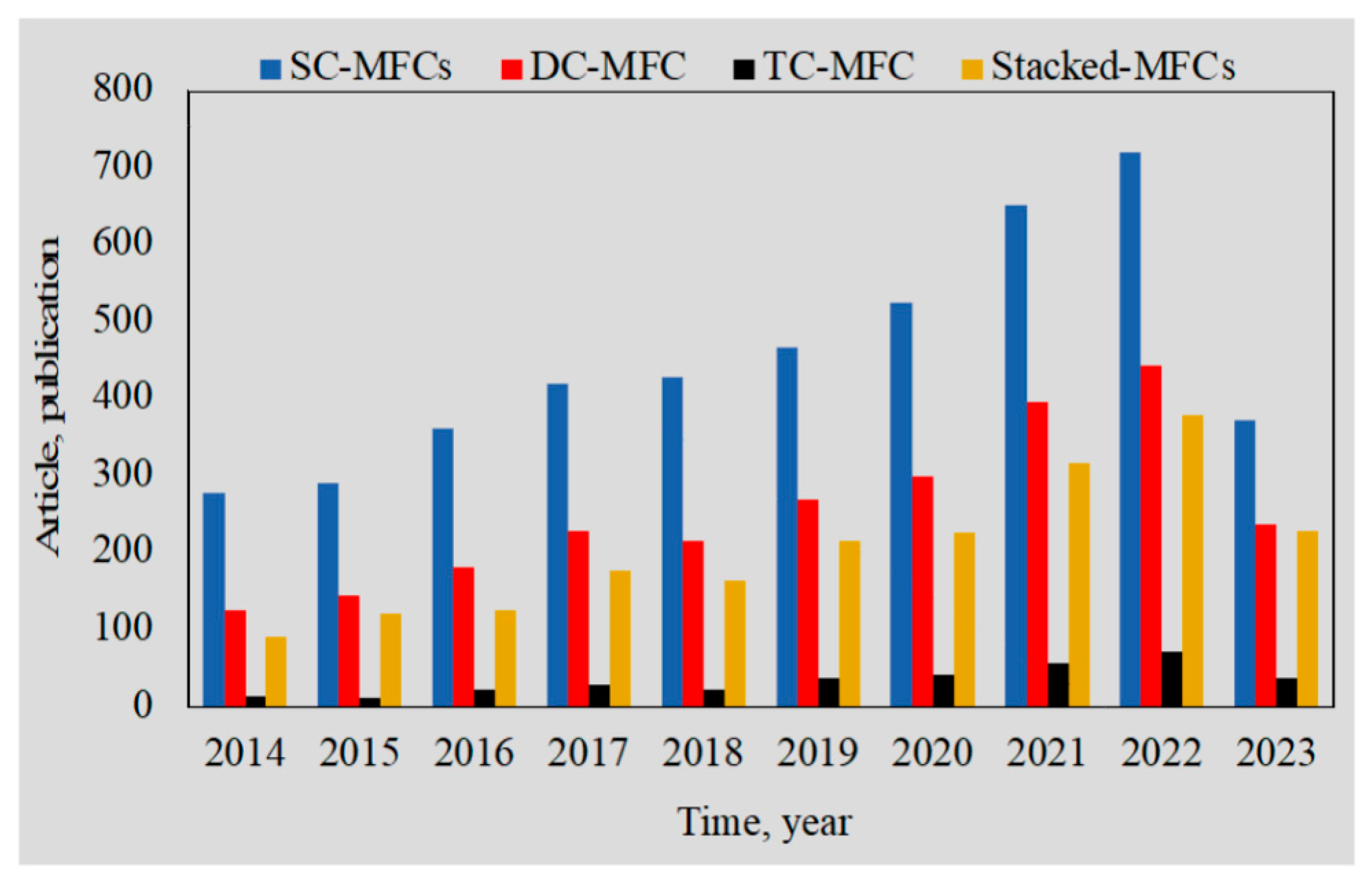
1. Introduction to microbial fuel cell technology
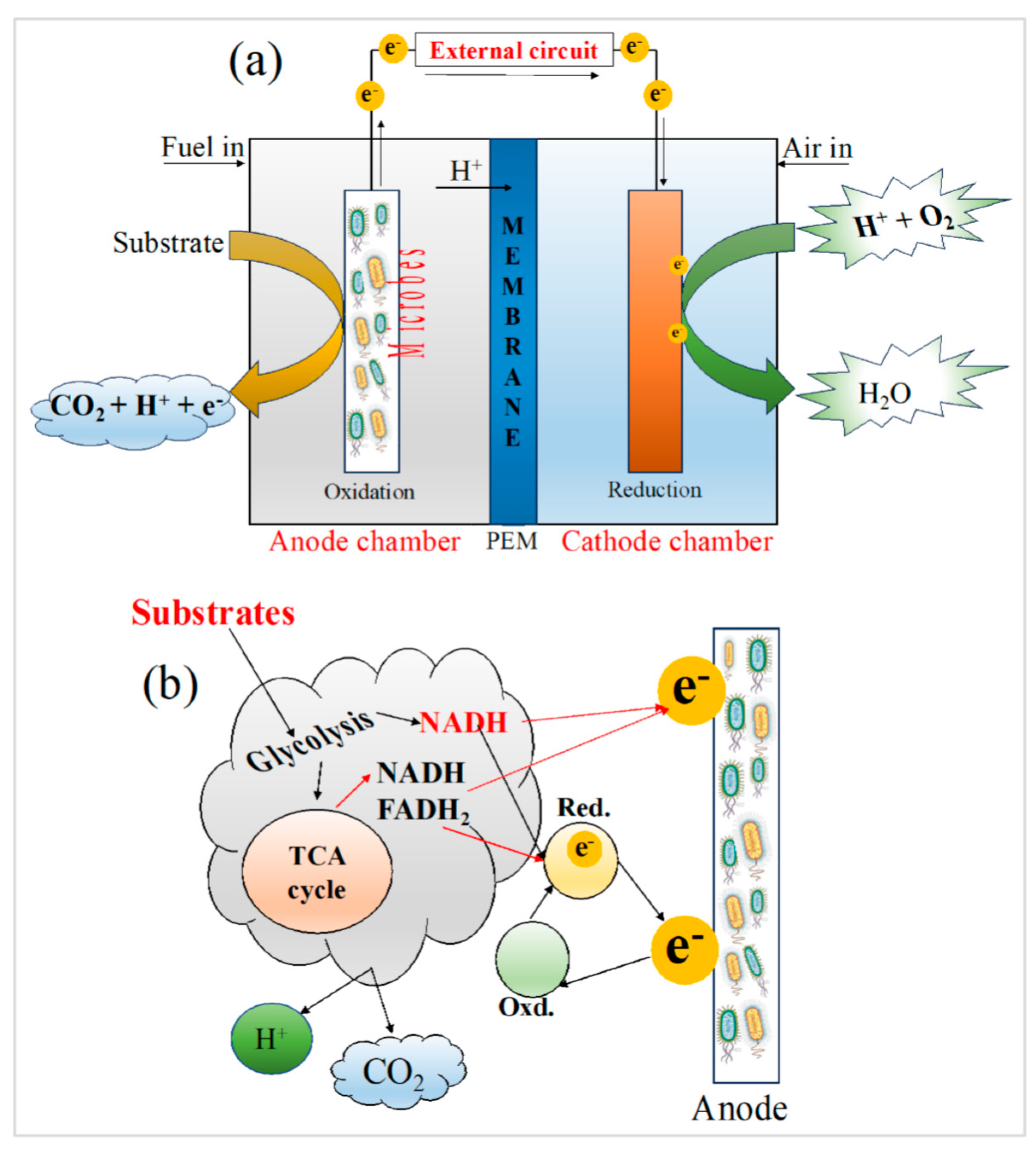
1.1. Designs, configurations, and operation
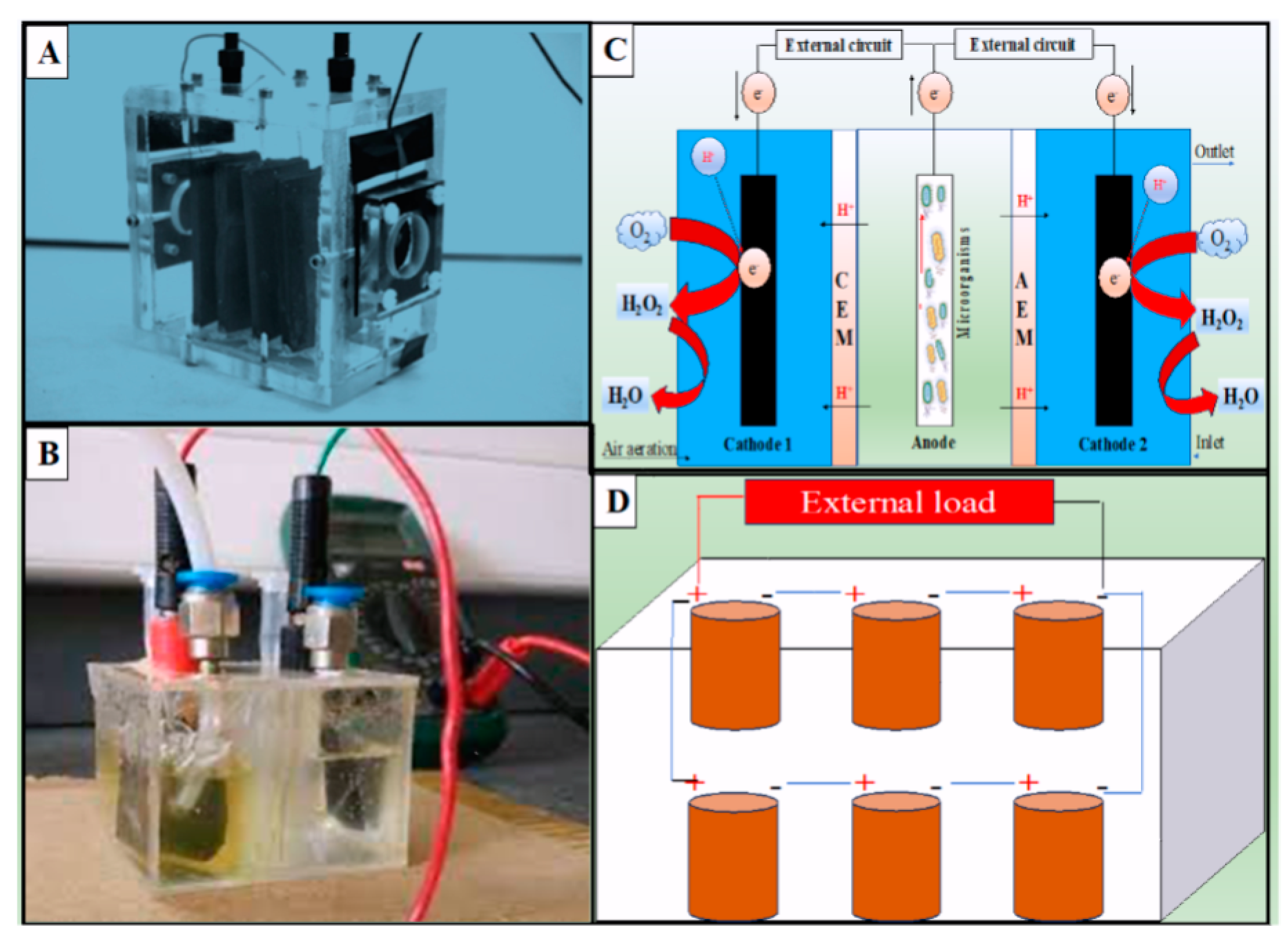
| Configuration of BES | Working volume (mL) |
Operation (days) |
Type of electrolyte | Removal efficiency (%) |
Maximum power generation |
Ref. |
|---|---|---|---|---|---|---|
| DC-MFC | 120 | N/A | Uranium-containing wastewater | 99.0 U(VI) | 269.5 mW/m2 | [57] |
| SC-MFC | 850 | ~ 30 | Activated sludge | N/A | 105 mW/m2 | [56] |
| DC-MFC | 1000 | N/A | Wastewater | 92 (Ni); 87 (Cd) | 722 mW/m3 | [58] |
| TC-MFC | 28 | ~ 2 (50 h) | Synthetic municipal wastewater | 80.0 (Iron); 22.1 (Sulfur) | 576.6; 184.8 mW/m2 | [59] |
| SC-MFC | 150 | 30 | Synthetic wastewaters | 89 (COD) | 450.36 mW/m2 | [60] |
| Stacked MFC | 37.5 | ~ 9 | Barley–shochu waste | 36.7 (COD) | 15.7 mW/m2 | [61] |
| Stacked MFC | 28 | N/A | Effluent | 16.9 (COD) | 1023; 1076 mW/m2 | [62] |
| Stacked MFC | N/A | N/A | Substrate | N/A | 21111 W/m3 | [63] |
| Stacked MFC | N/A | ~ 60 | Wastewater | 70.0 (Sulfide), 54.6 (COD) | 3.29 mA | [64] |
| SC-MFC | 80 | N/A | Wastewater | 83 (COD) | 548 mW/m2 | [65] |
| DC-MFC | 100-200 | N/A | Wastewater | 90 (COD); 40; 60 (orgN) | 1.69 A/m2 | [66] |
| SC-MFC | N/A | 18 | Wastewater | 81; 94 (COD) | 989 mv | [67] |
| DC-MFC | 118 | 30 | Sewage sludge | 99.08 (P) | ~ 40 mV | [68] |
| DC-MFC | 250 | 28 | Wastewater | 95.7; 94.7; 92.37 (COD) | 1696.56 mW/m2 | [69] |
| SC-MFC | 100 | N/A | Wastewater | 73.7 HCQ | 241 – 280 mW/m2 | [70] |
| DC-MFC | 300 | 30 | Wastewater | 70–88; 18–44 (COD) | 2.2; 44.6; 86.9 mW/m2 | [71] |
| DC-MFC | 125 (125 cm3) | 6 (144 h) | Wastewater | 99.16 (Cu+2) | 24.75 mW/m2 | [72] |
| TC-MFC | 28 | 8 | Wastewater | 86.2 (Cu+2) | 420 mW/m2 | [73] |
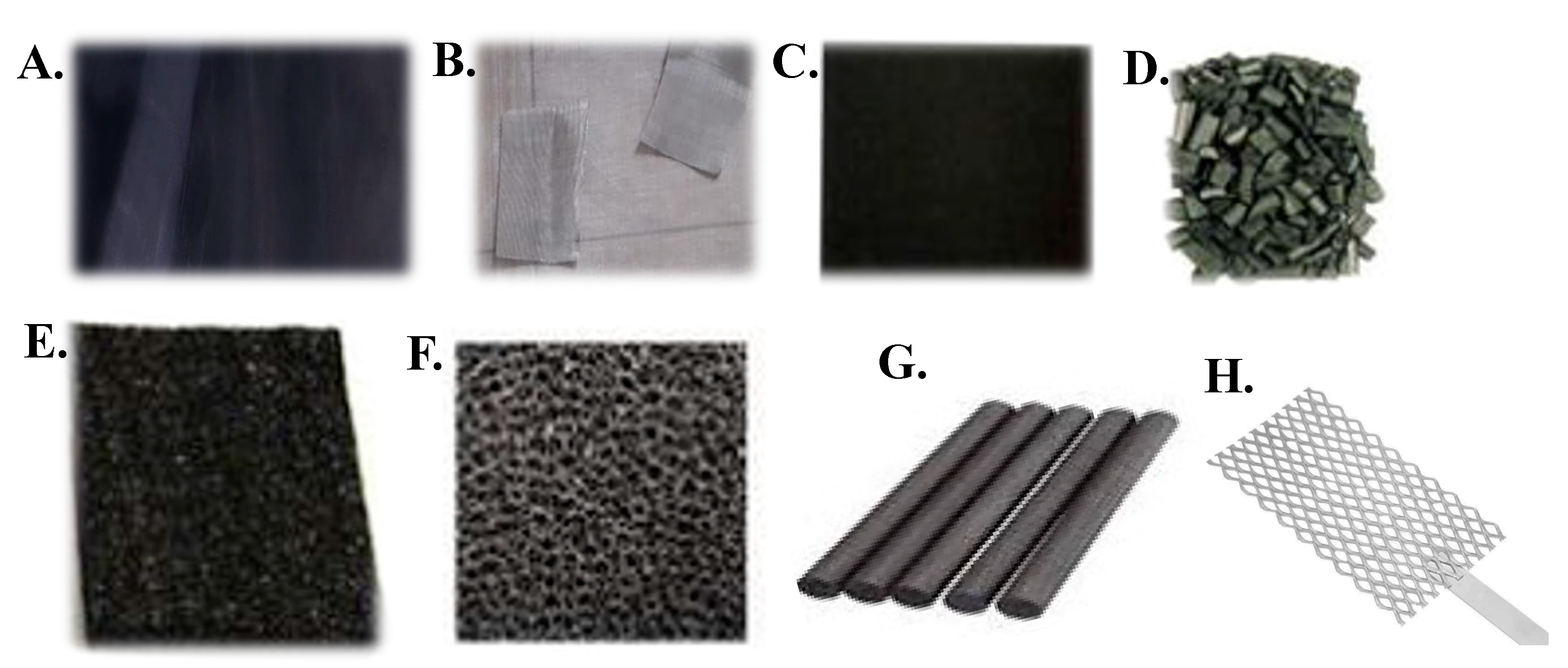
1.2. Electrode materials
1.3. Influence of microorganisms
2. Bioenergy production from MFCs
2.1. Bioelectricity production
| Configuration type | Electrode materials | Membrane type | Substrate | Working volume (mL) | Operation (days) |
Max. power generation |
Ref. | |
|---|---|---|---|---|---|---|---|---|
| Anode | Cathode | |||||||
| SC-MFC | Carbon felt (16 cm2) | Carbon felt (31 cm2) | Clayware | Synthetic wastewater | 150 | 30 | 995.73 mW/m3 | [110] |
| DC-MFC | Carbon fiber | Carbon fiber | SPEEK-goethite | N/A | N/A | N/A | 73.7 mW/m2 | [111] |
| T-MFC | Graphite rod | Carbon cloth coated Pt (200 cm2) | Nanocomposite | Sewage wastewater | 300 | 3 weeks | 138 mW/m2 | [97] |
| DC-MFC | Graphite | Graphite | Nation 117 | Activated strains | 500 | N/A | 12.82 mW/m2 | [112] |
| SMFC | Carbon-polymer composite | Carbon cloth (3 × 3 cm2) | N/A | Sediment from wastewater | 100 | 30 | 1056.6 W/m3 | [113] |
| MFC | Carbon fiber brushes | Carbon fiber brushes | Nafion 117 | Glucose, yeast, and MB | 800 | N/A | 5.55 W/m3 | [114] |
| SC-MFC | Carbon brush | Lignin-derived activated carbon | N/A | Sludge | 125 | N/A | 6.7 – 6.5 mW | [115] |
| C-MFC | Activated carbon coated carbon veil (30 mg/m2)/ pressed over stainless steel mesh | Activated carbon coated carbon veil (30 mg/m2)/ pressed over stainless steel mesh | Flat terracotta membrane (12.25 cm2) | Human urine and sludge | 12.5 | N/A | 492.85 μW | [32] |
| S-MFC | Graphite felt (7 × 7 × 0.4 cm) | Carbon cloth coated-Pt, plain carbon cloth, and graphite felt | N/A | Soil | N/A | ~ 50 | 87.3 mW/m2 | [116] |
| DC-MFC | Graphite filter | Stainless steel mesh | Carbon-ceramic composite | Wastewater | 5.3 (cm3) | N/A | 0.699 W/m3 | [117] |
|
SC-MFC |
Wired stainless steel 60 mesh | Wired stainless steel 60 mesh | Cylindrical terra- cotta pots | Textile effluent | N/A | N/A | 21–42 mW/m2 | [118] |
| SC-MFC | Graphite brush | graphite-based nanomaterials | N/A | Anaerobic mud | N/A | 30 | 2203 mW/m2 | [95] |
| SC-SMFC | Unidirectional Carbon Fiber (total area 81 cm2) | Unidirectional Carbon Fiber (total area 40.5 cm2 | N/A | Marine and fluvial sediments | 2000 | 30 | 70 mW/cm2 | [119] |
| SC-MFC | Graphite felt (thickness 10 mm, diameter 80 mm) | Graphite felt (thickness 10 mm, diameter 80 mm) | N/A | Oily sludge | 2000 | ~ 31 | 1277.90 mW m3 |
[120] |
| SC-MFC | Carbon cloth | Carbon cloth | Nafion 117 | Agro-waste | 200 | N/A | 590 mW/m2 | [96] |
2.2. Other types of energy production
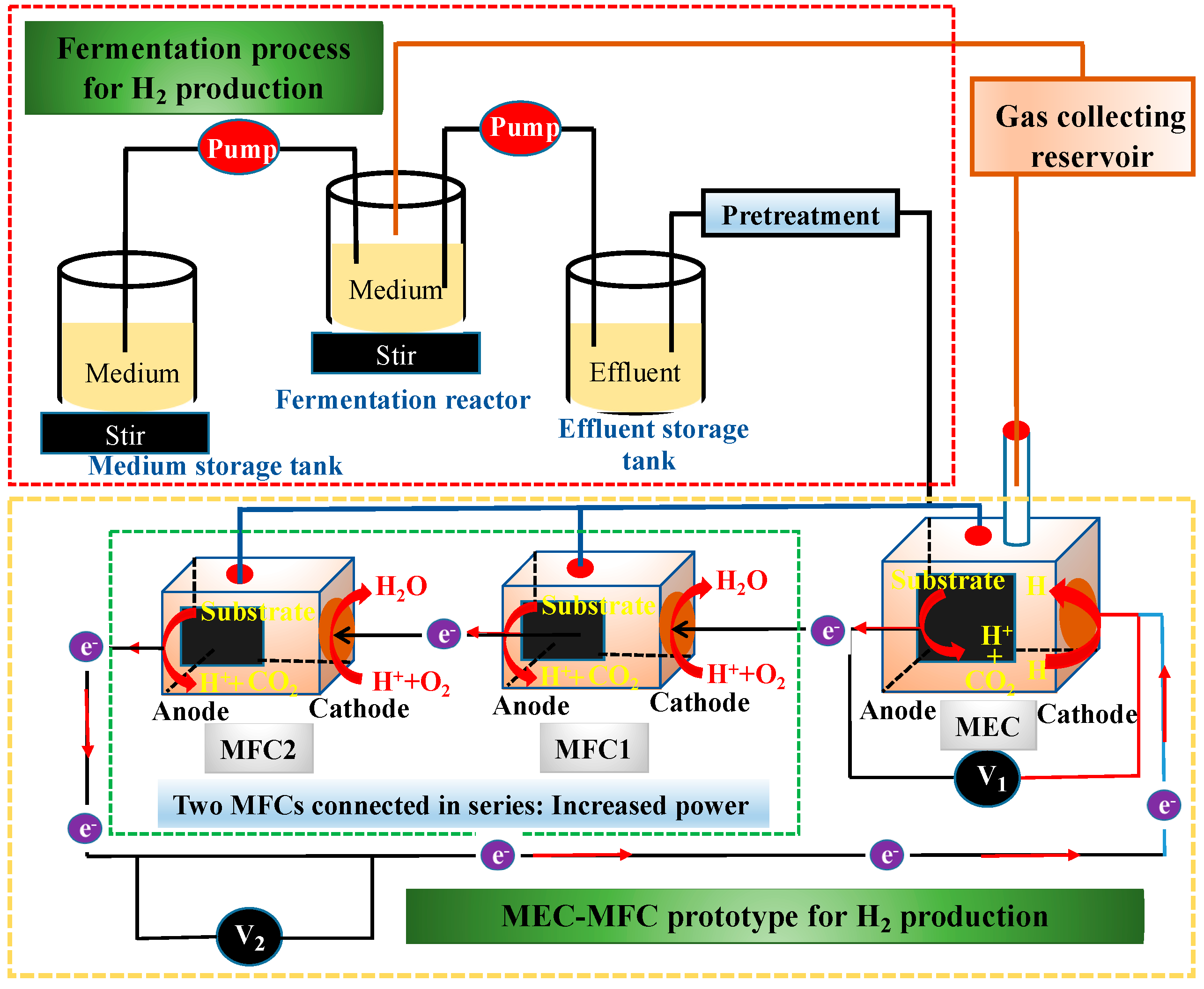
2.2.1. Methane (CH4) and Hydrogen (H2)
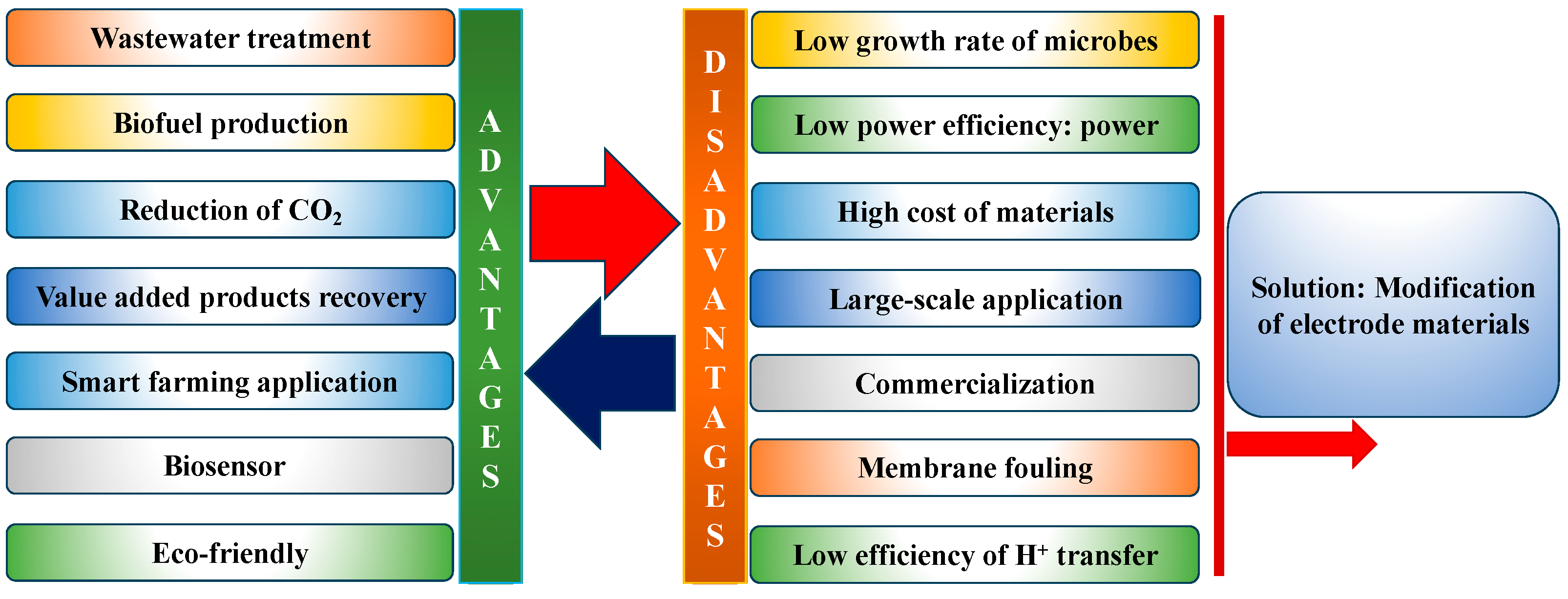
3. Applications and challenges of MFCs
4. Techno-economic and LCA of MFCs
5. Conclusions and future research direction
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, V.; Kundu, P. P. Biocatalysts in microbial fuel cells. Enzyme Microb. Technol. 2010, 47(5), 179-188. [CrossRef]
- Obileke, K.; Onyeaka, H.; Meyer, E. L.; Nwokolo, N. (2021). Microbial fuel cells, a renewable energy technology for bio-electricity generation: A mini-review. Electrochem. Commun. 2021, 125, 107003. [CrossRef]
- Sadeq, A. M.; Ismail, Z. Z. Sustainable application of tubular photosynthesis microbial desalination cell for simultaneous desalination of seawater for potable water supply associated with sewage treatment and energy recovery. Sci. Total Environ. 2023, 875, 162630. [CrossRef]
- Imoro, A. Z.; Mensah, M.; Buamah, R. Developments in the microbial desalination cell technology: A review. Water-Energy Nexus. 2021, 4, 76-87. [CrossRef]
- Jatoi, A. S.; Hashmi, Z.; Mazari, S. A.; Mubarak, N. M.; Karri, R. R.; Ramesh, S.; Rezakazemi, M. A comprehensive review of microbial desalination cells for present and future challenges. Desalination. 2022, 535, 115808. [CrossRef]
- Tanguay-Rioux, F.; Nwanebu, E.; Thadani, M.; Tartakovsky, B. On-line current control for continuous conversion of CO2 to CH4 in a microbial electrosynthesis cell. Biochem. Eng. J. 2023, 108965. [CrossRef]
- Li, Z.; Wu, R.; Chen, K.; Gu, W.; Zhang, Y. H. P.; Zhu, Z. Enzymatic biofuel cell-powered iontophoretic facial mask for enhanced transdermal drug delivery. Biosens. Bioelectron. 2023, 223, 115019. [CrossRef]
- Zhang, S.; Yang, C.; Jiang, Y.; Li, P.; Xia, C. A robust fluorine-containing ceramic cathode for direct CO2 electrolysis in solid oxide electrolysis cells. J. Energy Chem. 2023, 77, 300-309. [CrossRef]
- Pant, D.; Singh, A.; Van Bogaert, G.; Olsen, S. I.; Nigam, P. S.; Diels, L.; Vanbroekhoven, K. Bioelectrochemical systems (BES) for sustainable energy production and product recovery from organic wastes and industrial wastewaters. Rsc. Advances. 2012, 2(4), 1248-1263. [CrossRef]
- Apollon, W.; Kamaraj, S. K.; Silos-Espino, H.; Perales-Segovia, C.; Valera-Montero, L. L.; Maldonado-Ruelas, V. A.; Vázquez-Gutiérrez, M.A.; Ortiz-Medina, R.A.; Flores-Benítez, S.; Gómez-Leyva, J. F. Impact of Opuntia species plant bio-battery in a semi-arid environment: Demonstration of their applications. Appl. Energy. 2020, 279, 115788. [CrossRef]
- Apollon, W.; Valera-Montero, L. L.; Perales-Segovia, C.; Maldonado-Ruelas, V. A.; Ortiz-Medina, R. A.; Gómez-Leyva, J. F.; Vázquez-Gutiérrez, M.A.; Flores-Benítez, S.; Kamaraj, S. K. Effect of ammonium nitrate on novel cactus pear genotypes aided by biobattery in a semi-arid ecosystem. Sustain. Energy Technol. Assess. 2022, 49, 101730. [CrossRef]
- Ji, B.; Zhao, Y.; Yang, Y.; Li, Q.; Man, Y.; Dai, Y.; Fu, J.; Wei, T.; Tai, Y.; Zhang, X. Curbing per-and polyfluoroalkyl substances (PFASs): First investigation in a constructed wetland-microbial fuel cell system. Water Res. 2023, 230, 119530. [CrossRef]
- Potter, M. C. Electrical effects accompanying the decomposition of organic compounds. Proceedings of the royal society of London. 1911, 84(571), 260-276. [CrossRef]
- Logan, B.E.; Hamelers, B.; Rozendal, R.; Schröder, U.; Keller, J.; Freguia, S.; Aelterman, P.; Verstraete, W.; Rabaey, K. Microbial fuel cells: methodology and technology. Environ. Sci. Technol. 2006, 40 (17). [CrossRef]
- Pandey, P.; Shinde, V. N.; Deopurkar, R. L.; Kale, S. P.; Patil, S. A.; Pant, D. Recent advances in the use of different substrates in microbial fuel cells toward wastewater treatment and simultaneous energy recovery. Appl. Energy. 2016, 168, 706-723. [CrossRef]
- Apollon, W.; Luna-Maldonado, A. I.; Kamaraj, S. K.; Vidales-Contreras, J. A.; Rodríguez-Fuentes, H.; Gómez-Leyva, J. F.; Maldonado-Ruelas, V.A.; Ortiz-Medina, R. A. Self-sustainable nutrient recovery associated to power generation from livestock’s urine using plant-based bio-batteries. Fuel. 2023, 332, 126252. [CrossRef]
- Xu, X.; Zhao, Q.; Wu, M.; Ding, J.; Zhang, W. Biodegradation of organic matter and anodic microbial communities analysis in sediment microbial fuel cells with/without Fe(III) oxide addition. Bioresour. Technol. 2017, 225, 402–408. [CrossRef]
- Li, M.; Zhou, M.; Tian, X.; Tan, C.; McDaniel, C. T.; Hassett, D. J.; Gu, T. Microbial fuel cell (MFC) power performance improvement through enhanced microbial electrogenicity. Biotechnol. Adv. 2018, 36(4), 1316-1327. [CrossRef]
- Greenman, J.; Gajda, I.; You, J.; Mendis, B. A.; Obata, O.; Pasternak, G.; Ieropoulos, I. Microbial fuel cells and their electrified biofilms. Biofilm. 2021, 3, 100057. [CrossRef]
- Gude, V. G. Wastewater treatment in microbial fuel cells–an overview. J. Clean. Prod. 2016, 122, 287-307. [CrossRef]
- Dai, M.; Wu, Y.; Wang, J.; Lv, Z.; Li, F.; Zhang, Y.; Kong, Q. Constructed wetland-microbial fuel cells enhanced with iron carbon fillers for ciprofloxacin wastewater treatment and power generation. Chemosphere. 2022, 305, 135377. [CrossRef]
- Mohyudin, S.; Farooq, R.; Jubeen, F.; Rasheed, T.; Fatima, M.; Sher, F. Microbial fuel cells a state-of-the-art technology for wastewater treatment and bioelectricity generation. Environ. Res. 2022, 204, 112387. [CrossRef]
- Khandaker, S.; Das, S.; Hossain, M. T.; Islam, A.; Miah, M. R.; Awual, M. R. Sustainable approach for wastewater treatment using microbial fuel cells and green energy generation–A comprehensive review. J. Mol. Liq. 2021, 344, 117795. [CrossRef]
- Terbish, N.; Popuri, S. R.; Lee, C. H. Improved performance of organic–inorganic nanocomposite membrane for bioelectricity generation and wastewater treatment in microbial fuel cells. Fuel. 2023, 332, 126167. [CrossRef]
- Abubackar, H. N., Biryol, İ., & Ayol, A. Yeast industry wastewater treatment with microbial fuel cells: Effect of electrode materials and reactor configurations. Int. J. Hydrog. Energy. 2023, 48(33), 12424-12432. [CrossRef]
- Wang, Y.; He, C.; Li, W.; Zong, W.; Li, Z.; Yuan, L., Wang, G.; Mu, Y. High power generation in mixed-culture microbial fuel cells with corncob-derived three-dimensional N-doped bioanodes and the impact of N dopant states. J. Chem. Eng. 2020, 399, 125848. [CrossRef]
- Wang, X., Zhang, H., Ye, J., & Li, B. (2023). Atomically dispersed Fe–N4 moieties in porous carbon as efficient cathode catalyst for enhancing the performance in microbial fuel cells. Journal of Power Sources, 556, 232434. [CrossRef]
- Rusyn, I. Role of microbial community and plant species in performance of plant microbial fuel cells. Renew Sustain Energy Rev. 2021, 152, 111697. [CrossRef]
- Apollon, W.; Rusyn, I.; González-Gamboa, N.; Kuleshova, T.; Luna-Maldonado, A. I.; Vidales-Contreras, J. A.; Kamaraj, S. K. Improvement of zero waste sustainable recovery using microbial energy generation systems: A comprehensive review. Sci. Total Environ. 2022, 817, 153055. [CrossRef]
- Sharma, M.; Salama, E. S.; Thakur, N.; Alghamdi, H.; Jeon, B. H.; Li, X. Advances in the biomass valorization in bioelectrochemical systems: A sustainable approach for microbial-aided electricity and hydrogen production. Chem. Eng. J. 2023, 465, 142546. [CrossRef]
- Gadkari, S.; Fontmorin, J. M.; Yu, E.; Sadhukhan, J. Influence of temperature and other system parameters on microbial fuel cell performance: Numerical and experimental investigation. Chem. Eng. J. 2020, 388, 124176. [CrossRef]
- Rezk, H.; Olabi, A. G.; Abdelkareem, M. A.; Sayed, E. T. Artificial intelligence as a novel tool for enhancing the performance of urine fed microbial fuel cell as an emerging approach for simultaneous power generation and wastewater treatment. J. Taiwan Inst. Chem. Eng. 2023, 104726. [CrossRef]
- Wang, J.; Ren, K.; Zhu, Y.; Huang, J.; Liu, S. A review of recent advances in microbial fuel cells: Preparation, operation, and application. Biotech. 2022, 11(4), 44. [CrossRef]
- Yaqoob, A. A.; Ibrahim, M. N. M.; Umar, K. Electrode Material as Anode for Improving the Electrochemical Performance of Microbial Fuel Cells. In S. Haider, A. Haider, M. Khodaei, & L. Chen (Eds.), Energy Storage Battery Systems - Fundamentals and Applications. IntechOpen, 2021. [CrossRef]
- Palanisamy, G.; Jung, H.-Y.; Sadhasivam, T.; Kurkuri, M.D.; Kim, S.C.; Roh, S.-H. A comprehensive review on microbial fuel cell technologies: Processes, utilization, and advanced developments in electrodes and membranes. J. Clean. Prod. 2019, 221, 598-621. [CrossRef]
- Greenman, J.; Gajda, I.; You, J.; Mendis, B. A.; Obata, O.; Pasternak, G.; Ieropoulos, I. Microbial fuel cells and their electrified biofilms. Biofilm. 2021, 3, 100057. [CrossRef]
- Mukherjee, A.; Patel, V.; Shah, M. T.; Jadhav, D. A.; Munshi, N. S.; Chendake, A. D.; Pant, D. Effective power management system in stacked microbial fuel cells for onsite applications. J. Power Sources. 2022, 517, 230684. [CrossRef]
- Zhang, Y.; Mo, G.; Li, X.; Zhang, W.; Zhang, J.; Ye, J.; Huang, X., Yu, C. A graphene modified anode to improve the performance of microbial fuel cells. J. Power Sources. 2011, 196(13), 5402-5407. [CrossRef]
- Li, B.; Zhou, J.; Zhou, X.; Wang, X.; Li, B.; Santoro, C.; Grattieri, M.; Babanova, S.; Artyushkova, K.; Atanassov, P.; Schuler, A. J. Surface modification of microbial fuel cells anodes: approaches to practical design. Electrochim. Acta. 2014, 134, 116-126. [CrossRef]
- Ma, J.; Zhang, J.; Zhang, Y.; Guo, Q.; Hu, T.; Xiao, H.; Lu, W.; Jia, J. Progress on anodic modification materials and future development directions in microbial fuel cells. J. Power Sources. 2023, 556, 232486. [CrossRef]
- Wu, J.; Liu, R.; Dong, P.; Li, N.; He, W.; Feng, Y.; Liu, J. Enhanced electricity generation and storage by nitrogen-doped hierarchically porous carbon modification of the capacitive bioanode in microbial fuel cells. Sci. Total Environ. 2023, 858, 159688. [CrossRef]
- Kim, M.; Li, S.; Song, Y. E.; Park, S. Y.; Kim, H. I.; Jae, J.; Chung, I.; Kim, J. R. Polydopamine/polypyrrole-modified graphite felt enhances biocompatibility for electroactive bacteria and power density of microbial fuel cell. Chemosphere. 2023, 313, 137388. [CrossRef]
- Nawaz, A.; ul Haq, I.; Qaisar, K.; Gunes, B.; Raja, S. I.; Mohyuddin, K.; Amin, H. Microbial fuel cells: Insight into simultaneous wastewater treatment and bioelectricity generation. Process Saf. Environ. Prot. 2022, 161, 357-373. [CrossRef]
- Sato, C.; Paucar, N. E.; Chiu, S.; Mahmud, M. Z. I. M.; Dudgeon, J. Single-Chamber Microbial Fuel Cell with Multiple Plates of Bamboo Charcoal Anode: Performance Evaluation. Processes. 2021, 9(12), 2194. [CrossRef]
- Priya, R. L.; Ramachandran, T.; Suneesh, P. V. Fabrication and characterization of high power dual chamber E. coli microbial fuel cell. In IOP Conference Series: Mater. Sci. Eng. 2016, 149, 012215. [CrossRef]
- Choudhury, P.; Bhunia, B.; Mahata, N.; Bandyopadhyay, T. K. Optimization for the improvement of power in equal volume of single chamber microbial fuel cell using dairy wastewater. J. Indian Chem. Soc. 2022, 99(6), 100489. [CrossRef]
- Chaturvedi, A.; Dhillon, S. K.; Kundu, P. P. 1-D semiconducting TiO2 nanotubes supported efficient bimetallic Co-Ni cathode catalysts for power generation in single-chambered air-breathing microbial fuel cells. Sustain. Energy Technol. Assess. 2022, 53, 102479. [CrossRef]
- Zhai, D. D.; Fang, Z.; Jin, H.; Hui, M.; Kirubaharan, C. J.; Yu, Y. Y.; Yong, Y. C. Vertical alignment of polyaniline nanofibers on electrode surface for high-performance microbial fuel cells. Bioresour. Technol. 2019, 288, 121499. [CrossRef]
- Sumisha, A.; Haribabu, K. Modification of graphite felt using nano polypyrrole and polythiophene for microbial fuel cell applications-a comparative study. Int. J. Hydrog. Energy. 2018, 43(6), 3308-3316. [CrossRef]
- Liang, H.; Han, J.; Yang, X.; Qiao, Z.; Yin, T. Performance improvement of microbial fuel cells through assembling anodes modified with nanoscale materials. Nanomater Nanotechnol. 2022, 12, 18479804221132965. [CrossRef]
- Ouyang, T.; Liu, W.; Shi, X.; Li, Y.; Hu, X. Multi-criteria assessment and triple-objective optimization of a bio-anode microfluidic microbial fuel cell. Bioresour. Technol. 2023, 129193. [CrossRef]
- Chiranjeevi, P.; Patil, S. A. Strategies for improving the electroactivity and specific metabolic functionality of microorganisms for various microbial electrochemical technologies. Biotechnol Adv. 2020, 39, 107468. [CrossRef]
- Do, M. H.; Ngo, H. H.; Guo, W.; Chang, S. W.; Nguyen, D. D.; Deng, L.; Chen, Z.; Nguyen, T. V. Performance of mediator-less double chamber microbial fuel cell-based biosensor for measuring biological chemical oxygen. J. Environ. Manage. 2020, 276, 111279. [CrossRef]
- Yap, K. L.; Ho, L. N.; Guo, K.; Liew, Y. M.; Lutpi, N. A.; Azhari, A. W.; Thor, S.H.; Teoh, T.P., Oon, Y.S.; Ong, S. A. Exploring the potential of metal oxides as cathodic catalysts in a double chambered microbial fuel cell for phenol removal and power generation. J. Water Process Eng. 2023, 53, 103639. [CrossRef]
- Eslami, S.; Bahrami, M.; Zandi, M.; Fakhar, J.; Gavagsaz-Ghoachani, R.; Noorollahi, Y.; Phattanasak, M.; Nahid-Mobarakeh, B. Performance investigation and comparison of polypropylene to Nafion117 as the membrane of a dual-chamber microbial fuel cell. Clean. Mater. 2023, 8, 100184. [CrossRef]
- Papillon, J.,, Olivier, O.; Éric, M. "Scale up of single-chamber microbial fuel cells with stainless steel 3D anode: Effect of electrode surface areas and electrode spacing." Bioresour. Technol. Rep. 2021, 13, 100632. [CrossRef]
- Wu, X.; Lv, C.; Ye, J.; Li, M.; Zhang, X.; Lv, J.; Fang, Q.; Yu, S.; Xie, W. (2021). Glycine-hydrochloric acid buffer promotes simultaneous U (VI) reduction and bioelectricity generation in dual chamber microbial fuel cell. J Taiwan Inst. Chem. Eng. 2021, 127, 236-247. [CrossRef]
- Singh, A.; Kaushik, A. Removal of Cd and Ni with enhanced energy generation using biocathode microbial fuel cell: insights from molecular characterization of biofilm communities. J. Clean. Prod. 2021, 315, 127940. [CrossRef]
- Zheng, Y.; Wang, L.; Zhu, Y.; Li, X.; Ren, Y. A triple-chamber microbial fuel cell enabled to synchronously recover iron and sulfur elements from sulfide tailings. J. Hazard. Mater. 2021, 401, 123307. [CrossRef]
- Kumar, V.; Rudra, R.; Hait, S. Sulfonated polyvinylidene fluoride-crosslinked-aniline-2-sulfonic acid as ion exchange membrane in single-chambered microbial fuel cell. J. Environ. Chem. Eng. 2021, 9(6), 106467. [CrossRef]
- Fujimura, S.; Kamitori, K.; Kamei, I.; Nagamine, M.; Miyoshi, K.; Inoue, K. Performance of stacked microbial fuel cells with barley–shochu waste. J. Biosci. Bioeng. 2022, 133(5), 467-473. [CrossRef]
- Lin, N.; Yang, Q.; Feng, Y. Optimization of catalyst dosage and total volume for extendible stacked microbial fuel cell reactors using spacer. J. Power Sources. 2022, 517, 230697. [CrossRef]
- Shirkosh, M.; Hojjat, Y.; Mardanpour, M. M. Evaluation and optimization of the Zn-based microfluidic microbial fuel cells to power various electronic devices. Biosens. Bioelectron.: X., 2022, 12, 100254. [CrossRef]
- Liu, H.; Zhang, B.; Liu, Y.; Wang, Z.; Hao, L. Continuous bioelectricity generation with simultaneous sulfide and organics removals in an anaerobic baffled stacking microbial fuel cell. Int. J. Hydrog. 2015, 40(25), 8128-8136. [CrossRef]
- Kolubah, P. D., Mohamed, H. O., Ayach, M., Hari, A. R., Alshareef, H. N., Saikaly, P., ... & Castaño, P. (2023). W2N-MXene composite anode catalyst for efficient microbial fuel cells using domestic wastewater. Chemical Engineering Journal, 461, 141821. [CrossRef]
- Burns, M.; Qin, M. Ammonia recovery from organic nitrogen in synthetic dairy manure with a microbial fuel cell. Chemosphere. 2023, 325, 138388. [CrossRef]
- Adnan, M. Y.; Hassoon Ali, A. Investigating the Performance of Single Chamber Microbial Fuel Cell (SCMFC) as Sustainable-Innovative Technique for Electricity Generation and Wastewater Treatment. In Defect and Diffusion Forum. 2023, 425, 107-117). [CrossRef]
- Wang, L.; Tai, Y.; Zhao, X.; He, Q.; Hu, Z.; Li, M. Phosphorus recovery directly from sewage sludge as vivianite and simultaneous electricity production in a dual chamber microbial fuel cell. J. Environ. Chem. Eng. 2023, 110152. [CrossRef]
- Huang, S. J.; Dwivedi, K. A.; Kumar, S.; Wang, C. T.; Yadav, A. K. Binder-free NiO/MnO2 coated carbon based anodes for simultaneous norfloxacin removal, wastewater treatment and power generation in dual-chamber microbial fuel cell. Environ. Pollut. 2023, 317, 120578. [CrossRef]
- Wang, C.; Xing, Y.; Zhang, K. Zheng, H.; Zhang, Y. N.; Zhu, X.; Yuan, X.; Qu, J. Evaluation of photocathode coupling-mediated hydroxychloroquine degradation in a single-chamber microbial fuel cell based on electron transfer mechanism and power generation. J. Power Sources. 2023, 559, 232625. [CrossRef]
- Verma, M.; Mishra, V. Bioelectricity generation by microbial degradation of banana peel waste biomass in a dual-chamber S. cerevisiae-based microbial fuel cell. Biomass Bioenergy. 2023, 168, 106677. [CrossRef]
- Wang, H.; Chai, G.; Zhang, Y.; Wang, D.; Wang, Z.; Meng, H.; Jiang, C.; Dong, W.; Li, J.; Lin, Y.; Li, H. Copper removal from wastewater and electricity generation using dual-chamber microbial fuel cells with shrimp shell as the substrate. Electrochim. Acta. 2023, 141849. [CrossRef]
- Yang, Z.; Li, J.; Chen, F.; Xu, L.; Jin, Y.; Xu, S.; Wang, J.; Shen, X.; Zhang, L.; Song, Y. Bioelectrochemical process for simultaneous removal of copper, ammonium and organic matter using an algae-assisted triple-chamber microbial fuel cell. Sci. Total Environ. 2021, 798, 149327. [CrossRef]
- Senthilkumar, K.; Naveenkumar, M.; Ratnam, M. V.; Samraj, S. A review on scaling-up of microbial fuel cell: Challenges and opportunities. Scaling Up of Microbial Electrochemical Systems. 2022, 13-28. [CrossRef]
- Tan, K.L.; Reeves, S.; Hashemi, N.; Thomas, D.G.; Kavak, E.; Montazami, R.; Hashemi, N. Graphene as Flexible Electrode: Review of Fabrication Approaches. J. Mater.Chem. 2017, 5, 17777-17803. [CrossRef]
- Scott, K. An Introduction to Microbial Fuel Cells. Microbial Electrochemical and Fuel Cells. 2016, 3–27. [CrossRef]
- Liu, H.; Logan, B. E. Electricity generation using an air-cathode single chamber microbial fuel cell in the presence and absence of a proton exchange membrane. Environ. Sci. Technol. 2004, 38, 4040–4046. [CrossRef]
- Scott, K. Membranes and separators for microbial fuel cells. Woodhead Publishing. 2016, pp.153–178. [CrossRef]
- Rozendal, R. A.; Hamelers, H. V. M.; Rabaey, K.; Keller, J.; Buisman, C. J. N. Towards practical implementation of bioelectrochemical wastewater treatment. Trends Biotechnol. 2009, 26, 450–459. [CrossRef]
- Heijne, T. A.; Hamelers, H. V. M.; De Wilde, V.; Rozendal, R. A.; Buisman, C. J. N. A bipolar membrane combined with ferric iron reduction as an efficient cathode system in microbial fuel cells. Environ. Sci. Technol. 2006, 40, 5200–5205. [CrossRef]
- Pärnamäe, R.; Mareev, S.; Nikonenko, V.; Melnikov, S.; Sheldeshov, N.; Zabolotskii, V.; Hamelers, H.V.M.; Tedesco, M. Bipolar membranes: A review on principles, latest developments, and applications. J. Membr. Sci. 2021, 617, 118538. [CrossRef]
- Lefebvre, O.; Shen, Y.; Tan, Z.; Uzabiaga, A.; Chang, I. S.; Ng, H.Y. A comparison of membranes and enrichment strategies for microbial fuel cells. Bioresour. Technol. 2011, 102, 6291–6294. [CrossRef]
- Ghasemi, M.; Daud, W. R. W.; Ismail, A. F.; Jafari, Y.; Ismail, M.; Mayahi, A.; Othman, J. Simultaneous wastewater treatment and electricity generation by microbial fuel cell: performance comparison and cost investigation of using Nafion 117 and SPEEK as separators. Desalination. 2013, 325, 1–6. [CrossRef]
- Tang, H.; Guo, K.; Li, H.; Du, Z.; Tian, J. Microfiltration membrane performance in two-chamber microbial fuel cells. Biochem. Eng. J. 2010, 52, 194–198. [CrossRef]
- Dharmalingam, S.; Kugarajah, V.; Sugumar, M. Membranes for Microbial Fuel Cells. Microbial Electrochemical Technol. 2019, 143–194. [CrossRef]
- Sondhi, R.; Bhave, R.; Jung, G. Applications and benefits of ceramic membranes. Membr. Technol. 2003, 11, 5–8. [CrossRef]
- Kamaraj, S.-K.; Rivera, A. E.; Murugesan, S.; García-Mena, J.; Maya, O.; Frausto-Reyes, C.; Tapia-Ramírez, J., Espino, H.S.; Caballero-Briones, F. Electricity generation from Nopal biogas effluent using a surface modified clay cup (cantarito) microbial fuel cell. Heliyon. 2019, 5, e01506. [CrossRef]
- Yaqoob, A. A.; Ibrahim, M.; Rafatullah, M.; Chua, Y. S.; Ahmad, A.; Umar, K. Recent Advances in Anodes for Microbial Fuel Cells: An Overview. Materials. 2020, 13(9), 2078. [CrossRef]
- Apollon, W.; Luna-Maldonado, A. I.; Kamaraj, S. K.; Vidales-Contreras, J. A.; Rodríguez-Fuentes, H.; Gómez-Leyva, J. F.; Aranda-Ruíz, J. Progress and recent trends in photosynthetic assisted microbial fuel cells: A review. Biomass Bioenergy. 2021, 148, 106028. [CrossRef]
- Lovley, D. R. Bug juice: harvesting electricity with microorganisms. Nat. Rev. Microbiol. 2006, 4(7), 497-508. [CrossRef]
- Pant, D.; Singh, A.; Van Bogaert, G.; Olsen, S. I.; Nigam, P. S.; Diels, L.; Vanbroekhoven, K. Bioelectrochemical systems (BES) for sustainable energy production and product recovery from organic wastes and industrial wastewaters. Rsc. Adv. 2012, 2(4), 1248-1263. [CrossRef]
- Yang, Z.; Yang, A. Modelling the impact of operating mode and electron transfer mechanism in microbial fuel cells with two-species anodic biofilm. Biochem. Eng. J. 2020, 158, 107560. [CrossRef]
- Cao, Y.; Mu, H.; Liu, W.; Zhang, R.; Guo, J.; Xian, M.; Liu, H. Electricigens in the anode of microbial fuel cells: pure cultures versus mixed communities. Microb. Cell Factories. 2019, 18(1), 39. [CrossRef]
- Sherafatmand, M.; Ng, H. Y. Using sediment microbial fuel cells (SMFCs) for bioremediation of polycyclic aromatic hydrocarbons (PAHs). Bioresour. Technol. 2015, 195, 122–130. [CrossRef]
- Sathish, T.; Sathyamurthy, R.; Kumar, S. S.; Huynh, G. B.; Saravanan, R.; Rajasimman, M. Amplifying power generation in microbial fuel cells with cathode catalyst of graphite-based nanomaterials. Int. J. Hydrogen Energy. 2022. [CrossRef]
- Subran, N., Ajit, K., Krishnan, H., Pachiyappan, S., & Ramaswamy, P. Synthesis and performance of a cathode catalyst derived from areca nut husk in microbial fuel cell. Chemosphere. 2023, 312, 137303. [CrossRef]
- Sugumar, M.; Dharmalingam, S. Statistical assessment of operational parameters using optimized sulphonated titanium nanotubes incorporated sulphonated polystyrene ethylene butylene polystyrene nanocomposite membrane for efficient electricity generation in microbial fuel cell. Energy. 2022, 242, 123000. [CrossRef]
- Bensaida, K.; Maamoun, I.; Eljamal, R.; Falyouna, O.; Sugihara, Y.; Eljamal, O. New insight for electricity amplification in microbial fuel cells (MFCs) applying magnesium hydroxide coated iron nanoparticles. Energy Convers. Manag. 2021, 249, 114877. [CrossRef]
- Liu, H.; Cheng, S.; Logan, B. E. Production of electricity from acetate or butyrate using a single-chamber microbial fuel cell. Environ. Sci. Technol. 2005, 39(2), 658-662. [CrossRef]
- Khater, D.; El-Khatib, K. M.; Hazaa, M.; Hassan, R. Electricity generation using Glucose as substrate in microbial fuel cell. J. Environ. Sci. 2015, 2, 84-98.
- Hashmi, Z.; Jatoi, A. S.; Aziz, S.; Soomro, S. A.; Abbasi, S. A.; Usto, M. A.; Alam, M.S.; Anjum, A.; Iqbal, A.; Usman, M. T. Bio-assisted treatment of hazardous spent wash via microbial fuel cell. Environmental friendly approach. Biomass Convers. Biorefin. 2021, 1-9. [CrossRef]
- Arun, S.; Sinharoy, A.; Pakshirajan, K.; Lens, P. N. L. Algae based microbial fuel cells for wastewater treatment and recovery of value-added products. Renew. Sustain. Energy Rev. 2020, 132, 110041. [CrossRef]
- Zhang, Y.; Wang, J.-H.; Zhang, J.-T.; Chi, Z.-Y.; Kong, F.T.; Zhang, Q. The long overlooked microalgal nitrous oxide emission: Characteristics, mechanisms, and influencing factors in microalgae-based wastewater treatment scenarios. Sci. Total Environ. 2023, 856, 159153. [CrossRef]
- Shukla, M.; Kumar, S. Algal growth in photosynthetic algal microbial fuel cell and its subsequent utilization for biofuels. Renew. Sustain. Energy Rev. 2018, 82, 402- 414. [CrossRef]
- Mahmoud, R. H.; Samhan, F. A.; Ibrahim, M. K.; Ali, G. H.; Hassan, R. Y. Waste to energy conversion utilizing nanostructured Algal-based microbial fuel cells. Electrochem. Sci. Adv. 2021, e2100071. [CrossRef]
- Ribeiro, V. R.; Osório, H. D. D.; Ulrich, A. C.; Rizzetti, T. M.; Barrios, A. S.; de Cassia de Souza Schneider, R.; Benitez, L. B. The use of microalgae-microbial fuel cells in wastewater bioremediation and bioelectricity generation. J. Water Process. Eng. 2022, 48, 102882. [CrossRef]
- Yaqoob, A.A.; Guerrero–Barajas, C.; Ibrahim, M.N.M. et al. Local fruit wastes driven benthic microbial fuel cell: a sustainable approach to toxic metal removal and bioelectricity generation. Environ. Sci. Pollut. Res. 2022, 29, 32913–32928. [CrossRef]
- Du, H.; Li, F. Enhancement of solid potato waste treatment by microbial fuel cell with mixed feeding of waste activated sludge. J. Clean. Prod. 2017, 143, 336–344. [CrossRef]
- Sato, C.; Paucar, N. E.; Chiu, S.; Mahmud, M. Z.; Dudgeon, J. Single-Chamber Microbial Fuel Cell with Multiple Plates of Bamboo Charcoal Anode: Performance Evaluation. Processes. 2021, 9(12), 2194. [CrossRef]
- Suransh, J.; Mungray, A. K. Reduction in particle size of vermiculite and production of the low-cost earthen membrane to achieve enhancement in the microbial fuel cell performance. J. Environ. Chem. Eng. 2022, 10(6), 108787. [CrossRef]
- Saniei, N.; Ghasemi, N.; Zinatizadeh, A. A.; Zinadini, S.; Ramezani, M.; Derakhshan, A. A. Electricity generation enhancement in microbial fuel cell via employing a new SPEEK based proton exchange membrane modified by goethite nanoparticles functionalized with tannic acid and sulfanilic acid. Environ. Technol. Innov. 2022, 25, 102168. [CrossRef]
- Wang, J.; Huang, J.; Xiao, X.; Zhang, D.; Zhang, Z.; Zhou, Z.; Liu, S. (R)− 3-hydroxybutyrate production by Burkholderia cepacia in the cathode chamber of ethanol-producing microbial fuel cells. Biochem. Eng. J. 2022, 186, 108581. [CrossRef]
- Mejía-López, M.; Lastres, O.; Alemán-Ramirez, J. L.; Lobato-Peralta, D. R.; Verde, A.; Gámez, J. M.; de Paz, P.L.; Verea, L. Conductive carbon-polymer composite for bioelectrodes and electricity generation in a sedimentary microbial fuel cell. Biochem. Eng. J. 2023, 193, 108856. [CrossRef]
- Yuan, J.; Huang, H.; Chatterjee, S. G.; Wang, Z.; Wang, S. Effective factors for the performance of a co-generation system for bioethanol and electricity production via microbial fuel cell technology. Biochem. Eng. J. 2022, 178, 108309. [CrossRef]
- Poli, F.; Santoro, C.; Soavi, F. Improving microbial fuel cells power density using internal and external optimized, tailored and totally green supercapacitor. J. Power Sources. 2023, 564, 232780. [CrossRef]
- Nandy, A.; Farkas, D.; Pepió-Tárrega, B.; Martinez-Crespiera, S.; Borràs, E.; Avignone-Rossa, C.; Di Lorenzo, M. Influence of carbon-based cathodes on biofilm composition and electrochemical performance in soil microbial fuel cells. Environ. Sci. Ecotechnol. 2023, 16, 100276. [CrossRef]
- Sabina-Delgado, A.; Kamaraj, S. K.; Hernández-Montoya, V.; Cervantes, F. J. Novel carbon-ceramic composite membranes with high cation exchange properties for use in microbial fuel cell and electricity generation. Int. J. Hydrogen Energy. 2023. [CrossRef]
- Ravinuthala, S.; Anilbhai, B. V.; Settu, S. Fabrication of Low-cost, green Material-Based Microbial Fuel Cell for bioelectricity production through textile wastewater remediation. Mater. Today: Proc. 2023. [CrossRef]
- Feregrino-Rivas, M.; Ramirez-Pereda, B.; Estrada-Godoy, F.; Cuesta-Zedeño, L. F.; Rochín-Medina, J. J.; Bustos-Terrones, Y. A.; Gonzalez-Huitron, V. A. Performance of a sediment microbial fuel cell for bioenergy production: Comparison of fluvial and marine sediments. Biomass Bioenergy. 2023, 168, 106657. [CrossRef]
- Guo, H.; Huang, C.; Geng, X.; Jia, X.; Huo, H.; Yue, W. Influence of the original electrogenic bacteria on the performance of oily sludge Microbial Fuel Cells. Energy Rep. 2022, 8, 14374-14381. [CrossRef]
- Kadier, A.; Simayi, Y.; Abdeshahian, P.; Azman, N. F.; Chandrasekhar, K.; Kalil, M. S. A comprehensive review of microbial electrolysis cells (MEC) reactor designs and configurations for sustainable hydrogen gas production. Alex. Eng. J. 2016, 55(1), 427-443. [CrossRef]
- Hao, Y.; Zhang, X.; Du, Q.; Wang, H.; Ngo, H. H.; Guo, W.; Zhang, Y.; Long, T.; Qi, L. A new integrated single-chamber air-cathode microbial fuel cell-Anaerobic membrane bioreactor system for improving methane production and membrane fouling mitigation. J. Membr. Sci. 2022, 655, 120591. [CrossRef]
- Vu, H. T.; Min, B. Integration of submersible microbial fuel cell in anaerobic digestion for enhanced production of methane and current at varying glucose levels. Int. J. Hydrogen Energy. 2019, 44(14), 7574-7582. [CrossRef]
- Nguyen, P. K. T.; Das, G.; Kim, J.; Yoon, H. H. Hydrogen production from macroalgae by simultaneous dark fermentation and microbial electrolysis cell. Bioresour. Technol. 2020, 315, 123795. [CrossRef]
- Gebreslassie, T. R.; Nguyen, P. K. T.; Yoon, H. H.; Kim, J. Co-production of hydrogen and electricity from macroalgae by simultaneous dark fermentation and microbial fuel cell. Bioresour. Technol. 2021, 336, 125269. [CrossRef]
- Yun, W. H.; Yoon, Y. S.; Yoon, H. H.; Nguyen, P. K. T.; Hur, J. Hydrogen production from macroalgae by simultaneous dark fermentation and microbial electrolysis cell with surface-modified stainless steel mesh cathode. Int. J. Hydrogen Energy. 2021, 46(79), 39136-39145. [CrossRef]
- Li, W.; He, L.; Cheng, C.; Cao, G.; Ren, N. Effects of biochar on ethanol-type and butyrate-type fermentative hydrogen productions. Bioresour. Technol. 2020, 306, 123088. [CrossRef]
- Zhao, L.; Zhang, J.; Zhao, W.; Zang, L. Improved Fermentative hydrogen production with the addition of calcium-lignosulfonate-derived biochar. Energy Fuels. 2019, 33(8), 7406-7414. [CrossRef]
- Harun, K.; Adhikari, S.; Jahromi, H. Hydrogen production via thermocatalytic decomposition of methane using carbon-based catalysts. RSC. Adv. 2020, 10(67), 40882-40893. [CrossRef]
- Zhang, C.; He, P.; Liu, J; Zhou, X.; Li, X.; Lu, J.; Hou, B. Study on performance and mechanisms of anaerobic oxidation of methane-microbial fuel cells (AOM-MFCs) with acetate-acclimatizing or formate-acclimatizing electroactive culture. Bioelectrochemistry. 2023, 151, 108404. [CrossRef]
- Liu, J.; Yun, S.; Wang, K.; Liu, L.; An, J.; Ke, T.; Gao, T.; Zhang, X. Enhanced methane production in microbial electrolysis cell coupled anaerobic digestion system with MXene accelerants. Bioresource Technol. 2023, 380, 129089. [CrossRef]
- Durna Pişkin, E.; Genç, N. Multi response optimization of waste activated sludge oxidation and azo dye reduction in microbial fuel cell. Environ. Technol. 2023, 1-13. [CrossRef]
- Bhattacharya, R.; Bose, D.; Yadav, J.; Sharma, B.; Sangli, E.; Patel, A.; Mukherjee, A.; Singh, A. A. Bioremediation and bioelectricity from Himalayan rock soil in sediment-microbial fuel cell using carbon rich substrates. Fuel. 2023, 341, 127019. [CrossRef]
- Habibul, N.; Hu, Y.; Sheng, G. P. Microbial fuel cell driving electrokinetic remediation of toxic metal contaminated soils. J. Hazard. Mater. 2016, 318, 9-14. [CrossRef]
- Jabbar, N. M.; Alardhi, S. M.; Al-Jadir, T.; Dhahad, H. A. Contaminants Removal from Real Refinery Wastewater Associated with Energy Generation in Microbial Fuel Cell. J. Ecol. Eng. 2023, 24(1), 107-114. [CrossRef]
- Apollon, W.; Luna-Maldonado, A. I.; Vidales-Contreras, J. A.; Rodríguez-Fuentes, H.; Gómez-Leyva, J. F.; Kamaraj, S. K. Application of microbial fuel cells and other bioelectrochemical systems: a comparative study. In Microbial Fuel Cells: Emerging trends in electrochemical applications. IOP Publishing, 2022. [CrossRef]
- You, S.; Zhao, Q.; Zhang, J.; Jiang, J.; Zhao, S. A microbial fuel cell using permanganate as the cathodic electron acceptor. J. Power Sources. 2006, 162(2), 1409-1415. [CrossRef]
- Kim, B. H.; Chang, I. S.; Gadd, G. M. Challenges in microbial fuel cell development and operation. Appl. Microbiol. Biotechnol. 2007, 76, 485-494. [CrossRef]
- Logan, B. E.; Regan, J. M.. Electricity-producing bacterial communities in microbial fuel cells. Trends Microbiol. 2006, 14(12), 512-518. [CrossRef]
- Liu, H.; Cheng, S.; Logan, B. E. Power generation in fed-batch microbial fuel cells as a function of ionic strength, temperature, and reactor configuration. Environ. Sci. Technol. 2005, 39(14), 5488-5493. [CrossRef]
- Sabin, J. M.; Leverenz, H.; Bischel, H. N. Microbial fuel cell treatment energy-offset for fertilizer production from human urine. Chemospher. 2022, 294, 133594. [CrossRef]
- Sharma, R.; Kumari, R.; Pant, D.; Malaviya, P. Bioelectricity generation from human urine and simultaneous nutrient recovery: Role of Microbial Fuel Cells. Chemosphere. 2022, 292, 133437. [CrossRef]
- Walter, X. A.; You, J.; Gajda, I.; Greenman, J.; Ieropoulos, I. Impact of feedstock dilution on the performance of urine-fed ceramic and membrane-less microbial fuel cell cascades designs. J. Power Sources. 2023, 561, 232708. [CrossRef]
- Du, H.; Shao, Z. Synergistic effects between solid potato waste and waste activated sludge for waste-to-power conversion in microbial fuel cells. Appl. Energy. 2022, 314, 118994. [CrossRef]
- Ma, Y., Deng, D., Zhan, Y., Cao, L., & Liu, Y. (2022). A systematic study on self-powered microbial fuel cell based BOD biosensors running under different temperatures. Biochemical Engineering Journal, 180, 108372. [CrossRef]
- Nath, D.; Kallepalli, S.; Rao, L. T.; Dubey, S. K.; Javed, A.; Goel, S. Microfluidic paper microbial fuel cell powered by Shewanella putrefaciens in IoT cloud framework. Int. J. Hydrogen Energy. 2021, 46(4), 3230-3239. [CrossRef]
- Prasad, J.; Tripathi, R. K. Scale-up and control the voltage of sediment microbial fuel cell for charging a cell phone. Biosens.Bioelectron. 2021, 172, 112767. [CrossRef]
- Davidson, D. J. Exnovating for a renewable energy transition. Nat. Energy. 2019, 4(4), 254-256. [CrossRef]
- Liu, L.J.; Jiang, HD.; Liang, Q. M.; Creutzig, F.; Liao, H.; Yao, Y.F.; Qian, X.Y.; Ren, Z.Y.; Qing, J.; Cai, Q.R. Carbon emissions and economic impacts of an EU embargo on Russian fossil fuels. Nat. Clim. Chang. 2023, 13, 290–296. [CrossRef]
- Savla, N.; Pandit, S.; Verma, J. P.; Awasthi, A. K.; Sana, S. S.; Prasad, R. Techno-economical evaluation and life cycle assessment of microbial electrochemical systems: A review. Curr. Res. Green Sustain. Chem. 2021, 4, 100111. [CrossRef]
- Harnisch, F; Schröder, U. Selectivity versus mobility: Separation of anode and cathode in microbial bioelectrochemical systems. ChemSusChem. 2009, 2(10), 921-926. [CrossRef]
- Finkbeiner, M. The International Standards as the Constitution of Life Cycle Assessment: The ISO 14040 Series and its Offspring. In: Klöpffer, W. (eds) Background and Future Prospects in Life Cycle Assessment. LCA Compendium – The Complete World of Life Cycle Assessment. Springer. 2014, 85-106.
- Zhang, J.; Yuan, H.; Abu-Reesh, I. M.; He, Z.; Yuan, C. Life Cycle Environmental Impact Comparison of Bioelectrochemical Systems for Wastewater Treatment. Procedia CIRP. 2019, 80, 382– 388. [CrossRef]
- Alseroury, F. A. Microbial desalination cells: progress and impacts. J. Biochem. Technol. 2018, 9(1), 40.
- Mathuriya, A., S.; Hiloidhari, M.; Gware, P.; Singh, A.; Pant, D. Development and life cycle assessment of an auto circulating bioelectrochemical reactor for energy positive continuous wastewater treatment. Biores. Technol. 2020, 304, 122959. [CrossRef]
- Pant, D.; Singh, A.; Bogaert, G. V.; Alvarez-Gallego, Y.; Diels, L.; Vanbroekhoven, K. An introduction to the life cycle assessment (LCA) of bioelectrochemical systems (BES) for sustainable energy and product generation: relevance and key aspects. Renew. Sustain. Energy Rev. 2011, 15, 1305-1313. [CrossRef]
- Chin, M. Y.; Phuang, Z. X.; Hanafiah, M.; Zhang, Z.; Woon, K. S. Exploring the life cycle environmental performance of different microbial fuel cell configurations. Chem. Eng. Trans. 2021, 89, 175-180. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





