Submitted:
10 August 2023
Posted:
11 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
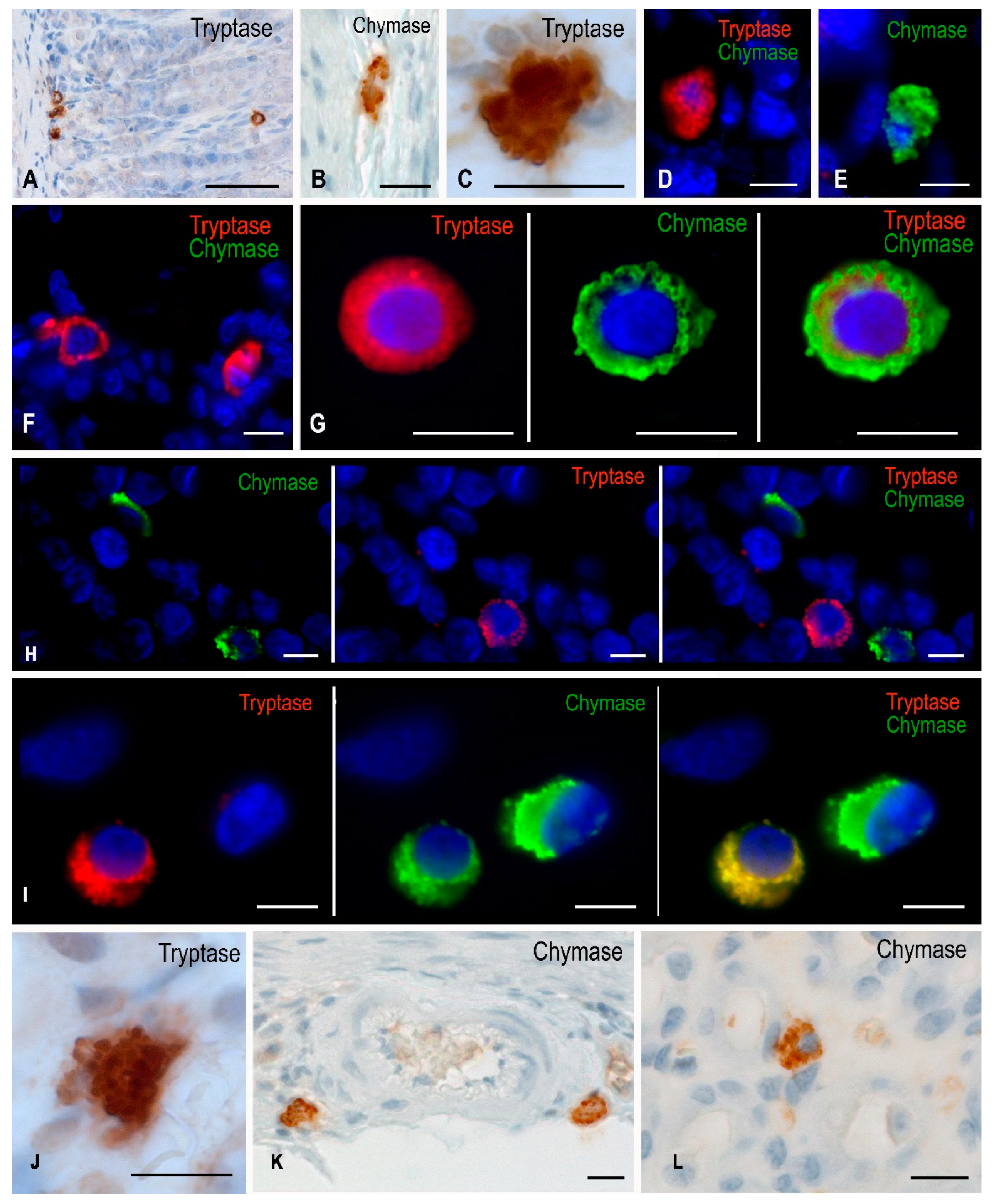
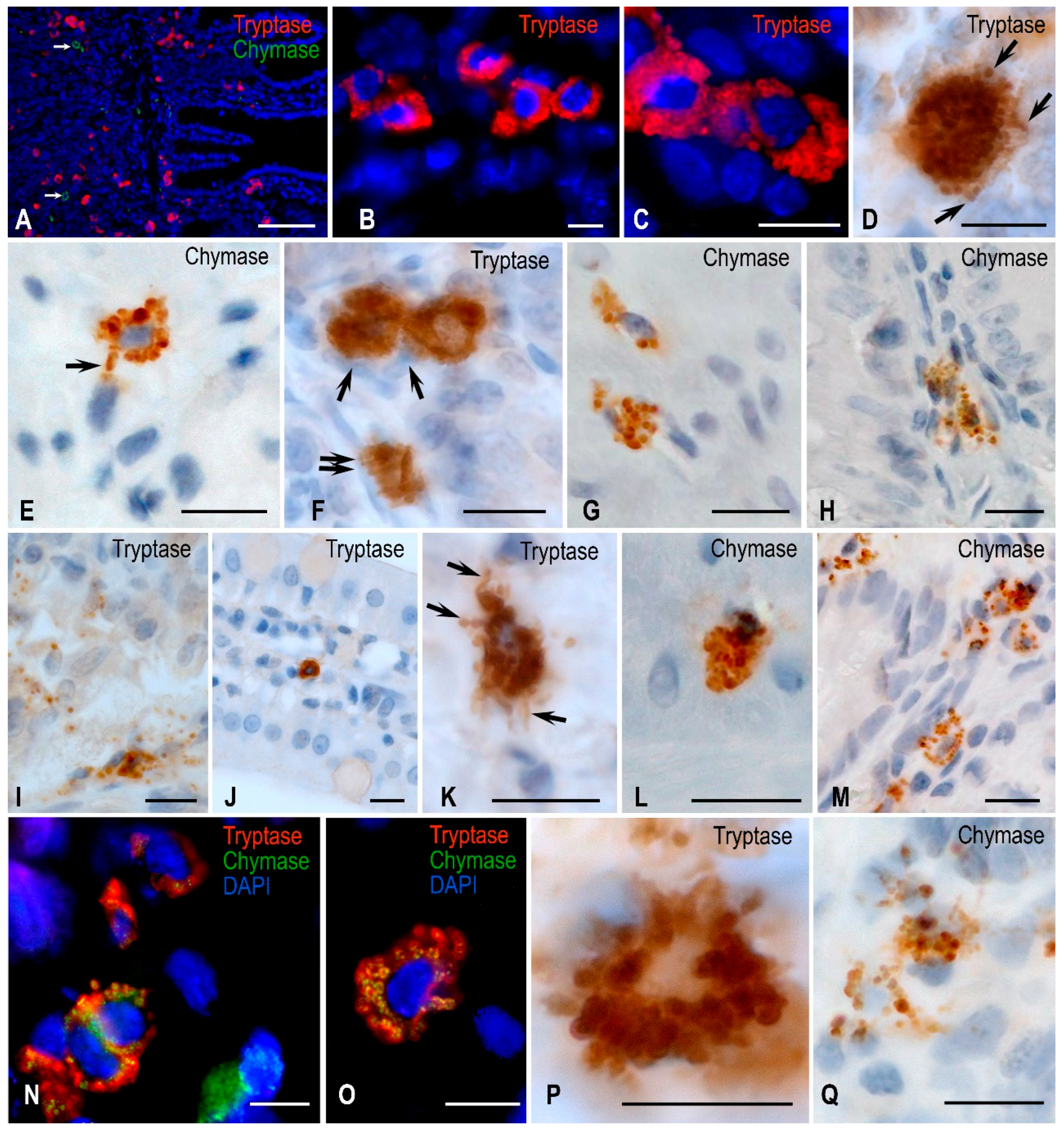
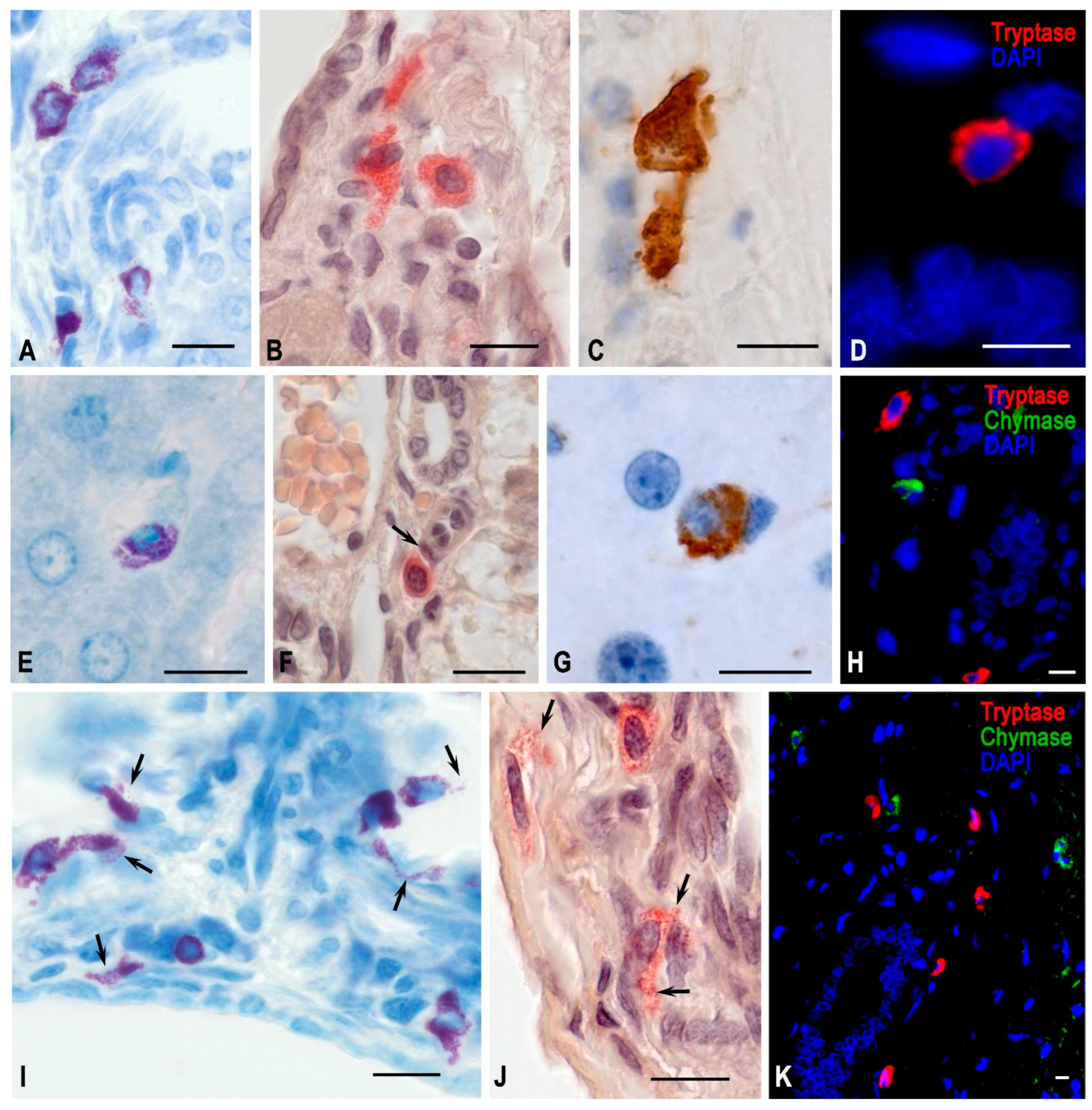
3. Discussion
4. Materials and methods
4.1. Experimental design
4.2. Histoprocessing
4.3. Tissue Probe Staining
4.4. Image acquisition
4.5. Statistical analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ISS | International Space Station |
| MC | Mast cell |
| LEO | Low Earth Orbit |
| ECM | Extracellular matrix |
| PAR-2 | Protease-activated receptor-2 |
| VEGF | Vascular endothelial growth factor |
| VIP | Vasoactive intestinal peptide |
| SP | Substance P |
| ICAM-1 | Intercellular adhesion molecule 1 |
| MMPs | Metalloproteinases |
References
- Loued-Khenissi, L.; Pfeiffer, C.; Saxena, R.; Adarsh, S.; Scaramuzza, D. Microgravity induces overconfidence in perceptual decision-making. Sci Rep 2023, 13, 9727. [Google Scholar] [CrossRef] [PubMed]
- Kandarpa, K.; Schneider, V.; Ganapathy, K. Human health during space travel: An overview. Neurol India 2019, 67, S176–S181. [Google Scholar] [CrossRef]
- Chancellor, J.C.; Scott, G.B.; Sutton, J.P. Space Radiation: The Number One Risk to Astronaut Health beyond Low Earth Orbit. Life 2014, 4, 491–510. [Google Scholar] [CrossRef] [PubMed]
- Sonnenfeld, G. Space flight, microgravity, stress, and immune responses. Adv Space Res 1999, 23, 1945–1953. [Google Scholar] [CrossRef]
- Bigley, A.B.; Agha, N.H.; Baker, F.L.; Spielmann, G.; Kunz, H.E.; Mylabathula, P.L.; Rooney, B.V.; Laughlin, M.S.; Mehta, S.K.; Pierson, D.L.; et al. NK cell function is impaired during long-duration spaceflight. J Appl Physiol (1985) 2019, 126, 842–853. [Google Scholar] [CrossRef] [PubMed]
- Elieh Ali Komi, D.; Wohrl, S.; Bielory, L. Mast Cell Biology at Molecular Level: A Comprehensive Review. Clin Rev Allergy Immunol 2020, 58, 342–365. [Google Scholar] [CrossRef] [PubMed]
- Elieh Ali Komi, D.; Bjermer, L. Mast Cell-Mediated Orchestration of the Immune Responses in Human Allergic Asthma: Current Insights. Clin Rev Allergy Immunol 2019, 56, 234–247. [Google Scholar] [CrossRef]
- Elieh Ali Komi, D.; Grauwet, K. Role of Mast Cells in Regulation of T Cell Responses in Experimental and Clinical Settings. Clin Rev Allergy Immunol 2018, 54, 432–445. [Google Scholar] [CrossRef]
- Elieh Ali Komi, D.; Shafaghat, F.; Kovanen, P.T.; Meri, S. Mast cells and complement system: Ancient interactions between components of innate immunity. Allergy 2020, 75, 2818–2828. [Google Scholar] [CrossRef]
- Komi, D.E.A.; Mortaz, E.; Amani, S.; Tiotiu, A.; Folkerts, G.; Adcock, I.M. The Role of Mast Cells in IgE-Independent Lung Diseases. Clin Rev Allergy Immunol 2020, 58, 377–387. [Google Scholar] [CrossRef]
- Elieh Ali Komi, D.; Ribatti, D. Mast cell-mediated mechanistic pathways in organ transplantation. Eur J Pharmacol 2019, 857, 172458. [Google Scholar] [CrossRef]
- Hernandez-Hernandez, L.; Sanz, C.; Garcia-Solaesa, V.; Padron, J.; Garcia-Sanchez, A.; Davila, I.; Isidoro-Garcia, M.; Lorente, F. Tryptase: Genetic and functional considerations. Allergol Immunopathol (Madr) 2012, 40, 385–389. [Google Scholar] [CrossRef]
- Caughey, G.H. Mast cell proteases as pharmacological targets. Eur J Pharmacol 2016, 778, 44–55. [Google Scholar] [CrossRef]
- Atiakshin, D.; Buchwalow, I.; Samoilova, V.; Tiemann, M. Tryptase as a polyfunctional component of mast cells. Histochem Cell Biol 2018, 149, 461–477. [Google Scholar] [CrossRef] [PubMed]
- Atiakshin, D.; Buchwalow, I.; Tiemann, M. Mast cell chymase: Morphofunctional characteristics. Histochem Cell Biol 2019, 152, 253–269. [Google Scholar] [CrossRef] [PubMed]
- Atiakshin, D.; Kostin, A.; Trotsenko, I.; Shishkina, V.; Tiemann, M.; Buchwalow, I. Carboxypeptidase A3 in the structure of the protease phenotype of mast cells: Cytophysiological aspects. RUDN Journal of Medicine 2022, 26, 9–33. [Google Scholar] [CrossRef]
- Atiakshin, D.; Kostin, A.; Trotsenko, I.; Samoilova, V.; Buchwalow, I.; Tiemann, M. Carboxypeptidase A3-A Key Component of the Protease Phenotype of Mast Cells. Cells 2022, 11, 570. [Google Scholar] [CrossRef]
- Komi, D.E.A.; Redegeld, F.A. Role of Mast Cells in Shaping the Tumor Microenvironment. Clin Rev Allergy Immunol 2020, 58, 313–325. [Google Scholar] [CrossRef]
- Hellman, L.; Akula, S.; Fu, Z.; Wernersson, S. Mast Cell and Basophil Granule Proteases—In Vivo Targets and Function. Front Immunol 2022, 13, 918305. [Google Scholar] [CrossRef]
- Trivedi, N.N.; Caughey, G.H. Mast cell peptidases: Chameleons of innate immunity and host defense. Am J Respir Cell Mol Biol 2010, 42, 257–267. [Google Scholar] [CrossRef]
- Elieh Ali Komi, D.; Shafaghat, F.; Christian, M. Crosstalk Between Mast Cells and Adipocytes in Physiologic and Pathologic Conditions. Clin Rev Allergy Immunol 2020, 58, 388–400. [Google Scholar] [CrossRef]
- Ronnberg, E.; Melo, F.R.; Pejler, G. Mast cell proteoglycans. J Histochem Cytochem 2012, 60, 950–962. [Google Scholar] [CrossRef] [PubMed]
- Elieh Ali Komi, D.; Jalili, A. The emerging role of mast cells in skin cancers: Involved cellular and molecular mechanisms. Int J Dermatol 2022, 61, 792–803. [Google Scholar] [CrossRef]
- Wintroub, B.U.; Kaempfer, C.E.; Schechter, N.M.; Proud, D. A human lung mast cell chymotrypsin-like enzyme. Identification and partial characterization. J Clin Invest 1986, 77, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Reilly, C.F.; Schechter, N.B.; Travis, J. Inactivation of bradykinin and kallidin by cathepsin G and mast cell chymase. Biochem Biophys Res Commun 1985, 127, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Franconi, G.M.; Graf, P.D.; Lazarus, S.C.; Nadel, J.A.; Caughey, G.H. Mast cell tryptase and chymase reverse airway smooth muscle relaxation induced by vasoactive intestinal peptide in the ferret. J Pharmacol Exp Ther 1989, 248, 947–951. [Google Scholar]
- Mizutani, H.; Schechter, N.; Lazarus, G.; Black, R.A.; Kupper, T.S. Rapid and specific conversion of precursor interleukin 1 beta (IL-1 beta) to an active IL-1 species by human mast cell chymase. J Exp Med 1991, 174, 821–825. [Google Scholar] [CrossRef]
- Raymond, W.W.; Ruggles, S.W.; Craik, C.S.; Caughey, G.H. Albumin is a substrate of human chymase. Prediction by combinatorial peptide screening and development of a selective inhibitor based on the albumin cleavage site. J Biol Chem 2003, 278, 34517–34524. [Google Scholar] [CrossRef]
- Raymond, W.W.; Cruz, A.C.; Caughey, G.H. Mast cell and neutrophil peptidases attack an inactivation segment in hepatocyte growth factor to generate NK4-like antagonists. J Biol Chem 2006, 281, 1489–1494. [Google Scholar] [CrossRef]
- Zhao, W.; Oskeritzian, C.A.; Pozez, A.L.; Schwartz, L.B. Cytokine production by skin-derived mast cells: Endogenous proteases are responsible for degradation of cytokines. J Immunol 2005, 175, 2635–2642. [Google Scholar] [CrossRef]
- Lees, M.; Taylor, D.J.; Woolley, D.E. Mast cell proteinases activate precursor forms of collagenase and stromelysin, but not of gelatinases A and B. Eur J Biochem 1994, 223, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Saarinen, J.; Kalkkinen, N.; Welgus, H.G.; Kovanen, P.T. Activation of human interstitial procollagenase through direct cleavage of the Leu83-Thr84 bond by mast cell chymase. J Biol Chem 1994, 269, 18134–18140. [Google Scholar] [CrossRef] [PubMed]
- Mulloy, B.; Lever, R.; Page, C.P. Mast cell glycosaminoglycans. Glycoconj J 2017, 34, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Morais, C.; Rajandram, R.; Blakeney, J.S.; Iyer, A.; Suen, J.Y.; Johnson, D.W.; Gobe, G.C.; Fairlie, D.P.; Vesey, D.A. Expression of protease activated receptor-2 is reduced in renal cell carcinoma biopsies and cell lines. PLoS ONE 2021, 16, e0248983. [Google Scholar] [CrossRef]
- Elieh Ali Komi, D.; Shafaghat, F.; Haidl, G. Significance of mast cells in spermatogenesis, implantation, pregnancy, and abortion: Cross talk and molecular mechanisms. Am J Reprod Immunol 2020, 83, e13228. [Google Scholar] [CrossRef]
- Ammendola, M.; Sacco, R.; Sammarco, G.; Luposella, M.; Patruno, R.; Gadaleta, C.D.; Sarro, G.D.; Ranieri, G. Mast Cell-Targeted Strategies in Cancer Therapy. Transfus Med Hemother 2016, 43, 109–113. [Google Scholar] [CrossRef]
- Singh, J.; Shah, R.; Singh, D. Targeting mast cells: Uncovering prolific therapeutic role in myriad diseases. Int Immunopharmacol 2016, 40, 362–384. [Google Scholar] [CrossRef]
- Vitte, J. Human mast cell tryptase in biology and medicine. Mol Immunol 2015, 63, 18–24. [Google Scholar] [CrossRef]
- Caughey, G.H. Mast cell tryptases and chymases in inflammation and host defense. Immunol Rev 2007, 217, 141–154. [Google Scholar] [CrossRef]
- Yu, M.; Tsai, M.; Tam, S.Y.; Jones, C.; Zehnder, J.; Galli, S.J. Mast cells can promote the development of multiple features of chronic asthma in mice. J Clin Invest 2006, 116, 1633–1641. [Google Scholar] [CrossRef]
- Krystel-Whittemore, M.; Dileepan, K.N.; Wood, J.G. Mast Cell: A Multi-Functional Master Cell. Front Immunol 2015, 6, 620. [Google Scholar] [CrossRef] [PubMed]
- Collier, I.E.; Legant, W.; Marmer, B.; Lubman, O.; Saffarian, S.; Wakatsuki, T.; Elson, E.; Goldberg, G.I. Diffusion of MMPs on the surface of collagen fibrils: The mobile cell surface-collagen substratum interface. PLoS ONE 2011, 6, e24029. [Google Scholar] [CrossRef]
- Elahirad, S.; Elieh Ali Komi, D.; Kiani, A.; Mohammadi-Noori, E.; Vaisi-Raygani, A.; Mozafari, H.; Bahrehmand, F.; Saidi, M.; Toupchi-Khosroshahi, V.; Salehi, N. Association of Matrix Metalloproteinase-2 (MMP-2) and MMP-9 Promoter Polymorphisms, Their Serum Levels, and Activities with Coronary Artery Calcification (CAC) in an Iranian Population. Cardiovasc Toxicol 2022, 22, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Zhou, G.; Hu, W. Carcinogenesis induced by space radiation: A systematic review. Neoplasia 2022, 32, 100828. [Google Scholar] [CrossRef]
- Barr, Y.R.; Bacal, K.; Jones, J.A.; Hamilton, D.R. Breast cancer and spaceflight: Risk and management. Aviat Space Environ Med 2007, 78, A26–A37. [Google Scholar] [PubMed]
- Komi, D.E.A.; Khomtchouk, K.; Santa Maria, P.L. A Review of the Contribution of Mast Cells in Wound Healing: Involved Molecular and Cellular Mechanisms. Clin Rev Allergy Immunol 2020, 58, 298–312. [Google Scholar] [CrossRef] [PubMed]
- Afonin, B.V. The hemodynamic mechanism determining the emergence of a hypersecretory state of the stomach in microgravity conditions. Aviakosmicheskaya i ekologicheskaya meditsina 2013, 47, 10–11. [Google Scholar]
- Molinari, J.F.; Scuri, M.; Moore, W.R.; Clark, J.; Tanaka, R.; Abraham, W.M. Inhaled tryptase causes bronchoconstriction in sheep via histamine release. Am J Respir Crit Care Med 1996, 154, 649–653. [Google Scholar] [CrossRef]
- Thomas, V.A.; Wheeless, C.J.; Stack, M.S.; Johnson, D.A. Human mast cell tryptase fibrinogenolysis: Kinetics, anticoagulation mechanism, and cell adhesion disruption. Biochemistry 1998, 37, 2291–2298. [Google Scholar] [CrossRef]
- Atiakshin, D.A.; Bykov, E.G.; Il’in, E.A.; Pashkov, A.N. [Tissue-specific reaction of the mucous coat of herbals’ small gut under the influence of spaceflight factors on board biosat “Foton M3”]. Aviakosm Ekolog Med 2011, 45, 25–30. [Google Scholar]
- Morey-Holton, E.; Globus, R.K.; Kaplansky, A.; Durnova, G. The hindlimb unloading rat model: Literature overview, technique update and comparison with space flight data. Adv Space Biol Med 2005, 10, 7–40. [Google Scholar] [CrossRef]
- Buchwalow, I.B.; Böcker, W. Immunohistochemistry: Basics and methods; Springer Science & Business Media, 2010. [Google Scholar]
- Naji-Haddadi, S.; Elieh-Ali-Komi, D.; Aghayan, S.; Asghari, R.; Rasouli, J. Investigation of p16 protein expression and its association with histopathologic parameters in breast cancer. Mol Biol Res Commun 2021, 10, 165–170. [Google Scholar] [CrossRef]
- Omelyanenko, N.P.; Slutsky, L.I. Connective tissue (histophysiology and biochemistry); RAS and RAMS ed.; Mironov, S.P., Ed.; Izvestia Publishing House: Moscow, 2009; Volume 1. [Google Scholar]
| Groups of animals | MC subpopulation | MC identification technique | |||||
|---|---|---|---|---|---|---|---|
| Tryptase MCs | Chymase MCs | ||||||
| Jejunum | Stomach | Liver | Jejunum | Stomach | Liver | ||
| Experiment “Rodentsiya” | |||||||
| VC | MSMC | 39.6 ± 2.2 | 4.4 ± 0.3 | 3.2 ± 0.4 | 2.6 ± 0.4 | Single | Single |
| CSMC | 2.5 ± 0.2 | 1.5 ± 0.2 | Single | 1.1 ± 0.2 | |||
| SE | MSMC | 42.4 ± 2.8 | 4.3 ± 0.3 | 2.9 ± 0.2 | 2.8 ± 0.2 | Single | Single |
| CSMC | 2.8 ± 0.3 | 1.4 ± 0.1 | 0.4 ± 0.0* | 1.3 ± 0.1 | |||
| SF | MSMC | 28.6 ± 1.1 | 2.6 ± 0.2 | 2.2 ± 0.2 | 7.8 ± 0.8 | 1.2 ± 0.1 | 1.8 ± 0.2 |
| CSMC | 2.1 ± 0.2 | 1.4 ± 0.2 | 2.2 ± 0.1 | 1.5 ± 0.1 | |||
| Simulated physiological effects of 12-day weightlessness | |||||||
| VC | MSMC | 36.8 ± 2.7 | 4.1 ± 0.7 | 2.9 ± 0.3 | 2.9 ± 0.2 | Single | Single |
| CSMC | 2.4 ± 0.3 | 1.4 ± 0.1 | Single | 1.2 ± 0.1 | |||
| AnOrtSusp | MSMC | 33.4 ± 2.5 | 4.8 ± 0.4 | 3.8 ± 0.2* | 3.6 ± 0.1* | 0.3 ± 0.1* | 0.8 ± 0.2* |
| CSMC | 2.9 ± 0.3 | 1.7 ± 0.2 | 0.5 ± 0.1* | 1.4 ± 0.1 | |||
| Groups of animals | Organs | MC mucosal subpopulation | MC connective tissue subpopulation | ||||
|---|---|---|---|---|---|---|---|
| MCTRY | MCCHYM | MCTRY+CHYM | MCTRY | MCCHYM | MCTRY+CHYM | ||
| Experiment “Rodentsiya” | |||||||
| VC | Jejunum | 89.8 ± 3.1 | 6.1 ± 0.2 | 4.1 ± 0.2 | 94.3 ± 3.3 | 3.8 ± 0.2 | 1.9 ± 0.1 |
| Stomach | 91.1 ± 2.3 | 3.2 ± 0.2 | 5.7 ± 0.4 | 45.4 ± 2.8 | 27.3 ± 3.4 | 27.3 ± 3.4 | |
| Liver1 | - | - | - | 97.2 ± 2.4 | 0.9 ± 0.2 | 1.9 ± 0.1 | |
| SE | Jejunum | 90.6 ± 4.4 | 6.0 ± 0.4 | 3.4 ± 0.3 | 84.8 ± 4.2* | 12.1 ± 1.1* | 3.1 ± 0.2* |
| Stomach | 89.4 ± 4.2 | 3.8 ± 0.3 | 6.8 ± 0.3 | 43.4 ± 4.2 | 26.3 ± 2.3 | 30.3 ± 2.5 | |
| Liver1 | - | - | - | 97.1 ± 3.3 | 0.7 ± 0.1 | 2.2 ± 0.3 | |
| SF | Jejunum | 65.7 ± 4.5*.** | 17.9 ± 1.1*.** | 16.4 ± 1.2*.** | 33.3 ± 2.8*.** | 34.9 ± 3.4*.** | 31.8 ± 2.5*.** |
| Stomach | 25.4 ± 0.2*.** | 28.4 ± 1.9*.** | 46.2 ± 3.3*.** | 24.2 ± 2.2*.** | 36.7 ± 3.6*.** | 39.1 ± 3.9*.** | |
| Liver1 | - | - | - | 21.3 ± 2.8*.** | 22.4 ± 1.2*.** | 56.3 ± 1.8*.** | |
| Simulated physiological effects of 12-day weightlessness | |||||||
| VC | Jejunum | 90.1 ± 3.1 | 5.4 ± 0.3 | 4.5 ± 0.5 | 93.4 ± 3.2 | 4.2 ± 0.3 | 2.4 ± 0.2 |
| Stomach | 87.6 ± 4.2 | 6.2 ± 0.5 | 6.2 ± 0.4 | 43.2 ± 3.1 | 25.7 ± 2.7 | 31.1 ± 1.4 | |
| Liver1 | - | - | - | 96.5 ± 2.9 | 1.5 ± 0.1 | 2.0 ± 0.3 | |
| AnOrtSusp | Jejunum | 79.5 ± 3.2* | 8.3 ± 0.4* | 12.2 ± 0.9* | 81.4 ± 4.5* | 9.2 ± 0.6* | 9.4 ± 0.5* |
| Stomach | 65.0 ± 3.5* | 12.6 ± 1.1* | 22.4 ± 2.0* | 26.2 ± 1.9* | 35.6 ± 3.1* | 38.2 ± 2.2* | |
| Liver1 | - | - | - | 76.3 ± 3.8* | 5.4 ± 0.6* | 18.3 ± 1.1* | |
| Antibodies | Host | Catalogue Nr. | Dilution | Source |
|---|---|---|---|---|
| Tryptase | Mouse monoclonal Ab | #ab2378 | 1:2000 | AbCam, United Kingdom |
| Mast cell Chymase/CHYMASE Antibody, Biotin Conjugated | Rabbit Polyclonal Ab | #bs-2353R-Biotin | 1:500 | Bioss |
| Antibodies and Other Reagents | Source | Dilution | Label |
|---|---|---|---|
| Goat anti-mouse IgG (#115-007-003) | Jackson ImmunoResearh | 1:13 | w/o |
| Cy3-conjugated AffinPure Goat Anti Mouse IgG (#115-165-166) | Jackson ImmunoResearh | 10 мг/1 мл PBS | Cy3 |
| Streptavidin (# S11223) | Invitrogen™ | 2 mg/mL solution | Alexa Fluor 488 |
| AmpliStain™ anti-Mouse 1-Step HRP (#AS-M1-HRP) | SDT GmbH, Baesweiler, Germany | ready-to-use | HRP |
| AmpliStain™ anti-Rabbit 1-Step HRP (#AS-R1-HRP) | SDT GmbH, Baesweiler, Germany | ready-to-use | HRP |
| 4′,6-diamidino-2-phenylindole (DAPI, #D9542-5MG) | Sigma, Hamburg, Germany | 5 µg/mL | w/o |
| VECTASHIELD®® Mounting Medium (#H-1000) | Vector Laboratories, Burlingame, CA, USA | ready-to-use | w/o |
| DAB Peroxidase Substrat Kit (#SK-4100) | Vector Laboratories, Burlingame, CA, USA | ready-to-use | DAB |
| Mayer’s hematoxylin (#MHS128) | Sigma-Aldrich | ready-to-use | w/o |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





