Submitted:
03 November 2023
Posted:
03 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
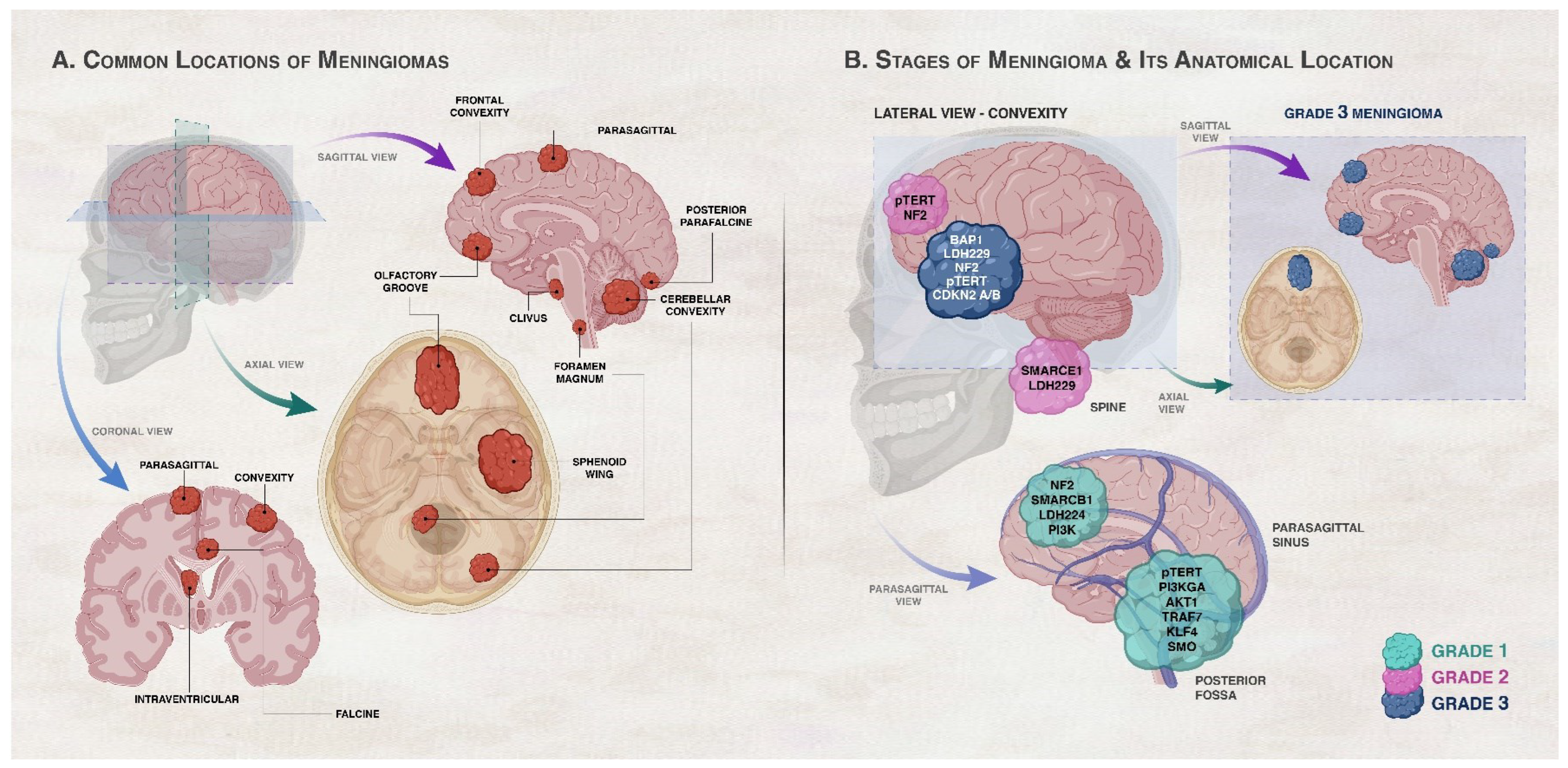
2. Grading of Meningiomas
3. Genomic Alterations and Epigenetic Modifications in Meningiomas
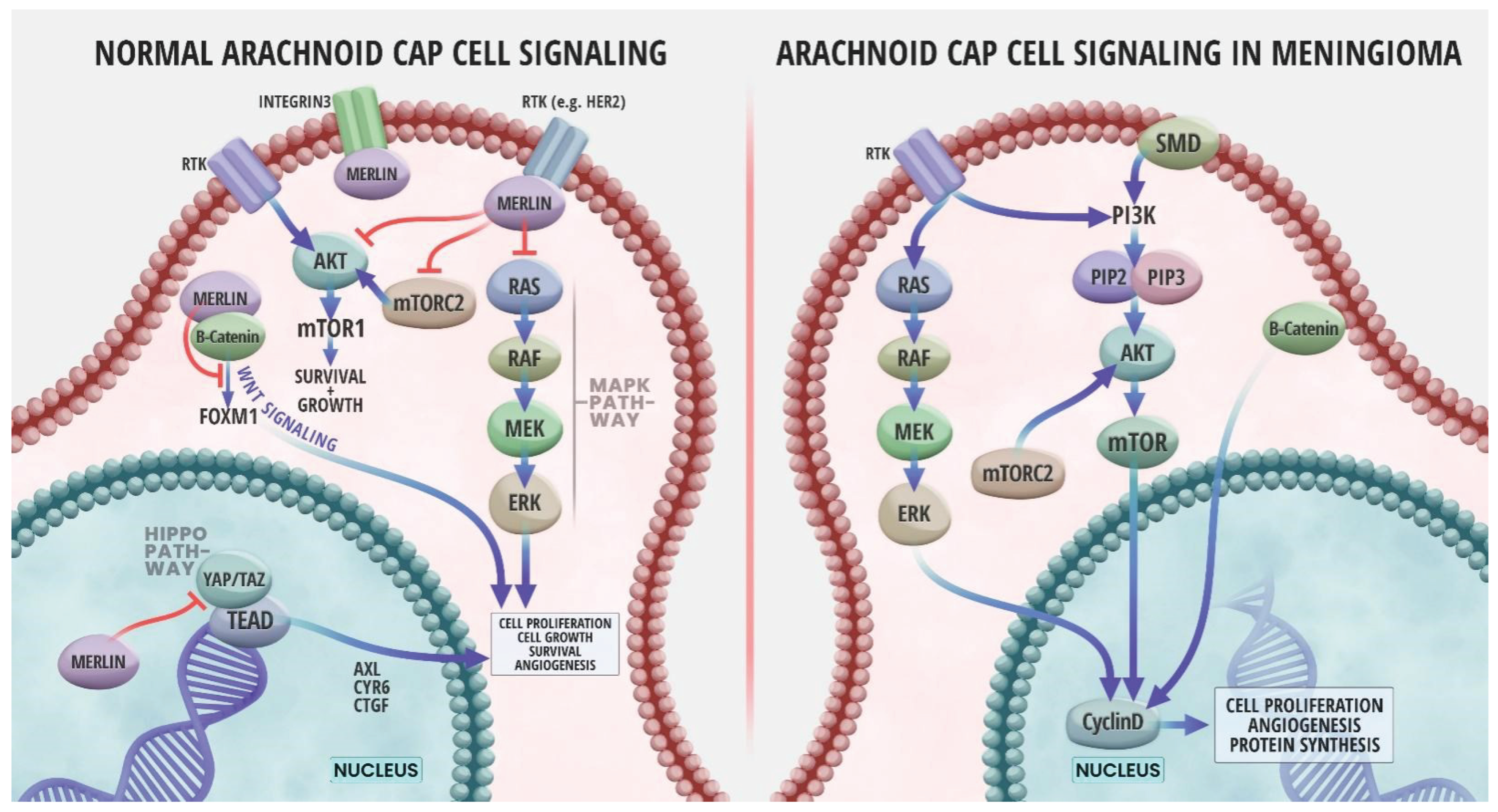
4. NF2/Merlin Signaling Pathways in Meningioma
5. Biomarkers of Meningiomas
5.1. Current Diagnosis and Prognosis
5.2. The Need for a Profile of Biomarkers of Different Types
5.3. Exploring Protein Biomarkers as Meningioma Biomarkers
- Serum Protein Biomarkers
- b.
- Cerebrospinal Fluid Protein Biomarkers
5.4. LncRNA and miRNA in Diagnosis and Prognosis of Meningiomas
6. Animal Models for Discovery of Meningioma Biomarkers
7. Conclusions
Acknowledgments
Conflicts of Interest
References
- Gittleman, H.R.; Ostrom, Q.T.; Rouse, C.D.; Dowling, J.A.; de Blank, P.M.; Kruchko, C.A.; Elder, J.B.; Rosenfeld, S.S.; Selman, W.R.; Sloan, A.E.; et al. Trends in central nervous system tumor incidence relative to other common cancers in adults, adolescents, and children in the United States, 2000 to 2010. Cancer 2015, 121, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Gittleman, H.; Liao, P.; Vecchione-Koval, T.; Wolinsky, Y.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary brain and other central nervous system tumors diagnosed in the United States in 2010–2014. Neuro-Oncology 2017, 19, v1–v88. [Google Scholar] [CrossRef] [PubMed]
- Flint-Richter, P.; Mandelzweig, L.; Oberman, B.; Sadetzki, S. Possible interaction between ionizing radiation, smoking, and gender in the causation of meningioma. Neuro-Oncology 2011, 13, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Schneider, B.; Pülhorn, H.; Röhrig, B.; Rainov, N.G. Predisposing conditions and risk factors for development of symptomatic meningioma in adults. Cancer Detection and Prevention 2005, 29, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Achey, R.L.; Gittleman, H.; Schroer, J.; Khanna, V.; Kruchko, C.; Barnholtz-Sloan, J.S. Nonmalignant and malignant meningioma incidence and survival in the elderly, 2005–2015, using the Central Brain Tumor Registry of the United States. Neuro-Oncology 2019, 21, 380–391. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathologica 2016, 131, 803–820. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.Y.; Park, C.K.; Park, S.H.; Kim, D.G.; Chung, Y.S.; Jung, H.W. Atypical and anaplastic meningiomas: prognostic implications of clinicopathological features. J Neurol Neurosurg Psychiatry 2008, 79, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro Oncol 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Loewenstern, J.; Rutland, J.; Gill, C.; Arib, H.; Pain, M.; Umphlett, M.; Kinoshita, Y.; McBride, R.; Donovan, M.; Sebra, R.; et al. Comparative genomic analysis of driver mutations in matched primary and recurrent meningiomas. Oncotarget 2019, 10, 3506–3517. [Google Scholar] [CrossRef]
- Dumanski, J.P.; Carlbom, E.; Collins, V.P.; Nordenskjöld, M. Deletion mapping of a locus on human chromosome 22 involved in the oncogenesis of meningioma. Proceedings of the National Academy of Sciences 1987, 84, 9275–9279. [Google Scholar] [CrossRef]
- Schneider, G.; Lutz, S.; Henn, W.; Zang, K.D.; Blin, N. Search for putative suppressor genes in meningioma: significance of chromosome 22. Human Genetics 1992, 88, 579–582. [Google Scholar] [CrossRef] [PubMed]
- Wellenreuther, R.; Kraus, J.A.; Lenartz, D.; Menon, A.G.; Schramm, J.; Louis, D.N.; Ramesh, V.; Gusella, J.F.; Wiestler, O.D.; von Deimling, A. Analysis of the neurofibromatosis 2 gene reveals molecular variants of meningioma. The American journal of pathology 1995, 146, 827–832. [Google Scholar] [PubMed]
- Gutmann, D.H.; Giordano, M.J.; Fishback, A.S.; Guha, A. Loss of merlin expression in sporadic meningiomas, ependymomas and schwannomas. Neurology 1997, 49, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Ruttledge, M.H.; Sarrazin, J.; Rangaratnam, S.; Phelan, C.M.; Twist, E.; Merel, P.; Delattre, O.; Thomas, G.; Nordenskjöld, M.; Collins, V.P.; et al. Evidence for the complete inactivation of the NF2 gene in the majority of sporadic meningiomas. Nat Genet 1994, 6, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Tabernero, M.D.; Espinosa, A.B.; Maíllo, A.; Sayagués, J.M.; Alguero, M.d.C.; Lumbreras, E.; Díaz, P.; Gonçalves, J.M.; Onzain, I.; Merino, M.; et al. Characterization of chromosome 14 abnormalities by interphase in situ hybridization and comparative genomic hybridization in 124 meningiomas: correlation with clinical, histopathologic, and prognostic features. American journal of clinical pathology 2005, 123, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Sayagués, J.M.; Tabernero, M.D.; Maíllo, A.; Espinosa, A.; Rasillo, A.; Díaz, P.; Ciudad, J.; López, A.; Merino, M.; Gonçalves, J.M.; et al. Intratumoral Patterns of Clonal Evolution in Meningiomas as Defined by Multicolor Interphase Fluorescence in Situ Hybridization (FISH). The Journal of Molecular Diagnostics 2004, 6, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Strickland, M.R.; Gill, C.M.; Nayyar, N.; D'Andrea, M.R.; Thiede, C.; Juratli, T.A.; Schackert, G.; Borger, D.R.; Santagata, S.; Frosch, M.P.; et al. Targeted sequencing of SMO and AKT1 in anterior skull base meningiomas. Journal of Neurosurgery 2017, 127, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Clark, V.E.; Harmancı, A.S.; Bai, H.; Youngblood, M.W.; Lee, T.I.; Baranoski, J.F.; Ercan-Sencicek, A.G.; Abraham, B.J.; Weintraub, A.S.; Hnisz, D.; et al. Recurrent somatic mutations in POLR2A define a distinct subset of meningiomas. Nature Genetics 2016, 48, 1253–1259. [Google Scholar] [CrossRef]
- Brastianos, P.K.; Horowitz, P.M.; Santagata, S.; Jones, R.T.; McKenna, A.; Getz, G.; Ligon, K.L.; Palescandolo, E.; Van Hummelen, P.; Ducar, M.D.; et al. Genomic sequencing of meningiomas identifies oncogenic SMO and AKT1 mutations. Nature Genetics 2013, 45, 285–289. [Google Scholar] [CrossRef]
- Sahm, F.; Bissel, J.; Koelsche, C.; Schweizer, L.; Capper, D.; Reuss, D.; Böhmer, K.; Lass, U.; Göck, T.; Kalis, K.; et al. AKT1E17K mutations cluster with meningothelial and transitional meningiomas and can be detected by SFRP1 immunohistochemistry. Acta Neuropathologica 2013, 126, 757–762. [Google Scholar] [CrossRef]
- Reuss, D.E.; Piro, R.M.; Jones, D.T.W.; Simon, M.; Ketter, R.; Kool, M.; Becker, A.; Sahm, F.; Pusch, S.; Meyer, J.; et al. Secretory meningiomas are defined by combined KLF4 K409Q and TRAF7 mutations. Acta Neuropathologica 2013, 125, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Clark, V.E.; Erson-Omay, E.Z.; Serin, A.; Yin, J.; Cotney, J.; Ozduman, K.; Avsar, T.; Li, J.; Murray, P.B.; Henegariu, O.; et al. Genomic analysis of non-NF2 meningiomas reveals mutations in TRAF7, KLF4, AKT1, and SMO. Science 2013, 339, 1077–1080. [Google Scholar] [CrossRef] [PubMed]
- Aldape, K.; Nejad, R.; Louis, D.N.; Zadeh, G. Integrating molecular markers into the World Health Organization classification of CNS tumors: a survey of the neuro-oncology community. Neuro Oncol 2017, 19, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Papaioannou, M.-D.; Djuric, U.; Kao, J.; Karimi, S.; Zadeh, G.; Aldape, K.; Diamandis, P. Proteomic analysis of meningiomas reveals clinically distinct molecular patterns. Neuro-Oncology 2019, 21, 1028–1038. [Google Scholar] [CrossRef] [PubMed]
- Nassiri, F.; Wang, J.Z.; Singh, O.; Karimi, S.; Dalcourt, T.; Ijad, N.; Pirouzmand, N.; Ng, H.K.; Saladino, A.; Pollo, B.; et al. Loss of H3K27me3 in meningiomas. Neuro Oncol 2021, 23, 1282–1291. [Google Scholar] [CrossRef] [PubMed]
- Katz, L.M.; Hielscher, T.; Liechty, B.; Silverman, J.; Zagzag, D.; Sen, R.; Wu, P.; Golfinos, J.G.; Reuss, D.; Neidert, M.C.; et al. Loss of histone H3K27me3 identifies a subset of meningiomas with increased risk of recurrence. Acta Neuropathologica 2018, 135, 955–963. [Google Scholar] [CrossRef]
- Bello, M.J.; Amiñoso, C.; Lopez-Marin, I.; Arjona, D.; Gonzalez-Gomez, P.; Alonso, M.E.; Lomas, J.; de Campos, J.M.; Kusak, M.E.; Vaquero, J.; et al. DNA methylation of multiple promoter-associated CpG islands in meningiomas: relationship with the allelic status at 1p and 22q. Acta Neuropathol 2004, 108, 413–421. [Google Scholar] [CrossRef]
- Barski, D.; Wolter, M.; Reifenberger, G.; Riemenschneider, M.J. Hypermethylation and transcriptional downregulation of the TIMP3 gene is associated with allelic loss on 22q12.3 and malignancy in meningiomas. Brain Pathol 2010, 20, 623–631. [Google Scholar] [CrossRef]
- Nazem, A.A.; Ruzevick, J.; Ferreira, M.J., Jr. Advances in meningioma genomics, proteomics, and epigenetics: insights into biomarker identification and targeted therapies. Oncotarget 2020, 11, 4544–4553. [Google Scholar] [CrossRef]
- Gritsch, S.; Batchelor, T.T.; Gonzalez Castro, L.N. Diagnostic, therapeutic, and prognostic implications of the 2021 World Health Organization classification of tumors of the central nervous system. Cancer 2022, 128, 47–58. [Google Scholar] [CrossRef]
- Kshettry, V.R.; Ostrom, Q.T.; Kruchko, C.; Al-Mefty, O.; Barnett, G.H.; Barnholtz-Sloan, J.S. Descriptive epidemiology of World Health Organization grades II and III intracranial meningiomas in the United States. Neuro-Oncology 2015, 17, 1166–1173. [Google Scholar] [CrossRef] [PubMed]
- Goldbrunner, R.; Minniti, G.; Preusser, M.; Jenkinson, M.D.; Sallabanda, K.; Houdart, E.; von Deimling, A.; Stavrinou, P.; Lefranc, F.; Lund-Johansen, M.; et al. EANO guidelines for the diagnosis and treatment of meningiomas. The Lancet Oncology 2016, 17, e383–e391. [Google Scholar] [CrossRef] [PubMed]
- Hashiba, T.; Hashimoto, N.; Izumoto, S.; Suzuki, T.; Kagawa, N.; Maruno, M.; Kato, A.; Yoshimine, T. Serial volumetric assessment of the natural history and growth pattern of incidentally discovered meningiomas. Journal of Neurosurgery 2009, 110, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Buerki, R.A.; Horbinski, C.M.; Kruser, T.; Horowitz, P.M.; James, C.D.; Lukas, R.V. An overview of meningiomas. Future Oncology 2018, 14, 2161–2177. [Google Scholar] [CrossRef] [PubMed]
- Chotai, S.; Schwartz, T.H. The Simpson Grading: Is It Still Valid? Cancers (Basel) 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- Surov, A.; Gottschling, S.; Bolz, J.; Kornhuber, M.; Alfieri, A.; Holzhausen, H.-J.; Abbas, J.; Kösling, S. Distant metastases in meningioma: an underestimated problem. Journal of Neuro-Oncology 2013, 112, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, T.; Aoki, M.; Kouzaki, Y.; Abe, H.; Imamura, N.; Iwasaki, A.; Inoue, T.; Nabeshima, K. WHO Grade I Meningioma Metastasis to the Lung 26 Years after Initial Surgery: A Case Report and Literature Review. NMC Case Report Journal 2019, 6, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Paix, A.; Waissi, W.; Antoni, D.; Adeduntan, R.; Noël, G. Visceral and bone metastases of a WHO grade 2 meningioma: A case report and review of the literature. Cancer/Radiothérapie 2017, 21, 55–59. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Duan, Y.; Ye, Y.; Xiao, L.; Mao, R. Subcutaneous Metastasis of Atypical Meningioma: Case Report and Literature Review. World Neurosurgery 2020, 138, 182–186. [Google Scholar] [CrossRef]
- Tsitsikov, E.N.; Hameed, S.; Tavakol, S.A.; Stephens, T.M.; Tsytsykova, A.V.; Garman, L.; Bi, W.L.; Dunn, I.F. Specific gene expression signatures of low grade meningiomas. Front Oncol 2023, 13, 1126550. [Google Scholar] [CrossRef]
- Harmancı, A.S.; Youngblood, M.W.; Clark, V.E.; Coşkun, S.; Henegariu, O.; Duran, D.; Erson-Omay, E.Z.; Kaulen, L.D.; Lee, T.I.; Abraham, B.J.; et al. Integrated genomic analyses of de novo pathways underlying atypical meningiomas. Nat Commun 2017, 8, 14433. [Google Scholar] [CrossRef]
- Patel, A.J.; Wan, Y.W.; Al-Ouran, R.; Revelli, J.P.; Cardenas, M.F.; Oneissi, M.; Xi, L.; Jalali, A.; Magnotti, J.F.; Muzny, D.M.; et al. Molecular profiling predicts meningioma recurrence and reveals loss of DREAM complex repression in aggressive tumors. Proc Natl Acad Sci U S A 2019, 116, 21715–21726. [Google Scholar] [CrossRef]
- Nassiri, F.; Liu, J.; Patil, V.; Mamatjan, Y.; Wang, J.Z.; Hugh-White, R.; Macklin, A.M.; Khan, S.; Singh, O.; Karimi, S.; et al. A clinically applicable integrative molecular classification of meningiomas. Nature 2021, 597, 119–125. [Google Scholar] [CrossRef]
- Bayley, J.C.t.; Hadley, C.C.; Harmanci, A.O.; Harmanci, A.S.; Klisch, T.J.; Patel, A.J. Multiple approaches converge on three biological subtypes of meningioma and extract new insights from published studies. Sci Adv 2022, 8, eabm6247. [Google Scholar] [CrossRef] [PubMed]
- Torp, S.H.; Solheim, O.; Skjulsvik, A.J. The WHO 2021 Classification of Central Nervous System tumours: a practical update on what neurosurgeons need to know-a minireview. Acta Neurochir (Wien) 2022, 164, 2453–2464. [Google Scholar] [CrossRef]
- Marastoni, E.; Barresi, V. Meningioma Grading beyond Histopathology: Relevance of Epigenetic and Genetic Features to Predict Clinical Outcome. Cancers (Basel) 2023, 15. [Google Scholar] [CrossRef]
- Pawloski, J.A.; Fadel, H.A.; Huang, Y.W.; Lee, I.Y. Genomic Biomarkers of Meningioma: A Focused Review. Int J Mol Sci 2021, 22. [Google Scholar] [CrossRef] [PubMed]
- Ruttledge, M.H.; Xie, Y.G.; Han, F.Y.; Giovannini, M.; Janson, M.; Fransson, I.; Werelius, B.; Delattre, O.; Thomas, G.; Evans, G.; et al. Physical mapping of the NF2/meningioma region on human chromosome 22q12. Genomics 1994, 19, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Ichimura, K.; Yuasa, Y. [Molecular biological analysis of neurofibromatosis type 2 gene]. Nihon Rinsho 1993, 51, 2462–2466. [Google Scholar]
- Cui, Y.; Ma, L.; Schacke, S.; Yin, J.C.; Hsueh, Y.P.; Jin, H.; Morrison, H. Merlin cooperates with neurofibromin and Spred1 to suppress the Ras-Erk pathway. Hum Mol Genet 2021, 29, 3793–3806. [Google Scholar] [CrossRef]
- Maggio, I.; Franceschi, E.; Tosoni, A.; Nunno, V.D.; Gatto, L.; Lodi, R.; Brandes, A.A. Meningioma: not always a benign tumor. A review of advances in the treatment of meningiomas. CNS Oncol 2021, 10, Cns72. [Google Scholar] [CrossRef] [PubMed]
- Moussalem, C.; Massaad, E.; Minassian, G.B.; Ftouni, L.; Bsat, S.; Houshiemy, M.N.E.; Alomari, S.; Sarieddine, R.; Kobeissy, F.; Omeis, I. Meningioma genomics: a therapeutic challenge for clinicians. J Integr Neurosci 2021, 20, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Hua, L.; Han, T.; Tian, M.; Wang, D.; Tang, H.; Sun, S.; Chen, H.; Cheng, H.; Zhang, T.; et al. The CREB-binding protein inhibitor ICG-001: a promising therapeutic strategy in sporadic meningioma with NF2 mutations. Neurooncol Adv 2020, 2, vdz055. [Google Scholar] [CrossRef] [PubMed]
- Youngblood, M.W.; Miyagishima, D.F.; Jin, L.; Gupte, T.; Li, C.; Duran, D.; Montejo, J.D.; Zhao, A.; Sheth, A.; Tyrtova, E.; et al. Associations of meningioma molecular subgroup and tumor recurrence. Neuro Oncol 2021, 23, 783–794. [Google Scholar] [CrossRef]
- Abedalthagafi, M.; Bi, W.L.; Aizer, A.A.; Merrill, P.H.; Brewster, R.; Agarwalla, P.K.; Listewnik, M.L.; Dias-Santagata, D.; Thorner, A.R.; Van Hummelen, P.; et al. Oncogenic PI3K mutations are as common as AKT1 and SMO mutations in meningioma. Neuro Oncol 2016, 18, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Bi, W.L.; Zhang, M.; Wu, W.W.; Mei, Y.; Dunn, I.F. Meningioma Genomics: Diagnostic, Prognostic, and Therapeutic Applications. Front Surg 2016, 3, 40. [Google Scholar] [CrossRef] [PubMed]
- Zotti, T.; Scudiero, I.; Vito, P.; Stilo, R. The Emerging Role of TRAF7 in Tumor Development. J Cell Physiol 2017, 232, 1233–1238. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Yuan, J.; Deng, Y.; Fan, X.; Shen, J. Emerging role of SWI/SNF complex deficiency as a target of immune checkpoint blockade in human cancers. Oncogenesis 2021, 10, 3. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.P.; Rand, K.N.; Molloy, P.L. Hypomethylation of repeated DNA sequences in cancer. Epigenomics 2010, 2, 245–269. [Google Scholar] [CrossRef]
- Robert, S.M.; Vetsa, S.; Nadar, A.; Vasandani, S.; Youngblood, M.W.; Gorelick, E.; Jin, L.; Marianayagam, N.; Erson-Omay, E.Z.; Günel, M.; et al. The integrated multiomic diagnosis of sporadic meningiomas: a review of its clinical implications. J Neurooncol 2022, 156, 205–214. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, K. Pan-cancer analysis of frequent DNA co-methylation patterns reveals consistent epigenetic landscape changes in multiple cancers. BMC Genomics 2017, 18, 1045. [Google Scholar] [CrossRef] [PubMed]
- Nassiri, F.; Mamatjan, Y.; Suppiah, S.; Badhiwala, J.H.; Mansouri, S.; Karimi, S.; Saarela, O.; Poisson, L.; Gepfner-Tuma, I.; Schittenhelm, J.; et al. DNA methylation profiling to predict recurrence risk in meningioma: development and validation of a nomogram to optimize clinical management. Neuro Oncol 2019, 21, 901–910. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Sharma, R.; Shukla, N.; Narwal, P.; Katiyar, A.; Mahajan, S.; Sahu, S.; Garg, A.; Sharma, M.C.; Suri, A.; et al. DNA methylation profiling of meningiomas highlights clinically distinct molecular subgroups. J Neurooncol 2023, 161, 339–356. [Google Scholar] [CrossRef]
- Choudhury, A.; Magill, S.T.; Eaton, C.D.; Prager, B.C.; Chen, W.C.; Cady, M.A.; Seo, K.; Lucas, C.G.; Casey-Clyde, T.J.; Vasudevan, H.N.; et al. Meningioma DNA methylation groups identify biological drivers and therapeutic vulnerabilities. Nat Genet 2022, 54, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Abbritti, R.V.; Polito, F.; Cucinotta, M.; Lo Giudice, C.; Caffo, M.; Tomasello, C.; Germanò, A.; Aguennouz, M. Meningiomas and Proteomics: Focus on New Potential Biomarkers and Molecular Pathways. Cancer Genomics Proteomics 2016, 13, 369–379. [Google Scholar] [PubMed]
- Sievers, P.; Hielscher, T.; Schrimpf, D.; Stichel, D.; Reuss, D.E.; Berghoff, A.S.; Neidert, M.C.; Wirsching, H.G.; Mawrin, C.; Ketter, R.; et al. CDKN2A/B homozygous deletion is associated with early recurrence in meningiomas. Acta Neuropathol 2020, 140, 409–413. [Google Scholar] [CrossRef]
- Zhi, F.; Zhou, G.; Wang, S.; Shi, Y.; Peng, Y.; Shao, N.; Guan, W.; Qu, H.; Zhang, Y.; Wang, Q.; et al. A microRNA expression signature predicts meningioma recurrence. Int J Cancer 2013, 132, 128–136. [Google Scholar] [CrossRef]
- Zhi, F.; Shao, N.; Li, B.; Xue, L.; Deng, D.; Xu, Y.; Lan, Q.; Peng, Y.; Yang, Y. A serum 6-miRNA panel as a novel non-invasive biomarker for meningioma. Scientific Reports 2016, 6, 32067. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, G.; Huang, H.; Li, H.; Lin, S.; Wang, Y. Differentially Expressed MicroRNAs in Radioresistant and Radiosensitive Atypical Meningioma: A Clinical Study in Chinese Patients. Front Oncol 2020, 10, 501. [Google Scholar] [CrossRef]
- Carneiro, V.; Cirino, M.; Panepucci, R.; Peria, F.; Tirapelli, D.; Colli, B.; Carlotti Jr, C.G. The Role of MicroRNA 181d as a Possible Biomarker Associated With Tumor Progression in Meningiomas. Cureus 2021, 13, e19158. [Google Scholar] [CrossRef]
- Ding, C.; Yi, X.; Xu, J.; Huang, Z.; Bu, X.; Wang, D.; Ge, H.; Zhang, G.; Gu, J.; Kang, D.; et al. Long Non-Coding RNA MEG3 Modifies Cell-Cycle, Migration, Invasion, and Proliferation Through AKAP12 by Sponging miR-29c in Meningioma Cells. Frontiers in Oncology 2020, 10. [Google Scholar] [CrossRef]
- Slavik, H.; Balik, V.; Kokas, F.Z.; Slavkovsky, R.; Vrbkova, J.; Rehulkova, A.; Lausova, T.; Ehrmann, J.; Gurska, S.; Uberall, I.; et al. Transcriptomic Profiling Revealed Lnc-GOLGA6A-1 as a Novel Prognostic Biomarker of Meningioma Recurrence. Neurosurgery 2022, 91, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Xing, H.; Wang, S.; Li, Q.; Ma, Y.; Sun, P. Long noncoding RNA LINC00460 targets miR-539/MMP-9 to promote meningioma progression and metastasis. Biomed Pharmacother 2018, 105, 677–682. [Google Scholar] [CrossRef] [PubMed]
- Eraky, A.M. Non-coding RNAs as Genetic Biomarkers for the Diagnosis, Prognosis, Radiosensitivity, and Histopathologic Grade of Meningioma. Cureus 2023, 15, e34593. [Google Scholar] [CrossRef] [PubMed]
- Murnyák, B.; Bognár, L.; Klekner, Á.; Hortobágyi, T. Epigenetics of Meningiomas. Biomed Res Int 2015, 2015, 532451. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Lin, D.; Cheng, L.; Tu, S.; Wu, H.; Xu, W.; Pan, Y.; Wang, X.; Zhang, J.; Shao, A. Is DNA Methylation a Ray of Sunshine in Predicting Meningioma Prognosis? Front Oncol 2020, 10, 1323. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Gao, Y.; Zhang, J.; Han, L.; Zhao, H. DNA methylation meningioma biomarkers: attributes and limitations. Front Mol Neurosci 2023, 16, 1182759. [Google Scholar] [CrossRef] [PubMed]
- Berghoff, A.S.; Hielscher, T.; Ricken, G.; Furtner, J.; Schrimpf, D.; Widhalm, G.; Rajky, U.; Marosi, C.; Hainfellner, J.A.; von Deimling, A.; et al. Prognostic impact of genetic alterations and methylation classes in meningioma. Brain Pathol 2022, 32, e12970. [Google Scholar] [CrossRef]
- Sahm, F.; Schrimpf, D.; Olar, A.; Koelsche, C.; Reuss, D.; Bissel, J.; Kratz, A.; Capper, D.; Schefzyk, S.; Hielscher, T.; et al. TERT Promoter Mutations and Risk of Recurrence in Meningioma. J Natl Cancer Inst 2016, 108. [Google Scholar] [CrossRef]
- Mirian, C.; Duun-Henriksen, A.K.; Juratli, T.; Sahm, F.; Spiegl-Kreinecker, S.; Peyre, M.; Biczok, A.; Tonn, J.C.; Goutagny, S.; Bertero, L.; et al. Poor prognosis associated with TERT gene alterations in meningioma is independent of the WHO classification: an individual patient data meta-analysis. J Neurol Neurosurg Psychiatry 2020, 91, 378–387. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, S.K.; Yoo, Y.C.; Park, N.H.; Park, D.B.; Yoo, J.S.; An, H.J.; Park, Y.M.; Cho, K.G. Proteome analysis of human cerebrospinal fluid as a diagnostic biomarker in patients with meningioma. Med Sci Monit 2012, 18, Br450–Br460. [Google Scholar] [CrossRef]
- Erkan, E.P.; Ströbel, T.; Dorfer, C.; Sonntagbauer, M.; Weinhäusel, A.; Saydam, N.; Saydam, O. Circulating Tumor Biomarkers in Meningiomas Reveal a Signature of Equilibrium Between Tumor Growth and Immune Modulation. Frontiers in Oncology 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Liu, C.; Xin, Y.; Zhang, A.; Zhou, Y.; Dong, D.; Ren, L. Evaluating EFEMP1 in Cerebrospinal Fluid and Serum as a Potential Diagnosis Biomarker for Meningiomas. Clin Lab 2017, 63, 1717–1722. [Google Scholar] [CrossRef] [PubMed]
- Anis, S.E.; Lotfalla, M.; Zain, M.; Kamel, N.N.; Soliman, A.A. Value of SSTR2A and Claudin - 1 in Differentiating Meningioma from Schwannoma and Hemangiopericytoma. Open Access Maced J Med Sci 2018, 6, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.; Baia, G.S.; Smith, J.S.; McDermott, M.W.; Bollen, A.W.; VandenBerg, S.R.; Lamborn, K.R.; Lal, A. Expression of the Hypoxia Marker Carbonic Anhydrase 9 Is Associated with Anaplastic Phenotypes in Meningiomas. Clinical Cancer Research 2007, 13, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Ijare, O.B.; Hambarde, S.; Brasil da Costa, F.H.; Lopez, S.; Sharpe, M.A.; Helekar, S.A.; Hangel, G.; Bogner, W.; Widhalm, G.; Bachoo, R.M.; et al. Glutamine anaplerosis is required for amino acid biosynthesis in human meningiomas. Neuro Oncol 2022, 24, 556–568. [Google Scholar] [CrossRef] [PubMed]
- Masalha, W.; Daka, K.; Woerner, J.; Pompe, N.; Weber, S.; Delev, D.; Krüger, M.T.; Schnell, O.; Beck, J.; Heiland, D.H.; et al. Metabolic alterations in meningioma reflect the clinical course. BMC Cancer 2021, 21, 211. [Google Scholar] [CrossRef] [PubMed]
- Safari Yazd, H.; Bazargani, S.F.; Fitzpatrick, G.; Yost, R.A.; Kresak, J.; Garrett, T.J. Metabolomic and Lipidomic Characterization of Meningioma Grades Using LC-HRMS and Machine Learning. J Am Soc Mass Spectrom 2023. [Google Scholar] [CrossRef] [PubMed]
- Bender, L.; Somme, F.; Ruhland, E.; Cicek, A.E.; Bund, C.; Namer, I.J. Metabolomic Profile of Aggressive Meningiomas by Using High-Resolution Magic Angle Spinning Nuclear Magnetic Resonance. J Proteome Res 2020, 19, 292–299. [Google Scholar] [CrossRef]
- Maas, S.L.N.; Stichel, D.; Hielscher, T.; Sievers, P.; Berghoff, A.S.; Schrimpf, D.; Sill, M.; Euskirchen, P.; Blume, C.; Patel, A.; et al. Integrated Molecular-Morphologic Meningioma Classification: A Multicenter Retrospective Analysis, Retrospectively and Prospectively Validated. J Clin Oncol 2021, 39, 3839–3852. [Google Scholar] [CrossRef]
- Driver, J.; Hoffman, S.E.; Tavakol, S.; Woodward, E.; Maury, E.A.; Bhave, V.; Greenwald, N.F.; Nassiri, F.; Aldape, K.; Zadeh, G.; et al. A molecularly integrated grade for meningioma. Neuro Oncol 2022, 24, 796–808. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Song, T.; Li, J.; Ao, F.; Gong, X.; Lu, Y.; Zhang, C.; Chen, L.; Liu, Y.; He, H.; et al. RAS Promotes Proliferation and Resistances to Apoptosis in Meningioma. Mol Neurobiol 2017, 54, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Pecina-Slaus, N. Merlin, the NF2 gene product. Pathol Oncol Res 2013, 19, 365–373. [Google Scholar] [CrossRef]
- Mawrin, C.; Sasse, T.; Kirches, E.; Kropf, S.; Schneider, T.; Grimm, C.; Pambor, C.; Vorwerk, C.K.; Firsching, R.; Lendeckel, U.; et al. Different activation of mitogen-activated protein kinase and Akt signaling is associated with aggressive phenotype of human meningiomas. Clin Cancer Res 2005, 11, 4074–4082. [Google Scholar] [CrossRef] [PubMed]
- G Mougel, G.M. , R Appay, A Querdray, C Roche, A Jijon, I Konstantinova, A Soude, T Graillon, A Barlier. A Hippo signaling pathway is strongly involved in meningioma tumorigenesis Neuro-Oncology 2022, 24. [Google Scholar] [CrossRef]
- Hong, A.W.; Meng, Z.; Plouffe, S.W.; Lin, Z.; Zhang, M.; Guan, K.L. Critical roles of phosphoinositides and NF2 in Hippo pathway regulation. Genes Dev 2020, 34, 511–525. [Google Scholar] [CrossRef] [PubMed]
- Baia, G.S.; Caballero, O.L.; Riggins, G.J. The Hippo signaling pathway and translational opportunities for brain cancers. CNS Oncol 2012, 1, 113–115. [Google Scholar] [CrossRef] [PubMed]
- Sievers, P.; Chiang, J.; Schrimpf, D.; Stichel, D.; Paramasivam, N.; Sill, M.; Gayden, T.; Casalini, B.; Reuss, D.E.; Dalton, J.; et al. YAP1-fusions in pediatric NF2-wildtype meningioma. Acta Neuropathol 2020, 139, 215–218. [Google Scholar] [CrossRef]
- Laraba, L.; Hillson, L.; de Guibert, J.G.; Hewitt, A.; Jaques, M.R.; Tang, T.T.; Post, L.; Ercolano, E.; Rai, G.; Yang, S.M.; et al. Inhibition of YAP/TAZ-driven TEAD activity prevents growth of NF2-null schwannoma and meningioma. Brain 2022. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, Y.; Zhou, C.; Mei, W.; Zeng, C. PI3K/Akt/mTOR Pathway and Its Role in Cancer Therapeutics: Are We Making Headway? Front Oncol 2022, 12, 819128. [Google Scholar] [CrossRef]
- Rong, R.; Tang, X.; Gutmann, D.H.; Ye, K. Neurofibromatosis 2 (NF2) tumor suppressor merlin inhibits phosphatidylinositol 3-kinase through binding to PIKE-L. Proc Natl Acad Sci U S A 2004, 101, 18200–18205. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Karas, P.J.; Hadley, C.C.; Bayley, V.J.; Khan, A.B.; Jalali, A.; Sweeney, A.D.; Klisch, T.J.; Patel, A.J. The Role of Merlin/NF2 Loss in Meningioma Biology. Cancers (Basel) 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Barresi, V.; Lionti, S.; La Rocca, L.; Caliri, S.; Caffo, M. High p-mTOR expression is associated with recurrence and shorter disease-free survival in atypical meningiomas. Neuropathology 2019, 39, 22–29. [Google Scholar] [CrossRef] [PubMed]
- James, M.F.; Han, S.; Polizzano, C.; Plotkin, S.R.; Manning, B.D.; Stemmer-Rachamimov, A.O.; Gusella, J.F.; Ramesh, V. NF2/merlin is a novel negative regulator of mTOR complex 1, and activation of mTORC1 is associated with meningioma and schwannoma growth. Mol Cell Biol 2009, 29, 4250–4261. [Google Scholar] [CrossRef]
- Shih, K.C.; Chowdhary, S.; Rosenblatt, P.; Weir, A.B., 3rd; Shepard, G.C.; Williams, J.T.; Shastry, M.; Burris, H.A., 3rd; Hainsworth, J.D. A phase II trial of bevacizumab and everolimus as treatment for patients with refractory, progressive intracranial meningioma. J Neurooncol 2016, 129, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Plotkin, S.R.; Kumthekar, P.; Wen, P.Y.; Barker, F.G.; Stemmer-Rachamimov, A.; Beauchamp, R.L.; Jordan, J.T.; Muzikansky, A.; Ramesh, V. Multi-center, single arm phase II study of the dual mTORC1/mTORC2 inhibitor vistusertib for patients with recurrent or progressive grade II-III meningiomas. Journal of Clinical Oncology 2021, 39, 2024. [Google Scholar] [CrossRef]
- Pecina-Slaus, N.; Kafka, A.; Lechpammer, M. Molecular Genetics of Intracranial Meningiomas with Emphasis on Canonical Wnt Signalling. Cancers (Basel) 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Lau, Y.K.; Murray, L.B.; Houshmandi, S.S.; Xu, Y.; Gutmann, D.H.; Yu, Q. Merlin is a potent inhibitor of glioma growth. Cancer Res 2008, 68, 5733–5742. [Google Scholar] [CrossRef] [PubMed]
- Spille, D.C.; Sporns, P.B.; Heß, K.; Stummer, W.; Brokinkel, B. Prediction of High-Grade Histology and Recurrence in Meningiomas Using Routine Preoperative Magnetic Resonance Imaging: A Systematic Review. World Neurosurg 2019, 128, 174–181. [Google Scholar] [CrossRef]
- Nowosielski, M.; Galldiks, N.; Iglseder, S.; Kickingereder, P.; von Deimling, A.; Bendszus, M.; Wick, W.; Sahm, F. Diagnostic challenges in meningioma. Neuro-Oncology 2017, 19, 1588–1598. [Google Scholar] [CrossRef]
- Tagle, P.; Villanueva, P.; Torrealba, G.; Huete, I. Intracranial metastasis or meningioma? Surgical Neurology 2002, 58, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Bendszus, M.; Warmuth-Metz, M.; Burger, R.; Klein, R.; Tonn, J.C.; Solymosi, L. Diagnosing dural metastases: the value of 1 H magnetic resonance spectroscopy. Neuroradiology 2001, 43, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Bourekas, E.C.; Wildenhain, P.; Lewin, J.S.; Tarr, R.W.; Dastur, K.J.; Raji, M.R.; Lanzieri, C.F. The dural tail sign revisited. AJNR. American journal of neuroradiology 1995, 16, 1514–1516. [Google Scholar] [PubMed]
- Wilms, G.; Lammens, M.; Marchal, G.; Demaerel, P.; Verplancke, J.; Van Calenbergh, F.; Goffin, J.; Plets, C.; Baert, A.L. Prominent dural enhancement adjacent to nonmeningiomatous malignant lesions on contrast-enhanced MR images. AJNR. American journal of neuroradiology 12 761–764.
- Huttner, H.B.; Bergmann, O.; Salehpour, M.; El Cheikh, R.; Nakamura, M.; Tortora, A.; Heinke, P.; Coras, R.; Englund, E.; Eyüpoglu, I.Y.; et al. Meningioma growth dynamics assessed by radiocarbon retrospective birth dating. EBioMedicine 2018, 27, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Narai, A.; Hermann, P.; Auer, T.; Kemenczky, P.; Szalma, J.; Homolya, I.; Somogyi, E.; Vakli, P.; Weiss, B.; Vidnyanszky, Z. Movement-related artefacts (MR-ART) dataset of matched motion-corrupted and clean structural MRI brain scans. Sci Data 2022, 9, 630. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.; Latif, S.; Asim, M.; Lee, B.-D.; Qadir, J. Retrospective Motion Correction in Multishot MRI using Generative Adversarial Network. Scientific Reports 2020, 10, 4786. [Google Scholar] [CrossRef] [PubMed]
- Harter, P.N.; Braun, Y.; Plate, K.H. Classification of meningiomas—advances and controversies. Chinese Clinical Oncology 2017, 6, S2. [Google Scholar] [CrossRef] [PubMed]
- Rogers, C.L.; Perry, A.; Pugh, S.; Vogelbaum, M.A.; Brachman, D.; McMillan, W.; Jenrette, J.; Barani, I.; Shrieve, D.; Sloan, A.; et al. Pathology concordance levels for meningioma classification and grading in NRG Oncology RTOG Trial 0539. Neuro-Oncology 2016, 18, 565–574. [Google Scholar] [CrossRef]
- Zhang, Q.; Jia, G.-J.; Zhang, G.-B.; Wang, L.; Wu, Z.; Jia, W.; Hao, S.-Y.; Ni, M.; Li, D.; Wang, K.; et al. A Logistic Regression Model for Detecting the Presence of Malignant Progression in Atypical Meningiomas. World Neurosurgery 2019, 126, e392–e401. [Google Scholar] [CrossRef]
- Parada, C.A.; Osbun, J.W.; Busald, T.; Karasozen, Y.; Kaur, S.; Shi, M.; Barber, J.; Adidharma, W.; Cimino, P.J.; Pan, C.; et al. Phosphoproteomic and Kinomic Signature of Clinically Aggressive Grade I (1.5) Meningiomas Reveals RB1 Signaling as a Novel Mediator and Biomarker. Clinical Cancer Research 2020, 26, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Hua, L.; Bian, L.; Chen, H.; Chen, L.; Cheng, H.; Dou, C.; Geng, D.; Hong, T.; Ji, H.; et al. Molecular diagnosis and treatment of meningiomas: an expert consensus (2022). Chinese Medical Journal 2022, 135, 1894–1912. [Google Scholar] [CrossRef]
- Al-Rashed, M.; Foshay, K.; Abedalthagafi, M. Recent Advances in Meningioma Immunogenetics. Front Oncol 2019, 9, 1472. [Google Scholar] [CrossRef] [PubMed]
- Sofela, A.A.; McGavin, L.; Whitfield, P.C.; Hanemann, C.O. Biomarkers for differentiating grade II meningiomas from grade I: a systematic review. Br J Neurosurg 2021, 35, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Raad, M.; El Tal, T.; Gul, R.; Mondello, S.; Zhang, Z.; Boustany, R.M.; Guingab, J.; Wang, K.K.; Kobeissy, F. Neuroproteomics approach and neurosystems biology analysis: ROCK inhibitors as promising therapeutic targets in neurodegeneration and neurotrauma. Electrophoresis 2012, 33, 3659–3668. [Google Scholar] [CrossRef] [PubMed]
- Ottens, A.K.; Kobeissy, F.H.; Fuller, B.F.; Liu, M.C.; Oli, M.W.; Hayes, R.L.; Wang, K.K. Novel neuroproteomic approaches to studying traumatic brain injury. Prog Brain Res 2007, 161, 401–418. [Google Scholar] [CrossRef] [PubMed]
- Kishida, Y.; Natsume, A.; Kondo, Y.; Takeuchi, I.; An, B.; Okamoto, Y.; Shinjo, K.; Saito, K.; Ando, H.; Ohka, F.; et al. Epigenetic subclassification of meningiomas based on genome-wide DNA methylation analyses. Carcinogenesis 2012, 33, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Olar, A.; Wani, K.M.; Wilson, C.D.; Zadeh, G.; DeMonte, F.; Jones, D.T.; Pfister, S.M.; Sulman, E.P.; Aldape, K.D. Global epigenetic profiling identifies methylation subgroups associated with recurrence-free survival in meningioma. Acta Neuropathol 2017, 133, 431–444. [Google Scholar] [CrossRef]
- Sahm, F.; Schrimpf, D.; Stichel, D.; Jones, D.T.W.; Hielscher, T.; Schefzyk, S.; Okonechnikov, K.; Koelsche, C.; Reuss, D.E.; Capper, D.; et al. DNA methylation-based classification and grading system for meningioma: a multicentre, retrospective analysis. Lancet Oncol 2017, 18, 682–694. [Google Scholar] [CrossRef]
- Liu, J.; Xia, C.; Wang, G. Multi-Omics Analysis in Initiation and Progression of Meningiomas: From Pathogenesis to Diagnosis. Frontiers in Oncology 2020, 10. [Google Scholar] [CrossRef]
- Agnihotri, S.; Suppiah, S.; Tonge, P.D.; Jalali, S.; Danesh, A.; Bruce, J.P.; Mamatjan, Y.; Klironomos, G.; Gonen, L.; Au, K.; et al. Therapeutic radiation for childhood cancer drives structural aberrations of NF2 in meningiomas. Nat Commun 2017, 8, 186. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Qian, J.; Ma, C. MPscore: A Novel Predictive and Prognostic Scoring for Progressive Meningioma. Cancers (Basel) 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.Z.; Bowman-Kirigin, J.A.; Desai, R.; Kang, L.-I.; Patel, P.R.; Patel, B.; Khan, S.M.; Bender, D.; Marlin, M.C.; Liu, J.; et al. Single-cell profiling of human dura and meningioma reveals cellular meningeal landscape and insights into meningioma immune response. Genome Medicine 2022, 14, 49. [Google Scholar] [CrossRef] [PubMed]
- Shi, J. Machine learning and bioinformatics approaches for classification and clinical detection of bevacizumab responsive glioblastoma subtypes based on miRNA expression. Sci Rep 2022, 12, 8685. [Google Scholar] [CrossRef] [PubMed]
- Kitano, Y.; Aoki, K.; Ohka, F.; Yamazaki, S.; Motomura, K.; Tanahashi, K.; Hirano, M.; Naganawa, T.; Iida, M.; Shiraki, Y.; et al. Urinary MicroRNA-Based Diagnostic Model for Central Nervous System Tumors Using Nanowire Scaffolds. ACS Applied Materials & Interfaces 2021, 13, 17316–17329. [Google Scholar] [CrossRef]
- Bhawal, R.; Oberg, A.L.; Zhang, S.; Kohli, M. Challenges and Opportunities in Clinical Applications of Blood-Based Proteomics in Cancer. Cancers 2020, 12, 2428. [Google Scholar] [CrossRef] [PubMed]
- Gomes Marin, J.F.; Nunes, R.F.; Coutinho, A.M.; Zaniboni, E.C.; Costa, L.B.; Barbosa, F.G.; Queiroz, M.A.; Cerri, G.G.; Buchpiguel, C.A. Theranostics in Nuclear Medicine: Emerging and Re-emerging Integrated Imaging and Therapies in the Era of Precision Oncology. Radiographics 2020, 40, 1715–1740. [Google Scholar] [CrossRef]
- Salgues, B.; Graillon, T.; Horowitz, T.; Chinot, O.; Padovani, L.; Taïeb, D.; Guedj, E. Somatostatin Receptor Theranostics for Refractory Meningiomas. Curr Oncol 2022, 29, 5550–5565. [Google Scholar] [CrossRef] [PubMed]
- Tubre, T.; Hacking, S.; Alexander, A.; Brickman, A.; Delalle, I.; Elinzano, H.; Donahue, J.E. Prostate-Specific Membrane Antigen Expression in Meningioma: A Promising Theranostic Target. J Neuropathol Exp Neurol 2022. [Google Scholar] [CrossRef]
- Zhang, D.; Ye, S.; Pan, T. The role of serum and urinary biomarkers in the diagnosis of early diabetic nephropathy in patients with type 2 diabetes. PeerJ 2019, 7, e7079. [Google Scholar] [CrossRef]
- Konstantinidou, A.E.; Givalos, N.; Gakiopoulou, H.; Korkolopoulou, P.; Kotsiakis, X.; Boviatsis, E.; Agrogiannis, G.; Mahera, H.; Patsouris, E. Caspase-3 immunohistochemical expression is a marker of apoptosis, increased grade and early recurrence in intracranial meningiomas. Apoptosis 2007, 12, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Ghantasala, S.; Pai, M.G.J.; Biswas, D.; Gahoi, N.; Mukherjee, S.; Kp, M.; Nissa, M.U.; Srivastava, A.; Epari, S.; Shetty, P.; et al. Multiple Reaction Monitoring-Based Targeted Assays for the Validation of Protein Biomarkers in Brain Tumors. Front Oncol 2021, 11, 548243. [Google Scholar] [CrossRef] [PubMed]
- Abou Khouzam, R.; Brodaczewska, K.; Filipiak, A.; Zeinelabdin, N.A.; Buart, S.; Szczylik, C.; Kieda, C.; Chouaib, S. Tumor Hypoxia Regulates Immune Escape/Invasion: Influence on Angiogenesis and Potential Impact of Hypoxic Biomarkers on Cancer Therapies. Front Immunol 2020, 11, 613114. [Google Scholar] [CrossRef] [PubMed]
- El-Benhawy, S.A.; Sakr, O.A.; Fahmy, E.I.; Ali, R.A.; Hussein, M.S.; Nassar, E.M.; Salem, S.M.; Abu-Samra, N.; Elzawawy, S. Assessment of Serum Hypoxia Biomarkers Pre- and Post-radiotherapy in Patients with Brain Tumors. J Mol Neurosci 2022. [Google Scholar] [CrossRef] [PubMed]
- Valvona, C.J.; Fillmore, H.L.; Nunn, P.B.; Pilkington, G.J. The Regulation and Function of Lactate Dehydrogenase A: Therapeutic Potential in Brain Tumor. Brain Pathol 2016, 26, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Fattahi, M.J.; Sedaghat, F.; Malekzadeh, M.; Nejat, A.A.; Poostkar, M.; Saberi, Y.; Taghipour, M.; Ghaderi, A. Endocan serum levels in patients with low- and high-grade meningiomas: does this biomarker have an indicative role? The Egyptian Journal of Neurology, Psychiatry and Neurosurgery 2021, 57, 92. [Google Scholar] [CrossRef]
- Atukeren, P.; Kunbaz, A.; Turk, O.; Kemerdere, R.; Ulu, M.O.; Turkmen Inanir, N.; Tanriverdi, T. Expressions of Endocan in Patients with Meningiomas and Gliomas. Dis Markers 2016, 2016, 7157039. [Google Scholar] [CrossRef]
- Shalaby, T.; Achini, F.; Grotzer, M.A. Targeting cerebrospinal fluid for discovery of brain cancer biomarkers. Journal of Cancer Metastasis and Treatment 2016, 2, 176–187. [Google Scholar] [CrossRef]
- Choudhary, R.; Elabbas, A.; Vyas, A.; Osborne, D.; Chigurupati, H.D.; Abbas, L.F.; Kampa, P.; M, H.F.; Sarwar, H.; Alfonso, M. Utilization of Cerebrospinal Fluid Proteome Analysis in the Diagnosis of Meningioma: A Systematic Review. Cureus 2021, 13, e20707. [Google Scholar] [CrossRef]
- Georgila, K.; Vyrla, D.; Drakos, E. Apolipoprotein A-I (ApoA-I), Immunity, Inflammation and Cancer. Cancers 2019, 11, 1097. [Google Scholar] [CrossRef] [PubMed]
- Rajagopal, M.U.; Hathout, Y.; MacDonald, T.J.; Kieran, M.W.; Gururangan, S.; Blaney, S.M.; Phillips, P.; Packer, R.; Gordish-Dressman, H.; Rood, B.R. Proteomic profiling of cerebrospinal fluid identifies prostaglandin D2 synthase as a putative biomarker for pediatric medulloblastoma: A pediatric brain tumor consortium study. Proteomics 2011, 11, 935–943. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Lv, S.; Zong, Z.; Wu, L.; Tang, X.; Kuang, W.; Zhang, P.; Li, X.; Fu, J.; Xiao, M.; et al. Cerebrospinal fluid biomarkers for brain tumor detection: clinical roles and current progress. Am J Transl Res 2020, 12, 1379–1396. [Google Scholar]
- Wang, P.; Piao, Y.; Zhang, X.; Li, W.; Hao, X. The concentration of CYFRA 21-1, NSE and CEA in cerebro-spinal fluid can be useful indicators for diagnosis of meningeal carcinomatosis of lung cancer. Cancer Biomark 2013, 13, 123–130. [Google Scholar] [CrossRef] [PubMed]
- O'Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front Endocrinol (Lausanne) 2018, 9, 402. [Google Scholar] [CrossRef] [PubMed]
- Kopkova, A.; Sana, J.; Fadrus, P.; Slaby, O. Cerebrospinal fluid microRNAs as diagnostic biomarkers in brain tumors. Clin Chem Lab Med 2018, 56, 869–879. [Google Scholar] [CrossRef] [PubMed]
- Slavik, H.; Balik, V.; Vrbkova, J.; Rehulkova, A.; Vaverka, M.; Hrabalek, L.; Ehrmann, J.; Vidlarova, M.; Gurska, S.; Hajduch, M.; et al. Identification of Meningioma Patients at High Risk of Tumor Recurrence Using MicroRNA Profiling. Neurosurgery 2020, 87, 1055–1063. [Google Scholar] [CrossRef] [PubMed]
- Gareev, I.; Beylerli, O.; Liang, Y.; Xiang, H.; Liu, C.; Xu, X.; Yuan, C.; Ahmad, A.; Yang, G. The Role of MicroRNAs in Therapeutic Resistance of Malignant Primary Brain Tumors. Frontiers in Cell and Developmental Biology 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Urbschat, S.; Landau, B.; Bewersdorf, N.C.; Schuster, C.; Wagenpfeil, G.; Schulz-Schaeffer, W.J.; Oertel, J.; Ketter, R. MicroRNA 200a as a histologically independent marker for meningioma recurrence: Results of a four microRNA panel analysis in meningiomas. Cancer Med 2022. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Kang, M.H.; Kim, J.H. Diverse Effects of Exosomes on COVID-19: A Perspective of Progress From Transmission to Therapeutic Developments. Front Immunol 2021, 12, 716407. [Google Scholar] [CrossRef] [PubMed]
- Saugstad, J.A.; Lusardi, T.A.; Van Keuren-Jensen, K.R.; Phillips, J.I.; Lind, B.; Harrington, C.A.; McFarland, T.J.; Courtright, A.L.; Reiman, R.A.; Yeri, A.S.; et al. Analysis of extracellular RNA in cerebrospinal fluid. J Extracell Vesicles 2017, 6, 1317577. [Google Scholar] [CrossRef] [PubMed]
- Negroni, C.; Hilton, D.A.; Ercolano, E.; Adams, C.L.; Kurian, K.M.; Baiz, D.; Hanemann, C.O. GATA-4, a potential novel therapeutic target for high-grade meningioma, regulates miR-497, a potential novel circulating biomarker for high-grade meningioma. EBioMedicine 2020, 59, 102941. [Google Scholar] [CrossRef] [PubMed]
- Ricklefs, F.L.; Maire, C.L.; Wollmann, K.; Dührsen, L.; Fita, K.D.; Sahm, F.; Herold-Mende, C.; von Deimling, A.; Kolbe, K.; Holz, M.; et al. Diagnostic potential of extracellular vesicles in meningioma patients. Neuro Oncol 2022, 24, 2078–2090. [Google Scholar] [CrossRef] [PubMed]
- Mattick, J.S.; Amaral, P.P.; Carninci, P.; Carpenter, S.; Chang, H.Y.; Chen, L.-L.; Chen, R.; Dean, C.; Dinger, M.E.; Fitzgerald, K.A.; et al. Long non-coding RNAs: definitions, functions, challenges and recommendations. Nature Reviews Molecular Cell Biology 2023. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Lan, Y.; Xie, A.; Shi, J.; Zhao, H.; Xu, L.; Zhu, S.; Luo, T.; Zhao, T.; Xiao, Y.; et al. Comprehensive analysis of long noncoding RNA (lncRNA)-chromatin interactions reveals lncRNA functions dependent on binding diverse regulatory elements. J Biol Chem 2019, 294, 15613–15622. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Ge, Y.; Wang, D.; Liu, Q.; Sun, S.; Hua, L.; Deng, J.; Luan, S.; Cheng, H.; Xie, Q.; et al. LncRNA-IMAT1 Promotes Invasion of Meningiomas by Suppressing KLF4/hsa-miR22-3p/Snai1 Pathway. Mol Cells 2022, 45, 388–402. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Ren, J.; Ma, J.; Wu, J.; Zhang, R.; Yuan, H.; Han, X. LINC00702/miR-4652-3p/ZEB1 axis promotes the progression of malignant meningioma through activating Wnt/beta-catenin pathway. Biomed Pharmacother 2019, 113, 108718. [Google Scholar] [CrossRef] [PubMed]
- Rana, M.W.; Pinkerton, H.; Thornton, H.; Nagy, D. Heterotransplantation of human glioblastoma multiforme and meningioma to nude mice. Proc Soc Exp Biol Med 1977, 155, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Jensen, R.L.; Leppla, D.; Rokosz, N.; Wurster, R.D. Matrigel augments xenograft transplantation of meningioma cells into athymic mice. Neurosurgery 1998, 42, 130–135. [Google Scholar] [CrossRef] [PubMed]
- McCutcheon, I.E.; Friend, K.E.; Gerdes, T.M.; Zhang, B.M.; Wildrick, D.M.; Fuller, G.N. Intracranial injection of human meningioma cells in athymic mice: an orthotopic model for meningioma growth. J Neurosurg 2000, 92, 306–314. [Google Scholar] [CrossRef]
- Boetto, J.; Peyre, M.; Kalamarides, M. Mouse Models in Meningioma Research: A Systematic Review. Cancers (Basel) 2021, 13. [Google Scholar] [CrossRef]
- Püttmann, S.; Senner, V.; Braune, S.; Hillmann, B.; Exeler, R.; Rickert, C.H.; Paulus, W. Establishment of a benign meningioma cell line by hTERT-mediated immortalization. Lab Invest 2005, 85, 1163–1171. [Google Scholar] [CrossRef] [PubMed]
- van Furth, W.R.; Laughlin, S.; Taylor, M.D.; Salhia, B.; Mainprize, T.; Henkelman, M.; Cusimano, M.D.; Ackerley, C.; Rutka, J.T. Imaging of murine brain tumors using a 1.5 Tesla clinical MRI system. Can J Neurol Sci 2003, 30, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Baia, G.S.; Dinca, E.B.; Ozawa, T.; Kimura, E.T.; McDermott, M.W.; James, C.D.; VandenBerg, S.R.; Lal, A. An orthotopic skull base model of malignant meningioma. Brain Pathol 2008, 18, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Castle, K.D.; Chen, M.; Wisdom, A.J.; Kirsch, D.G. Genetically engineered mouse models for studying radiation biology. Transl Cancer Res 2017, 6, S900–S913. [Google Scholar] [CrossRef] [PubMed]
- Peyre, M.; Salaud, C.; Clermont-Taranchon, E.; Niwa-Kawakita, M.; Goutagny, S.; Mawrin, C.; Giovannini, M.; Kalamarides, M. PDGF activation in PGDS-positive arachnoid cells induces meningioma formation in mice promoting tumor progression in combination with Nf2 and Cdkn2ab loss. Oncotarget 2015, 6, 32713–32722. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, M.; Suzuki, S.O.; Yamashima, T.; Fukui, M.; Iwaki, T. Prostaglandin D synthase (beta-trace) in meningeal hemangiopericytoma. Mod Pathol 2001, 14, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Urade, Y.; Kitahama, K.; Ohishi, H.; Kaneko, T.; Mizuno, N.; Hayaishi, O. Dominant expression of mRNA for prostaglandin D synthase in leptomeninges, choroid plexus, and oligodendrocytes of the adult rat brain. Proc Natl Acad Sci U S A 1993, 90, 9070–9074. [Google Scholar] [CrossRef] [PubMed]
- Yamashima, T.; Sakuda, K.; Tohma, Y.; Yamashita, J.; Oda, H.; Irikura, D.; Eguchi, N.; Beuckmann, C.T.; Kanaoka, Y.; Urade, Y.; et al. Prostaglandin D synthase (beta-trace) in human arachnoid and meningioma cells: roles as a cell marker or in cerebrospinal fluid absorption, tumorigenesis, and calcification process. J Neurosci 1997, 17, 2376–2382. [Google Scholar] [CrossRef] [PubMed]
- von Werder, A.; Seidler, B.; Schmid, R.M.; Schneider, G.; Saur, D. Production of avian retroviruses and tissue-specific somatic retroviral gene transfer in vivo using the RCAS/TVA system. Nat Protoc 2012, 7, 1167–1183. [Google Scholar] [CrossRef]
- Kalamarides, M.; Niwa-Kawakita, M.; Leblois, H.; Abramowski, V.; Perricaudet, M.; Janin, A.; Thomas, G.; Gutmann, D.H.; Giovannini, M. Nf2 gene inactivation in arachnoidal cells is rate-limiting for meningioma development in the mouse. Genes Dev 2002, 16, 1060–1065. [Google Scholar] [CrossRef]
- Kalamarides, M.; Stemmer-Rachamimov, A.O.; Takahashi, M.; Han, Z.Y.; Chareyre, F.; Niwa-Kawakita, M.; Black, P.M.; Carroll, R.S.; Giovannini, M. Natural history of meningioma development in mice reveals: a synergy of Nf2 and p16(Ink4a) mutations. Brain Pathol 2008, 18, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Peyre, M.; Stemmer-Rachamimov, A.; Clermont-Taranchon, E.; Quentin, S.; El-Taraya, N.; Walczak, C.; Volk, A.; Niwa-Kawakita, M.; Karboul, N.; Giovannini, M.; et al. Meningioma progression in mice triggered by Nf2 and Cdkn2ab inactivation. Oncogene 2013, 32, 4264–4272. [Google Scholar] [CrossRef] [PubMed]
- Mandara, M.T.; Ricci, G.; Rinaldi, L.; Sarli, G.; Vitellozzi, G. Immunohistochemical identification and image analysis quantification of oestrogen and progesterone receptors in canine and feline meningioma. J Comp Pathol 2002, 127, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Michelhaugh, S.K.; Guastella, A.R.; Varadarajan, K.; Klinger, N.V.; Parajuli, P.; Ahmad, A.; Sethi, S.; Aboukameel, A.; Kiousis, S.; Zitron, I.M.; et al. Development of patient-derived xenograft models from a spontaneously immortal low-grade meningioma cell line, KCI-MENG1. J Transl Med 2015, 13, 227. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Park, K.J.; Ryu, B.K.; Park, D.H.; Kong, D.S.; Chong, K.; Chae, Y.S.; Chung, Y.G.; Park, S.I.; Kang, S.H. Forkhead box M1 (FOXM1) transcription factor is a key oncogenic driver of aggressive human meningioma progression. Neuropathol Appl Neurobiol 2020, 46, 125–141. [Google Scholar] [CrossRef] [PubMed]
- Saydam, O.; Shen, Y.; Würdinger, T.; Senol, O.; Boke, E.; James, M.F.; Tannous, B.A.; Stemmer-Rachamimov, A.O.; Yi, M.; Stephens, R.M.; et al. Downregulated microRNA-200a in meningiomas promotes tumor growth by reducing E-cadherin and activating the Wnt/beta-catenin signaling pathway. Mol Cell Biol 2009, 29, 5923–5940. [Google Scholar] [CrossRef] [PubMed]
- Tuchen, M.; Wilisch-Neumann, A.; Daniel, E.A.; Baldauf, L.; Pachow, D.; Scholz, J.; Angenstein, F.; Stork, O.; Kirches, E.; Mawrin, C. Receptor tyrosine kinase inhibition by regorafenib/sorafenib inhibits growth and invasion of meningioma cells. Eur J Cancer 2017, 73, 9–21. [Google Scholar] [CrossRef]
- Juratli, T.A.; Prilop, I.; Saalfeld, F.C.; Herold, S.; Meinhardt, M.; Wenzel, C.; Zeugner, S.; Aust, D.E.; Barker, F.G., 2nd; Cahill, D.P.; et al. Sporadic multiple meningiomas harbor distinct driver mutations. Acta Neuropathol Commun 2021, 9, 8. [Google Scholar] [CrossRef]
| Biomarker Type | Known Biomarkers | Study design | Clinical Use | Correlation with Grade | Description of Marker Usage & its Effects | Reference and Year |
|---|---|---|---|---|---|---|
| Genomics | NF2, TRAF7,AKT1, SMO, PIK3CA | Review | Diagnosis/ Therapy | - | - | [29], 2020 |
| SMARCB1 | Review | Diagnosis/ Therapy | Grade 1, 2 | Genetic risk factor for sporadic multiple meningiomas | [29], 2020 | |
| KLF4 | Review | Diagnosis/ Therapy | Grade 1 | Downregulated in anaplastic Meningioma | [29], 2020; [65], 2017 | |
| CDKN2A/B homozygous deletion | Cohort of 528 meningioma patients | Diagnostic/ Prognostic | Grade 3> Garde 2; absent in Grade 1 | Faster progression to recurrence. Higher mortality. | [66], 2020 | |
| miRNA | miR-29c-3p and miR-219-5p | A study of 50 meningioma patients training set and 60 meningioma patients validation set compared to normal adjacent tissue | Diagnosis, Prognosis, Therapy Response | Grade 1>2>3 | Downregulation associated with advanced clinical stages of meningioma and significant correlation with higher recurrence rates | [67], 2013 |
| miR-190a | A study of 50 meningioma patients training set and 60 meningioma patients validation set compared to normal adjacent tissue | Prognosis | Grade 1<2<3 | Upregulation associated with advanced clinical stages of meningioma, independent of other clinicopathological factors | [67], 2013 | |
| miR-17-5p, miR-199a, miR-190a, miR-186-5p, miR-155-5p, miR-22-3p, miR-24-3p, miR- 26b-5p, miR-27a-3p, miR-27b-3p, miR-96-5p,miR-146a-5p | A study of 50 meningioma patients training set and 60 meningioma patients validation set compared to normal adjacent tissue | Diagnosis, Prognosis, Histological grade & Radiosensitivity | - | Significantly upregulated in meningioma samples | [67], 2013 | |
| miR-219-5p, miR-106a-5p, miR-375, and miR-409-3p | 20 pre-operative meningiomas and 20 healthy controls as discovery set. Candidate miRNAs were validated individually in another 210 meningioma and 210 healthy controls. | Non-invasive Diagnostic/Prognostic | miR-219-5p: Grade 3>2>1 | Serum levels of the miRNA panel significantly increased in meningioma cases. Serum levels of miR-219-5p positively correlated with higher meningioma grade | [68], 2016 | |
| miR-197 and miR-224 | 20 pre-operative meningiomas and 20 healthy controls as discovery set. Candidate miRNAs were validated individually in another 210 meningioma and 210 healthy controls. | Non-invasive Diagnostic/Prognostic | - | Serum levels significantly decreased in meningioma cases. High serum miR-409-3p and low miR-224 expression significantly correlated with higher recurrence rates | [68], 2016 | |
| Upregulation of miR-4286, miR-4695-5p, miR-6732-5p, miR-6855-5p, miR-7977, miR-6765-3p, miR-6787-5p and downregulation of miR-1275, miR-30c-1-3p, miR-4449, miR-4539, miR-4684-3p, miR-6129, miR-6891-5p | Study of 55 atypical meningioma patients (43 radiosensitive and 12 radioresistant meningiomas) and 6 six arachnoid samples as control | Prognosis/ response to radiotherapy | Grade 2 | 14 miRNAs are significantly dysregulated in meningioma. Prediction of individual sensitivity to radiotherapy. in patients resistant to radiotherapy. Dysregulated miRNAs enriched in fatty acid biosynthesis and metabolism and TGF-β signaling pathways | [69], 2020 | |
| miR-181d | Study collected meningioma tissues and plasma of 40 meningioma patients (16 Grade 1, 16 rade 2, and 8 Grade 3 patients) | Non-invasive Diagnostic/Prognostic | Grade 1<2<3 | Associated with tumor progression In plasma and tumor tissues | [70], 2021 | |
| LncRNA | LncRNA-LINC00460 | A study of tissues from 32 meningioma patients and 5 normal control cases, in addition to in vitro studies in meningioma cell lines | Diagnosis | Grade 2<3 | Upregulated in Meningioma tissues and malignant cell lines | [71], 2020 |
| ISLR2, Lnc-GOLGA6A-1, AMH, and Grade 1>2 | A study of 64 meningioma patients (with and without recurrence and of different WHO grades were subjected to RNA-seq; 90 samples validated by RT-qPCR | Prognosis and Pathogenesis | Lnc-MAST4-5: Grade 1>2, 3 | ISLR2, Lnc-GOLGA6A-1, and AMH Associated with recurrence risk | [72], 2022 | |
| Lnc-00460 | A study of 33 of human meningeoma tumor tissues and 10 normal meninges tissues, in addition to meningioma cell lines | Diagnosis | - | Upregulated in meningeoma tissues and cell lines | [73], 2018 | |
| LncRNA- NUP210, LncRNA-SPIRE2, LncRNA-SLC7A1, and LncRNA-DMTN | Review | Diagnosis, Prognosis | - | Upregulated In meningioma. Target microRNA-195 | [74], 2023 | |
| Epigenetic | TIMP3, HOXA7,HOXA9, HOXA10, | Review | Prognosis | - | Hypermethylation associated with tumor progression & malignant transformation | [75], 2015; [76], 2020; [77], 2023 |
| TRAF7, KLF4, NF2, TRAKL, ARID1A, and AKT1 | Retrospective analysis of formalin-fixed paraffin-embedded sections of 126 meningioma patients of different grades | Prognosis | - | Aberrant DNA methylation of these genes may be involved in the development and progression of meningioma | [78], 2022; [77], 2023 | |
| TIMP3, CDKN 2A, NDRG2 | Review | Prognosis | - | Faster recurrence | [76], 2020; [77], 2023 | |
| TP73, RSSF1A, MAL2 | Review | Prognosis | - | Hypermethylation increases risk of malignancy | [76], 2020, [77], 2023 | |
| H3K27me3 histone modification | Retrospective study of 232 meningioma patients | Diagnosis/ Prognosis | Grade 1< 2< 3 | Loss of H3K27me3 methylation patterns correlated with high recurrence. | [26], 2018 | |
| Mutations in hTERT gene promoter | Study of 252 meningioma patients | Diagnosis/ Prognosis | Grade 3 (aggressive) | Presence of hTERT promoter mutations means shorter time to progression. | [79], 2016 | |
| Mutations in hTERT gene promoter | Meta-analysis of 8 clinical trials. | Diagnosis/ Prognosis | Grade 1< 2< 3 | Presence of hTERT promoter mutations resulted in higher recurrence rates, and mortality. This was a better prediction than WHO grading system. | [80], 2019 | |
| Proteomic | APO-E, APO-J | Proteomic analysis of CSF from 4 meningioma patients and 4 patients with a non-brain. | Diagnosis | Grade 2 | Tumor progression marker | [81], 2012 |
| PTGDS | Clinical Study | Diagnosis | Grade 1 | Associated with higher-grade and early recurrence in intracranial Meningioma | [82], 2019 | |
| Cspase-3, Amphiregulin, VEFG-D | Screening Cohort followed by a validation set of meningioma tissues and serum | Non-invasive diagnosis and prognosis | Grade 1<2, 3 | The 3 proteins may constitute a panel that correlates with meningioma progression | [82], 2019 | |
| EFEMP1 | A study of CSF and serum of 45 meningioma patients and 30 healthy controls | Diagnosis | CSF and serum EFEMP1 levels significantly higher meningioma patients | [83], 2017 | ||
| Histological | SSTR2A, Claudin-1 | 35 meningioma, 10 intracranial schwannoma, and 10f hemangiopericytoma cases | Diagnosis | SSTRA:Grade 1,2>36768Claudin-1: Grade 1,2< 3 | Distinguishes meningioma from Schwannoma & Hemangiopericytoma. | [84], 2018 |
| CA9 | Immunohistochemistry of paraffin-embedded sections of 25 Grade 1, 17 Grade 2, and 20 Grade 3 meningioma | Prognosis | Grade 3 | Associated with higher-grade histology & common in recurrent tumors. | [85], 2007 | |
| Metabolomic | Alanine, Glutamine/Glutamate | 1H NMR of 23 Grade 1 and 10 Grade-II meningioma tissues. | Diagnosis/ Prognosis | Glutamine metabolism: Grade 1>2 | Predominantly elevated in Grade-2 meningiomas. | [86], 2022 |
| Glycine/Serine | Validation of 43 meningioma patients. | Diagnosis/ Prognosis | Grade 1>2>3 | Grade 1 associated with lower proliferation and longer progression-free survival. | [87], 2021 | |
| Choline/Tryptophan | Validation of 43 meningioma patients | Diagnosis/ Prognosis | Grade 2, 3>I | Higher Tryptophan/ Choline associated with shorter progression free survival. Similar incidence of Grade 1, 2, and 3 | [87], 2021, | |
| Sphingolipid Galactosyl Ceramide, | Discovery using LC-MS/MS and validation in 85 meningioma biopsies of different grades | Diagnosis/ Prognosis | Grade 2, 3 > Grade 1 | Higher levels in WHO Grade 2 and 3 than Grade 1 | [88] , 2023 | |
| High acetate, threonine, N-acetyl-lysine, hydroxybutyrate, myoinositol, ascorbate, and total choline and a low aspartate, glucose, isoleucine, valine, adenosine, arginine and alanine | Metabolomics analysis by HRMAS NMR of 62 human meningioma samples | Diagnostic/ Prognostic | Aggressive Grade 1 and Grade 2 have similar metabolic signature to Grade 3 | Poor prognosis and high proliferation and histological grade | [89], 2020 | |
| Integrated systems of molecular/ histological biomarkers | WHO grade, methylation class, and absence of chromosomes 1p, 6q, and 14q | Retrospective and prospective multi-center clinical study of 514 meningiomas and validation in 471 samples | Diagnosis/ Prognosis | Nine points scoring system. Final scores of 3–5→ low risk of recurrence; 3-5 → intermediate risk; and score 6–9→ high risk of recurrence. | [90], 2021 | |
| Mitotic index, CDKN2A/B homologous deletion, and alterations of copy number of specific chromosomes | Discovery cohort of 527 meningiomas and a validation set of 172 meningiomas | Diagnosis/ Prognosis | Points scoring system. Final scores of 0–1→ low risk of recurrence; 2-3→ intermediate risk; and score 4 or more→ high risk of recurrence. | [91], 2022 |
| Mouse Model | Advantages | Limitations |
|---|---|---|
| Heterotopic xenograft model | Very reliable in term of tumor take rates. | Lacks the key components of the meningiomas specific microenvironment. |
| Orthotopic xenograft model |
|
|
| Genetically Engineered Mouse Models (GEMM) |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





