1. Introduction
The cement industry, according to the World Cement Association (WCA), is responsible for 5 to 6% of global carbon dioxide emissions [
1]. This association requires a significant reduction in the carbon footprint of the cement industry. One approach to this is to partially move away from cement in favor of "mineral additions", which can often be waste products from other industries. However, substituting cement with mineral additions such as silica fume strongly modifies the rheological and mechanical properties of concrete [
2,
3]. Therefore, in some cases compositions of concrete mixes have to be adjusted with regards to these additives in order to maintain the quality and parameters of concrete. The use of polycarboxylate ether superplasticizers (PCEs) is absolutely necessary for concretes that contain mineral additives, e.g. silica fume, in order to make these additives "work". The compatibility of PCEs with silica fume is currently the subject of many studies [
4,
5,
6,
7].
PCEs are currently the most effective at reducing the water content (up to 40%) and improving the workability and fluidity of concrete when compared to other types of superplasticizers. PCEs are copolymers and have a comb structure [
8,
9]. The main chain of a PCE carries a negatively charged carboxylic group (COO-), which promotes the adsorption of the polymer onto the positively charged surfaces of cement particles or individual hydrate phases, primarily ettringite [Ca3Al(OH)6 12 H2O]2(SO4)3 2 H2O [
10]. This occurs based on the electrostatic interaction. While being adsorbed, the polymer promotes the dispersion of the cement particles due to the steric hindrance produced by its side chains containing polyethylene glycol (PEG) structures [
11,
12,
13,
14,
15,
16,
17,
18,
19,
20,
21]. As a result, the water entrapped in the agglomerated binder particles can be released and the increasing of the flowability and reduction of the viscosity of cement paste [
22,
23,
24,
25].
The adsorption and dispersing capacity of PCEs in cement-based materials depends on their chemical structure, as well as their molecular weight, carboxylic groups, density and length of the side chain [
26,
27,
28].
Some PCEs are incompatible with silica fume in high-performance concretes, which in turn can result in a loss of slump and poor rheological behavior. This is due to prior adsorption of the PCE on the surface of the silica fume [
22,
29,
30,
31] which in turn increases the dispersion of the SF particles. Therefore, the adverse effect of PCEs on cement hydration could be mitigated by adding SF, which provides additional surfaces for PCE absorption, and, as such, reduces PCE adsorption on cement surfaces [
6].
To the best of our knowledge, the effectiveness of PCEs in high-performance concrete (HPC) containing silica fume (SF) as a partial replacement of cement has not been fully investigated. There are several knowledge gaps regarding this matter, especially concerning the interaction of SF and PCE (with a different molecular weight and carboxyl density) in HPC. To fill this research gap, the authors of this paper conducted studies of the interaction between PCE and silica fume in cement slurries, as well as in high-performance concretes (HPC) that have a low w/b ratio. Three PCEs with different molecular compositions were used to study their impact on the properties of concretes (HPC). Therefore, the fluidity and mechanical properties of HPC were evaluated, and the impact of the PCE on the shear stresses, plastic viscosity and zeta potential of cement slurries (containing 10% silica fume as a cement substitute) was also measured.
2. Materials and methods
2.1. Materials
2.1.1. Superplasticizer
Three types of superplasticizers produced in Algeria were used: Polyflow SR 3600 - designated as PCE1, Polyflow SR 5400 - designated as PCE2, and Medaflow 30 - designated as PCE3.
The three polymers are new-generation, non-chlorinated, and are based on modified polycarboxylic ether.
The molecular weight (Mn, Mw) and the polydispersity indices (PDI) of the superplasticizers were measured using multi-detector steric exclusion chromatography (SEC) (C2P2, France). The analytical conditions are presented in
Table 1.
The characteristics of the superplasticizers, according to their technical data sheet, are presented in
Table 2.
2.1.2. Portland cement and silica fume
The Portland cement (PC) that was used in the experimental study was CEM I 52.5 R. It is commercially produced by the Lafarge Company, the manufacturing site of which is located in the city of Msila (in the east region of Algeria).
The micro silica fume (SF) sample was obtained from Granitex, Algeria. Its marketing name is Medaplast HP, and its specific surface is 23000 m
2/kg. The chemical composition of the PC and SF are presented in
Table 3.
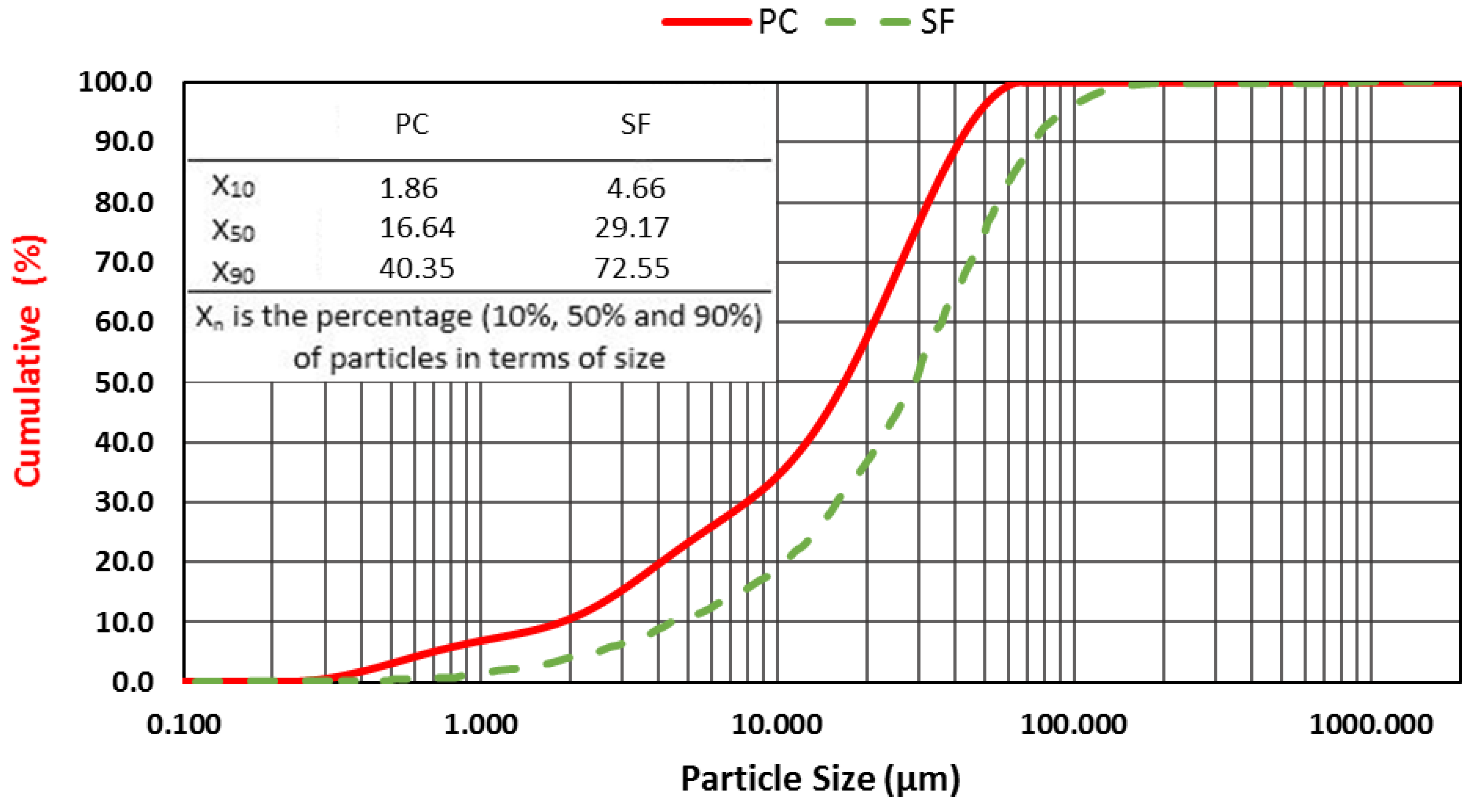
The particle size distributions of the PC and SF were prepared using a Malvern Mastersizer 2000 analyzer (liquid:Hydro, 2000MU). The results are presented in
Figure 1.
2.1.3. Aggregates
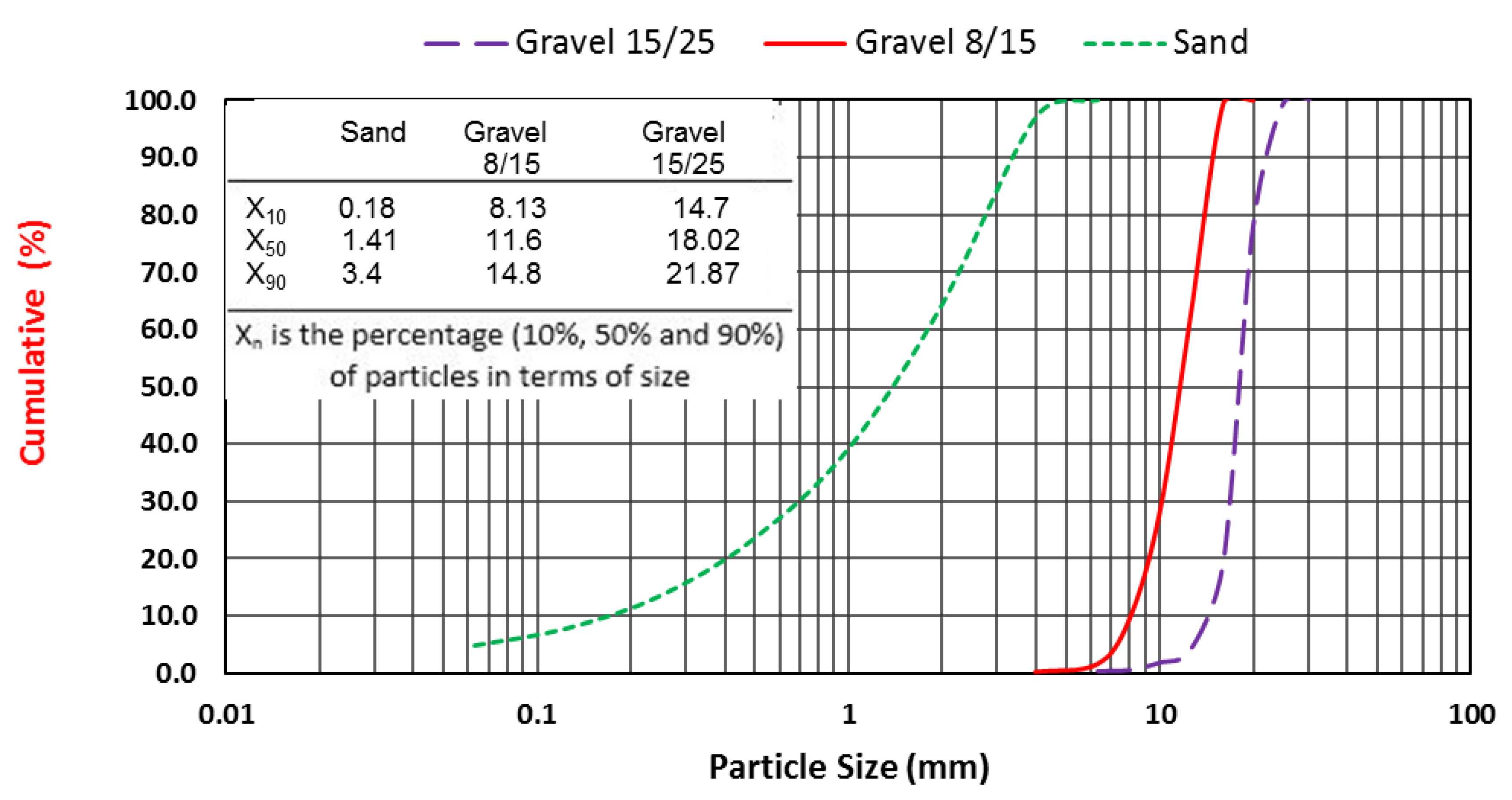
The properties of the aggregates are summarized in
Table 4, and their particle size distribution curves are given in
Figure 2.
2.2. Experimental measurements
2.2.1. Evaluation of the content of carboxyl groups using the titration method
The acid-base titration method was used to measure the content of carboxyl groups in each polymer sample [
32]. The measurements involved the determination of the amount of NaOH solution, which in turn was expressed as the product of its molar concentration and the volume that was needed to titrate a sample. The determined amount of NaOH (number of moles) is directly related to the content of carboxyl groups in polymers, because COO- and Na+ ions, which come from carboxyl groups and NaOH, react with each other in a stoichiometric ratio. Therefore, the more NaOH is needed to titrate the sample, the more carboxyl groups the sample contains. The content of carboxyl groups can then be calculated as follows:
where:
C(COO−) = content of carboxyl groups (mmol/100 g);
CNaOH = molar concentration of NaOH (mmol/L);
VNaOH = volume of the NaOH solution used (L); and m = weight of sample.
2.2.2. The composition of the concrete mix, and the performances of the polymers in the high-performance concrete (HPC)
The recipe of the concrete was determined on the basis of the Dreux-Gorisse method while taking into account the ratio of its components (water, cement, sand, and aggregates), it is shown in
Table 5. The prepared concrete had a mechanical compressive strength of 55 MPa, and a slump of 175 mm. The manufactured concrete mixes had a constant water to binder ratio of 0.35. The amount of PCE added to the concrete (in the presence of SF), in relation to the binder, was adopted based on viscosimetric measurements (using a VT 550 viscometer). The ratio was assumed to be PCE/b = 1.75%.
2.2.3. Fluidity
The slump test was used to evaluate the fluidity of the concrete mix in the case of all the formed HPCs. The dosage of superplasticizer (PC/b) was 1.75%, and it was determined using the VT 550 viscometer. The initial fluidity of the HPC was measured 5 min after mixing all the components. The concrete mix was then placed in a container, which was covered with a plastic film in order to prevent any water evaporation. The second slump test measurement was made after 1 hour of concrete maturation. In each case, the concrete mix was stirred one minute before taking the measurements.
2.2.4. Rheology
In order to evaluate the rheology of the cement paste containing 10% of SF (characterized by a Blaine specific surface of 4650 cm2/g) as a partial replacement of cement, the water to-binder ratio (w/b) was assumed to be 0.35. In this case, for an amount of water equal to 35 g, the mix contains 90 g of cement and 10 g of SF, as well as an added superplasticizer (PCE) in an amount of 0 - 2.5 wt% of the total cement-based binder. The test was performed at the temperature of 20 ± 1 ℃.
The rheology of the cement paste was evaluated using a VT 550 viscometer, which is a rotational viscometer. Using this viscometer, the following parameters of cement pastes can be measured: shear stresses (τ) and viscosities (µ), which are obtained as a function of shear rates (𝛾̇).
2.2.5. Zeta potential
The zeta potentials of the cement pastes (containing PCEs at saturation dosages) were determined using a Malvern ZETASIZER 2000. In a standard experiment, one-centimeter cube of the cementitious suspension is diluted in 30 cm3 of distilled water, after which 5 ml of this suspension is injected into the analyzer.
2.2.6. Compressive strength
In order to determine the compressive strength, six cylindrical concrete specimens (150× 300) mm2 were prepared according to the EN 206-1 protocol. These specimens were kept for 24 h in molds.
After demolding, all the specimens were stored in tap water at 20 ± 1℃ for 3, 7, and 28 days.
3. Results and discussion
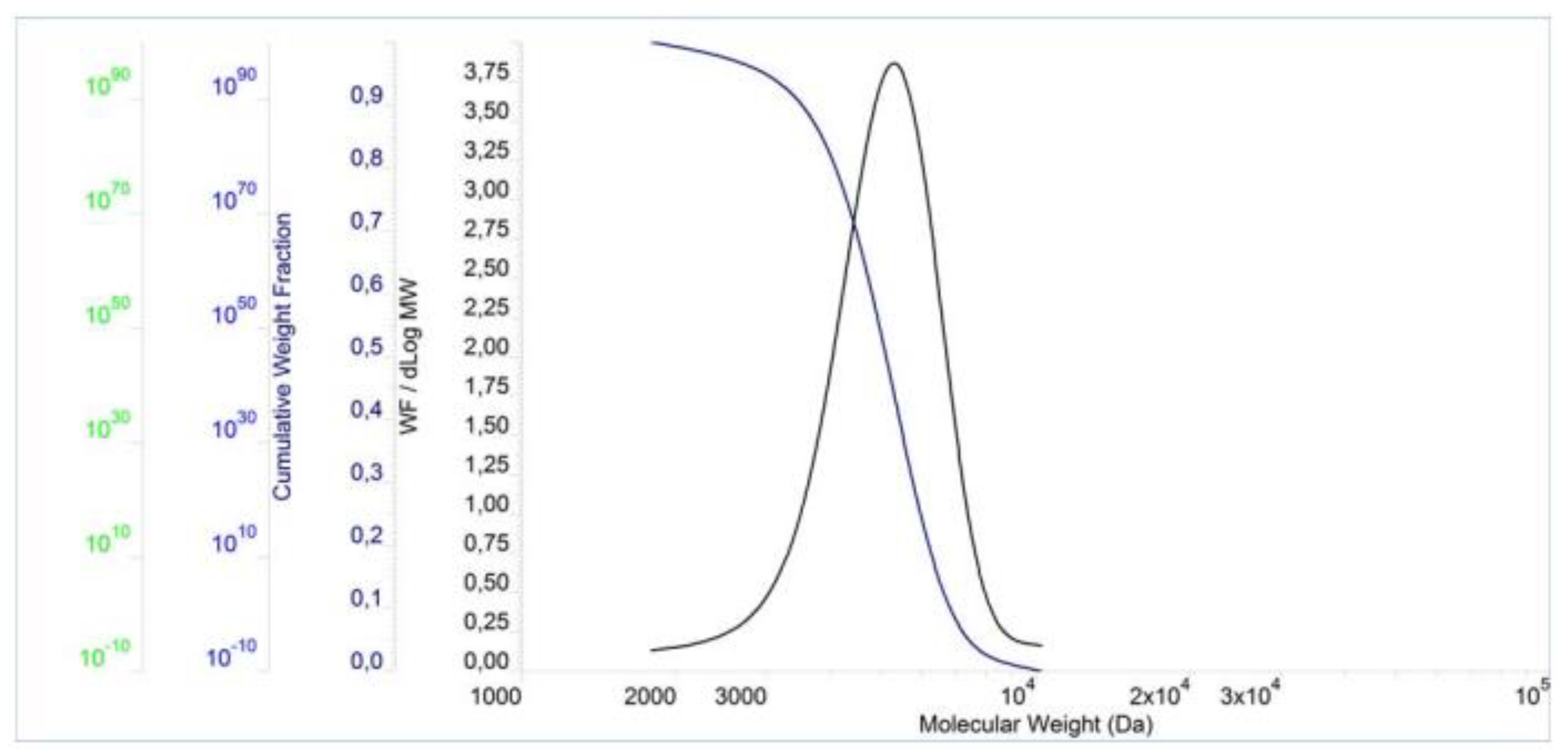
3.1. Molecular weight analysis
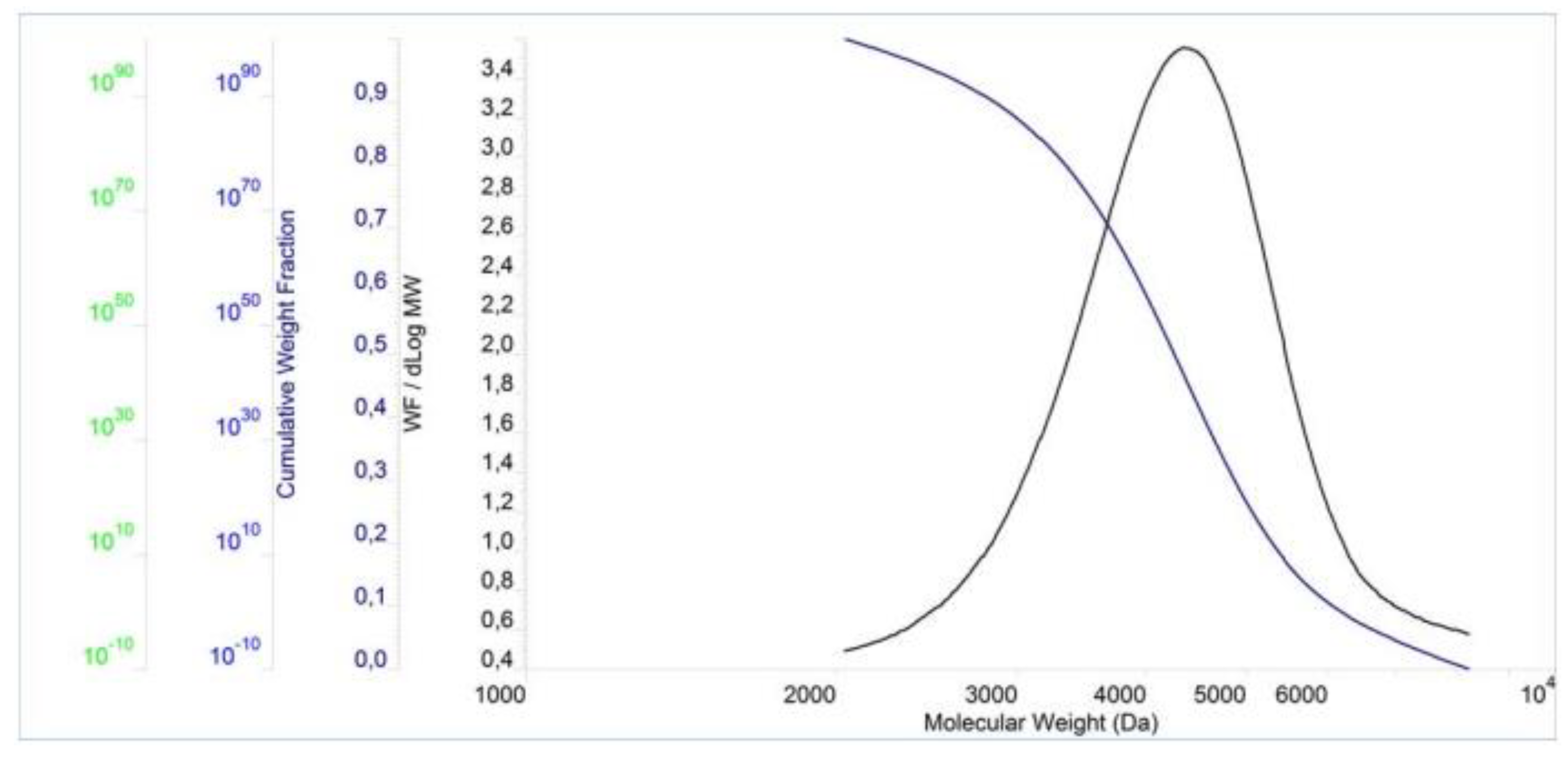
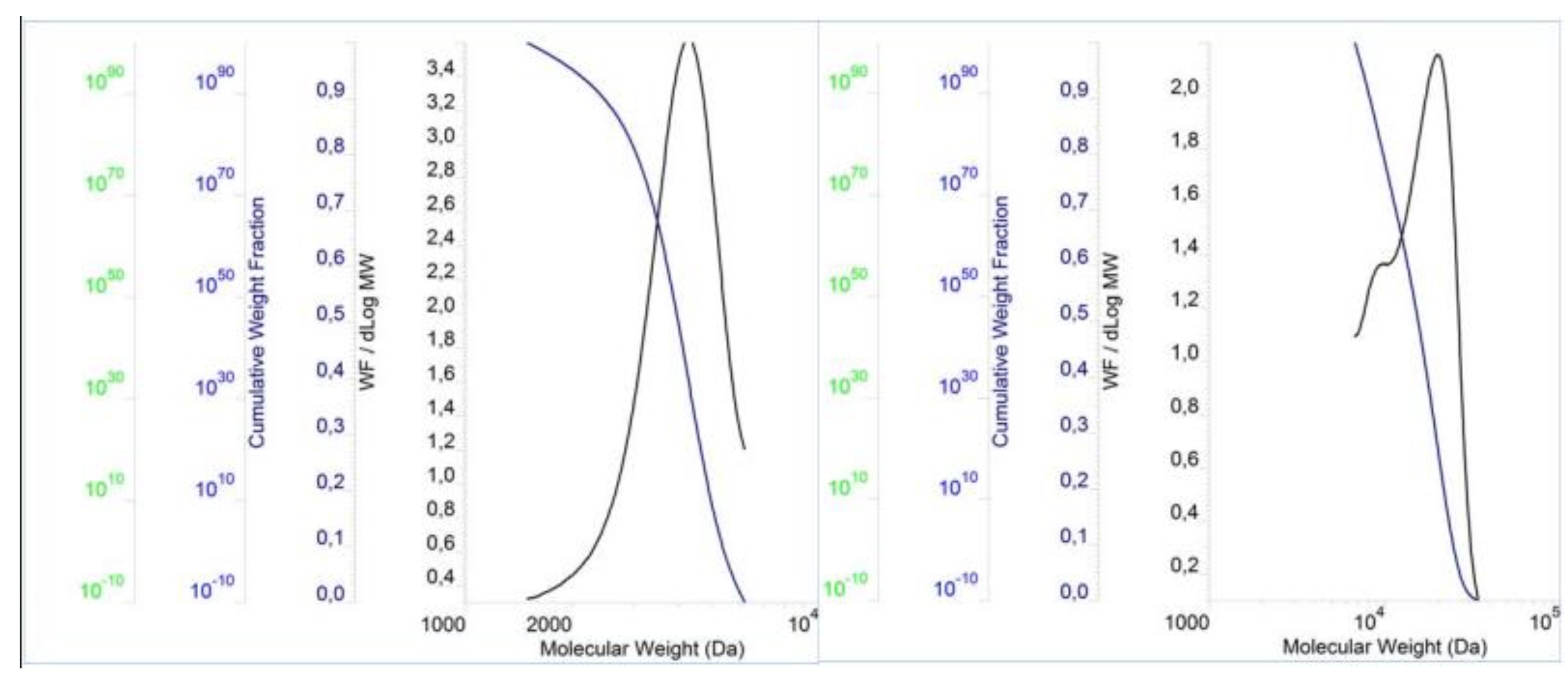
The molecular properties of the PCEs are shown in
Figure 3,
Figure 4 and
Figure 5 and
Table 2. The result show that the PDI values for these polymers are slightly above 1.00, which in turn indicates a small dispersion of molecular weights in the polymers. Furthermore, the PDI value of PCE3 is the closest to 1 when compared to the other two polymers. This means that PCE3 consists of the most similar macromolecules (regarding their length), which therefore have a similar molar mass. In terms of molecular weight, expressed both as Mn and Mw, PCE2 obtained the highest values, while PCE1 the lowest ones.
3.2. Analysis of the content of the carboxyl groups
The content of the carboxyl groups of the PCEs is shown in
Table 2. This content directly influences the carboxyl density of the PCEs. It should be mentioned that both of these values increase simultaneously – the increase of one causes a rise of the other. Therefore, PCE3 has the highest carboxyl density, whereas PCE1 has the lowest one. This is due to the fact that the content of the carboxyl groups in PCE3 and PCE1, expressed for 1 g of the polymer, amounts to 1.95 and 1.80 mmol, respectively.
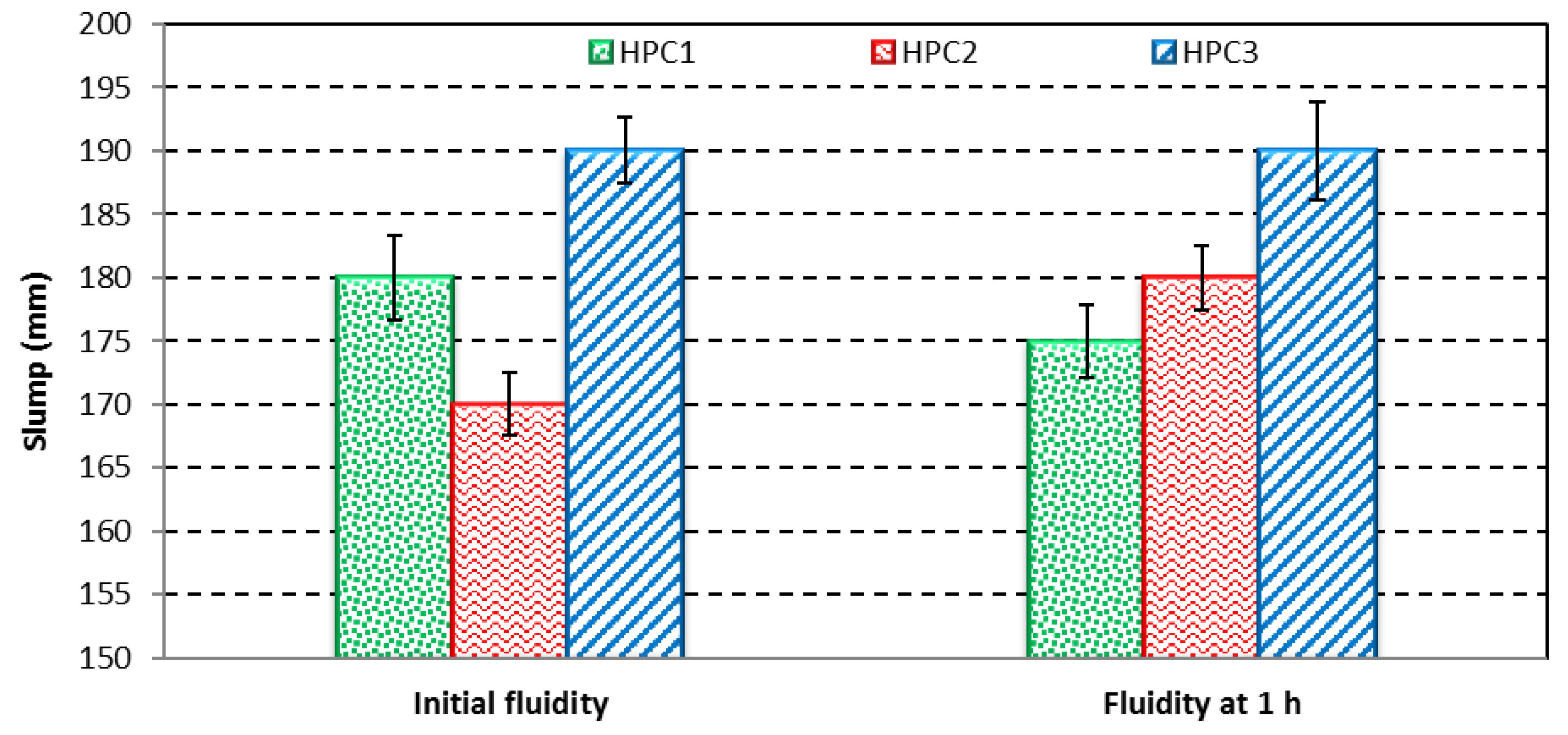
3.3. Fluidity
The results of the initial fluidity (slump) and the fluidity assessed after 1 hour (slump retention) are shown in
Figure 6.
PCE3 with the highest charge density and a moderate molecular weight exhibited a higher value of initial fluidity and a higher value of fluidity after 1 h when compared to PCE1 and PCE2. The COO− group on the backbone of the PCE was adsorbed on the surface of cement particles by complexation with Ca2+ or by electrostatic attraction. An increase in the number of COO– groups increases the adsorption capacity of PCEs [
32]. It means that the flowability of the cement paste increased with a rise in the content of the COO− groups. Therefore, very good compatibility was found between PCE3 and the SF, as they both retain the fluidity of the cement pastes after 1 hour.
PCE1 with the lowest carboxylic density and the lowest molecular weight exhibited a poor slump and poor slump retention due to the prior adsorption of the PCE on the silica fume surfaces [
22,
29]. The SF provided additional surfaces for the adsorption of the PCE, and consequently reduced PCE adsorption on the cement surfaces. Moreover, it reduced the performance of the concrete, and it can therefore be stated that there is an incompatibility between PCE1 and the SF.
PCE2 (with the highest molecular weight) had a bad initial slump and the highest slump retention. The amount of the absorbed PCE on the surface of the cement paste increased with a decrease in molecular weight, and therefore the PCE with a high molecular weight significantly delayed the hydration of the cement. This is due to the fact that the main chain of PCEs adsorbs on different cement particles simultaneously, making them become agglomerated, and in turn hindering the hydration process [
33].
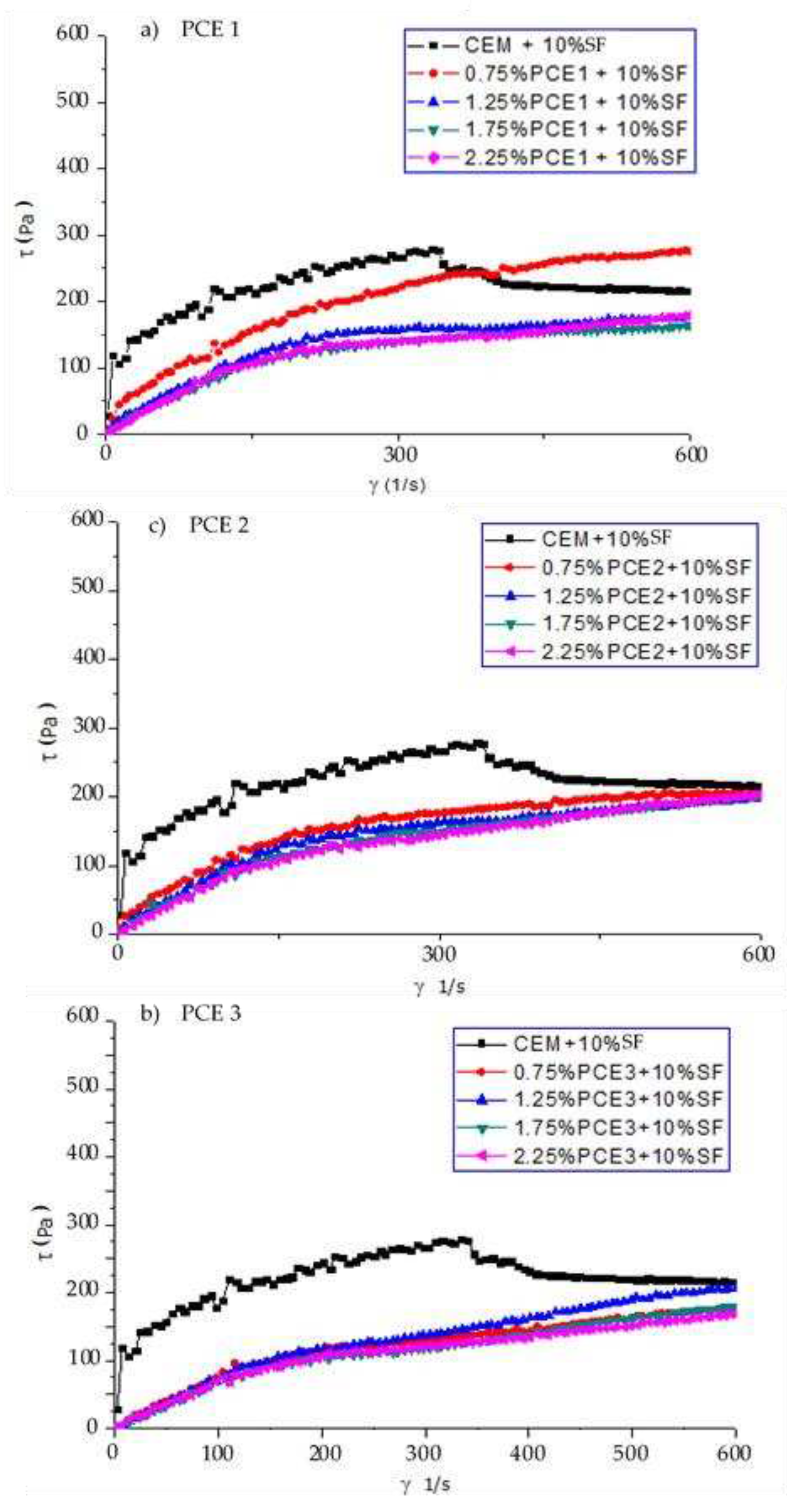
3.4. Rheology
The saturation point is the value of dosage beyond which the superplasticizer has no effect on the rheological properties of the cement paste. It was determined using the viscometer VT 550. Each of the polymers (PCE1, 2 and 3) had the same saturation point value, i.e. 1.75 at the tested water to binder ratio (w/b).
Figure 7 and
Figure 8 show that the shear stress and viscosity decreased with an increase in the dosage of the superplasticizer. The cement pastes became more fluid, with their flow then approaching the characteristics of the Newtonian flow.
The shear stress and viscosity depend on the applied stress. The shear stress increases with a rise in the shear rate, whereas the viscosity decreases with an increase in the shear rate. After adding the PCEs, the yield stress reduced [
34,
35]. The cement paste with the PCE that has a moderate molecular weight and a high content of the carboxylic groups exhibits the lowest shear stress and the lowest plastic viscosity. The carboxylic density and molecular weight of PCEs has a great effect on their dispersing performance. As the carboxylic density increases, the dispersing capability of a PCE improves [
10]. PCE3 had the highest carboxylic density and a moderate molecular weight. Due to this, it exhibited the best adsorption behavior on cement grains, which in turn resulted in the lowest viscosity and shear stress of the samples with this polymer.. PCE3, which had the highest anionic charge density and a moderate molecular weight, was more compatible with the SF when compared to PCE1 and PCE2.
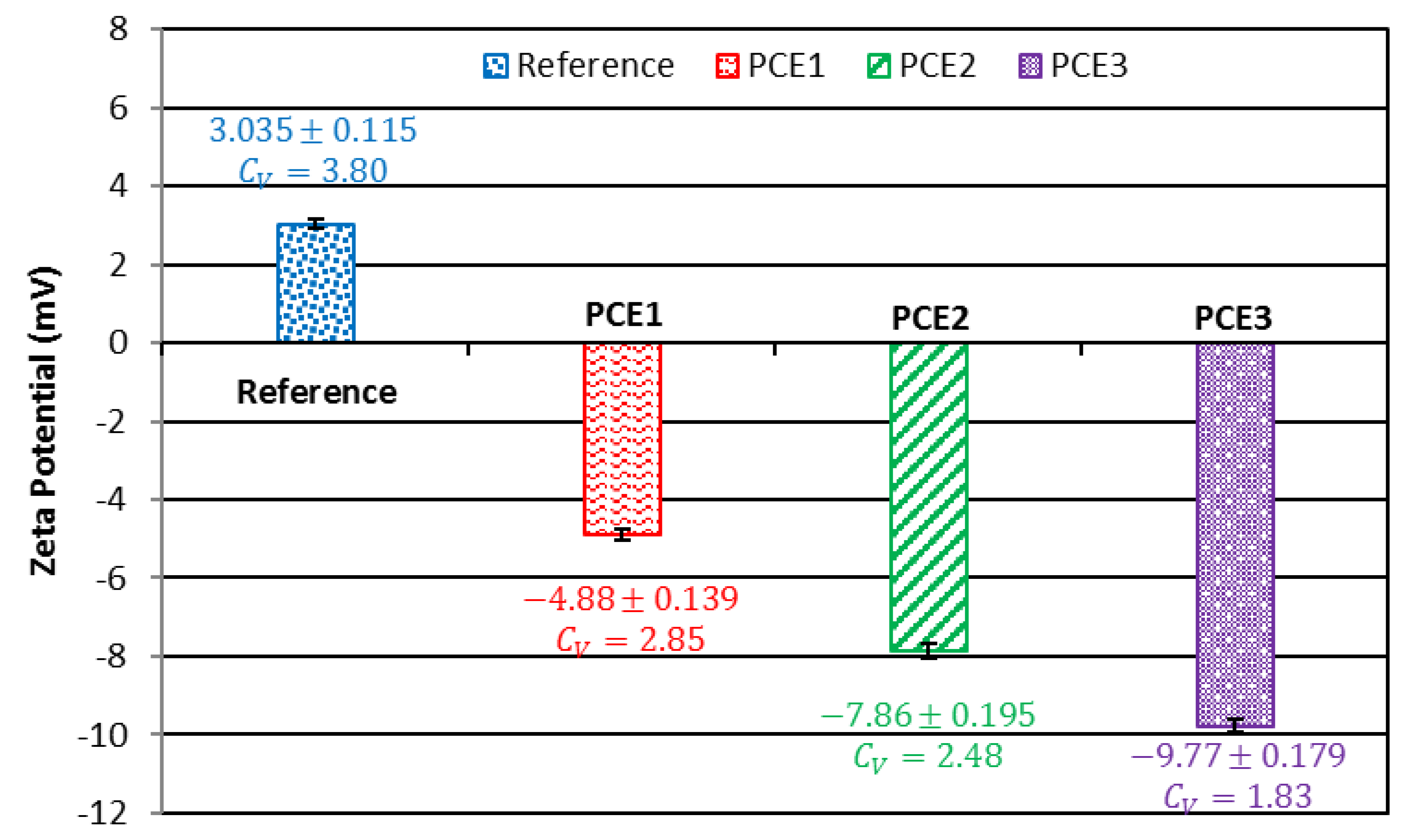
3.5. Zeta potential
The Zeta potentials of the cement pastes prepared with the addition of the PCEs (with different carboxylic densities and molecular weights) are shown in
Figure 9.
The order of the zeta potentials is as follows: reference sample <PCE1<PCE2<PCE3.This indicates that there is a good correlation between the zeta potential and carboxylic density. The results show that the zeta potential of the cement paste initially exhibited a positive value (3.035 mV), but then changed to a negative value after adding the PCE. This is because there is adsorption of the anionic groups (COO-) from the PCE on the surface of the hydration products of cementitious materials (via electrostatic adsorption by complexation with Ca2+).
PCE3 had a high carboxyl content (1.95 mmol/g) and a moderate molecular weight, whereas its polydipersity index (5 157 Da, 1.081) presented a high absolute value of zeta potential in the cement paste (9.7735 mV). This is due to it having the best adsorption capacity with regards to cement particles.
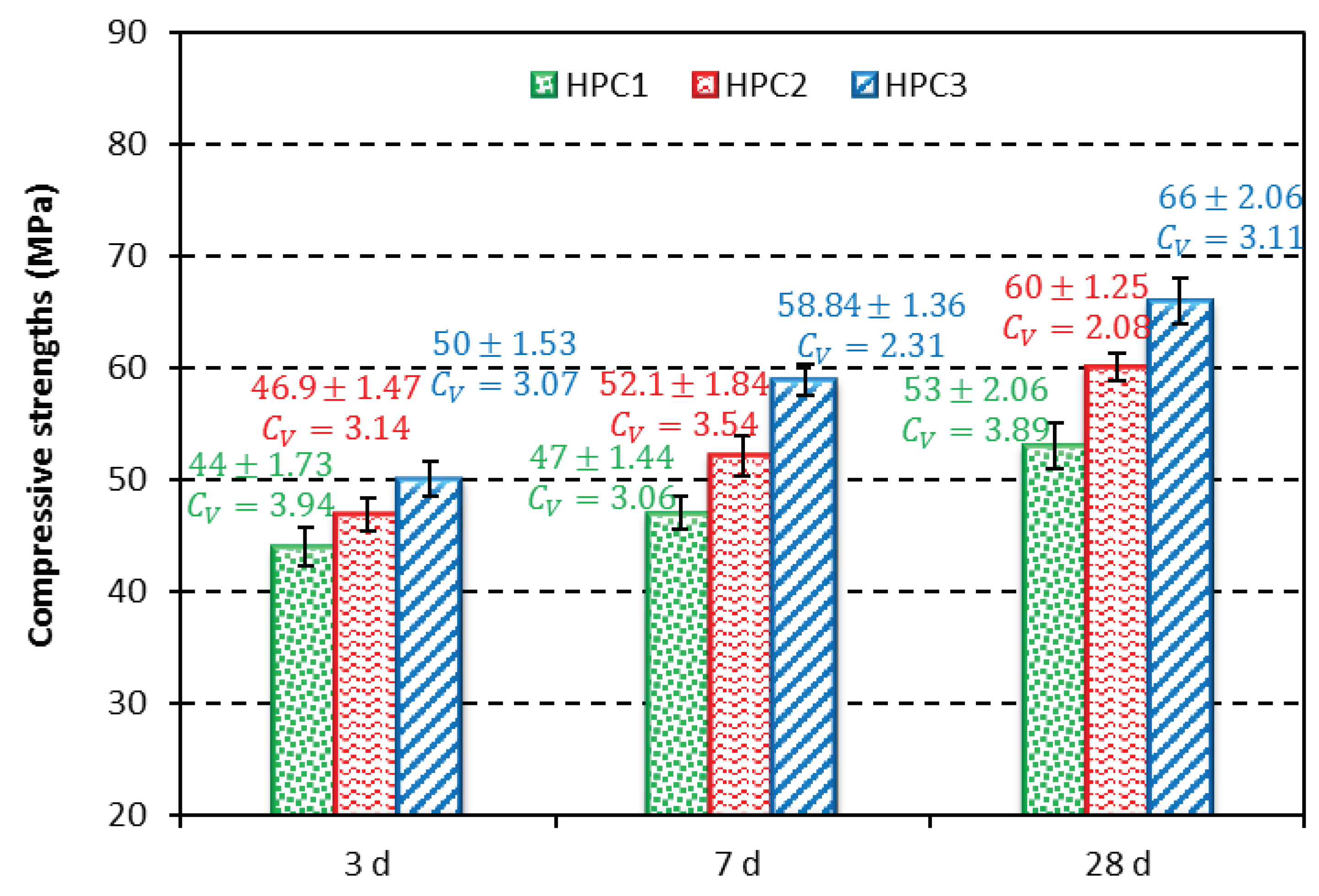
3.6. Compressive strength
The compressive strength of the HPC with PCEs is shown in
Figure 10. The compressive strength values of the hardened HPC were found to be the highest for the samples containing PCE3. The compressive strength values obtained for these samples, measured after 3, 7 and 28 curing days, were 50, 58.8 and 66 MPa, respectively. Generally, PCEs with high zeta potential exhibit good compressive strength values, which in turn are correlated with a better adsorption of superplasticizer molecules [
36].
4. Conclusions
The PCE with a moderate molecular weight and the highest content of the carboxylic groups has the best dispersion (high value of zeta potential), the lowest viscosity, and the highest compressive strength of hardened HPC. Therefore, it can be considered to be compatible with SF. Such compatibility was not observed in the case of the PCE with the low carboxyl density, which was due to the fact that this PCE was adsorbed on the SF, and thus reduced the ability of the cement particles to disperse. Therefore, the workability of the HPC was also reduced.
There is a clear relationship between the zeta potential and rheological properties of cement paste and the anionic charge density and molecular weight of PCEs. A good correlation between zeta potential, carboxylic density and compressive strength was also found.
The results from the retention slump tests show that a PCE with a high molecular weight can be applied to precast concrete, the consistency of which has to be retained over a longer period of time (until this concrete is delivered to a construction site).
The molecular weight of polycarboxylate superplasticizer has a great impact on the properties of cementitious systems. Therefore, PCE3 can be seen to be a perfect polymer, in which all the macromolecules have the same length and the same molar mass (PDI close to 1). This in turn makes it the most efficient admixture, which was proved by the results of the conducted research.
References
- World Cement Association Urges Climate Action. Available online: https://unfccc.int/news/world-cement-association-urges-climate-action (accessed on 17 February 2023).
- Mazloom, M.; Ramezanianpour, A.A.; Brooks, J.J. Effect of silica fume on mechanical properties of high-strength concrete. Cement and Concrete Composites 2004, 26, 347–357. [Google Scholar] [CrossRef]
- Saad, M.; Abo-El-Enein, S.A.; Hanna, G.B.; Kotkata, M.F. Effect of temperature on physical and mechanical properties of concrete containing silica fume. Cement and Concrete Research 1996, 26, 669–675. [Google Scholar] [CrossRef]
- Plank, J.; Schrorfl, C.; Gruber, M.; Lesti, M.; Sieber, R. Effectiveness of Polycarboxylate Superplasticizers in Ultra-High Strength Concrete: The Importance of PCE Compatibility with Silica Fume. Journal of Advanced Concrete Technology 2009, 5–12. [Google Scholar] [CrossRef]
- Hommer, H. Interaction of Polycarboxylate Ether with Silica Fume. Journal of the European Ceramic Society 2009, 1847–1853. [Google Scholar] [CrossRef]
- Meng, W.; Kumar, A.; Khayat, K. Effect of silica fume and slump-retaining polycarboxylate-based dispersant on the development of properties of portland cement paste. Cement and Concrete Composites 2019, 181–190. [Google Scholar] [CrossRef]
- Meng, W.; Lunkad, P.; Kumar, A.; Khayat, K.H. Influence of silica fume and polycarboxylate ether dispersant on hydration mechanisms of cement. Journal of Physical Chemistry C 2016, 120, 26814–26823. [Google Scholar] [CrossRef]
- Harichane, A.; Benmounah, A. Influence of Polycarboxylic Ether-based Superplasticizers (PCE) on the Rheological Properties of Cement Pastes. Journal of Materials and Engreening Structures 2021, 325–339. [Google Scholar]
- Erzengin, S.G.; Kaya, K.; Perçinözkorucuklu, S. , et al. The properties of cement systems superplasticized with methacrylic ester-based polycarboxylates. Construction and Building Materials 2018, 166, 96–109. [Google Scholar] [CrossRef]
- Lei, L.; Hirata, T.; Plank, J. 40 years of PCE superplasticizers - History, current state-of-the-art and an outlook. Cement and Concrete Research 2022, 157, 106826. [Google Scholar]
- Marchon, D.; Boscaro, F.; Flatt, R.J. First steps to the molecular structure optimization of polycarboxylate ether superplasticizers: mastering fluidity and retardation. Cement and Concrete Research 2019, 115, 116–123. [Google Scholar] [CrossRef]
- Zhonga, K.; Shaoa, Q.; Xua, H.; Weib, J. Effects of core-shell polycarboxylate superplasticizer on the fluidity and hydration behavior of cement paste. Colloids and Surfaces A 2020, 590, 124464. [Google Scholar]
- Ma, B.; Qi, H.; Tan, H.; Su, Y.; Li, X.; Liu, X.; Li, C.; Zhang, T. Effect of aliphatic-based superplasticizer on rheological performance of cement paste plasticized by polycarboxylate superplasticizer. Construction and Building Materials 2020, 233, 117181. [Google Scholar] [CrossRef]
- Conte, T.; Plank, J. Impact of molecular structure and composition of polycarboxylate comb polymers on the flow properties of alkali-activated slag. Cement and Concrete Research 2019, 116, 95–101. [Google Scholar] [CrossRef]
- Wang, X.; Yang, Y.; Shu, X.; Ran, Q.; Liu, J. Effects of polycarboxylate architecture on flow behaviour of cement paste. Advances in Cement Research 2019, 1–10. [Google Scholar] [CrossRef]
- Sha, S.; Wang, M.; Shi, C.; Xiao, Y. Influence of the structures of Polycarboxylate superplasticizer on its performance in cement-based materials-a review. Construction and Building Materials 2020, 233, 117257. [Google Scholar] [CrossRef]
- Kai, K.; Heng, Y.; Yingbin, W. Effect of chemical structure on dispersity of Polycarboxylate superplasticiser in cement paste. Advances in Cement Research 2019, 1–9. [Google Scholar] [CrossRef]
- Ezzat, M.; Xu, X.; El Cheikh, K.; Lesage, K.; Hoogenboom, R.; De Schutter, G. Structure property relationships for polycarboxylate ether superplasticizers by means of RAFT polymerization. Journal of colloid and interface science 2019, 553, 788–797. [Google Scholar] [CrossRef]
- Cook, R.; Ma, H.; Kumar, A. Mechanism of tricalcium silicate hydration in the presence of polycarboxylate polymers. SN Applied Sciences 2019, 1, 145. [Google Scholar] [CrossRef]
- Feng, H.; Feng, Z.; Wang, W.; Deng, Z.; Zheng, B. Impact of polycarboxylate superplasticizers (PCEs) with novel molecular structures on fluidity, rheological behavior and adsorption properties of cement mortar. Construction and Building Materials 2021, 292, 123285. [Google Scholar] [CrossRef]
- Li, R.; Lei, L.; Plank, J. Impact of metakaolin content and fineness on the behavior of calcined clay blended cements admixed with HPEG PCE superplasticizer. Cement and Concrete Composites 2022, 133, 104654. [Google Scholar] [CrossRef]
- Zhang, Q.; Shu, X.; Yang, Y.; Wang, X.; Liu, J.; Ran, Q. Preferential adsorption of superplasticizer on cement/silica fume and its effect on rheological properties of UHPC. Construction and Building Materials 2022, 129519. [Google Scholar] [CrossRef]
- Li, P.P.; Yu, Q.L.; Brouwers, H.J.H. Effect of PCE-type superplasticizer on early-age behaviour of ultra-high performance concrete (UHPC). Constr. Build. Mater. 2017, 153, 740–750. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, D.J.; Ryu, G.S.; Koh, K.T. Tensile behavior of Ultra High Performance Hybrid Fiber Reinforced Concrete, Cem. Concr. Compos. 2012, 34, 172–184. [Google Scholar] [CrossRef]
- Wang, M.; Yao, H. Effects of polycarboxylate ether grafted silica fume on flowability, rheological behavior and mechanical properties of cement-silica fume paste with low water-binder ratio ; Cons and Buil Mat 272. [CrossRef]
- Zhang, Y.; Kong, X. Correlations of the dispersing capability of NSF and PCE types of superplasticizer and their impacts on cement hydration with the adsorption in fresh cement pastes. Cement and concrete research 2015, 69, 1–9. [Google Scholar] [CrossRef]
- Zhang, Y.R.; Kong, X.M.; Lu, Z.B.; Lu, Z.C.; Hou, S.S. Effects of the charge characteristics of polycarboxylate superplasticizers on the adsorption and the retardation in cement pastes. Cement and Concrete Research 2015, 67, 184–196. [Google Scholar] [CrossRef]
- Yang, H.; Plank, J.; Sun, Z. Investigation on the optimal chemical structure of methacrylate ester based polycarboxylate superplasticizers to be used as cement grinding aid under laboratory conditions: Effect of anionicity, side chain length and dosage on grinding efficiency, mortar workability and strength development. Construction and Building Materials 2019, 224, 1018–1025. [Google Scholar]
- Schröfl, C.; Gruber, M.; Plank, J. Preferential adsorption of polycarboxylate superplasticizers on cement and silica fume in ultra-high performance concrete (UHPC). Cement and Concrete Research 2012, 42, 1401–1408. [Google Scholar] [CrossRef]
- Ji, Y.I.; Cahyadi, J.H. Effects of densified silica fume on microstructure and compressive strength of binary cement pastes. Cement Concr. Res. 2003, 33, 1543–1548. [Google Scholar]
- Mitchell, D.R.G.; Hinczak, I.; Day, R.A. Interaction of silica fume with calcium hydroxide solutions and hydrated cement pastes. Cement Concr. Res. 1998, 28, 1571–1584. [Google Scholar] [CrossRef]
- Ran, Q.; Liu, J.; Yang, Y.; Shu, X.; Zhang, J.; Mao, Y. Effect of molecular weight of polycarboxylate superplasticizer on its dispersion, adsorption, and hydration of a cementitious system. Journal of Materials in Civil Engineering 2016, 28, 04015184. [Google Scholar] [CrossRef]
- Papo, A.; Piani, L. Effect of various superplasticizers on the rheological properties of Portland cement pastes. Cement and Concrete Research 2004, 34, 2097–2101. [Google Scholar] [CrossRef]
- Hallal, A.; Kadri, E.H.; Ezziane, K.; Kadri, A.; Khelafi, H. Combined effect of mineral admixtures with superplasticizers on the fluidity of the blended cement paste. Construction and Building Materials 2010, 24, 1418–1423. [Google Scholar] [CrossRef]
- Ferrari, L.; Kaufmann, J.; Winnefeld, F.; Plank, J. Multi-method approach to study influence of superplasticizers on cement suspensions. Cement and Concrete Research 2011, 41, 1058–1066. [Google Scholar] [CrossRef]
- Plank, J.; Sachsenhauser, B. Impact of molecular structure on zeta potential and adsorbed conformation of α-allyl-ω-methoxypolyethylene glycol-maleic anhydride superplasticizers. Journal of advanced concrete technology 2006, 4, 233–239. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).