Submitted:
07 August 2023
Posted:
09 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
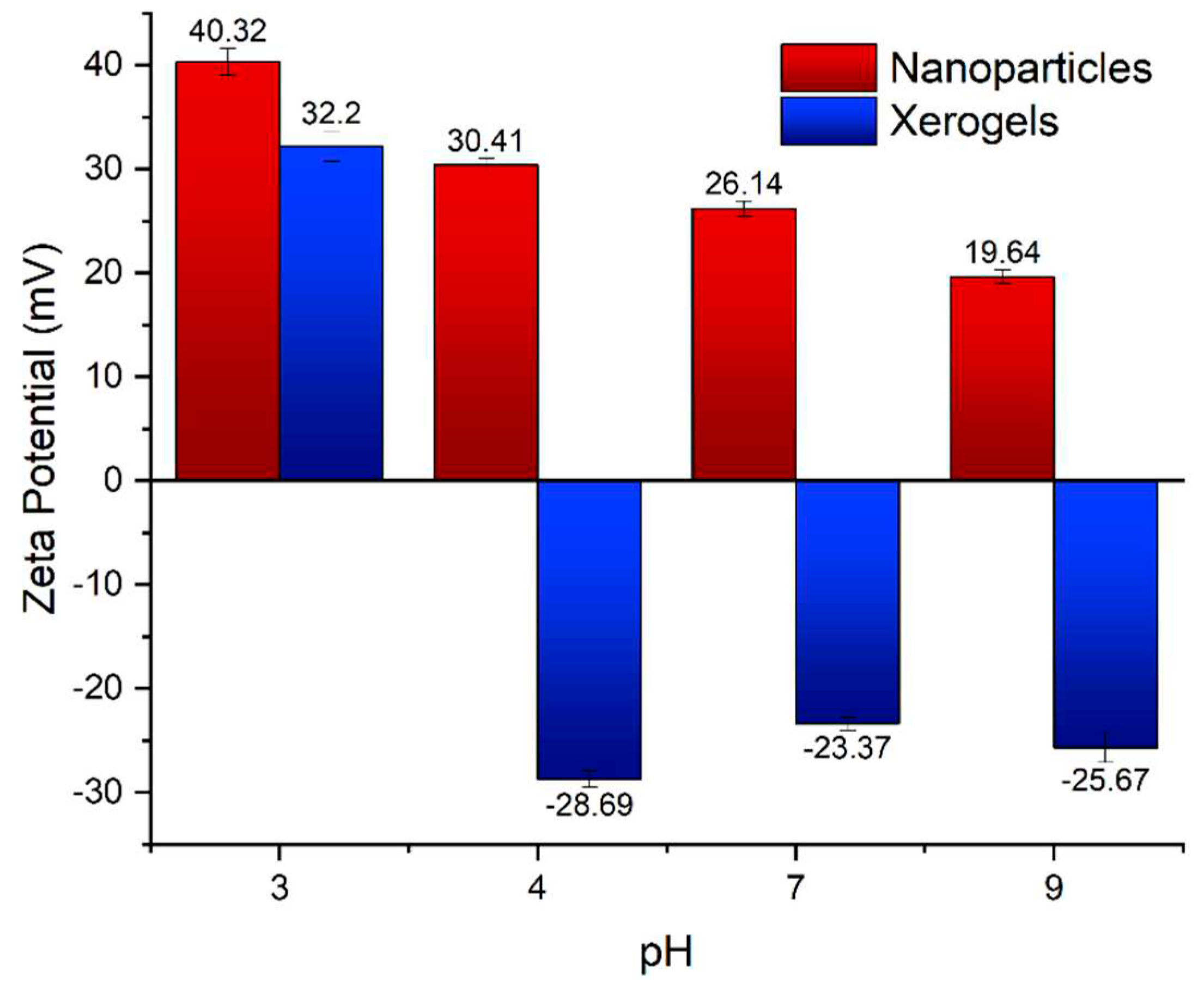
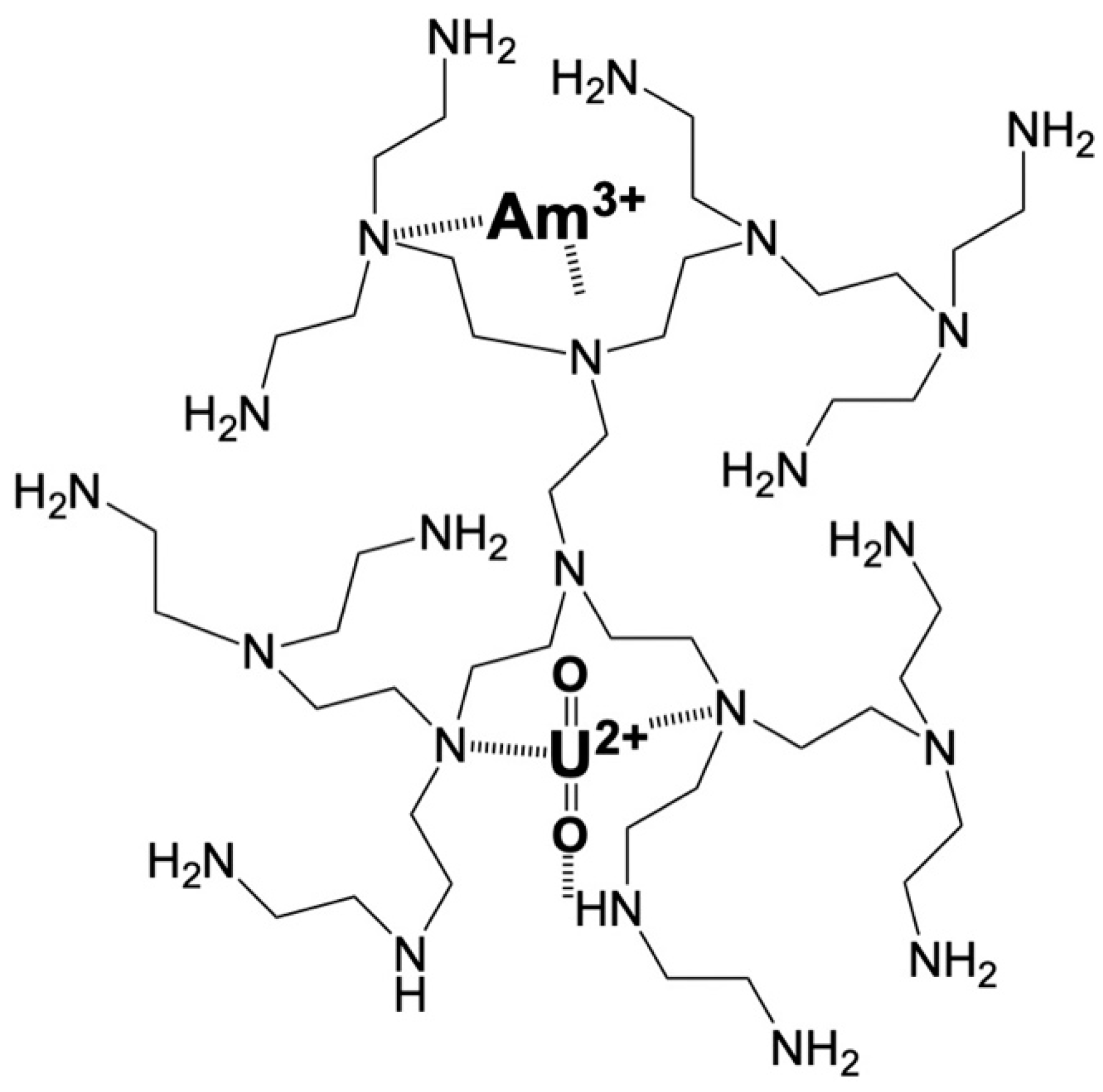
2.1. Xerogel and Nanoparticle Characteristics
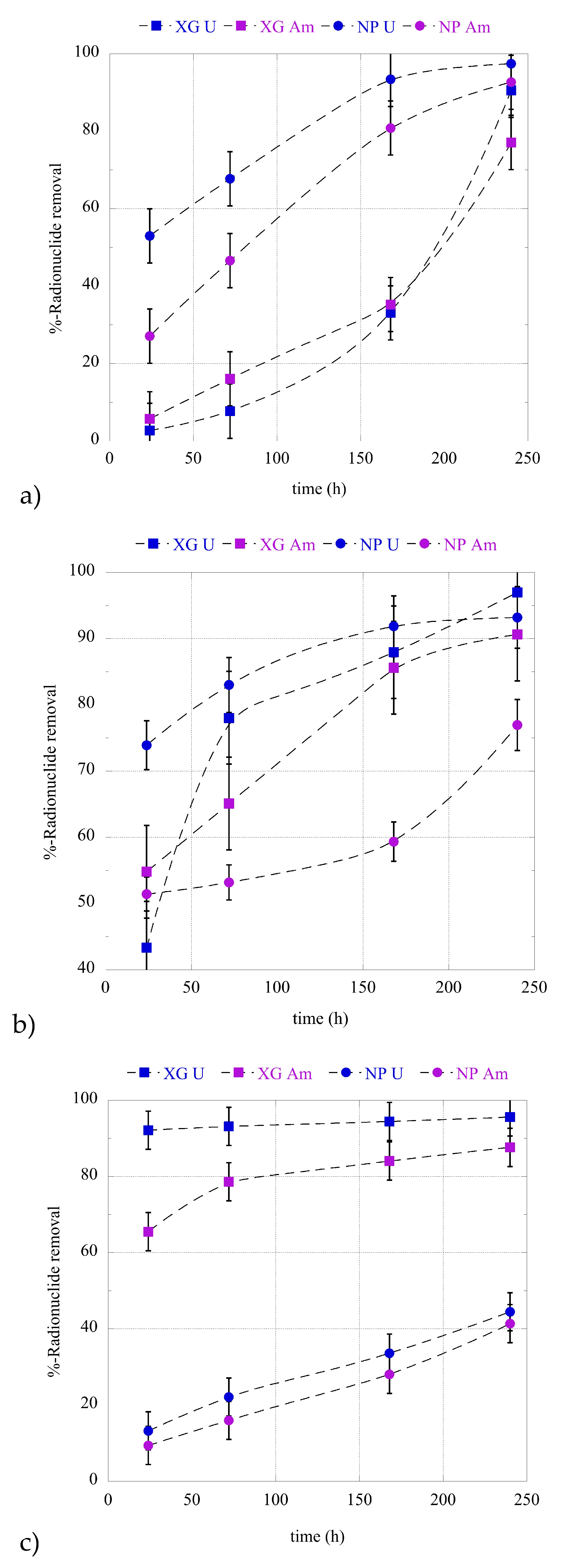
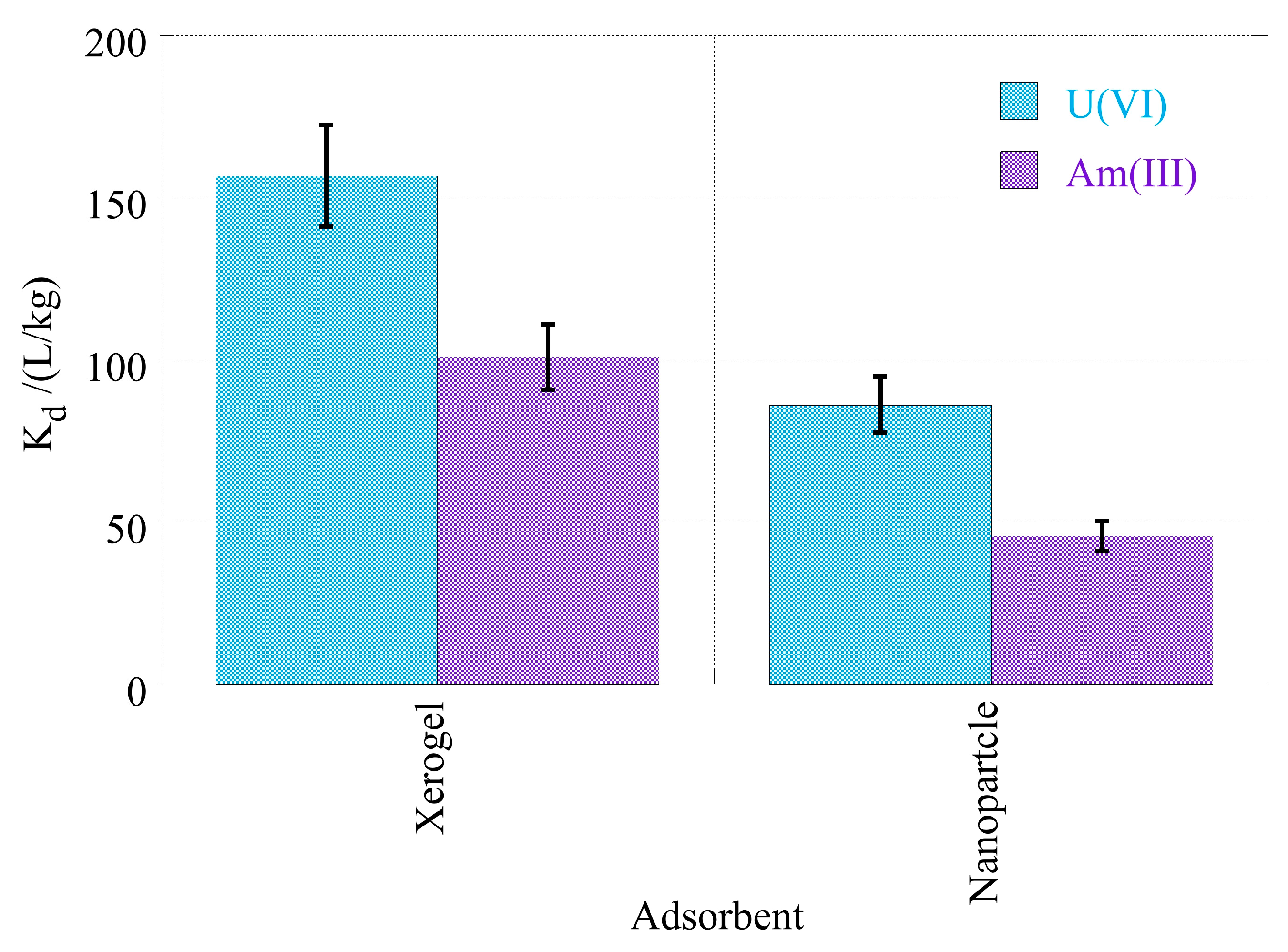
2.2. Adsorption Kinetics
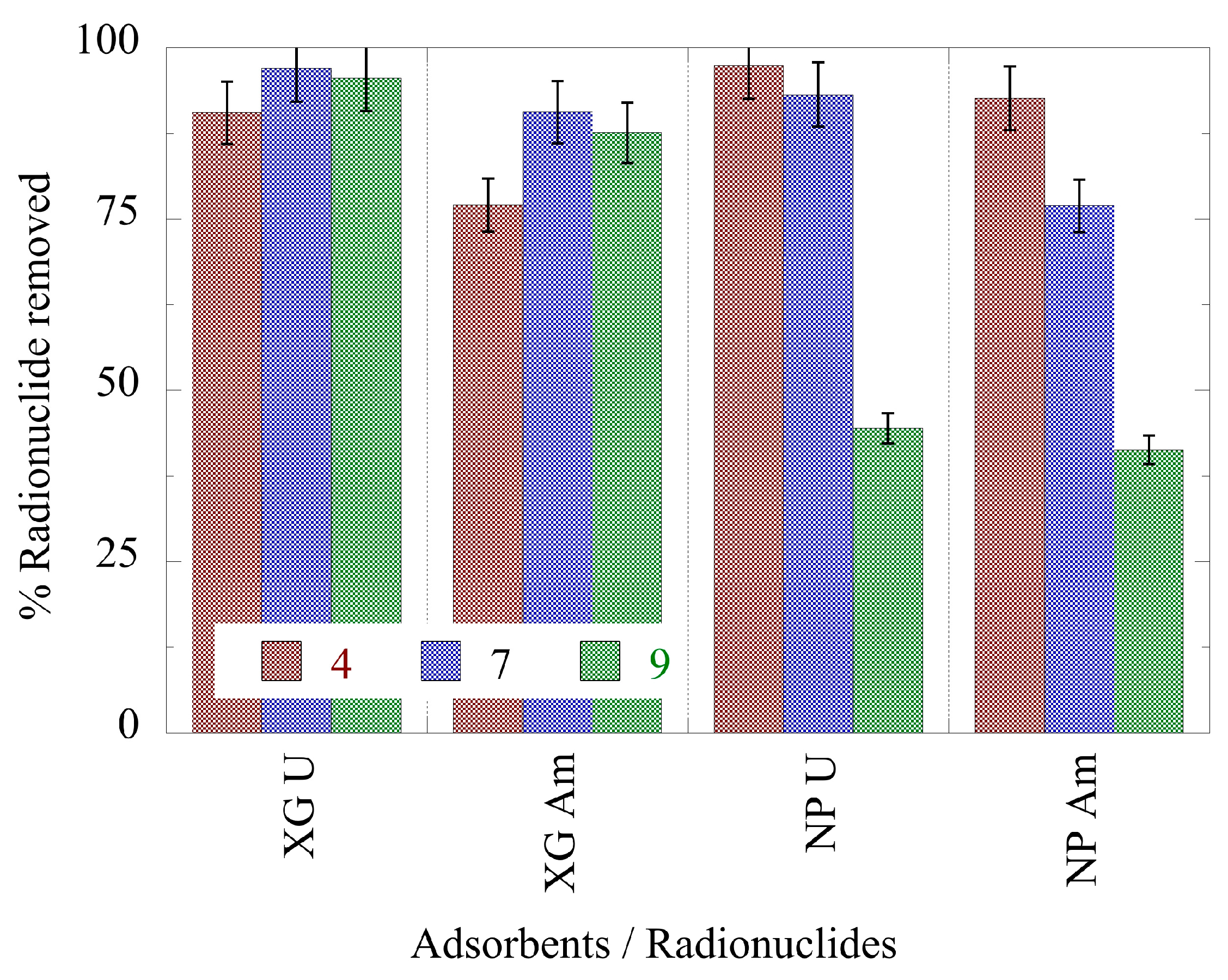
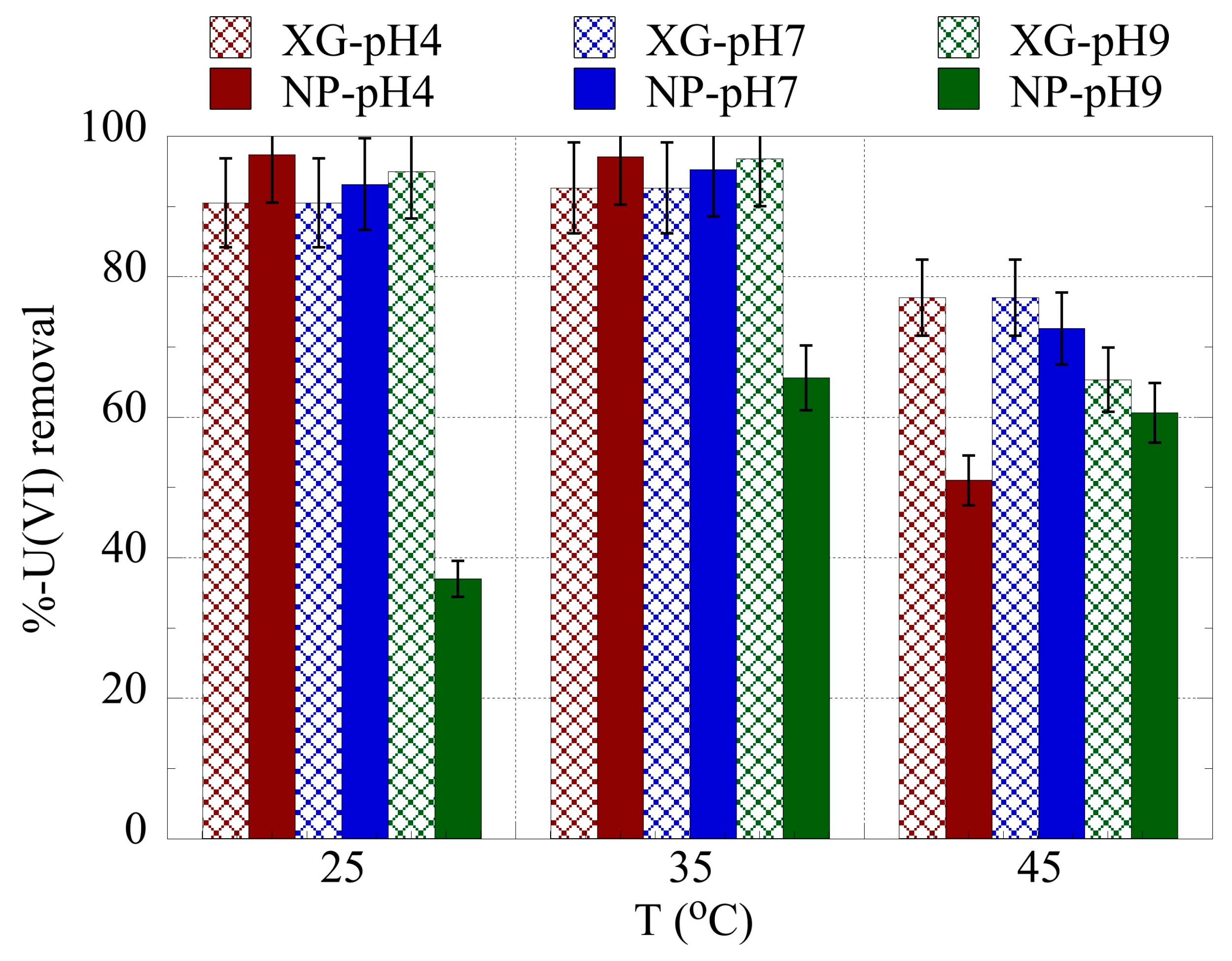
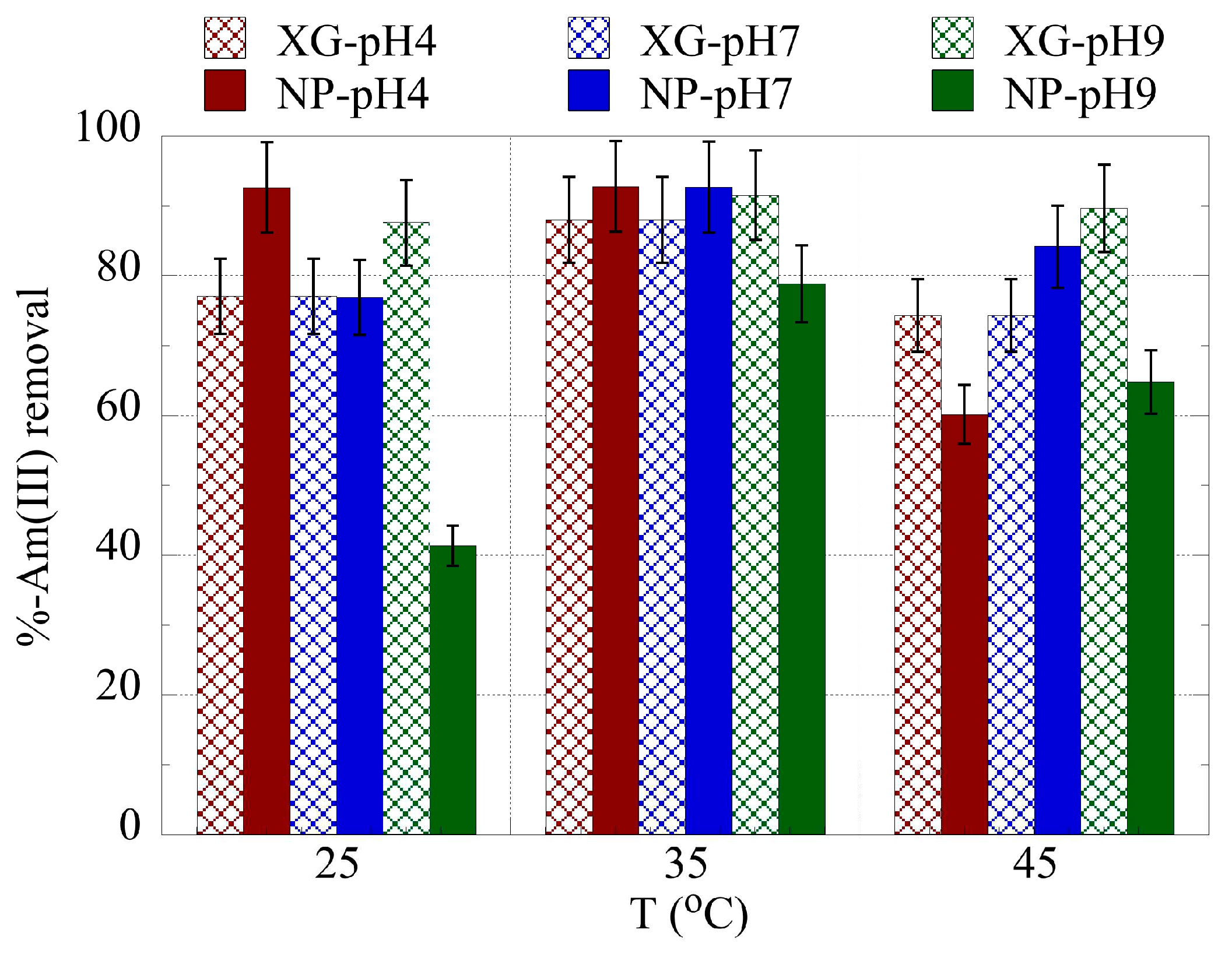
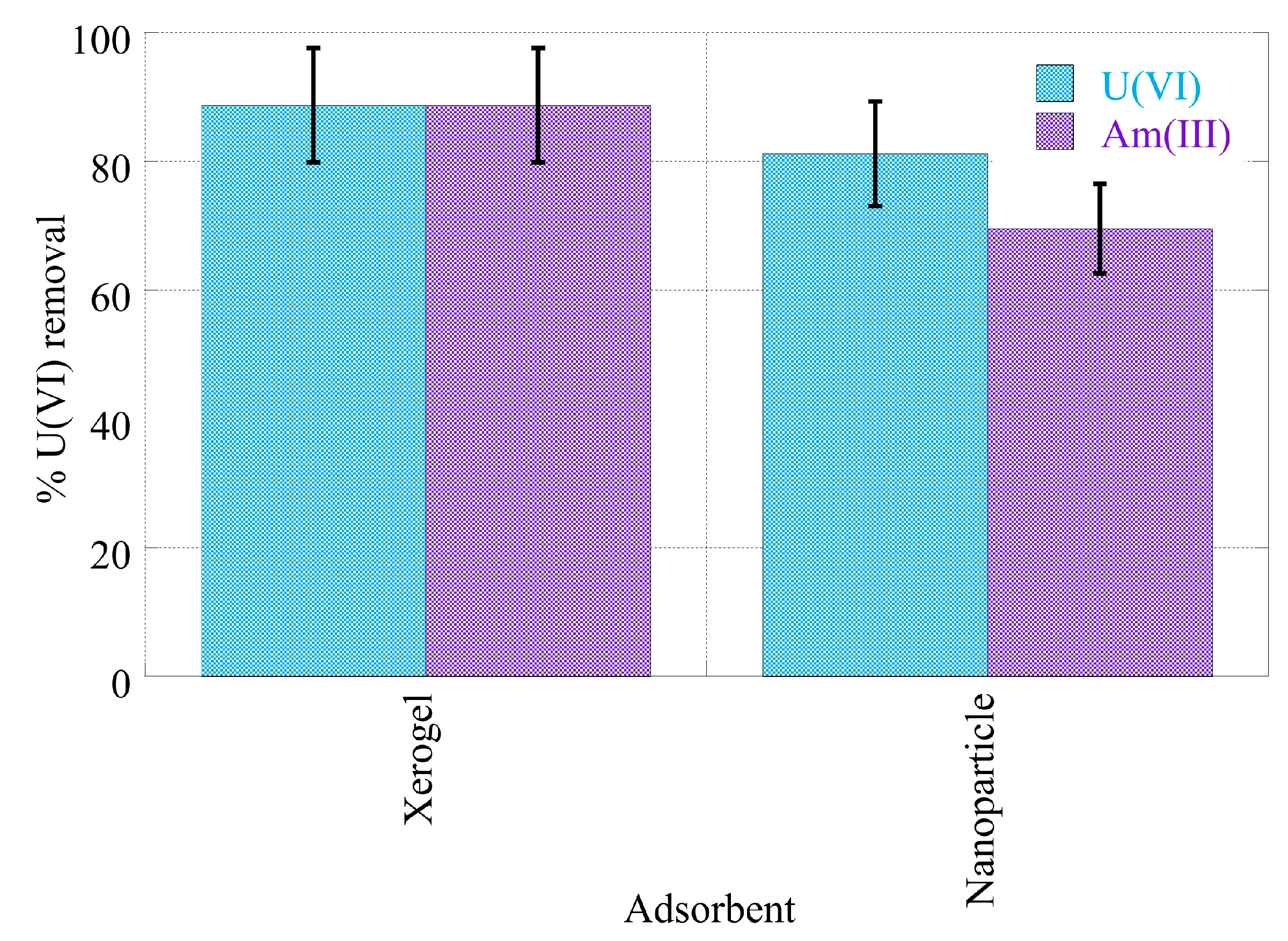
2.3. pH effect on the actinide binding efficiency
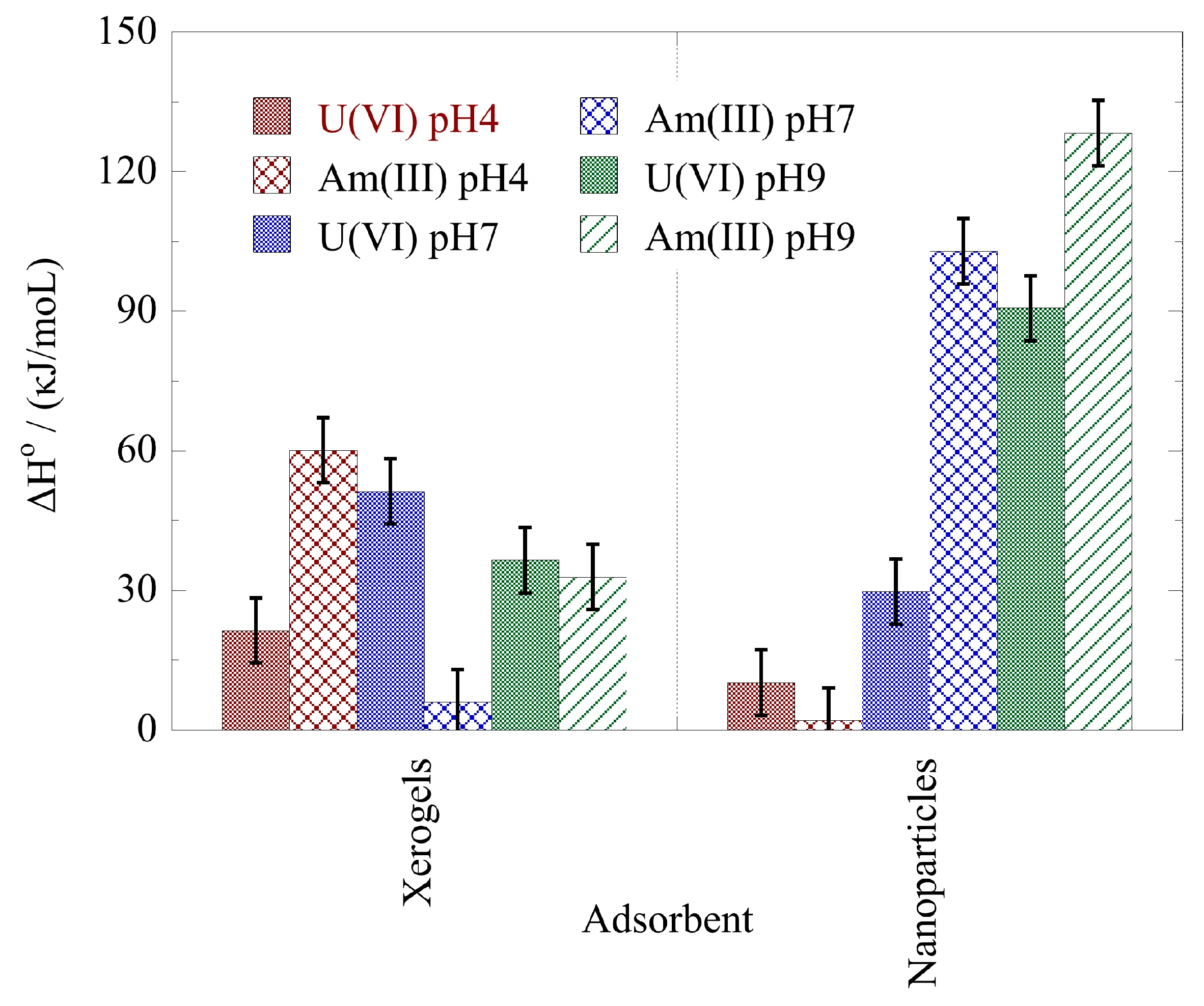
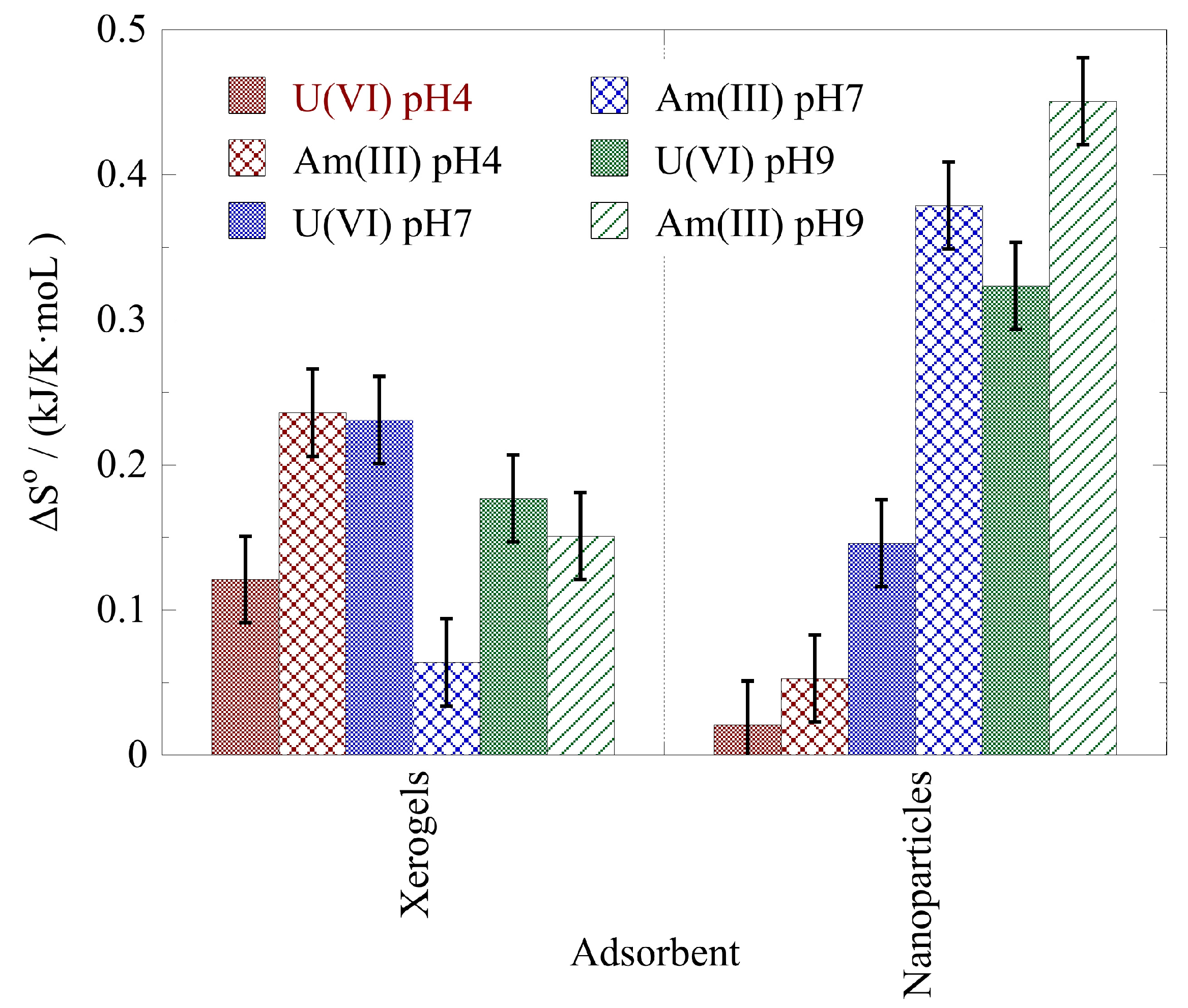
2.4. Adsorption Thermodynamics
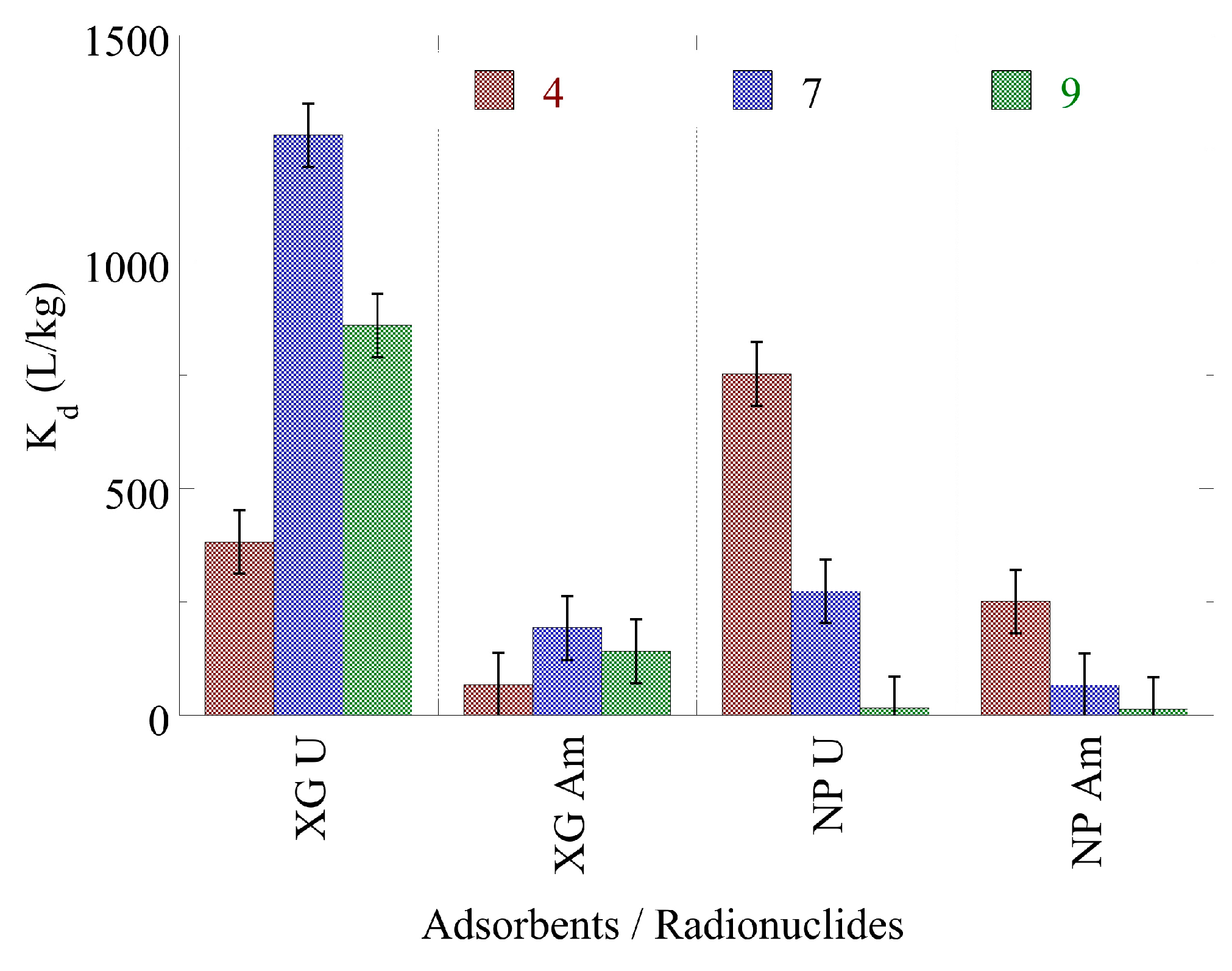
2.5. Radionuclide Interaction in Seawater
3. Conclusions
- Hybrid silica-hyperbranched poly(ethylene imine) nanoparticles and xerogels present relatively high removal efficiency at pH 4 and pH 7 (> 70%) for Am(III) and U(VI).
- Generally, the adsorption process is relatively slow due to very low radionuclide concentrations and is governed by the actinide diffusion from the bulk solution to the composite surface.
- The actinide binding by the NP and XG composites is favored by increasing temperature indicating an endothermic and entropy-driven binding reaction.
- Compared to other adsorbents, which have been investigated regarding the removal of the studied actinide ions, both composites show far higher removal efficiency from laboratory and seawater samples, which is for xerogels almost 90% and for nanoparticles about 80% for uranium and 70% for americium.
- The simple derivatization of NP and XG to increase the selectivity towards specific actinides and other metal ions along with their easy implementation in water treatment technologies, could make these materials attractive candidates for the decontamination of actinide-contaminated waters, including seawaters.
4. Experimental section
4.1. Synthesis of the Composite Silica-PEI 750,000 Nanoparticles
4.2. Synthesis of the Silica-PEI 750,000 Xerogels
4.3. Adsorption Experiments
Author Contributions
Funding
Acknowledgments
References
- Choppin, G.R.; Jensen, M.P. Actinides in Solution: Complexation and Kinetics. In The Chemistry of the Actinide and Transactinide Elements; Morss, L.R., Edel-stein, N.M., Fuger, J., Eds.; Springer: Dordrecht, 2008. [Google Scholar] [CrossRef]
- Seaborg, G.T. Uranium. In The Encyclopedia of the Chemical Elements; Reinhold Book Corporation: Skokie, Illinois, 1968; pp. 773–786. [Google Scholar]
- Pashalidis, I.; Czerwinski, K.R.; Fanghänel, T.; Kim, J.I. Solid-Liquid Phase Equilibria of Pu(VI) and U(VI) in Aqueous Carbonate Systems. Determination of Stability Constants. Radiochim. Acta 1997, 76, 55–62. [Google Scholar] [CrossRef]
- Ioannidis, I.; Xenofontos, A.; Anastopoulos, I.; Pashalidis, I. Americium Sorption by Microplastics in Aqueous Solutions. Coatings. 2022, 12, 1452. [Google Scholar] [CrossRef]
- Runde, W.; Meinrath, G.; Kim, J.I. A study of solid-liquid phase equilibria of trivalent lanthanide and actinide ions in carbonate systems. Radiochim. Acta 1992, 58–59, 93–100. [Google Scholar] [CrossRef]
- Ioannidis, I.; Kinigopoulou, V.; Giannakoudakis, D.A.; Arkas, M.; Anastopoulos, I.; Triantafyllidis, K.S.; Pashalidis, I. Microplastics and disposable face masks as “Trojan Horse” for radionuclides pollution in water bodies–A review with emphasis on the involved interactions. Sustainable Chemistry for the Environment. 2023, 100005. [Google Scholar] [CrossRef]
- Ioannidis, I.; Pashalidis, I.; Raptopoulos, G.; Paraskevopoulou, P. Radioactivity/Radionuclide (U-232 and Am-241) Removal from Waters by Polyurea-Crosslinked Alginate Aerogels in the Sub-Picomolar Concentration Range. Gels. 2023, 9, 211. [Google Scholar] [CrossRef]
- Philippou, K.; Savva, I.; Pashalidis, I. Uranium(VI) Binding by Pine Needles Prior and after Chemical Modification. J. Radioanal. Nucl. Chem. 2018, 318, 2205–2211. [Google Scholar] [CrossRef]
- Liatsou, I.; Michail, G.; Demetriou, M.; Pashalidis, I. Uranium Binding by Biochar Fibres Derived from Luffa Cylindrica after Controlled Surface Oxidation. J. Radioanal. Nucl. Chem. 2017, 311, 871–875. [Google Scholar] [CrossRef]
- Hadjittofi, L.; Pashalidis, I. Uranium Sorption from Aqueous Solutions by Activated Biochar Fibres Investigated by FTIR Spectroscopy and Batch Experiments. J. Radioanal. Nucl. Chem. 2015, 304, 897–904. [Google Scholar] [CrossRef]
- Stasi, C.; Georgiou, E.; Ioannidis, I.; Pashalidis, I. Uranium Removal from Laboratory and Environmental Waters by Oxidised Biochar Prepared from Palm Tree Fibres. J. Radioanal. Nucl. Chem. 2022, 331, 375–381. [Google Scholar] [CrossRef]
- Bhalara, P.D.; Punetha, D.; Balasubramanian, K.A. Review of Potential Remediation Techniques for Uranium(VI) Ion Retrieval from Contaminated Aqueous Environment. J. Environ. Chem. Eng. 2014, 2, 1621–1634. [Google Scholar] [CrossRef]
- Ioannidis, I.; Anastopoulos, I.; Giannakopoulos, K.; Arkas, M.; Dosche, C.; Pashalidis, I. A comprehensive investigation on the sorption of U (VI) and Eu (III) by polyamide microplastics: Surface-assisted microparticle formation. J. Mol. Liquids 2022, 368, 120757. [Google Scholar] [CrossRef]
- Yin, J.; Yang, S.; He, W.; Zhao, T.; Li, C.; Hua, D. Biogene-Derived Aerogels for Simultaneously Selective Adsorption of Uranium(VI) and Strontium(II) by Co-Imprinting Method. Sep. Purif. Technol. 2021, 271, 118849. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Milojković, J.V.; Tsigkou, K.; Zafiri, C.; Lopičić, Z.R.; Kornaros, M.; Pashalidis, I. A Nappies Management By-Product for the Treatment of Uranium-Contaminated Waters. J. Hazard. Mater. 2021, 404, 124147. [Google Scholar] [CrossRef] [PubMed]
- Liatsou, I.; Savva, I.; Vasile, E.; Vekas, L.; Marinica, O.; Mpekris, F.; Pashalidis, I.; Krasia-Christoforou, T. Magnetoresponsive Polymer Networks as Adsorbents for the Removal of U(VI) Ions from Aqueous Media. Eur. Polym. J. 2017, 97, 138–146. [Google Scholar] [CrossRef]
- Paschalidou, P.; Liatsou, I.; Pashalidis, I.; et al. Effect of surface and textural characteristics on uranium adsorption by nanoporous titania. J. Radioanal. Nucl. Chem. 2017, 314, 1141–1147. [Google Scholar] [CrossRef]
- Konstantinou, M.; Demetriou, A.; Pashalidis, I. Adsorption of hexavalent uranium on dunite. Global NEST Journal 2007, 9, 229–236. [Google Scholar] [CrossRef]
- Guo, H.; Mei, P.; Xiao, J.; Huang, X.; Ishag, A.; Sun, Y. Carbon Materials for Extraction of Uranium from Seawater. Chemosphere 2021, 278, 130411. [Google Scholar] [CrossRef]
- Panagiotou, N.; Liatsou, I.; Pournara, A.; Angeli, G.K.; Giappa, R.M.; Tylianakis, E.; Manos, M.J.; Froudakis, G.E.; Trikalitis, P.N.; Pashalidis, I.; et al. Water-Stable 2-D Zr MOFs with Exceptional UO22+ Sorption Capability. J. Mater. Chem. A. 2020, 8, 1849–1857. [Google Scholar] [CrossRef]
- Liu, H.; Fu, T.; Mao, Y. Metal–Organic Framework-Based Materials for Adsorption and Detection of Uranium(VI) from Aqueous Solution. ACS Omega 2022, 7, 14430–14456. [Google Scholar] [CrossRef]
- Koppula, S.; Manabolu Surya, S.; Katari, N.K.; Dhami, P.S.; Sivasankaran Nair, R.K. Mesoporous MOF Composite for Efficient Removal of Uranium, Methyl Orange, Methylene Blue, and Congo Red Dyes from Aqueous Solutions. Appl. Organomet. Chem. 2022, 36, e6554. [Google Scholar] [CrossRef]
- Li, N.; Yang, L.; Wang, D.; Tang, C.; Deng, W.; Wang, Z. High-Capacity Amidoxime-Functionalized β-Cyclodextrin/Graphene Aerogel for Selective Uranium Capture. Environ. Sci. Technol. 2021, 55, 9181–9188. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhang, L.; Chen, F.; Wang, H.; Wang, Q.; Liang, K. Efficiency and Mechanism of Adsorption of Low-Concentration Uranium from Water by a New Chitosan/Aluminum Sludge Composite Aerogel. Dalton Trans. 2020, 49, 3209–3221. [Google Scholar] [CrossRef] [PubMed]
- Georgiou, E.; Raptopoulos, G.; Papastergiou, M.; Paraskevopoulou, P.; Pashalidis, I. Extremely Efficient Uranium Removal from Aqueous Environments with Polyurea-Cross-Linked Alginate Aerogel Beads. ACS Applied Polymer Materials. 2022, 4, 920–928. [Google Scholar] [CrossRef]
- Georgiou, E.; Raptopoulos, G.; Anastopoulos, I.; Giannakoudakis, D.A.; Arkas, M.; Paraskevopoulou, P.; Pashalidis, I. Uranium Removal from Aqueous Solutions by Aerogel-Based Adsorbents-A Critical Review. Nanomaterials. 2023, 13, 363. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, K.; Hadjiyiannis, P.; Liatsou, I.; Pashalidis, I. U(VI) Adsorption by Biochar Fiber-MnO2 Composites. J. Radioanal. Nucl. Chem. 2019, 320, 425–432. [Google Scholar] [CrossRef]
- Philippou, K.; Anastopoulos, I.; Dosche, C.; Pashalidis, I. Synthesis and Characterization of a Novel Fe3O4-Loaded Oxidized Biochar from Pine Needles and Its Application for Uranium Removal. Kinetic, Thermodynamic, and Mechanistic Analysis. J. Environ. Manag. 2019, 252, 109677. [Google Scholar] [CrossRef]
- Philippou, K.; Christou, C.N.; Socoliuc, V.; Vekas, L.; TanasΡÉ, E.; Miclau, M.; Pashalidis, I.; Krasia-Christoforou, T. Superparamagnetic Polyvinylpyrrolidone/Chitosan/Fe3O4 Electrospun Nanofibers as Effective U(VI) Adsorbents. J. Appl. Polym. Sci. 2021, 138, 50212. [Google Scholar] [CrossRef]
- Guo, D.; Song, X.; Zhang, L.; Chen, W.; Chu, D.; Tan, L. Recovery of Uranium (VI) from Aqueous Solutions by the Polyethyleneimine-Functionalized Reduced Graphene Oxide/Molybdenum Disulfide Composition Aerogels. J. Taiwan Inst. Chem. Eng. 2020, 106, 198–205. [Google Scholar] [CrossRef]
- Huang, Z.; Li, Z.; Zheng, L.; Zhou, L.; Chai, Z.; Wang, X.; Shi, W. Interaction Mechanism of Uranium(VI) with Three-Dimensional Graphene Oxide-Chitosan Composite: Insights from Batch Experiments, IR, XPS, and EXAFS Spectroscopy. Chem. Eng. J. 2017, 328, 1066–1074. [Google Scholar] [CrossRef]
- Buhleier, E.; Wehner, W.; Vogtle, F. "Cascade"- and "nonskid-chain-like" syntheses of molecular cavity topologies. Synthesis. 1978, 155–158. [Google Scholar] [CrossRef]
- de Gennes, P.G.; Hervet, H. Statistics of "starburst" polymers. J. Phys. Lett. 1983, 44, 351–360. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Fréchet, J.M. Discovery of dendrimers and dendritic polymers: A brief historical perspective. J. Polym. Sci. Part A: Polym. Chem. 2002, 40, 2719–2728. [Google Scholar] [CrossRef]
- Douloudi, M.; Nikoli, E.; Katsika, T.; Arkas, M. Dendritic polymers for water resources remediation. In Novel Materials for Environmental Remediation Applications; Elsevier: 2023; pp. 435-490. [CrossRef]
- Arkas, M.; Tsiourvas, D.; Paleos, C.M. Functional dendritic polymers for the development of hybrid materials for water purification. Macromol. Mater. Eng. 2010, 295, 883–898. [Google Scholar] [CrossRef]
- Vöegtle, F.; Gestermann, S.; Hesse, R.; Schwierz, H.; Windisch, B. Functional dendrimers. Prog. Polym. Sci. 2000, 25, 987–1041. [Google Scholar] [CrossRef]
- Caminade, A.M.; Turrin, C.O.; Laurent, R.; Ouali, A.; Delavaux-Nicot, B. (Eds.) Dendrimers: Towards Catalytic, Material, and Biomedical Uses; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Arkas, M.; Eleades, L.; Paleos, C.M.; Tsiourvas, D. Alkylated hyperbranched polymers as molecular nanosponges for the purification of water from polycyclic aromatic hydrocarbons. J. Appl. Polym. Sci. 2005, 97, 2299–2305. [Google Scholar] [CrossRef]
- Arkas, M.; Anastopoulos, I.; Giannakoudakis, D.A.; Pashalidis, I.; Katsika, T.; Nikoli, E.; Panagiotopoulos, R.; Fotopoulou, A.; Vardavoulias, M.; Douloudi, M. Catalytic Neutralization of water pollutants mediated by dendritic polymers. Nanomaterials 2022, 12. [Google Scholar] [CrossRef]
- Arkas, M.; Panagiotaki, K.; Kitsou, I.; Petrakli, F. Dendritic Polymer—Enhanced Ultrafiltration. In Nanoscale Materials in Water Purification; Elsevier: 2019; pp. 111-152. [CrossRef]
- Ilaiyaraja, P.; Deb, A.S.; Ponraju, D.; Ali, S.M.; Venkatraman, B. Surface Engineering of PA-MAM-SDB Chelating Resin with Diglycolamic Acid (DGA) Functional Group for Efficient Sorption of U(VI) and Th(IV) from Aqueous Medium. J. Hazard. Mater. 2017, 328, 1–11. [Google Scholar] [CrossRef]
- Ilaiyaraja, P.; Deb, A.K.S.; Sivasubramanian, K.; Ponraju, D.; Venkatraman, B. Adsorption of Uranium from Aqueous Solution by PAMAM Dendron Functionalized Styrene Divinylbenzene. J. Hazard. Mater. 2013, 250, 155–166. [Google Scholar] [CrossRef]
- Ilaiyaraja, P.; Deb, A.K.S.; Ponraju, D.; Venkatraman, B. Xanthate Functionalized PAMAM Dendrimer (XFPD) Chelating Ligand for Treatment of Radioactive Liquid Wastes. J. Environ. Chem. Eng. 2015, 3, 1047–1054. [Google Scholar] [CrossRef]
- Priyadarshini, N.; Ilaiyaraja, P. Adsorption of U(VI) and Th(IV) from Simulated Nuclear Waste Using PAMAM and DGA Functionalized PAMAM Dendron Grafted Styrene Divinylbenzene Chelating Resins. Chem. Pap. 2019, 73, 2879–2884. [Google Scholar] [CrossRef]
- Ardoin, N.; Astruc, D. Molecular trees: From syntheses towards applications. Bull. Soc. Chim. Fr. 1995, 9, 875–909. [Google Scholar]
- Bosman, D.A.; Janssen, H.M.; Meijer, E.W. About dendrimers: Structure, physical properties, applications. Chem. Rev. 1999, 99, 1665–1688. [Google Scholar] [CrossRef] [PubMed]
- Dvornic, P.R.; Tomalia, D.A. November. Starburst® Dendrimers: A Conceptual Approach to Nanoscopic Chemistry and Architecture. In Macromolecular Symposia; Springer: Heidelberg/Berlin, Germany, 1994, 88, 123–148. [Google Scholar] [CrossRef]
- Tully, D.C.; Fréchet, J.M. Dendrimers at Surfaces and Interfaces: Chemistry and Applications. Chem. Commun. 2001, 14, 1229–1239. [Google Scholar] [CrossRef]
- Zeng, F.; Zimmerman, S.C. Dendrimers in Supramolecular Chemistry: From Molecular Recognition to Self-Assembly. Chem. Rev. 1997, 97, 1681–1712. [Google Scholar] [CrossRef]
- Jikei, M.; Kakimoto, M.A. Hyperbranched Polymers: A Promising New Class of Materials. Prog. Polym. Sci. 2001, 26, 1233–1285. [Google Scholar] [CrossRef]
- Kim, Y.H. Hyperbranched Polymers 10 Years After. J. Polym. Sci. Part A Polym. Chem. 1998, 36, 1685–1698. [Google Scholar] [CrossRef]
- Malmström, E.; Hult, A. Hyperbranched Polymers. J. Macromol. Sci. Part C Polym. Rev. 1997, 37, 555–579. [Google Scholar] [CrossRef]
- Sunder, A.; Heinemann, J.; Frey, H. Controlling the Growth of Polymer Trees: Concepts and Perspectives for Hyperbranched Polymers. Chem. A Eur. J. 2000, 6, 2499–2506. [Google Scholar] [CrossRef]
- Voit, B.I. Hyperbranched Polymers: A Chance and a Challenge. Comptes Rendus Chim. 2003, 6, 821–832. [Google Scholar] [CrossRef]
- Yates, C.R.; Hayes, W. Synthesis and Applications of Hyperbranched Polymers. Eur. Polym. J. 2004, 40, 1257–1281. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, S.; Weng, Z.; Gao, C. Hyperbranched Polymers: Advances from Synthesis to Applications. Chem. Soc. Rev. 2015, 44, 4091–4130. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Sun, J.; Zhu, H.; Wu, H.; Zhang, H.; Gu, Z.; Luo, K. Recent Advances in Development of Dendritic Polymer-Based Nanomedicines for Cancer Diagnosis. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2021, 13, 1670. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Mou, Q.; Wang, D.; Zhu, X.; Yan, D. Dendritic Polymers for Theranostics. Theranostics 2016, 6, 930. [Google Scholar] [CrossRef]
- Korake, S.; Shaikh, A.; Salve, R.; Gajbhiye, K.R.; Gajbhiye, V.; Pawar, A. Biodegradable Dendritic Boltorn™ Nano-constructs: A Promising Avenue for Cancer Theranostics. Int. J. Pharm. 2021, 594, 120177. [Google Scholar] [CrossRef]
- German, N.; Popov, A.; Ramanavicius, A.; Ramanaviciene, A. Development and Practical Application of Glucose Biosensor Based on Dendritic Gold Nanostructures Modified by Conducting Polymers. Biosensors 2022, 12, 641. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, A.; Armada, M.P.G.; Losada, J.; Villena, C.; Alonso, B.; Casado, C.M. Amperometric Biosensors for NADH Based on Hyperbranched Dendritic Ferrocene Polymers and Pt Nanoparticles. Sens. Actuators B Chem. 2014, 190, 111–119. [Google Scholar] [CrossRef]
- Arkas, M.; Kythreoti, G.; Favvas, E.P.; Giannakopoulos, K.; Mouti, N.; Arvanitopoulou, M.; Athanasiou, A.; Douloudi, M.; Nikoli, E. Vardavoulias, M.; et al. Hydrophilic Antimicrobial Coatings for Medical Leathers from Silica-Dendritic Polymer-Silver Nanoparticle Composite Xerogels. Textiles 2022, 2, 464–485. [Google Scholar] [CrossRef]
- Marcos, M.; Martín-Rapún, R.; Omenat, A.; Serrano, J.L. Highly Congested Liquid Crystal Structures: Dendrimers, Dendrons, Dendronized and Hyperbranched Polymers. Chem. Soc. Rev. 2007, 36, 1889–1901. [Google Scholar] [CrossRef]
- Tsiourvas, D.; Arkas, M. Columnar and Smectic Self-Assembly Deriving from Non-Ionic Amphiphilic Hyperbranched Polyethylene Imine Polymers and Induced by Hydrogen Bonding and Segregation into Polar and Non-Polar Parts. Polymer 2013, 54, 1114–1122. [Google Scholar] [CrossRef]
- Arkas, M.; Kitsou, I.; Gkouma, A.; Papageorgiou, M. The Role of Hydrogen Bonds in the Mesomorphic Behaviour of Supramolecular Assemblies Organized in Dendritic Architectures. Liq. Cryst. Rev. 2019, 7, 60–105. [Google Scholar] [CrossRef]
- Paleos, C.M.; Tsiourvas, D.; Sideratou, Z.; Tziveleka, L.A. Drug Delivery Using Multifunctional Dendrimers and Hyperbranched Polymers. Expert Opin. Drug Deliv. 2010, 7, 1387–1398. [Google Scholar] [CrossRef]
- Douloudi, M.; Nikoli, E.; Katsika, T.; Vardavoulias, M.; Arkas, M. Dendritic Polymers as Promising Additives for the Manufacturing of Hybrid Organoceramic Nanocomposites with Ameliorated Properties Suitable for an Extensive Diversity of Applications. Nanomaterials 2020, 11, 19. [Google Scholar] [CrossRef] [PubMed]
- Tsetsekou, A., Arkas, M., Kritikaki, A., Simonetis, S. and Tsiourvas, D., 2008. Optimization of Hybrid Hyperbranched Polymer/Ceramic Filters for the Efficient Absorption of Polyaromatic Hydrocarbons from Water. Journal of Membrane Science, 311(1-2), pp.128-135. [CrossRef]
- Pang, Y.; Zeng, G.; Tang, L.; Zhang, Y.; Liu, Y.; Lei, X.; Li, Z.; Zhang, J.; Xie, G. PEI-Grafted Magnetic Porous Powder for Highly Effective Adsorption of Heavy Metal Ions. Desalination 2011, 281, 278–284. [Google Scholar] [CrossRef]
- Arkas, M.; Tsiourvas, D.; Paleos, C.M. Organosilicon Dendritic Networks in Porous Ceramics for Water Purification. Chem. Mater. 2005, 17, 3439–3444. [Google Scholar] [CrossRef]
- Allabashi, R.; Arkas, M.; Hörmann, G.; Tsiourvas, D. Removal of Some Organic Pollutants in Water Employing Ceramic Membranes Impregnated with Cross-Linked Silylated Dendritic and Cyclodextrin Polymers. Water Research. 2007, 41, 476–486. [Google Scholar] [CrossRef]
- Kroger, N.; Deutzmann, R.; Sumper, M. Polycationic Peptides from Diatom Biosilica that Direct Silica Nanosphere Formation. Science. 1999, 286, 1129–1132. [Google Scholar] [CrossRef]
- Arkas, M.; Tsiourvas, D. Organic/Inorganic Hybrid Nanospheres Based on Hyperbranched Poly(ethylene imine) Encapsulated into Silica for the Sorption of Toxic Metal Ions and Polycyclic Aromatic Hydrocarbons from Water. J. Hazard. Mater. 2009, 170, 35–42. [Google Scholar] [CrossRef]
- Arkas, M.; Giannakopoulos, K.; Favvas, E.P.; Papageorgiou, S.; Theodorakopoulos, G.V.; Giannoulatou, A.; Vardavoulias, M.; Giannakoudakis, D.A.; Triantafyllidis, K.S.; Georgiou, E.; et al. Comparative Study of the U(VI) Adsorption by Hybrid Silica-Hyperbranched Poly(ethylene imine) Nanoparticles and Xerogels. Nanomaterials 2023, 13, 1794. [Google Scholar] [CrossRef]
- Philippou, M.; Pashalidis, I.; Theocharis, C.R. Uranium Isotope (U-232) Removal from Waters by Biochar Fibers: An Adsorption Study in the Sub-Picomolar Concentration Range. Molecules 2022, 27, 6765. [Google Scholar] [CrossRef]
- Philippou, M.; Pashalidis, I.; Kalderis, D. Removal of 241Am from Aqueous Solutions by Adsorption on Sponge Gourd Biochar. Molecules 2023, 28, 2552. [Google Scholar] [CrossRef] [PubMed]
- Konstantinou, M.; Pashalidis, I. Speciation and Spectrophotometric Determination of Uranium in Seawater. Mediterr. Mar. Sci. 2004, 5, 55–60. [Google Scholar] [CrossRef]
- Knecht, M.R.; Wright, D.W. Amine-Terminated Dendrimers as Biomimetic Templates for Silica Nanosphere Formation. Langmuir 2004, 20, 4728–4732. [Google Scholar] [CrossRef] [PubMed]
- Knecht, M.R.; Sewell, S.L.; Wright, D.W. Size Control of Dendrimer-Templated Silica. Langmuir 2005, 21, 2058–2061. [Google Scholar] [CrossRef]
- Jensen, L.K.; Jensen, H.E.; Blirup-Plum, S.A.; Bue, M.; Hanberg, P.; Kvich, L.; Aalbæk, B.; López, Y.; Soto, S.M.; Douloudi, M.; Papageorgiou, M. Coating of Bone Implants with Silica, Hyperbranched Poly(ethyleneimine), and Gentamicin Prevents Development of Osteomyelitis in a Porcine Model. Materialia 2022, 24, 101473. [Google Scholar] [CrossRef]
- Kiliari, T.; Pashalidis, I. Simplified Alpha-Spectroscopic Analysis of Uranium in Natural Waters after Its Separation by Cation-Exchange. Radiat. Meas. 2010, 45, 966–968. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




