1. Introduction
Cardiomyopathies are a heterogeneous group of diseases that affects the myocardium, resulting in mechanical and/or electrical dysfunction [
1]. DCM is a myocardial condition characterized by structural and functional abnormalities that lead to left, right, or biventricular dilation, without any underlying pressure or volume overload (e.g., systemic hypertension or valvular disease) or coronary artery disease (CAD) [
2].
Advancements in treatment, familial and preparticipation screening, and individualized follow-up have significantly improved the prognosis of DCM [
1]. Survival free from death and transplantation has risen to more than 80% in 8 years [
2].
Despite its complex nature, clinical management of DCM remains challenging. It is currently one of the most common heart failure (HF) phenotypes with an indication for transplantation [
3]. DCM is thought to be the final presentation of a wide spectrum of pathogenic processes and genetic interactions [
2]. Therefore, a better understanding of these pathophysiological complexities is crucial to optimize targeted therapy and obtain a more accurate prognosis.
2. Epidemiology
The estimated prevalence of DCM varies between 1:250 and 1:2500 with an incidence of 5 to 7 cases per 100,000 person-years [
4]. Variations in prevalence can be attributed to underdiagnosis and differences in patient selection in clinical trials. DCM predominantly affects younger individuals, with a higher prevalence in men (3:1 ratio). Some cohorts have shown a threefold increased risk of DCM in black individuals, who also face twice the mortality risk compared to other groups [
5]. DCM accounts for 12-35% of heart failure with reduced ejection fraction (HFrEF) cases in clinical trials, with patients typically experiencing symptoms 10 years earlier than those with other HF phenotypes, in addition to more severe symptoms and lower left ventricular ejection fraction (LVEF) [
3].
3. Cardiomyopathies Classification
The classification of cardiomyopathies has been challenging over the years. In 1957, Wallace Brigden defined cardiomyopathy as an isolated myocardial disease unrelated to CAD [
6]. In 1961, Goodwin refined this concept, describing cardiomyopathy as a myocardial disease of unknown etiology, not associated with CAD, and capable of affecting the endocardium and pericardium as well [
7]. Subsequently, in 1980, the World Health Organization (WHO) introduced subtypes of cardiomyopathies based on morphological and physiological characteristics, such as left ventricular (LV) hypertrophy or dilation [
8]. In 1995, the WHO added a new entity to the classification, known as arrhythmogenic right ventricular cardiomyopathy (ARVC) [
9]. In 2008, the European Society of Cardiology (ESC) emphasized the differentiation between genetic and non-genetic types of DCM [
10].
Attempts to classify cardiomyopathies have presented several limitations, since each of them has prioritized a specific characteristic of these diseases [
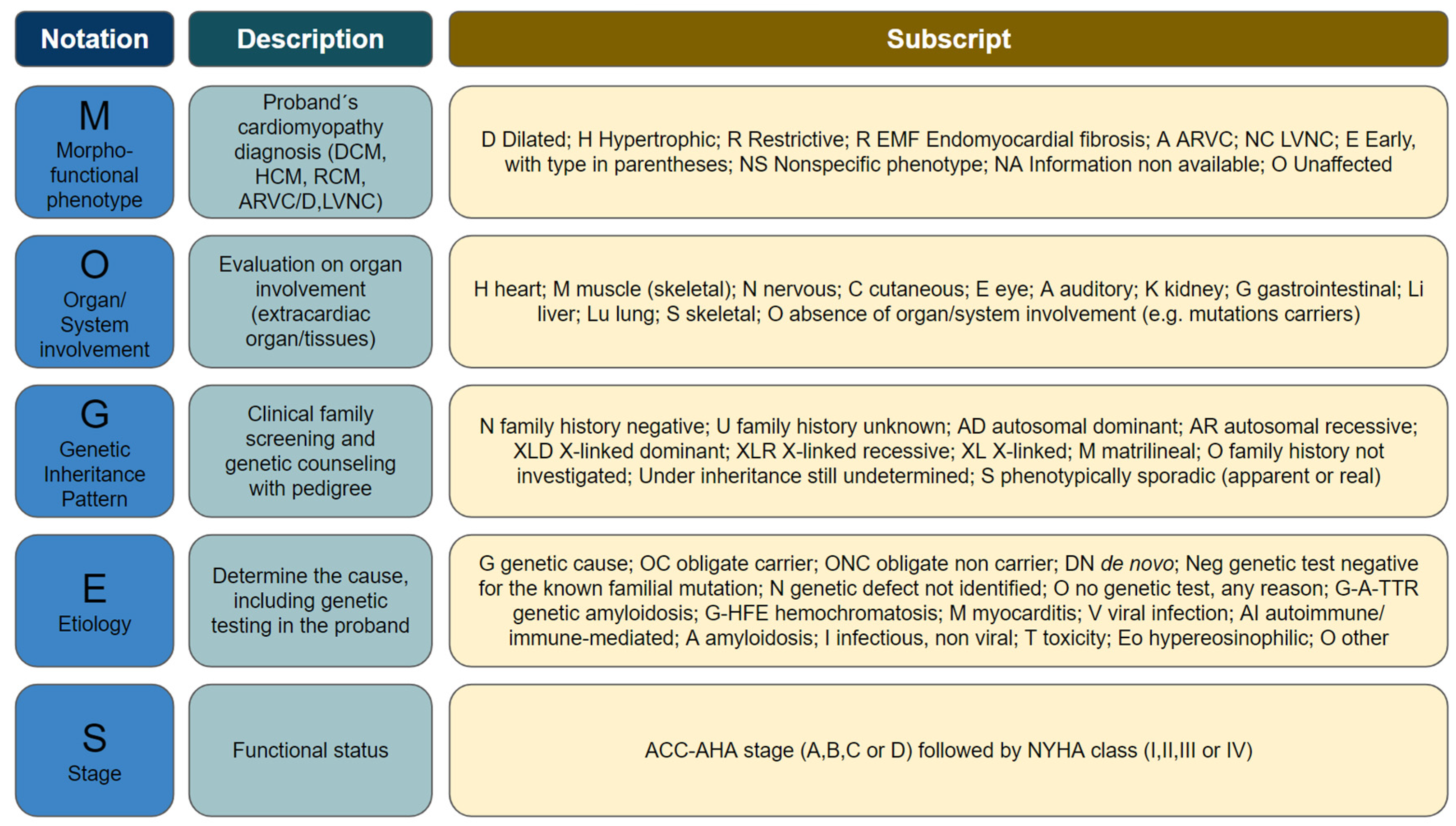
11]. In 2014, the WHO proposed a new classification called MOGES (
Table 1). Although broader, this classification is less commonly used in clinical practice. MOGES encompasses 5 domains: (M) refers to the morphofunctional phenotype (e.g., dilated, hypertrophic, restrictive, non-compaction, etc.); (O) refers to organic involvement (e.g., LV, right ventricle, biventricular, muscles, nerves, skin, etc.); (G) refers to the genetic inheritance pattern, when identified (e.g., autosomal dominant, autosomal recessive, X-linked, etc.); (E) refers to etiology (e.g., genetic, myocarditis, autoimmune, amyloidosis, etc.); (S) refers to functional staging as defined by the New York Heart Association (NYHA) classification [
12].
4. Etiology
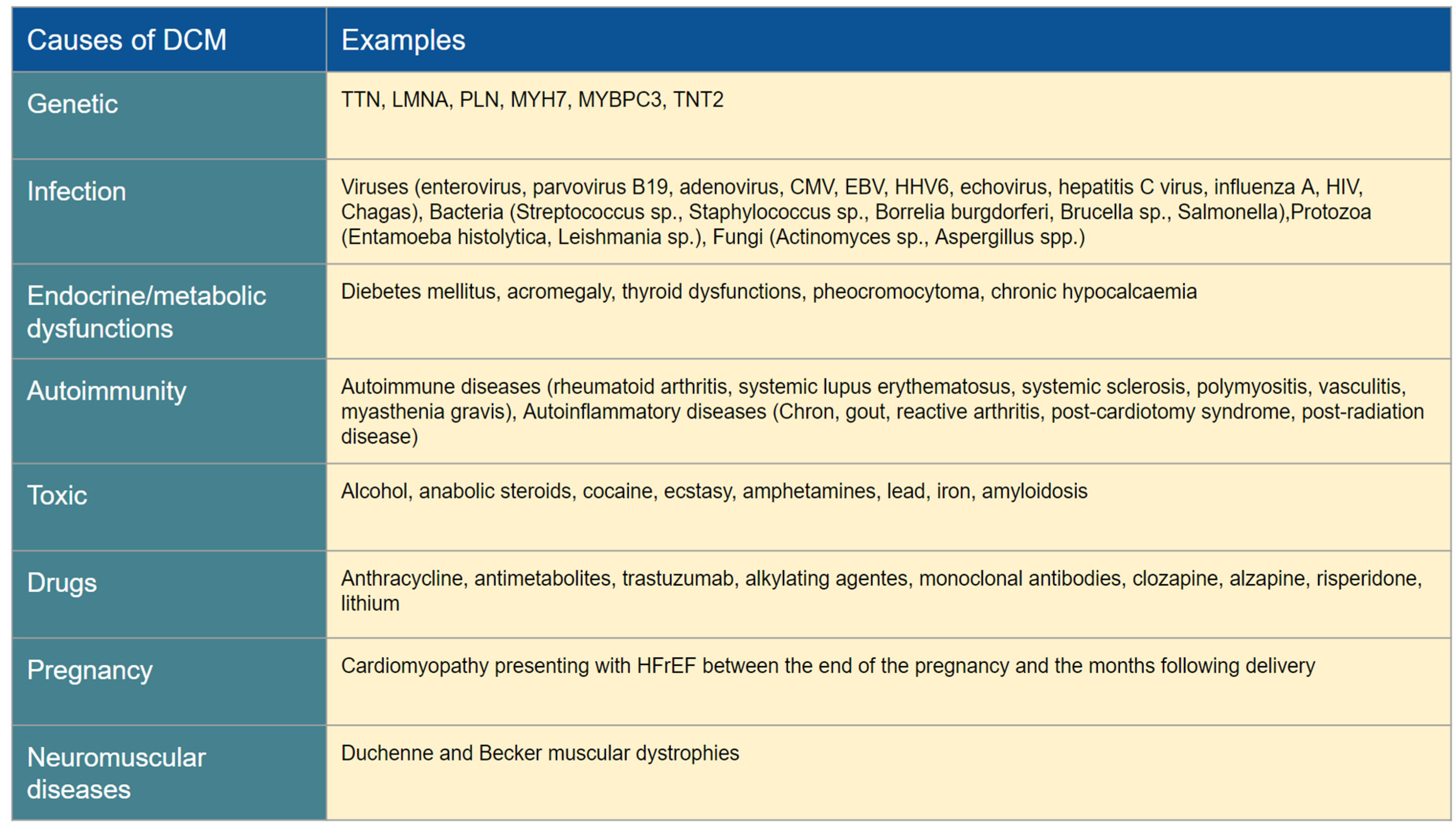
DCM is characterized by a multifaceted phenotype influenced by a diverse range of etiological factors, both genetic and acquired, which often coexist rather than be mutually exclusive [
2]. These factors collectively contribute to the development of typical morphological changes associated with DCM, such as enlarged atria and ventricles, reduced ventricular wall thickness, and functional mitral and tricuspid regurgitations [
3]. Before classifying DCM as "idiopathic", it is essential to perform a thorough investigation to rule out possible secondary causes, as described in
Table 2.
4.1. Acquired
4.1.1. Infection
Infectious agents trigger an inflammatory response, leading to the activation of cytokines and an exacerbated immunologic response, culminating in ventricular dilation and dysfunction [
13]. Approximately 50% of cases of DCM show evidence of myocardial inflammation, which can be caused by infections, autoimmune diseases, and toxic agents [
14]. Animal studies have demonstrated a three-phase inflammatory response in heart muscle following a viral infection. Initially, there is a direct cytotoxic effect, followed by immune activation involving both innate and acquired immunity. In the subacute phase, the mechanisms of molecular mimicry guided by T lymphocytes and autoantibodies predominate [
3]. The most common viruses associated with myocarditis are enteroviruses (such as Coxsackievirus B2, accounting for up to 50% of cases), parvovirus B19, adenoviruses, herpesviruses, and Epstein-Barr virus [
4]. Additionally, human immunodeficiency virus (HIV) is known to be related to DCM, as this phenotype is frequently found in HIV-positive patients [
4]. With specific reference to the SARS-CoV-2 virus, multiple cardiac manifestations have been described, such as elevations in troponin and B-type natriuretic peptide (BNP), as well as documented edema and/or fibrosis in cardiac magnetic resonance (CMR) imaging [
15]. Myocardial inflammation in COVID-19 may increase the risk of heart failure and progression to DCM [
16]. In Chagas disease, the parasitic infestation leads to diffuse fibrosis, microcirculatory damage, and autoimmune mechanisms [
3].
4.1.2. Immune-mediated diseases
The core of the myocardial damage in these cases is the formation of autoantibodies and immune complexes, associated with a genetic background, leading to fibrosis and the development of DCM [
3].
4.1.3. Drugs and toxins
Agents such as ethanol, chemotherapy drugs, and other toxins can lead to myocardial damage and left ventricular (LV) dysfunction through several mechanisms, including neurohormonal activation, altered calcium homeostasis, oxidative stress, and direct cytotoxicity [
1,
3]. Some agents cause acute damage, while others may take years of exposure to induce myocardial dysfunction (e.g., anthracyclines) [
17]. The potential for recovery is also determined by the specific agent; for example, alcohol-related damage may be reversible, while anthracycline toxicity is believed to be irreversible [
17].
4.1.4. Peripartum
This is a rare and potentially lethal cause that develops from the last trimester of gestation to the first months following delivery [
18]. It is associated with multiple factors, including African descent, older age, multiparity, hypertension, preeclampsia, obesity, and genetic factors (pathogenic variants in genes such as titin (TTN) and myosin binding protein C3 (MYBPC3) [
19]. Together, these factors result in excessive oxidative stress, activation of cathepsin D, and the formation of a prolactin fragment with direct cardiotoxic properties [
3].
4.2. Genetic
The field of precision medicine has been rapidly expanding in recent years, shedding light on the etiological evaluation of DCM. This progress has rendered the term "idiopathic DCM" less frequent, while also providing valuable clinical insights for family screening, management, and prognosis.
DCM is known for its genetic heterogeneity, with many described inheritance patterns [
20]. Over 50 genes have been associated with DCM, although the significance of some associations remains uncertain [
21]. Moreover, it is not uncommon to observe more than one pathogenic variant related to the same specific type of DCM, and conversely, a single pathogenic variant may be related to multiple phenotypes of cardiomyopathy and/or channelopathy [
22,
23].
Familial DCM is defined when two or more family members meet the criteria for DCM, or when the proband meets the criteria for DCM and has a first-degree relative who experienced sudden cardiac death before the age of 35 years [
24]. DCM is recognized as familial in 20-30% of cases [
25]. Genetic test yield ranges from 15 to 25% in unselected patients with DCM to 20 to 40% in familial DCM [
24,
26]. The yield is higher in syndromic DCM, especially in patients with muscular dystrophies, conduction defects, and hearing loss [
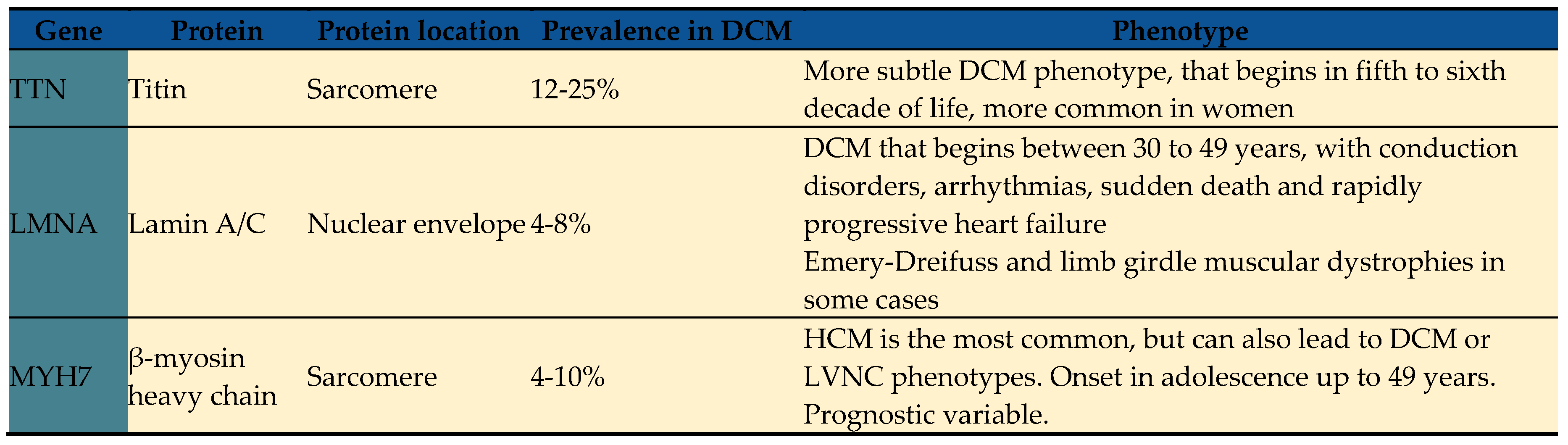
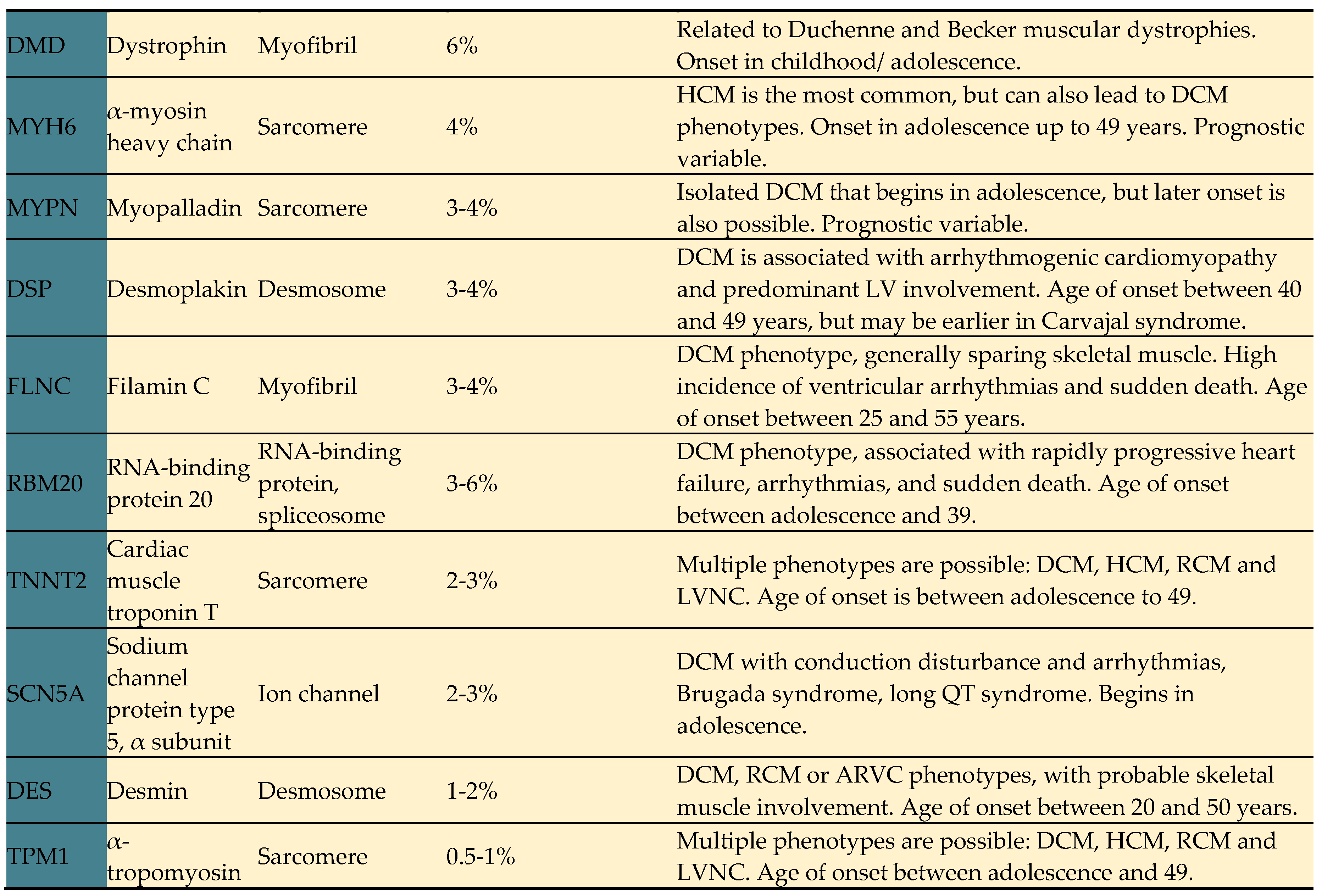
24]. DCM-associated genes are related to several biological pathways, including sarcomere components, cytoskeletal and desmosomal proteins, mitochondrial proteins, and ion channels amongst others. Key genes strongly associated with DCM are summarized in
Table 3 [
27].
Certain red flags in the family history of the proband should prompt further genetic investigation, including cases of sudden cardiac death, unexplained syncope, seizures, heart transplantation, pacemaker, or implantable cardioverter-defibrillator placement in a relative aged <60 years, myopathy, drowning of an experienced swimmer, and unexplained motor vehicle accidents [
24].
Pathogenic variants implicated in DCM can be grouped according to their cellular and molecular involvement in genes encoding cytoskeletal, sarcomeric, desmosomal, nuclear membrane and RNA binding proteins.
4.2.1. Sarcomeric genes
These genes code for sarcomeric proteins involved in myocardial contractility and cellular anchoring. The main representatives are TTN, myosin, actin, troponin, and tropomyosin [
1]. Pathogenic variants in these genes are the most frequent genetic cause of DCM, but they can also manifest as hypertrophic and other phenotypes [
1,
24]. TTN, the largest sarcomeric protein in the myocardium, modulates contraction and myocardial rigidity [
5]. Most TTN pathogenic variants are considered truncating, while some data suggest that missense variants may be less harmful [
28]. Variants located in the A band have a higher probability of causing disease [
29], whereas those in the I band have a lesser pathogenic potential [
29].
TTN variants are estimated to be responsible for 12-28% of sporadic DCM and up to 25% of familial DCM cases, inherited in an autosomal-dominant pattern [
24,
30]. Patients with TTN cardiomyopathy typically present clinical symptoms in the fifth to sixth decade of life and have a similar prognosis to idiopathic DCM [
24,
31]. However, compared to other genetic causes of DCM, TTN-associated DCM appears to have a more subtle disease presentation [
32]. Recent data also reveal that TTN variants are associated with DCM previously related only to toxins, indicating the integration of genetic and environmental factors in this phenotype's development [
33]. For example, TTN variants are found in 10% of women with peripartum DCM [
34].
Classically linked to hypertrophic cardiomyopathy (HCM), MYBPC3, and myosin heavy chain 7 (MYH7) variants also contribute to DCM [
24]. Other sarcomeric variants, such as myosin heavy chain 6 (MYH6), troponin T2 cardiac type (TNNT2), troponin I3 cardiac type (TNNI3), tropomyosin alpha-1 chain (TPM1), myosin light chain-2 (MYL2), myosin light chain-3 (MYL3), actin alpha cardiac muscle 1 (ACTC1), actinin alpha 2 (ACTN2), ankyrin repeat domain containing (ANKRD), cysteine and glycine rich protein 3 (CSRP3), LIM domain binding 3 (LDB3), myozenin-2 (MYOZ2), titin-Cap (TCAP), vinculin (VCL), and myopalladin (MYPN), have been described in DCM [
24].
4.2.2. Laminopathies
Lamin A and lamin C isoforms of the gene LMNA are a nuclear envelope protein that supports cellular integrity [
24]. Pathogenic variants in LMNA are found in up to 8% of DCM cases and have an autosomal dominant inheritance pattern [
24,
35]. Depending on the variant's location within the LMNA gene, it can lead to Emery-Dreifuss and limb-girdle muscular dystrophies, characterized by cardiac manifestations and progressive skeletal muscle wasting [
24]. Patients with DCM and LMNA variants often exhibit conduction disorders, atrial and ventricular arrhythmias, and rapid progression to end-stage heart failure requiring advanced therapies [
36]. Sudden cardiac death is also prevalent in these patients, even before ventricular dysfunction develops, with a mortality rate of up to 46% compared to controls [
37,
38]. Therefore, recent guidelines have become more permissive in recommending implantable cardioverter-defibrillator (ICD) placement in patients with DCM and LMNA variants, with specific risk factors for sudden cardiac death [
39].
4.2.3. Myofibrillar genes
This group of genes codes for intermediate filaments, which are essential for myofibril structural integrity, force transmission, and cellular signaling [
24]. Key cytoskeleton proteins include filamins, dystrophin, and desmin [
1]. Pathogenic variants in these genes may not only cause DCM but also lead to muscle dystrophies [
1].
Pathogenic variants in desmin (DES) are inherited in an autosomal-dominant manner and may result in both skeletal and cardiac myopathies, sometimes overlapping, with cardiac involvement manifesting as dilated or restrictive cardiomyopathy and/or arrhythmogenic phenotypes [
40].
Dystrophin (DMD) pathogenic variants, which have X-linked inheritance, are also associated with skeletal muscle dystrophies (Duchenne and Becker) and have a high frequency of cardiac involvement [
41]. Truncating variants in the filamin C gene (FLNC) cause a cardiac-specific phenotype, sparing the skeletal muscle [
24]. FLNC variants are responsible for 3 to 4% of DCM cases and are associated with a broad spectrum of DCM phenotypes, often with a high incidence of ventricular arrhythmias, even without overt ventricular remodeling, increasing the risk of sudden death [
42]. Therefore, ICD should be considered as primary prophylaxis in patients with FLNC variants, especially if other risk factors are present (e.g., non-sustained ventricular tachycardia [NSVT], LVEF <45%) [
43].
Variants within the BAG3 gene are also implicated in the progression of DCM and severe muscular dystrophy in children [
44,
45].
4.2.4. Desmosomal genes
Desmosomes are proteins that constitute intercalated discs, responsible for intercellular signaling and mechanical functions [
24]. Pathogenic variants in desmosome coding genes have been described in both arrhythmogenic cardiomyopathies and DCM [
46]. Approximately 13% of DCM cases are associated with desmosome mutations [
46]. The most prevalent pathogenic variants causing DCM are in desmoplakin (DSP) and plakophilin 2 (PKP2) desmosomal genes [
24]. The Carvajal syndrome, related to a homozygous or compound heterozygous desmoplakin pathogenic variant, is associated with a rapidly progressing form of DCM, heart failure onset during adolescence, and sudden cardiac death [
47]. Truncating variants in desmoplakin have strong evidence of pathogenicity and represent 15% of inherited arrhythmogenic cardiomyopathies, with a predominant LV involvement [
48,
49].
4.2.5. Other genes
Pathogenic variants in the RNA binding motif protein 20 (RBM20) gene, which regulates splicing of titin and other genes, are responsible for 3 to 6% of DCM cases, leading to rapidly progressive advanced heart failure, atrial and ventricular arrhythmias like LMNA-associated DCM, and sudden death [
50,
51]. However, further data is needed to guide prognostic stratification for these patients [
5]. Tafazzin (TAZ) gene variants are also related to the DCM phenotype, ranging from a milder to more severe disease. The TAZ gene encodes the Taz1p acyltransferase involved in the metabolism of cardiolipin, an important phospholipid in the inner membranes of mitochondria. Barth syndrome is a disorder that is caused by mutations in TAZ gene, leading to disturbance in phospholipid metabolism which results in DCM, neutropenia, skeletal myopathy, growth deficiency and lactic acidosis. [
52]. Additionally, variants in sodium voltage-gated channel alpha subunit 5 (SCN5A), a gene associated with cardiac sodium channels, may not only cause Brugada and long QT syndromes, but also the DCM phenotype, conduction disturbances, and arrhythmias [
53].
Researchers and clinicians can better understand the complex genetic landscape underlying DCM and potentially develop targeted therapies or risk stratification strategies for affected individuals by grouping pathogenic variants according to their cellular and molecular involvement.
5. Diagnostic Evaluation
The diagnosis of DCM can be established based on clinical symptoms or incidentally discovered during routine examinations. Additionally, family screening may identify affected individuals after diagnosing a proband within the family. The clinical presentation of DCM ranges from isolated arrhythmic events to fully developed signs and symptoms of HF [
54,
55].
Typically, DCM is diagnosed in individuals between 20 and 50 years of age [
55]. Diagnostic evaluation involves a complete history and physical examination, and a variety of tests, including electrocardiography (ECG), Holter monitoring, echocardiography, CMR, genetic testing, and, when necessary, endomyocardial biopsy (EMB).
5.1. History and Physical Examination
DCM patients often present with symptoms of heart failure, such as dyspnea, orthopnea, paroxysmal nocturnal dyspnea (PND), bendopnea, and edema. Classical physical findings may include a third heart sound, rales, jugular venous distension, abdominojugular reflux, and edema [
56]. Although certain physical examination findings may suggest underlying systolic dysfunction and a possible dilated phenotype, they are not routinely used in clinical practice because of their limited accuracy in targeting treatment. Instead, more accessible tests are employed to better understand the disease.
Clinical findings should be used in a probabilistic manner, with pretest estimations and proper modification of probabilities based on the power and reliability of the clinical findings. The Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness (ESCAPE) trial showed that orthopnea presents good sensitivity (86%), but low specificity (25%), while rales greater than 1/3 of the chest had low sensitivity (15%), but high specificity (89%) [
57]. Similarly, PND had the highest positive likelihood ratio (LR) for HF (2.6) in patients presenting with dyspnea. Regarding physical findings, third heart sound, abdominojugular reflux and jugular venous distension had the highest LR (11, 6.4 and 5.1, respectively) [
58].
The clinical evaluation also plays a role in suspecting an underlying genetic cause of DCM. "Red flags" such as mental disorders, deafness, visual impairment, ataxia, muscular weakness, and certain dermatologic conditions (
café-au-lait spots, pigmentation, and palmoplantar keratoderma) may raise suspicion of genetic involvement [
58]. A detailed three-generation family history, including inquiries about possible sudden cardiac deaths and cardiac diseases, can provide valuable insights [
54,
56,
59,
60].
5.2. Electrocardiogram (ECG)
ECG is a powerful diagnostic tool that can show changes that precede the clinical manifestations of cardiomyopathy, as well as provide information on etiology and prognosis. It is often nonspecific, showing typical findings of left ventricular hypertrophy and branch blocks [
61,
62]. Abnormal Q waves and T wave inversions may also be found, but are less common [
62]. Interestingly, a red flag approach can also be useful with the help of ECG, searching for typical signs of genetic causes. Sinus node dysfunction and AV blocks may raise suspicion for LMNA variants [
59,
62]. A short PR interval has been reported to be present in 35% of patients with DMC variants. On the other hand, low voltages have been described in patients with FLNC and PLN variants [
62]. Nongenetic causes may also manifest typical ECG abnormalities. Patients with Chagas disease often have right bundle branch block and left anterior fascicular block [
63]. Tachycardia-induced DCM may present with atrial flutter or atrial fibrillation on the ECG [
62]. ECG may also show prognostic factors, and T wave alternans and a fragmented QRS seem to be predictors of arrhythmic events [
64].
5.3. Holter Monitoring
Holter monitoring is essential for assessing the presence and burden of ventricular and atrial arrhythmias, which can be more common in some DCM phenotypes, such as FLNC variants [
62]. Conduction disturbances, including atrioventricular (AV) block and sinus node disease, may also be evaluated, and they can correlate with the genetic background [
59,
62]. Moreover, Holter monitoring can help detect tachycardia-induced DCM in patients with frequent premature ventricular beats [
65].
5.4. Echocardiography
Echocardiography is crucial for detecting and quantifying the DCM phenotype and evaluating other structural diseases. DCM patients typically have dilated chambers with normal wall thickness and reduced LVEF [
66]. Biventricular dysfunction may also be present in some cases. Although regional abnormalities of ventricular wall movement may raise the suspicion of CAD, they may also occur in other conditions, such as Chagas disease and myocarditis [
61,
63]. Advanced techniques, such as global longitudinal strain (GLS) of LV and speckle tracking strain (STS), have been increasingly used for early detection of ventricular dysfunction, especially in family screening and in patients with normal LVEF [
67].
Verdonschot et al. evaluated the prevalence of systolic dysfunction using global longitudinal strain (GLS) and its prognostic value in relatives of DCM. Relatives of DCM patients had a significantly higher prevalence of systolic dysfunction detected by GLS despite normal LVEF compared with control subjects. Abnormal GLS was associated with LVEF deterioration, cardiac hospitalization, and death [
68]. Additionally, Raafs et al. also demonstrated that GLS is an independent and incremental predictor of adverse outcome, which exceeded LVEF in patients with optimally treated DCM [
69].
5.5. Cardiac Magnetic Resonance Imaging (CMR)
CMR is increasingly incorporated in the evaluation of cardiomyopathies. Useful information from this method includes the presence and pattern of fibrosis (late gadolinium enhancement - LGE), presence of edema and inflammation and extracellular volume quantification [
70]. In addition, CMR may aid in detecting specific etiologies, such as iron deposits [
71]. Peterson et al. showed no difference in diagnostic yield for specific etiologies in patients with nonischemic cardiomyopathy [
72]. However, it is important to note that the selective group had a relatively high incidence of CMR use (24%), as in this group of patients they should have CMR performed if infiltrative disease, ARVC, pericardial adult congenital heart disease was suspected. or pericardial, leaving the final decision at the discretion of the attending physician. Although a negative study, in an as-treated analysis, more specific diagnoses were found in the routine CMR group.
5.6. Genetic Testing
Knowledge the genetic basis of DCM is crucial for phenotypic manifestation, counseling, and follow-up. Genetic testing may involve a broad panel of culprit variants, or a targeted search based on clinical suspicion [
59]. The interpretation of results is complex, as variants can be classified as pathogenic, likely pathogenic, variants of uncertain significance, likely benign, or benign. Variant classification may change over time with new evidence [
73].
The European Society of Cardiology (ESC) Working Group on Myocardial and Pericardial diseases outlined indications for genetic testing that include patients with a confirmed diagnosis of DCM with therapeutic, family counseling and prognostic implications. Red flags described earlier may further prompt genetic testing. Additionally, relatives of affected individuals with a known disease-causing variant may be offered genetic testing for counseling, preparticipation evaluation, and individualized follow-up [
74,
75]. However, genetic testing may have negative consequences, and proper counseling by an expert team is essential [
74].
5.7. Whole Exome Sequencing
Recent studies have shown that whole exome sequencing (WES) has a high diagnostic yield in achieving a molecular diagnosis of rare Mendelian disorders [
76,
77]. Ramchand et al. showed that WES is an effective diagnostic tool for patients with dilated cardiomyopathy. Using WES with stringent criteria for classifying genomic variants, the authors identified pathogenic or likely pathogenic variants were in 12% of the study population [
78]. D'Agostino et al. identified 70 pathogenic or likely pathogenic variants and 1240 variants of uncertain clinical significance through WES performed on 15 patients affected by DCM [
79]. However, WES is not effective for all DCM variants. Mak et al. showed that the diagnostic yield of WES for DCM was no better than existing commercial gene panels [
80].
5.8. Endomyocardial Biopsy (EMB)
EMB is reserved for severe or rapidly progressing cases of DCM and situations where other diagnostic methods have not confirmed a specific diagnosis, potentially affecting management decisions [
61,
81,
82,
83]. Specific indications for EMB according to guidelines on heart failure include suspected conditions with specific treatments, such as giant cell or eosinophilic myocarditis, sarcoidosis, and certain storage diseases [
61]. Proper histologic, immunohistochemistry evaluations, and cardiotropic virus search should be conducted for accurate diagnosis.
6. Prognosis prediction
Data on the long-term mortality and associated prognostic factors in patients with DCM are scarce. Xu et al. demonstrated that the 15-year survival rate in DCM patients is 34% [
84]. The independent predictors of 15-year all-cause mortality were age ≥70 years, LVEF ≤35%, and systolic blood pressure >120 mmHg [
84].
Clinical prediction models allow estimating the prognosis of patients with DCM. The Seattle Heart Failure Model (SHFM) [
85], the Meta-Analysis Global Group in Chronic (MAGGIC) risk score [
86], and the Barcelona bio-HF risk calculator [
87] allow estimating the risk of death in patients with DCM. Other scores, such as The Madrid Genotype Score, allow predicting the probability for a positive genetic test result in DCM patients, with a C-statistic in the external validation cohort of 0.74 (95% CI: 0.71-0.78) [
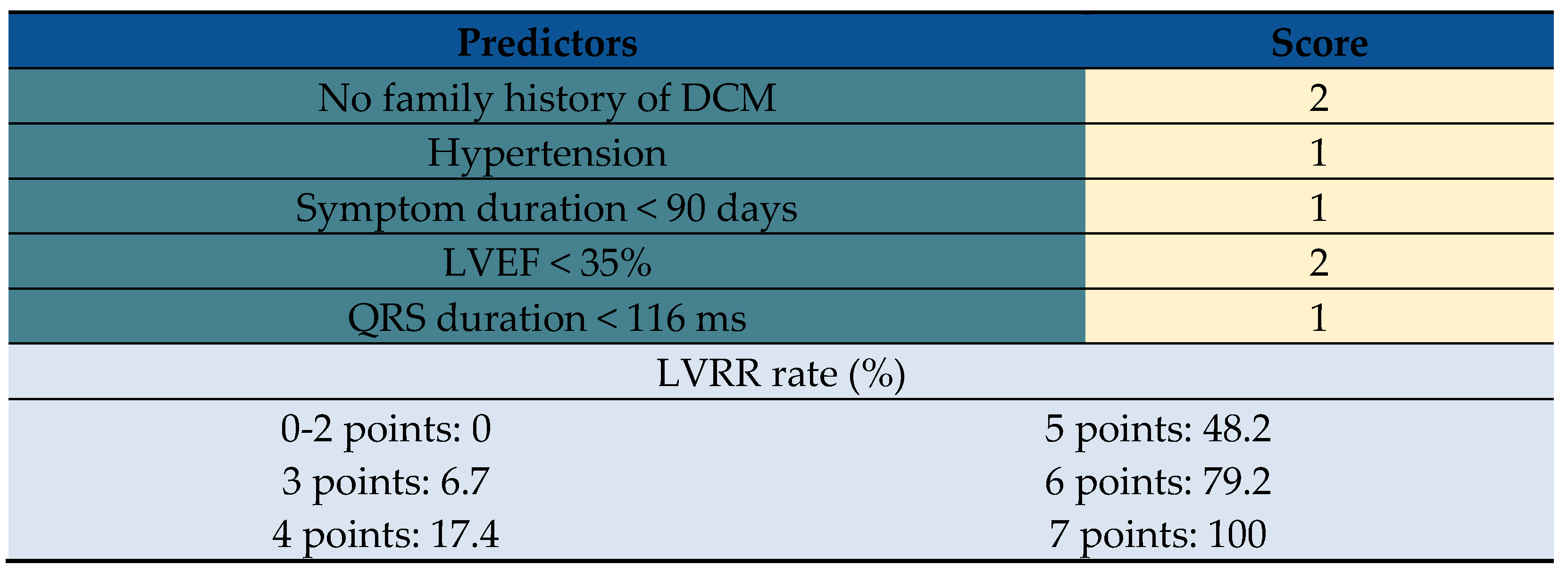
88]. In turn, the Left Ventricular Reverse remodeling (LVRR) predicting score has been used to predict LVRR in patients with DCM [
89]. Predictors including hypertension, no family history of DCM, symptom duration <90 days, LVEF <35%, and QRS duration <116 ms and the score of >5 is a reliable predictor independently from LGE on CMR or myocardial fibrosis (
Table 4).
Patients with DCM are at high risk for life-threatening ventricular arrhythmias (LTVA). The risk is higher in younger patients with hypertension, prior (non-)sustained ventricular arrhythmia, decreased left ventricular ejection fraction, left ventricular dilatation, late gadolinium enhancement, and genetic mutations (PLN, LMNA, and FLNC) [
90].
The DCM-SVA score allows predicting life-threatening arrhythmia in DCM patients. The score can improve decision making in primary prevention of sudden cardiac death and significantly reduce ICD implantations [
91].
However, a critical view of risk scores in patients with DCM should be considered, since patients with DCM are typically younger than other heart failure etiologies and, therefore, are significantly less burdened with comorbidity. Furthermore, a substantial number of DCM patients (between 30 to 50%) undergo LVRR, which critically improves prognosis, and DCM patients respond better to CRT than ischemic HF patients.
7. Management
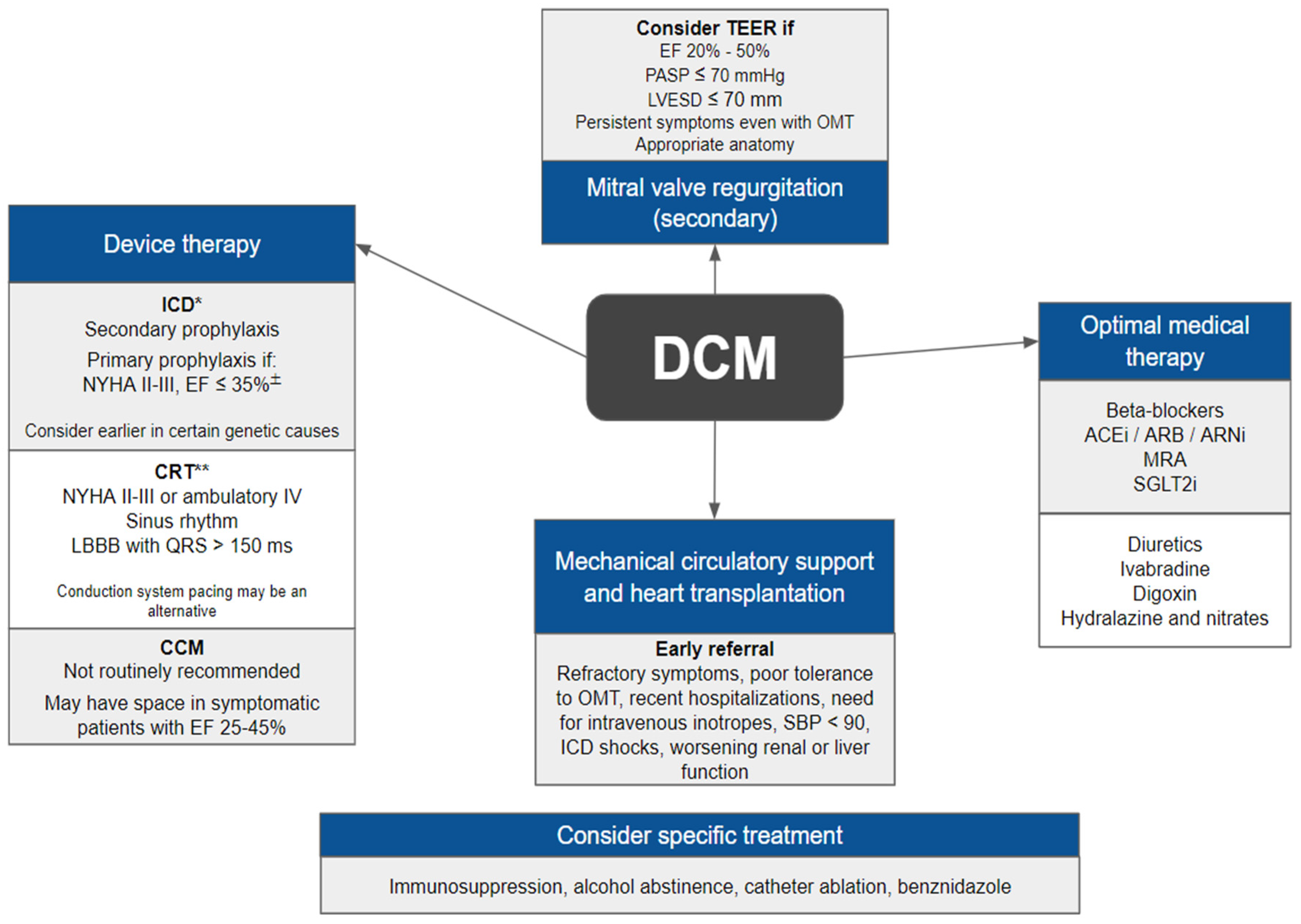
The management of patients with DCM should be aligned with current guidelines for heart failure, which includes non-pharmacological measures, optimized drug therapy and, when indicated, implantation of devices, structural interventions, and heart transplantation (
Figure 1).
Pharmacologic therapy remains the cornerstone of disease-modifying treatments. While this review will address established pharmacologic treatments, the focus will be on emerging interventions and specific therapies for genetic causes of DCM.
7.1. Medical Treatment
The current optimal medical therapy for DCM includes beta-blockers, angiotensin receptor-neprilysin inhibitors/angiotensin-converting enzyme inhibitors/angiotensin receptor blockers, mineralocorticoid receptor antagonists, and sodium-glucose cotransporter-2 inhibitors. Additionally, diuretics, ivabradine, digoxin, hydralazine, and nitrates may be used. Disease-modifying drugs should be initiated and gradually titrated to their maximum tolerated doses [
61].
7.2. Device Therapy
Implantable cardiac devices play a crucial role in the management of different phenotypes of heart failure by treating life-threatening arrhythmias and improving morbidity and survival. There are different types of devices, each with a slightly different function. They fall into three categories: implantable cardioverter defibrillator (ICD), pacemakers, cardiac resynchronisation therapy (CRT), cardiac contractility modulation (CCM), and ventricular assist devices (VADs).
7.2.1. Implantable cardioverter defibrillator
ICDs are generally recommended for patients who have experienced an aborted sudden cardiac death or poorly tolerated ventricular tachycardia (VT), provided there are no reversible causes and a life expectancy of over one year [
75,
92]. They are also indicated as primary prophylaxis in high-risk individuals, particularly symptomatic patients (New York Heart Association [NYHA] II-III) with an EF ≤35% despite three months of optimal medical therapy [
61,
75,
92]. Furthermore, ICD should be considered in DCM patients with sustained monomorphic VT, even if well tolerated [
92]. The field of precision medicine has led to specific ICD indications for genetic-related DCM. The 2022 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death have recommended ICD for DCM patients with a 5-year estimated risk ≥10% and a manifest cardiac phenotype (NSVT, LVEF <50%, or atrioventricular [AV] conduction delay) [
92]. The 2017 American Heart Association (AHA) guidelines also have suggested ICD for primary prevention in patients with specific genetic mutations [
39]. Similarly, the 2019 The Heart Rhythm Society expert consensus has recommended lower and specific thresholds for ICD indication for primary prophylaxis in patients with certain genetic mutations [
75].
7.2.2. Cardiac resynchronization therapy
In patients with heart failure and a low LVEF, resynchronization therapy may be indicated to reduce mortality and morbidity [
61,
92]. Traditional candidates for CRT are those symptomatic (NYHA II-III or ambulatory NYHA IV), in sinus rhythm, with a QRS >150 ms and a left bundle branch block pattern. However, patients not fulfilling all these criteria might also be candidates, with a lower grade of recommendation [
61,
92]. Patients with ventricular dysfunction who require a permanent pacemaker usually receive CRT to prevent pacemaker-induced dyssynchrony [
61]. Typically, CRT is achieved by pacing both the right and the left ventricle. However, new stimulation techniques have been developed and will possibly become better CRT alternatives, the so-called conduction system pacing (CSP). CSP consists of a “physiologic” stimulation of either the His bundle or the left bundle branch area [
93,
94]. His bundle pacing (HBP) is not as consolidated as biventricular pacing for CRT; therefore, guidelines still recommend it as an alternative [
92,
94]. Nevertheless, some data point to a promising future for CSP. Although there are no randomized controlled clinical trials (RCTs) comparing CSP with biventricular pacing, a recent observational study compared the results of patients with indication of CRT undergoing biventricular pacing or HBP [
93]. The study showed that patients undergoing HBP had a reduction in the primary outcome of death or hospitalization for HF, in addition to achieving narrower QRS durations.
7.2.3. Cardiac contractility modulation
This innovative device employs high-energy delivery to the right ventricular septal wall during the absolute refractory period, leading to functional improvement through metabolic modulation of the heart [
95]. The Evaluate Safety and Efficacy of the OPTIMIZER System in Subjects with Moderate-to-Severe Heart Failure (FIX-HF-5C) trial demonstrated improved functional measures and reduced cardiovascular death or heart failure-related hospitalizations in symptomatic patients with an LVEF between 25% and 45% and a QRS duration <130 ms [
96]. Although CCM is not routinely recommended in heart failure guidelines due to insufficient evidence, it is a promising device for treating patients with DCM [
61,
92].
7.3. Mitral Valve Regurgitation
Mitral regurgitation (MR) is common in DCM patients and is related with worse outcomes [
97]. Patients with DCM and mitral regurgitation do not necessarily have primary valve disease. MR in these patients is primarily a function of LV geometric changes. In other words, these patients usually have normal valve and subvalvular apparatus, but the papillary muscle displacement and annular dilation generate coaptation failure [
98,
99].
Treatment decisions in these patients are not straightforward, and evidence is conflicting. Patients should always receive optimal medical treatment as usual, and interventional procedures are reserved for specific patients [
98,
99].
Two RCTs evaluated transcatheter mitral valve repair in patients with LV dysfunction and secondary MR. While the Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients with Functional Mitral Regurgitation (COAPT) trial reported significant reductions in hospitalizations for HF and all-cause mortality in 24 months [
100], the Percutaneous Repair with the MitraClip Device for Severe Functional/Secondary Mitral Regurgitation (MITRA-FR) trial showed no significant difference between groups [
101]. The differences may be attributed to patient characteristics, including LV volumes and severity of MR, highlighting the importance of careful patient selection for transcatheter edge-to-edge repair (TEER) [
98,
99].
Guidelines provide further guidance on how to proceed in such challenging situations. The 2020 AHA valvular diseases guidelines recommend transcatheter edge-to-edge repair (TEER) in patients with an LVEF between 20% and 50%, a LV end-systolic dimension ≤70 mm, a pulmonary artery systolic pressure ≤70 mmHg and an anatomy suitable for the procedure (evaluated with the aid of a transesophageal echocardiogram) [
98]. The ESC guidelines also recommend TEER in symptomatic patients not eligible for surgery and with predictors of good response [
99].
Valve replacement surgery is not usually indicated in this scenario, but it is an option in patients with a progressive increase in LV size or decrease in LVEF, after evaluation by the Heart Team [
98,
99].
7.4. Etiology-Driven Treatment
Patients with specific causes of DCM may benefit from etiology-guided treatments, such as immunosuppressants or antiprotozoals.
Certain inflammatory conditions, including giant cell myocarditis, eosinophilic myocarditis, immune checkpoint inhibitor-associated myocarditis, cardiac sarcoidosis, and rheumatic carditis, may warrant immunosuppressive therapy [
102,
103]. Research indicates that immunosuppressants could improve LV function in certain patients with active lymphocytic myocarditis [
104].
Virus-targeted drugs are not indicated in the usual treatment for DCM, but there has been some interest in their potential role in virus-associated cardiomyopathy. The Betaferon in chronic viral cardiomyopathy (BICC) study evaluated the role of interferon-β in patients with heart failure and viral presence in the myocardium confirmed by EMB [
105]. This study randomized 143 patients and compared interferon-β to placebo. Patients in the treatment group had better NYHA functional class and quality of life measures and treatment was well-tolerated. However, this treatment is not yet addressed in current guidelines, likely due to the paucity of evidence.
Infectious causes of DCM, such as Chagas disease, might also require tailored treatments. Benznidazole has been suggested for specific scenarios, although the evidence from a relevant RCT remains inconclusive [
63,
106].
Patients with tachycardiomyopathy may benefit from catheter ablation, which could lead to the recovery of cardiac function [
61,
65].
Lastly, patients with toxic-associated DCM should have their exposures controlled. For example, patients with alcohol-induced DCM should abstain from alcohol and cardiotoxic chemotherapy should be reviewed and discussed with the oncologist [
61].
7.5. Gene therapy
Gene therapy is the insertion of a gene into a human cell to treat a disease. The usual delivery system involves vectors that are often of viral origin, such as retroviruses, adenoviruses, adeno-associated viruses. Several potential gene therapy targets in patients with HF have been identified from experiments using transgenic mouse models. These targets include sarcoplasmic/endoplasmic reticulum Ca2+ ATPase 2a (SERCA2a), phospholamban, G protein-coupled receptor kinase 2, protein phosphatase 1, adenylate cyclase, among others.
Three different gene therapy strategies are currently used: 1) Gene replacement therapy (GRT), in which a wild-type gene is expressed by a promoter within a viral vector to replace gene function in the setting of a gene-loss variant. function, 2) Gene silencing therapy, commonly used to reduce the expression of a mutated gene and applies primarily to nonsense variants (i.e., single nucleotide substitutions) that alter protein function, 3) Direct genome editing, using CRISPR-Cas9 (biotechnological genomic editing tools), a targeted DNA cleavage can be directed to a precise location in the genome determined by the unique sequence of a guide RNA [
107].
7.6. Induced pluripotent stem cells
The advent of induced pluripotent stem cells (iPSCs) provides the development of disease-specific cellular models and, with that, the investigation of underlying mechanisms and optimization of therapy. Sun et al. showed that cardiomyocytes derived from iPSCs from DCM patients exhibited altered regulation of calcium ion (Ca
2+), when compared to control healthy individuals in the same family cohort, decreased contractility, and abnormal distribution of sarcomeric α-actinin when stimulated with a β-adrenergic agonist [
108].
Lee et al. demonstrated that the effect of PTC124, also known as ataluren (an acid which promotes the readthrough of premature but not normal termination codons in HEK293 cells), is codon selective [
109]. The authors used cardiomyocytes derived from human induced pluripotent stem cells carrying different LMNA mutations in patients with Lamin A/C (LMNA)-related cardiomyopathy to confirm your findings.
However, human iPSC-derived cardiomyocytes have some limitations, such as 1) Genomic instability; 2) Heterogeneity of iPSC-derived cardiomyocytes; 3) Cellular, molecular, and functional differences of adult ventricular cardiomyocytes and iPSC-derived cardiomyocytes [
110].
7.7. Mechanical Circulatory Support and Heart Transplantation
Patients with advanced heart failure due to DCM should be referred to specialized centers capable of providing appropriate treatment. Mechanical circulatory support and/or heart transplantation can offer improved survival and quality of life [
111,
112,
113].
Guideline-based thresholds for referral include refractory symptoms, poor tolerance for optimal medical therapy, recent hospitalizations, need for intravenous inotropes, low systolic blood pressure (<90 mmHg), high heart rate, and receipt of ICD shocks, among other criteria [
61,
93].
8. Conclusions
DCM is a complex and multi-faceted condition. Advances in genetic research, optimal medical treatments, and the development of innovative device-based techniques have significantly expanded our diagnostic and management capabilities for patients with DCM. These advancements have brought about a wealth of resources to better understand and address the challenges posed by this condition. As we continue to enhance our understanding and refine our approaches, we can look forward to improved outcomes and a better quality of life for individuals affected by DCM.
9. Future Directions
A significant knowledge gap persists regarding pathophysiology, diagnosis, and management of DCM patients. Therefore, future research should focus on these two domains: (1) The development and improvement of sequencing technology to define the DCM genome will improve the diagnosis, prevention, and therapy of DCM; (2) A better understanding of genetic testing could lead to the correct treatment of asymptomatic genetic DCM carriers and the development of specific gene therapies.
Author Contributions
Conceptualization, V.C.M., L.L.H.d.O. and T.L.S.; methodology, V.C.M., V.M.J., L.L.H.d.O. and T.L.S.; software, L.M.A.d.C., V.C.M. and L.L.H.d.O.; validation, R.G.R., J.A.G.O.L., L.M.A.d.C., F.F., J.E.K. and P.R.S.; formal analysis, L.M.A.d.C. and T.L.S.; writing—original draft preparation, V.C.M., L.L.H.d.O. and T.L.S.; writing—review and editing, L.M.A.d.C. and T.L.S.; visualization, T.L.S.; supervision, T.L.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Orphanou, N.; Papatheodorou, E.; Anastasakis, A. Dilated cardiomyopathy in the era of precision medicine: latest concepts and developments. Heart Fail. Rev. 2022, 27, 1173–1191. [Google Scholar] [CrossRef]
- Sinagra, G.; Elliott, P.M.; Merlo, M. Dilated cardiomyopathy: so many cardiomyopathies! Eur. Heart J. 2020, 41, 3784–3786. [Google Scholar] [CrossRef]
- Seferović, P.M.; Polovina, M.; Bauersachs, J.; Arad, M.; Ben Gal, T.; Lund, L.H.; Felix, S.B.; Arbustini, E.; Caforio, A.L.P.; Farmakis, D.; et al. Heart failure in cardiomyopathies: a position paper from the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2019, 21, 553–576. [Google Scholar] [CrossRef] [PubMed]
- Reichart, D.; Magnussen, C.; Zeller, T.; Blankenberg, S. Dilated cardiomyopathy: from epidemiologic to genetic phenotypes: A translational review of current literature. J. Intern. Med. 2019, 286, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Njoroge, J.N.; Mangena, J.C.; Aribeana, C.; Parikh, V.N. Emerging Genotype-Phenotype Associations in Dilated Cardiomyopathy. Curr. Cardiol. Rep. 2022, 24, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Bridgen, W. Uncommon myocardial diseases; the non-coronary cardiomyopathies. Lancet 1957, 273, 1179–1184. [Google Scholar]
- Goodwin, J.F.; Gordon, H.; Hollman, A.; Bishop, M.B. Clinical aspects of cardiomyopathy. Br. Med. J. 1961, 1, 69–79. [Google Scholar] [CrossRef]
- Report of the WHO/ISFC task force on the definition and classification of cardiomyopathies. Br. Heart J. 1980, 44, 672–673. [CrossRef]
- Richardson, P.; McKenna, W.; Bristow, M.; Maisch, B.; Mautner, B.; O'Connell, J.; Olsen, E.; Thiene, G.; Goodwin, J.; Gyarfas, I.; et al. Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the Definition and Classification of cardiomyopathies. Circulation 1996, 93, 841–842. [Google Scholar]
- Elliott, P.; Andersson, B.; Arbustini, E.; Bilinska, Z.; Cecchi, F.; Charron, P.; Dubourg, O.; Kühl, U.; Maisch, B.; McKenna, W.J.; et al. Classification of the cardiomyopathies: a position statement from the European Society Of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2008, 29, 270–276. [Google Scholar] [CrossRef]
- Elliott, P. Towards a New Classification of Cardiomyopathies. Curr. Cardiol. Rep. 2023, 25, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Arbustini, E.; Narula, N.; Tavazzi, L.; Serio, A.; Grasso, M.; Favalli, V.; Bellazzi, R.; Tajik, J.A.; Bonow, R.O.; Fuster, V.; et al. The MOGE(S) classification of cardiomyopathy for clinicians. J. Am. Coll. Cardiol. 2014, 64, 304–318, Erratum in: Bonow, R.D. J. Am. Coll. Cardiol. 2014, 64(11), 1186. [Google Scholar] [CrossRef] [PubMed]
- Monda, E.; Palmiero, G.; Rubino, M.; Verrillo, F.; Amodio, F.; Di Fraia, F.; Pacileo, R.; Fimiani, F.; Esposito, A.; Cirillo, A.; et al. Molecular Basis of Inflammation in the Pathogenesis of Cardiomyopathies. Int. J. Mol. Sci. 2020, 21, 6462. [Google Scholar] [CrossRef] [PubMed]
- Kühl, U.; Pauschinger, M.; Noutsias, M.; Seeberg, B.; Bock, T.; Lassner, D.; Poller, W.; Kandolf, R.; Schultheiss, H.P. High prevalence of viral genomes and multiple viral infections in the myocardium of adults with "idiopathic" left ventricular dysfunction. Circulation 2005, 111, 887–893. [Google Scholar] [CrossRef]
- Puntmann, V.O.; Carerj, M.L.; Wieters, I.; Fahim, M.; Arendt, C.; Hoffmann, J.; Shchendrygina, A.; Escher, F.; Vasa-Nicotera, M.; Zeiher, A.M.; et al. Outcomes of Cardiovascular Magnetic Resonance Imaging in Patients Recently Recovered From Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 1265–1273, Erratum in: JAMA Cardiol. 2020, 5(11), 1308. [Google Scholar] [CrossRef]
- Arentz, M.; Yim, E.; Klaff, L.; Lokhandwala, S.; Riedo, F.X.; Chong, M.; Lee, M. Characteristics and Outcomes of 21 Critically Ill Patients With COVID-19 in Washington State. JAMA 2020, 323, 1612–1614. [Google Scholar] [CrossRef]
- Volkova, M.; Russell, R. Anthracycline cardiotoxicity: prevalence, pathogenesis and treatment. Curr. Cardiol. Rev. 2012, 7, 214–220. [Google Scholar] [CrossRef]
- Sliwa, K.; Hilfiker-Kleiner, D.; Petrie, M.C.; Mebazaa, A.; Pieske, B.; Buchmann, E.; Regitz-Zagrosek, V.; Schaufelberger, M.; Tavazzi, L.; van Veldhuisen, D.J.; et al.; Heart Failure Association of the European Society of Cardiology Working Group on Peripartum Cardiomyopathy Current state of knowledge on aetiology, diagnosis, management, and therapy of peripartum cardiomyopathy: a position statement from the Heart Failure Association of the European Society of Cardiology Working Group on peripartum cardiomyopathy. Eur. J. Heart Fail. 2010, 12, 767–778. [Google Scholar] [CrossRef]
- European Society of Gynecology (ESG); Association for European Paediatric Cardiology (AEPC); German Society for Gender Medicine (DGesGM); Regitz-Zagrosek, V. ; Blomstrom Lundqvist, C.; Borghi, C.; Cifkova, R.; Ferreira, R.; Foidart, J.M.; Gibbs, J.S.; Gohlke-Baerwolf, C.; Gorenek, B.; et al; ESC Committee for Practice Guidelines. ESC Guidelines on the management of cardiovascular diseases during pregnancy: the Task Force on the Management of Cardiovascular Diseases during Pregnancy of the European Society of Cardiology (ESC). Eur. Heart J. 2011, 32, 3147–3197. [Google Scholar]
- Arbustini, E.; Morbini, P.; Pilotto, A.; Gavazzi, A.; Tavazzi, L. Genetics of idiopathic dilated cardiomyopathy. Herz 2000, 25, 156–160. [Google Scholar] [CrossRef]
- van Spaendonck-Zwarts, K.Y.; van Rijsingen, I.A.; van den Berg, M.P.; Lekanne Deprez, R.H.; Post, J.G.; van Mil, A.M.; Asselbergs, F.W.; Christiaans, I.; van Langen, I.M.; Wilde, A.A.; et al. Genetic analysis in 418 index patients with idiopathic dilated cardiomyopathy: overview of 10 years' experience. Eur. J. Heart Fail. 2013, 15, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Haas, J.; Frese, K.S.; Peil, B.; Kloos, W.; Keller, A.; Nietsch, R.; Feng, Z.; Müller, S.; Kayvanpour, E.; Vogel, B.; et al. Atlas of the clinical genetics of human dilated cardiomyopathy. Eur. Heart J. 2015, 36, 1123–1135a. [Google Scholar] [CrossRef] [PubMed]
- Elliott, P.M.; Anastasakis, A.; Asimaki, A.; Basso, C.; Bauce, B.; Brooke, M.A.; Calkins, H.; Corrado, D.; Duru, F.; Green, K.J.; et al. Definition and treatment of arrhythmogenic cardiomyopathy: an updated expert panel report. Eur. J. Heart Fail. 2019, 21, 955–964. [Google Scholar] [CrossRef]
- Rosenbaum, A.N.; Agre, K.E.; Pereira, N.L. Genetics of dilated cardiomyopathy: practical implications for heart failure management. Nat. Rev. Cardiol. 2020, 17, 286–297. [Google Scholar] [CrossRef] [PubMed]
- Petretta, M.; Pirozzi, F.; Sasso, L.; Paglia, A.; Bonaduce, D. Review and meta-analysis of the frequency of familial dilated cardiomyopathy. Am. J. Cardiol. 2011, 108, 1171–1176. [Google Scholar] [CrossRef]
- Gigli, M.; Merlo, M.; Graw, S.L.; Barbati, G.; Rowland, T.J.; Slavov, D.B.; Stolfo, D.; Haywood, M.E.; Dal Ferro, M.; Altinier, A.; et al. Genetic Risk of Arrhythmic Phenotypes in Patients with Dilated Cardiomyopathy. J. Am. Coll. Cardiol. 2019, 74, 1480–1490. [Google Scholar] [CrossRef]
- Walsh, R.; Thomson, K.L.; Ware, J.S.; Funke, B.H.; Woodley, J.; McGuire, K.J.; Mazzarotto, F.; Blair, E.; Seller, A.; Taylor, J.C.; et al. Reassessment of Mendelian gene pathogenicity using 7,855 cardiomyopathy cases and 60,706 reference samples. Genet. Med. 2017, 19, 192–203. [Google Scholar] [CrossRef]
- Akinrinade, O.; Heliö, T.; Lekanne Deprez, R.H.; Jongbloed, J.D.H.; Boven, L.G.; van den Berg, M.P.; Pinto, Y.M.; Alastalo, T.P.; Myllykangas, S.; Spaendonck-Zwarts, K.V.; et al. Relevance of Titin Missense and Non-Frameshifting Insertions/Deletions Variants in Dilated Cardiomyopathy. Sci. Rep. 2020, 9, 4093, Erratum in: Sci. Rep. 2020, 10(1), 17264. [Google Scholar] [CrossRef]
- Deo, R.C. Alternative splicing, internal promoter, nonsense-mediated decay, or all three: explaining the distribution of truncation variants in titin. Circ. Cardiovasc. Genet. 2016, 9, 419–425. [Google Scholar] [CrossRef]
- Akhtar, M.M.; Lorenzini, M.; Cicerchia, M.; Ochoa, J.P.; Hey, T.M.; Sabater Molina, M.; Restrepo-Cordoba, M.A.; Dal Ferro, M.; Stolfo, D.; Johnson, R.; et al. Clinical Phenotypes and Prognosis of Dilated Cardiomyopathy Caused by Truncating Variants in the TTN Gene. Circ. Heart Fail. 2020, 13, e006832. [Google Scholar] [CrossRef]
- Haggerty, C.M.; Damrauer, S.M.; Levin, M.G.; Birtwell, D.; Carey, D.J.; Golden, A.M.; Hartzel, D.N.; Hu, Y.; Judy, R.; Kelly, M.A.; et al. Genomics-First Evaluation of Heart Disease Associated With Titin-Truncating Variants. Circulation 2019, 140, 42–54. [Google Scholar] [CrossRef]
- Tayal, U.; Newsome, S.; Buchan, R.; Whiffin, N.; Halliday, B.; Lota, A.; Roberts, A.; Baksi, A.J.; Voges, I.; Midwinter, W.; et al. Phenotype and Clinical Outcomes of Titin Cardiomyopathy. J. Am. Coll. Cardiol. 2017, 70, 2264–2274. [Google Scholar] [CrossRef]
- Pirruccello, J.P.; Bick, A.; Chaffin, M.; Aragam, K.G.; Choi, S.H.; Lubitz, S.A.; Ho, C.Y.; Ng, K.; Philippakis, A.; Ellinor, P.T.; et al. Titin Truncating Variants in Adults Without Known Congestive Heart Failure. J. Am. Coll. Cardiol. 2020, 75, 1239–1241. [Google Scholar] [CrossRef]
- Goli, R.; Li, J.; Brandimarto, J.; Levine, L.D.; Riis, V.; McAfee, Q.; DePalma, S.; Haghighi, A.; Seidman, J.G.; Seidman, C.E.; et al.; IMAC-2 and IPAC Investigators; Arany, Z Genetic and Phenotypic Landscape of Peripartum Cardiomyopathy. Circulation 2021, 143, 1852–1862. [Google Scholar] [CrossRef] [PubMed]
- Crasto, S.; My, I.; Di Pasquale, E. The Broad Spectrum of LMNA Cardiac Diseases: From Molecular Mechanisms to Clinical Phenotype. Front. Physiol. 2020, 11, 761. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Baldinger, S.H.; Gandjbakhch, E.; Maury, P.; Sellal, J.M.; Androulakis, A.F.; Waintraub, X.; Charron, P.; Rollin, A.; Richard, P.; et al. Long-Term Arrhythmic and Nonarrhythmic Outcomes of Lamin A/C Mutation Carriers. J. Am. Coll. Cardiol. 2016, 68, 2299–2307. [Google Scholar] [CrossRef] [PubMed]
- van Rijsingen, I.A.; Nannenberg, E.A.; Arbustini, E.; Elliott, P.M.; Mogensen, J.; Hermans-van Ast, J.F.; van der Kooi, A.J.; van Tintelen, J.P.; van den Berg, M.P.; Grasso, M.; et al. Gender-specific differences in major cardiac events and mortality in lamin A/C mutation carriers. Eur. J. Heart Fail. 2013, 15, 376–384. [Google Scholar] [CrossRef] [PubMed]
- van Rijsingen, I.A.; Arbustini, E.; Elliott, P.M.; Mogensen, J.; Hermans-van Ast, J.F.; van der Kooi, A.J.; van Tintelen, J.P.; van den Berg, M.P.; Pilotto, A.; Pasotti, M.; et al. Risk factors for malignant ventricular arrhythmias in lamin a/c mutation carriers a European cohort study. J. Am. Coll. Cardiol. 2012, 59, 493–500. [Google Scholar] [CrossRef]
- Al-Khatib, S.M.; Stevenson, W.G.; Ackerman, M.J.; Bryant, W.J.; Callans, D.J.; Curtis, A.B.; Deal, B.J.; Dickfeld, T.; Field, M.E.; Fonarow, G.C.; et al. 2017 AHA/ACC/HRS Guideline for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation 2018, 138, e210–e271. [Google Scholar]
- Hershberger, R.E.; Hedges, D.J.; Morales, A. Dilated cardiomyopathy: the complexity of a diverse genetic architecture. Nat. Rev. Cardiol. 2013, 10, 531–547. [Google Scholar] [CrossRef]
- Politano, L.; Nigro, V.; Nigro, G.; Petretta, V.R.; Passamano, L.; Papparella, S.; Di Somma, S.; Comi, L.I. Development of cardiomyopathy in female carriers of Duchenne and Becker muscular dystrophies. JAMA 1996, 275, 1335–1338. [Google Scholar] [CrossRef]
- Begay, R.L.; Graw, S.L.; Sinagra, G.; Asimaki, A.; Rowland, T.J.; Slavov, D.B.; Gowan, K.; Jones, K.L.; Brun, F.; Merlo, M.; et al. Filamin C Truncation Mutations are Associated with Arrhythmogenic Dilated Cardiomyopathy and Changes in the Cell-Cell Adhesion Structures. JACC Clin. Electrophysiol. 2018, 4, 504–514. [Google Scholar]
- Gigli, M.; Stolfo, D.; Graw, S.L.; Merlo, M.; Gregorio, C.; Nee Chen, S.; Dal Ferro, M.; PaldinoMD, A.; De Angelis, G.; Brun, F.; et al. Phenotypic Expression, Natural History, and Risk Stratification of Cardiomyopathy Caused by Filamin C Truncating Variants. Circulation 2021, 144, 1600–1611. [Google Scholar] [CrossRef]
- Brauch, K.M.; Karst, M.L.; Herron, K.J.; de Andrade, M.; Pellikka, P.A.; Rodeheffer, R.J.; Michels, V.V.; Olson, T.M. Mutations in ribonucleic acid binding protein gene cause familial dilated cardiomyopathy. J. Am. Coll. Cardiol. 2009, 54, 930–941. [Google Scholar] [CrossRef]
- Selcen, D.; Muntoni, F.; Burton, B.K.; Pegoraro, E.; Sewry, C.; Bite, A.V.; Engel, A.G. Mutation in BAG3 causes severe dominant childhood muscular dystrophy. Ann. Neurol. 2009, 65, 83–89. [Google Scholar] [CrossRef]
- Brun, F.; Barnes, C.V.; Sinagra, G.; Slavov, D.; Barbati, G.; Zhu, X.; Graw, S.L.; Spezzacatene, A.; Pinamonti, B.; Merlo, M.; et al.; Familial Cardiomyopathy Registry Titin and desmosomal genes in the natural history of arrhythmogenic right ventricular cardiomyopathy. J. Med. Genet. 2014, 51, 669–676. [Google Scholar] [CrossRef]
- Yermakovich, D.; Sivitskaya, L.; Vaikhanskaya, T.; Danilenko, N.; Motuk, I. Novel desmoplakin mutations in familial Carvajal syndrome. Acta Myol. 2018, 37, 263–266. [Google Scholar]
- Wang, W.; Murray, B.; Tichnell, C.; Gilotra, N.A.; Zimmerman, S.L.; Gasperetti, A.; Scheel, P.; Tandri, H.; Calkins, H.; James, C.A. Clinical characteristics and risk stratification of desmoplakin cardiomyopathy. Europace 2022, 24, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.D.; Lakdawala, N.K.; Papoutsidakis, N.; Aubert, G.; Mazzanti, A.; McCanta, A.C.; Agarwal, P.P.; Arscott, P.; Dellefave-Castillo, L.M.; Vorovich, E.E.; et al. Desmoplakin Cardiomyopathy, a Fibrotic and Inflammatory Form of Cardiomyopathy Distinct From Typical Dilated or Arrhythmogenic Right Ventricular Cardiomyopathy. Circulation 2020, 141, 1872–1884. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Morales, A.; Gonzalez-Quintana, J.; Norton, N.; Siegfried, J.D.; Hofmeyer, M.; Hershberger, R.E. Identification of novel mutations in RBM20 in patients with dilated cardiomyopathy. Clin. Transl. Sci. 2010, 3, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Hey, T.M.; Rasmussen, T.B.; Madsen, T.; Aagaard, M.M.; Harbo, M.; Mølgaard, H.; Møller, J.E.; Eiskjær, H.; Mogensen, J. Pathogenic RBM20-variants are associated with a severe disease expression in male patients with dilated cardiomyopathy. Circ. Heart Fail. 2019, 12, e005700. [Google Scholar] [CrossRef]
- Ichida, F.; Tsubata, S.; Bowles, K.R.; Haneda, N.; Uese, K.; Miyawaki, T.; Dreyer, W.J.; Messina, J.; Li, H.; Bowles, N.E.; et al. Novel gene mutations in patients with left ventricular noncompaction or Barth syndrome. Circulation 2001, 103, 1256–1263. [Google Scholar] [CrossRef]
- Olson, T.M.; Michels, V.V.; Ballew, J.D.; Reyna, S.P.; Karst, M.L.; Herron, K.J.; Horton, S.C.; Rodeheffer, R.J.; Anderson, J.L. Sodium channel mutations and susceptibility to heart failure and atrial fibrillation. JAMA 2005, 293, 447–454. [Google Scholar] [CrossRef]
- Japp, A.G.; Gulati, A.; Cook, S.A.; Cowie, M.R.; Prasad, S.K. The Diagnosis and Evaluation of Dilated Cardiomyopathy. J. Am. Coll. Cardiol. 2016, 67, 2996–3010. [Google Scholar] [CrossRef]
- Schultheiss, H.P.; Fairweather, D.; Caforio, A.L.P.; Escher, F.; Hershberger, R.E.; Lipshultz, S.E.; Liu, P.P.; Matsumori, A.; Mazzanti, A.; McMurray, J.; et al. Dilated cardiomyopathy. Nat. Rev. Dis. Primers 2019, 5, 32. [Google Scholar] [CrossRef]
- Thibodeau, J.T.; Drazner, M.H. The Role of the Clinical Examination in Patients With Heart Failure. JACC Heart Fail. 2018, 6, 543–551. [Google Scholar] [CrossRef]
- Drazner, M.H.; Hellkamp, A.S.; Leier, C.V.; Shah, M.R.; Miller, L.W.; Russell, S.D.; Young, J.B.; Califf, R.M.; Nohria, A. Value of clinician assessment of hemodynamics in advanced heart failure: the ESCAPE trial. Circ. Heart Fail. 2008, 1, 170–177. [Google Scholar] [CrossRef]
- Wang, C.S.; FitzGerald, J.M.; Schulzer, M.; Mak, E.; Ayas, N.T. Does This Dyspneic Patient in the Emergency Department Have Congestive Heart Failure? JAMA 2005, 294, 1944–1956. [Google Scholar] [CrossRef]
- Rapezzi, C.; Arbustini, E.; Caforio, A.L.; Charron, P.; Gimeno-Blanes, J.; Heliö, T.; Linhart, A.; Mogensen, J.; Pinto, Y.; Ristic, A.; et al. Diagnostic work-up in cardiomyopathies: bridging the gap between clinical phenotypes and final diagnosis. A position statement from the ESC Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2013, 34, 1448–1458. [Google Scholar] [CrossRef]
- Tester, D.J.; Medeiros-Domingo, A.; Will, M.L.; Ackerman, M.J. Unexplained drownings and the cardiac channelopathies: a molecular autopsy series. Mayo Clin. Proc. 2011, 86, 941–947. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al.; ESC Scientific Document Group 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Finocchiaro, G.; Merlo, M.; Sheikh, N.; De Angelis, G.; Papadakis, M.; Olivotto, I.; Rapezzi, C.; Carr-White, G.; Sharma, S.; Mestroni, L.; et al. The electrocardiogram in the diagnosis and management of patients with dilated cardiomyopathy. Eur. J. Heart Fail. 2020, 22, 1097–1107. [Google Scholar] [CrossRef] [PubMed]
- Marin-Neto, J.A.; Rassi, A.Jr.; Oliveira, G.M.M.; Correia, L.C.L.; Ramos Júnior, A.N.; Luquetti, A.O.; Hasslocher-Moreno, A.M.; Sousa, A.S.; Paola, A.A.V.; Sousa, A.C.S.; et al. SBC Guideline on the Diagnosis and Treatment of Patients with Cardiomyopathy of Chagas Disease - 2023. Arq. Bras. Cardiol. 2023, 120, e20230269. [Google Scholar] [CrossRef]
- Goldberger, J.J.; Subačius, H.; Patel, T.; Cunnane, R.; Kadish, A.H. Sudden cardiac death risk stratification in patients with nonischemic dilated cardiomyopathy. J. Am. Coll. Cardiol. 2014, 63, 1879–1889. [Google Scholar] [CrossRef]
- Takemoto, M.; Yoshimura, H.; Ohba, Y.; Matsumoto, Y.; Yamamoto, U.; Mohri, M.; Yamamoto, H.; Origuchi, H. Radiofrequency catheter ablation of premature ventricular complexes from right ventricular outflow tract improves left ventricular dilation and clinical status in patients without structural heart disease. J. Am. Coll. Cardiol. 2005, 45, 1259–1265. [Google Scholar] [CrossRef]
- Jan, M.F.; Tajik, A.J. Modern imaging techniques in cardiomyopathies. Circ. Res. 2017, 121, 874–891. [Google Scholar] [CrossRef]
- Verdonschot, J.A.J.; Merken, J.J.; Brunner-La Rocca, H.P.; Hazebroek, M.R.; Eurlings, C.G.M.J.; Thijssen, E.; Wang, P.; Weerts, J.; van Empel, V.; Schummers, G.; et al. Value of Speckle Tracking-Based Deformation Analysis in Screening Relatives of Patients With Asymptomatic Dilated Cardiomyopathy. JACC Cardiovasc. Imaging 2020, 13, 549–558. [Google Scholar]
- Verdonschot, J.A.J.; Merken, J.J.; Brunner-La Rocca, H.P.; Hazebroek, M.R.; Eurlings, C.G.M.J.; Thijssen, E.; Wang, P.; Weerts, J.; van Empel, V.; Schummers, G.; et al. Value of Speckle Tracking-Based Deformation Analysis in screening relatives of patients with asymptomatic dilated cardiomyopathy. JACC Cardiovasc. Imaging 2020, 13, 549–558. [Google Scholar]
- Raafs, A.G.; Boscutti, A.; Henkens, M.T.H.M.; van den Broek, W.W.A.; Verdonschot, J.A.J.; Weerts, J.; Stolfo, D.; Nuzzi, V.; Manca, P.; Hazebroek, M.R.; et al. Global Longitudinal Strain is Incremental to Left Ventricular Ejection Fraction for the Prediction of Outcome in Optimally Treated Dilated Cardiomyopathy Patients. J. Am. Heart Assoc. 2022, 11, e024505. [Google Scholar] [CrossRef]
- Ismail, T.F.; Strugnell, W.; Coletti, C.; Božić-Iven, M.; Weingärtner, S.; Hammernik, K.; Correia, T.; Küstner, T. Cardiac MR: From Theory to Practice. Front. Cardiovasc. Med. 2022, 9, 826283. [Google Scholar] [CrossRef]
- Anderson, L.J.; Holden, S.; Davis, B.; Prescott, E.; Charrier, C.C.; Bunce, N.H.; Firmin, D.N.; Wonke, B.; Porter, J.; Walker, J.M.; et al. Cardiovascular T2-star (T2*) magnetic resonance for the early diagnosis of myocardial iron overload. Eur. Heart J. 2001, 22, 2171–2179. [Google Scholar] [CrossRef] [PubMed]
- Paterson, D.I.; Wells, G.; Erthal, F.; Mielniczuk, L.; O'Meara, E.; White, J.; Connelly, K.A.; Knuuti, J.; Radja, M.; Laine, M.; et al. ; IMAGE-HF Investigators. OUTSMART HF: A Randomized Controlled Trial of Routine Versus Selective Cardiac Magnetic Resonance for Patients With Nonischemic Heart Failure (IMAGE-HF 1B). Circulation 2020, 141, 818–827. [Google Scholar] [PubMed]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al.; ACMG Laboratory Quality Assurance Committee Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef] [PubMed]
- Charron, P.; Arad, M.; Arbustini, E.; Basso, C.; Bilinska, Z.; Elliott, P.; Helio, T.; Keren, A.; McKenna, W.J.; Monserrat, L.; et al.; European Society of Cardiology Working Group on Myocardial and Pericardial Diseases Genetic counselling and testing in cardiomyopathies: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2010, 31, 2715–2726. [Google Scholar] [CrossRef]
- Towbin, J.A.; McKenna, W.J.; Abrams, D.; Ackerman, M.J.; Calkins, H.; Darrieux, F.C.C.; Daubert, J.P.; Chillou, C.; DesPasquale, E.C.; Desai, M.Y.; et al. 2019 HRS expert consensus statement on evaluation, risk stratification, and management of arrhythmogenic cardiomyopathy. Heart Rhythm 2019, 16, e301–e372. [Google Scholar] [CrossRef]
- Yang, Y.; Muzny, D.M.; Reid, J.G.; Bainbridge, M.N.; Willis, A.; Ward, P.A.; Braxton, A.; Beuten, J.; Xia, F.; Niu, Z.; et al. Clinical whole-exome sequencing for the diagnosis of mendelian disorders. N. Engl. J. Med. 2013, 369, 1502–1511. [Google Scholar] [CrossRef]
- Stark, Z.; Tan, T.Y.; Chong, B.; Brett, G.R.; Yap, P.; Walsh, M.; Yeung, A.; Peters, H.; Mordaunt, D.; Cowie, S.; et al.; Melbourne Genomics Health A, Gaff, C ; White, S.M. A prospective evaluation of whole-exome sequencing as a first-tier molecular test in infants with suspected monogenic disorders. Genet. Med. 2016, 18, 1090–1096. [Google Scholar] [CrossRef]
- Ramchand, J.; Wallis, M.; Macciocca, I.; Lynch, E.; Farouque, O.; Martyn, M.; Phelan, D.; Chong, B.; Lockwood, S.; Weintraub, R.; et al. Prospective Evaluation of the Utility of Whole Exome Sequencing in Dilated Cardiomyopathy. J. Am. Heart Assoc. 2020, 9, e013346. [Google Scholar] [CrossRef]
- D'Agostino, Y.; Palumbo, D.; Rusciano, M.R.; Strianese, O.; Amabile, S.; DiRosa, D.; DeAngelis, E.; Mistletoe, V.; Russian, F.; Alexandrova, E.; et al. Whole-Exome Sequencing Revealed New Candidate Genes for Human Dilated Cardiomyopathy. Diagnostics (Basel) 2022, 12, 2411. [Google Scholar] [CrossRef]
- Mak, T.S.H.; Lee, Y.K.; Tang, C.S.; Hai, J.S.H.; Ran, X.; Sham, PC; Tse, H. F. Coverage and diagnostic yield of Whole Exome Sequencing for the Evaluation of Cases with Dilated and Hypertrophic Cardiomyopathy. Sci. Rep. 2018, 8, 10846. [Google Scholar] [CrossRef]
- Pinto, Y.M.; Elliott, P.M.; Arbustini, E.; Adler, Y.; Anastasakis, A.; Böhm, M.; Duboc, D.; Gimeno, D.; de Groote, P.; Imazio, M.; et al. Proposal for a revised definition of dilated cardiomyopathy, hypokinetic nondilated cardiomyopathy, and its implications for clinical practice: a position statement of the ESC Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2016, 37, 1850–1858. [Google Scholar] [CrossRef] [PubMed]
- Cooper, L.T.; Baughman, K.L.; Feldman, A.M.; Frustaci, A.; Jessup, M.; Kuhl, U.; Levine, G.N.; Narula, J.; Starling, R.C.; Towbin, J.; et al.; American Heart Association; American College of Cardiology; European Society of Cardiology; Heart Failure Society of America; Heart Failure Association of the European Society of Cardiology The role of endomyocardial biopsy in the management of cardiovascular disease: a scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology. Endorsed by the Heart Failure Society of America and the Heart Failure Association of the European Society of Cardiology. J. Am. Coll. Cardiol. 2007, 50, 1914–1931. [Google Scholar]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e895–e1032. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.R.; Han, M.M.; Yang, Y.Z.; Wang, X.; Hou, D.Y.; Meng, X.C.; Wang, H.; Zhao, W.S.; Zhang, L.; Xu, L. Fifteen-year mortality and prognostic factors in patients with dilated cardiomyopathy: persistent standardized application of drug therapy and strengthened management may bring about encouraging change in an aging society. J. Geriatr. Cardiol. 2022, 19, 335–342. [Google Scholar] [PubMed]
- Levy, W.C.; Mozaffarian, D.; Linker, D.T.; Sutradhar, S.C.; Anker, S.D.; Cropp, A.B.; Anand, I.; Maggioni, A.; Burton, P.; Sullivan, M.D.; et al. The Seattle Heart Failure Model: prediction of survival in heart failure. Circulation 2006, 113, 1424–1433. [Google Scholar] [CrossRef] [PubMed]
- Pocock, S.J.; Ariti, C.A.; McMurray, J.J.; Maggioni, A.; Køber, L.; Squire, I.B.; Swedberg, K.; Dobson, J.; Poppe, K.K.; Whalley, G.A.; et al.; Meta-Analysis Global Group in Chronic Heart Failure Predicting survival in heart failure: a risk score based on 39 372 patients from 30 studies. Eur. Heart J. 2013, 34, 1404–1413. [Google Scholar] [CrossRef] [PubMed]
- Lupón, J.; De Antonio, M.; Vila, J.; Peñafiel, J.; Galán, A.; Zamora, E.; Urrutia, A.; Bayes-Genis, A. Development of a novel heart failure risk tool: The Barcelona bio-heart failure risk calculator (BCN bio-HF calculator). PLoS ONE 2014, 9, e85466. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Lopez, L.; Ochoa, J.P.; Royuela, A.; Verdonschot, J.A.J.; Dal Ferro, M.; Espinosa, M.A.; Sabater-Molina, M.; Gallego-Delgado, M.; Larrañaga-Moreira, J.M.; Garcia-Pinilla, J.M.; et al. Clinical Risk Score to Predict Pathogenic Genotypes in Patients With Dilated Cardiomyopathy. J. Am. Coll. Cardiol. 2022, 80, 1115–1126. [Google Scholar] [CrossRef]
- Kimura, Y.; Okumura, T.; Morimoto, R.; Kazama, S.; Shibata, N.; Oishi, H.; Araki, T.; Mizutani, T.; Kuwayama, T.; Hiraiwa, H.; et al. A clinical score for predicting left ventricular reverse remodeling in patients with dilated cardiomyopathy. ESC Heart Fail. 2021, 8, 1359–1368. [Google Scholar] [CrossRef]
- Sammani, A.; Kayvanpour, E.; Bosman, L.P.; Sedaghat-Hamedani, F.; Proctor, T.; Gi, W.T.; Broezel, A.; Jensen, K.; Katus, H.A.; Te Riele, A.S.J.M.; et al. Predicting sustained ventricular arrhythmias in dilated cardiomyopathy: a meta-analysis and systematic review. ESC Heart Fail. 2020, 7, 1430–1441. [Google Scholar] [CrossRef]
- Kayvanpour, E.; Sammani, A.; Sedaghat-Hamedani, F.; Lehmann, D.H.; Broezel, A.; Koelemenoglu, J.; Chmielewski, P.; Curjol, A.; Socie, P.; Miersch, T.; et al. A novel risk model for predicting potentially life-threatening arrhythmias in non-ischemic dilated cardiomyopathy (DCM-SVA risk). Int. J. Cardiol. 2021, 339, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Zeppenfeld, K.; Tfelt-Hansen, J.; de Riva, M.; Winkel, B.G.; Behr, E.R.; Blom, N.A.; Charron, P.; Corrado, D.; Dagres, N.; de Chillou, C.; et al.; ESC Scientific Document Group 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur. Heart J. 2022, 43, 3997–4126. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraman, P.; Zalavadia, D.; Haseeb, A.; Dye, C.; Madan, N.; Skeete, J.R.; Vipparthy, S.C.; Young, W.; Ravi, V.; Rajakumar, C.; et al. Clinical outcomes of conduction system pacing compared to biventricular pacing in patients requiring cardiac resynchronization therapy. Heart Rhythm 2022, 19, 1263–1271. [Google Scholar] [CrossRef] [PubMed]
- Glikson, M.; Nielsen, J.C.; Kronborg, M.B.; Michowitz, Y.; Auricchio, A.; Barbash, I.M.; Barrabés, J.A.; Boriani, G.; Braunschweig, F.; Brignole, M.; et al.; ESC Scientific Document Group 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Eur. Heart J. 2021, 42, 3427–3520. [Google Scholar] [CrossRef] [PubMed]
- Borggrefe, M.; Mann, D.L. Cardiac Contractility Modulation in 2018. Circulation 2018, 138, 2738–2740. [Google Scholar] [CrossRef]
- Abraham, W.T.; Kuck, K.H.; Goldsmith, R.; Lindenfeld, J.; Reddy, V.Y.; Carson, P.E.; Mann, D.L.; Saville, B.; Parise, H.; Chan, R.; et al. A Randomized Controlled Trial to Evaluate the Safety and Efficacy of Cardiac Contractility Modulation. JACC Heart Fail. 2018, 6, 874–883. [Google Scholar] [CrossRef]
- Vallejo Garcia, V.; Gonzalez Calle, D.; Castro Garay, J.C.; Garcia Monsalvo, J.; Rodriguez, J.B.; Martin, J.G.; Laffond, A.E.; Nunez Garcia, J.; Morales, J.C.E.; Cruz Gonzalez, I.; Barreiro Perez, M.; Sanchez Fernandez, P.L.; et al. Functional mitral regurgitation in dilated cardiomyopathy: clinical implications and prognosis. Eur. Heart, J. 2021, 22, jeaa356361. [Google Scholar] [CrossRef]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P. 3rd.; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 143, e35–e71. [Google Scholar]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al.; ESC/EACTS Scientific Document Group 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef]
- Stone, G.W.; Lindenfeld, J.; Abraham, W.T.; Kar, S.; Lim, D.S.; Mishell, J.M.; Whisenant, B.; Grayburn, P.A.; Rinaldi, M.; Kapadia, S.R.; et al.; COAPT Investigators Transcatheter Mitral-Valve Repair in Patients with Heart Failure. N. Engl. J. Med. 2018, 379, 2307–2318. [Google Scholar] [CrossRef]
- Obadia, J.F.; Messika-Zeitoun, D.; Leurent, G.; Iung, B.; Bonnet, G.; Piriou, N.; Lefèvre, T.; Piot, C.; Rouleau, F.; Carrié, D.; et al.; MITRA-FR Investigators Percutaneous Repair or Medical Treatment for Secondary Mitral Regurgitation. N. Engl. J. Med. 2018, 379, 2297–2306. [Google Scholar] [CrossRef]
- Caforio, A.L.P.; Pankuweit, S.; Arbustini, E.; Basso, C.; Gimeno-Blanes, J.; Felix, S.B.; Fu, M.; Heliö, T.; Heymans, S.; Jahns, R.; et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013, 34, 2636–2648. [Google Scholar] [CrossRef] [PubMed]
- Montera, M.W.; Marcondes-Braga, F.G.; Simões, M.V.; Moura, L.A.Z.; Fernandes, F.; Mangine, S.; Oliveira Júnior, A.C.; Souza, A.L.A.A.G.; Ianni, B.M.; Rochitte, C.E.; et al. Brazilian Society of Cardiology Guideline on Myocarditis - 2022. Arq. Bras. Cardiol. 2022, 119, 143–211. [Google Scholar] [CrossRef]
- Frustaci, A.; Russo, M.A.; Chimenti, C. Randomized study on the efficacy of immunosuppressive therapy in patients with virus-negative inflammatory cardiomyopathy: the TIMIC study. Eur. Heart J. 2009, 30, 1995–2002. [Google Scholar] [CrossRef] [PubMed]
- Schultheiss, H.P.; Piper, C.; Sowade, O.; Waagstein, F.; Kapp, J.F.; Wegscheider, K.; Groetzbach, G.; Pauschinger, M.; Escher, F.; Arbustini, E.; et al. Betaferon in chronic viral cardiomyopathy (BICC) trial: Effects of interferon-β treatment in patients with chronic viral cardiomyopathy. Clin. Res. Cardiol. 2016, 105, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Morillo, C.A.; Marin-Neto, J.A.; Avezum, A.; Sosa-Estani, S.; Rassi, A.Jr.; Rosas, F.; Villena, E.; Quiroz, R.; Bonilla, R.; Britto, C.; et al.; BENEFIT Investigators Randomized Trial of Benznidazole for Chronic Chagas' Cardiomyopathy. N. Engl. J. Med. 2015, 373, 1295–1306. [Google Scholar] [CrossRef]
- Helms, A.S.; Thompson, A.D.; Day, S.M. Translation of New and Emerging Therapies for Genetic Cardiomyopathies. JACC Basic Transl. Sci. 2021, 7, 70–83. [Google Scholar]
- Sun, N.; Yazawa, M.; Liu, J.; Han, L.; Sanchez-Freire, V.; Abilez, O.J.; Navarrete, E.G.; Hu, S.; Wang, L.; Lee, A.; et al. Patient-specific induced pluripotent stem cells as a model for familial dilated cardiomyopathy. Sci. Transl. Med. 2012, 4, 130ra47. [Google Scholar] [CrossRef]
- Lee, Y.K.; Lau, Y.M. , Cai, Z.J.; Lai, W.H.; Wong, L.Y.; Tse, H.F.; Ng, K.M.; Siu, C.W. Modeling Treatment Response for Lamin A/C Related Dilated Cardiomyopathy in Human Induced Pluripotent Stem Cells. J. Am. Heart Assoc. 2017, 6, e005677. [Google Scholar] [CrossRef]
- Brodehl, A.; Ebbinghaus, H.; Deutsch, M.A.; Gummert, J.; Gartner, A.; Ratnavadivel, S.; Milting, H. Human Induced Pluripotent Stem-Cell-Derived Cardiomyocytes as Models for Genetic Cardiomyopathies. Int. J. Mol. Sci. 2019, 20, 4381. [Google Scholar] [CrossRef]
- Mehra, M.R.; Goldstein, D.J.; Cleveland, J.C.; Cowger, J.A.; Hall, S.; Salerno, C.T.; Naka, Y.; Horstmanshof, D.; Chuang, J.; Wang, A.; et al. Five-Year Outcomes in Patients With Fully Magnetically Levitated vs Axial-Flow Left Ventricular Assist Devices in the MOMENTUM 3 Randomized Trial. JAMA 2022, 328, 1233–1242. [Google Scholar] [CrossRef] [PubMed]
- Grady, K.L.; Naftel, D.C.; Myers, S.; Dew, M.A.; Weidner, G.; Spertus, J.A.; Idrissi, K.; Lee, H.B.; McGee, E.C.; Kirklin, J.K. Change in health-related quality of life from before to after destination therapy mechanical circulatory support is similar for older and younger patients: analyses from INTERMACS. J. Heart Lung Transplant. 2015, 34, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Khush, K.K.; Hsich, E.; Potena, L.; Cherikh, W.S.; Chambers, D.C.; Harhay, M.O.; Hayes, D.Jr.; Perch, M.; Sadavarte, A.; Toll, A.; et al.; International Society for Heart and Lung Transplantation The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-eighth adult heart transplantation report - 2021; Focus on recipient characteristics. J. Heart Lung Transplant. 2021, 40, 1035–1049. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).