Submitted:
16 August 2023
Posted:
17 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
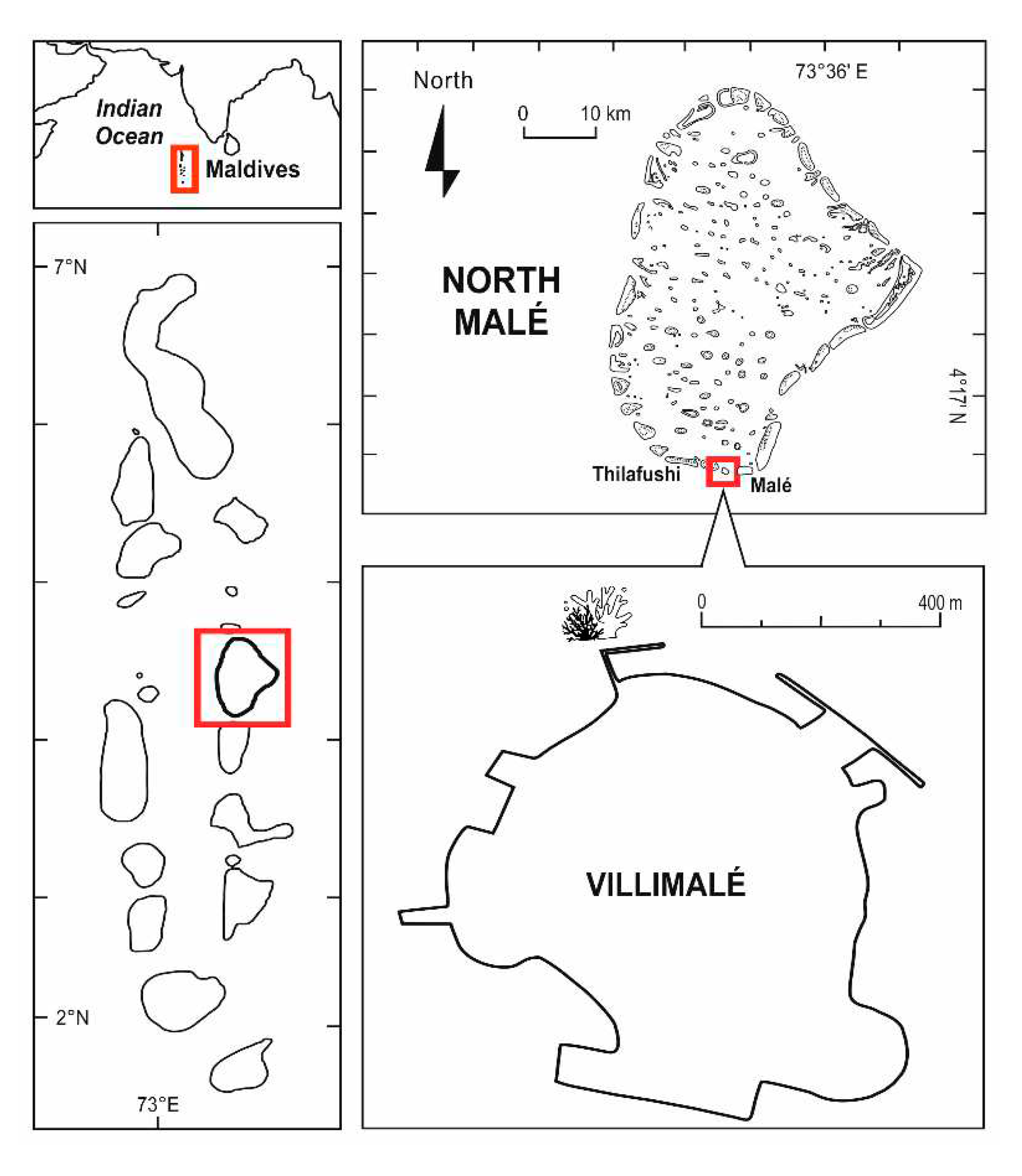
2.1. Study area and field activities
2.2. Data collection and analysis
3. Results
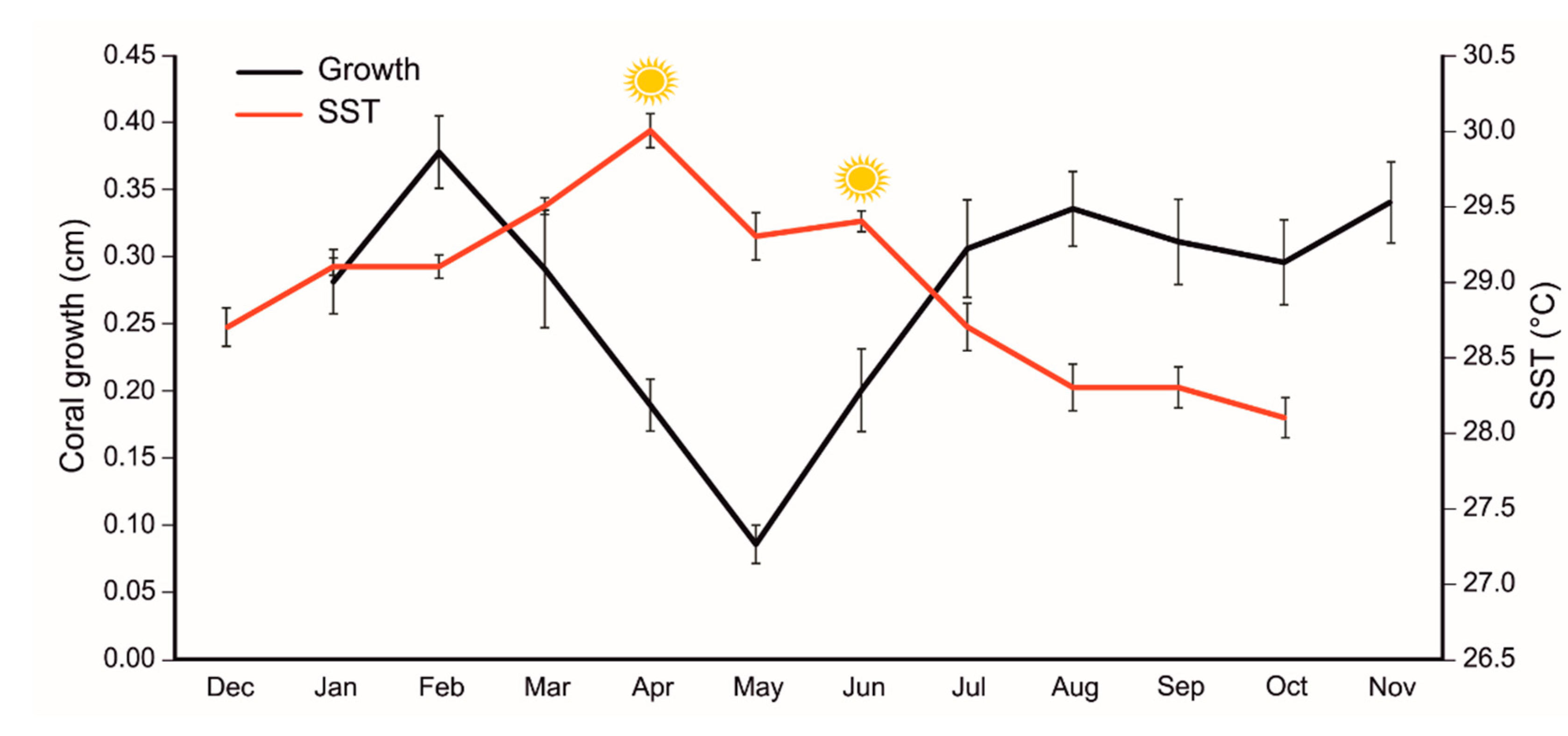
3.1. Growth rate and thermal anomaly
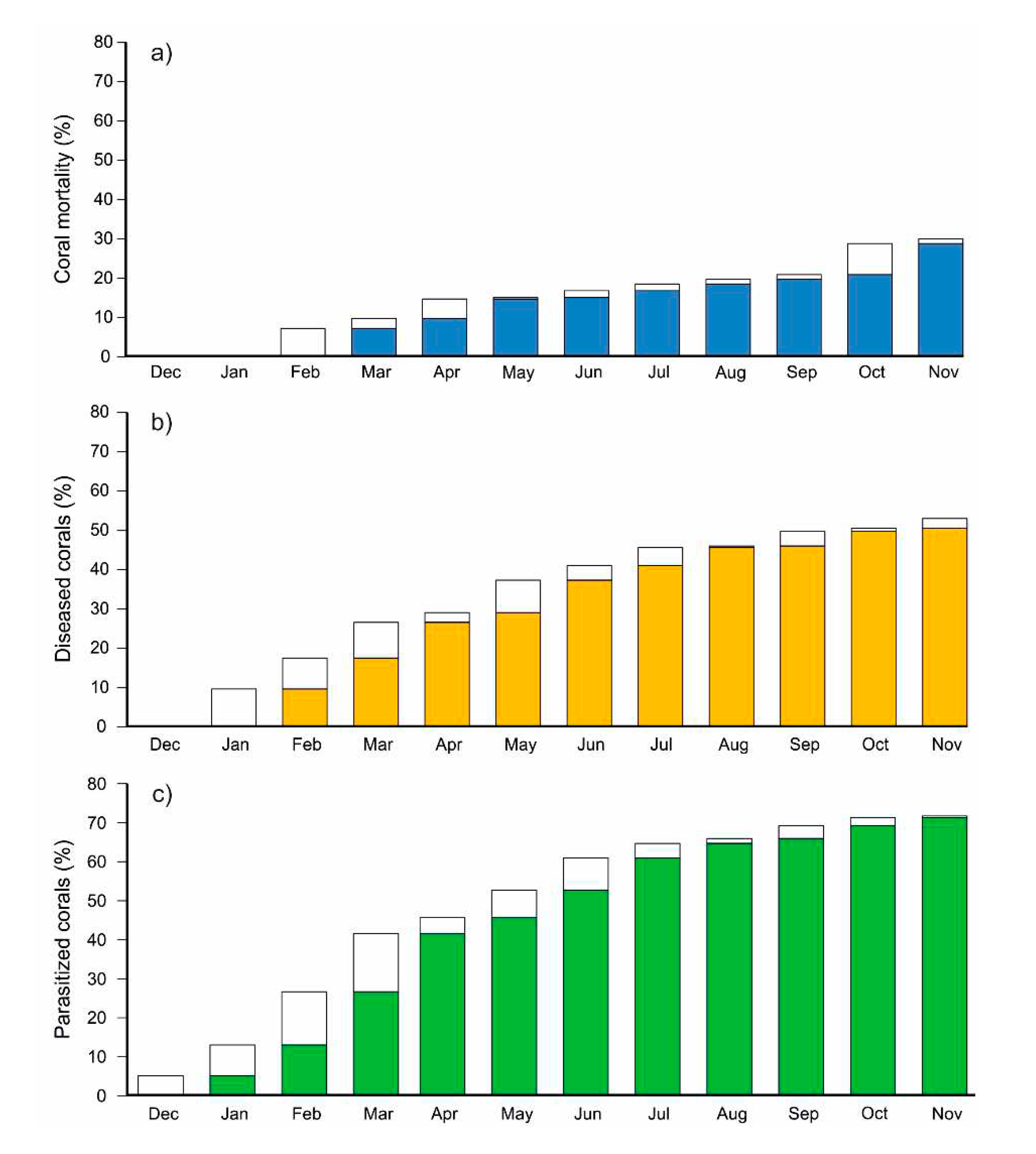
3.2. Survival rate, spreading of diseases and parasites
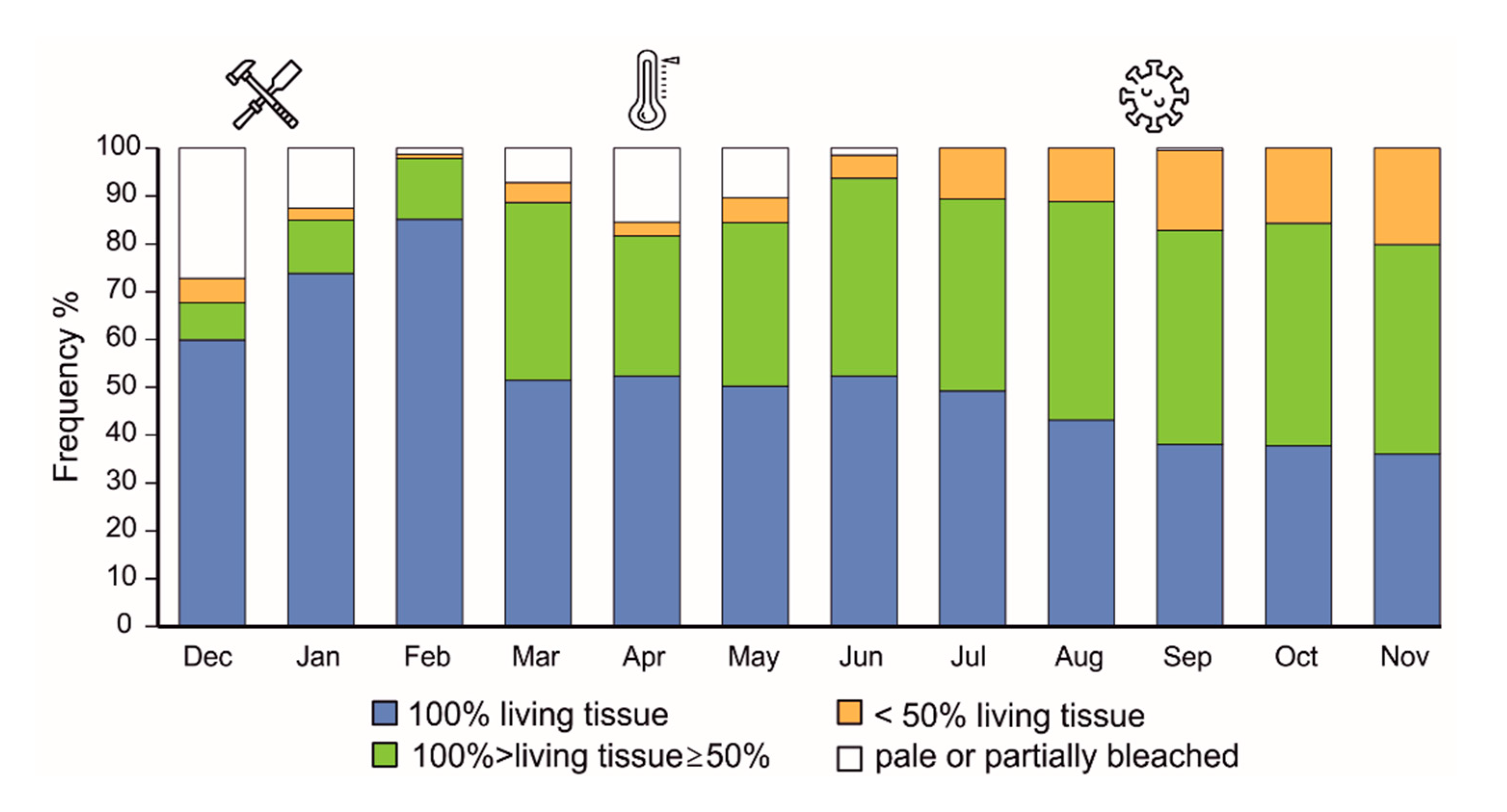
3.3. Health condition of coral fragments
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shaver, E.C.; Silliman, B.R. Time to cash in on positive interactions for coral restoration. PeerJ 2017, 5, e3499. [Google Scholar] [CrossRef] [PubMed]
- Silveira, C.B.; Cavalcant, G.S.; Walter, J.M.; Silva-Lima, A.W.; Dinsdale, E.A.; Bourne David, G.; Thompson, C.C.; Thompson, F.L. Microbial processes driving coral reef organic carbon flow. FEMS Microbiol. Rev. 2017, 41, 575–595. [Google Scholar] [CrossRef] [PubMed]
- Reaka-Kudla, M.L. The global biodiversity of coral reefs: a comparison with rain forests, In Biodiversity II: Understanding and protecting our biological resources., eds Reaka-Kudla, M.L., Wilson, D.E., Wilson E.O.; Publisher: Joseph Henry/National Academy Press: Washington, D.C, 1997; pp. 83–108. [Google Scholar]
- Heery, E.C.; Hoeksema, B.W.; Browne, N.K.; Reimer, J.D.; Ang, P.O.; Huang, D.; Friess, D.A.; Chou, L.M.; Loke, L.H.L.; Saksena-Taylor, P.; Alsagoff, N.; Yeemin, T.; Sutthacheep, M.; Vo, S.T.; Bos, A.R.; Gumanao, G.S.; Syed Hussein, M.A.; Waheed, Z.; Lane, D.J.W.; Johan, O.; Kunzmann, A.; Jompa, J.; Suharsono; Taira, D. ; Bauman, A.G.; Todd, P.A. Urban coral reefs: degradation and resilience of hard coral assemblages in coastal cities of East and Southeast Asia. Mar. Pollut. Bull. 2018, 135, 654–681. [Google Scholar] [CrossRef] [PubMed]
- Olguín-López, N.; Gutiérrez-Chavez, C.; Hérnández-Elizárraga, V.H.; Ibarra-Alvarado, C; Rojas-Molina, A. Coral reef bleaching: an ecological and biological overview. In Corals in a Changing World, eds Carmenza, D., Edisson, T.C.; IntechOpen, 2018. https://doi.org/10.5772/intechopen.69685. Available online: https://www.intechopen.com/chapters/56347 (Accessed on 12/08/2023).
- Cinner, J. Coral reef livelihoods. COSUST 2014, 7, 65–71. [Google Scholar] [CrossRef]
- Heron, S.F.; Eakin, C.M.; Douvere, F.; Anderson, K.L.; Day, J.C.; Geiger, E.; Hoegh-Guldberg, O.; Van Hooidonk, R.; Hughes, T.; Marshall, P.; Obura, D.O. Impacts of climate change on world heritage coral reefs: A first global scientific assessment. UNESCO World Heritage Centre 2017, National Oceanic and Atmospheric Administration. Available online: https://repository.library.noaa.gov/view/noaa/16386 (Accessed on 12/08/2023).
- Reguero, B.G.; Beck, M.W.; Agostini, V.N.; Kramer, P.; Hancock, B. Coral reefs for coastal protection: A new methodological approach and engineering case study in Grenada. J. Environ. Manag. 2018, 210, 146–161. [Google Scholar] [CrossRef]
- Westoby, R.; McNamara, K.E. Fear, grief, hope and action. Nat. Clim. Chang. 2019, 9, 500–501. [Google Scholar] [CrossRef]
- Morrison, T.H.; Hughes, T.P.; Adger, W. N, Brown, K.; Barnett, J.; Lemos, M.C. Save reefs to rescue all ecosystems. Nature 2019, 573, 333–336. [Google Scholar] [CrossRef]
- Montefalcone, M.; Morri, C.; Bianchi, C.N. Influence of local pressures on Maldivian coral reef resilience following repeated bleaching events, and recovery perspectives. Front. Mar. Sci. 2020, 7, 587. [Google Scholar] [CrossRef]
- Pancrazi, I.; Ahmed, H.; Cerrano, C.; Montefalcone, M. Synergic effect of global thermal anomalies and local dredging activities on coral reefs of the Maldives. Mar. Pollut. Bull. 2020, 160, 111585. [Google Scholar] [CrossRef]
- Jaap, W.C. Coral reef restoration. Ecol. Eng. 2000, 15, 345–364. [Google Scholar] [CrossRef]
- United Nations decade in ecosystem restoration 2021-2030. Available online: https://www.decadeonrestoration.org/about-un-decade (Accessed on 12/08/2023).
- Boström-Einarsson, L.; Babcock, R.C.; Bayraktarov, E.; Ceccarelli, D.; Cook, N.; Ferse Sebastian, C.A.; Hancock, B.; Harrison, P.; Hein, M.; Shaver, E.; Smith, A.; Suggett, D.; Stewart-Sinclair, P.J.; Vardi, T.; McLeod, I.M.; Bianchi, C.N. Coral restoration - A systematic review of current methods, successes, failures and future directions. PLoS One 2020, 15, e0226631. [Google Scholar] [CrossRef] [PubMed]
- Rinkevich, B. Rebuilding coral reefs: does active reef restoration lead to sustainable reefs? COSUST 2014, 7, 28–36. [Google Scholar] [CrossRef]
- Possingham, H.P.; Bode, M.; Klein, C.J. Optimal conservation outcomes require both restoration and protection. PLoS boil. 2015, 13, e1002052. [Google Scholar] [CrossRef]
- Westoby, R.; Becken, S.; Laria, A.P. Perspectives on the human dimensions of coral restoration. Reg. Environ. Change 2020, 20, 1–13. [Google Scholar] [CrossRef]
- Bowden-Kerby, A. Coral transplantation and restocking to accelerate the recovery of coral reef habitats and fisheries resources within no-take marine protected areas: hands-on approaches to support community-based coral reef management. ITMEMS 2003, AquaDocs.org. Available online: https://aquadocs.org/handle/1834/849 (Accessed on 12/08/2023).
- Rinkevich, B. Management of coral reefs: We have gone wrong when neglecting active reef restoration. Mar. Pollut. Bull. 2008, 56, 1821–1824. [Google Scholar] [CrossRef] [PubMed]
- Dehnert, I.; Galli, P.; Montano, S. Ecological impacts of coral gardening outplanting in the Maldives. Restor. Ecol. 2022, 31, e13783. [Google Scholar] [CrossRef]
- Morgan, K.M.; Kench, P.S. Reef to island sediment connections on a Maldivian carbonate platform: using benthic ecology and biosedimentary depositional facies to examine island-building potential. Earth Surf. Process. Landf. 2016, 41, 1815–1825. [Google Scholar] [CrossRef]
- Dhunya, A.; Huang, Q.; Aslam, A. Coastal habitats of Maldives: status, trends, threats, and potential conservation strategies. Int. J. Sci. Eng. Res. 2017, 8, 47–62. [Google Scholar]
- Giampiccoli, A.; Muhsin, B.A.; Mtapuri, O. Community-based tourism in the case of the Maldives. Geoj. Tour. Geosites 2020, 29, 428–439. [Google Scholar] [CrossRef]
- The World Bank IBRD-IDA. Maldives-wetland conservation and coral reef monitoring for adaptation to climate change project. Available online: https://documents.worldbank.org/en/publication/documents-reports/documentdetail/621291468283182902/maldives-wetlands-conservation-and-coral-reef-monitoring-for-adaptation-to-climate-change-project-restructuring (Accessed on 12/08/2023).
- Wadey, M. , Brown, S.; Nicholls, R.J.; Haigh, I. Coastal flooding in the Maldives: an assessment of historic events and their implications. Nat. Hazards 2017, 89, 131–159. [Google Scholar] [CrossRef]
- Dijkstra, H.A. The ENSO phenomenon: theory and mechanisms. Adv. Geosci. 2006, 6, 3–15. [Google Scholar] [CrossRef]
- Montefalcone, M.; Morri, C.; Bianchi, C.N. Long-term change in bioconstruction potential of Maldivian coral reefs following extreme climate anomalies. Glob. Change Biol. 2018, 24, 5629–5641. [Google Scholar] [CrossRef] [PubMed]
- Maldives bureau of statistics, Ministry of national planning housing & infrastructure. Available online: https://statisticsmaldives.gov.mv/census-in-2022/ (Accessed on 12/08/2023) & .
- Munavvar, R. Tragedy of the Maldives. Of fleeting paradise, enduring world power and a great sense of responsibility. The Edition Section “Climate Change” 2019. Available online: https://edition.mv/comic_of_the_day/12528 (Accessed on 12/08/2023).
- Montano, S.; Strona, G.; Seveso, D.; Maggioni, D.; Galli, P. Widespread occurrence of coral diseases in the central Maldives. Mar. Freshw. Res. 2016, 67, 1253–1262. [Google Scholar] [CrossRef]
- Harvell, C.D.; Mitchell, C.E.; Ward, J.R.; Altizer, S.; Dobson, A.P.; Ostfeld, R.S.; Samuel, M.D. Climate warming and disease risks for terrestrial and marine biota. Science 2002, 296, 2158–2162. [Google Scholar] [CrossRef]
- Miller, A.W.; Richardson, L.L. Emerging coral diseases: a temperature-driven process? Mar. Ecol. 2014, 36, 278–291. [Google Scholar] [CrossRef]
- Sokolow, S. Effects of a changing climate on the dynamics of coral infectious disease: a review of the evidence. Dis. Aquat. Org. 2009, 87, 5–18. [Google Scholar] [CrossRef]
- Barton, J.A.; Bourne, D.G.; Humphrey, C.; Hutson, K.S. Parasites and coral-associated invertebrates that impact coral health. Rev. Aquac. 2020, 12. [Google Scholar] [CrossRef]
- Montano, S. The extraordinary importance of coral-associated fauna. Diversity 2020, 12, 357. [Google Scholar] [CrossRef]
- Hein, M.Y.; Birtles, A.; Willis, B.L.; Gardiner, N.; Beeden, R.; Marshall, N.A. Coral restoration: Socio-ecological perspectives of benefits and limitations. Biol. Conserv. 2019, 229, 14–25. [Google Scholar] [CrossRef]
- Boskalis. Available online: https://boskalis.com/press/press-releases-and-company-news/boskalis-acquires-eur-120-million-contract-for-development-and-climate-adaptive-measures-for-gulhifalhu-in-the-maldives (Accessed on 12/08/2023).
- The Maldives journal fourth pillar of democracy at work. Available online: https://themaldivesjournal.com/28744 (Accessed on 12/08/2023).
- Majeedha, M. State of the Environment 2016. Ministry of Environment, Climate Change and Technology Republic of Maldives 2017. Available online: https://www.environment.gov.mv/v2/en/download/4270 (Accessed on 12/08/2023).
- Peterson, C. Assessment of solid waste management practices and its vulnerability to climate risks in Maldives Tourism Sector. Report submitted to Ministry of Tourism, Arts and Culture, Malé Republic of Maldives, 2013. Available online: chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://archive.tourism.gov.mv/downloads/tap/2014/Solid_Waste.pdf (Accessed on 12/08/2023).
- NOAA satellite and informative service, National Environmental Satellite, Data, and Information Service (NESDIS), Coral Reef Watch. Available online: https://coralreefwatch.noaa.gov/product/vs/gauges/maldives.php (Accessed on 12/08/2023).
- Schopmeyer, S.A.; Lirman, D.; Bartels, E.; Gilliam, D.S.; Goergen, E.A.; Griffin, S.P.; Johnson, M.E.; Lustic, C.; Maxwell, K.; Walter, C.S. Regional restoration benchmarks for Acropora cervicornis. Coral Reefs 2017, 36, 1047–1057. [Google Scholar] [CrossRef]
- Dehnert, I.; Saponari, L.; Isa, V.; Seveso, D.; Galli, P.; Montano, S. Exploring the performance of mid-water lagoon nurseries for coral restoration in the Maldives. Restor. Ecol. 2021, 30, e13600. [Google Scholar] [CrossRef]
- Cooper, T.F.; Gilmour, J.P.; Fabricius, K.E. Bioindicators of changes in water quality on coral reefs: review and recommendations for monitoring programmes. Coral Reefs 2009, 28, 589–606. [Google Scholar] [CrossRef]
- Browne, N.K.; Tay, J.K.; Low, J.; Larson, O.; Todd, P.A. Fluctuations in coral health of four common inshore reef corals in response to seasonal and anthropogenic changes in water quality. Mar. Environ. Res. 2015, 105, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Muller, E.M.; Sartor, C.; Alcaraz, N.I.; Van Woesik, R. Spatial epidemiology of the stony-coral-tissue-loss disease in Florida. Front. Mar. Sci. 2020, 7, 163. [Google Scholar] [CrossRef]
- Sutherland, W.J.; Clout, M.; Depledge, M.; Dicks, L.V.; Dinsdale, J.; Entwistle, A.C.; Fleishman, E; Gibbons, D. W.; Keim B.; Lickorish, F.A.; Monk, K.A.; Ockendon, N.; Peck, L.S.; Pretty, J.; Rockström, J.; Spalding, M.D.; Tonneijck, F.H.; Wintle, B.C. A horizon scan of global conservation issues for 2015. Trends Ecol. Evol. 2015, 30, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Mizuta, R.; Arakawa, O.; Ose, T.; Kusunoki, S.; Endo, H.; Kitoh, A. Classification of CMIP5 future climate responses by the tropical sea surface temperature changes. Sola 2014, 10, 167–171. [Google Scholar] [CrossRef]
- Vidal-Martínez, V.M.; Pech, D.; Sures, B.; Purucker, S.T.; Poulin, R. Can parasites really reveal environmental impact? Trends Parasitol. 2010, 26, 44–51. [Google Scholar] [CrossRef]
- Marcogliese, D.J. Parasites of the superorganism: Are they indicators of ecosystem health? Int. J. Parasitol. 2005, 35, 705–716. [Google Scholar] [CrossRef]
- Sandin, S.A.; Smith, J.E.; DeMartini, E.E.; Dinsdale, E.A.; Donner, S.D.; Friedlander, A.M.; Konotchick, T.; Malay, M.; Maragos, J.E.; Obura, D.; Pantos, O.; Paulay, G.; Richie, M.; Rohwer, F.; Schroeder, R.E.; Walsh, S.; Jackson, J.B.C.; Knowlton, N.; Sala, E. Baselines and degradation of coral reefs in the Northern Line Islands. PLoS One 2008, 3, e1548. [Google Scholar] [CrossRef]
- González-Díaz, P.; González-Sansón, G.; Aguilar Betancourt, C.; Álvarez Fernández, S.; Perera Pérez, O.; Hernández Fernández, L.; Rodríguez, V.M.F.; Caballero, Y.C. , Armenteros, M.; de la Guardia Llanso, E. Status of Cuban coral reefs. Bull. Mar. Sci. 2018, 94, 229–247. [Google Scholar] [CrossRef]
- Dean, A.J.; Church, E.K.; Loder, J.; Fielding, K.S.; Wilson, K.A. How do marine and coastal citizen science experiences foster environmental engagement? J. Environ. Manag. 2018, 213, 409–416. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




