1. Introduction
In recent years, solar panels have played a significant role in reducing global warming by generating clean and emission-free electricity from the sun, thus reducing reliance on greenhouse-gas-producing fossil fuels [
1]. Solar energy is considered the fuel of the future due to its potential for meeting increasing electricity demand and reducing greenhouse gas emissions
. As a result, the mass production of solar panels using different technologies has increased in recent decades, and the production of new generations of solar panels is expected to continue [
1,
2]. However, despite the positive impact of solar panels, their production also has negative consequences, including the generation of large amounts of waste. The average lifetime of a PV panel is 25 years, and given their worldwide production, it is anticipated that there will be a significant amount of waste generated annually. According to the International Renewable Energy Agency (IRENA) report, apart from considering the generation of a large amount of waste during the manufacturing process of solar cells [
3,
4], an estimated 80 million tons of PV waste will be generated by 2050 [
5].
The issue of solar panel waste is significant from two perspectives. Firstly, these wastes contain lead, cadmium, and other harmful chemicals that can cause significant health and environmental hazards [
6,
7]. Secondly, these wastes are considered valuable due to their high content of valuable metals [
7,
8,
9,
10]. Therefore, the life cycle assessment (LCA) should be applied to evaluate all aspects of the environmental impacts, energy consumption and production, and emissions during the entire life cycle of solar panel technology. LCA is a feasible method that can be used as an environmental management tool to consider the positive ecological effects of solar panels due to the decrease in carbon emissions and energy consumption. This should also evaluate the potential cradle-to-grave life cycle impacts of solar panels after their service life, as it is uncertain what will happen to this massive amount of solar panel waste.
Moreover, in addition to the issue of waste management, the recovery of metals from these wastes and their reuse should also be considered. Although few LCA studies have explored the recycling of PV technologies, some have investigated the production and use of PV technologies [
11,
12], and energy consumption due to PV recycling [
13]. The main factors affecting end-of-life panels' waste management are self-take-back collection, recycling facilities, and material recovery [
14].
The most commonly used PV panels are crystalline silicon and thin-film PV cells. The former accounts for around 80% of the market share, but this is decreasing due to the increased capacity and the shortage of production costs [
15]. Conversely, the lower production costs and optimum efficiency of thin-film panels are driving their growth in the overall photovoltaic market [
15,
16,
17]. However, the handling and waste management of these extensive ranges of used solar panels that contain Cd, Se, Pb, and other environmentally hazardous metals pose an environmental concern. Additionally, there is another concern about the fate of these sources of valuable metals. These two perspectives will force industries and governments to plan for PV waste safe disposal or recycling shortly.
Although many researchers have studied the recycling processes of used solar panels [
18,
19,
20,
21,
22]., only two processes have been established on an industrial scale. The recycling of C-Si and CdTe thin-film modules is operated by Deutsche Solar and First Solar, respectively [
23,
24,
25]. The first step in the recycling process is the separation of the modules, followed by the separation of the non-metal and metal parts. Many separation methods have been developed for C-Si [
26,
27,
28,
29], CdTe [30-32], and Copper-indium-gallium-selenide (CIGS) [
18,
33,
34], most of which include physical, mechanical, and chemical processes. This step involves the elimination of glass, Al, and plastic, as well as the separation of metal and non-metal parts from solar panels. The second recycling processes are different for different types of solar panels. The recycling processes for silicone solar modules typically involve delamination and metal extraction. The solar cell electrodes and interconnected ribbons, made of silver, aluminum, and copper, are dissolved into aqueous media for recycling [
35,
36]. Two of the most advanced processes developed worldwide are the Full Recovery End-of-Life Photovoltaic (FRELP) and Baseline processes [
37].
Recovery of cadmium and tellurium from cadmium telluride PVs is difficult due to the low content of the semiconductor [
38]. There are many hydrometallurgical recycling processes for CdTe as well as acid dissolution and subsequent precipitation [
39], cementation [
40]electroplating [
41], and ion exchange [
42,
43]. A recycling process for CdTe PVs based on a sequence of mechanical steps rather than wet-chemical techniques has also been proposed [
44].
In recent years, researchers have shown significant interest in end-of-life CIGS panels due to the presence of gallium and indium [
45,
46]. Several recycling processes such as mechanical techniques [
47], wet chemical process [
48], electrochemical method [
49], and leaching and electrolyzing of metals have been developed [
50]. Xiang Li and colleagues have reported an effective separation process by an alkaline agent [
51]. They found selective alkali leaching is feasible to separate indium and gallium effectively as pure In
2O
3 and Ga
2O
3 separately. The recycling of copper, indium, and gallium from thin-film solar panels has also been reported [
52], where the H
2SO
4 is used as a leaching agent. In many researchers, the separation of indium and gallium was studied by solvent extraction and ion exchange following the leaching step [
53].
On the topic of recycling, there is an important point that using a single treatment or recycling process is not usually practical for recycling all waste of a particular type [
54]. Given the variety of solar panels on the market and the need for economically feasible recycling processes, an investigation of a general procedure for the extraction of metals from the metallic parts of various solar panels is suggested in this work. The recovery of metals through the co-processing of all types of solar panels was examined. To do this, the metallic and non-metallic parts from Si, CdTe, and CIGS solar panels were separated and subjected to hydrometallurgical processes. This section is the initial step of the suggested flowsheet and includes the separation of metallic and non-metallic parts and comprehensive leaching studies of copper and other base metals. Copper is the main component of solar PV systems due to its thermal and electrical conductivity, and other base metals are also used in solar PV systems. Many hydrometallurgical methods have been studied for the dissolution of copper and other base metals; most of these studies used two or more leaching agents in two or more stages [
55,
56,
57,
58,
59]. The simultaneous leaching of copper and solder alloy was studied by a new method from PCB waste with HBF
4 as a leaching agent [
60]. After the leaching step, there were different methods for the separation of copper from other metals including solvent extraction [
60,
61,
62,
63,
64], precipitation [
65,
66], and cementation [
67]. In the second step, the recovery of gallium and indium by chemical leaching has been proposed. The optimum conditions for the selective extraction of copper, the further leaching step of other base metals, and the extraction of indium and gallium have been investigated.
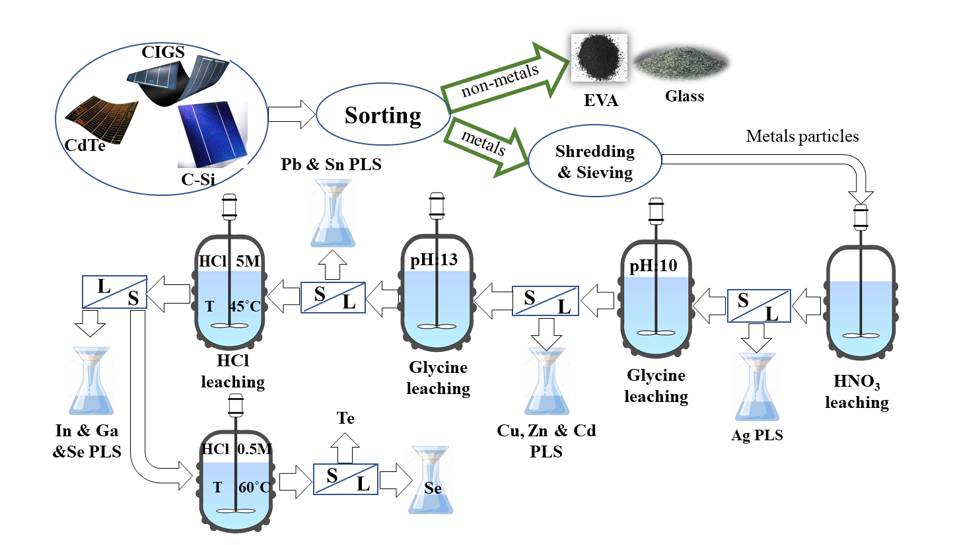
The study presented in this paper proposes a comprehensive and innovative approach for the extraction of valuable metals through the co-processing of all types of solar panels, which is a significant contribution to the field of solar panel recycling. By utilizing glycine, an eco-friendly leaching agent, in the initial step of the process, this recycling method is not only environmentally friendly but also economically feasible. In many studies, it was proven that the leaching of base metals has been successfully conducted by glycine as a dissolution agent [
68,
69,
70,
71]. The study also provides insights into the leaching behavior of various metals under different conditions, allowing for the selective extraction of different metals from solar panels. Furthermore, the paper presents a general flowsheet for the recycling of all types of solar panels, which has the potential to be implemented globally in the future, considering its economic and environmental benefits. The study highlights the importance of developing comprehensive recycling methods for spent solar panels to ensure the effectiveness of solar PV technology and reduce its environmental impact. This approach appears to be a significant contribution to the field of solar panel recycling, and it could potentially pave the way for future research and development in this area.
5. Conclusions
Life cycle assessment is used to evaluate the environmental effects of solar panel technology. To ensure the effectiveness of solar PV technology, it is necessary to consider all environmental consequences of the significant growth of solar PV production. Comprehensive methods should be developed to recycle spent solar panels after their end of life to protect the environment and achieve economic added value. Advanced research has been initiated to recover metals from various types of solar panels, and this paper focuses on the leaching of base metals, which is the initial step of future research in this field.
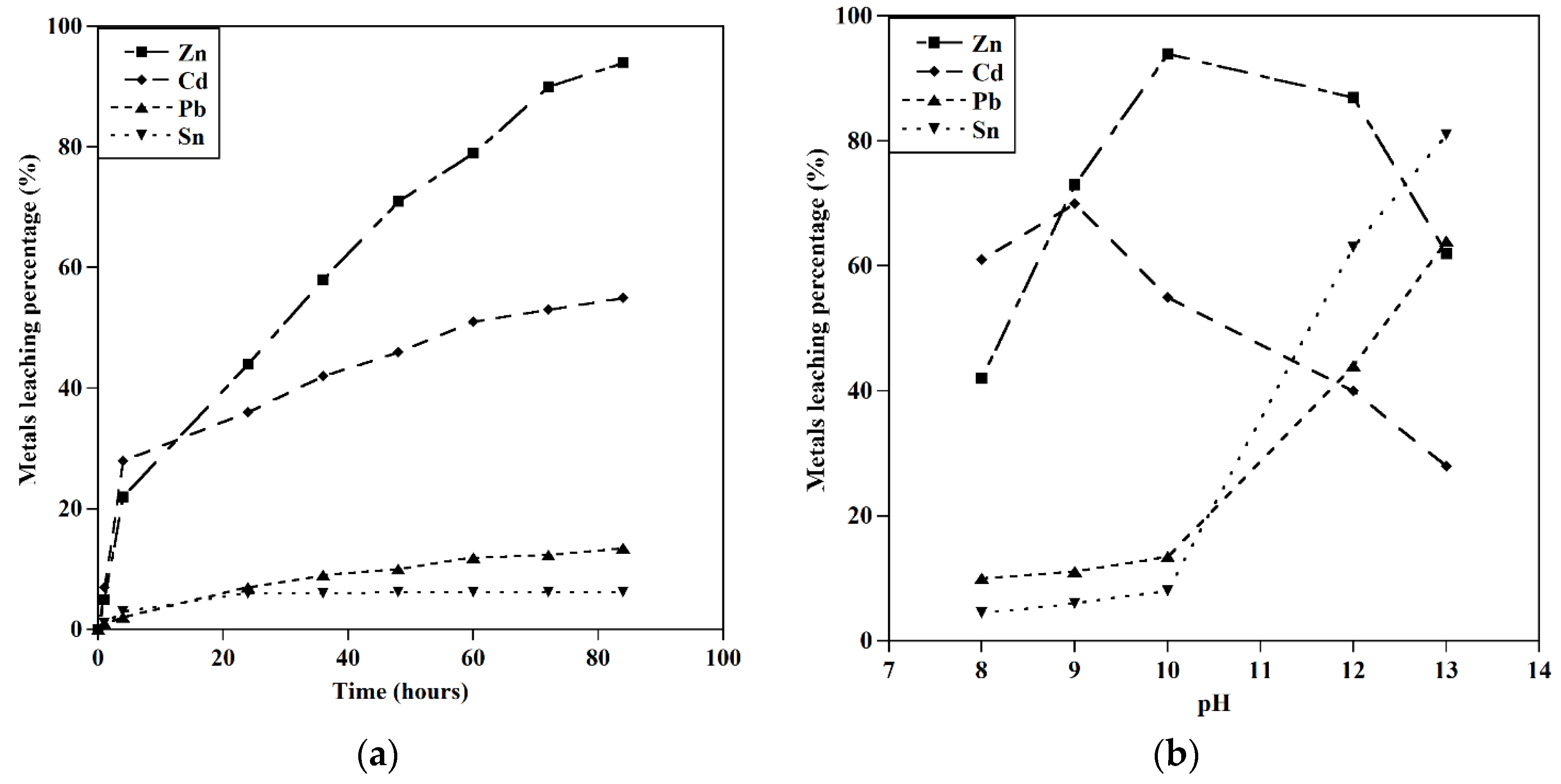
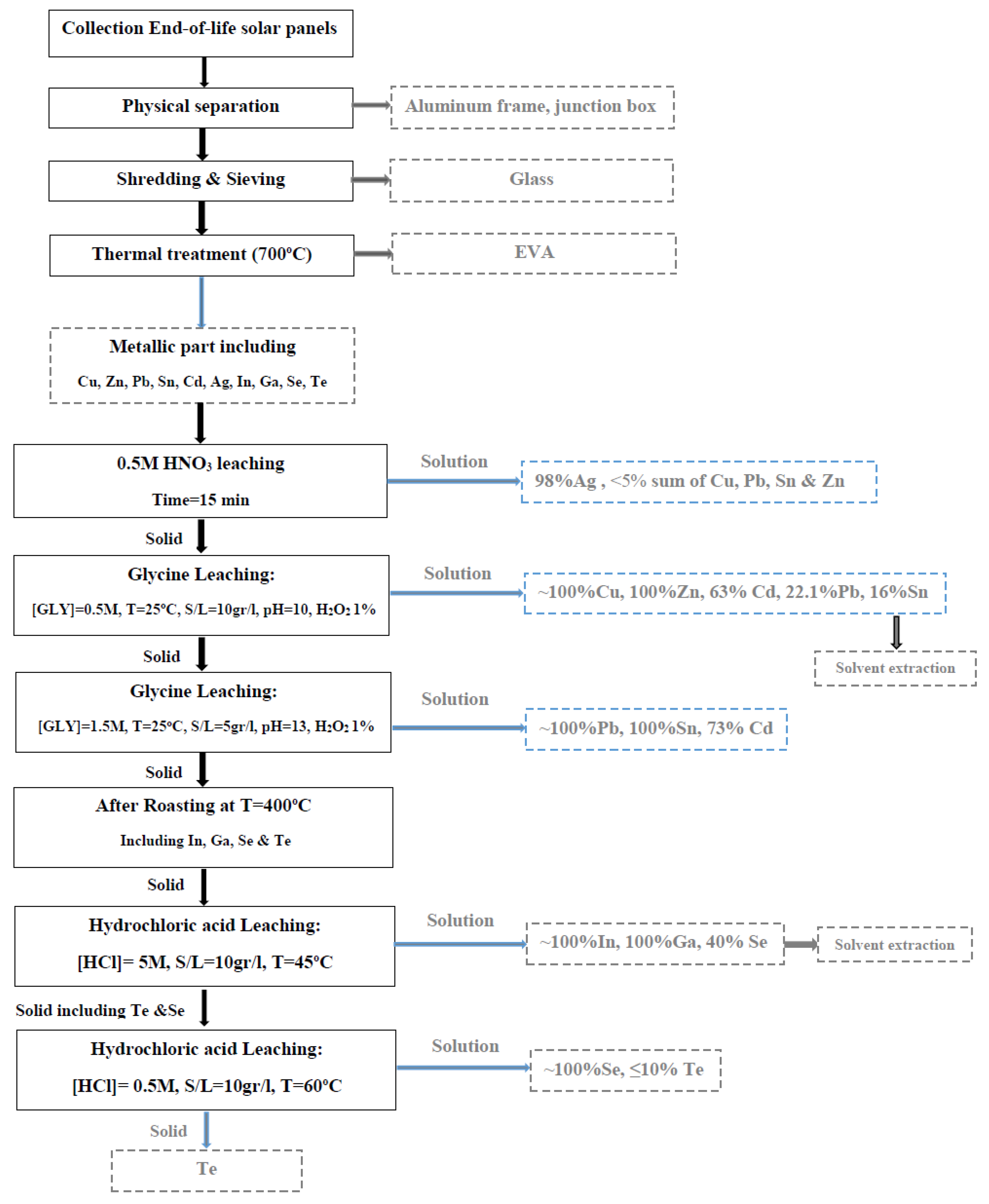
This study presents an innovative approach for extracting metals (Cu, Ag, Cd, Te, Se, In, Ga, Sn, Pb, Zn) from all kinds of solar panels. The base metals extraction was a prerequisite for recovering precious metals from end-of-life solar panels. The initial step after preparation was the dissolution of silver using a 0.5 M HNO3 solution. The leaching behavior of copper and other metals in an alkaline glycine solution was then studied under various conditions. The optimal conditions for the selective extraction of copper and zinc were found to be a glycine concentration of 0.5M, an S/L ratio of 10 gr/l, and an initial pH value of 10. To achieve the highest extraction of other metals (Cd, Sn, Pb), the leaching procedure was performed at different initial pHs, and the optimized conditions for the extraction of Cu, Pb, Cd, Zn, and Sn were studied.
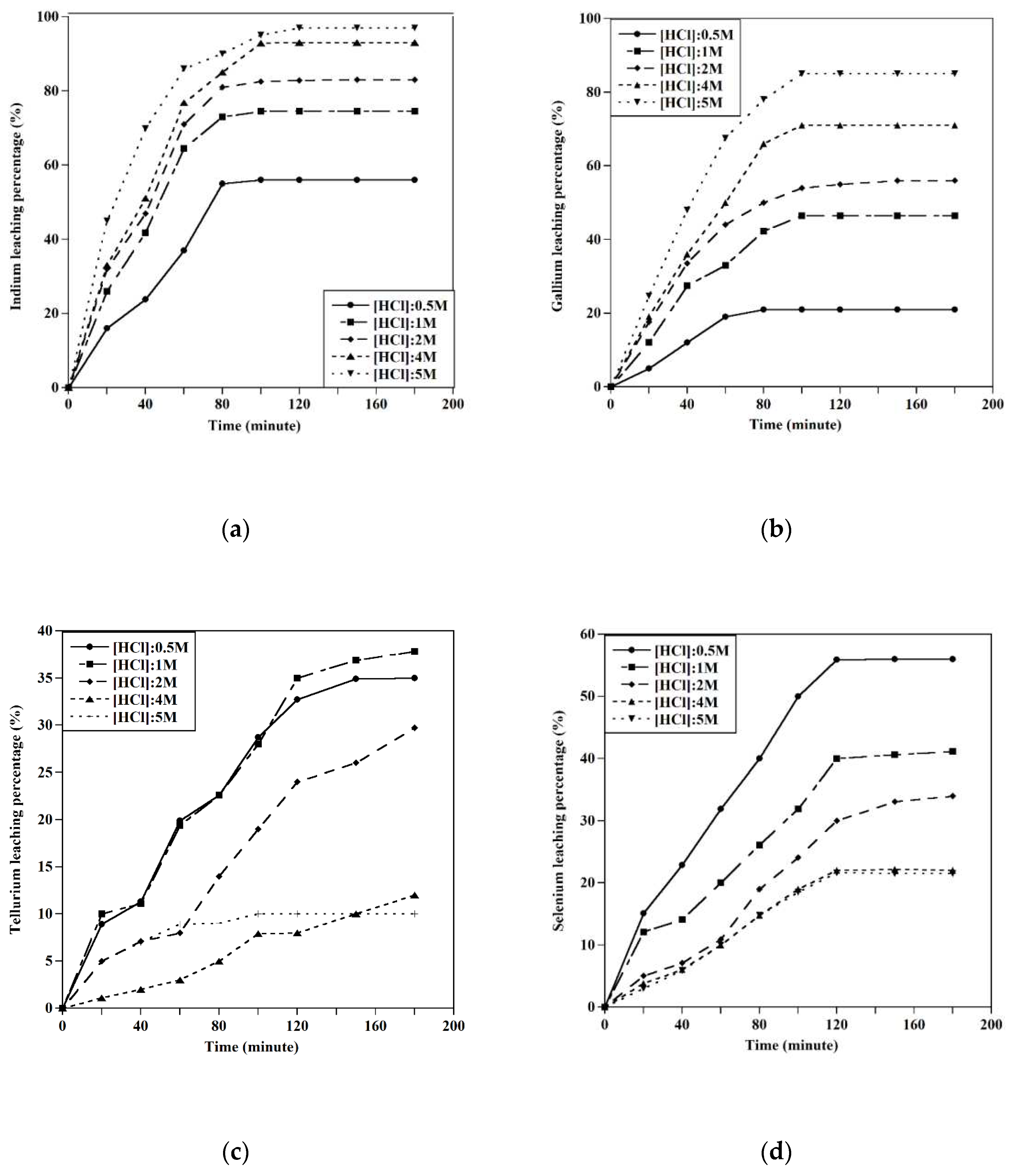
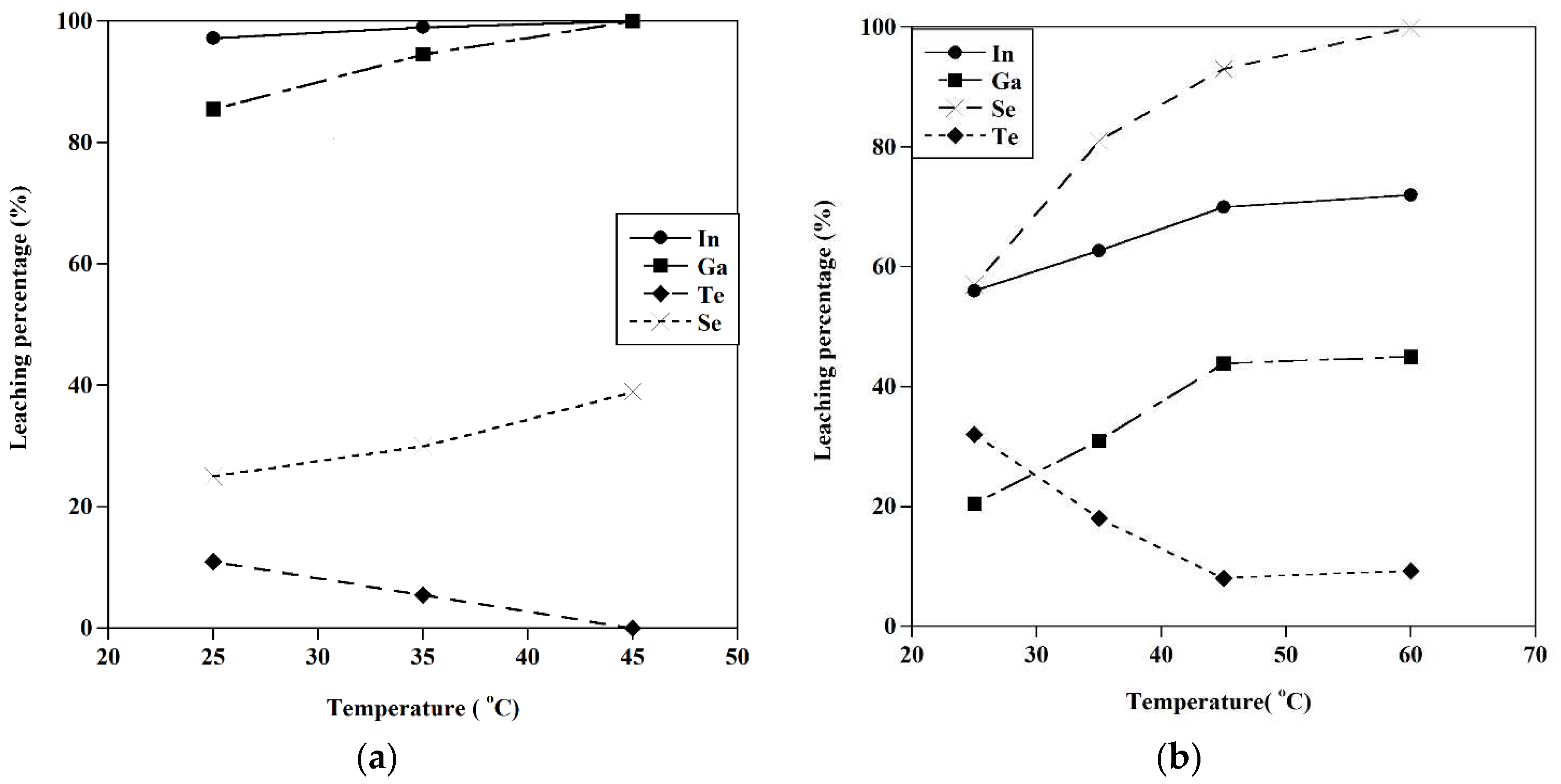
In the final step, the dissolution of indium, gallium, selenium, and tellurium was studied using HCl acid under different conditions. The indium and gallium were recovered at experimental conditions of [HCl]: 5M, T=45°C, and S/L: 10gr/l, achieving recoveries of about 100. By decreasing the acid concentration to 0.5M, the extraction of selenium was conducted at about 100% at a temperature of 60 ˚C, and completely, the separation of tellurium and selenium happened.
This study demonstrates an innovative approach for extracting metals from all types of solar panels. A comprehensive method for recycling spent solar panels must be developed to ensure the effectiveness of solar PV technology and reduce its environmental impact. The research on recovering metals from different types of solar panels is ongoing, and this paper presents the leaching step of metals, paving the way for future research in this field. Our hope is by conducting kinetic studies, the time of experiments can be optimized. Also, by controlling pH and temperature during the glycine leaching process, we can improve the efficiency of the leaching process. The solvent extraction method can separate copper, Zinc, and cadmium from the glycine solution and indium and gallium from the final solution.
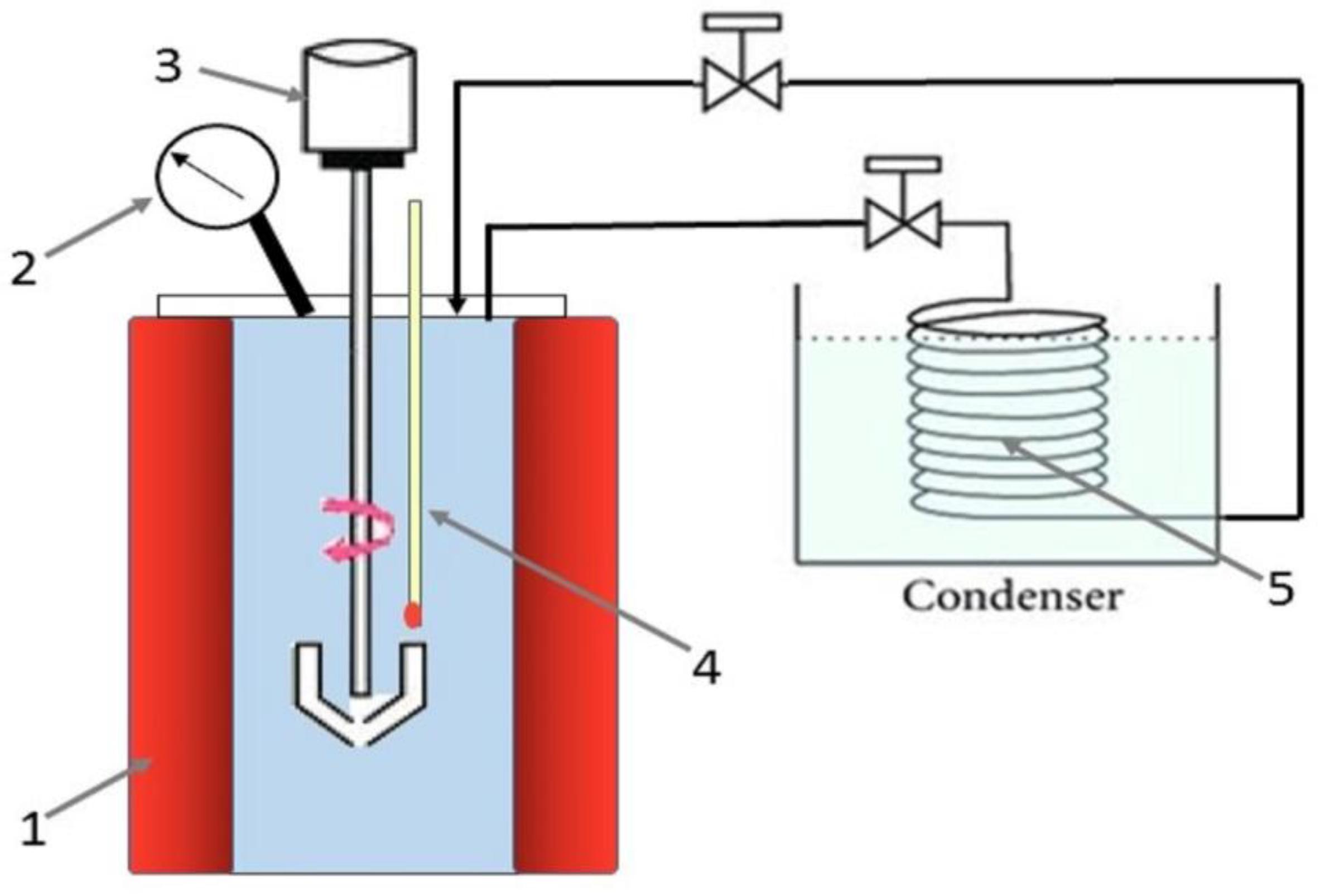
Figure 1.
Schematic diagram of the lab-scale autoclave reactor 1-Electric heater, 2-Pressure sensor, 3- Stirrer, 4- Temperature sensor, 5- Condenser.
Figure 1.
Schematic diagram of the lab-scale autoclave reactor 1-Electric heater, 2-Pressure sensor, 3- Stirrer, 4- Temperature sensor, 5- Condenser.
Figure 2.
The leaching behavior glycine concentration=0.5 M, particle size≤1 mm, T=25°C, S/L = 20gr/l.
Figure 2.
The leaching behavior glycine concentration=0.5 M, particle size≤1 mm, T=25°C, S/L = 20gr/l.
Figure 3.
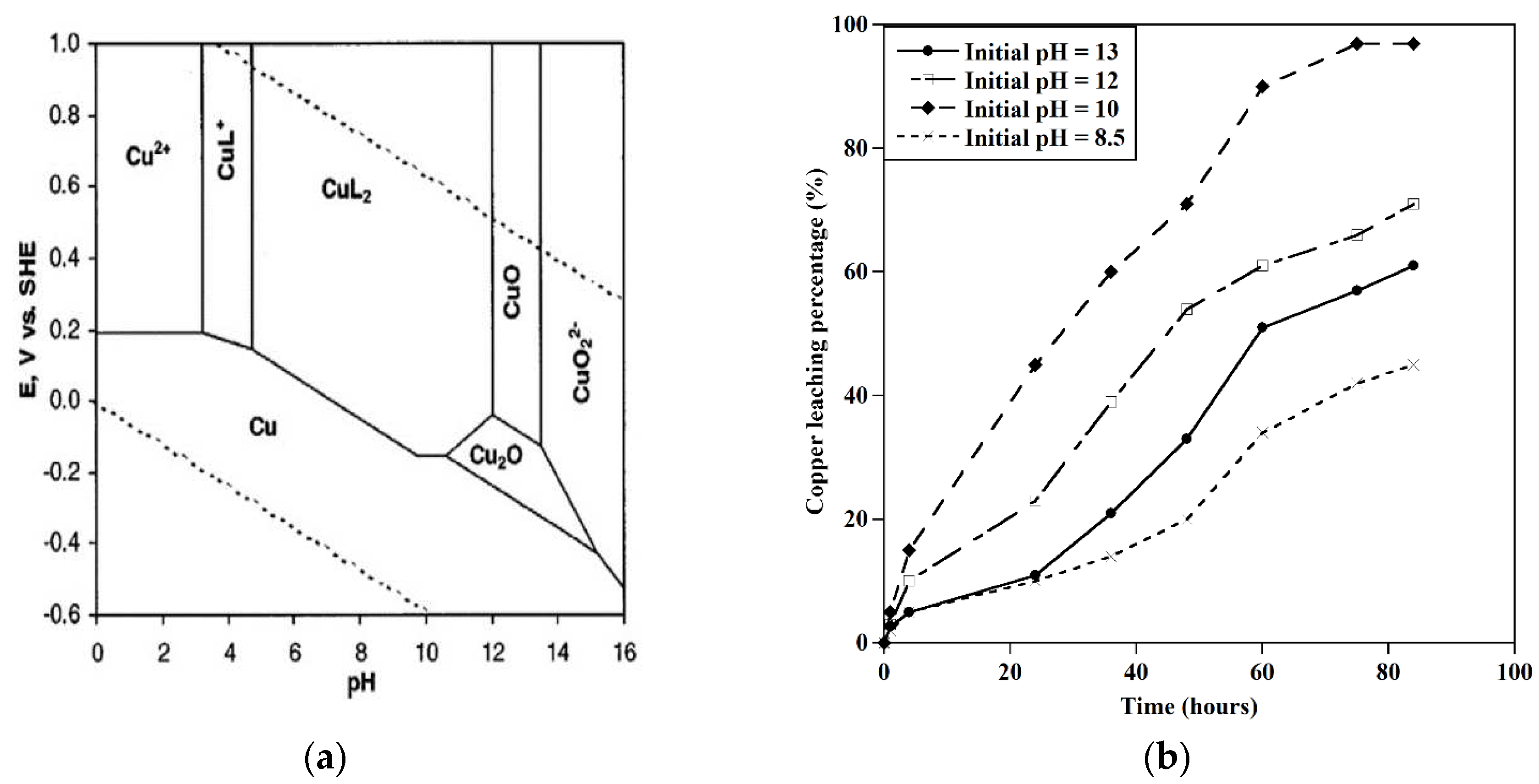
a) Potential/pH diagram for the Cu-water-glycine system at 25 ˚C and 1 atm[
77]. b) Effect of initial pH on the extraction of Cu glycine concentration=0.5 M, S/L: 20 gr/l, particle size=1mm, T=25 ˚C.
Figure 3.
a) Potential/pH diagram for the Cu-water-glycine system at 25 ˚C and 1 atm[
77]. b) Effect of initial pH on the extraction of Cu glycine concentration=0.5 M, S/L: 20 gr/l, particle size=1mm, T=25 ˚C.
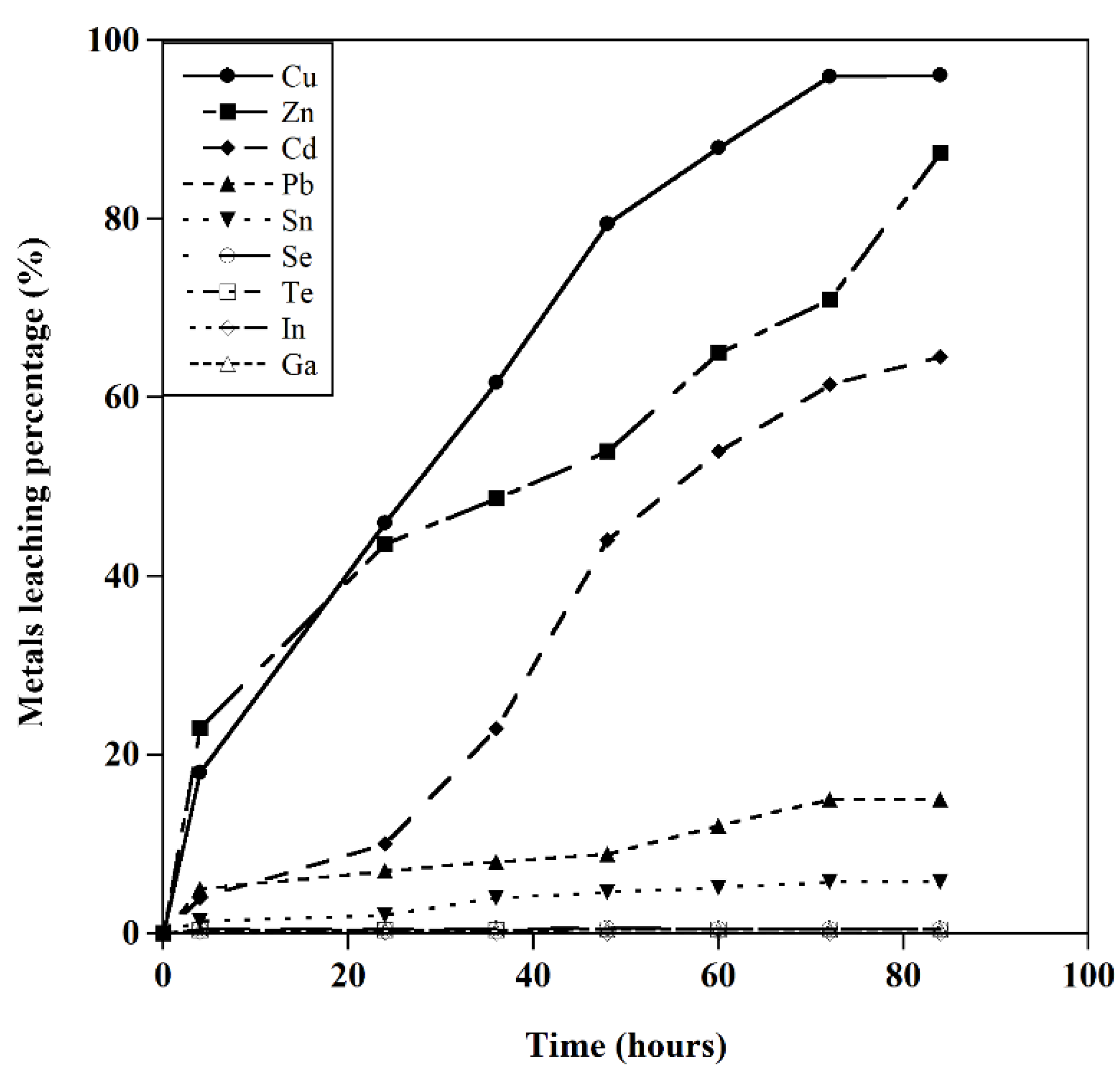
Figure 4.
-a) The leaching behavior of metals (Glycine concentration=0.5M, T=25 ˚C, S/L: 20 gr/l, initial pH= 10, particle size=1mm), b) Effect of pH on the leaching behavior of metals (Glycine concentration=0.5M, T=25 ˚C, S/L: 20 gr/l, particle size=1mm).
Figure 4.
-a) The leaching behavior of metals (Glycine concentration=0.5M, T=25 ˚C, S/L: 20 gr/l, initial pH= 10, particle size=1mm), b) Effect of pH on the leaching behavior of metals (Glycine concentration=0.5M, T=25 ˚C, S/L: 20 gr/l, particle size=1mm).
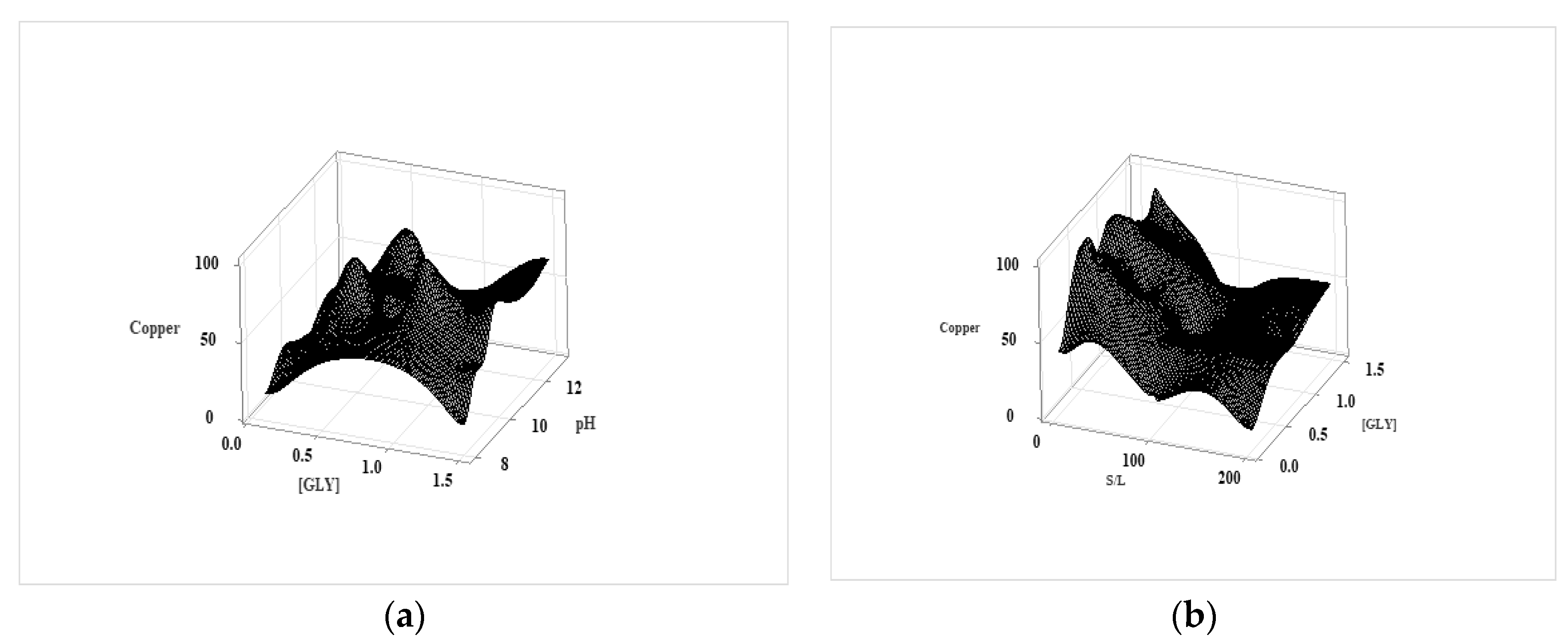
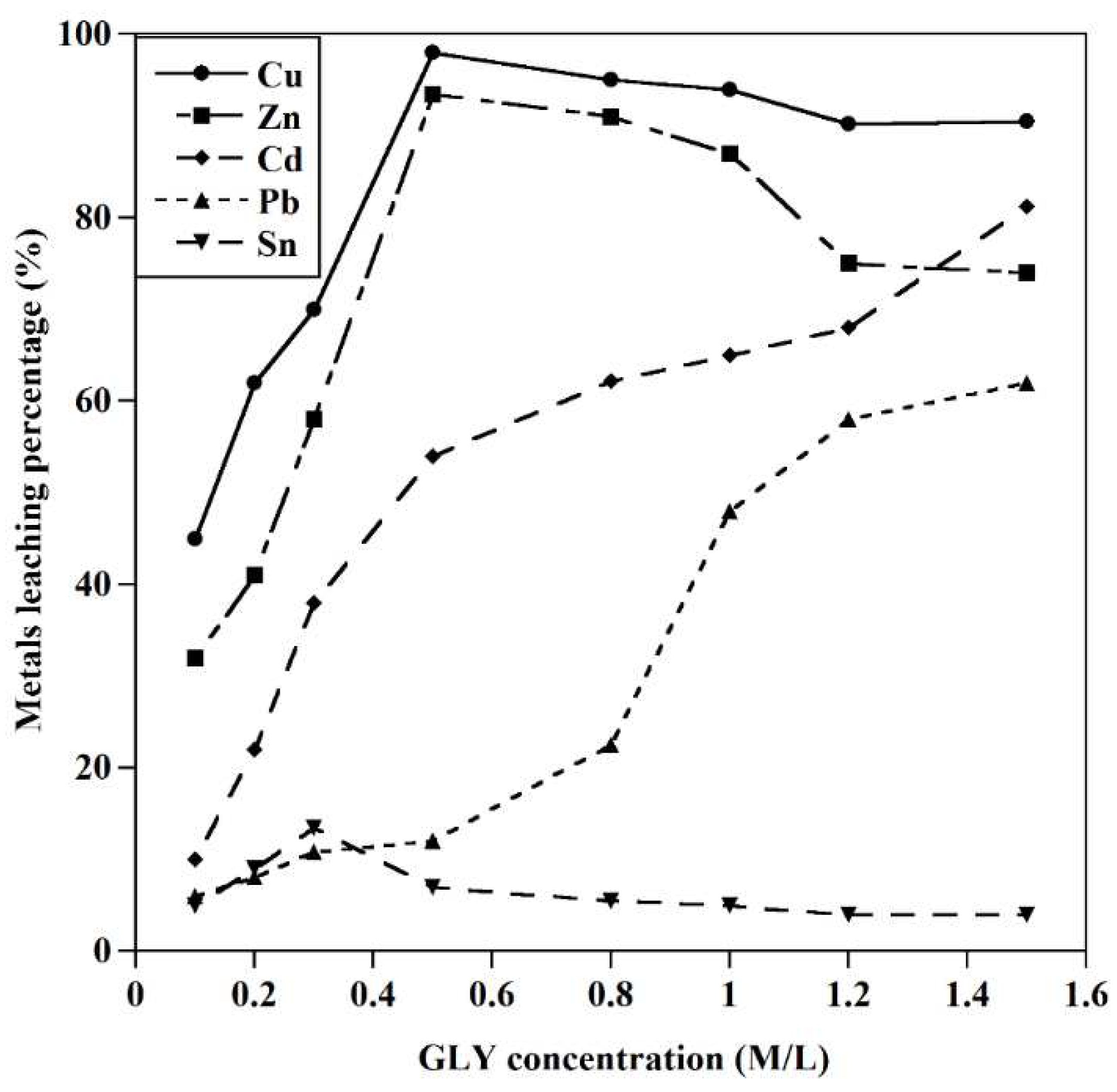
Figure 5.
The effect of [GLY] concentration on the leaching behavior of metals (T=25 ˚C, S/L: 20gr/l, initial pH=10).
Figure 5.
The effect of [GLY] concentration on the leaching behavior of metals (T=25 ˚C, S/L: 20gr/l, initial pH=10).
Figure 6.
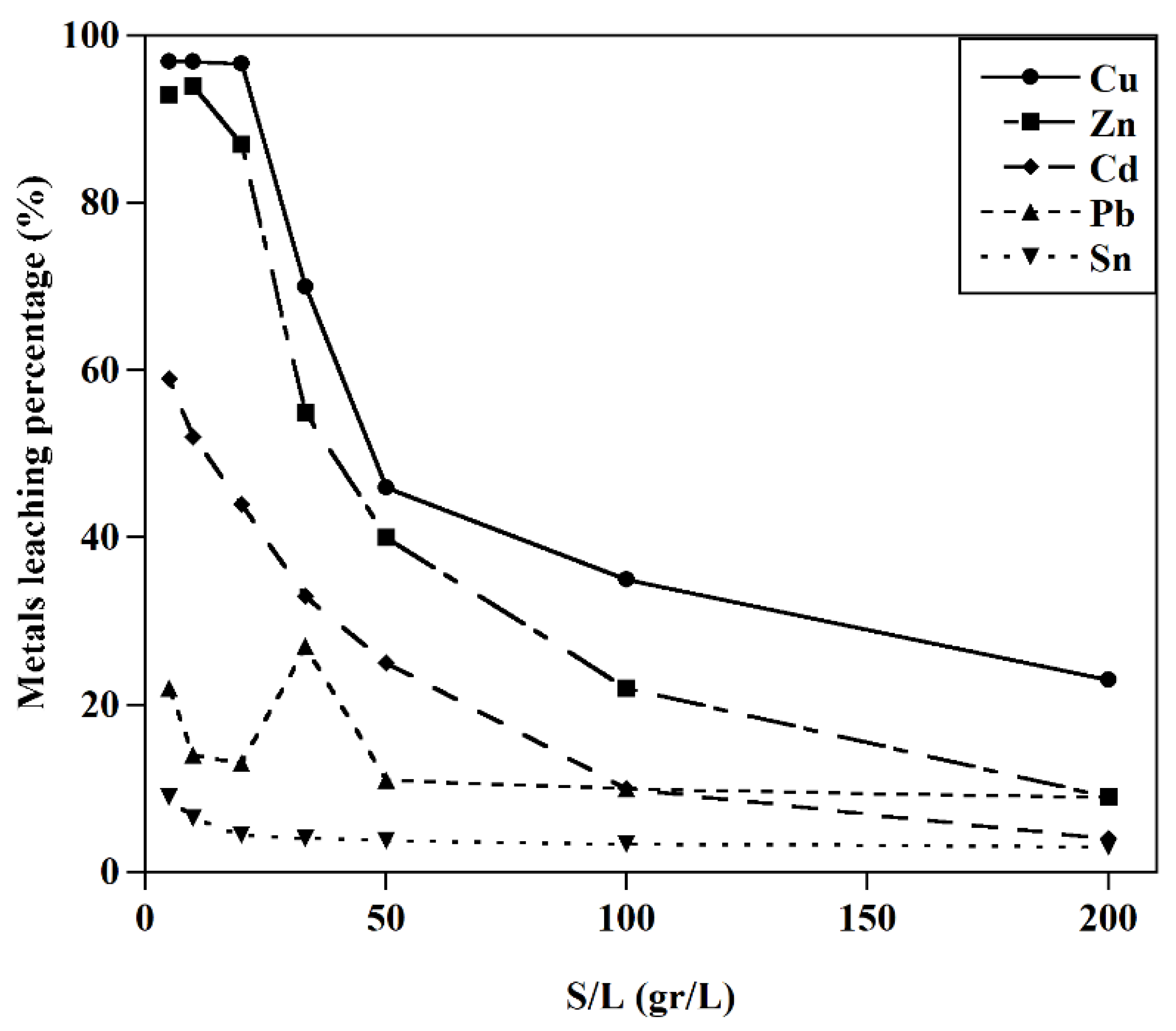
The effect of S/L on the leaching behavior of metals (T=25 ˚C, [GLY]:0.5M, initial pH=10).
Figure 6.
The effect of S/L on the leaching behavior of metals (T=25 ˚C, [GLY]:0.5M, initial pH=10).
Figure 7.
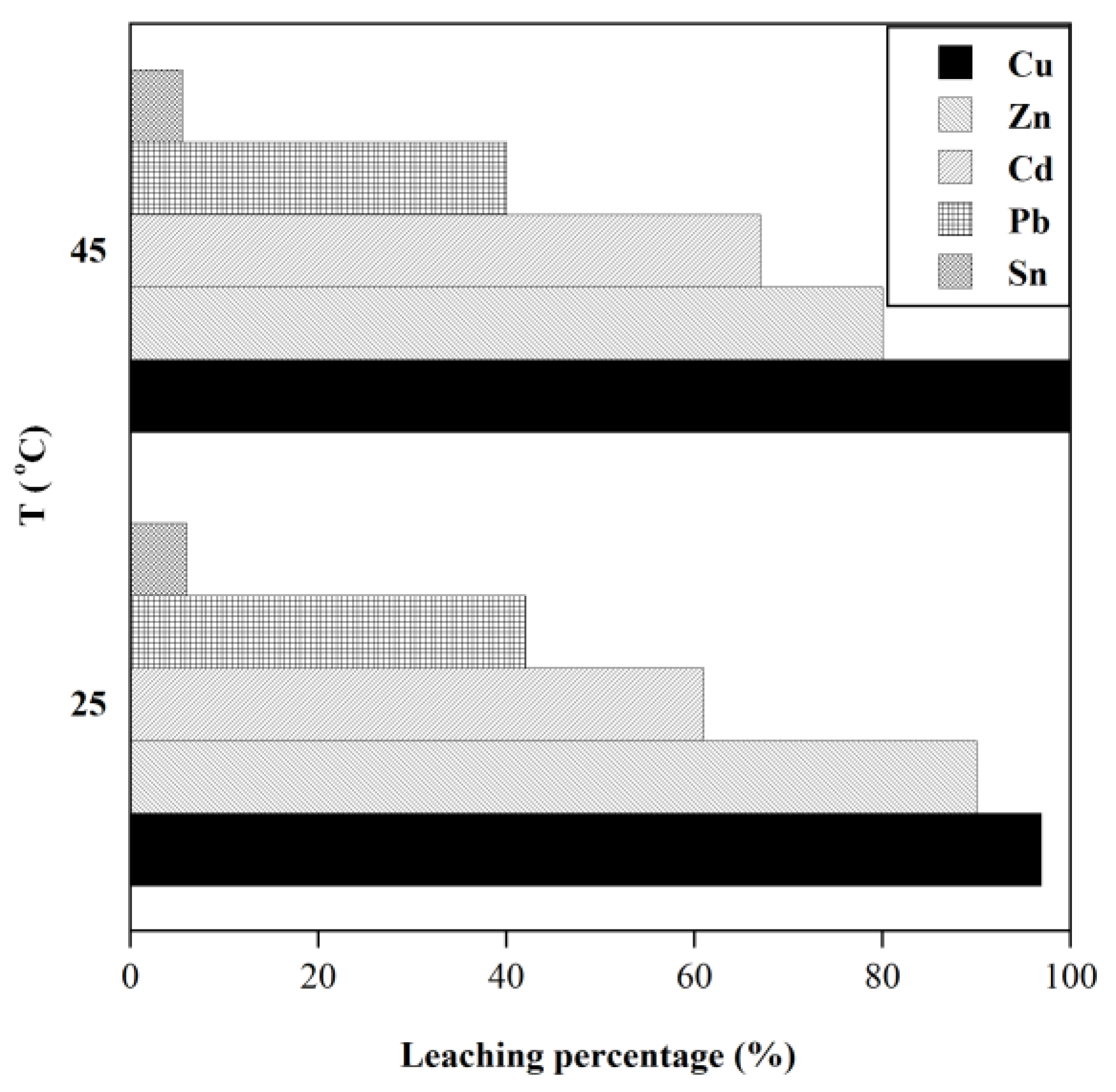
Effect of temperature on the extraction of metals for 72 h ([GLY]=0.5 M, S/L:20 gr/l, initial pH=10).
Figure 7.
Effect of temperature on the extraction of metals for 72 h ([GLY]=0.5 M, S/L:20 gr/l, initial pH=10).
Figure 8.
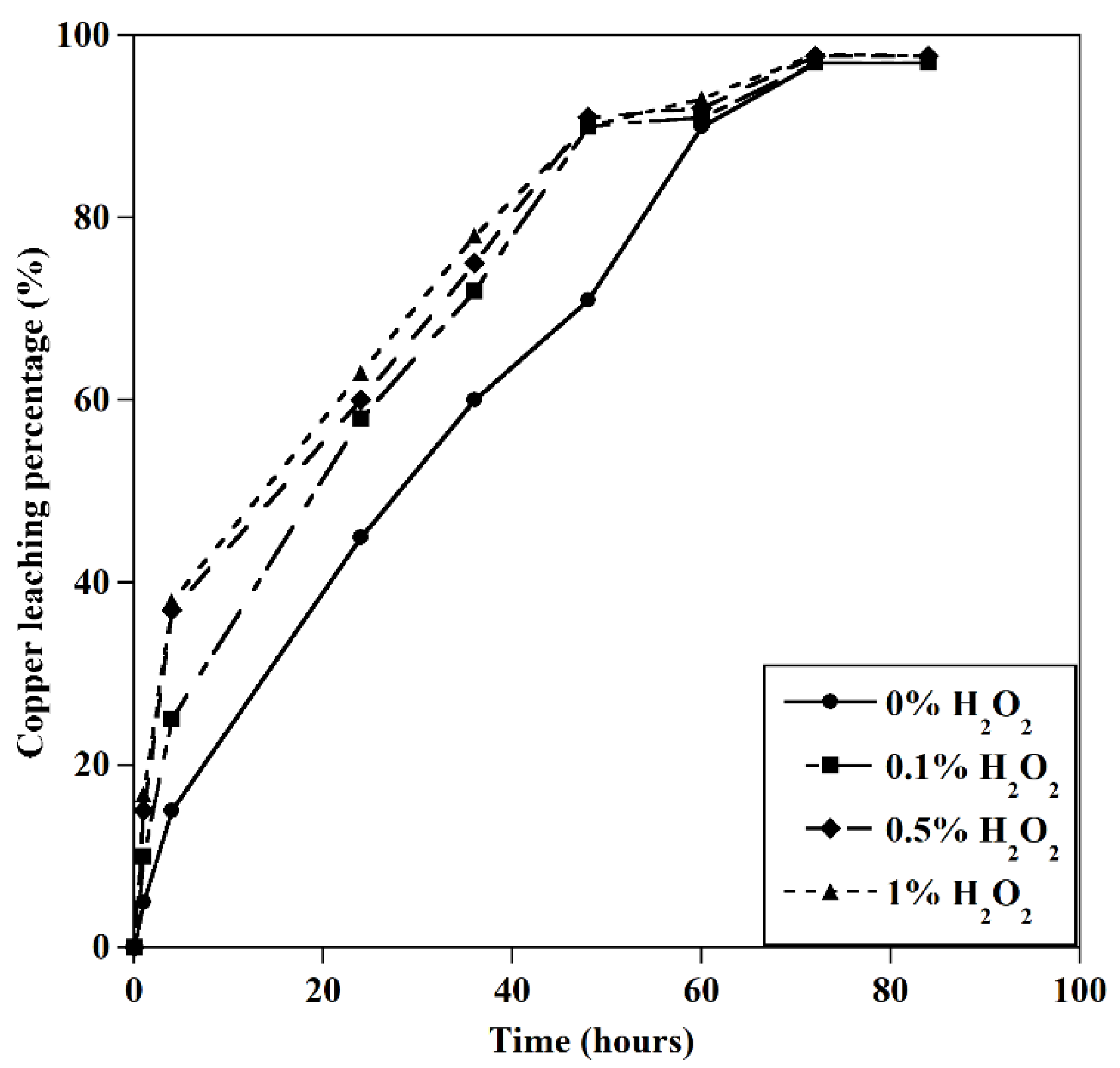
The effect of H2O2 on the leaching of copper (T=25 ˚C, [GLY]=0.5M, S/L: 5 gr/l, initial pH=10).
Figure 8.
The effect of H2O2 on the leaching of copper (T=25 ˚C, [GLY]=0.5M, S/L: 5 gr/l, initial pH=10).
Figure 9.
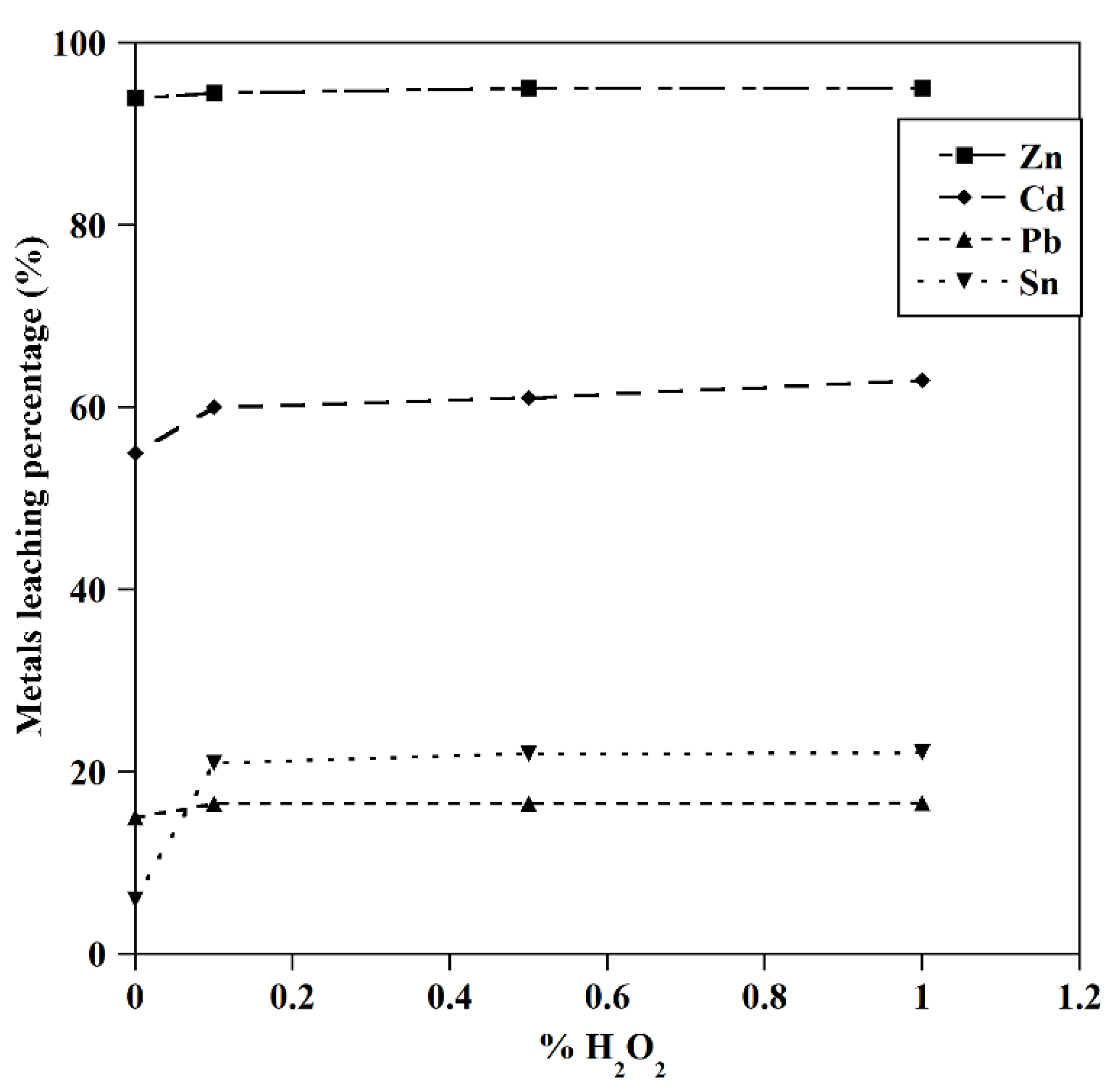
The effect of H2O2 on the leaching of metals (T=25 ˚C, [GLY]=0.5 M, S/L: 5 gr/l, initial pH=10).
Figure 9.
The effect of H2O2 on the leaching of metals (T=25 ˚C, [GLY]=0.5 M, S/L: 5 gr/l, initial pH=10).
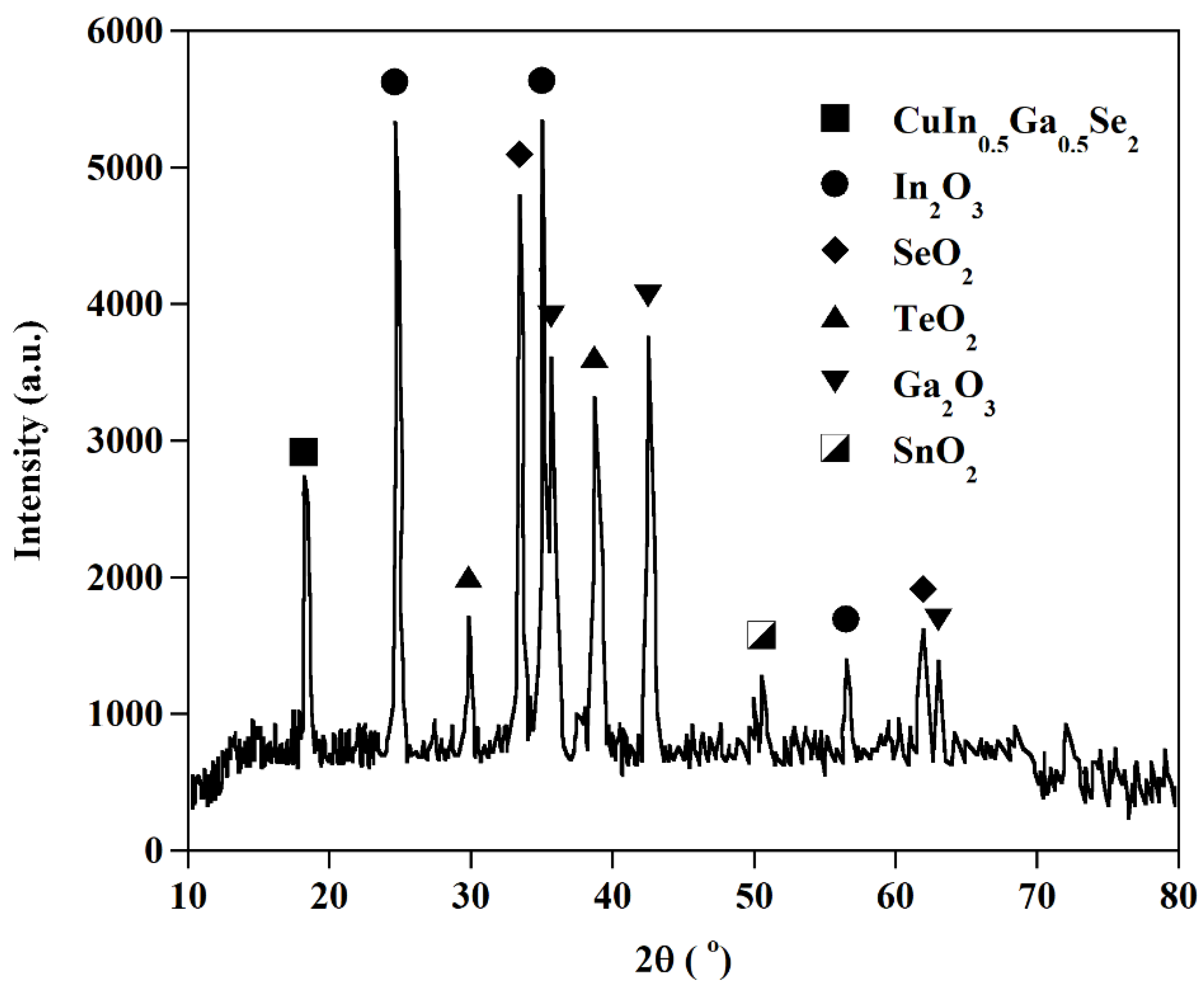
Figure 11.
XRD pattern of glycine leaching residue after oxidation roasting.
Figure 11.
XRD pattern of glycine leaching residue after oxidation roasting.
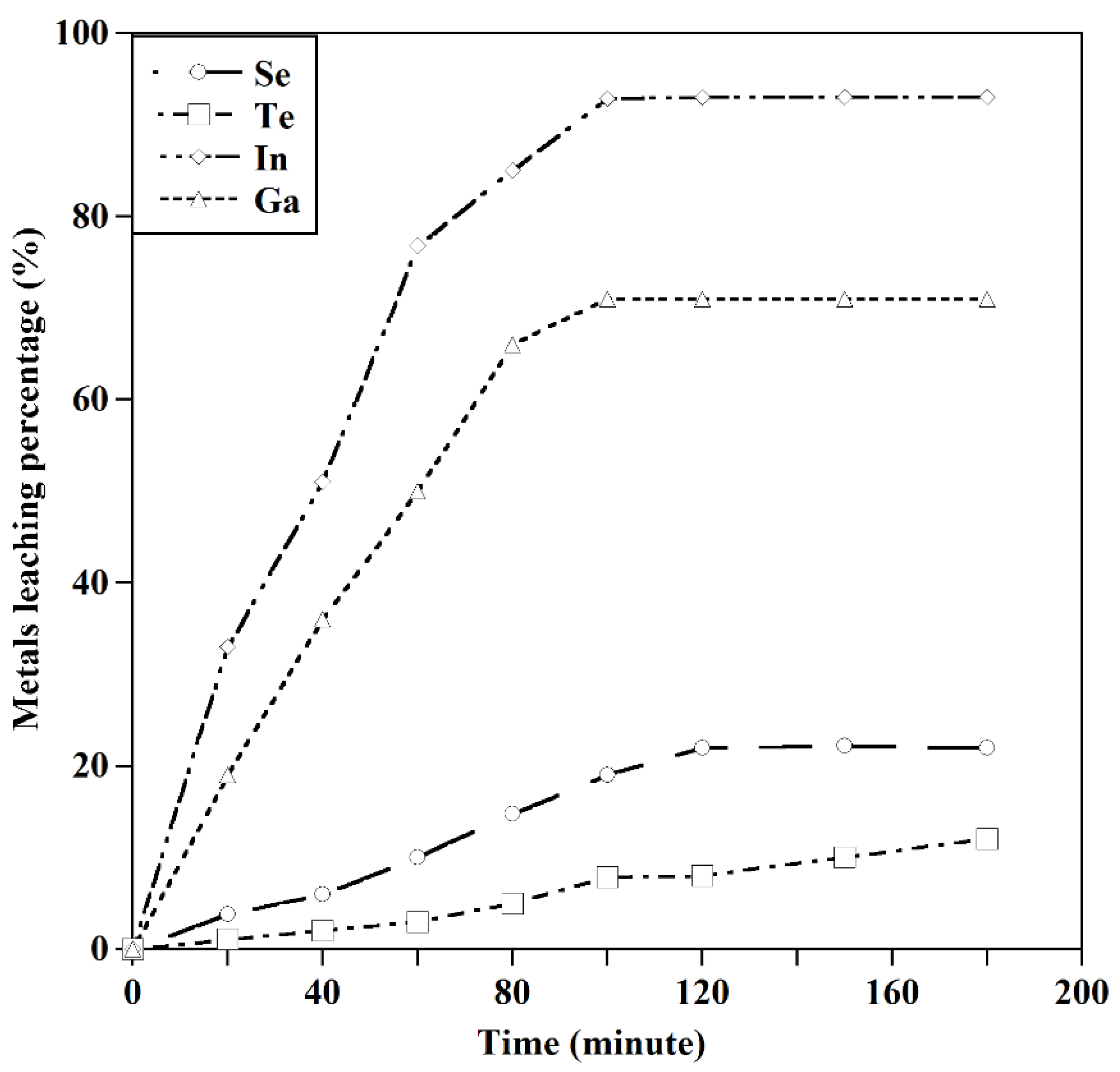
Figure 12.
The leaching behavior of In, Ga, Se, and Te ([HCL]:4 M, T=250C, S/L:10 gr/l).
Figure 12.
The leaching behavior of In, Ga, Se, and Te ([HCL]:4 M, T=250C, S/L:10 gr/l).
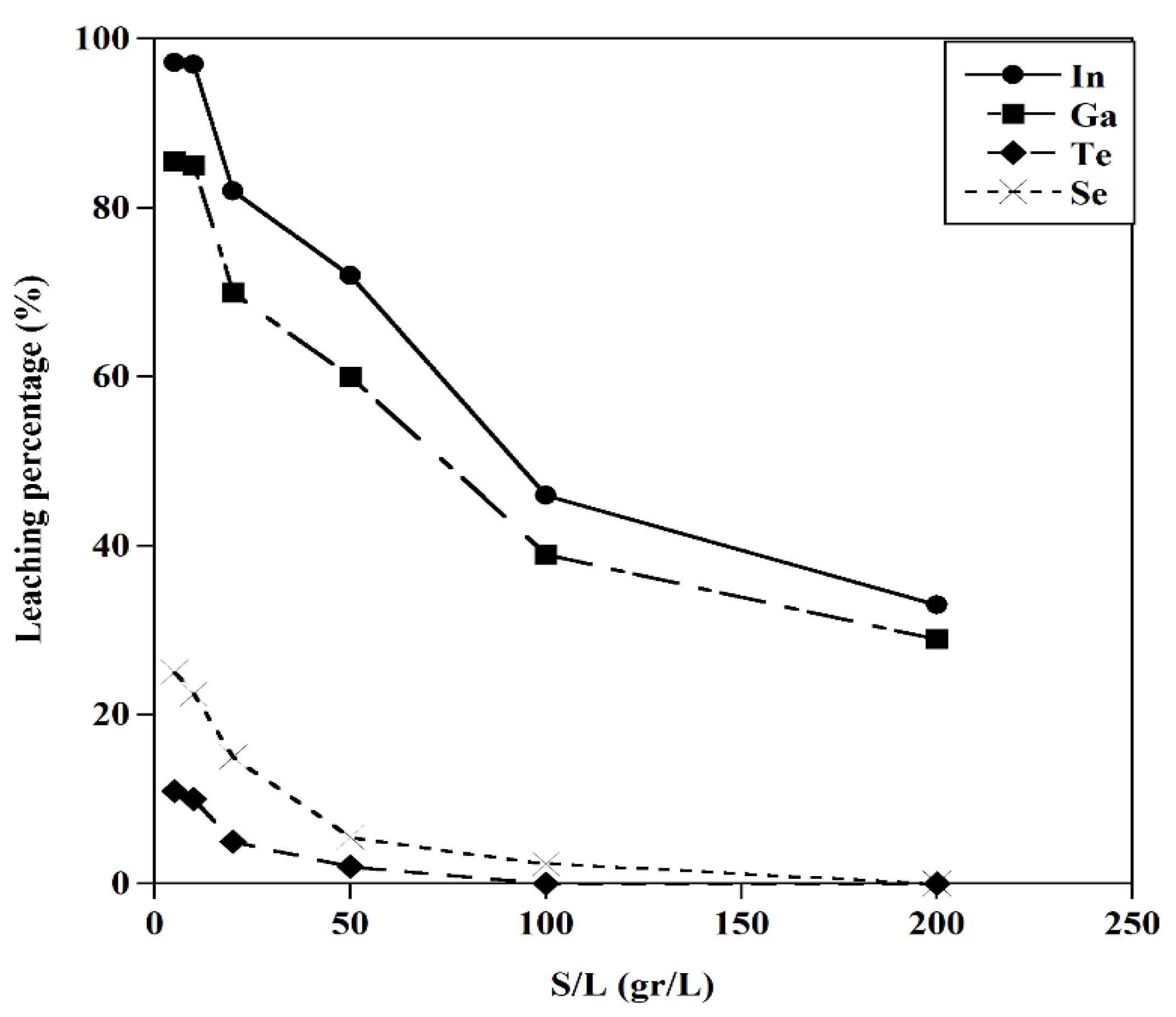
Figure 14.
The effect of S/L on the leaching behavior of metals (T=25 ˚C, [HCl]:5M, t=120 min).
Figure 14.
The effect of S/L on the leaching behavior of metals (T=25 ˚C, [HCl]:5M, t=120 min).
Figure 15.
The effect of temperature on the metal extraction a) [HCl]=5M, S/L=10 gr/l, b) [HCl]=0.5 M, S/L=10gr/l, t=120 min.
Figure 15.
The effect of temperature on the metal extraction a) [HCl]=5M, S/L=10 gr/l, b) [HCl]=0.5 M, S/L=10gr/l, t=120 min.
Figure 16.
The general flowsheet for recycling all types of solar panels.
Figure 16.
The general flowsheet for recycling all types of solar panels.
Table 1.
The XRF results of major metal contents of the sample (Prepared in this study by using end-of-life solar panels and alloy layers).
Table 1.
The XRF results of major metal contents of the sample (Prepared in this study by using end-of-life solar panels and alloy layers).
| |
Cu |
Pb |
Sn |
Cd |
Zn |
Ag |
Se |
In |
Ga |
Te |
| Metal content (Wt%) |
38 |
1.9 |
1.1 |
0.81 |
5.9 |
0.56 |
0.12 |
0.09 |
0.08 |
0.51 |
Table 2.
Factors and their levels.
Table 2.
Factors and their levels.
| Factor |
Name |
Unit |
Minimum |
Maximum |
Coded Low |
Coded High |
| A |
[GLY] |
M |
0.10 |
1.50 |
-1 ↔ 0.10 |
+1 ↔ 1.50 |
| B |
S/L |
gr/l |
5.0 |
200 |
-1 ↔ 5.0 |
+1 ↔ 200 |
| C |
pH |
|
8.0 |
13.0 |
-1 ↔ 8.0 |
+1 ↔ 13.0 |
| D |
H2O2
|
% |
0.0 |
1.0 |
-1 ↔ 0.0 |
+1 ↔ 1.0 |
Table 3.
Stability constants of copper glycinate species at 25 ˚C and 1 atm [
77].
Table 3.
Stability constants of copper glycinate species at 25 ˚C and 1 atm [
77].
| Reaction |
Stability Constant |
| Cu2++2(NH2CH2COO)-=Cu(NH2CH2COO)2
|
15.64 |
| Cu2++(NH2CH2COO)-=Cu(NH2CH2COO)+
|
8.57 |
| Cu++2(NH2CH2COO)-=[Cu(NH2CH2COO)2]-
|
10.1 |
| Cu(NH2CH2COO)++H+= Cu(NH3CH2COO)2+
|
2.92 |
| (NH2CH2COO)-+ H+= Cu(NH3CH2COO) |
9.778 |
| H(NH2CH2COO)+ H+=H2(NH2CH2COO)+
|
2.350 |