Submitted:
14 August 2023
Posted:
15 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
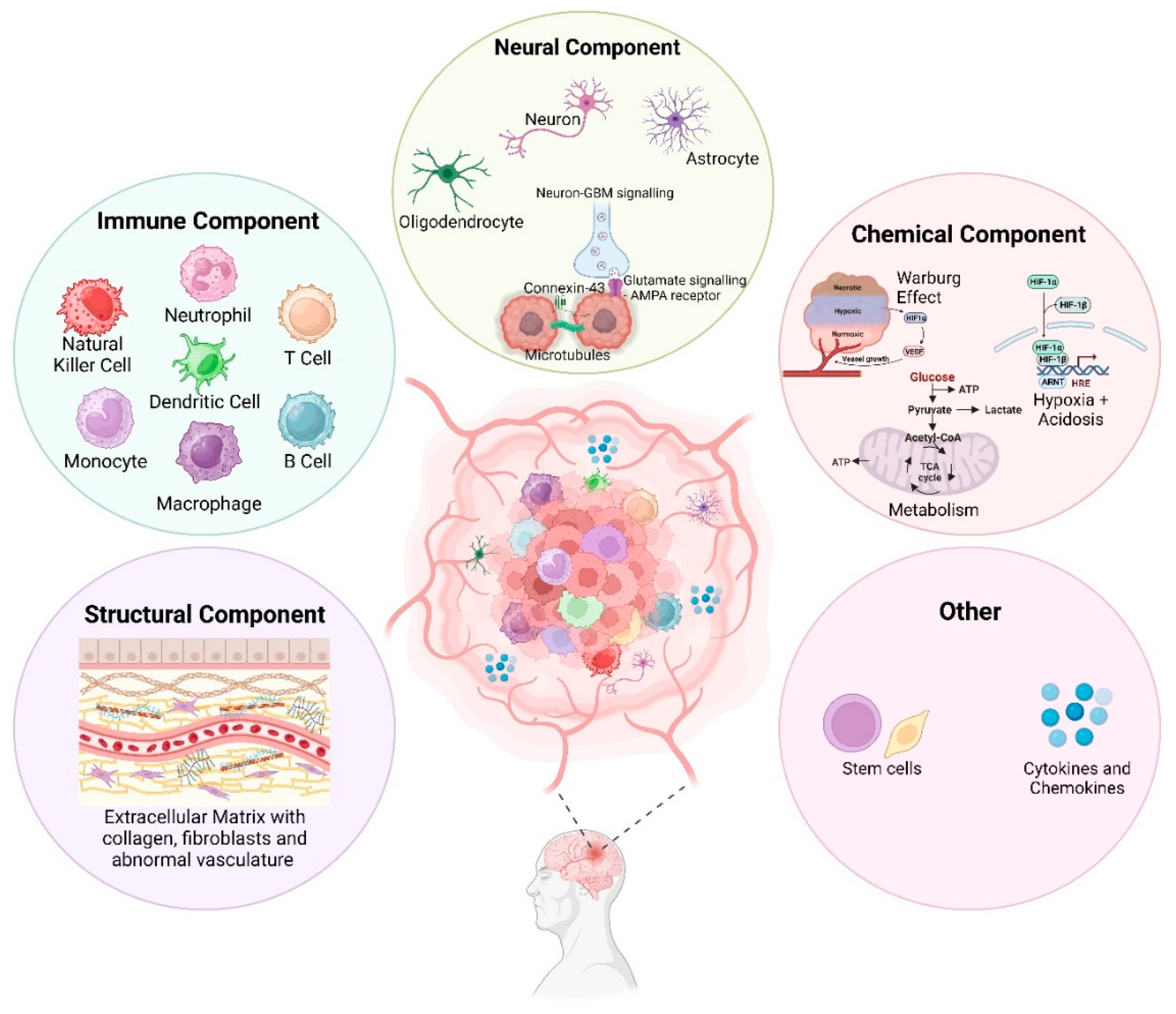
2. Complexity of the GBM TME
2.1. Structural Component
2.1.1. Extracellular Matrix (ECM)
2.1.2. Integrins
2.2. Immune Component
2.2.1. Tumour Associated Macrophages (TAMs)
2.2.2. Dendritic Cells (DCs)
2.2.3. Neutrophils
2.2.4. Tumour-Infiltrating Lymphocytes (TILs)
2.2.5. Natural Killer (NK) Cells
2.3. Neural Component
2.3.1. Astrocytes
2.3.2. Neurons
2.3.3. Oligodendrocytes
2.3.4. Glial Cells
2.3.5. Paracrine Interactions
2.4. Chemical Component
2.4.1. Tumour Acidosis
2.4.2. Hypoxia
2.5. Glioblastoma Stem Cells (GSCs)
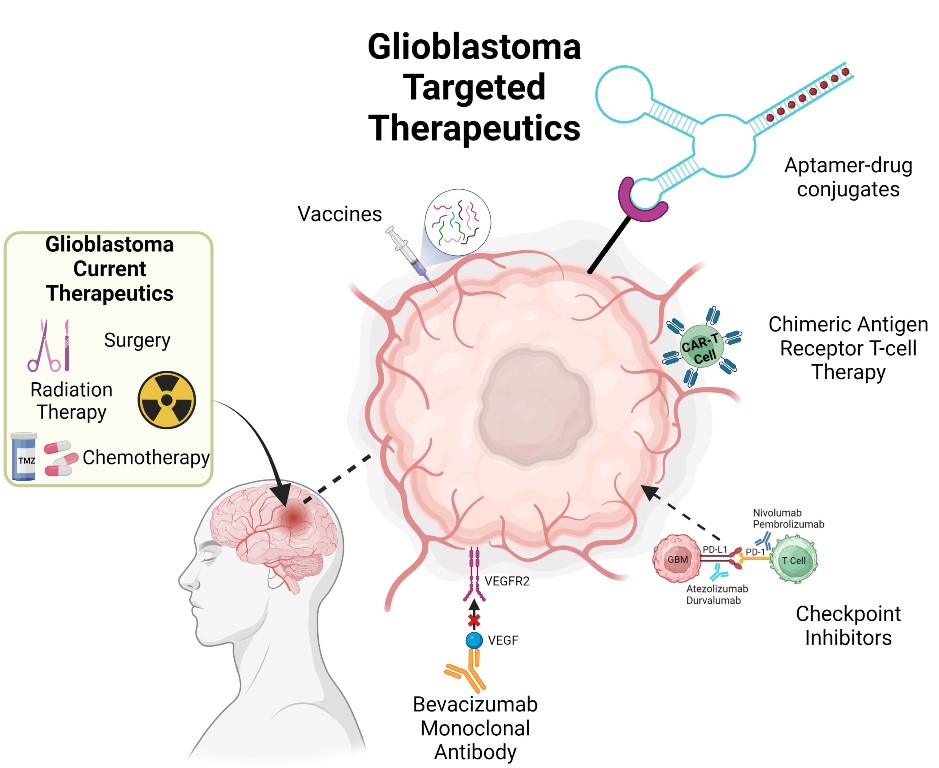
3. Immunotherapies Targeting the TME
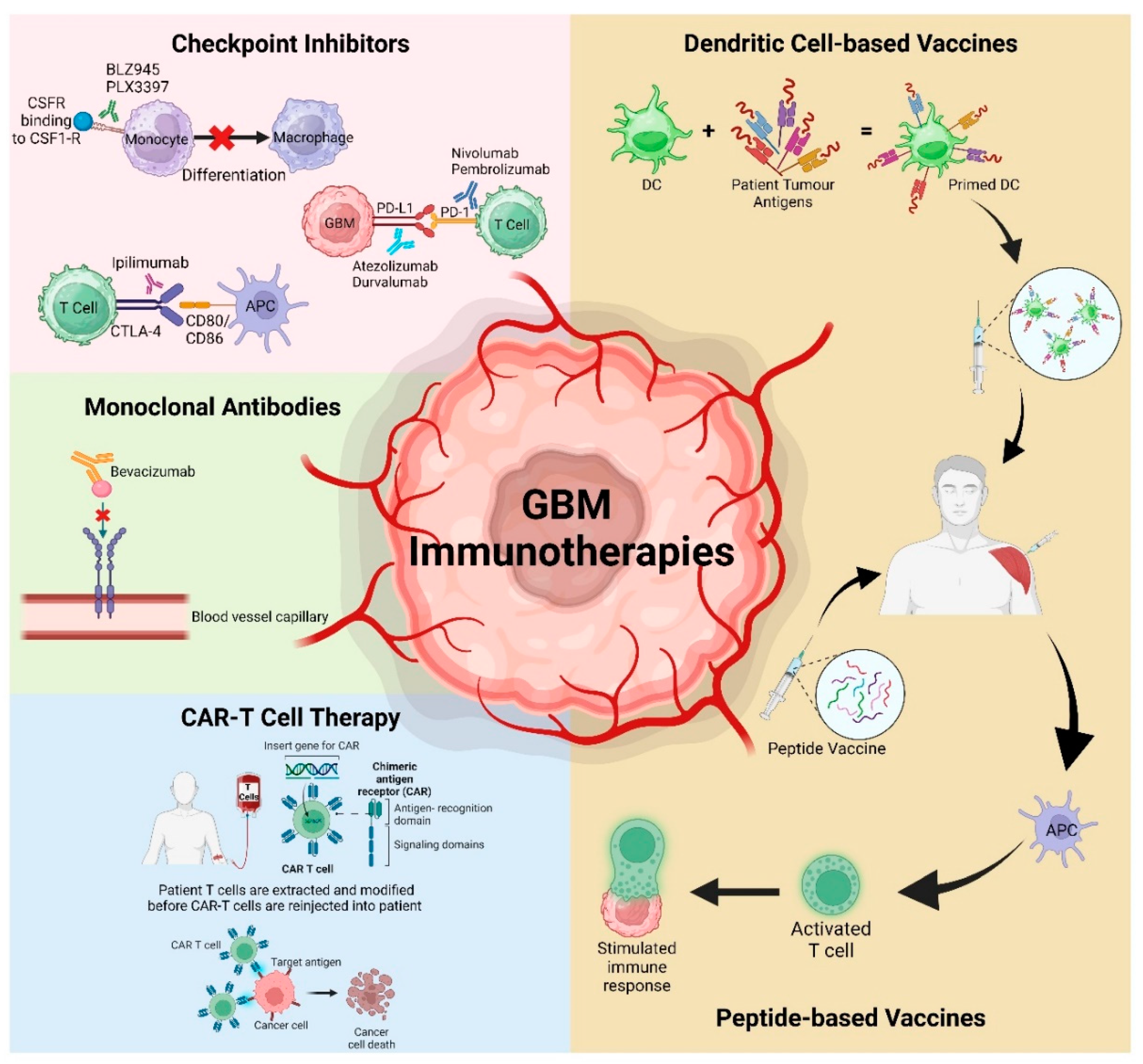
3.1. Checkpoint Inhibitors
3.1.1. Colony Stimulating Factor-1 Receptor (CSF-1R)
3.1.2. Programmed Cell Death Protein-1 (PD1) and Its Ligand PD-L1
3.1.3. Cytotoxic T-Lymphocyte Associated Protein 4 (CTLA-4)
3.2. Chimeric Antigen Receptor T (CAR-T) Cell Therapy
3.3. Vaccinations
3.4. Monoclonal Antibodies (mAbs)
4. Aptamers- Novel Therapeutics Option for GBM
4.1. Tenascin-C
4.2. Cluster of Differentiation-133 (CD-133)
4.3. Epidermal Growth Factor Receptor (EGFR)
4.4. Platelet-Derived Growth Factor Receptor (PDGFR)
4.5. Ephrin Receptor Tyrosine Kinase (Eph Receptors)
4.6. Vascular Endothelia Growth Factor (VEGF)
4.7. Stromal-Derived Factor-1 (SDF-1)
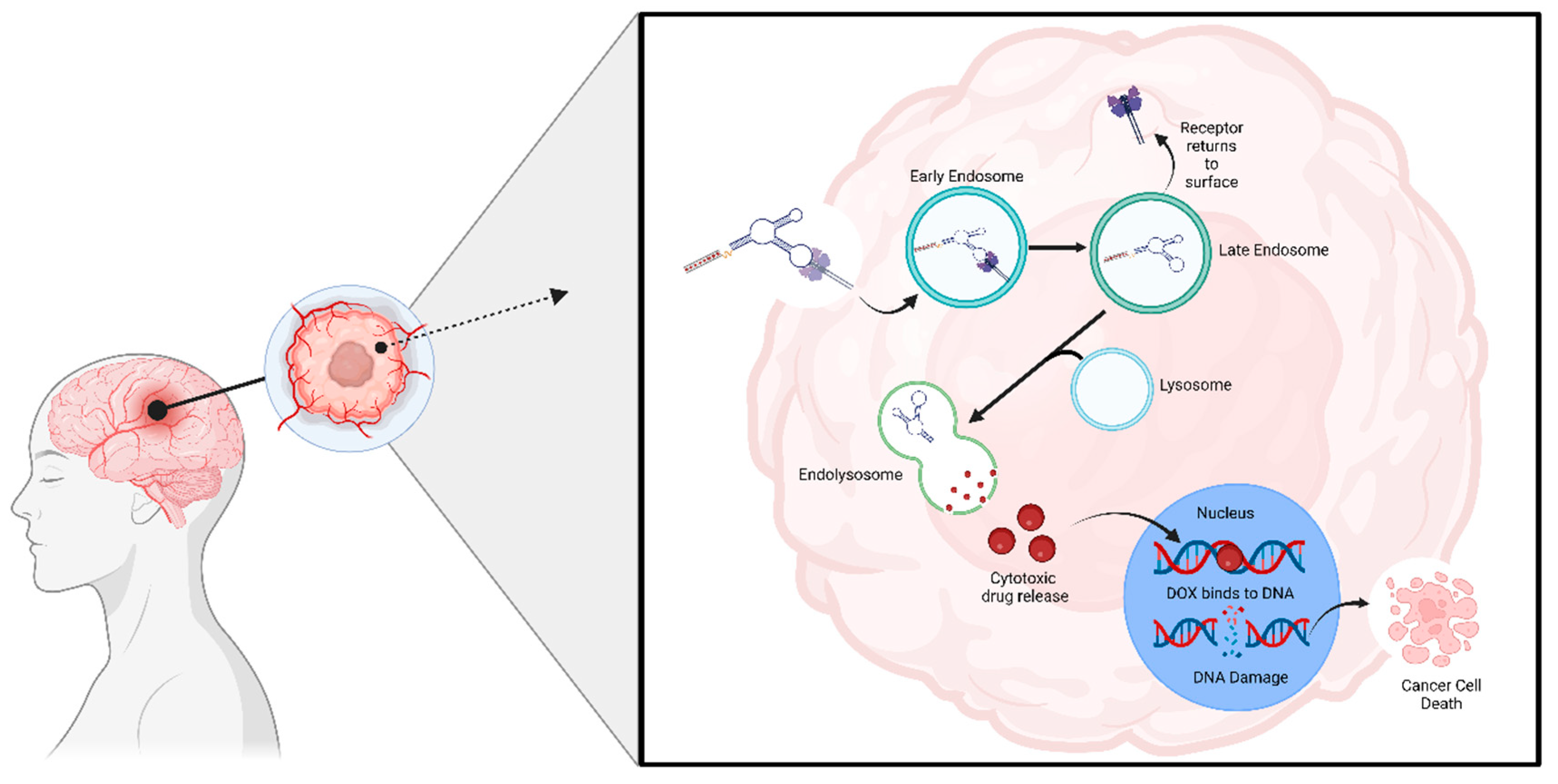
4.8. Aptamers as a Drug Carrier
4.8.1. GMT-3 Aptamer
4.8.2. AS1411 Aptamer
4.8.3. AS1411 and GS24 Aptamers
4.8.4. GMT8 and Gint4.T Aptamers
4.8.5. PDGFRβ Aptamer
4.8.6. Aptamer 32
4.8.7. GL21.T and Gint4.T Aptamers
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Ostrom, Q.T.; Price, M.; Neff, C.; Cioffi, G.; Waite, K.A.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2015–2019. J Neuro-Oncol 2022, 24, v1–v95. [Google Scholar] [CrossRef]
- Chen, W.; Wang, Y.; Zhao, B.; Liu, P.; Liu, L.; Wang, Y.; Ma, W. Optimal Therapies for Recurrent Glioblastoma: A Bayesian Network Meta-Analysis. Front Oncol 2021, 11, 641878. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, S.; Dinesan, M.; Ajayakumar, T. Survival and quality of life analysis in glioblastoma multiforme with adjuvant chemoradiotherapy: a retrospective study. Rep Pract Oncol Radiother 2022, 27, 1026–1036. [Google Scholar] [CrossRef] [PubMed]
- Sloan, A.E.; Gilbert, M.R.; Zhang, P.; Aldape, K.D.; Wu, J.; Rogers, L.R.; Wen, P.Y.; Barani, I.J.; Iwamoto, F.M.; Raval, R.R.; et al. NRG BN002: Phase I study of checkpoint inhibitors anti-CTLA-4, anti-PD-1, the combination in patients with newly diagnosed glioblastoma. 2018, 36, 2053–2053. [CrossRef]
- Nayak, L.; Molinaro, A.M.; Peters, K.; Clarke, J.L.; Jordan, J.T.; de Groot, J.; Nghiemphu, L.; Kaley, T.; Colman, H.; McCluskey, C.; et al. Randomized Phase II and Biomarker Study of Pembrolizumab plus Bevacizumab versus Pembrolizumab Alone for Patients with Recurrent Glioblastoma. Clin Cancer Res 2021, 27, 1048–1057. [Google Scholar] [CrossRef] [PubMed]
- Schalper, K.A.; Rodriguez-Ruiz, M.E.; Diez-Valle, R.; Lopez-Janeiro, A.; Porciuncula, A.; Idoate, M.A.; Inoges, S.; de Andrea, C.; Lopez-Diaz de Cerio, A.; Tejada, S.; et al. Neoadjuvant nivolumab modifies the tumor immune microenvironment in resectable glioblastoma. Nat Med 2019, 25, 470–476. [Google Scholar] [CrossRef]
- Reardon, D.A.; Brandes, A.A.; Omuro, A.; Mulholland, P.; Lim, M.; Wick, A.; Baehring, J.; Ahluwalia, M.S.; Roth, P.; Bahr, O.; et al. Effect of Nivolumab vs Bevacizumab in Patients With Recurrent Glioblastoma: The CheckMate 143 Phase 3 Randomized Clinical Trial. JAMA Oncol 2020, 6, 1003–1010. [Google Scholar] [CrossRef]
- Ahluwalia, M.S.; Rauf, Y.; Li, H.; Wen, P.Y.; Peereboom, D.M.; Reardon, D.A. Randomized phase 2 study of nivolumab (nivo) plus either standard or reduced dose bevacizumab (bev) in recurrent glioblastoma (rGBM). Clin Oncol 2021, 39, 2015–2015. [Google Scholar] [CrossRef]
- Omuro, A.; Vlahovic, G.; Lim, M.; Sahebjam, S.; Baehring, J.; Cloughesy, T.; Voloschin, A.; Ramkissoon, S.H.; Ligon, K.L.; Latek, R.; et al. Nivolumab with or without ipilimumab in patients with recurrent glioblastoma: results from exploratory phase I cohorts of CheckMate 143. J Neuro-Oncol 2018, 20, 674–686. [Google Scholar] [CrossRef]
- Lim, M.; Weller, M.; Idbaih, A.; Steinbach, J.; Finocchiaro, G.; Raval, R.R.; Ansstas, G.; Baehring, J.; Taylor, J.W.; Honnorat, J.; et al. Phase III trial of chemoradiotherapy with temozolomide plus nivolumab or placebo for newly diagnosed glioblastoma with methylated MGMT promoter. J Neuro-Oncol 2022, 24, 1935–1949. [Google Scholar] [CrossRef]
- Cloughesy, T.; Finocchiaro, G.; Belda-Iniesta, C.; Recht, L.; Brandes, A.A.; Pineda, E.; Mikkelsen, T.; Chinot, O.L.; Balana, C.; Macdonald, D.R.; et al. Randomized, Double-Blind, Placebo-Controlled, Multicenter Phase II Study of Onartuzumab Plus Bevacizumab Versus Placebo Plus Bevacizumab in Patients With Recurrent Glioblastoma: Efficacy, Safety, and Hepatocyte Growth Factor and O(6)-Methylguanine-DNA Methyltransferase Biomarker Analyses. J Clin Oncol 2017, 35, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Cher, L.; Nowak, A.; Iatropoulos, G.; Lee, W.S.; Lee, S.Y.; Shim, S.R.; Yoo, J.J.N.O. ACTR-75. A multicenter, 3-arm, open-label, phase IIa clinical trial to evaluate safety and efficacy of Tanibirumab (VEGFR2 mAB), in patients with recurrent GBM assessed with K-trans and initial area under the gadolinium concentration-time curve (IAUGC). J Neuro-Oncol 2017, 19, 17. [Google Scholar] [CrossRef]
- Reardon, D.A.; Kaley, T.J.; Dietrich, J.; Clarke, J.L.; Dunn, G.; Lim, M.; Cloughesy, T.F.; Gan, H.K.; Park, A.J.; Schwarzenberger, P.; et al. Phase II study to evaluate safety and efficacy of MEDI4736 (durvalumab) + radiotherapy in patients with newly diagnosed unmethylated MGMT glioblastoma (new unmeth GBM). Clin Oncol 2019, 37, 2032–2032. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, J.; Yang, X.; Liu, Y.; Zou, C.; Lv, W.; Chen, C.; Cheng, K.K.; Chen, T.; Chang, L.J.; et al. Safety and antitumor activity of GD2-Specific 4SCAR-T cells in patients with glioblastoma. Mol Cancer 2023, 22, 3. [Google Scholar] [CrossRef] [PubMed]
- Goff, S.L.; Morgan, R.A.; Yang, J.C.; Sherry, R.M.; Robbins, P.F.; Restifo, N.P.; Feldman, S.A.; Lu, Y.C.; Lu, L.; Zheng, Z.; et al. Pilot Trial of Adoptive Transfer of Chimeric Antigen Receptor-transduced T Cells Targeting EGFRvIII in Patients With Glioblastoma. J Immunother 2019, 42, 126–135. [Google Scholar] [CrossRef]
- Wick, W.; Dietrich, P.-Y.; Kuttruff, S.; Hilf, N.; Frenzel, K.; Admon, A.; van der Burg, S.H.; von Deimling, A.; Gouttefangeas, C.; Kroep, J.R.; et al. GAPVAC-101: First-in-human trial of a highly personalized peptide vaccination approach for patients with newly diagnosed glioblastoma. Clin Oncol 2018, 36, 2000–2000. [Google Scholar] [CrossRef]
- Kodysh, J.; Rubinsteyn, A.; Blazquez, A.; Mandeli, J.; Bhardwaj, N.; Hormigo, A.J.N.-O. CTIM-17. phase I study of the safety and immunogenicity of personalized neoantigen vaccines and tumor treating fields in patients with newly diagnosed glioblastoma. J Neuro-Oncol 2020, 22, ii36. [Google Scholar] [CrossRef]
- Hu, J.L.; Omofoye, O.A.; Rudnick, J.D.; Kim, S.; Tighiouart, M.; Phuphanich, S.; Wang, H.; Mazer, M.; Ganaway, T.; Chu, R.M.; et al. A Phase I Study of Autologous Dendritic Cell Vaccine Pulsed with Allogeneic Stem-like Cell Line Lysate in Patients with Newly Diagnosed or Recurrent Glioblastoma. Clin Cancer Res 2022, 28, 689–696. [Google Scholar] [CrossRef]
- Migliorini, D.; Dutoit, V.; Allard, M.; Grandjean Hallez, N.; Marinari, E.; Widmer, V.; Philippin, G.; Corlazzoli, F.; Gustave, R.; Kreutzfeldt, M.; et al. Phase I/II trial testing safety and immunogenicity of the multipeptide IMA950/poly-ICLC vaccine in newly diagnosed adult malignant astrocytoma patients. J Neuro-Oncol 2019, 21, 923–933. [Google Scholar] [CrossRef]
- Weller, M.; Butowski, N.; Tran, D.D.; Recht, L.D.; Lim, M.; Hirte, H.; Ashby, L.; Mechtler, L.; Goldlust, S.A.; Iwamoto, F.; et al. Rindopepimut with temozolomide for patients with newly diagnosed, EGFRvIII-expressing glioblastoma (ACT IV): a randomised, double-blind, international phase 3 trial. Lancet Oncol 2017, 18, 1373–1385. [Google Scholar] [CrossRef]
- Liau, L.M.; Ashkan, K.; Tran, D.D.; Campian, J.L.; Trusheim, J.E.; Cobbs, C.S.; Heth, J.A.; Salacz, M.; Taylor, S.; D'Andre, S.D.; et al. First results on survival from a large Phase 3 clinical trial of an autologous dendritic cell vaccine in newly diagnosed glioblastoma. J Transl Med 2018, 16, 142. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. J Neuro-Oncol 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Ruan, Y.; Wei, F.; Qin, G.; Mo, X.; Wang, X.; Zou, D. Identification of three glioblastoma subtypes and a six-gene prognostic risk index based on the expression of growth factors and cytokines. Am J Transl Res 2020, 12, 4669–4682. [Google Scholar] [PubMed]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Prager, B.C.; Bhargava, S.; Mahadev, V.; Hubert, C.G.; Rich, J.N. Glioblastoma Stem Cells: Driving Resilience through Chaos. Trends in Cancer 2020, 6, 223–235. [Google Scholar] [CrossRef]
- Hambardzumyan, D.; Bergers, G. Glioblastoma: Defining Tumor Niches. Trends in Cancer 2015, 1, 252–265. [Google Scholar] [CrossRef]
- Janjua, T.I.; Rewatkar, P.; Ahmed-Cox, A.; Saeed, I.; Mansfeld, F.M.; Kulshreshtha, R.; Kumeria, T.; Ziegler, D.S.; Kavallaris, M.; Mazzieri, R.; et al. Frontiers in the treatment of glioblastoma: Past, present and emerging. Adv Drug Deliv Rev 2021, 171, 108–138. [Google Scholar] [CrossRef]
- Sarafraz, M.; Nakhjavani, M.; Shigdar, S.; Christo, F.C.; Rolfe, B. Modelling of mass transport and distribution of aptamer in blood-brain barrier for tumour therapy and cancer treatment. Eur J Pharma Biopharm 2022, 173, 121–131. [Google Scholar] [CrossRef]
- Sharma, P.; Aaroe, A.; Liang, J.; Puduvalli, V.K. Tumor microenvironment in glioblastoma: Current and emerging concepts. J Neuro-Oncol Adv 2023, 5, vdad009. [Google Scholar] [CrossRef]
- BioRender. Available online: https://www.biorender.com/ (accessed on 8 August).
- Hambardzumyan, D.; Gutmann, D.H.; Kettenmann, H. The role of microglia and macrophages in glioma maintenance and progression. Nat Neurosci 2016, 19, 20–27. [Google Scholar] [CrossRef]
- Fanelli, G.N.; Grassini, D.; Ortenzi, V.; Pasqualetti, F.; Montemurro, N.; Perrini, P.; Naccarato, A.G.; Scatena, C. Decipher the Glioblastoma Microenvironment: The First Milestone for New Groundbreaking Therapeutic Strategies. Genes (Basel) 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Mohiuddin, E.; Wakimoto, H. Extracellular matrix in glioblastoma: opportunities for emerging therapeutic approaches. Am J Cancer Res 2021, 11, 3742–3754. [Google Scholar] [PubMed]
- Yan, T.; Chen, X.; Zhan, H.; Yao, P.; Wang, N.; Yang, H.; Zhang, C.; Wang, K.; Hu, H.; Li, J.; et al. Interfering with hyaluronic acid metabolism suppresses glioma cell proliferation by regulating autophagy. Cell Death Dis 2021, 12, 486. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Wang, K.; Li, J.; Hu, H.; Yang, H.; Cai, M.; Liu, R.; Li, H.; Wang, N.; Shi, Y.; et al. Suppression of the hyaluronic acid pathway induces M1 macrophages polarization via STAT1 in glioblastoma. Cell Death Dis 2022, 8, 193. [Google Scholar] [CrossRef]
- Malfanti, A.; Catania, G.; Degros, Q.; Wang, M.; Bausart, M.; Preat, V. Design of Bio-Responsive Hyaluronic Acid-Doxorubicin Conjugates for the Local Treatment of Glioblastoma. Pharmaceutics 2022, 14. [Google Scholar] [CrossRef]
- Nandhu, M.S.; Behera, P.; Bhaskaran, V.; Longo, S.L.; Barrera-Arenas, L.M.; Sengupta, S.; Rodriguez-Gil, D.J.; Chiocca, E.A.; Viapiano, M.S. Development of a Function-Blocking Antibody Against Fibulin-3 as a Targeted Reagent for Glioblastoma. Clin Cancer Res 2018, 24, 821–833. [Google Scholar] [CrossRef]
- Armento, A.; Ehlers, J.; Schotterl, S.; Naumann, U. Molecular Mechanisms of Glioma Cell Motility. In Glioblastoma, De Vleeschouwer, S., Ed.; Codon Publications Brisbane (AU), 2017.
- Cobb, D.A.; de Rossi, J.; Liu, L.; An, E.; Lee, D.W. Targeting of the alpha(v) beta(3) integrin complex by CAR-T cells leads to rapid regression of diffuse intrinsic pontine glioma and glioblastoma. J Immunother Cancer 2022, 10. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Guo, Q.; Guan, G.F.; Cheng, W.; Cheng, P.; Wu, A.H. Integrin Beta 5 Is a Prognostic Biomarker and Potential Therapeutic Target in Glioblastoma. Front Oncol 2019, 9, 904. [Google Scholar] [CrossRef]
- Franovic, A.; Elliott, K.C.; Seguin, L.; Camargo, M.F.; Weis, S.M.; Cheresh, D.A. Glioblastomas require integrin alphavbeta3/PAK4 signaling to escape senescence. Cancer Res 2015, 75, 4466–4473. [Google Scholar] [CrossRef]
- Gerstner, E.R.; Ye, X.; Duda, D.G.; Levine, M.A.; Mikkelsen, T.; Kaley, T.J.; Olson, J.J.; Nabors, B.L.; Ahluwalia, M.S.; Wen, P.Y.; et al. A phase I study of cediranib in combination with cilengitide in patients with recurrent glioblastoma. J Neuro-Oncol 2015, 17, 1386–1392. [Google Scholar] [CrossRef]
- Gilbert, M.R.; Kuhn, J.; Lamborn, K.R.; Lieberman, F.; Wen, P.Y.; Mehta, M.; Cloughesy, T.; Lassman, A.B.; Deangelis, L.M.; Chang, S.; et al. Cilengitide in patients with recurrent glioblastoma: the results of NABTC 03-02, a phase II trial with measures of treatment delivery. J Neuro-Oncol 2012, 106, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Nabors, L.B.; Fink, K.L.; Mikkelsen, T.; Grujicic, D.; Tarnawski, R.; Nam, D.H.; Mazurkiewicz, M.; Salacz, M.; Ashby, L.; Zagonel, V.; et al. Two cilengitide regimens in combination with standard treatment for patients with newly diagnosed glioblastoma and unmethylated MGMT gene promoter: results of the open-label, controlled, randomized phase II CORE study. J Neuro-Oncol 2015, 17, 708–717. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Hegi, M.E.; Gorlia, T.; Erridge, S.C.; Perry, J.; Hong, Y.K.; Aldape, K.D.; Lhermitte, B.; Pietsch, T.; Grujicic, D.; et al. Cilengitide combined with standard treatment for patients with newly diagnosed glioblastoma with methylated MGMT promoter (CENTRIC EORTC 26071-22072 study): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 2014, 15, 1100–1108. [Google Scholar] [CrossRef] [PubMed]
- Crivii, C.B.; Bosca, A.B.; Melincovici, C.S.; Constantin, A.M.; Marginean, M.; Dronca, E.; Sufletel, R.; Gonciar, D.; Bungardean, M.; Sovrea, A. Glioblastoma Microenvironment and Cellular Interactions. Cancers (Basel) 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- Di Nunno, V.; Franceschi, E.; Tosoni, A.; Gatto, L.; Bartolini, S.; Brandes, A.A. Glioblastoma Microenvironment: From an Inviolable Defense to a Therapeutic Chance. Front Oncol 2022, 12, 852950. [Google Scholar] [CrossRef]
- Poon, C.C.; Gordon, P.M.K.; Liu, K.; Yang, R.; Sarkar, S.; Mirzaei, R.; Ahmad, S.T.; Hughes, M.L.; Yong, V.W.; Kelly, J.J.P. Differential microglia and macrophage profiles in human IDH-mutant and -wild type glioblastoma. Oncotarget 2019, 10, 3129–3143. [Google Scholar] [CrossRef]
- Roesch, S.; Rapp, C.; Dettling, S.; Herold-Mende, C. When Immune Cells Turn Bad-Tumor-Associated Microglia/Macrophages in Glioma. Int J Mol Sci 2018, 19. [Google Scholar] [CrossRef]
- Ochocka, N.; Segit, P.; Walentynowicz, K.A.; Wojnicki, K.; Cyranowski, S.; Swatler, J.; Mieczkowski, J.; Kaminska, B. Single-cell RNA sequencing reveals functional heterogeneity of glioma-associated brain macrophages. Nat Commun 2021, 12, 1151. [Google Scholar] [CrossRef]
- Sorensen, M.D.; Dahlrot, R.H.; Boldt, H.B.; Hansen, S.; Kristensen, B.W. Tumour-associated microglia/macrophages predict poor prognosis in high-grade gliomas and correlate with an aggressive tumour subtype. Neuropathol Appl Neurobiol 2018, 44, 185–206. [Google Scholar] [CrossRef]
- Zhou, W.; Ke, S.Q.; Huang, Z.; Flavahan, W.; Fang, X.; Paul, J.; Wu, L.; Sloan, A.E.; McLendon, R.E.; Li, X.; et al. Periostin secreted by glioblastoma stem cells recruits M2 tumour-associated macrophages and promotes malignant growth. Nat Cell Biol 2015, 17, 170–182. [Google Scholar] [CrossRef]
- Azambuja, J.H.; Ludwig, N.; Yerneni, S.S.; Braganhol, E.; Whiteside, T.L. Arginase-1+ Exosomes from Reprogrammed Macrophages Promote Glioblastoma Progression. Int J Mol Sci 2020, 21. [Google Scholar] [CrossRef] [PubMed]
- Akkari, L.; Bowman, R.L.; Tessier, J.; Klemm, F.; Handgraaf, S.M.; de Groot, M.; Quail, D.F.; Tillard, L.; Gadiot, J.; Huse, J.T.; et al. Dynamic changes in glioma macrophage populations after radiotherapy reveal CSF-1R inhibition as a strategy to overcome resistance. Sci Transl Med 2020, 12. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.R.; Kumari, N.; Thi Vu, H.; Kim, H.; Park, C.-K.; Choi, S.H. Increased Antiangiogenic Effect by Blocking CCL2-dependent Macrophages in a Rodent Glioblastoma Model: Correlation Study with Dynamic Susceptibility Contrast Perfusion MRI. Sci Rep 2019, 9, 11085. [Google Scholar] [CrossRef] [PubMed]
- Pinton, L.; Masetto, E.; Vettore, M.; Solito, S.; Magri, S.; D'Andolfi, M.; Del Bianco, P.; Lollo, G.; Benoit, J.P.; Okada, H.; et al. The immune suppressive microenvironment of human gliomas depends on the accumulation of bone marrow-derived macrophages in the center of the lesion. J Immunother Cancer 2019, 7, 58. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Jackson, C.; Kim, T.; Choi, J.; Lim, M. A Characterization of Dendritic Cells and Their Role in Immunotherapy in Glioblastoma: From Preclinical Studies to Clinical Trials. Cancers (Basel) 2019, 11. [Google Scholar] [CrossRef]
- Wang, J.; Liu, P.; Xin, S.; Wang, Z.; Li, J. Nrf2 suppresses the function of dendritic cells to facilitate the immune escape of glioma cells. Exp Cell Res 2017, 360, 66–73. [Google Scholar] [CrossRef]
- Yu, J.; Sun, H.; Cao, W.; Song, Y.; Jiang, Z. Research progress on dendritic cell vaccines in cancer immunotherapy. Exp Hematol Oncol 2022, 11, 3. [Google Scholar] [CrossRef]
- Gabrusiewicz, K.; Rodriguez, B.; Wei, J.; Hashimoto, Y.; Healy, L.M.; Maiti, S.N.; Thomas, G.; Zhou, S.; Wang, Q.; Elakkad, A.; et al. Glioblastoma-infiltrated innate immune cells resemble M0 macrophage phenotype. JCI Insight 2016, 1. [Google Scholar] [CrossRef]
- Yee, P.P.; Wei, Y.; Kim, S.Y.; Lu, T.; Chih, S.Y.; Lawson, C.; Tang, M.; Liu, Z.; Anderson, B.; Thamburaj, K.; et al. Neutrophil-induced ferroptosis promotes tumor necrosis in glioblastoma progression. Nat Commun 2020, 11, 5424. [Google Scholar] [CrossRef]
- Liang, J.; Piao, Y.; Holmes, L.; Fuller, G.N.; Henry, V.; Tiao, N.; de Groot, J.F. Neutrophils promote the malignant glioma phenotype through S100A4. Clin Cancer Res 2014, 20, 187–198. [Google Scholar] [CrossRef]
- Chang, Y.; Cai, X.; Syahirah, R.; Yao, Y.; Xu, Y.; Jin, G.; Bhute, V.J.; Torregrosa-Allen, S.; Elzey, B.D.; Won, Y.Y.; et al. CAR-neutrophil mediated delivery of tumor-microenvironment responsive nanodrugs for glioblastoma chemo-immunotherapy. Nat Commun 2023, 14, 2266. [Google Scholar] [CrossRef] [PubMed]
- Kmiecik, J.; Poli, A.; Brons, N.H.; Waha, A.; Eide, G.E.; Enger, P.O.; Zimmer, J.; Chekenya, M. Elevated CD3+ and CD8+ tumor-infiltrating immune cells correlate with prolonged survival in glioblastoma patients despite integrated immunosuppressive mechanisms in the tumor microenvironment and at the systemic level. J Neuroimmunol 2013, 264, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Lohr, J.; Ratliff, T.; Huppertz, A.; Ge, Y.; Dictus, C.; Ahmadi, R.; Grau, S.; Hiraoka, N.; Eckstein, V.; Ecker, R.C.; et al. Effector T-cell infiltration positively impacts survival of glioblastoma patients and is impaired by tumor-derived TGF-beta. Clin Cancer Res 2011, 17, 4296–4308. [Google Scholar] [CrossRef]
- Woroniecka, K.; Chongsathidkiet, P.; Rhodin, K.; Kemeny, H.; Dechant, C.; Farber, S.H.; Elsamadicy, A.A.; Cui, X.; Koyama, S.; Jackson, C.; et al. T-Cell Exhaustion Signatures Vary with Tumor Type and Are Severe in Glioblastoma. Clin Cancer Res 2018, 24, 4175–4186. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, X.; Guo, R.; Wang, P. CD4(+)Foxp3(-) type 1 regulatory T cells in glioblastoma multiforme suppress T cell responses through multiple pathways and are regulated by tumor-associated macrophages. Int J Biochem Cell Biol 2016, 81, 1–9. [Google Scholar] [CrossRef]
- Lazarova, M.; Steinle, A. Impairment of NKG2D-Mediated Tumor Immunity by TGF-beta. Front Immunol 2019, 10, 2689. [Google Scholar] [CrossRef]
- Friebel, E.; Kapolou, K.; Unger, S.; Nunez, N.G.; Utz, S.; Rushing, E.J.; Regli, L.; Weller, M.; Greter, M.; Tugues, S.; et al. Single-Cell Mapping of Human Brain Cancer Reveals Tumor-Specific Instruction of Tissue-Invading Leukocytes. Cell 2020, 181, 1626–1642. [Google Scholar] [CrossRef]
- Shaim, H.; Shanley, M.; Basar, R.; Daher, M.; Gumin, J.; Zamler, D.B.; Uprety, N.; Wang, F.; Huang, Y.; Gabrusiewicz, K.; et al. Targeting the alphav integrin/TGF-beta axis improves natural killer cell function against glioblastoma stem cells. J Clin Invest 2021, 131. [Google Scholar] [CrossRef]
- Wang, J.; Toregrosa-Allen, S.; Elzey, B.D.; Utturkar, S.; Lanman, N.A.; Bernal-Crespo, V.; Behymer, M.M.; Knipp, G.T.; Yun, Y.; Veronesi, M.C.; et al. Multispecific targeting of glioblastoma with tumor microenvironment-responsive multifunctional engineered NK cells. Proc Natl Acad Sci U S A 2021, 118. [Google Scholar] [CrossRef]
- McCutcheon, S.; Spray, D.C. Glioblastoma-Astrocyte Connexin 43 Gap Junctions Promote Tumor Invasion. Mol Cancer Res 2022, 20, 319–331. [Google Scholar] [CrossRef]
- Venkataramani, V.; Yang, Y.; Schubert, M.C.; Reyhan, E.; Tetzlaff, S.K.; Wissmann, N.; Botz, M.; Soyka, S.J.; Beretta, C.A.; Pramatarov, R.L.; et al. Glioblastoma hijacks neuronal mechanisms for brain invasion. Cell 2022, 185, 2899–2917 e2831. [Google Scholar] [CrossRef] [PubMed]
- Pei, Z.; Lee, K.C.; Khan, A.; Erisnor, G.; Wang, H.Y. Pathway analysis of glutamate-mediated, calcium-related signaling in glioma progression. Biochem Pharmacol 2020, 176, 113814. [Google Scholar] [CrossRef] [PubMed]
- MacVicar, B.A.; Newman, E.A. Astrocyte regulation of blood flow in the brain. Cold Spring Harb Perspect Biol 2015, 7. [Google Scholar] [CrossRef] [PubMed]
- Watkins, S.; Robel, S.; Kimbrough, I.F.; Robert, S.M.; Ellis-Davies, G.; Sontheimer, H. Disruption of astrocyte-vascular coupling and the blood-brain barrier by invading glioma cells. Nat Commun 2014, 5, 4196. [Google Scholar] [CrossRef]
- Kim, J.K.; Jin, X.; Sohn, Y.W.; Jin, X.; Jeon, H.Y.; Kim, E.J.; Ham, S.W.; Jeon, H.M.; Chang, S.Y.; Oh, S.Y.; et al. Tumoral RANKL activates astrocytes that promote glioma cell invasion through cytokine signaling. Cancer Lett 2014, 353, 194–200. [Google Scholar] [CrossRef]
- Wang, X.; Jia, L.; Jin, X.; Liu, Q.; Cao, W.; Gao, X.; Yang, M.; Sun, B. NF-κB inhibitor reverses temozolomide resistance in human glioma TR/U251 cells. Oncol Lett 2015, 9, 2586–2590. [Google Scholar] [CrossRef]
- Ugbode, C.I.; Smith, I.; Whalley, B.J.; Hirst, W.D.; Rattray, M. Sonic hedgehog signalling mediates astrocyte crosstalk with neurons to confer neuroprotection. J Neurochem 2017, 142, 429–443. [Google Scholar] [CrossRef]
- Hung, H.C.; Liu, C.C.; Chuang, J.Y.; Su, C.L.; Gean, P.W. Inhibition of Sonic Hedgehog Signaling Suppresses Glioma Stem-Like Cells Likely Through Inducing Autophagic Cell Death. Front Oncol 2020, 10, 1233. [Google Scholar] [CrossRef]
- Ko, H.Y.; Chung, J.-I.; Kim, D.; Park, Y.M.; Jo, H.H.; Lee, S.; Kim, S.Y.; Kim, J.; Chun, J.-H.; Han, K.-S.J.B.A. Visualizing reactive astrogliosis extends survival in glioblastoma patients. BioRxiv 2021, 14. [Google Scholar]
- Edwards, L.A.; Woolard, K.; Son, M.J.; Li, A.; Lee, J.; Ene, C.; Mantey, S.A.; Maric, D.; Song, H.; Belova, G.; et al. Effect of brain- and tumor-derived connective tissue growth factor on glioma invasion. J Natl Cancer Inst 2011, 103, 1162–1178. [Google Scholar] [CrossRef]
- Song, Z.B.; Yang, H.P.; Xu, A.Q.; Zhan, Z.M.; Song, Y.; Li, Z.Y. Connective tissue growth factor as an unfavorable prognostic marker promotes the proliferation, migration, and invasion of gliomas. Chin Med J (Engl) 2020, 133, 670–678. [Google Scholar] [CrossRef] [PubMed]
- Jin, P.; Shin, S.H.; Chun, Y.S.; Shin, H.W.; Shin, Y.J.; Lee, Y.; Kim, D.; Nam, D.H.; Park, J.W. Astrocyte-derived CCL20 reinforces HIF-1-mediated hypoxic responses in glioblastoma by stimulating the CCR6-NF-kappaB signaling pathway. Oncogene 2018, 37, 3070–3087. [Google Scholar] [CrossRef] [PubMed]
- Biasoli, D.; Sobrinho, M.F.; da Fonseca, A.C.C.; de Matos, D.G.; Romão, L.; de Moraes Maciel, R.; Rehen, S.K.; Moura-Neto, V.; Borges, H.L.; Lima, F.R.S. Glioblastoma cells inhibit astrocytic p53-expression favoring cancer malignancy. Oncogenesis 2014, 3, e123–e123. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Carlsson, R.; Ambjorn, M.; Hasan, M.; Badn, W.; Darabi, A.; Siesjo, P.; Issazadeh-Navikas, S. PD-L1 expression by neurons nearby tumors indicates better prognosis in glioblastoma patients. J Neurosci 2013, 33, 14231–14245. [Google Scholar] [CrossRef]
- Qiu, X.Y.; Hu, D.X.; Chen, W.Q.; Chen, R.Q.; Qian, S.R.; Li, C.Y.; Li, Y.J.; Xiong, X.X.; Liu, D.; Pan, F.; et al. PD-L1 confers glioblastoma multiforme malignancy via Ras binding and Ras/Erk/EMT activation. Biochim Biophys Acta Mol Basis Dis 2018, 1864, 1754–1769. [Google Scholar] [CrossRef]
- Litak, J.; Mazurek, M.; Grochowski, C.; Kamieniak, P.; Rolinski, J. PD-L1/PD-1 Axis in Glioblastoma Multiforme. Int J Mol Sci 2019, 20. [Google Scholar] [CrossRef]
- Kawashima, T.; Yashiro, M.; Kasashima, H.; Terakawa, Y.; Uda, T.; Nakajo, K.; Umaba, R.; Tanoue, Y.; Tamrakar, S.; Ohata, K. Oligodendrocytes Up-regulate the Invasive Activity of Glioblastoma Cells via the Angiopoietin-2 Signaling Pathway. Anticancer Res 2019, 39, 577–584. [Google Scholar] [CrossRef]
- Felcht, M.; Luck, R.; Schering, A.; Seidel, P.; Srivastava, K.; Hu, J.; Bartol, A.; Kienast, Y.; Vettel, C.; Loos, E.K.; et al. Angiopoietin-2 differentially regulates angiogenesis through TIE2 and integrin signaling. J Clin Invest 2012, 122, 1991–2005. [Google Scholar] [CrossRef]
- Oliveira, A.I.; Anjo, S.I.; Vieira de Castro, J.; Serra, S.C.; Salgado, A.J.; Manadas, B.; Costa, B.M. Crosstalk between glial and glioblastoma cells triggers the "go-or-grow" phenotype of tumor cells. Cell Commun Signal 2017, 15, 37. [Google Scholar] [CrossRef]
- Papa, S.; Choy, P.M.; Bubici, C. The ERK and JNK pathways in the regulation of metabolic reprogramming. Oncogene 2019, 38, 2223–2240. [Google Scholar] [CrossRef]
- Gao, L.; Liu, J.; Xu, P.; Deng, G.; Liu, B.; Yuan, F.; Tan, Y.; Sun, Q.; Xu, Y.; Zhang, H.; et al. AKT Inhibitor SC66 Inhibits Proliferation and Induces Apoptosis in Human Glioblastoma Through Down-Regulating AKT/beta-Catenin Pathway. Front Pharmacol 2020, 11, 1102. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Zhou, L.; Lim, Y.; Yang, M.; Zhu, Y.H.; Li, Z.W.; Zhou, F.H.; Xiao, Z.C.; Zhou, X.F. Mature BDNF promotes the growth of glioma cells in vitro. Oncol Rep 2013, 30, 2719–2724. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Zhou, L.I.; Lim, Y.; Yang, M.; Zhu, Y.H.; Li, Z.W.; Fu, D.L.; Zhou, X.F. Mature brain-derived neurotrophic factor and its receptor TrkB are upregulated in human glioma tissues. Oncol Lett 2015, 10, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Zhou, L.; Yang, M.; Lim, Y.; Zhu, Y.H.; Fu, D.L.; Li, Z.W.; Zhong, J.H.; Xiao, Z.C.; Zhou, X.F. ProBDNF and its receptors are upregulated in glioma and inhibit the growth of glioma cells in vitro. J Neuro-Oncol 2013, 15, 990–1007. [Google Scholar] [CrossRef]
- Liu, S.; Jiang, T.; Zhong, Y.; Yu, Y. miR-210 inhibits cell migration and invasion by targeting the brain-derived neurotrophic factor in glioblastoma. J Cell Biochem 2019, 120, 11375–11382. [Google Scholar] [CrossRef]
- Zheng, B.; Chen, T. MiR-489-3p inhibits cell proliferation, migration, and invasion, and induces apoptosis, by targeting the BDNF-mediated PI3K/AKT pathway in glioblastoma. Open Life Sci 2020, 15, 274–283. [Google Scholar] [CrossRef]
- Venkatesh, H.S.; Johung, T.B.; Caretti, V.; Noll, A.; Tang, Y.; Nagaraja, S.; Gibson, E.M.; Mount, C.W.; Polepalli, J.; Mitra, S.S.; et al. Neuronal Activity Promotes Glioma Growth through Neuroligin-3 Secretion. Cell 2015, 161, 803–816. [Google Scholar] [CrossRef]
- Venkatesh, H.S.; Tam, L.T.; Woo, P.J.; Lennon, J.; Nagaraja, S.; Gillespie, S.M.; Ni, J.; Duveau, D.Y.; Morris, P.J.; Zhao, J.J.; et al. Targeting neuronal activity-regulated neuroligin-3 dependency in high-grade glioma. Nat 2017, 549, 533–537. [Google Scholar] [CrossRef]
- Liu, R.; Qin, X.P.; Zhuang, Y.; Zhang, Y.; Liao, H.B.; Tang, J.C.; Pan, M.X.; Zeng, F.F.; Lei, Y.; Lei, R.X.; et al. Glioblastoma recurrence correlates with NLGN3 levels. Cancer Medicine 2018, 7, 2848–2859. [Google Scholar] [CrossRef]
- Faubert, B.; Solmonson, A.; DeBerardinis, R.J. Metabolic reprogramming and cancer progression. Science 2020, 368. [Google Scholar] [CrossRef]
- Caldwell, J.C. Alterations in cell proliferation, cell death, or nutrient supply. In Tumour Site Concordance and Mechanisms of Carcinogenesis, Baan, R.A., Stewart, B.W., Straif, K., Eds.; IARC Scientific Publications; International Agency for Research on Cancer Lyon (FR), 2019.
- Duan, K.; Liu, Z.J.; Hu, S.Q.; Huo, H.Y.; Xu, Z.R.; Ruan, J.F.; Sun, Y.; Dai, L.P.; Yan, C.B.; Xiong, W.; et al. Lactic acid induces lactate transport and glycolysis/OXPHOS interconversion in glioblastoma. Biochem Biophys Res Commun 2018, 503, 888–894. [Google Scholar] [CrossRef]
- Larionova, T.D.; Bastola, S.; Aksinina, T.E.; Anufrieva, K.S.; Wang, J.; Shender, V.O.; Andreev, D.E.; Kovalenko, T.F.; Arapidi, G.P.; Shnaider, P.V.; et al. Alternative RNA splicing modulates ribosomal composition and determines the spatial phenotype of glioblastoma cells. Nat Cell Biol 2022, 24, 1541–1557. [Google Scholar] [CrossRef] [PubMed]
- Park, J.M.; Josan, S.; Jang, T.; Merchant, M.; Watkins, R.; Hurd, R.E.; Recht, L.D.; Mayer, D.; Spielman, D.M. Volumetric spiral chemical shift imaging of hyperpolarized [2-(13) c]pyruvate in a rat c6 glioma model. Magn Reson Med 2016, 75, 973–984. [Google Scholar] [CrossRef] [PubMed]
- Pillai, S.R.; Damaghi, M.; Marunaka, Y.; Spugnini, E.P.; Fais, S.; Gillies, R.J. Causes, consequences, and therapy of tumors acidosis. Cancer Metastasis Rev 2019, 38, 205–222. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, J.; Dong, W.; Liu, X.; Yang, C.; Wang, D.; Xue, Y.; Ruan, X.; Liu, L.; Wang, P.; et al. The MBNL1/circNTRK2/PAX5 pathway regulates aerobic glycolysis in glioblastoma cells by encoding a novel protein NTRK2-243aa. Cell Death Dis 2022, 13, 767. [Google Scholar] [CrossRef]
- Huang, Y.C.; Cheng, M.L.; Tang, H.Y.; Huang, C.Y.; Chen, K.M.; Wang, J.S. Eccentric Cycling Training Improves Erythrocyte Antioxidant and Oxygen Releasing Capacity Associated with Enhanced Anaerobic Glycolysis and Intracellular Acidosis. Antioxidants (Basel) 2021, 10. [Google Scholar] [CrossRef]
- Jones, W.; Bianchi, K. Aerobic glycolysis: beyond proliferation. Front Immunol 2015, 6, 227. [Google Scholar] [CrossRef]
- Soeda, A.; Park, M.; Lee, D.; Mintz, A.; Androutsellis-Theotokis, A.; McKay, R.D.; Engh, J.; Iwama, T.; Kunisada, T.; Kassam, A.B.; et al. Hypoxia promotes expansion of the CD133-positive glioma stem cells through activation of HIF-1alpha. Oncogene 2009, 28, 3949–3959. [Google Scholar] [CrossRef]
- Li, Z.; Bao, S.; Wu, Q.; Wang, H.; Eyler, C.; Sathornsumetee, S.; Shi, Q.; Cao, Y.; Lathia, J.; McLendon, R.E.; et al. Hypoxia-inducible factors regulate tumorigenic capacity of glioma stem cells. Cancer Cell 2009, 15, 501–513. [Google Scholar] [CrossRef]
- John, S.; Sivakumar, K.C.; Mishra, R. Extracellular Proton Concentrations Impacts LN229 Glioblastoma Tumor Cell Fate via Differential Modulation of Surface Lipids. Front Oncol 2017, 7, 20. [Google Scholar] [CrossRef]
- Zervantonakis, I.K.; Hughes-Alford, S.K.; Charest, J.L.; Condeelis, J.S.; Gertler, F.B.; Kamm, R.D. Three-dimensional microfluidic model for tumor cell intravasation and endothelial barrier function. Proc Natl Acad Sci U S A 2012, 109, 13515–13520. [Google Scholar] [CrossRef] [PubMed]
- Cong, L.; Ran, F.A.; Cox, D.; Lin, S.; Barretto, R.; Habib, N.; Hsu, P.D.; Wu, X.; Jiang, W.; Marraffini, L.A.; et al. Multiplex genome engineering using CRISPR/Cas systems. Science 2013, 339, 819–823. [Google Scholar] [CrossRef] [PubMed]
- Kahlon, A.S.; Alexander, M.; Kahlon, A.; Wright, J. Lactate levels with glioblastoma multiforme. Proc (Bayl Univ Med Cent) 2016, 29, 313–314. [Google Scholar] [CrossRef] [PubMed]
- Yamini, B.; Lyne, S.; Driscoll, R.; Bernal, G.; Wu, L.; NIcholas, M.; Chmura, S.; Collins, J.; Park, D.; Pytel, P.; et al. CTNI-47. interim results of NCT03011671: a multi-institutional phase i study of acetazolamide with temozolomide in adults with newly diagnosed mgmt-methylated malignant glioma. J Neuro-Oncol 2021, 23, vi70–vi70. [Google Scholar] [CrossRef]
- Stadlbauer, A.; Kinfe, T.M.; Eyupoglu, I.; Zimmermann, M.; Kitzwogerer, M.; Podar, K.; Buchfelder, M.; Heinz, G.; Oberndorfer, S.; Marhold, F. Tissue Hypoxia and Alterations in Microvascular Architecture Predict Glioblastoma Recurrence in Humans. Clin Cancer Res 2021, 27, 1641–1649. [Google Scholar] [CrossRef]
- Wang, D.; Lu, Y.; Li, X.; Mei, N.; Wu, P.Y.; Geng, D.; Wu, H.; Yin, B. Evaluation of HIF-1α Expression in a Rat Glioma Model Using Intravoxel Incoherent Motion and R2* Mapping. Front Oncol 2022, 12, 902612. [Google Scholar] [CrossRef]
- Macharia, L.W.; Muriithi, W.; Heming, C.P.; Nyaga, D.K.; Aran, V.; Mureithi, M.W.; Ferrer, V.P.; Pane, A.; Filho, P.N.; Moura-Neto, V. The genotypic and phenotypic impact of hypoxia microenvironment on glioblastoma cell lines. BMC Cancer 2021, 21, 1248. [Google Scholar] [CrossRef]
- Voss, D.M.; Sloan, A.; Spina, R.; Ames, H.M.; Bar, E.E. The Alternative Splicing Factor, MBNL1, Inhibits Glioblastoma Tumor Initiation and Progression by Reducing Hypoxia-Induced Stemness. Cancer Res 2020, 80, 4681–4692. [Google Scholar] [CrossRef]
- Inukai, M.; Hara, A.; Yasui, Y.; Kumabe, T.; Matsumoto, T.; Saegusa, M. Hypoxia-mediated cancer stem cells in pseudopalisades with activation of hypoxia-inducible factor-1alpha/Akt axis in glioblastoma. Hum Pathol 2015, 46, 1496–1505. [Google Scholar] [CrossRef]
- Monteiro, A.R.; Hill, R.; Pilkington, G.J.; Madureira, P.A. The Role of Hypoxia in Glioblastoma Invasion. Cells 2017, 6. [Google Scholar] [CrossRef]
- Bowman-Kirigin, J.A.; Desai, R.; Saunders, B.T.; Wang, A.Z.; Schaettler, M.O.; Liu, C.J.; Livingstone, A.J.; Kobayashi, D.K.; Durai, V.; Kretzer, N.M.; et al. The Conventional Dendritic Cell 1 Subset Primes CD8+ T Cells and Traffics Tumor Antigen to Drive Antitumor Immunity in the Brain. Cancer Immunol Res 2023, 11, 20–37. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Cao, B.; Liang, X.; Lu, S.; Luo, H.; Wang, Z.; Wang, S.; Jiang, J.; Lang, J.; Zhu, G. Microenvironmental oxygen pressure orchestrates an anti- and pro-tumoral gammadelta T cell equilibrium via tumor-derived exosomes. Oncogene 2019, 38, 2830–2843. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Morales, R.T.; Qian, W.; Wang, H.; Gagner, J.P.; Dolgalev, I.; Placantonakis, D.; Zagzag, D.; Cimmino, L.; Snuderl, M.; et al. Hacking macrophage-associated immunosuppression for regulating glioblastoma angiogenesis. Biomaterials 2018, 161, 164–178. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.L.; Wang, C.C.; Lin, Y.J.; Wu, C.P.; Hsieh, C.H. Cycling hypoxia induces chemoresistance through the activation of reactive oxygen species-mediated B-cell lymphoma extra-long pathway in glioblastoma multiforme. J Transl Med 2015, 13, 389. [Google Scholar] [CrossRef]
- Cui, J.; Zhang, Q.; Song, Q.; Wang, H.; Dmitriev, P.; Sun, M.Y.; Cao, X.; Wang, Y.; Guo, L.; Indig, I.H.; et al. Targeting hypoxia downstream signaling protein, CAIX, for CAR T-cell therapy against glioblastoma. J Neuro-Oncol 2019, 21, 1436–1446. [Google Scholar] [CrossRef]
- Ruiz-Ontanon, P.; Orgaz, J.L.; Aldaz, B.; Elosegui-Artola, A.; Martino, J.; Berciano, M.T.; Montero, J.A.; Grande, L.; Nogueira, L.; Diaz-Moralli, S.; et al. Cellular plasticity confers migratory and invasive advantages to a population of glioblastoma-initiating cells that infiltrate peritumoral tissue. Stem Cells 2013, 31, 1075–1085. [Google Scholar] [CrossRef]
- Cheng, L.; Huang, Z.; Zhou, W.; Wu, Q.; Donnola, S.; Liu, J.K.; Fang, X.; Sloan, A.E.; Mao, Y.; Lathia, J.D.; et al. Glioblastoma stem cells generate vascular pericytes to support vessel function and tumor growth. Cell 2013, 153, 139–152. [Google Scholar] [CrossRef]
- D'Alessio, A.; Proietti, G.; Lama, G.; Biamonte, F.; Lauriola, L.; Moscato, U.; Vescovi, A.; Mangiola, A.; Angelucci, C.; Sica, G. Analysis of angiogenesis related factors in glioblastoma, peritumoral tissue and their derived cancer stem cells. Oncotarget 2016, 7, 78541–78556. [Google Scholar] [CrossRef]
- Lemée, J.-M.; Clavreul, A.; Menei, P. Intratumoral heterogeneity in glioblastoma: don't forget the peritumoral brain zone. J Neuro-Oncol 2015, 17, 1322–1332. [Google Scholar] [CrossRef]
- Wang, X.; Prager, B.C.; Wu, Q.; Kim, L.J.Y.; Gimple, R.C.; Shi, Y.; Yang, K.; Morton, A.R.; Zhou, W.; Zhu, Z.; et al. Reciprocal Signaling between Glioblastoma Stem Cells and Differentiated Tumor Cells Promotes Malignant Progression. Cell Stem Cell 2018, 22, 514–528.e515. [Google Scholar] [CrossRef]
- Ma, T.; Hu, C.; Lal, B.; Zhou, W.; Ma, Y.; Ying, M.; Prinos, P.; Quinones-Hinojosa, A.; Lim, M.; Laterra, J.; et al. Reprogramming Transcription Factors Oct4 and Sox2 Induce a BRD-Dependent Immunosuppressive Transcriptome in GBM-Propagating Cells. Cancer Res 2021, 81, 2457–2469. [Google Scholar] [CrossRef] [PubMed]
- Kreatsoulas, D.; Bolyard, C.; Wu, B.X.; Cam, H.; Giglio, P.; Li, Z. Translational landscape of glioblastoma immunotherapy for physicians: guiding clinical practice with basic scientific evidence. J Hematol Oncol 2022, 15, 80. [Google Scholar] [CrossRef] [PubMed]
- Rong, L.; Li, N.; Zhang, Z. Emerging therapies for glioblastoma: current state and future directions. J Exp Clin Cancer Res 2022, 41, 142. [Google Scholar] [CrossRef] [PubMed]
- Quail, D.F.; Bowman, R.L.; Akkari, L.; Quick, M.L.; Schuhmacher, A.J.; Huse, J.T.; Holland, E.C.; Sutton, J.C.; Joyce, J.A. The tumor microenvironment underlies acquired resistance to CSF-1R inhibition in gliomas. Science 2016, 352, aad3018. [Google Scholar] [CrossRef] [PubMed]
- Almahariq, M.F.; Quinn, T.J.; Kesarwani, P.; Kant, S.; Miller, C.R.; Chinnaiyan, P. Inhibition of Colony-Stimulating Factor-1 Receptor Enhances the Efficacy of Radiotherapy and Reduces Immune Suppression in Glioblastoma. In Vivo 2021, 35, 119–129. [Google Scholar] [CrossRef]
- Stafford, J.H.; Hirai, T.; Deng, L.; Chernikova, S.B.; Urata, K.; West, B.L.; Brown, J.M. Colony stimulating factor 1 receptor inhibition delays recurrence of glioblastoma after radiation by altering myeloid cell recruitment and polarization. J Neuro-Oncol 2016, 18, 797–806. [Google Scholar] [CrossRef]
- Butowski, N.; Colman, H.; De Groot, J.F.; Omuro, A.M.; Nayak, L.; Wen, P.Y.; Cloughesy, T.F.; Marimuthu, A.; Haidar, S.; Perry, A.; et al. Orally administered colony stimulating factor 1 receptor inhibitor PLX3397 in recurrent glioblastoma: an Ivy Foundation Early Phase Clinical Trials Consortium phase II study. J Neuro-Oncol 2016, 18, 557–564. [Google Scholar] [CrossRef]
- Berghoff, A.S.; Kiesel, B.; Widhalm, G.; Rajky, O.; Ricken, G.; Wohrer, A.; Dieckmann, K.; Filipits, M.; Brandstetter, A.; Weller, M.; et al. Programmed death ligand 1 expression and tumor-infiltrating lymphocytes in glioblastoma. J Neuro-Oncol 2015, 17, 1064–1075. [Google Scholar] [CrossRef]
- Bouffet, E.; Larouche, V.; Campbell, B.B.; Merico, D.; de Borja, R.; Aronson, M.; Durno, C.; Krueger, J.; Cabric, V.; Ramaswamy, V.; et al. Immune Checkpoint Inhibition for Hypermutant Glioblastoma Multiforme Resulting From Germline Biallelic Mismatch Repair Deficiency. Clin Oncol 2016, 34, 2206–2211. [Google Scholar] [CrossRef]
- Johanns, T.M.; Miller, C.A.; Dorward, I.G.; Tsien, C.; Chang, E.; Perry, A.; Uppaluri, R.; Ferguson, C.; Schmidt, R.E.; Dahiya, S.; et al. Immunogenomics of Hypermutated Glioblastoma: A Patient with Germline POLE Deficiency Treated with Checkpoint Blockade Immunotherapy. Cancer Discov 2016, 6, 1230–1236. [Google Scholar] [CrossRef]
- Ott, P.A.; Bang, Y.J.; Piha-Paul, S.A.; Razak, A.R.A.; Bennouna, J.; Soria, J.C.; Rugo, H.S.; Cohen, R.B.; O'Neil, B.H.; Mehnert, J.M.; et al. T-Cell-Inflamed Gene-Expression Profile, Programmed Death Ligand 1 Expression, and Tumor Mutational Burden Predict Efficacy in Patients Treated With Pembrolizumab Across 20 Cancers: KEYNOTE-028. J Clin Oncol 2019, 37, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Cloughesy, T.F.; Mochizuki, A.Y.; Orpilla, J.R.; Hugo, W.; Lee, A.H.; Davidson, T.B.; Wang, A.C.; Ellingson, B.M.; Rytlewski, J.A.; Sanders, C.M.; et al. Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nat Med 2019, 25, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Huang, J.; Liu, X.; Cheng, Q.; Luo, C.; Liu, Z. CTLA-4 correlates with immune and clinical characteristics of glioma. Cancer Cell Int 2020, 20, 7. [Google Scholar] [CrossRef]
- Kuwana, Y.; Asakura, Y.; Utsunomiya, N.; Nakanishi, M.; Arata, Y.; Itoh, S.; Nagase, F.; Kurosawa, Y. Expression of chimeric receptor composed of immunoglobulin-derived V regions and T-cell receptor-derived C regions. Biochem BiophysRes Commun 1987, 149, 960–968. [Google Scholar] [CrossRef]
- Styczyński, J. A brief history of CAR-T cells: from laboratory to the bedside. Acta Haematol Polonica 2020, 51, 2–5. [Google Scholar] [CrossRef]
- Wang, X.; Lu, J.; Guo, G.; Yu, J. Immunotherapy for recurrent glioblastoma: practical insights and challenging prospects. Cell Death Dis 2021, 12, 299. [Google Scholar] [CrossRef]
- Bagley, S.J.; Desai, A.S.; Linette, G.P.; June, C.H.; O'Rourke, D.M. CAR T-cell therapy for glioblastoma: recent clinical advances and future challenges. J Neuro-Oncol 2018, 20, 1429–1438. [Google Scholar] [CrossRef] [PubMed]
- O'Rourke, D.M.; Nasrallah, M.P.; Desai, A.; Melenhorst, J.J.; Mansfield, K.; Morrissette, J.J.D.; Martinez-Lage, M.; Brem, S.; Maloney, E.; Shen, A.; et al. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci Transl Med 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.E.; Badie, B.; Barish, M.E.; Weng, L.; Ostberg, J.R.; Chang, W.C.; Naranjo, A.; Starr, R.; Wagner, J.; Wright, C.; et al. Bioactivity and Safety of IL13Rα2-Redirected Chimeric Antigen Receptor CD8+ T Cells in Patients with Recurrent Glioblastoma. Clin Cancer Res 2015, 21, 4062–4072. [Google Scholar] [CrossRef]
- Brown, C.E.; Alizadeh, D.; Starr, R.; Weng, L.; Wagner, J.R.; Naranjo, A.; Ostberg, J.R.; Blanchard, M.S.; Kilpatrick, J.; Simpson, J.; et al. Regression of Glioblastoma after Chimeric Antigen Receptor T-Cell Therapy. N Engl J Med 2016, 375, 2561–2569. [Google Scholar] [CrossRef]
- Brown, N.F.; Carter, T.J.; Ottaviani, D.; Mulholland, P. Harnessing the immune system in glioblastoma. Br J Cancer 2018, 119, 1171–1181. [Google Scholar] [CrossRef] [PubMed]
- Bloch, O.; Crane, C.A.; Fuks, Y.; Kaur, R.; Aghi, M.K.; Berger, M.S.; Butowski, N.A.; Chang, S.M.; Clarke, J.L.; McDermott, M.W.; et al. Heat-shock protein peptide complex-96 vaccination for recurrent glioblastoma: a phase II, single-arm trial. J Neuro-Oncol 2014, 16, 274–279. [Google Scholar] [CrossRef] [PubMed]
- De Vleeschouwer, S.; Fieuws, S.; Rutkowski, S.; Van Calenbergh, F.; Van Loon, J.; Goffin, J.; Sciot, R.; Wilms, G.; Demaerel, P.; Warmuth-Metz, M.; et al. Postoperative adjuvant dendritic cell-based immunotherapy in patients with relapsed glioblastoma multiforme. Clin Cancer Res 2008, 14, 3098–3104. [Google Scholar] [CrossRef] [PubMed]
- Westphal, M.; Heese, O.; Steinbach, J.P.; Schnell, O.; Schackert, G.; Mehdorn, M.; Schulz, D.; Simon, M.; Schlegel, U.; Senft, C.; et al. A randomised, open label phase III trial with nimotuzumab, an anti-epidermal growth factor receptor monoclonal antibody in the treatment of newly diagnosed adult glioblastoma. Eur J Cancer 2015, 51, 522–532. [Google Scholar] [CrossRef]
- Diaz, R.J.; Ali, S.; Qadir, M.G.; De La Fuente, M.I.; Ivan, M.E.; Komotar, R.J. The role of bevacizumab in the treatment of glioblastoma. J Neuro-Oncol 2017, 133, 455–467. [Google Scholar] [CrossRef]
- Nakhjavani, M.; Shigdar, S. Future of PD-1/PD-L1 axis modulation for the treatment of triple-negative breast cancer. Pharm Res 2022, 175, 106019. [Google Scholar] [CrossRef]
- Bukari, B.; Samarasinghe, R.M.; Noibanchong, J.; Shigdar, S.L. Non-Invasive Delivery of Therapeutics into the Brain: The Potential of Aptamers for Targeted Delivery. Biomedicines 2020, 8, 120. [Google Scholar] [CrossRef]
- Giles, B.; Samarasinghe, R.M.; Shigda, S. Rising to the challenge: recent aptamer-conjugate success in treating glioblastoma. Aptamers 2022, 6, 28–37. [Google Scholar]
- Macdonald, J.; Denoyer, D.; Henri, J.; Jamieson, A.; Burvenich, I.J.; Pouliot, N.; Shigdar, S. Bifunctional aptamer–doxorubicin conjugate crosses the blood–brain barrier and selectively delivers its payload to EpCAM-positive tumor cells. Nucleic Acid Thera 2020, 30, 117–128. [Google Scholar] [CrossRef]
- Macdonald, J.; Henri, J.; Roy, K.; Hays, E.; Bauer, M.; Veedu, R.N.; Pouliot, N.; Shigdar, S. EpCAM Immunotherapy versus Specific Targeted Delivery of Drugs. Cancers (Basel) 2018, 10. [Google Scholar] [CrossRef]
- Hicke, B.J.; Marion, C.; Chang, Y.-F.; Gould, T.; Lynott, C.K.; Parma, D.; Schmidt, P.G.; Warren, S. Tenascin-C aptamers are generated using tumor cells and purified protein. J Bio Chem 2001, 276, 48644–48654. [Google Scholar] [CrossRef] [PubMed]
- Hicke, B.J.; Stephens, A.W.; Gould, T.; Chang, Y.-F.; Lynott, C.K.; Heil, J.; Borkowski, S.; Hilger, C.-S.; Cook, G.; Warren, S. Tumor targeting by an aptamer. J Nuclear Med 2006, 47, 668–678. [Google Scholar]
- Li, K.; Deng, J.; Jin, H.; Yang, X.; Fan, X.; Li, L.; Zhao, Y.; Guan, Z.; Wu, Y.; Zhang, L. Chemical modification improves the stability of the DNA aptamer GBI-10 and its affinity towards tenascin-C. Org Biomol Chem 2017, 15, 1174–1182. [Google Scholar] [CrossRef] [PubMed]
- Kemper, K.; Sprick, M.R.; de Bree, M.; Scopelliti, A.; Vermeulen, L.; Hoek, M.; Zeilstra, J.; Pals, S.T.; Mehmet, H.; Stassi, G. The AC133 epitope, but not the CD133 protein, is lost upon cancer stem cell differentiation. Cancer Res 2010, 70, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Yuan, X.; Zeng, Z.; Tunici, P.; Ng, H.; Abdulkadir, I.R.; Lu, L.; Irvin, D.; Black, K.L.; Yu, J.S. Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol Cancer 2006, 5, 67. [Google Scholar] [CrossRef] [PubMed]
- Gambelli, F.; Sasdelli, F.; Manini, I.; Gambarana, C.; Oliveri, G.; Miracco, C.; Sorrentino, V. Identification of cancer stem cells from human glioblastomas: growth and differentiation capabilities and CD133/prominin-1 expression. Cell Bio Int 2012, 36, 29–38. [Google Scholar] [CrossRef]
- Kang, D.; Wang, J.; Zhang, W.; Song, Y.; Li, X.; Zou, Y.; Zhu, M.; Zhu, Z.; Chen, F.; Yang, C.J. Selection of DNA aptamers against glioblastoma cells with high affinity and specificity. PLoS One 2012. [Google Scholar] [CrossRef]
- Wang, T.; Philippovich, S.; Mao, J.; Veedu, R.N. Efficient epidermal growth factor receptor targeting oligonucleotide as a potential molecule for targeted cancer therapy. Int J Mol Sci 2019, 20, 4700. [Google Scholar] [CrossRef]
- Liu, Y.; Kuan, C.-T.; Mi, J.; Zhang, X.; Clary, B.M.; Bigner, D.D.; Sullenger, B.A. Aptamers selected against the unglycosylated EGFRvIII ectodomain and delivered intracellularly reduce membrane-bound EGFRvIII and induce apoptosis. Bio Chem 2009, 390, 137–144. [Google Scholar] [CrossRef]
- Tan, Y.; Shi, Y.-s.; Wu, X.-d.; Liang, H.-y.; Gao, Y.-b.; Li, S.-j.; Zhang, X.-m.; Wang, F.; Gao, T.-m. DNA aptamers that target human glioblastoma multiforme cells overexpressing epidermal growth factor receptor variant III in vitro. Acta Pharmacologica Sinica 2013, 34, 1491–1498. [Google Scholar] [CrossRef]
- Zhang, X.; Liang, H.; Tan, Y.; Wu, X.; Li, S.; Shi, Y. A U87-EGFRvIII cell-specific aptamer mediates small interfering RNA delivery. Biomed Rep 2014, 2, 495–499. [Google Scholar] [CrossRef]
- Tang, J.; Huang, N.; Zhang, X.; Zhou, T.; Tan, Y.; Pi, J.; Pi, L.; Cheng, S.; Zheng, H.; Cheng, Y. Aptamer-conjugated PEGylated quantum dots targeting epidermal growth factor receptor variant III for fluorescence imaging of glioma. Int J Nanomed 2017, 12, 3899. [Google Scholar] [CrossRef]
- Camorani, S.; Crescenzi, E.; Colecchia, D.; Carpentieri, A.; Amoresano, A.; Fedele, M.; Chiariello, M.; Cerchia, L. Aptamer targeting EGFRvIII mutant hampers its constitutive autophosphorylation and affects migration, invasion and proliferation of glioblastoma cells. Oncotarget 2015, 6, 37570. [Google Scholar] [CrossRef]
- Zhang, X.; Peng, L.; Liang, Z.; Kou, Z.; Chen, Y.; Shi, G.; Li, X.; Liang, Y.; Wang, F.; Shi, Y. Effects of aptamer to U87-EGFRvIII cells on the proliferation, radiosensitivity, and radiotherapy of glioblastoma cells. Mol Thera-Nucleic Acids 2018, 10, 438–449. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Liang, Y.; Zhong, X.; Liang, Z.; Tian, Y.; Li, S.; Liang, J.; Wang, R.; Zhong, Y.; Shi, Y. Aptamer-conjugated gold nanoparticles targeting epidermal growth factor receptor variant III for the treatment of glioblastoma. Int J Nanomed 2020, 1363–1372. [Google Scholar] [CrossRef]
- Yoon, S.; Wu, X.; Armstrong, B.; Habib, N.; Rossi, J.J. An RNA aptamer targeting the receptor tyrosine kinase PDGFRα induces anti-tumor effects through STAT3 and p53 in glioblastoma. Mol Thera-Nucleic Acids 2019, 14, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Camorani, S.; Esposito, C.L.; Rienzo, A.; Catuogno, S.; Iaboni, M.; Condorelli, G.; De Franciscis, V.; Cerchia, L. Inhibition of receptor signaling and of glioblastoma-derived tumor growth by a novel PDGFRβ aptamer. Mol Thera 2014, 22, 828–841. [Google Scholar] [CrossRef] [PubMed]
- Xiao, T.; Xiao, Y.; Wang, W.; Tang, Y.Y.; Xiao, Z.; Su, M. Targeting EphA2 in cancer. J Hematol Oncol 2020, 13, 1–17. [Google Scholar] [CrossRef]
- Miao, H.; Gale, N.W.; Guo, H.; Qian, J.; Petty, A.; Kaspar, J.; Murphy, A.J.; Valenzuela, D.M.; Yancopoulos, G.; Hambardzumyan, D. EphA2 promotes infiltrative invasion of glioma stem cells in vivo through cross-talk with Akt and regulates stem cell properties. Oncogene 2015, 34, 558–567. [Google Scholar] [CrossRef]
- Binda, E.; Visioli, A.; Giani, F.; Lamorte, G.; Copetti, M.; Pitter, K.L.; Huse, J.T.; Cajola, L.; Zanetti, N.; DiMeco, F. The EphA2 receptor drives self-renewal and tumorigenicity in stem-like tumor-propagating cells from human glioblastomas. Cancer Cell 2012, 22, 765–780. [Google Scholar] [CrossRef]
- Affinito, A.; Quintavalle, C.; Esposito, C.L.; Roscigno, G.; Giordano, C.; Nuzzo, S.; Ricci-Vitiani, L.; Scognamiglio, I.; Minic, Z.; Pallini, R. Targeting ephrin receptor tyrosine kinase A2 with a selective aptamer for glioblastoma stem cells. Mol Thera-Nucleic Acids 2020, 20, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Amero, P.; Esposito, C.L.; Rienzo, A.; Moscato, F.; Catuogno, S.; De Franciscis, V. Identification of an interfering ligand aptamer for EphB2/3 receptors. Nucleic Acid Thera 2016, 26, 102–110. [Google Scholar] [CrossRef]
- Verhoeff, J.J.; Stalpers, L.J.; Claes, A.; Hovinga, K.E.; Musters, G.D.; Vandertop, W.P.; Richel, D.J.; Leenders, W.P.; Van Furth, W.R. Tumour control by whole brain irradiation of anti-VEGF-treated mice bearing intracerebral glioma. Eur J Cancer 2009, 45, 3074–3080. [Google Scholar] [CrossRef] [PubMed]
- Kioi, M.; Vogel, H.; Schultz, G.; Hoffman, R.M.; Harsh, G.R.; Brown, J.M. Inhibition of vasculogenesis, but not angiogenesis, prevents the recurrence of glioblastoma after irradiation in mice. J Clin Invest 2010, 120, 694–705. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-C.; Alomran, R.; Chernikova, S.B.; Lartey, F.; Stafford, J.; Jang, T.; Merchant, M.; Zboralski, D.; Zöllner, S.; Kruschinski, A.; et al. Blockade of SDF-1 after irradiation inhibits tumor recurrences of autochthonous brain tumors in rats. J Neuro-oncology 2013, 16, 21–28. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Glioblastoma Treatment With Irradiation and Olaptesed Pegol (NOX-A12) in MGMT Unmethylated Patients (GLORIA). Available online: https://clinicaltrials.gov/ct2/show/NCT04121455 (accessed on 25 October).
- Bayrac, A.T.; Sefah, K.; Parekh, P.; Bayrac, C.; Gulbakan, B.; Oktem, H.A.; Tan, W. In vitro selection of DNA aptamers to glioblastoma multiforme. ACS Chem Neuro 2011, 2, 175–181. [Google Scholar] [CrossRef]
- Bayraç, A.T.; Akça, O.E.; Eyidoğan, F.İ.; Öktem, H.A. Target-specific delivery of doxorubicin to human glioblastoma cell line via ssDNA aptamer. J Biosci 2018, 43, 97–104. [Google Scholar] [CrossRef]
- Luo, Z.; Yan, Z.; Jin, K.; Pang, Q.; Jiang, T.; Lu, H.; Liu, X.; Pang, Z.; Yu, L.; Jiang, X. Precise glioblastoma targeting by AS1411 aptamer-functionalized poly (l-γ-glutamylglutamine)–paclitaxel nanoconjugates. J Colloid Interface Sci 2017, 490, 783–796. [Google Scholar] [CrossRef]
- Fu, W.; You, C.; Ma, L.; Li, H.; Ju, Y.; Guo, X.; Shi, S.; Zhang, T.; Zhou, R.; Lin, Y. Enhanced efficacy of temozolomide loaded by a tetrahedral framework DNA nanoparticle in the therapy for glioblastoma. ACS Appl Materials Interfaces 2019, 11, 39525–39533. [Google Scholar] [CrossRef]
- Shi, S.; Fu, W.; Lin, S.; Tian, T.; Li, S.; Shao, X.; Zhang, Y.; Zhang, T.; Tang, Z.; Zhou, Y. Targeted and effective glioblastoma therapy via aptamer-modified tetrahedral framework nucleic acid-paclitaxel nanoconjugates that can pass the blood brain barrier. Nanomed: Nanotech, Bio Med 2019, 21, 102061. [Google Scholar] [CrossRef]
- Esposito, C.L.; Nuzzo, S.; Ibba, M.L.; Ricci-Vitiani, L.; Pallini, R.; Condorelli, G.; Catuogno, S.; de Franciscis, V. Combined targeting of glioblastoma stem-like cells by neutralizing RNA-bio-drugs for STAT3. Cancers 2020, 12, 1434. [Google Scholar] [CrossRef] [PubMed]
- Esposito, C.L.; Nuzzo, S.; Catuogno, S.; Romano, S.; de Nigris, F.; de Franciscis, V. STAT3 gene silencing by aptamer-siRNA chimera as selective therapeutic for glioblastoma. Mol Thera-Nucleic Acids 2018, 10, 398–411. [Google Scholar] [CrossRef]
- Anton, J.; Sudibio, S.; Handoko, H.; Permata, T.B.M.; Kodrat, H.; Nuryadi, E.; Sofyan, H.; Susanto, E.; Mulyadi, R.; Aman, R.A. Overexpression of c-Met is Associated with Poor Prognosis in Glioblastoma Multiforme: A Systematic Review and Meta-Analyses. Asian Pacific J Cancer Prev: APJCP 2021, 22, 3075. [Google Scholar] [CrossRef]
- Esposito, C.L.; Nuzzo, S.; Kumar, S.A.; Rienzo, A.; Lawrence, C.L.; Pallini, R.; Shaw, L.; Alder, J.E.; Ricci-Vitiani, L.; Catuogno, S. A combined microRNA-based targeted therapeutic approach to eradicate glioblastoma stem-like cells. J Controlled Release 2016, 238, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, J.; Denoyer, D.; Henri, J.; Jamieson, A.; Burvenich, I.J.G.; Pouliot, N.; Shigdar, S. Bifunctional Aptamer-Doxorubicin Conjugate Crosses the Blood-Brain Barrier and Selectively Delivers Its Payload to EpCAM-Positive Tumor Cells. Nucleic Acid Ther 2020, 30, 117–128. [Google Scholar] [CrossRef] [PubMed]
| Phase | Therapeutic Target Interventions | Identifier | Outcome | Ref |
|---|---|---|---|---|
| Checkpoint Inhibitors | ||||
| I | 3mg/kg ipilimumab (Arm A) vs 3mg/kg nivolumab (Arm B) vs 1mg/kg ipilimumab + 3mg/kg nivolumab (Arm C) vs expansion cohort for adult GBM. | NCT02311920 | Overall treatment well tolerated – 16% reported grade 4 events: no grade 5. No dose-limiting toxicity in arm C. At median 7.1-month follow-up, 32% experienced progression, 26% (8) died – 7 from progression, 1 from pulmonary embolism. | [4] |
| I | 200mg pembrolizumab via IV every 3 weeks + 10mg/kg bevacizumab via IV fortnightly (Arm A) vs 200mg pembrolizumab via IV every 3 weeks for adults with primary or secondary GBM. | NCT02337491 | MOS: 8.8 months (Arm A); 10.3 months (Arm B). PFS (6-months): 26% (Arm A); 6.7% (Arm B). Objective response rates: 20% (Arm A); 0% (Arm B). Pembrolizumab is ineffective as monotherapy and concurrently with bevacizumab. | [5] |
| II | 3mg nivolumab pre-surgery and every 2 weeks post-surgery until toxicity or disease progression (Paediatrics and adults). | NCT02550249 | PFS: 4.1 months and MOS: 7.3 months. Safe, well-tolerated; minimal adverse events. PD-1 blockade alone is not sufficient to prevent disease relapse. | [6] |
| II | 3mk/kg nivolumab (Arm A) vs 10mg/kg bevacizumab following standard RT and TMZ for adult rGBM. | NCT02017717 | MOS: comparable between nivolumab and bevacizumab treatments (9.8 vs 10.0 months). 12-month OS: 42% (both arms). Grade 3/4 events similar between nivolumab (18.1%) and bevacizumab (15.2%). No end point reached | [7] |
| II | 240mg IV nivolumab with either 10mg/kg bevacizumab (Arm A) vs 3mg/kg low-dose bevacizumab (Arm B) for adult rGBM. | NCT03452579 | MOS: significantly greater for arm A in patients >60 years (10.6 vs 5.9 months). No difference between treatment arms in patients <60 years old (8.0 vs 12.4 months). | [8] |
| III | Standard RT + 240mg every two weeks (8 cycles), 480mg every 4 weeks of nivolumab (Arm A) vs standard RT + 75mg/m2 during RT of TMZ and 150-200mg/m2/day for on day 5 of 28-day cycle (Arm B) for adult GBM | NCT02617589 | MOS: 13.4 months (arm A); 14.9 months (arm B). Median progression-free: 6 months (arm A); 6.2 months (arm B). Response rates: 7.8% (arm A); 7.2% (arm B). Grade 3/4 treatment-related events: 21.9% (arm A); 25.1% (arm B). | [9] |
| III | 240mg nivolumab fortnightly x8 then 480mg monthly + standard RT over 6 weeks + 75mg/m2 daily during RT and 150-200mg/m2/day on days 1-5 of 28-day cycle x6 (Arm A) vs placebo + RT + same dosage TMZ (Arm B) for MGMT or indeterminant MGMT positive adult GBM | NCT02667587 | MOS: 28.9 months (arm A); 32.1% (arm B). PFS: 10.6 months (arm A); 10.3 months (arm B). Grade 3/4 treatment-related events: 52.4% (arm A); 33.6% (arm B). Nivolumab did not improve patient survival | [10] |
| Monoclonal Antibodies | ||||
| II | 15mg/kg onartuzumab + bevacizumab every 3 weeks (Arm A) vs placebo + bevacizumab (Arm B). | NCT01632228 | PFS: 3.9 months (Arm A) vs 2.9 months (Arm B). MOS: 8.8 months (Arm A) vs 12.6 months (Arm B). No clinical benefit; 38.5% (Arm A) and 35.9% patients (Arm B) experienced grade 3 and above adverse events. | [11] |
| II | 8mg/kg days 1, 8, 15/q28 tanibirumab (Arm A) vs 12mg/kg days 1,8,15/q28 tanibirumab vs 12mg/kg weekly tanibirumab (Arm C). | NCT03033524 | No dose-limiting toxicities or grade 3/4 adverse events reported; half patients had secondary recurrence. One quarter of patients had stable disease. | [12] |
| II | Standard RT + 10mg/kg durvalumab every 2 weeks in unmethylated GBM patients | NCT02336165 | MOS: 15.1 months; 24 of 40 patients alive 12-months post treatment; durvalumab well tolerated in combination, effective; treatment-related adverse events – 14 (35%) patients experienced ≥ grade 3 events. | [13] |
| CAR-T cell therapy | ||||
| I | IV GD2-specific 4SCAR-T cells vs IV and IC GD2-specific 4SCAR-T cells. | NCT03170141 | Safe and well tolerated; half patients (4) showed partial response (3-24 months), 3 patients – progressive disease 6-23 months, 1 with stable disease 4 months post-infusion. MOS: 10 months (entire cohort- 8). | [14] |
| I/II | Nonmyeloablative preparative chemotherapy – 2x days 60mg/kg cyclophosphamide, 5x days 25mg/m2 fludarabine, following day 6.3x106 – 2.6x1010 anti-EGFRvIII-CAR T cell infusion + 72,000 IU/kg IL-2 IV administered every 8 hours to tolerance. | NCT01454596 | PFS: 13 months (IQR: 1.1-1.9); MOS: 6.9 months (IQR: 2.8-10). No clinically meaningful response in GBM patients. At higher dosage, one mortality, two patients experienced severe hypoxia | [15] |
| Vaccines | ||||
| I | APVAC1 + GM-CSF + poly-ICLC in 1st cycle TMZ (Arm A). APVAC2 in 4th cycle TMZ | NCT02149225 | PFS: 14.2 months; MOS: 29 months. Adverse events mostly from injection site – 2 patients anaphylaxis, one with grade 4 cerebral oedema. | [16] |
| I | Post standard of care: poly-ICLC vaccine (up to 14x – fortnightly for 2 months, monthly thereafter) with TTF (Arm A) or without (Arm B). | NCT03223103 | After follow-up: 9 patients alive 25 months post-vaccine, 8 patients disease-free. Minimal adverse events from vaccine | [17] |
| I | Newly diagnosed GBM Patients (Arm A): Weekly DC vaccine for 4 weeks, maintained every 8 weeks + RT + concurrent/adjuvant TMZ. Recurrent GBM (Arm B): DC vaccine + bevacizumab. | NCT02010606 | Arm A PFS: 8.75 months; MOS: 20.36 months. Arm B GBM PFS: 3.23 months, 6-months PFR: 24%, MOS: 11.97 months. No serious adverse events related to vaccine. | [18] |
| I/II | Chemoradiotherapy + IMA950 vaccine intradermally + poly-ICLC intramuscularly | NCT01920191 | Safe and immunogenic. Greater immune response (63.2% vs 36.8%) with single peptide vs multiple peptides. MOS: 19 months. 4 patients grade 4 oedemas, one possibly vaccine related; 22% patients (4) experienced pseudoprogression | [19] |
| III | 500µg Rindopepimut EGFRvIII vaccine with either 150µg GM-CSF (Arm A) vs 100µg keyhole limpet haemocyanin (Arm B) concurrently with standard TMZ | NCT01480479 | MOS: 20.1 months (Arm A) vs 20.0 months (Arm B). Serious adverse events in both groups eg: seizures, brain oedema. Failed to improve survival. | [20] |
| III | DCVAX-L + TMZ (Arm A) vs placebo + TMZ post-surgery and chemotherapy for adult GBM – cross-over trial design. | NCT00045968 | Intent-to-treat population = 331; MOS: 23.1 months. 90% received DCVAX-L. MGMT patients: MOS = 34.7 months, 3-year survival = 46.4%. Of cohort, 223 survived ≥ 30 months – 44 of these lived ≥ 36 months (MOS: 88.2 months). Grade 3/4 events: 2.1% of 331 patients. | [21] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





